Abstract
Objective:
To study the effect of Lactobacillus sp. A-2 metabolites on viability of CAL-27 cells and apoptosis in CAL-27 cells.
Methods:
Lactobacillus sp. A-2 metabolites 1 and 2 (LM1 and LM2) were obtained by culturing Lactobacillus sp. A-2 in reconstituted whey medium and whey-inulin medium; the cultured CAL-27 cells were treated with different concentrations of LM1 and LM2 (0, 3, 6, 12, 24, 48 mg/mL) and assayed by methyl thiazolyltetrazolium (MTT) method; morphological changes of apoptotic cell were observed under fluorescence microscopy by acridine orange (Ao) fluorescent staining; flow cytometry method (FCM) and agarose gel electrophoresis were used to detect the apoptosis of CAL-27 cells treated LM1 and LM2.
Results:
The different concentrations of LM1 and LM2 could restrain the growth of CAL-27 cells, and in a dose-dependent manner; the apoptosis of CAL-27 cells was obviously induced and was time-dependent.
Conclusions:
Viability of CAL-27 cells was inhibited by Lactobacillus sp. A-2 metabolites; Lactobacillus sp. A-2 metabolites could induce CAL-27 cells apoptosis; study on the bioactive compounds in the Lactobacillus sp. A-2 metabolites and their molecular mechanism is in progress.
Lactobacillus ; Metabolism; Milk protein; Oral cancer; Apoptosis
INTRODUCTION
Ninety-five percent of head and neck cancers are squamous cell carcinomas. Carcinoma of tongue represents 25% to 50% of all cases of oral squamous cell carcinoma, with the lateral border and the anterior two thirds of the tongue being the most commonly affected locations33- Dantas DD, Ramos CC, Costa AL, Souza LB, Pinto LP. Clinicalpathological parameters in squamous cell carcinoma of the tongue. Braz Dent J. 2003;14:22-5.,88- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-49.,1616- Tanaka T, Tanaka M, Tanaka T. Oral carcinogenesis and oral cancer chemoprevention: a review. Patholog Res Int. 2011;2011:431246.. An estimated 400,000 new cases of cancer of the lip and oral cavity (ICD-10 C00-C08) and pharynx (excluding the nasopharynx) were diagnosed across the world in 2008 (3% of the total)55- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2: Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon (France): IARC Press; 2010..
The etiology of oral cancer includes many factors, such as tobacco, alcohol, betel quid and so on, while the consumption of fruit and vegetables is helpful to reduce the risk of oral cancer1717- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309-16.. Consumption of fermented milk correlates with a reduced risk of numerous cancers1212- Parodi PW. A role for milk proteins and their peptides in cancer prevention. Curr Pharm Des. 2007;13:813-28.. It has been reported that Lactobacillus spp. and its metabolites have antimicrobial, antioxidant, anti-caries and anti-tumor function77- He X, Lux R, Kuramitsu HK, Anderson MH, Shi W. Achieving probiotic effects via modulating oral microbial ecology. Adv Dent Res. 2009;21(1):53-6.,1010- Kumar M, Nagpal R, Verma V, Kumar A, Kaur N, Hemalatha R, et al. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev. 2012;71(1):23-34.,1313- Patel S, Goyal A. Evolving roles of probiotics in cancer prophylaxis and therapy. Probiotics & Antimicro Prot. 2013;5:59-67.,1515- Shmuely H, Domniz N, Cohen D. Probiotics in the prevention of colorectal cancer. Curr Colorectal Cancer Rep. 2013;9:31-6..
In this experiment, we have tried to study the effect of Lactobacillus sp. A-2 metabolites, obtained by culturing Lactobacillus sp. A-2 in reconstituted whey medium and whey-inulin medium, on growth and apoptosis of human tongue squamous cell carcinoma CAL-27 cell lines so as to understand the possible role of Lactobacillus sp. A-2 metabolites against cancer and provide experimental basis for screening new anti-tumor bioactive agent from natural source.
MATERIAL AND METHODS
Strain, cell line and chemicals
Lactobacillus sp. A-2 was obtained from Research Centre of Microecological Engineering Technology, Qiqihar Medical University, Heilongjiang province, the People's Republic of China. Cell line CAL-27 was obtained from Cell Resource Center, IBMS, CAMS/PUMC. Cell culture medium DMEM (GIBCO, Invitrogen Corporation, New York, USA), fetal bovine serum and Acridine orange dye (Shanghai Sangon Biological Engineering Technology and Service Co., Ltd, Shanghai, China), MTT (Amresco Inc. Solon, Ohio, USA), annexin V-FITC/PI double staining apoptosis detection kit (Nanjing KGI biotechnology Co., Ltd, Nanjing, Jiangsu, China) were purchased.
Culture media for Lactobacillus sp. A-2
MRS (DeMan rogosa-sharpe) medium
Soya peptone 10 g, beef powder 10 g, yeast extract 5 g, glucose 20 g, Tween 801 mL, dipotassium hydrogen phosphate 2 g, sodium acetate 5 g, ammonium citrate 2 g, magnesium sulfate, 200 mg, manganese sulfate 50 mg, solid agar supplemented medium 15 g. Water added to make a total of 1 L, pH 6.0-6.5, 121ºC, sterilized for 15 min.
Reconstituted whey medium
Demineralised whey powder 50 g/L, calcium carbonate, 20 g/L, pH 6.0-6.5, 121ºC sterilized for 15 min.
Whey-inulin medium
Demineralised whey powder 50 g/L, calcium carbonate, 20 g/L, Inulin 10 g/L, pH 6.0-6.5, 121ºC sterilized for 15 min.
Preparation of LM1 and LM2
The strain of Lactobacillus sp. A-2 in MRS was cultured for 3 times in a row. The culture was centrifuged and washed twice with saline solution containing 10 mmol/L CaCl2. The supernatant was discarded. Bacterial cells suspension was prepared using the above mentioned saline solution. The optical density (OD) of the suspension was determined at 600 nm with UV/VIS spectrophotometer. The bacterial cells were inoculated in reconstituted whey medium and whey-inulin medium with final cell concentration of 1 OD/mL, cultured at 40ºC for 48 h. The culture was centrifuged, and supernatant was collected. The supernatant was dried by a freeze-drying method. The dried powder obtained from whey recovery medium was assigned as Lactobacillus sp. A-2 metabolite 1 (LM1), while that obtained from whey-inulin medium was assigned Lactobacillus sp. A-2 metabolite 2 (LM2). LM1 and LM2 were stored at -20ºC until use.
Cell culture
Human tongue squamous cell carcinoma CAL-27 cells were cultured in DMEM containing 10% fetal bovine serum at 37ºC in a humidified atmosphere of 5% CO2. Adherent cells monolayer was grown to 80%-90% confluence and then passaged using 0.05% trypsin digestion solution.
Assessment of cell viability
In the log phase of cell cycle, the cells were collected and seeded in a 96-well plate at a density of 1.0×104 cells/mL, each well containing 0.1 mL. They were treated with LM1 (0, 3, 6, 12, 24 and 48 mg/mL), cultured for 72 h, after which cell viability was measured using MTT assay. Paclitaxel was used for positive control and culture medium for blank. MTT was added to each well, incubated for 2 h, and the supernatant was discarded. Precipitates were dissolved in 150 µL DMSO and oscillated for 5-10 min to dissolve the crystals. Optical density was measured at 570 nm using a plate reader. Cell growth inhibition was measured using following formula:
Cell growth inhibition rate (%)=(1-value of experimental group/value of control group) × 100%
MTT assay for LM2 was performed and calculated in the similar way.
Assessment of cell morphology
CAL-27 cells in a concentration of 2.2×105 cells/mL were seeded in a cover slip, placed in a 35 mm Petri dish, and cultured for 24 h. Then, 36 mg/mL of LM1 and LM2 were added in an experimental group. Culture medium was used for blank control and cultured for 16 h. Subsequently, cover slips were removed and fixed (methanol/glacial acetate 3:1, at 4ºC) for 5 minutes, stained with Acridine orange, washed with PBS (pH 4.8-6), and immediately placed in a fluorescence microscope for observation.
Assessment apoptosis by flow cytometry
In a 35 mm Petri dish, 2 mL of cell suspension in a log phase with a density of 1.5×106 cells/mL were seeded. Cells were incubated at 37ºC in a humidified atmosphere with 5% CO2 overnight, than the media were replaced with fresh media containing 36 mg/mL of LM1 and LM2, and incubated for 12 h. The treated cells were washed twice with PBS, detached using 0.05% of EDTA-free trypsin solution, subjected to be stained by annexin V and propidium iodine (PI) following manufacturer's instructions and the cells analyzed by flow cytometry.
DNA gel electrophoresis for apoptotic cells
CAL-27 cells with a density of 3×106 cells/mL were seeded in a 35 mm diameter Petri dish and cultured for 24 h, and then treated with 36 mg/mL of LM1 and LM2 for 0, 12, 18, 24 and 30 h. Cells were collected and lysed in a lysis buffer (10 μmol/mL Tris-HCl, 10 μmol/mL EDTA, 0.5% Triton X-100, pH 8.0) at 4ºC for 10 minutes. 2 µL RNase A (20 mg/mL) and 2 mL proteinase K (20 mg/mL) were added and incubated at 37ºC for 60 min. NaCl-isopropanolol was added. The mixtures were stored at -20ºC overnight and centrifuged at 7,200 x g for 15 min. The extracted DNA was resuspended. DNA gel electrophoresis was performed using 1% agarose gel. DNA ladders were photographed under UV light.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software package. Analysis of variance for independent samples between groups was executed and the results were expressed as mean ± standard deviation (SD) and P<0.05 was considered to be significant.
RESULTS
Effect of LM1 and LM2 on the viability of CAL-27
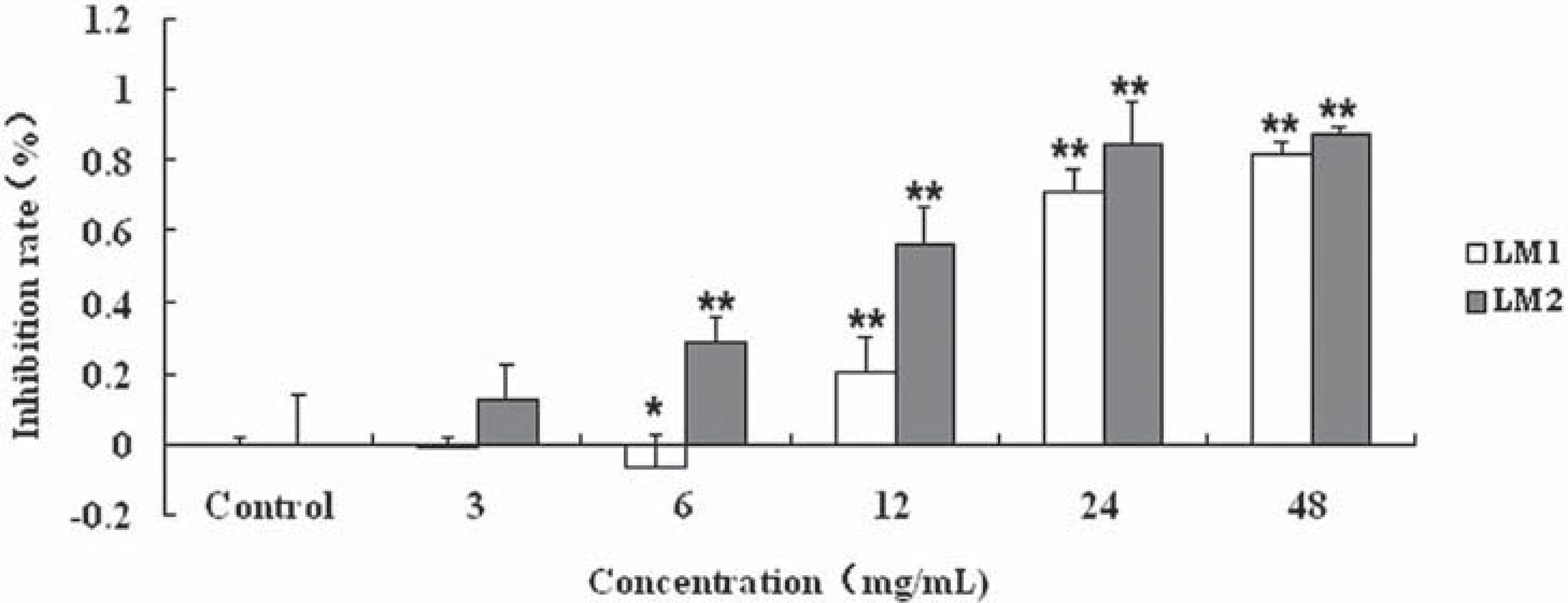
CAL-27 cells were used for investigating the effect of LM1 and LM2 on the viability of human tongue squamous cells CAL-27 by MTT assay in vitro. It was shown that 3-48 mg/mL LM1 or LM2 inhibited cell growth in a dose-dependent manner (Figure 1). The half maximal inhibitory concentration (IC50) for LM1 and LM2 was found to be 15.1 mg/mL and 10.27 mg/mL respectively. It was shown that LM1 and LM2 were able to induce cytotoxicity in CAL-27 cells. Nevertheless, no statistically significant difference in cytotoxicity to CAL-27 was found between LM1 and LM2 (P>0.05).
Effect of LM1 and LM2 on viability of CAL-27 cells. Cells were cultured in DMEM+10% FBS with different concentrations of LM1 and LM2 for 72 h. Inhibition rates were determined as described in Material and Methods. Each point is mean ± Standard Deviation of three experiments.*p<0.01 compared to control (0 mg/mL)
Effect of LM1 and LM2 on cell morphology
To evaluate whether the cytotoxic effects induced by LM1 and LM2 were caused by apoptosis, nuclear morphology was observed using Acridine orange staining under fluorescence microscope. After being treated with LM1 and LM2 at a 36 mg/mL concentration for 16 h, cell morphology became abnormal with cell nuclear shrinkage, and chromatin condensation was induced to occur in the treated CAL-27 cells (Figure 2). It was suggested that the number of apoptotic cells significantly increased in comparison with control.
Morphological change of CAL 27 cells with apoptotic features. CAL-27 cells were treated with LM1 and LM2 for 16 h. The cells were stained by Ao and then photographed using a fluorescent microscope (x200). The arrow indicates apoptotic cells
Effect of LM1 and LM2 on apoptosis in CAL-27
To investigate the apoptosis-inducing effects of LM1 and LM2, CAL-27 cells treated with a concentration of 36 mg/mL LM1 for 10 h and LM2 for 12 h were analyzed by annexin V and PI staining on a flow cytometer. The induction of apoptosis in CAL-27 cells was determined by a flow cytometer (Figure 3). For LM1, the percentages of early apoptotic CAL-27 cells was 12.8%, while the percentages of the late cells was 47.3%; for LM2, the percentages of early apoptotic CAL-27 cells was 11.22%, while the percentages of the late cells was 69.48%.
Effect of LM1 and LM2 on apoptosis of CAL-27 cells. A: blank control group; B: CAL-27 cells were treated with LM1 for 10 h; C: CAL-27 cells were treated with LM2 for 12 h. After staining with annexin V and PI, the cells were subjected to flow cytometry analysis
Effect of LM1 and LM2 on DNA fragment in CAL-27
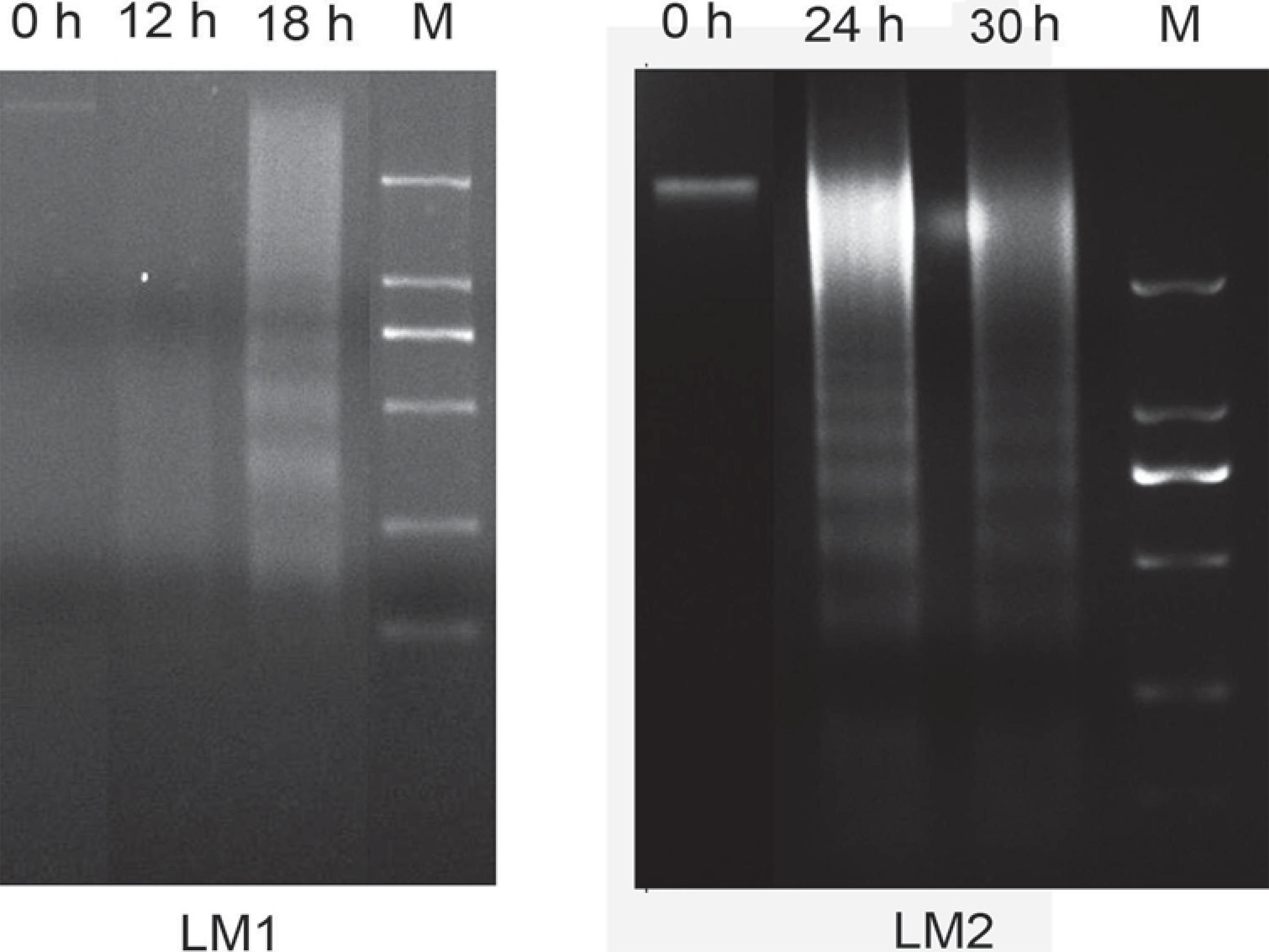
By using agarose gel electrophoresis, significant DNA ladders were detected after CAL-27 cells were exposed to 36 mg/mL of LM1 and LM2 for a different time period (Figure 4). These results confirmed that LM1 and LM2 were able to induce late apoptosis in CAL-27 cells.
Effect of LM1 and LM2 on DNA fragment in CAL-27. CAL-27 cells were treated with LM1 and LM2 for different time periods and then collected. DNA was separated, followed by DNA gel electrophoresis (M, Marker: 2,000 bp, 1,000 bp, 750 bp, 250 bp, 100 bp)
DISCUSSION
Apoptosis refers to programmed cell death. In the process of apoptosis, there were changes in the morphology of the cells, such as chromatin condensation and a reduction in cell volume, associated with fragmentation of DNA into nucleosomal size fragments of 180-200 bp or multiples thereof in many systems, and apoptotic bodies were formed11- Cohen GM, Sun XM, Snowden RT, Dinsdale D, Skilleter DN. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992;286:331-4..
Whey proteins and inulin are natural products with bioactivities. β-Lactoglobulin (BLG-58%)and a-lactalbumin (ALA-13%) are major proteins in whey. β-Lactoglobulin is the main source of sulfur amino acid and has immune modulatory effects, while a-lactalbumin forms complexes with oleic acid named HAMLET/BAMLET (human/bovine a-lactalbumin made lethal to tumor cell). The protein-liquid complexes selectively induce apoptosis in tumor cells1414- Pihlanto A. Whey proteins and peptides: emerging properties to promote health. Nutrafoods. 2011;10:29-42.. Meanwhile, whey protein also contains a large number of biologically active peptides and amino acids which are released through degradation of microbial fermentation99- Korhonen HJ. Bioactive milk proteins and peptides: from science to functional applications. Aust J Dairy Tech. 2009;64:16-25.,1111- Madureira AR, Pereira CI, Gomes AM, Pintado ME, Malcata FX. Bovine whey proteins - overview on their main biological properties. Food Res Int. 2007;40:1197-211.. Inulin and its hydrolyzed products, fructooligosaccharides (FOS), used as potent prebiotics and dietary fibers, have many more beneficial functions, such as increasing growth and activity of bifidobacteria, reducing concentration of serum cholesterol and reducing risks of colon cancer and intestinal infections22- Cooper PD, Barclay TG, Ginic-Markovic M, Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23(10):1164-74.,44- Dominguez AL, Rodrigues LR, Lima NM, Teixeira JA. An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol. 2014;7:324-37.,66- Gustaw W, Kordowska-Wiater M, Koziol J. The influence of selected prebiotics on the growth of lactic acid bacteria for bioyoghurt production. Acta Sci Pol Technol Aliment. 2011;10(4):455-66..
In this study, the source of Lactobacillus sp. A-2 is yogurt, from which it is separated and screened out. It is an anaerobic organism. The strain could grow in reconstituted whey medium and whey-inulin medium. The metabolites, LM1 and LM2, which contain a number of peptides, amino acids, short chain fatty acids, lactic acid, butyric acid and certain other chemicals, were obtained after incubation for 48 h at 40ºC. There is much less research on prevention of oral cancer using those metabolites.
In this experiment, we used human tongue squamous cell carcinoma CAL-27 cell line culture model to evaluate the effect of LM1 and LM2 on cancer cells, which provides theoretical and experimental basis for the use of probiotic metabolites in prevention and treatment of oral cancer. It was demonstrated that both LM1 and LM2 inhibited the growth of CAL-27 cells and induced apoptosis in CAL-27 cells in vitro. The cytotoxic effect of LM1 and LM2 on CAL-27 cells is similar. It was suggested that inulin had no synergistic effect in inhibiting the growth of CAL-27 in whey medium. Screening new anti-tumor bioactive agent from LM1 or LM2 remains to be further studied.
CONCLUSION
Lactobacillus sp. A-2 metabolites in reconstituted whey medium and whey-inulin medium have a potential role in the inhibition of growth and induction of apoptosis of human tongue squamous cell carcinoma CAL-27 cells in vitro.
ACKNOWLEDGMENTS
This study was supported by grants from the Natural Science Foundation of Heilongjiang Province (H201357), Research Project of Jiamusi University (L2012-034).
REFERENCES
-
1- Cohen GM, Sun XM, Snowden RT, Dinsdale D, Skilleter DN. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992;286:331-4.
-
2- Cooper PD, Barclay TG, Ginic-Markovic M, Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23(10):1164-74.
-
3- Dantas DD, Ramos CC, Costa AL, Souza LB, Pinto LP. Clinicalpathological parameters in squamous cell carcinoma of the tongue. Braz Dent J. 2003;14:22-5.
-
4- Dominguez AL, Rodrigues LR, Lima NM, Teixeira JA. An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol. 2014;7:324-37.
-
5- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2: Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon (France): IARC Press; 2010.
-
6- Gustaw W, Kordowska-Wiater M, Koziol J. The influence of selected prebiotics on the growth of lactic acid bacteria for bioyoghurt production. Acta Sci Pol Technol Aliment. 2011;10(4):455-66.
-
7- He X, Lux R, Kuramitsu HK, Anderson MH, Shi W. Achieving probiotic effects via modulating oral microbial ecology. Adv Dent Res. 2009;21(1):53-6.
-
8- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-49.
-
9- Korhonen HJ. Bioactive milk proteins and peptides: from science to functional applications. Aust J Dairy Tech. 2009;64:16-25.
-
10- Kumar M, Nagpal R, Verma V, Kumar A, Kaur N, Hemalatha R, et al. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev. 2012;71(1):23-34.
-
11- Madureira AR, Pereira CI, Gomes AM, Pintado ME, Malcata FX. Bovine whey proteins - overview on their main biological properties. Food Res Int. 2007;40:1197-211.
-
12- Parodi PW. A role for milk proteins and their peptides in cancer prevention. Curr Pharm Des. 2007;13:813-28.
-
13- Patel S, Goyal A. Evolving roles of probiotics in cancer prophylaxis and therapy. Probiotics & Antimicro Prot. 2013;5:59-67.
-
14- Pihlanto A. Whey proteins and peptides: emerging properties to promote health. Nutrafoods. 2011;10:29-42.
-
15- Shmuely H, Domniz N, Cohen D. Probiotics in the prevention of colorectal cancer. Curr Colorectal Cancer Rep. 2013;9:31-6.
-
16- Tanaka T, Tanaka M, Tanaka T. Oral carcinogenesis and oral cancer chemoprevention: a review. Patholog Res Int. 2011;2011:431246.
-
17- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309-16.
Publication Dates
-
Publication in this collection
Jul-Aug 2014
History
-
Received
27 Nov 2013 -
Reviewed
04 Apr 2014 -
Accepted
06 May 2014