Abstract
Clinicians tend to make reductions in glass ionomer power/liquid (P/L) ratios since some materials are difficult to mix and flow into small cavities, grooves or pits. In general, changing the P/L ratio decreases the physical and mechanical properties of conventional glass ionomer cements (GICs) and resin modified glass ionomer cements (RMGICs), but alterations seem to depend on their composition. OBJECTIVE: To determine the influence of P/L ratio on the radiodensity and diametral tensile strength (DTS) of glass ionomer cements. MATERIAL AND METHODS: There were 2 factors under study: P/L ratio (manufacturer's recommended P/L ratio and a 50% reduced P/L ratio), and materials (Vitro Molar, Vitro Fil, Vitro Cem conventional GICs and Vitro Fil LC, Ortho Glass LC RMGICs. Five 1-mm-thick samples of each material-P/L ratio were produced for radiodensity evaluation. Samples were x-ray exposed onto Digora phosphor plate and radiodensity was obtained using the software Digora for Windows 2.5 Rev 0. For DTS, five (4.0x8.0 mm) cylinder samples of each material were tested (0.5 mm/min). Data were subjected to one- and two-way ANOVA (5x2) followed by Tukey's HSD test, or Kruskal-Wallis and Dunn's method. For paired comparisons, t-test or Mann-Whitney test were used (α=0.05). RESULTS: There was a significant interaction (P=0.001) for the studied factors (materials vs. P/L ratio). Reduced P/L ratio resulted in significantly lower DTS for the RMGICs, but radiodensity was affected for all materials (P<0.05). CONCLUSIONS: Reduced P/L ratio affected properties of the tested glass ionomer cements. RMGICs were more susceptible to lower values of DTS, but radiodensity decreased for all materials following P/L ratio reduction.
Tensile strength; Glass ionomer cements; Radiography; Microscopy; electron; scanning
ORIGINAL ARTICLE
Influence of powder/liquid ratio on the radiodensity and diametral tensile strength of glass ionomer cements
Rodrigo Borges FonsecaI; Carolina Assaf BrancoII; Paulo Sérgio QuagliattoIV; Luciano de Souza GonçalvesIII; Carlos José SoaresIV; Hugo Lemes CarloV; Lourenço Correr-SobrinhoVI
IDDS, MS, PhD, Professor at Federal University of Goiás, Dental School, Restorative Dentistry Area, Goiânia, GO, Brazil
IIDDS, MS, PhD student, Ribeirão Preto Dental School, São Paulo University, Ribeirão Preto, SP, Brazil
IIIDDS, MS, PhD student, Department of Dental Materials, Piracicaba Dental School, State University of Campinas, Piracicaba, SP, Brazil
IVDDS, MS, PhD, Professor, Department of Operative Dentistry and Dental Materials, Dental School, Federal University of Uberlândia, Uberlândia, MG, Brazil
VDDS, MS, PhD, Professor, Department of Restorative Dentistry, Dental School, Federal University of Pernambuco, Recife, PE, Brazil
VIDDS, MS, PhD, Professor, Department of Dental Materials, Piracicaba Dental School, State University of Campinas, Piracicaba, SP, Brazil
Corresponding address Corresponding address: Rodrigo B. Fonseca Faculdade de Odontologia - Universidade Federal de Goiás Praça Universitária esquina com 1ª. Avenida, s/n, Setor Universitário Goiânia - GO - Brasil - 74605-220 Phone: +55-62-32096325 - Fax: +55-62-32096054 e-mail: rbfonseca.ufg@gmail.com
ABSTRACT
Clinicians tend to make reductions in glass ionomer power/liquid (P/L) ratios since some materials are difficult to mix and flow into small cavities, grooves or pits. In general, changing the P/L ratio decreases the physical and mechanical properties of conventional glass ionomer cements (GICs) and resin modified glass ionomer cements (RMGICs), but alterations seem to depend on their composition.
OBJECTIVE: To determine the influence of P/L ratio on the radiodensity and diametral tensile strength (DTS) of glass ionomer cements.
MATERIAL AND METHODS: There were 2 factors under study: P/L ratio (manufacturer's recommended P/L ratio and a 50% reduced P/L ratio), and materials (Vitro Molar, Vitro Fil, Vitro Cem conventional GICs and Vitro Fil LC, Ortho Glass LC RMGICs. Five 1-mm-thick samples of each material-P/L ratio were produced for radiodensity evaluation. Samples were x-ray exposed onto Digora phosphor plate and radiodensity was obtained using the software Digora for Windows 2.5 Rev 0. For DTS, five (4.0x8.0 mm) cylinder samples of each material were tested (0.5 mm/min). Data were subjected to one- and two-way ANOVA (5x2) followed by Tukey's HSD test, or Kruskal-Wallis and Dunn's method. For paired comparisons, t-test or Mann-Whitney test were used (α=0.05).
RESULTS: There was a significant interaction (P=0.001) for the studied factors (materials vs. P/L ratio). Reduced P/L ratio resulted in significantly lower DTS for the RMGICs, but radiodensity was affected for all materials (P<0.05).
CONCLUSIONS: Reduced P/L ratio affected properties of the tested glass ionomer cements. RMGICs were more susceptible to lower values of DTS, but radiodensity decreased for all materials following P/L ratio reduction.
Key words: Tensile strength. Glass ionomer cements. Radiography. Microscopy, electron, scanning.
INTRODUCTION
Glass ionomer cements have wide spread clinical indications, being used as bases, liners, luting agents, and temporary and restorative dental materials17. The reasons for such a useful clinical versatility are related to the constant fluoride release6, adhesion to dental tissues and base metals11,12,25, biocompatibility and low coefficient of thermal expansion15,23,25. Resin-modified glass ionomer cements (RMGICs) extended these clinical applications since they present better mechanical properties due to the polymeric nature24, overcoming some shortcomings of conventional GICs, such as surface crazing during dehydration, brittleness and low fracture strength22.
The setting reaction of glass ionomers depends on composition. Conventional GICs set by mans of an acid-base reaction in which a polyacid attacks powder particles, forming a mass with some interstitial spaces. According to Nicholson16 (1998), GICs consist of interpenetrating networks of inorganic and organic components forming a matrix in which particles of unreacted glass are embedded. In addition to this ionic cure, RMGICs have also a resinous polymer-based reaction due to the presence of visible light-polymerizable components14. Both reactions are processed at the same time; however, light polymerization results in a rapid initial set while the acid-base reaction, which proceeds normally from the point of mixing, slows down or becomes virtually inhibited by network formation after irradiation26.
Several aspects have been studied in order to improve the mechanical and physical properties of GICs, such as composition25, viscoelastic properties24, fracture toughness15,24, powder particle size reduction15 radiodensity9,10,20, strength6,11 and powder/liquid (P/L) ratio9. Composition seems to be the most important factor which affects the radiopacity of dental materials9,10. In addition, the material thickness9,10, x-ray beam angulation, methodology21, type of x-ray film, age of developing and fixing solutions4, and alteration in P/L ratio9 can also have an influence. The first radiopaque GICs were cermet-containing or metal reinforced cements, in which metals were responsible by high radiodensity levels16. After that, the addition of radiopaque fillers resulted on sufficiently radiopaque GICs5, which could be detected against a background of enamel and dentin, facilitating the evaluation of many clinical situations.
The diametral tensile strength (DTS) is one of the most common and useful mechanical properties of GICs, being extensively found in many studies18,23-25. Depending on the clinical application, GICs should be strong enough to resist stresses generated within it when loaded with occlusal forces, as it is truth for luting materials11. The type of material, filler size and proportion, addition of metals, composition and P/L ratio are factors which affect strength of GICs7,11,15,23-25. Increased P/L ratios tend to increase DTS of GICs11. Mechanically, RMGICs generally exhibit substantial plastic deformation in compression and conventional GICs display brittle fracture24. Clinicians tend to make reductions in glass ionomer P/L ratios since some materials are difficult to mix13 and flow into small cavities, grooves or pits2. In general, changing the P/L ratio decreases the physical and mechanical properties of conventional GICs and RMGICs depending on the reduction of powder per volume15.
There is the chance of altering the DTS and radiodensity of GICs due to reduced P/L ratios, which could virtually lessen the longevity of clinical procedures. Then, the aim of this study was to determine the influence of a 50% reduction in P/L ratio on the DTS and radiodensity of different conventional GICs and RMGICs.
MATERIAL AND METHODS
Five GICs (DFL, Rio de Janeiro, Brazil) with different clinical indications were employed in this study. Material applications, commercial names, batch numbers and composition are listed in Figure 1. Two tests were performed in order to analyze the effect of reducing the P/L ratio of different GICs: Radiodensity and DTS. Groups were formed according to 2 experimental factors under study: materials, with 5 levels (Figure 1) and P/L ratio, with 2 levels, the manufacturer's recommended P/L ratio and a 50% reduced P/L ratio. Samples were fabricated at room temperature according to each experimental method, as described below. Chemical cured materials were allowed to set during the period recommended by each manufacturer. Light-cured materials were photoactivated for 40 s with a halogen curing unit (XL3000; 3M ESPE, St. Paul, MN, USA) at 850 mW/cm2.
Radiodensity Test
Five 1-mm-thick ring-shaped standard samples were fabricated for each experimental group by mixing the materials according to the manufacturers' instructions and inserting them in a 1-mm-thick stainless steel mold with diameter of 4.0 mm. After removal of the samples from the mold, the thickness was checked with a digital caliper in order to fit 1.0 mm (±0.1 mm). An aluminum stepwedge, ranging from 1.0 mm to 9.0 mm in thickness, served as a control.
The samples were positioned onto a phosphor plate and radiographic exposure was performed for 0.2 s at 70 kV and 10 mA, with a source-to-sample distance of 40 cm using an x-ray machine (GE 1000; General Electric, Milwaukee, WI, USA). Three exposures were done for each sample. The radiographs were transferred from the phosphor plate to the computer via a Digora scanner (Digora Optime, Soredex, Helsinki, Finland).
The radiodensity (in pixels) of the samples was determined with the resident software provided by the manufacturer. The Digora system has a windows-based software, Digora for Windows 2.5 Rev 0 (Soredex, Helsinki, Finland), which is capable to measure density curves of digital radiographies obtained by x-ray impregnation on the image phosphor plate. The radiodensity of each radiographed structure or material was obtained by clicking with the software cursor right above the digital image. Each digital image had it radiodensity measured immediately after scanning, without any modification in contrast or brightness. This software shows data concerning the highest and the lowest radiodensity of the sample, and an average value, which was considered to be the sample's initial radiodensity. Since each sample was subjected to three exposures the sample's final radiodensity was considered to be the mean of those values.
DTS Test
Five cylindrical specimens of each experimental group (n=5) were prepared for the DTS test according to ADA specification no. 27. A 4.0 mm long and 8.0 mm diameter aluminum mold was used. Three minutes after filling the molds with mixed cements, samples were kept in 100% humidity and 37ºC for 1 h, followed by gentle removal from the mold and immersion in distilled water for 23 h prior to testing. A compressive load was applied on the diametral surface of the samples to obtain the DTS at a crosshead speed of 0.5 mm/min in a universal testing machine (Instron 4411; Instron Testing Instruments, Canton, MA, USA).
Scanning Electron Microscopy Analysis (SEM)
For observations of materials characteristics after complete setting, SEM analysis was accomplished in samples from Ortho Glass LC and Vitro Cem experimental groups. Fractured samples were sputter-coated with gold (MED 010; Balzers Union, Balzers, Liechtenstein) and observed with a scanning electron microscope (DSM 940A; Zeiss, Oberkoshen, Germany).
Statistical Analysis
Statistical analysis was performed in accordance with the results of Shapiro-Wilk test of normal distribution, with parametric or non-parametric tests, for each variable. One-way ANOVA followed by Tukey's Honestly Significant Difference (HSD) test was employed for DTS and the Kruskal-Wallis and Dunn's method for radiodensity, using the SPSS 12.0 for Windows statistical software (SPSS Inc., Chicago, IL, USA). Two-way ANOVA (5×2) followed by Tukey's HSD test, with a general linear model procedure, was also used to analyze the interaction between materials and P/L ratio. For paired comparisons, independent samples t-test was used for DTS and Mann-Whitney Test for Radiodensity. The aluminum step was compared to each group by ANOVA and Dunnett's 2-sided test. For all tests, groups were considered statistically different at ±=09.05.
RESULTS
Tables 1 and 2 show the results of DTS test (in MPa) and radiodensity measurements (in pixels), respectively, together with the statistical analysis. Radiodensity means and standard deviations are presented only to facilitate the understanding. Since data were not normally distributed, the sum of the ranks (non-parametric analysis) is also provided. There was a significant interaction (P=0.001) for the factors under study (materials vs. P/L ratio). Thus, a separate analysis was accomplished for the interactions.
DTS analysis for both levels of P/L ratios showed that both RMGICs presented similarly higher values than GICs (P<0.05; Table 1). Radiodensity analysis for manufacturer's recommended P/L groups showed that two GICs (Vitro Molar and Vitro Fil) were more radiopaque than the other three. For the 50% reduced P/L groups, Vitro Fil and Vitro Molar were again the most radiopaque materials, but the last was similar to Vitro Cem, and this one was similar to the RMGICs (Table 2).
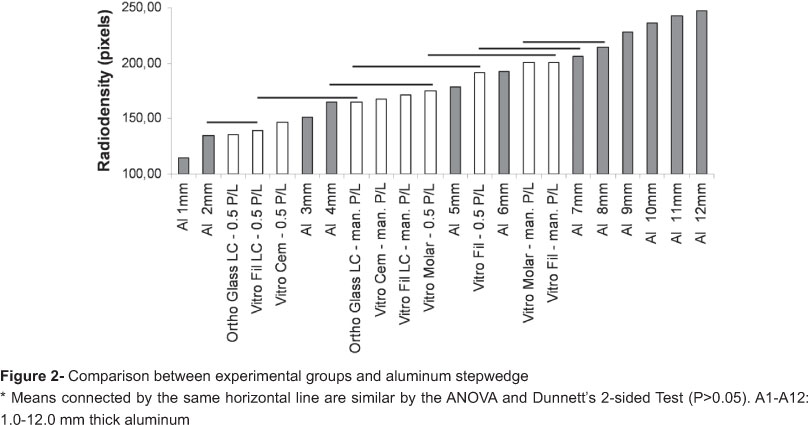
Within each material, pairwise DTS analysis by t-test showed that reducing the P/L ratio resulted in a significant reduction in strength just for Ortho Glass LC and Vitro Fil LC, with 27.1% and 46.6% of reduction, respectively. Pairwise radiodensity analysis by the Mann-Whitney Test showed that reducing the P/L ratio resulted in a significant reduction in radiodensity for all materials. Figure 2 presents the comparison between the aluminum stepwedge and experimental groups. Figure 3 shows a radiographic image of the groups and the aluminum stepwedge. During mixing of the cements, it was noted that Vitro Molar and Vitro Fil were difficult to mix at the manufacturer's recommended P/L and the mixed material resulted in a rough mass. At 50% reduced P/L ratio, it was easier to mix these materials and a smooth mass was obtained.
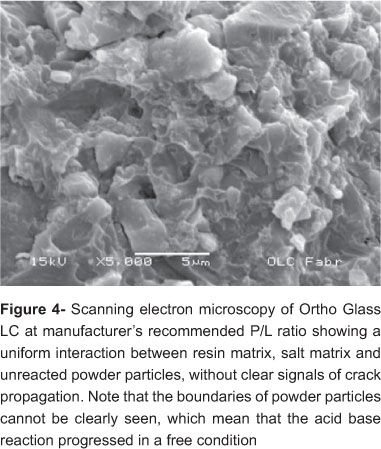
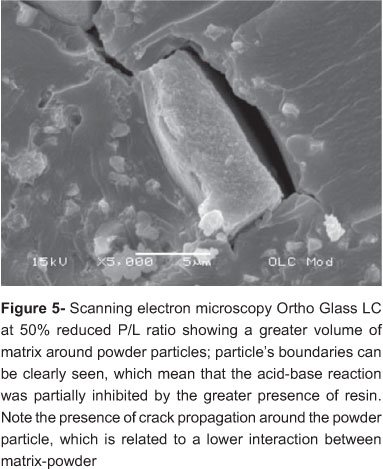
Figures 4 and 5 are SEM images of Ortho Glass LC at the manufacturer's recommended P/L and the 50% reduced P/L ratio, respectively. Figures 6 and 7 of Vitro Cem at the manufacturer's recommended P/L ratio and 50% reduced P/L ratio, respectively. For the RMGICs, reduction in liquid at 50% P/L resulted in poor interaction between matrix and powder particles with clear signs of crack development. For the conventional GICs, reduction in liquid at 50% P/L ratio resulted in a greater presence of salt matrix and its close interaction with powder particles without the development of cracks, which could be seen at the manufacturer's recommended P/L.
DISCUSSION
Several factors can have an influence on conventional GIC and RMGIC properties, but the P/L ratio9,13 is the one that relies on the responsibility of clinicians11. The hypothesis driven on this study was confirmed with the observed alterations in DTS and radiodensity of GICs and RMGICs due to changes in P/L ratio. The assumption that radiodensity depends more on the composition of dental materials than the type of material5 was confirmed and the DTS results are in general agreement with other studies23,24, who reported that the RMGICs exhibited higher DTS than GICs. Composition, setting reactions, materials' maturation and microstructures seemed to be the principal reasons for these observations.
Materials tested with the same thickness mostly vary in radiodensity by the influence of composition5. X-ray beam interactions with the matter are always directly proportional to either the atomic number of the absorber or to its electric density8; then depending on the atomic composition and density of each atom in the matter a radiographic image will be differently influenced. The addition of chemical elements with high atomic numbers such as zinc, strontium, zirconium, barium and lanthanum result in more radiopaque materials5,9,10. RMGICs are not always radiopaque10 and GICs are usually radioluscent9,10.
Vitro Molar and Vitro Fil, which present barium/FeO and strontium/FeO, respectively, in their composition, were the most radiopaque materials, when mixed in the manufacturer's recommended P/L ratio. However, even with the presence of strontium/FeO in the other materials, their radiodensity were similarly lower than the previous mentioned ones, which mean these components are present in relatively lower quantities. With the reduced P/L ratio, Vitro Fil and Vitro Molar still presented the highest radiodensity levels, but Vitro Cem was similar to Vitro Molar and the RMGICs were similar to Vitro Cem. Vitro Molar is a restorative GIC and is more viscous than Vitro Cem. The higher viscosity and the apparent presence of more quantities of radiopaque fillers would render higher radiodensity, but with the reduction in P/L ratio, these differences disappeared. Since organic components do not seem to offer radiopaque characteristics to dental materials5, the observed lower radiodensity for RMGICs at 50% reduced P/L ratio is a direct cause of the increase in liquid per volume. Within each material, the reduction in P/L ratio significantly reduced the radiodensity of all materials in this study (Table 2), and this also may be a direct result of the increase in liquid per volume. Since all materials employed on the present study, irrespective of the P/L ratio being similar or more radiopaque than A2 or A3 aluminum steps (Figure 2), they could eventually be detected against an enamel background (good clinical property)5,9,10.
Generally, chain displacement can occur at sufficiently high stress in deformable polymers, than in brittle polymeric and ceramic materials, which undergo crack propagation at high stress3,19. Mitsuhashi, et al.15 (2003) showed that when the P/L ratio is decreased and the matrix volume increased as a consequence, the characteristics of the resin matrix are significantly emphasized for the RMGICs. It is expected that greater presence of resin in RMGICs, by reduction of P/L ratio, would render a material with more viscoelastic behavior, greater strain capacity and possibly greater resistance to stress development by load application. However, even if a more viscoelastic behavior is expected, the tendency for generating materials which withstand less load application is real because in the cement mixtures, the volume fraction of the matrix, which has weak mechanical strength, increases at lower P/L ratios. Irrespective of the P/L ratio, this study showed that RMGICs' strength was always higher than that of conventional GICs (Table 1), possibly due to the greater expected viscoelastic behavior of RMGICs and the higher cohesive strength of resin matrix versus salt matrix23. On the other hand, DTS was reduced only for RMGICs when 50% reduced P/L ratio was employed (Table 1).
When comparing RMGICs and GICs, Yamazaki, et al.24 (2006) showed they possess similar viscoelastic behavior, irrespective of the polymeric character of RMGICs. Then, it is possible that resin and salt matrixes act similarly, in these materials, with a higher probability of enabling plastic deformation for the former23. However, Mitsuhashi, et al.15 (2003) found that the fracture toughness of the RMGICs is not greatly influenced by the P/L ratio, as it is for GICs. Only at high reductions in P/L ratio, the fracture toughness started to decrease for RMGICs15. With lower fracture toughness materials became more brittle, reducing plastic behavior and also the resistance against crack propagation15. Thus, differently from GICs, a significant alteration in RMGICs properties is only expected with high reduction of powder amount, because the acid-base reaction of the powder particle and liquid is critical for many physical properties of hardened materials15. The SEM analysis showed an integrated microstructure for RMGICs with manufacturer's recommended P/L ratio (Fig 3), but the same was not true for 50% reduced P/L ratio, which showed a greater presence of matrix per volume, unreacted particles and crack development, recognized as a signal of the jeopardized interaction between matrix and powder particles (Figure 5). The high reduction in P/L ratio, as performed in the present study, resulted in significantly lower DTS values just for RMGICs, and not for conventional GICs (Table 1). In spite of that, for all materials, a decrease in DTS occurred with the reduction in P/L ratio. There are two possibilities to explain this result. One is that limitations of the experimental design did not allow differences between manufacturer's recommended P/L ratio and 50% reduced P/L ratio of the materials to be observed. Larger sample sizes may possibly be necessary to demonstrate statistically significant differences. The other possibility is closely related to the setting reactions of each type of material.
For light-cured GICs, the maturation of cure by chemical setting has an important influence on the physical properties of the hardened materials15 because an increase in the overall properties is observed with material's maturation25. More integrated microstructures with better glass particle-polymer matrix bonding, results in higher values of DTS23, which could be seen in manufacturer's recommended P/L ratio (Figure 2). Adusei, et al.1 (2004) showed that the use of silanated glass in polyacid-modified resin composites results in a decrease in flexural strength because not all particles seem to participate of the setting reaction. This leads to a less cohesive matrix, and the same situation is expected on RMGICs, which leads to the conclusion that there is an important role of the salt part of the matrix in the overall strength of these materials1.
It is speculated, from the DTS results of the present study that a high reduction in P/L ratio leads to a more fluid mass, where the completion of the chemical reaction is reduced through the polymerization of the resinous part, as previously stated by Yelamanchili and Darvel26 (2008). Even if the chemical reaction is generally able to keep going after light polymerization14, it seems a greater volume of resin by reduction in P/L ratio, entraps polyacid molecules, and the so important chemical reaction reduces in effectiveness. According to Peutzfeldt, et al.17 (1997) HEMA molecule crosslinking keeps the carboxylate groups of different polyacid chains too far apart to be crosslinked via Ca+2 as will normally happen without resin. Since chemical and light irradiated network formation compete26, but with 50% reduced P/L ratio the chemical reaction is slowed down, strength of final product will be negatively influenced. In addition, the higher proportion of the resinous part does not assure better mechanical properties because light irradiation results in a temperature rise, potentially permitting a higher value of the "auto-limiting" glass-transition temperature, with a consequent negative effect of degree of conversion26. Figure 5 shows an apparent ineffective interaction between particles and matrix as a result of the "HEMA blocking effect".
The assumption that both the salt matrix and the resinous matrix have a determinant relationship in the overall strength of RMGICs1,26 was confirmed in this study, but needs more specific studies to be proved. The reduction in P/L ratio for GICs did not result in significantly lower DTS, which mean that the polyacid is able to react with normally unreacted glass particles and the greater presence of salt matrix does not significantly reduces strength. SEM analysis showed a lower presence of salt matrix in manufacturer's recommended P/L ratio (Figure 6) and clear signs of cracks around unreacted powder particles, which mean a high tendency for brittle characteristics in these ionomers. At 50% reduced P/L ratio (Figure 7) a greater presence of salt matrix can be seen, with smaller particles and no signals of cracks. These observations led to the assumption that even if a better interaction between matrix and powder particles is seen when more liquid is added to the mixture, GICs' DTS can be considered more stable when alterations in P/L ratio are performed, since different morphological structures are formed and can resist differently to load application. However, the reduction in absolute DTS means for Vitro Molar and Vitro Fil should be taken into consideration, meaning that more salt matrix can possibly result in poor mechanical properties with time. Further studies are necessary to prove this hypothesis. The use of the manufacturers' recommended P/L ratio is always advisable since properties will be preserved.
CONCLUSIONS
From the results of this study it is possible to conclude that: 1. Alterations in the P/L ratio should be avoided since a decrease in radiodensity and DTS was seen; 2. RMGICs had a significant decrease in DTS, but all materials were affected in their radiodensity due to alterations in P/L ratio; 3. The DTS of RMGICs seems to be highly influenced by structural organization (organic molecules and inorganic particles), while radiodensity is affected by chemical composition.
ACKNOWLEDGEMENTS
Authors are grateful to DFL for full donation of the materials used in this study, and to CAPES-Brazil (Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior) for Rodrigo B. Fonseca PhD program support.
Received: February 18, 2009
Modification: September 6, 2009
Accepted: October 9, 2009
- 1- Adusei GO, Deb S, Nicholson JW. The role of the ionomer glass component in polyacid-modified composite resin dental restorative materials. J Mater Sci Mater Med. 2004;15(7):751-4.
- 2- Celiberti P, Lussi A. Penetration ability and microleakage of a fissure sealant applied on artificial and natural enamel fissure caries. J Dent. 2007;35(1):59-67.
- 3- Craig R. Restorative dental materials. St. Louis: Elsevier; 2001.
- 4- el-Mowafy OM, Benmergui C. Radiopacity of resin-based inlay luting cements. Oper Dent. 1994;19(1):11-5.
- 5- Fonseca RB, Branco CA, Soares PV, Correr-Sobrinho L, Haiter-Neto F, Fernandes-Neto AJ, et al. Radiodensity of base, liner and luting dental materials. Clin Oral Investig. 2006;10(2):114-8.
- 6- Forsten L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials. 1998;19(6):503-8.
- 7- Gladys S, Van Meerbeek B, Braem M, Lambrechts P, Vanherle G. Comparative physico-mechanical characterization of new hybrid restorative materials with conventional glass-ionomer and resin composite restorative materials. J Dent Res. 1997;76(4):883-94.
- 8- Goaz PW, White SC. Oral radiology. St. Louis: C.V. Mosby; 1987.
- 9- Hara AT, Serra MC, Haiter-Neto F, Rodrigues AL Jr. Radiopacity of esthetic restorative materials compared with human tooth structure. Am J Dent. 2001;14(6):383-6.
- 10- Hara AT, Serra MC, Rodrigues AL Jr. Radiopacity of glass-ionomer/composite resin hybrid materials. Braz Dent J. 2001;12(2):85-9.
- 11- Hibino Y, Kuramochi K, Hoshino T, Moriyama A, Watanabe Y, Nakajima H. Relationship between the strength of glass ionomers and their adhesive strength to metals. Dent Mater. 2002;18(7):552-7.
- 12- Hotz P, McLean JW, Sced I, Wilson AD. The bonding of glass ionomer cements to metal and tooth substrates. Br Dent J. 1977;142(2):41-7.
- 13- Irie M, Tjandrawinata R, Suzuki K, Watts DC. Root-surface gap-formation with RMGIC restorations minimized by reduced P/L ratio of the first increment and delayed polishing. Dent Mater. 2006;22(5):486-97.
- 14- McCabe JF. Resin-modified glass-ionomers. Biomaterials. 1998;19(6):521-7.
- 15- Mitsuhashi A, Hanaoka K, Teranaka T. Fracture toughness of resin-modified glass ionomer restorative materials: effect of powder/liquid ratio and powder particle size reduction on fracture toughness. Dent Mater. 2003;19(8):747-57.
- 16- Nicholson JW. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19(6):485-94.
- 17- Peutzfeldt A, García-Godoy F, Asmussen E. Surface hardness and wear of glass ionomers and compomers. Am J Dent. 1997;10(1):15-7.
- 18- Piwowarczyk A, Ottl P, Lauer HC, Büchler A. Laboratory strength of glass ionomer cement, compomers, and resin composites. J Prosthodont. 2002;11(2):86-91.
- 19- Rosen SL. Fundamental principles of polymeric materials. New York: John Wiley & Sons; 1993.
- 20- Sidhu SK, Shah PM, Chong BS, Pitt Ford TR. Radiopacity of resin-modified glass-ionomer restorative cements. Quintessence Int. 1996;27(9):639-43.
- 21- Turgut MD, Attar N, Onen A. Radiopacity of direct esthetic restorative materials. Oper Dent. 2003;28(5):508-14.
- 22- Uno S, Finger WJ, Fritz U. Long-term mechanical characteristics of resin-modified glass ionomer restorative materials. Dent Mater. 1996;12(1):64-9.
- 23- Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000;16(2):129-38.
- 24- Yamazaki T, Schricker SR, Brantley WA, Culbertson BM, Johnston W. Viscoelastic behavior and fracture toughness of six glass-ionomer cements. J Prosthet Dent. 2006;96(4):266-72.
- 25- Yap AU, Pek YS, Cheang P. Physico-mechanical properties of a fast-set highly viscous GIC restorative. J Oral Rehabil. 2003;30(1):1-8.
- 26- Yelamanchili A, Darvell BW. Network competition in a resin-modified glass-ionomer cement. Dent Mater. 2008;24(8):1065-9.
Corresponding address:
Publication Dates
-
Publication in this collection
14 Jan 2011 -
Date of issue
Dec 2010
History
-
Received
18 Feb 2009 -
Accepted
09 Oct 2009 -
Reviewed
06 Sept 2009