Abstract
Red ceramics, due to the low compressive strength and high porosity, make it difficult to use it as an artificial aggregate in the production of mortars and concretes. However, since it has a silicon-rich composition, the ceramic material from blocks and tiles has been studied as a possible supplementary cementitious material in concrete and also as a raw material in alkali-activated binders. This paper aims to evaluate the content of calcium hydroxide (portlandite) fixed in the alkali activation reaction and the microstructure of red clay waste from construction and demolition waste (CDW) and hydrated lime mixtures, varying the atomic ratio between the silicon and the calcium. The results indicated that higher availability of lime is directly related to the content of hydrated compounds and its porosity. The increase in the silicon/calcium ratio resulted in a reduction of available lime content by 40% and an increase, in volume, of micropores by 7%.
Keywords:
Construction and demolition waste; Red clay waste; Alkali activation; Supplementary cementitious materials; Porosity
Resumo
A cerâmica vermelha, devido à baixa resistência à compressão e alta porosidade, dificulta sua utilização como agregado artificial na produção de argamassas e concretos. No entanto, por possuir uma composição rica em silício, o material cerâmico dos blocos e telhas foi estudado como possível adição pozolânica no concreto e também como matéria-prima em ligantes álcali-ativados. O objetivo deste trabalho é avaliar o teor de hidróxido de cálcio (portlandita) fixado na reação de álcali ativação e a microestrutura das misturas formadas pelos resíduos de cerâmica vermelha provenientes de resíduos de construção e demolição (RCD) e cal hidratada, variando a razão atômica entre os silício e cálcio das misturas. Os resultados indicam que uma maior disponibilidade de cal está diretamente relacionada ao teor de compostos hidratados e à porosidade. O aumento da relação silício/cálcio resultou em uma redução no teor de cal disponível em 40% e em um aumento, em volume, de microporos em 7%.
Palavras-chave:
Resíduo de construção e demolição; Resíduo de cerâmica vermelha; Álcali-ativação; Pozolana; Porosidade
Introduction
The concern with construction waste in Brazil started only in the late '80s (ANGULO et al., 2009ANGULO, S. C. et al. Chemical-mineralogical characterization of C&D waste recycled aggregates from São Paulo, Brazil. Waste Management, v. 29, n. 2, p. 721-730, 2009.) and it is estimated that the volume generated in the cities in 2018 reaches 0.585 kg.hab-1.day-1 and about 50% of solid waste generated nationwide is classified as construction and demolition waste (CDW) (ASSOCIAÇÃO…, 2019ASSOCIAÇÃO BRASILEIRA DE EMPRESAS DE LIMPEZA PÚBLICA E RESÍDUOS ESPECIAIS. Panorama dos sólidos no Brasil 2018/2019. Panorama dos Resíduos Sólidos no Brasil. São Paulo, 2019. Disponível em:Disponível em:https://abrelpe.org.br/panorama/ . Acesso em: 20 out. 2020.
https://abrelpe.org.br/panorama/...
).
The first CDW recycling plants in Brazil were installed by the city hall of São Paulo-SP (1991), Londrina-PR (1993) and Belo Horizonte-MG (1994) (MIRANDA; ANGULO; CARELI, 2009MIRANDA, L. F. R.; ANGULO, S. C.; CARELI, E. D. A reciclagem de resíduos de construção e demolição no Brasil: 1986-2008. Ambiente Construído, Porto Alegre, v. 9, n. 1, p. 57-71, jan./mar. 2009.). In 2020, according to the Brazilian association for recycling of civil construction and demolition waste (ABRECON), there are 350 CDW recycling plants in Brazil. After processing by these plants, some of these residues are classified and marketed as mixed recycled aggregates, that is, they present in its composition ceramic materials mainly from bricks and tiles. These two components, associated with the excavation soil, are defined by "red fraction" and makeup to 48% of the total volume of the mixed recycled aggregates (ANGULO et al., 2009ANGULO, S. C. et al. Chemical-mineralogical characterization of C&D waste recycled aggregates from São Paulo, Brazil. Waste Management, v. 29, n. 2, p. 721-730, 2009.; SILVA et al., 2018SILVA, N. M. et al. Quebra do grão em Resíduos de Construção Civil (RCC) induzida pelo processo de compactação. Ambiente Construído, Porto Alegre, v. 18, n. 1, p. 281-298, jan./mar. 2018.). The mixed recycled aggregates classification in Brazil is defined by NBR 15116 (ABNT, 2004ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 15116: agregados reciclados de resíduos sólidos da construção civil: utilização em pavimentação e preparo de concreto sem função estrutural: requisitos. Rio de Janeiro, 2004.), stating that this material needs to present more than 90% of fragments from Portland cement composites, as opposed to the concrete recycled aggregates, composed of less than 90% of Portland cement-based materials.
The presence of red ceramics in the recycled aggregates is undesirable because of its high porosity, which results in low compressive strength in concrete and mortars (PEREIRA-DE-OLIVEIRA; CASTRO-GOMES; SANTOS, 2012PEREIRA-DE-OLIVEIRA, L. A.; CASTRO-GOMES, J. P.; SANTOS, P. M. S. The potential pozzolanic activity of glass and red-clay ceramic waste as cement mortars components. Construction and Building Materials, v. 31, p. 197-203, 2012.). The most obvious consequence of the porosity is the need to increase the water content in concrete and mortars, which reflects a demand for higher consumption of cement to keep the w/c ratio constant. This procedure causes an increase in the cost of concrete and mortar and an increase in environmental impact due to the amount of embedded CO2 in the cement (AMORIM et al., 1999AMORIM, L. V. et al. Avaliação da atividade pozolânica de resíduos cerâmicos na construção civil. In: CONGRESSO BRASILEIRO DE CERÂMICA, 43., Florianópolis, 1999. Anais [...] Florianópolis, 1999.). Thus, mixed recycled aggregates have limited application to concrete and mortars with lower compressive strength (FACHINI et al., 2010FACHINI, D. et al. Comportamento no estado fresco de aglomerante alternativo a base de fração vermelha de RCD, cal, silica ativa e cimento Portland. In: ENCONTRO NACIONAL DE TECNOLOGIA DO AMBIENTE CONSTRUÍDO, 13., Canela, 2010. Anais [...] Canela, 2010.; JOHN; AGOPYAN, 2000JOHN, V.; AGOPYAN, V. Reciclagem de resíduos da construção. In: SEMINÁRIO - RECICLAGEM DE RESÍDUOS SÓLIDOS DOMICILIARES, São Paulo, 2000. Anais [...] São Paulo: CETESB, 2000.; PATRICIO et al., 2013PATRICIO, S. M. R. et al. Blocos solo-cal utilizando resíduo da construção civil. Cerâmica, v. 59, n. 349, p. 27-33, 2013.).
Possible efficient processes for separating ceramic components in mixed recycled aggregates, especially between red and gray fractions, are still not feasible. Although there are already processing plants for recycled aggregates using more sophisticated separation processes, most of the mixed recycled aggregates are separated manually, which justifies the level of contamination of the mixed recycled aggregates by ceramic, provided by standard (ANGULO et al., 2013ANGULO, S. C. et al. Separação óptica do material cerâmico dos agregados mistos de resíduos de construção e demolição. Ambiente Construído, Porto Alegre, v. 13, n. 2, p. 61-73, abr./jun. 2013.). Besides, there is not yet an application for the red ceramic residue that is economically attractive. On the contrary, in the recycling plants, red ceramics is considered a liability, and a reason to overtax users whose waste is contaminated with ceramics. Thus, a proposal to add value to the mixed recycled aggregates red ceramics is to modify them chemically (ÂNGULO; ZORDAN; JOHN, 2001ANGULO, S. C. et al. Chemical-mineralogical characterization of C&D waste recycled aggregates from São Paulo, Brazil. Waste Management, v. 29, n. 2, p. 721-730, 2009.; FACHINI et al., 2010FACHINI, D. et al. Comportamento no estado fresco de aglomerante alternativo a base de fração vermelha de RCD, cal, silica ativa e cimento Portland. In: ENCONTRO NACIONAL DE TECNOLOGIA DO AMBIENTE CONSTRUÍDO, 13., Canela, 2010. Anais [...] Canela, 2010.).
One of the alternatives of chemical modification of the ceramic residue is the comminution followed by combination with the calcium hydroxide, whose reaction results in a binder by the alkali-activation process employing the direct combination with hydrated lime or as a pozzolanic addition to Portland cement to react with the calcium hydroxide formed after the hydration reactions of the silicate phases (C3S and C2S) (CORDEIRO et al., 2009CORDEIRO, G. C. et al. Influência da substituição parcial de cimento por cinza ultrafina da casca de arroz com elevado teor de carbono nas propriedades do concreto. Ambiente Construído, v. 9, n. 4, p. 99-107, out./dez. 2009.; DUXSON et al., 2007DUXSON, P. et al. Geopolymer technology: the current state of the art. Journal of Materials Science, v. 42, n. 9, p. 2917-2933, 2007.; MEHTA; MONTEIRO, 2013MEHTA, P. K.; MONTEIRO, P. J. M. Concrete: microstructure, properties, and materials. 4. ed. Berkeley: McGraw-Hill Education, 2013.). This chemical reactivity is essential because the ceramic materials consist of a siliceous structure similar to other mineral additions which, in the presence of calcium hydroxide and water, resulting in reaction products with good mechanical properties (CASTRO et al., 2017CASTRO, A. L. et al. Caracterização de cimentos compostos com resíduo da indústria de cerâmica vermelha. Cerâmica, v. 63, n. 365, p. 65-76, 2017.; GARCIA et al., 2015GARCIA, E. et al. Avaliação da atividade pozolânica dos resíduos de cerâmica vermelha produzidos nos principais polos ceramistas do Estado de S. Paulo. Cerâmica, v. 61, n. 358, p. 251-258, 2015.; PAN et al., 2002PAN, Z. et al. Hydration products of alkali-activated slag-red mud cementitious material. Cement and Concrete Research, v. 32, n. 3, p. 357-362, 2002.).
Some studies have already been carried out to evaluate the possibility of using the ceramic material and clays as pozzolan material (BARATA; ANGÉLICA, 2012BARATA, M. S.; ANGÉLICA, R. S. Caracterização dos resíduos cauliníticos das indústrias de mineração de caulim da amazônia como matéria-prima para produção de pozolanas de alta reatividade. Cerâmica, v. 58, n. 345, p. 36-42, 2012.; CASTRO et al., 2017CASTRO, A. L. et al. Caracterização de cimentos compostos com resíduo da indústria de cerâmica vermelha. Cerâmica, v. 63, n. 365, p. 65-76, 2017.; CORDEIRO; DÉSIR, 2010CORDEIRO, G. C.; DÉSIR, J. M. Potencial de argila caulinítica de Campos dos Goytacazes, RJ, na produção de pozolana para concreto de alta resistência. Cerâmica, v. 56, n. 337, p. 71-76, 2010.; GARCIA et al., 2015GARCIA, E. et al. Avaliação da atividade pozolânica dos resíduos de cerâmica vermelha produzidos nos principais polos ceramistas do Estado de S. Paulo. Cerâmica, v. 61, n. 358, p. 251-258, 2015.; PICANÇO; ANGÉLICA; BARATA, 2011PICANÇO, M. S.; ANGÉLICA, R. S.; BARATA, M. S. Avaliação preliminar do emprego de arenito zeolítico da região nordeste do Brasil como material pozolânico para cimento Portland. Cerâmica, v. 57, n. 344, p. 467-473, 2011.). However, part of the studies in the area of alkali-activated binders and geopolymers do not evaluate how the availability of silicon and calcium influences the reaction, even if these elements, together with aluminum, are the main components in the reaction products that generate mechanical resistance in the compositions. The researchers, mostly, use alkaline bases that are more electropositive, facilitating the integration into the newly formed structure, such as sodium or potassium silicate (BEZERRA et al., 2019BEZERRA, A. C. S. et al. Alkaline activation of high-calcium ash and iron ore tailings and their recycling potential in building materials. Ambiente Construído, Porto Alegre, v. 19, n. 3, p. 99-112, jul./set. 2019.; DUXSON et al., 2007DUXSON, P. et al. Geopolymer technology: the current state of the art. Journal of Materials Science, v. 42, n. 9, p. 2917-2933, 2007.; LI; SUN; LI, 2010LI, C.; SUN, H.; LI, L. A review: the comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cement and Concrete Research, v. 40, n. 9, p. 1341-1349, 2010.; PALOMO; GRUTZECK; BLANCO, 1999PALOMO, A.; GRUTZECK, M. W.; BLANCO, M. T. Alkali-activated fly ashes: a cement for the future. Cement and Concrete Research, v. 29, n. 8, p. 1323-1329, 1999.; TORGAL; GOMES; JALALI, 2007TORGAL, F. P.; GOMES, J. P. C.; JALALI, S. Argamassas antigas: reacção pozolânica ou activação alcalina? In: Congresso Nacional de Argamassas de Construção - “Sob a Égide da Reabilitação”, 2., Lisboa, 2007. Anais [...] Lisboa: APFAC, 2007.). However, due to the high cost of these bases, its use is not feasible for incorporation in residues as the ceramic material for the production of a binder by alkali-activation in civil construction on a large scale. Thus, even with less mechanical resistance, it is possible to combine the calcium hydroxide with the red ceramic residue, since it is a material already used in civil construction and low cost when compared to the sodium and potassium bases.
The possibility of adding market value to the red ceramic waste from mixed recycled aggregates could lead to the reduction of the environmental impact and the generation of new business. Some benefits from this type of study may be: reducing the consumption of non-renewable natural resources; reduce areas needed for landfills; add value to ceramic waste, reduce energy consumption during the binder production process, and reduce pollution through mitigation of CO2 emissions (ÂNGULO; ZORDAN; JOHN, 2001ÂNGULO, S. C.; ZORDAN, S. E.; JOHN, V. M. Desenvolvimento sustentável e a reciclagem de resíduos na construção civil. In: SEMINÁRIO DE DESENVOLVIMENTO SUSTENTÁVEL E A RECICLAGEM NA CONSTRUÇÃO CIVIL EM ANAIS DO INSTITUTO BRASILEIRO DE CONCRETO COMITÊ TÉCNICO, 206., São Paulo, 2001. Anais [...] São Paulo, 2001.; JOHN; AGOPYAN, 2000JOHN, V.; AGOPYAN, V. Reciclagem de resíduos da construção. In: SEMINÁRIO - RECICLAGEM DE RESÍDUOS SÓLIDOS DOMICILIARES, São Paulo, 2000. Anais [...] São Paulo: CETESB, 2000.).
In this context, this paper aims to evaluate the calcium hydroxide (portlandite) consumption and the microstructure of the compositions formed by the red ceramic waste from mixed recycled aggregates and hydrated lime, varying the atomic relation between silicon and calcium. The novelty of this research is the evaluation of the portlandite consumption and the formation of hydrated products using X-ray diffractometry and how the micro-porosity of the samples can be affected by the availability of base content and SiO2/CaO ratio of the compositions, even for samples with similar mechanical strengths.
Alkali-activation reaction
Pozzolanic materials are defined as siliceous or silicon-aluminum materials which on its own have little or no agglomerating activity but which, when finely divided and in the presence of water, react with the calcium hydroxide at room temperature to form compounds with binding properties - as defined by NBR 12653 (ABNT, 2014ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 12653: materiais pozolânicos:requisitos. Rio de Janeiro, 2014.) and C618 (AMERICAN…, 2019AMERICAN SOCIETY FOR TESTING AND MATERIALS. C618: standard specification for coal fly ash and raw or calcined natural pozzolan for use in concrete. West Conshohocken, 2019.). In this way, it can be understood that a pozzolan needs to be activated by an alkaline base (mostly Ca+2, Na+1 and K+1), called the alkali-activation reaction, to develop mechanical resistance.
Alkali-activation is a chemical process that allows the transformation of amorphous structures (partially or totally amorphous and/or metastable), such as pozzolans, into a compact and cementitious material. One of the most common types of alkali-activation is the use of calcined clays (such as metakaolin), fly ash and granulated blast furnace slag as mineral additions to Portland cement (PALOMO; GRUTZECK; BLANCO, 1999PALOMO, A.; GRUTZECK, M. W.; BLANCO, M. T. Alkali-activated fly ashes: a cement for the future. Cement and Concrete Research, v. 29, n. 8, p. 1323-1329, 1999.; VARGAS et al., 2007VARGAS, A. S. et al. Cinzas volantes álcali-ativadas com solução combinada de NaOH e Ca(OH)2. Revista Matéria, v. 12, n. 3, p. 8, 2007.).
Alkaline activation of silicon materials with calcium occurs in a solution of high alkalinity and mainly produces structures of hydrated calcium silicate (C-S-H) similar to the hydrated Portland cement. The pozzolanic reactions in some types of Portland cement (between pozzolans and calcium hydroxide resulting from the hydration process of the calcium silicate phases) and the chemical stabilization of soils with lime can be classified in this category (LI; SUN; LI, 2010LI, C.; SUN, H.; LI, L. A review: the comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cement and Concrete Research, v. 40, n. 9, p. 1341-1349, 2010.; TORGAL; GOMES; JALALI, 2007TORGAL, F. P.; GOMES, J. P. C.; JALALI, S. Argamassas antigas: reacção pozolânica ou activação alcalina? In: Congresso Nacional de Argamassas de Construção - “Sob a Égide da Reabilitação”, 2., Lisboa, 2007. Anais [...] Lisboa: APFAC, 2007.). Therefore, pozzolanic reactions in Portland cement is considered an alkaline activation with calcium hydroxide.
Since antiquity, ground ceramics are used as pozzolans (TORGAL; GOMES; JALALI, 2007TORGAL, F. P.; GOMES, J. P. C.; JALALI, S. Argamassas antigas: reacção pozolânica ou activação alcalina? In: Congresso Nacional de Argamassas de Construção - “Sob a Égide da Reabilitação”, 2., Lisboa, 2007. Anais [...] Lisboa: APFAC, 2007.), and alkali-activation can also be performed with red ceramics from previously-calcined building residues (SEVERO et al., 2013SEVERO, C. G. S. et al. Ativação alcalina de resíduos minerais com NaOH. Revista Eletrônica de Materiais e Processos, v. 8, n. 2, p. 106-109, 2013.). The main factors that influence the reaction kinetics between calcium hydroxide and a pozzolanic material are: silicon and amorphous material content, lime/pozzolan ratio of the composition, time and curing temperature, the specific surface area of the materials, the water/solids ratio of the composition and the type of activator, if used.
The two major types of the alkali-activation reaction are: "high calcium", in which materials rich in calcium and silicon generate a C-S-H (hydrated calcium silicate) gel similar to that obtained during the hydration of Portland cement; and "low calcium" when the activated materials mainly contain aluminum and silicon in the composition and the formation of a three-dimensional inorganic alkaline polymer, a N-A-S-H gel (alkaline aluminosilicate hydrate) occurs. Some authors consider that the product of the second type of reaction can also be called a geopolymer and for this reaction a sodium or potassium base is required (CABRERA; ROJAS, 2001CABRERA, J.; ROJAS, M. F. Mechanism of hydration of the metakaolin-lime-water system. Cement and Concrete Research, v. 31, n. 2, p. 177-182, 2001.; HEWLETT, 2003HEWLETT, P. C. Lea’s chemistry of cement and concrete. 5. ed. Oxford: Butterworth-Heinemann Elsevier, 2003.; PACHECO-TORGAL et al., 2015PACHECO-TORGAL, F. et al. Handbook of alkali-activated cements, mortars and concretes. Cambridge: Woodhead Publishing, 2015.).
It is important to highlight that, in the development of new materials, studies related to durability are of fundamental importance, since the alkali-activated material may have deficiencies in is durability properties (HUSEIEN; SHAH, 2020HUSEIEN, G. F.; SHAH, K. W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Construction and Building Materials, v. 235, 2020.; IDIR; CYR; PAVOINE, 2020IDIR, R.; CYR, M.; PAVOINE, A. Investigations on the durability of alkali-activated recycled glass. Construction and Building Materials, v. 236, 2020.; MUNDRA et al., 2020MUNDRA, S. et al. Modelling chloride transport in alkali-activated slags. Cement and Concrete Research, v. 130, 2020.). For this reason, it is emphasized the importance of studies of the microstructure of alkali-activated binders, especially porosity, since this characteristic will exert an influence on the mechanical resistance, the penetration of aggressive agents, the absorption of water and will also accelerate the carbonation reaction (FACHINI et al., 2010FACHINI, D. et al. Comportamento no estado fresco de aglomerante alternativo a base de fração vermelha de RCD, cal, silica ativa e cimento Portland. In: ENCONTRO NACIONAL DE TECNOLOGIA DO AMBIENTE CONSTRUÍDO, 13., Canela, 2010. Anais [...] Canela, 2010.).
Materials and methods
In this section, it will be described the materials used in the research, the calculation of the compositions, the mixing process, the molding of the samples, and the applied characterization techniques.
Characterization of raw materials
The red ceramics waste used in this research was obtained at the CDW recycling plant in the Brazilian city of Ponta Grossa. After receiving the material, the ceramic waste was milled and sieved in a 75μm mesh. The red ceramics are composed of the crushing of ceramic material such as blocks and tiles, as well as soil from excavations. The dolomitic hydrated lime used in this paper was a CH-III type.
The specific surface value was obtained by the Blaine method NBR NM 76 (ABNT, 1998ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR NM 76: cimento Portland: determinação da finura pelo método de permeabilidade ao ar (Método de Blaine). Rio de Janeiro, 1998.), which consists of estimating the area of the particles by comparing them with a reference sample by measuring the air permeability of the material. The measurement of the actual density of solids was determined by the helium pycnometer D6226 (AMERICAN…, 2015AMERICAN SOCIETY FOR TESTING AND MATERIALS. D6226: standard test method for open cell content of rigid cellular plastics. West Conshohocken, 2015.).
The chemical composition of the raw materials and compositions was determined by X-ray fluorescence spectrometry (XRF Axios Max, PANalytical). The mineralogical analysis was performed with the aid of an X-ray diffractometer (Shimatzu XRD-7000, 30 kV - 30 mA). The test parameters were: scan angle 5° to 75°, step of 0,02° and scan speed of 1º/min. Peak indexing was performed based on 2003 data from the International Center of Diffraction Data (INTERNATIONAL…, 2003INTERNATIONAL CENTRE FOR DIFFRACTION DATA. JCPDS International Centre for Diffraction Data: Crystalography database. Newtown Square, 2003.), phase quantification was performed by profile fitting method and amorphous content determined by the degree of crystallinity (DOC) of the material (MADSEN; SCARLETT; KERN, 2011MADSEN, I. C.; SCARLETT, N. V. Y.; KERN, A. Description and survey of methodologies for the determination of amorphous content via X-ray powder diffraction. Zeitschrift fur Kristallographie, v. 226, n. 12, p. 944-955, 2011.).
Compositions study
For the evaluation of the reactivity of the red ceramics waste, 3 compositions were prepared with hydrated lime, based on studies by authors who used clay, ceramics or pozzolans with lime hydrated in alkali-activation reactions (AMORIM et al., 1999AMORIM, L. V. et al. Avaliação da atividade pozolânica de resíduos cerâmicos na construção civil. In: CONGRESSO BRASILEIRO DE CERÂMICA, 43., Florianópolis, 1999. Anais [...] Florianópolis, 1999.; CABRERA; ROJAS, 2001CABRERA, J.; ROJAS, M. F. Mechanism of hydration of the metakaolin-lime-water system. Cement and Concrete Research, v. 31, n. 2, p. 177-182, 2001.; DERING et al., 2012DERING, E. et al. Estudo do comportamento mecânico e reatividade de aglomerante alternativo a base de fração vermelha de resíduo de construção e demolição, cal, sílica ativa e cimento portland. In: ENCONTRO NACIONAL DE TECNOLOGIA DO AMBIENTE CONSTRUÍDO, 14., Juiz de Fora, 2012. Anais [...] Juiz de Fora, 2012.; FERRAZ et al., 2012FERRAZ, E. et al. Utilização do ensaio de Chapelle modificado para avaliação da reactividade pozolânica de metacaulinos. In: CONGRESSO PORTUGUÊS DE ARGAMASSAS, 4.; ETICS, Coimbra, 2012. Anais [...]Coimbra, 2012.; GARCIA et al., 2015GARCIA, E. et al. Avaliação da atividade pozolânica dos resíduos de cerâmica vermelha produzidos nos principais polos ceramistas do Estado de S. Paulo. Cerâmica, v. 61, n. 358, p. 251-258, 2015.; GARCIA; CABRASL JUNIOR; CHOTOLI, 2014GARCIA, E.; CABRAL JUNIOR, M.; CHOTOLI, F. F. Resíduo de Cerâmica Vermelha ( RCV ): uma alternativa como material pozolânico. Cerâmica Industrial, v. 19, n. 4, p. 31-38, 2014.; HEWLETT, 2003HEWLETT, P. C. Lea’s chemistry of cement and concrete. 5. ed. Oxford: Butterworth-Heinemann Elsevier, 2003.). As the majority of the studies presented only the proportion between the components, the results of the chemical analysis and the compositions used by those authors were used to calculate the most favorable molar ratios between SiO2 and CaO for the development of mechanical resistance in those works.
As the range of SiO2/CaO ratios of the studies ranged from 1.20 to 2.12, it was decided to study three molar ratios within this range, as presented in Table 1. In this way, it was expected to evaluate the influence of the chemical composition in the development of the alkali-activation reaction.
The compositions were prepared with the dispersion of lime in water for 60 seconds on a slow speed mixer. The red ceramics were added gradually at low speed for 30 seconds and finally the paste was mixed for a further 60 seconds at high speed, similar to the times prescribed by NBR NM 43 (ABNT, 2002ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR NM 43: cimento Portland: determinação da pasta de consistência normal. Rio de Janeiro, 2002.) for the Portland cement pastes.
All compositions shown in Table 1 were mixed maintaining the fixed ratio of water to dry components at 0.42. This amount of water was determined for composition C1 to obtain 6 mm in the standard consistency test, according to NBR NM 43 (ABNT, 2002ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR NM 43: cimento Portland: determinação da pasta de consistência normal. Rio de Janeiro, 2002.). In this test a standard weight rod penetrates the paste and must stop at 6 mm from the base, thus defining the amount of water required to obtain the standard consistency. For the other compositions, it was recorded the consistency index obtained, with no correction on the amount of water.
Evaluation methods for the pastes
The molding of the specimens was performed in a split PVC cylinder with dimensions of (16x32) mm. Three test specimens were molded for each age with densification in two layers of 30 strokes each, using a 5 mm diameter cylindrical glass rod.
The samples were demolded after 24 hours and submerged cured in water saturated with lime until the ages of 3, 7, 28 and 56 days. Samples were rectified and tested by axial compression as prescribed by ABNT NBR 7215 (ABNT, 1996ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 7215: cimento Portland: determinação da resistência à compressão. Rio de Janeiro, 1996.) in a universal test machine (EMIC, DL 30,000).
The compositions were characterized by X-ray diffractometry under the same conditions as the components. The reaction was analyzed at the initial ages (3 and 7 days) and more advanced ages (28 and 56 days). After these analyses, the samples were submerged in isopropyl alcohol for 24 hours, vacuum filtered in a Büchner funnel and dried at 40°C to stop the hydration of the samples.
The free (remaining) lime content was determined on the hydrated samples (3, 7, 28 and 56 days) after they had milled, sieved in a 75μm mesh and dispersed in distilled water. About 1g of the sample was titrated in triplicate with 0.1 M solution of hydrochloric acid, and phenolphthalein as an indicator (LAVAT; TREZZA; POGGI, 2009LAVAT, A. E.; TREZZA, M. A.; POGGI, M. Characterization of ceramic roof tile wastes as pozzolanic admixture. Waste Management, v. 29, n. 5, p. 1666-1674, 2009.).
To complement the microstructural evaluation, the water immersion index void ratio NBR 9778 (ABNT, 2005ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 9778: argamassa e concreto endurecidos: determinação da absorção de água, índice de vazios e massa específica. Rio de Janeiro, 2005.) was performed at 7, 28 and 56 days. And mercury intrusion porosimetry (MIP) according to D4404 (AMERICAN…, 2018AMERICAN SOCIETY FOR TESTING AND MATERIALS. D4404: standard test method for determination of pore volume and pore volume distribution of soil and rock by mercury porosimetry. West Conshohocken, 2018.) (Quantachrome, Poremaster 33) was evaluated in the 56-day old samples of the compositions C1, C2 and C3, to demonstrate the effect of the different molar ratios on the porosity of the samples.
Results and discussion
In this section the results obtained in the pastes with different molar ratios are presented, as well as the statistical analysis and correlations found.
Components characterization
The specific mass of hydrated lime was 2.47 g/cm3 and the Blaine specific area was 1.43m²/g. The value of the specific mass and the Blaine specific area of the red ceramics were 2.65 g/cm³ and 0.67 m²/g, respectively. Table 2 shows the results of the chemical characterization of both raw materials.
According to the values presented in Table 2, the concentrations of CaO and MgO are adequate because it is a dolomitic lime, and can it be classified, according to NBR 7175 (ABNT, 2003ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 7175: cal hidratada para argamassas: requisitos. Rio de Janeiro, 2003.), as CH-III type. The loss on ignition refers to the loss of mass related to chemically combined water and carbon dioxide from the sample by the degradation of hydroxyl and possible carbonates of hydrated lime.
For the red ceramic waste, it can be observed in Table III that the amounts of SiO2, Fe2O3 and Al2O3 account for 78.0% of the total, a higher value than the minimum stipulated for natural and artificial pozzolans according to NBR 12653 (ABNT, 2014ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 12653: materiais pozolânicos:requisitos. Rio de Janeiro, 2014.), although physical test would still be required. It is important to note that, even considering the chemical parameters of NBR 12653 (ABNT, 2014ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 12653: materiais pozolânicos:requisitos. Rio de Janeiro, 2014.), the pozzolanic activity depends on the presence of amorphous material in the sample, which was detected by X-ray diffraction (HOPPE FILHO et al., 2017HOPPE FILHO, J. et al. Atividade pozolânica de adições minerais para cimento Portland (Parte I): índice de atividade pozolânica (IAP) com cal, difração de raios-X (DRX), termogravimetria (TG/DTG) e Chapelle modificado. Revista Matéria, v. 22, p. 1-18, 2017., 2013aHOPPE FILHO, J. et al. Atividade pozolânica de adições minerais para cimento Portland: índice de atividade pozolânica (IAP) e difração de raios X (DRX). In: CONGRESSO BRASILEIRO DO CONCRETO, 55., Gramado, 2013. Anais [...] Gramado, 2013a.). The SO3 content is below the maximum limit of 4%, and the presence of alkali in the form of Na2O was not detected.
The presence of SiO2 is related to silicates (micas and feldspars) and to free silica (quartz). Al2O3 is an element that is commonly present in clay minerals such as kaolinite, illite, smectite (montmorillonite) and feldspar. Fe2O3 may be present in the form of hematite, pyrite or goethite and is responsible for the reddish coloration characteristic of tiles and ceramic blocks, as well as some types of soils (MINERAIS…., 2009MINERAIS DO PARANÁ S/A. Avaliação do Potencial de argilas para uso em cerâmicas vermelhas. Programa de Desenvolvimento da Indústria de Cerâmica Vermelha no Estado do Paraná - RELATÓRIO FINAL. Curitiba, 2009.). X-ray diffraction analysis of the components used is presented in Figure 1 and Figure 2. The quantitative phase analysis and amorphous content are presented in Table 3.
Based on the indexed peaks in the XRD test and the quantitative analysis, it is possible to observe in Figure 1 and Table 3 that the hydrated lime presented Portlandite (P) and Brucite (B) phases, related to calcium and magnesium hydroxides, respectively, commonly found in dolomitic limestones (PACHECO-TORGAL et al., 2015PACHECO-TORGAL, F. et al. Handbook of alkali-activated cements, mortars and concretes. Cambridge: Woodhead Publishing, 2015.). The result also indicated the presence of the Calcite (C) phase, indicating possible carbonation in the sample or incomplete lime calcination. The phase quantification is coherent to the loss on ignition for the hydrated lime. It was also found a low-intensity peak of Quartz (Q), that is coherent to the chemical composition according to Table 2 that indicated a 1.3% of SiO2, possibly present due to external contamination during its fabrication.
For the red ceramic waste, Figure 2 shows the predominance of Illite (I), and small concentrations of Quartz (Q), and Hematite (H). Similar crystalline phases were observed on the industrial ceramic block, mud brick and sanitary ceramic waste evaluated by Trezza et al. (2017TREZZA, M. A. et al. Portland blended cements: demolition ceramic waste management. Materiales de Construcción, v. 67, n. 325, p. 1-8, 2017.). It was not possible to find characteristic peaks of 1:1 mullite and cristobalite (crystalline silica), normally formed only at high temperatures, indicating that the heat treatment of the ceramic block did not occur above 1000 °C (CARNEIRO; ANGÉLICA, 2003CARNEIRO, B. S.; ANGÉLICA, R. S. Caracterização mineralógica e geoquímica e estudo das transformações de fase do caulim duro da região do Rio Capim, Pará. Cerâmica, v. 49, p. 237-244, 2003.; FRÍAS et al., 2008FRÍAS, M. et al. Influencia de la activación de un residuo arcilloso de la industria papelera en el comportamiento de matrices de cemento. Materiales de Construcción, v. 58, n. 292, p. 67-79, 2008.; SANTOS et al., 2006SANTOS, H. S. et al. Estudo por microscopia eletrônica das transformações durante a queima de argilas altamente aluminosas brasileiras. Cerâmica, v. 52, p. 125-137, 2006.). The illite and kaolinite phases are highlighted by some authors as the compounds responsible for the alkali-activation reaction, and the existence of an amorphous halo in the diffractogram indicates the reactive capacity of the material (GONÇALVES; TOLEDO FILHO; FAIRBAIRN, 2006GONÇALVES, J. P.; TOLEDO FILHO, R. D.; FAIRBAIRN, E. M. R. Estudo da hidratação de pastas de cimento Portland contendo resíduo cerâmico por meio de análise térmica. Ambiente Construído, v. 6, n. 4, p. 83-94, out./dez. 2006.; MATIAS; FARIA; TORRES, 2014MATIAS, G.; FARIA, P.; TORRES, I. Lime mortars with heat treated clays and ceramic waste: a review. Construction and Building Materials, v. 73, p. 125-136, 2014.).
The presence of a low-intensity amorphous halo (grey area in Figure 2) between 20° and 35° (2θ) is common in the indexation of materials with amorphous content. The amorphous content of the red ceramic waste was estimated at 58%, indicating a considerable reactivity potential. This amorphous content is common in supplementary cementitious materials such as silica fume, metakaolin and blast furnace slag (GONÇALVES; TOLEDO FILHO; FAIRBAIRN, 2006GONÇALVES, J. P.; TOLEDO FILHO, R. D.; FAIRBAIRN, E. M. R. Estudo da hidratação de pastas de cimento Portland contendo resíduo cerâmico por meio de análise térmica. Ambiente Construído, v. 6, n. 4, p. 83-94, out./dez. 2006.; MEDEIROS et al., 2015MEDEIROS, M. H. F. et al. Pozolanas de elevada reatividade: uma avaliação crítica do ensaio de Índice de Atividade Pozolânica (IAP) com cal usando Difração de Raios X. Ambiente Construído, v. 15, n. 3, p. 19-29, jul./set. 2015.; MINERAIS…, 2009MINERAIS DO PARANÁ S/A. Avaliação do Potencial de argilas para uso em cerâmicas vermelhas. Programa de Desenvolvimento da Indústria de Cerâmica Vermelha no Estado do Paraná - RELATÓRIO FINAL. Curitiba, 2009.; SEVERO et al., 2013SEVERO, C. G. S. et al. Ativação alcalina de resíduos minerais com NaOH. Revista Eletrônica de Materiais e Processos, v. 8, n. 2, p. 106-109, 2013.).
Consistency index
The values of the consistency index of the obtained pastes were 6mm, 3mm and 1mm for the compositions C1, C2 and C3, respectively. From the obtained results, it is possible to observe that the reduction of the quantity of lime resulted in a reduction of the consistency index, for the same water/dry components relation. This behavior is due to the high specific area of the hydrated lime, which requires a greater amount of water increasing the consistency of the composition.
Nevertheless, it was possible to mold the samples without the use of a plasticizer additive or to change the amount of water in the pastes, which could cause the variation in the pore quantity and, consequently, reduction in the compressive strength.
Compressive strength
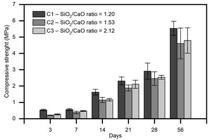
The behavior of the axial compressive strength as a function of the curing time of the compositions is shown in Figure 3.
After performing a multivariate statistical analysis of two factors (ANOVA) at a significance level of 5%, it was possible to prove that only age influenced the results obtained (p < 0.05). Hence, the SiO2/CaO ratio did not influence the mechanical strength of the samples. Figure 3 shows that the compositions at 28 days had a 520% increase in the strength values from 7 days and a 980% increase at 56 days.
Pereira-de-Oliveira, Castro-Gomes e Santos (2012) also observed that additions of up to 25% of tiles and ground ceramic blocks in Portland cement pastes do not generate a significant change in the axial compressive strength of the pastes. Thus, it is possible that the range that was chosen for this study (1.2 < SiO2/CaO < 2.12), based on previous studies, had already provided optimization of the compositions and the best ratio interval for the development for the reaction. Frías et al. (2008FRÍAS, M. et al. Influencia de la activación de un residuo arcilloso de la industria papelera en el comportamiento de matrices de cemento. Materiales de Construcción, v. 58, n. 292, p. 67-79, 2008.) evaluated different calcinated temperatures and mix proportions and reached similar strength results as those obtained in this paper.
X-ray diffractometry
Figure 4 shows the result of diffraction data for the 3 pastes compositions at 56 days. In the diffractograms it is possible to observe the presence of the anhydrous components Portlandite (P), Calcite (C), Quartz (Q), Brucite (B), Ilite (I), Hematite (H); and the hydrated components Hydrated Calcium Silicate (S) and Hydrated Calcium Monocarboaluminate (M).
From Figure 4 it is possible to observe the disappearing of the Portlandite main peak at 34.09º (2θ) from C1 to C3 at 56 days. Therefore, the availability of portlandite content changed among the compositions, however, a higher hydroxide concentration did not improve the reaction mechanics since strength differences were not observed and there were no visible changes in the Hydrated Calcium Silicate (S) main peak intensity at 29.50º (2θ). The XRD test was performed completely at the curing ages of 3, 7, 28 and 56 days and Figure 5 shows the variation of the intensity of the main peak for the elements: portlandite and hydrated monocarboaluminate over time.
The reduction in the intensity of the Portlandite peak, observed in all samples, is related to the consumption of this compound in the alkali-reaction resulting in hydrated compounds formation (ADORNO et al., 2018ADORNO, C. S. et al. Effects of the addition of red ceramic, limestone filler and rice husk ash in alkali silica reaction. Journal of Building Pathology and Rehabilitation, v. 3, n. 1, p. 1-11, 2018.; TREZZA et al., 2017TREZZA, M. A. et al. Portland blended cements: demolition ceramic waste management. Materiales de Construcción, v. 67, n. 325, p. 1-8, 2017.), although they do not present variations in the diffractograms as a function of the compositions. It can be observed in Figure 5 that the reduction of the Portlandite in compositions C1, C2 and C3 were, respectively, 45%, 47% and 70%. Therefore, the consumption of Portlandite is more evident in composition 3, which contained less availability of lime. Thus, it is possible that excess lime in the other compositions leads to a slower formation of hydrated compounds (HOPPE FILHO et al., 2013bHOPPE FILHO, J. et al. High-volume fly ash concrete with and without hydrated lime: chloride diffusion coefficient from accelerated test. Journal of Materials in Civil Engineering, v. 25, n. 3, p. 411-418, 2013b.). It was not possible to observe significant variations in the carbonation process since the calcite peaks at 29.42º (2θ) are proportional to the mass content of lime in the compositions (Table 1), that already had some calcite phases. Although hydrated monocarboaluminate formation was similar for the 3 compositions (average 42% increase), it is possible to form hydrated compounds of the C-S-H type with amorphous characteristics (not detectable in the XRD test) in pozzolanic reactions (HEWLETT, 2003HEWLETT, P. C. Lea’s chemistry of cement and concrete. 5. ed. Oxford: Butterworth-Heinemann Elsevier, 2003.; TAYLOR, 1997TAYLOR, H. F. W. Cement chemistry. 2. ed. London: Thomas Telford, 1997.).
Free lime content
From the titration (quantitative chemical analysis) of the samples, it was possible to confirm the consumption of the base (calcium and magnesium hydroxide) as shown in Figure 6. For the initial time (0 days) the theoretical calculation of the base content in the compositions was carried out. Here, the presence of the magnesium hydroxide is considered since the magnesium ion can also be chemically bonded during titration, however, during the XRD analysis no magnesium hydrated phases, such as hydrotalcite, were found.
Figure 6 shows that at the initial age the compositions have decreasing rates of base content. This is consistent because the lime content in the compositions is reduced in the same order (C1 > C2 > C3). Throughout the curing time, it is possible to observe the Brucite and Portlandite base consumption in the order of 70, 75 and 90% for compositions C1, C2 and C3, respectively. Thus, this consumption corroborates the hypothesis of the formation of hydrated siliceous structures, confirming the trend of the results of Figure 5. However, it is important to state that with the simultaneous solubilization of Brucite and Portlandite, the test may overestimate the portlandite content for dolomitic limes.
The same behavior was observed by Sánchez de Rojas et al. (2001)SÁNCHEZ DE ROJAS, M. I. et al. Investigación sobre la actividad puzolánica de materiales de desecho procedentes de arcilla cocida. Materiales de Construcción, v. 51, n. 261, p. 45-52, 2001. in the Fratini test performed on calcined clays. Lavat, Trezza and Poggi (2009LAVAT, A. E.; TREZZA, M. A.; POGGI, M. Characterization of ceramic roof tile wastes as pozzolanic admixture. Waste Management, v. 29, n. 5, p. 1666-1674, 2009.), in turn, observed accentuated drop in calcium hydroxide content in ceramic powder compositions with hydrated lime solution up to 7 days, with little variation up to 28 days. In the present study, consumption of Portlandite continued to occur markedly up to 28 days. This behavior indicates that the reaction presents a kinetic similar to that of Portland cement, with reaction progression significantly at initial ages and stabilization at 28 days, and can be explained by the stabilization of the pH and electric conductivity of the solutions (TASHIMA et al., 2014TASHIMA, M. M. et al. New method to assess the pozzolanic reactivity of mineral admixtures by means of pH and electrical conductivity measurements in lime:pozzolan suspensions. Materiales de Construcción, v. 64, n. 316, p. 1-12, 2014.).
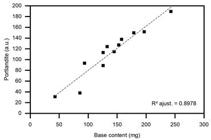
The difference observed between the works is expected, since in aqueous solution the ability of association of the compounds is facilitated. The reduction in the content of calcium hydroxide at 56 days for composition 3 (90% reduction) is also observed, due to the marked reduction of the remaining free lime content, shown in Figure 6, indicating a considerable consumption of alkali in the system by a higher content of ceramic. Figure 7 shows the linear correlation between the 100% intensity peaks attributed to Portlandite (34.098°) in the X-ray diffraction test and the titrated alkali content in the pastes.
In Figure 7, a good correlation (R² = 89.78%) between the peak intensity of Portlandite and the titrated hydroxide content in the samples is shown, indicating good X-ray diffraction test accuracy in a possible quantification of the element. Therefore, the monitoring of the main peak of the Portlandite diffraction data could help to evaluate its content and the hydration mechanism in alkali-activated binders.
Index void ratio and porosity
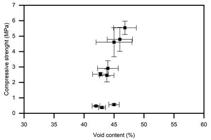
The relation between the index void ratios and the axial compressive strength is presented in Figure 8 and the pore size distribution by mercury intrusion porosimetry (MIP) is shown in Figure 9.
Figure 8 shows that the index void ratio test did not present statistically significant differences between the compositions (significance level of 5% in the ANOVA test). It can be noticed differences only between 7, 28 and 56 days. However, since the immersion void index test is capable of measuring only the capillary pores, there may be variations in the micropores of the structure that have not been measured. Figure 9 shows that the variation in the distribution of the average pore size occurred more strongly in the micropores volume, with a diameter smaller than 50nm (MEHTA; MONTEIRO, 2013MEHTA, P. K.; MONTEIRO, P. J. M. Concrete: microstructure, properties, and materials. 4. ed. Berkeley: McGraw-Hill Education, 2013.) as a function of the relation molar ratio of the compositions.
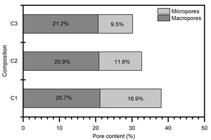
Figure 10 shows that the total porosity obtained in the intrusion test of samples C1, C2 and C3 was 38%, 32% and 30%, respectively. However, it can be noticed a variation in the micropores volume.
The change in the micropores can be related to the higher consumption of lime content observed in composition 3 (C3), with the formation of products inside the pores, reducing its average size. Although it was not observed the formation of crystalline hydrated phases during the DRX test, it is possible the formation of amorphous C-S-H and other hydrated phases that could explain the lower porosity. Micropores cannot influence the resistance of Portland cement composites, but they influence mass transport or diffusion capacity (MEHTA; MONTEIRO, 2013MEHTA, P. K.; MONTEIRO, P. J. M. Concrete: microstructure, properties, and materials. 4. ed. Berkeley: McGraw-Hill Education, 2013.). Thus, the same mechanical strength of the compositions evaluated is related to the same macro-porosity of the material.
Conclusion
Based on the results, it can be stated that it is possible to develop a pozzolanic reaction from ceramic waste, since the red ceramic residue from the mixed recycled aggregate was reactive with hydrated lime for the formation of hydrated phases, with development of resistance to axial compression of 5.5 MPa at 56 days.
The SiO2/CaO ratio had no statistically significant influence on the development of axial compression strength. However, in the molar range evaluated, the increase in silicon/calcium ratio resulted in a reduction in the non-combined lime content by 40% and a reduction in micropore volume by 7%, while the macropores remained unchanged.
X-ray diffraction and titration were efficient in the evaluation of the compounds consumed during hydration, being a parameter indicative of the evolution of reactions involving red ceramics, lime and water.
Thus, the alkali-activation reaction is capable of chemically altering a residue that is currently considered an environmental liability but could be used as a binder in the production of mortars and low strength compounds.
References
- ASSOCIAÇÃO BRASILEIRA DE EMPRESAS DE LIMPEZA PÚBLICA E RESÍDUOS ESPECIAIS. Panorama dos sólidos no Brasil 2018/2019. Panorama dos Resíduos Sólidos no Brasil. São Paulo, 2019. Disponível em:Disponível em:https://abrelpe.org.br/panorama/ Acesso em: 20 out. 2020.
» https://abrelpe.org.br/panorama/ - ADORNO, C. S. et al Effects of the addition of red ceramic, limestone filler and rice husk ash in alkali silica reaction. Journal of Building Pathology and Rehabilitation, v. 3, n. 1, p. 1-11, 2018.
- AMERICAN SOCIETY FOR TESTING AND MATERIALS. C618: standard specification for coal fly ash and raw or calcined natural pozzolan for use in concrete. West Conshohocken, 2019.
- AMERICAN SOCIETY FOR TESTING AND MATERIALS. D4404: standard test method for determination of pore volume and pore volume distribution of soil and rock by mercury porosimetry. West Conshohocken, 2018.
- AMERICAN SOCIETY FOR TESTING AND MATERIALS. D6226: standard test method for open cell content of rigid cellular plastics. West Conshohocken, 2015.
- AMORIM, L. V. et al Avaliação da atividade pozolânica de resíduos cerâmicos na construção civil. In: CONGRESSO BRASILEIRO DE CERÂMICA, 43., Florianópolis, 1999. Anais [...] Florianópolis, 1999.
- ANGULO, S. C. et al Chemical-mineralogical characterization of C&D waste recycled aggregates from São Paulo, Brazil. Waste Management, v. 29, n. 2, p. 721-730, 2009.
- ANGULO, S. C. et al Separação óptica do material cerâmico dos agregados mistos de resíduos de construção e demolição. Ambiente Construído, Porto Alegre, v. 13, n. 2, p. 61-73, abr./jun. 2013.
- ÂNGULO, S. C.; ZORDAN, S. E.; JOHN, V. M. Desenvolvimento sustentável e a reciclagem de resíduos na construção civil. In: SEMINÁRIO DE DESENVOLVIMENTO SUSTENTÁVEL E A RECICLAGEM NA CONSTRUÇÃO CIVIL EM ANAIS DO INSTITUTO BRASILEIRO DE CONCRETO COMITÊ TÉCNICO, 206., São Paulo, 2001. Anais [...] São Paulo, 2001.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 12653: materiais pozolânicos:requisitos. Rio de Janeiro, 2014.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 15116: agregados reciclados de resíduos sólidos da construção civil: utilização em pavimentação e preparo de concreto sem função estrutural: requisitos. Rio de Janeiro, 2004.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 7175: cal hidratada para argamassas: requisitos. Rio de Janeiro, 2003.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 7215: cimento Portland: determinação da resistência à compressão. Rio de Janeiro, 1996.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 9778: argamassa e concreto endurecidos: determinação da absorção de água, índice de vazios e massa específica. Rio de Janeiro, 2005.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR NM 43: cimento Portland: determinação da pasta de consistência normal. Rio de Janeiro, 2002.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR NM 76: cimento Portland: determinação da finura pelo método de permeabilidade ao ar (Método de Blaine). Rio de Janeiro, 1998.
- BARATA, M. S.; ANGÉLICA, R. S. Caracterização dos resíduos cauliníticos das indústrias de mineração de caulim da amazônia como matéria-prima para produção de pozolanas de alta reatividade. Cerâmica, v. 58, n. 345, p. 36-42, 2012.
- BEZERRA, A. C. S. et al Alkaline activation of high-calcium ash and iron ore tailings and their recycling potential in building materials. Ambiente Construído, Porto Alegre, v. 19, n. 3, p. 99-112, jul./set. 2019.
- CABRERA, J.; ROJAS, M. F. Mechanism of hydration of the metakaolin-lime-water system. Cement and Concrete Research, v. 31, n. 2, p. 177-182, 2001.
- CARNEIRO, B. S.; ANGÉLICA, R. S. Caracterização mineralógica e geoquímica e estudo das transformações de fase do caulim duro da região do Rio Capim, Pará. Cerâmica, v. 49, p. 237-244, 2003.
- CASTRO, A. L. et al Caracterização de cimentos compostos com resíduo da indústria de cerâmica vermelha. Cerâmica, v. 63, n. 365, p. 65-76, 2017.
- CORDEIRO, G. C.; DÉSIR, J. M. Potencial de argila caulinítica de Campos dos Goytacazes, RJ, na produção de pozolana para concreto de alta resistência. Cerâmica, v. 56, n. 337, p. 71-76, 2010.
- CORDEIRO, G. C. et al Influência da substituição parcial de cimento por cinza ultrafina da casca de arroz com elevado teor de carbono nas propriedades do concreto. Ambiente Construído, v. 9, n. 4, p. 99-107, out./dez. 2009.
- DERING, E. et al Estudo do comportamento mecânico e reatividade de aglomerante alternativo a base de fração vermelha de resíduo de construção e demolição, cal, sílica ativa e cimento portland. In: ENCONTRO NACIONAL DE TECNOLOGIA DO AMBIENTE CONSTRUÍDO, 14., Juiz de Fora, 2012. Anais [...] Juiz de Fora, 2012.
- DUXSON, P. et al Geopolymer technology: the current state of the art. Journal of Materials Science, v. 42, n. 9, p. 2917-2933, 2007.
- FACHINI, D. et al Comportamento no estado fresco de aglomerante alternativo a base de fração vermelha de RCD, cal, silica ativa e cimento Portland. In: ENCONTRO NACIONAL DE TECNOLOGIA DO AMBIENTE CONSTRUÍDO, 13., Canela, 2010. Anais [...] Canela, 2010.
- FERRAZ, E. et al Utilização do ensaio de Chapelle modificado para avaliação da reactividade pozolânica de metacaulinos. In: CONGRESSO PORTUGUÊS DE ARGAMASSAS, 4.; ETICS, Coimbra, 2012. Anais [...]Coimbra, 2012.
- FRÍAS, M. et al Influencia de la activación de un residuo arcilloso de la industria papelera en el comportamiento de matrices de cemento. Materiales de Construcción, v. 58, n. 292, p. 67-79, 2008.
- GARCIA, E. et al Avaliação da atividade pozolânica dos resíduos de cerâmica vermelha produzidos nos principais polos ceramistas do Estado de S. Paulo. Cerâmica, v. 61, n. 358, p. 251-258, 2015.
- GARCIA, E.; CABRAL JUNIOR, M.; CHOTOLI, F. F. Resíduo de Cerâmica Vermelha ( RCV ): uma alternativa como material pozolânico. Cerâmica Industrial, v. 19, n. 4, p. 31-38, 2014.
- GONÇALVES, J. P.; TOLEDO FILHO, R. D.; FAIRBAIRN, E. M. R. Estudo da hidratação de pastas de cimento Portland contendo resíduo cerâmico por meio de análise térmica. Ambiente Construído, v. 6, n. 4, p. 83-94, out./dez. 2006.
- HEWLETT, P. C. Lea’s chemistry of cement and concrete. 5. ed. Oxford: Butterworth-Heinemann Elsevier, 2003.
- HOPPE FILHO, J. et al High-volume fly ash concrete with and without hydrated lime: chloride diffusion coefficient from accelerated test. Journal of Materials in Civil Engineering, v. 25, n. 3, p. 411-418, 2013b.
- HOPPE FILHO, J. et al Atividade pozolânica de adições minerais para cimento Portland: índice de atividade pozolânica (IAP) e difração de raios X (DRX). In: CONGRESSO BRASILEIRO DO CONCRETO, 55., Gramado, 2013. Anais [...] Gramado, 2013a.
- HOPPE FILHO, J. et al Atividade pozolânica de adições minerais para cimento Portland (Parte I): índice de atividade pozolânica (IAP) com cal, difração de raios-X (DRX), termogravimetria (TG/DTG) e Chapelle modificado. Revista Matéria, v. 22, p. 1-18, 2017.
- HUSEIEN, G. F.; SHAH, K. W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Construction and Building Materials, v. 235, 2020.
- IDIR, R.; CYR, M.; PAVOINE, A. Investigations on the durability of alkali-activated recycled glass. Construction and Building Materials, v. 236, 2020.
- INTERNATIONAL CENTRE FOR DIFFRACTION DATA. JCPDS International Centre for Diffraction Data: Crystalography database. Newtown Square, 2003.
- JOHN, V.; AGOPYAN, V. Reciclagem de resíduos da construção. In: SEMINÁRIO - RECICLAGEM DE RESÍDUOS SÓLIDOS DOMICILIARES, São Paulo, 2000. Anais [...] São Paulo: CETESB, 2000.
- LAVAT, A. E.; TREZZA, M. A.; POGGI, M. Characterization of ceramic roof tile wastes as pozzolanic admixture. Waste Management, v. 29, n. 5, p. 1666-1674, 2009.
- LI, C.; SUN, H.; LI, L. A review: the comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cement and Concrete Research, v. 40, n. 9, p. 1341-1349, 2010.
- MADSEN, I. C.; SCARLETT, N. V. Y.; KERN, A. Description and survey of methodologies for the determination of amorphous content via X-ray powder diffraction. Zeitschrift fur Kristallographie, v. 226, n. 12, p. 944-955, 2011.
- MATIAS, G.; FARIA, P.; TORRES, I. Lime mortars with heat treated clays and ceramic waste: a review. Construction and Building Materials, v. 73, p. 125-136, 2014.
- MEDEIROS, M. H. F. et al Pozolanas de elevada reatividade: uma avaliação crítica do ensaio de Índice de Atividade Pozolânica (IAP) com cal usando Difração de Raios X. Ambiente Construído, v. 15, n. 3, p. 19-29, jul./set. 2015.
- MEHTA, P. K.; MONTEIRO, P. J. M. Concrete: microstructure, properties, and materials. 4. ed. Berkeley: McGraw-Hill Education, 2013.
- MINERAIS DO PARANÁ S/A. Avaliação do Potencial de argilas para uso em cerâmicas vermelhas. Programa de Desenvolvimento da Indústria de Cerâmica Vermelha no Estado do Paraná - RELATÓRIO FINAL. Curitiba, 2009.
- MIRANDA, L. F. R.; ANGULO, S. C.; CARELI, E. D. A reciclagem de resíduos de construção e demolição no Brasil: 1986-2008. Ambiente Construído, Porto Alegre, v. 9, n. 1, p. 57-71, jan./mar. 2009.
- MUNDRA, S. et al Modelling chloride transport in alkali-activated slags. Cement and Concrete Research, v. 130, 2020.
- PACHECO-TORGAL, F. et al Handbook of alkali-activated cements, mortars and concretes. Cambridge: Woodhead Publishing, 2015.
- PALOMO, A.; GRUTZECK, M. W.; BLANCO, M. T. Alkali-activated fly ashes: a cement for the future. Cement and Concrete Research, v. 29, n. 8, p. 1323-1329, 1999.
- PAN, Z. et al Hydration products of alkali-activated slag-red mud cementitious material. Cement and Concrete Research, v. 32, n. 3, p. 357-362, 2002.
- PATRICIO, S. M. R. et al Blocos solo-cal utilizando resíduo da construção civil. Cerâmica, v. 59, n. 349, p. 27-33, 2013.
- PEREIRA-DE-OLIVEIRA, L. A.; CASTRO-GOMES, J. P.; SANTOS, P. M. S. The potential pozzolanic activity of glass and red-clay ceramic waste as cement mortars components. Construction and Building Materials, v. 31, p. 197-203, 2012.
- PICANÇO, M. S.; ANGÉLICA, R. S.; BARATA, M. S. Avaliação preliminar do emprego de arenito zeolítico da região nordeste do Brasil como material pozolânico para cimento Portland. Cerâmica, v. 57, n. 344, p. 467-473, 2011.
- SÁNCHEZ DE ROJAS, M. I. et al Investigación sobre la actividad puzolánica de materiales de desecho procedentes de arcilla cocida. Materiales de Construcción, v. 51, n. 261, p. 45-52, 2001.
- SANTOS, H. S. et al Estudo por microscopia eletrônica das transformações durante a queima de argilas altamente aluminosas brasileiras. Cerâmica, v. 52, p. 125-137, 2006.
- SEVERO, C. G. S. et al Ativação alcalina de resíduos minerais com NaOH. Revista Eletrônica de Materiais e Processos, v. 8, n. 2, p. 106-109, 2013.
- SILVA, N. M. et al Quebra do grão em Resíduos de Construção Civil (RCC) induzida pelo processo de compactação. Ambiente Construído, Porto Alegre, v. 18, n. 1, p. 281-298, jan./mar. 2018.
- TASHIMA, M. M. et al New method to assess the pozzolanic reactivity of mineral admixtures by means of pH and electrical conductivity measurements in lime:pozzolan suspensions. Materiales de Construcción, v. 64, n. 316, p. 1-12, 2014.
- TAYLOR, H. F. W. Cement chemistry. 2. ed. London: Thomas Telford, 1997.
- TORGAL, F. P.; GOMES, J. P. C.; JALALI, S. Argamassas antigas: reacção pozolânica ou activação alcalina? In: Congresso Nacional de Argamassas de Construção - “Sob a Égide da Reabilitação”, 2., Lisboa, 2007. Anais [...] Lisboa: APFAC, 2007.
- TREZZA, M. A. et al Portland blended cements: demolition ceramic waste management. Materiales de Construcción, v. 67, n. 325, p. 1-8, 2017.
- VARGAS, A. S. et al Cinzas volantes álcali-ativadas com solução combinada de NaOH e Ca(OH)2. Revista Matéria, v. 12, n. 3, p. 8, 2007.
Publication Dates
-
Publication in this collection
13 Nov 2020 -
Date of issue
Jan-Mar 2021
History
-
Received
11 Nov 2019 -
Accepted
31 May 2020