Abstract
OBJECTIVE: The aim of this study was to compare subgingival irrigation with tetracycline hydrochloride (TTC-HCL) as adjunctive treatment to scaling and root planning (SRP) on induced periodontitis in rats. MATERIAL AND METHODS: In 60 rats, periodontal disease was ligature-induced at the mandibular left first molar. After 7 days, the ligature was removed and all animals were submitted to SRP, and divided into 2 groups according to the following treatment: C (n=30) - subgingival irrigation with 1 mL of saline; T (n=30) - subgingival irrigation with 1 mL of TTC-HCL (50 mg/mL). Ten animals in each group were euthanized at 7, 15 and 30 days posttreatment. The histometric values were statistically analyzed (p<0.05). RESULTS: In the histometric analysis, at 7, 15 and 30 days, Group T (0.72±0.05 mm², 0.57±0.14 mm², 0.62±0.07 mm²), showed less bone loss (p<0.05) than Group C (1.35±0.25 mm²; 1.40±0.31 mm²; 1.29±0.27 mm²), respectively. CONCLUSIONS: Subgingival irrigation with TTC-HCL was an effective adjunctive treatment for periodontal disease induced in rats.
Irrigation; Periodontal diseases; Periodontitis; Rats; Tetracycline
ORIGINAL ARTICLES
Experimental periodontal disease treatment by subgingival irrigation with tetracycline hydrochloride in rats
Leandro Araújo FernandesI; Thiago Marchi MartinsI; Juliano Milanezi De AlmeidaI; Maria José Hitomi NagataII; Leticia Helena TheodoroIII; Valdir Gouveia GarciaII; Alvaro Francisco BoscoII
IDDS, MSc, Graduate student, Department of Surgery and Integrated Clinic, Araçatuba Dental School, São Paulo State University, Araçatuba, SP, Brazil
IIDDS, MSc, PhD, Full Professor, Department of Surgery and Integrated Clinic, Araçatuba Dental School, São Paulo State University, Araçatuba, SP, Brazil
IIIPhD, Department of Periodontics, Barretos Dental School, University Center of Educational Foundation of Barretos, Barretos, SP, Brazil
Corresponding address Corresponding address: Leandro Araújo Fernandes Rua Alameda das Azaléias - Número 23 - Bairro Parque das Orquídeas CEP - 37800-000 - Guaxupé, MG - Brasil e-mail: leandroataunesp@ig.com.br
ABSTRACT
OBJECTIVE: The aim of this study was to compare subgingival irrigation with tetracycline hydrochloride (TTC-HCL) as adjunctive treatment to scaling and root planning (SRP) on induced periodontitis in rats.
MATERIAL AND METHODS: In 60 rats, periodontal disease was ligature-induced at the mandibular left first molar. After 7 days, the ligature was removed and all animals were submitted to SRP, and divided into 2 groups according to the following treatment: C (n=30) - subgingival irrigation with 1 mL of saline; T (n=30) - subgingival irrigation with 1 mL of TTC-HCL (50 mg/mL). Ten animals in each group were euthanized at 7, 15 and 30 days posttreatment. The histometric values were statistically analyzed (p<0.05).
RESULTS: In the histometric analysis, at 7, 15 and 30 days, Group T (0.72±0.05 mm2, 0.57±0.14 mm2, 0.62±0.07 mm2), showed less bone loss (p<0.05) than Group C (1.35±0.25 mm2; 1.40±0.31 mm2; 1.29±0.27 mm2), respectively.
CONCLUSIONS: Subgingival irrigation with TTC-HCL was an effective adjunctive treatment for periodontal disease induced in rats.
Key words: Irrigation. Periodontal diseases. Periodontitis. Rats. Tetracycline.
INTRODUCTION
Periodontal disease is an infectious disease that results in destruction and degradation of the periodontal tissues by the local action of periodontopathogenic microorganisms. These microorganisms release substances that strictly injury periodontal tissues, in addition to inducing tissue destruction by inflammatory and immunologic responses of the host31.
The treatment of periodontal disease is based on pathogenic microbiota reduction by scaling and root planning13. However, mechanical therapy used alone can fail to eliminate pathogenic bacteria that are placed into the soft tissue, and also in inaccessible areas to periodontal instruments, such as furcation area and root depression1. Because of these limitations, several studies have demonstrated clinical improvement with the administration of either a systemic or local antimicrobial agent4,15,21,27. Local administration of antimicrobial agents within periodontal pockets has emerged as an adjuvant factor to conventional mechanical therapy, specifically to sites with periodontitis that have not provided remission towards the initial treatment. One of the local administration systems of antimicrobial agents is subgingival irrigation.
A relevant property for an antimicrobial agent to be effective when used by means of local administration is the substantivity27. Chlorhexidine digluconate (CHX) has demonstrated to have substantivity with regard to enamel and oral mucosa20. Nevertheless, current studies have indicated that this substantivity can have a short term, with antimicrobial activity of only 24 h26. Clinical evidences related to subgengival irrigation with CHX have shown the inefficiency of this application5. Pavia, et al.18 (2003), in a literature meta-analysis, reports that clinical studies on subgingival irrigation with TTC-HCL have depicted different results, stating that those divergences can occur due to methodological differences used. Baker, et al.3 (1983) demonstrated that TTC-HCL solution is strongly adsorbed by dental surface, with adsorption of 100 times more when compared to other antimicrobial agents, being subsequently released in its active form. Other studies have addressed the occurrence of antimicrobial release from dentin and enamel impregnated with TTC-HCL4,26. In addition, it has been indicated that tetracycline is adsorbed and subsequently desorbed from dentin, maintaining its antimicrobial activity3,22. These findings have led to widespread use of tetracycline treatment on root surfaces in periodontal therapy. However, TTC-HCL concentration ranges from 0.5% to 200% and application periods vary between 0.5 and 10 min14.
Several studies report the occurrence of limited clinical and antimicrobial effects when subgingival irrigation was carried out as adjuvant therapy to SRP17, whereas other papers depicted relevant clinical results4,18. Stabholz, et al.27 (1993) demonstrated that teeth submitted to subgingival irrigation with TTC held on antimicrobial activity for over 15 days, where tetracycline substantivity and desorption from the root surface was concentration-dependent. In infected sites that did not respond to conventional mechanical therapy, or in areas of recurrent periodontal pockets, the association between slow-releasing antimicrobial agents and SRP may prevent from the need for surgical periodontal therapy6.
TTC-HCL has been one of the most studied antibiotics on local release. In addition to its antimicrobial activity against the pathogenic microflora32, it has also been demonstrated that TTC-HCL inhibits collagenase9 and the osteoclastic function, stimulates osteoblastic bone formation, regulates angiogenesis20, and when associated with fibronectin increases the fibroblast insertion over radicular structure29.
Most of studies found in literature about the local action of tetracycline as adjuvant in periodontitis mechanical treatment are of clinical and/or microbiological nature18. Thus, the objective of the present study was to evaluate histologically and histometrically the effectiveness of TTC-HCL irrigation upon the treatment of induced periodontitis in rats.
MATERIAL AND METHODS
Animals
This study was conducted on 60 adult male Wistar rats (120 to 140 g). The animals were housed in plastic cages with access to food and water ad libitum. Prior to surgical procedures, all animals were allowed to acclimatize to the laboratory environment for a period of 5 days. All protocols described below were approved by the Institutional Review Board of Araçatuba Dental School, São Paulo State University, Araçatuba, SP, Brazil (Protocol #22/07).
Experimental Design (Figure 1)
Protocol of experimental periodontal disease
General anesthesia was obtained by association of ketamine (0.4 mL/kg) and xylazine (0.2 mL/kg) via intramuscular injection. The mandibular left first molar of each animal was selected to receive the cotton ligature in submarginal position in order to induce experimental periodontitis12,23.
Groups
After 7 days, the ligature was removed and all the animals were submitted to scaling and root planing (SRP) with manual mine five curettes 13-14 (Hu-Friedy Co. Inc., Chicago, IL, USA) through 10 distal-mesial strokes and then were randomly allocated by use a computer-generated table to the Groups C (Control) and T (Tetracycline Irrigation). For better standardization, the animal 1 was the first choice, followed by 2 and 3, respectively. Thus, the animals were randomly assigned to one of the two Groups (30 animals/Group): according to the following treatment: Group C (30 animals): - irrigation with 1 mL of saline solution; Group T (30 animals): irrigation with 1 mL of TTC-HCL (Tetraclin® 500 mg, Lab. Teuto Bras. São Paulo, SP, Brazil.) at a concentration of 50 mg/mL. Saline solution and TTC-HCL were deposited into the periodontal pocket slowly (30 s) using a syringe (1 mL) and an insulin needle (13 mm x 0.45 mm) (Becton Dickinson Ind. Ltd, Curitiba, PR, Brazil) without bevel.
TTC-HCL solutions were prepared by dissolving the content of 500 mg TTC-HCL capsules into 10 mL distilled sterile water. Any capsule filler particles were filtered away.
Experimental periods
Ten animals from each group euthanized killed at 7, 15 and 30 days after the treatment of periodontal disease with the administration of a lethal dose of thiopental (150 mg/Kg) (Cristália, Ltd, Itapira, SP, Brazil). Jaws were removed and fixed in 10% neutral formalin for 48 h.
Laboratory procedures
The specimens were demineralized in a solution with equal parts of 50% formic acid and 20% sodium citrate for 15 days. Paraffin serial sections (6 µm) were obtained in mesiodistal direction and stained with hematoxylin and eosin (H&E) or Masson's Trichromic (MT).
Intraexaminer Reproducibility
Before histometric analyses were performed, the examiner was trained by double measurements of 15 specimens, with a one-week interval. Paired t-test statistics was run and no differences were observed in the mean values for comparison (p=0.52).
Histological and Histometric Analysis
Sections dyed by H&E were analyzed under light microscopy to establish the bone loss and characteristics of periodontal ligament in the furcation region of first molars. Collagen fibers were analyzed in sections dyed by MT.
The area of bone loss in the furcation region was histometrically determined using an image analysis system (Image Tool, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA). After excluding the first and last sections where the furcation region was evident, 5 equidistant sections of each specimen block were selected and captured by a digital camera coupled to a light microscope. The area (mm2) of bone loss in the furcation region was determined histometrically16. One blinded, trained examiner selected the sections for the histometric and histological analysis. Another masked, calibrated examiner conducted the histometric analysis. Mean values were averaged and compared statistically.
Statistical Analysis
The hypothesis that there were no differences in bone loss rate in the furcation region between treatment groups was tested by Bioestat 3.0 software (Bioestat, Windows 1995, Sonopress Brazilian Industry, Manaus, AM, Brazil). After checking data normality through Shapiro-Wilk test, an intragroup and intergroup analysis was carried out with a two-way analysis of variance (ANOVA; p<0.05). When ANOVA detected statistic difference, the multiple comparisons were assessed by Tukey's test (p<0.05).
RESULTS
Histological analysis
Group C
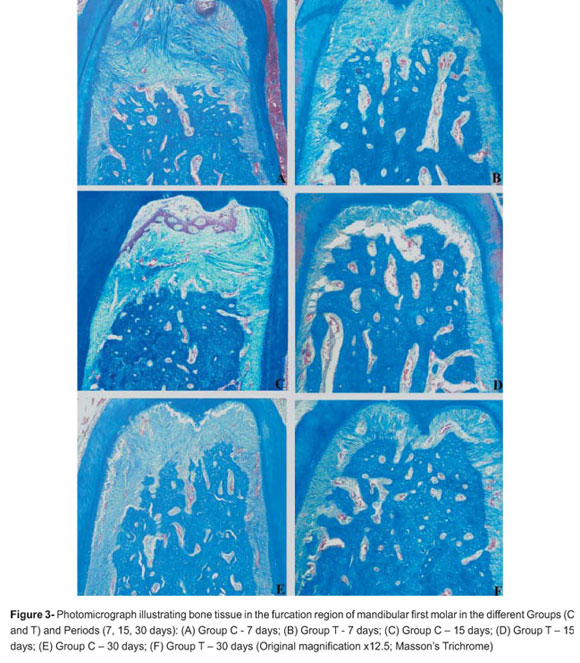
At 7 and 15 days, most specimens of Group C showed connective tissue with a high number of neutrophils in degeneration, bone tissue with thin bone trabeculae and resorption areas. These changes were also observed at 30 days (Figure 2).
Group T
At 7 and 15 days, in most specimens of Group T, the periodontal ligament was found to be intact, organized with parallel collagen fibers and showed no inflammatory infiltrate. The bone tissue showed organization with thick bone trabeculae and no signs of resorption. The cementum surface did not present resorption areas. All these changes mentioned above were also observed at 30 days (Figure 2).
Histometric analysis
Histometric data are shown in Table 1. In the intergroup histometric analysis, animals of Group T (0.72±0.05 mm2, 0.57±0.14 mm2, 0.62±0.07 mm2), showed less bone loss (p<0.05) than animals of Group C (1.35±0.25 mm2; 1.40±0.31 mm2; 1.29±0.27 mm2) at 7, 15 and 30 days, respectively (Figure 3).
DISCUSSION
The present study evaluated the influence of TTC-HCL subgingival irrigation as an adjuvant therapy in conventional periodontal treatment in rats submitted to experimentally induced periodontal disease.
It has been clearly shown that periodontitis is an infectious disease10, and that a current concept for treating periodontitis is primarily found on eliminating the infection by SRP13. However, this therapy can often be challenged due to the complex and unfavorable radicular morphology, especially in sites of deep periodontal pockets19. In these cases, several studies have shown the presence of remnants of Tannarella forsythensis and mainly Aggregactibacter actinomycetemcomitans in periodontal pockets after non-surgical periodontal therapy28.
Due to these limitations, antibiotic therapy has been used as an additional treatment of bacterial infections, noticeably in specific types of periodontal disease7. The administration of systemic antibiotic therapy can be done at therapeutic concentrations in infected sites, it may not cause satisfactory clinical and microbiological responses either when associated to SRP8. Sigusch, et al.24 (2001) reported that the administration of systemic antibiotics, even when preceded by complete removal of supra and subgingival irritants, did not lead to satisfactory overcomes with regard to the reduction of probing depth, to the clinical attachment gain, as well as to bacterial elimination, unless a re-instrumentation of the affected sites would be performed in an additional session. Furthermore, systemic administration of antibiotics, depending on the doses applied, may increase bacterial resistance, interact with other drugs, and cause several adverse effects25. However, recent studies have reported significant advantages when systemic antibiotics are combined with SRP in the treatment of smokers with chronic periodontitis15 or in the treatment of patients with localized aggressive periodontitis2.
TTC-HCL has been one of the most investigated antimicrobial agents considering its local release and its important property of featuring substantivity by its affinity with the radicular surface26. In addition to this fact, this drug has demonstrated to be effective against the periodontopathogenic microbiota32, inhibiting collagenase9,25 as well as increasing fibroblast attachment to dental structure when associated with fibronectin29.
The application method of TTC-HCL in the present study was trans-operatory subgingival irrigation after SRP, which was idealized because it allows the irrigating solution to reach subgingival microflora of the periodontal pocket apical portion, regardless of the probing depth11. Although there have been no studies conducted to compare the several application methods of TTC-HCL, some authors have considered that TTC-HCL fibers may improve drug release, as it is kept for a long period of time in a given site and does not require re-administration to maintain therapeutic levels18,21. On the other hand, there was no statistically significant difference in the number of bacteria between the SRP plus tetracycline fibers or with SRP only33.
Histologically, a reorganized periodontal ligament was observed in Group T specimens in the overall experimental periods, with a predomination of oblique collagen fibers and fibroblasts instead of intense inflammatory infiltrate, such as that found in Group C. Moreover, bone tissue was found to be organized, with thicker bone trabeculae compared to Group C specimens. These histological findings strongly suggests that the antiinflammatory properties of TTC-HCL can suppress the activity of polymorphonuclear cells8, as it blocks the synthesis of prostaglandin E2 through inhibition of phospholipase A230, and provide colonization and fibroblastic attachment adjacent to the dentin when followed by an application of fibronectin29.
In the analysis of histometric results, Group T animals presented less significant bone loss when compared to Group C animals in all experimental periods. The results of the present study suggested that subgingival irrigation with TTC-HCL (50 mg/mL) demonstrated positive effects, restoring supportive periodontal tissues in the furcation region. These findings are in accordance with other study, which demonstrate the properties of TTC-HCL of inhibiting bone resorption through inhibition of colagenase activity and modifying the osteoclastic effects9. Nevertheless, no positive results were observed in another study when subgingival irrigation with TTC-HCL (50 mg/mL) was carried out as adjuvant to SRP17. Christersson, et al.4 (1993) demonstrated that a simple irrigation with TTC-HCL (100 mg/mL) in humans, associated with SRP in sites with periodontitis, resulted in significantly greater attachment when compared to SRP alone. This divergence in the results could be associated with the methodology used with regard to the drug, such as: concentration, exposition time on radicular surface and local temperature where the solution was prepared, which could interfere in its dissolution18. Thus, further investigations are needed to reach to an agreement about the real effectiveness of the currently used methodologies.
CONCLUSION
Within the limits of the present study, it was possible to conclude that the subgingival irrigation with TTC-HCL used as adjunctive therapy to SRP in the treatment of periodontal disease induced in rats, resulted in significantly less bone loss in the furcation when compared to SRP alone. In addition, others experimental studies should be realized to effort the ideal application mode and concentration of the TTC-HCL to use in the periodontal clinical procedures.
Received: March 27, 2009
Modification: April 9, 2010
Accepted: May 21, 2010
- 1- Adriaens PA, Edwards CA, De Boever JA, Loesche WJ. Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol. 1988;59(8):493-503.
- 2- Akincibay H, Orsal SO, Sengün D, Tözüm TF. Systemic administration of doxycycline versus metronidazole plus amoxicillin in the treatment of localized aggressive periodontitis: a clinical and microbiologic study. Quintessence Int. 2008;39(2):33-9.
- 3- Baker PJ, Evans RT, Coburn RA, Genco RJ. Tetracycline and its derivatives strongly bind to and are released from the tooth surface in active form. J Periodontol. 1983;54(10):580-5.
- 4- Christersson LA, Norderyd OM, Puchalsky CS. Topical application of tetracycline-HCl in human periodontitis. J Clin Periodontol. 1993;20(2):88-95.
- 5- Cosyn J, De Bruyn H, Sabzevar MM. Subgingival application of chlorhexidine in the treatment of periodontitis. Rev Belge Med Dent. 2007;62(4):176-82.
- 6- Etienne D. Locally delivered antimicrobials for the treatment of chronic periodontitis. Oral Dis. 2003;9(sp. issue 1):45-50.
- 7- Flemmig TF, Milián E, Karch H, Klaiber, B. Differential clinical treatment outcome after systemic metronidazole and amoxicillin in patients harboring Actinobacillus actinomycetemcomitans and/or Porphyromonas gingivalis J Clin Periodontol. 1998;25(5):380-7.
- 8- Gabler WL, Creamer HR. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res. 1991;26(1):52-8.
- 9- Golub LM, Ramamurthy N, McNamara TF, Gomes B, Wolff M, Casino A, et al. Tetracyclines inhibit tissue collagenase activity. A new mechanism in the treatment of periodontal disease. J Periodontal Res. 1984;19(6):651-5.
- 10- Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5(1):78-111.
- 11- Hardy JH, Newman HN, Strahan JD. Direct irrigation and subgingival plaque. J Clin Periodontol. 1982;9(1):57-65.
- 12- Johnson IH. Effects of local irritation and dextran sulphate administration on the periodontium of the rat. J Periodontal Res. 1975;10(6):332-45.
- 13- Kaldahl WB, Kalkwarf KL, Patil KD. A review of longitudinal studies that compared periodontal therapies. J Periodontol. 1993;64(4):243-53.
- 14- Madison JG 3rd, Hokett SD. The effects of different tetracyclines on the dentin root surface of instrumented, periodontally involved human teeth: a comparative scanning electron microscope study. J Periodontol. 1997;68(8):739-45.
- 15- Matarazzo F, Figueiredo LC, Cruz SE, Faveri M, Feres M. Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2008;35(10):885-96.
- 16- Nociti FH Jr., Nogueira-Filho GR, Primo MT, Machado MA, Tramontina VA, Barros SP, et al. The influence of nicotine on the bone loss rate in ligature induced periodontitis. A histometric study in rats. J Periodontol. 2000;71(9):1460-4
- 17- Nylund K, Egelberg J. Antimicrobial irrigation of periodontal furcation lesions to supplement oral hygiene instruction and root debridement. J Clin Periodontol. 1990;17(2):90-5.
- 18- Pavia M, Nobile, CG, Angelillo IF. Meta-analysis of local tetracycline in treating chronic periodontitis. J Periodontol. 2003;74(6):916-32.
- 19- Ramfjord SP, Caffesse RG, Morrison EC, Hill RW, Kerry GJ, Appleberry EA, et al. 4 modalities of periodontal treatment compared over 5 years. J Clin Periodontol. 1987;14(8):445-52.
- 20- Rawal SY, Rawal YB. Non-antimicrobial properties of tetracyclines-dental and medical implications. West Indian Med J. 2001;50(2):105-8.
- 21- Rodrigues RM, Gonçalves C, Souto R, Feres-Filho EJ, Uzeda M, Colombo AP. Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J Clin Periodontol. 2004;31(6):420-7.
- 22- Rolla G, Löe H, Schiott CR. Retention of chlorhexidine in the human oral cavity. Arch Oral Biol. 1971;16(9):1109-16.
- 23- Rovin S, Costich ER, Gordon HA. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J Periodontal Res. 1966;1(3):193-204.
- 24- Sigusch B, Beier M, Klinger G, Pfister W, Glockmann EA. 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J Periodontol. 2001;72(3):275-83.
- 25- Slots J, Rams TE. Antibiotics in periodontal therapy: advantages and disadvantages. J Clin Periodontol. 1990;17(7):479-93.
- 26- Stabholz A, Kettering J, Aprecio R, Zimmerman G, Baker PJ, Wikesjö UM. Antimicrobial properties of human dentin impregnated with tetracycline HCl or chlorhexidine. An in vitro study. J Clin Periodontol. 1993;20(8):557-62.
- 27- Stabholz A, Kettering J, Aprecio R, Zimmerman G, Baker PJ, Wikesjö UM. Retention of antimicrobial activity by human root surfaces after in situ subgingival irrigation with tetracycline HCl or chlorhexidine. J Periodontol. 1993;64(2):137-41.
- 28- Takamatsu N, Yano K, He T, Umeda M, Ishikawa I. Effect of initial periodontal therapy on the frequency of detecting Bacteroides forsythus, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans J Periodontol. 1999;70(6):574-80.
- 29- Terranova VP, Franzetti LC, Hic S, DiFlorio RM, Lyall RM, Wikesjö UME, et al. A biochemical approach to periodontal regeneration: tetracycline treatment of dentin promotes fibroblast adhesion and growth. J Periodontal Res. 1986;21(4):330-7.
- 30- Vadas P, Greewald RA, Street RT, Pruzanski W. Inhibition of synovial fluid phospholipase A2 activity by 2 tetracycline derivatives, minocycline and doxycycline. Arthritis Rheum. 1991;34(1):5160.
- 31- Van Dyke TE. The etiology and pathogenesis of periodontitis revisited. J Appl Oral Sci. 2009;17(1):ii
- 32- Walker CB, Gordon JM, McQuilkin SJ, Niebloom TA, Socransky SS. Tetracycline: levels of achievable in gingival crevice fluid and in vitro effect on subgingival organisms. Part II. Susceptibilities of periodontal bacteria. J Periodontol. 1981;52(10):613-6.
- 33- Wong MY, Lu CL, Liu CM, Hou LT. Microbiological response of localized sites with recurrent periodontitis in maintenance patients treated with tetracycline fibers. J Periodontol. 1999;70(8):861-8.
Publication Dates
-
Publication in this collection
14 Jan 2011 -
Date of issue
Dec 2010
History
-
Received
27 Mar 2009 -
Accepted
21 May 2010