Abstract
Cotesia glomerata is a natural enemy of the vegetable plague Ascia monuste orseis and preferably parasites 2nd, 3rd and 4th instar larvae. Parasitism effects on the haemolymph protein profile of Ascia monuste orseis larvae from the 2nd to 7th days were analyzed both qualitatively and quantitatively by SDS-PAGE and Coomassie-Blue binding methods. Quantitative analysis showed a progressive increase in the protein content of about 6.5 and 12.5 times in parasitized and non-parasitized larvae from the 2nd to 5th days, respectively. On the 6th day, a decrease in protein content was observed in both groups, although this decrease was significantly less than the control group that continued to metamorphosis. Meanwhile, parasitized larvae had one more day (7th day) in their larval period to complete parasitoid development, justified by the fact that parasitoid is koinobiont and allows host feeding. On this day, a drastic increase in protein content was detected when the parasitoids left the host. The SDS-PAGE showed proteins of high molecular weight (>120 kDa) on the 5th day of the non-parasitized larvae when they entered pre-pupa stage and on the 7th day of parasitized larvae. Proteins with MW lower than 62 kDa and higher than 27 kDa were absent on the 5th day in control larvae (pre-pupa phase), but present in parasitized larvae. This could indicate a possible relation between these proteins and the host juvenile hormone. Therefore, the presence of C. glomerata influences Ascia monuste orseis development, but its own physiological development is apparently independent of the host, which tends to die when parasitism succeeds.
Ascia monuste orseis; Cotesia glomerata; electrophoretic protein profile; haemolymph; natural enemy; parasitism; SDS-PAGE
ORIGINAL PAPER
Haemolymph electrophoretic pattern of Ascia monuste orseis larvae (Lepidoptera: pieridae) parasitized by Cotesia glomerata (Hymenoptera: braconidae)
M. ScagliaI; M. R. Brochetto-BragaI; J. Chaud-NettoI; N. GobbiII
IDepartment of Biology, Instituto Básico, Universidade Estadual Paulista (UNESP), Rio Claro, São Paulo, Brasil
IIDepartment of Ecology,Instituto Básico, Universidade Estadual Paulista (UNESP), Rio Claro, São Paulo, Brasil
Address to correspondence Address to correspondence M. R. BROCHETTO-BRAGA Departmento de Biologia, Instituto de Biociências, Universidade Estadual Paulista (UNESP), Rio Claro Caixa postal 199, 13506-900, Rio Claro, São Paulo, Brasil mrbbraga@rc.unesp.br
ABSTRACT
Cotesia glomerata is a natural enemy of the vegetable plague Ascia monuste orseis and preferably parasites 2nd, 3rd and 4th instar larvae. Parasitism effects on the haemolymph protein profile of Ascia monuste orseis larvae from the 2nd to 7th days were analyzed both qualitatively and quantitatively by SDS-PAGE and Coomassie-Blue binding methods. Quantitative analysis showed a progressive increase in the protein content of about 6.5 and 12.5 times in parasitized and non-parasitized larvae from the 2nd to 5th days, respectively. On the 6th day, a decrease in protein content was observed in both groups, although this decrease was significantly less than the control group that continued to metamorphosis. Meanwhile, parasitized larvae had one more day (7th day) in their larval period to complete parasitoid development, justified by the fact that parasitoid is koinobiont and allows host feeding. On this day, a drastic increase in protein content was detected when the parasitoids left the host. The SDS-PAGE showed proteins of high molecular weight (>120 kDa) on the 5th day of the non-parasitized larvae when they entered pre-pupa stage and on the 7th day of parasitized larvae. Proteins with MW lower than 62 kDa and higher than 27 kDa were absent on the 5th day in control larvae (pre-pupa phase), but present in parasitized larvae. This could indicate a possible relation between these proteins and the host juvenile hormone. Therefore, the presence of C. glomerata influences Ascia monuste orseis development, but its own physiological development is apparently independent of the host, which tends to die when parasitism succeeds.
Keywords: Ascia monuste orseis, Cotesia glomerata, electrophoretic protein profile, haemolymph, natural enemy, parasitism, SDS-PAGE.
INTRODUCTION
Ascia monuste orseis is one of the most important plagues of vegetables. The larvae of this Lepidoptera feed on leaves of several brassicaceous, causing serious damage to these crops.
Biological control of this plague can be very efficient when it is done by Cotesia glomerata, a gregarious koinobiont endoparasitoid that deposits its eggs in 2nd, 3rd or 4th instar Ascia larvae, where its own larvae develop. The preferential instar for host oviposition by Cotesia females and the rate of food consumption by parasitized and non-parasitized larvae of Ascia monuste orseis have been determined by Gobbi et al. (11) and Gobbi and Cunha (10).
Studies about parasite-host relationship revealed that the parasitoid is attracted to the host mainly by chemical substances exhaled from damaged fresh leaves and products from salivary glands and silk of the host (18). Faced with the need to control several pierids, Sato (16,17) demonstrated the efficiency of Cotesia glomerata parasitism in Pieris rapae crucivora, P. napi japonica, and P. napi nesis with mortality rates of 100%, 100%, and 40%, respectively. Pieris melete was also tested but showed a strong defense reaction, encapsulating the parasitoid eggs just as observed with Diatraea saccharalis parasitized by Cotesia flavipes (3,4). According to Dover et al. (7) parasitism is efficiency related to a polydnavirus located in the calyx cells, where it is transcribed and amplified. It is a normal component of the oviduct microflora of the female parasitoid, and a mutualist vertically transmitted (generation to generation) with an essential role in reproduction and survival of this braconid. The parasitoid eggs, deposited in the larvae hemocele overlaid with polydnavirus oviduct secretions, and venom are the components that protect them against the host encapsulating reaction. This endoparasitoid defense apparatus will cause qualitative and quantitative changes in host haemolymph protein profile. Studies carried out with Heliothis virescens larvae revealed that the haemolymph protein content quickly increased during the 5th day of life in both parasitized and non-parasitized larvae. Meanwhile, no significant increase in total proteins was observed in larvae that received an injection of calyx cells fluid from parasitoid (7).
Dushay and Beckage (8) demonstrated that the effects of the complex polydnavirus- venom, which affect the development and immunity of Manduca sexta, are different in some aspects. The endocrine/neuroendocrine system is more sensitive, being quickly brought into action in favor of the parasitoid, in spite of the host immunological system. This demonstrates that parasite-host interaction is continuously influenced by selective pressure for survival of both organisms. In a host, not all the endoparasitoids survive, but selective pressure indicates that the direction of both survival strategies seems to favor the parasitoids (22).
The purpose of this research was to evaluate the alterations of haemolymph protein profile in larvae of Ascia monuste orseis parasitized by Cotesia glomerata and to correlate these alterations with the physiological events leading to successful parasitism in the system.
MATERIALS AND METHODS
Insects
This study was carried out at the Ecology and Biology laboratories of the Rio Claro Institute of Biosciences (UNESP). Leaves of Brassica oleracea with Ascia monuste orseis eggs were collected and transferred to a glass recipient with water in the bottom until the emergence of larvae (approximately 72 hours after oviposition). The newly emerged larvae were introduced into a plastic box of 900cm3 containing Brassica oleracea leaves and maintained at 28 ± 2°C and 60 ± 10% RH. Fourth instar larvae were parasitized by Cotesia glomerata and their development was followed during the 5th instar. Then, haemolymphs of at least three parasitized larvae per day, from the 2nd to the 7th days, were extracted and analyzed. The same number of non-parasitized larvae from the 2nd to the 6th days had their haemolymphs collected and used as control (all experiments were performed in triplicate).
Chemicals
All compounds were obtained from Sigma Chemical Co. (St. Louis, Mo.).
Collection and preparation of haemolymph extracts
The haemolymph of each larva was collected under stereomicroscopy, using a capillary introduced into the 2nd or the 3rd abdominal segment. For this procedure, the larvae were maintained in Petri dishes cooled at 4°C. Soon after extraction, the haemolymph was transferred to eppendorf tubes containing a few crystals of glutathione to avoid melanization. The samples were diluted (1:1) with EDTA saline buffer (5 mM EDTA, 0.15 M KCl in 0.05 M NaPO4 pH 6.0) and centrifuged (15 min., 14,000xg, 4°C) to remove hemocytes. The supernatants were used as protein source.
Protein concentration in the haemolymph during parasitism
The dye binding method described by Sedmak and Grossberg (19) was used to determine haemolymph protein concentration.
Electrophoresis
Gradient SDS polyacrylamide gels (5-20%) were performed in a discontinuous buffer system (Tris-HCl/Tris-glycine), according to the Hames and Rickwood method (13). Samples (50mg of total protein, 5-10ml) already denatured were loaded in each 5mm-wide slot. Gels (10 X 15 X 1.5 cm) were run at constant voltage (150 V) at room temperature for about 2 hours. After electrophoresis, the gels were stained with 0.1% Coomassie blue G-250 in acetic acid: methanol: water (1: 3: 6, v: v: v), and distained with the same solution without the dye. Dalton Mark IV (Sigma) was used as a molecular weight (MW) marker; the protein band MW being determined by the RF method.
Statistical Analysis
The Student t test was used to evaluate significant differences between the mean values of haemolymph total protein content from parasitized and non-parasitized larvae.
RESULTS
The effect of Cotesia glomerata oviposition in the course of the Ascia monuste orseis development was both quantitatively and qualitatively evaluated. The quantitative analysis was performed by determining changes in total protein levels, and the qualitative analysis by determining changes in electrophoretic protein profile by SDS-PAGE.
Analysis of total protein levels
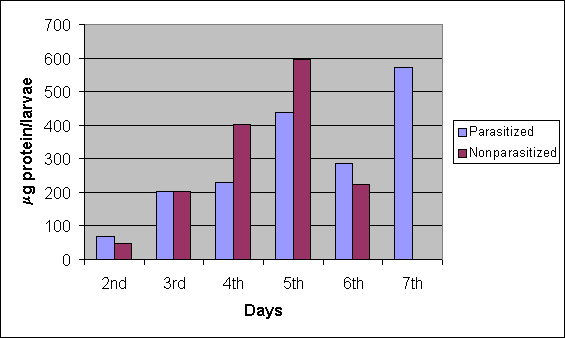
Haemolymph protein content from both parasitized and non-parasitized (control group) larvae increased progressively from the 2nd to the 5th days by about 6.5 and 12.5 times, respectively (Figure 1). However, on the 2nd day, the mean protein content value was lower in the controls (47.79 ± SD 3.21mg) than the parasitized group (67.42 ± SD 1.61mg) (t = 9.48; P<0.001), but the next day (3rd day), these values were similar in both groups (202.3 ± SD 4.35mg and 202.77 ± SD 2.58mg, respectively) t = 0.16; 0.0386 < P < 0.0767; (P = 0.0577). On the 4th and 5th days, the mean values obtained from controls were significantly higher in relation to parasitized groups, being respectively 400.65 ± SD 8.94mg and 299.37 ± SD 3.63 mg; t = 30.74; P< 0.001 and 596.12 ± SD 5.30mg to 436.65 ± SD 3.98mg; t = 41.69; P< 0.001. On the 6th day, a decrease in protein content was seen in both groups (224.02 ± SD 2.99mg and 285.45 ± SD 4.27mg; t = 20.45; P< 0.001), although this decrease had been significantly lower than the control group that continued to metamorphosis. Finally, a drastic increase in protein content (571.66 ± SD 7.56mg) was detected on the 7th day in parasitized larvae, when the parasitoids left the host.
Haemolymph protein content (mg/ larvae) in Ascia monuste orseis after parasitism by Cotesia glomerata from the 2nd to 7th days. Non-parasitized larvae served as controls.
Electrophoretic protein profile
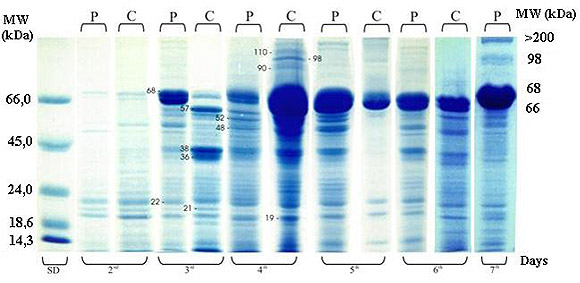
Haemolymph protein profile in parasitized (P) and non-parasitized (control group-C) larvae is shown in Figure 2. Higher levels of protein synthesis were observed both in the control and parasitized larvae from the 3rd to the 5th days. In our experiment, proteins were detected in the range of < 14.3 kDa to > 110 kDa. Proteins in the range of >14.3 to 66 kDa changed greatly in band intensity in all samples, and especially in control larvae from elevated levels in the 4th day to almost total absence on the 5th day. These proteins showed two bands of 57 and 36 kDa, much more evident in the control than parasitized larvae of the 3rd day. In addition, protein bands of 66 and 68 kDa were more intense on the 4th day in controls and on the 5th and the 7th days in parasitized larvae. Proteins of high molecular weight (MW) - 90, 98, 110, and > 110 kDa - were detected with high levels of band intensity only on the 4th day of the controls and the 7th day of parasitized larvae. After the 6th day, all control larvae continued to metamorphosis while parasitized larvae stayed one more day in their larval stage. Haemolymph analysis from this day showed no remarkable change on the electrophoretic protein profile in relation to the all other days, but the presence of the high molecular weight proteins (98 and > 110 kDa).
Electrophoretic pattern (5-20% gradient SDS-PAGE) of Ascia monuste orseis haemolymph from control (C) and parasitized larvae (P) by Cotesia glomerata. The larvae were stung on the 3rd instar by a parasitoid wasp, and haemolymph was collected from the 2nd through the 7th days later. Samples of 50mg of total protein were applied in each lane. Standard molecular weight (SD) is on the left. The marks on the gel point out specific bands of interest (see text).
DISCUSSION
Content of haemolymph total protein
Haemolymph protein content of the 4th and 5th day larvae post-parasitism was lower than control (Figure 1). This difference was due to the endoparasitoid maximum growth on the 2nd larval stage (L2), when part of the host nutrients was supplied to the parasitoids. C. glomerata is a parasitoid koinobiont that allows its host development. A koinobiont parasitoid has the ability to acquire specific nutrients at different instars of its host in order to get its own biomass (25). On the 6th day of the period preceding metamorphosis, the non-parasitized larvae presented low protein content in relation to parasitized larvae. However, the parasitized larvae had one additional day in their period of development in comparison to the controls. In addition, a significant increase in haemolymph protein content was observed before releasing of parasitoid larvae on the 7th day. Presumably, this additional day in the host larval stage could be advantageous to the gregarious parasitoid, since it keeps the host feeding longer, resulting in beneficial effects on its own nutrition.
In general, all braconid parasitoids reduce the growth and development of their typical host and prolong larval stage. For example, C. congregata inhibits feeding and growth of its host M. sexta (1). In the same way, Pseudaletia squax parasitized by Glyptapanteles museibeki consumes less food than non-parasitized larvae (6). However, Slansky (21) determined that Pieris rapae larvae parasitized by C. glomerata increased in total mass faster than non-parasitized larvae. In addition, Gobbi et al. (11) found that parasitized larvae of A. m. orseis consumed significantly more food than non-parasitized larvae in all tested instars.
Gobbi and Cunha (10) observed that the larval period of Cotesia glomerata in A. m. orseis was 11 days, but Dalhaman and Vinson (5) found that its embryogenesis and larval period were 3 and l0 days, respectively. Harvey et al. (14) compared the growth of C. rubecola in Pieris rapae and in P. brassica and observed that development of both parasitoids was completed only in the host that could grow after parasitism. These authors concluded that P. rapae was the best host for C. rubecola because it provided the best nutritional, physiological, and biochemical conditions.
In studies with Spodoptera littoralis parasitized in the later stages by Chelonus inanitus, Grossniklaus-Burgin et al. (12) observed that host growth and development occur in contrast with earlier instar. In addition to other results, the authors proposed that these effects are caused by polydnavirus and venom from parasitoid. In Pseudaletia separata parasitized by C. kariyai, the altered development is due to a disruption in the endocrinal process induced by polydnavirus and venom, which is not observed in control larvae with normal metamorphosis (from larvae to chrysalids) (23). Most of these alterations on the host growth and development have been associated with a reduction in the haemolymph protein concentration as detected in H. virescens - C. sonorensis system; in this case being a decrease specifically caused by a reduction in the synthesis of storage proteins from body fat (20).
All these above effects have been considered evidence of host regulation by the parasitoid, and the factors associated with it are believed to benefit the parasitoid (9).
Electrophoretic protein profile
Haemolymph protein profile of A. m. orseis larvae showed a progressive increase in the protein synthesis revealed by increase of band intensity following larva development (Figure 2). However, in the control larvae some bands were absent, weak, or more intense in relation to those observed in parasitized larvae by C. glomerata. For example, in the control larvae of the 3rd day, the 66 kDa protein band was weak but the 36 and 38 kDa protein bands were very intense. In these larvae of the 4th and 5th days, the major protein components were observed (Figure 2) in accordance with the increase in total protein content (Figure 1). In addition, the highest level of synthesis of proteins - MW 90, 98, and 110 kDa - also occurred in this period and these proteins only were again observed on the 7th day at less intensity. Maybe, they were associated with the parasitoid release on the subsequent day.
The small amounts of proteins in the range of > 14.3 and 66 kDa in control larvae of the 5th day must be associated with the pre-metamorphosis process, since on the 7th day all control larvae became chrysalids. The lack of these proteins was also observed in enzymatic electrophoretic profiles of the same extract.
Haemolymph proteins with MW around 42 and 71 kDa have been related to the class of juvenile hormone binding proteins in various Lepidoptera species (15,24). Production of specific proteins in the range of 70-80 kDa was also induced in Heliothis virescens haemolymph through either the injection of small amounts of Microplitis croceipes teratocytes or teratocyte secreted proteins (TSP) (26). Beckage et al. (2) found that in the M. sexta - C. congregata system proteins of 56 kDa and 60 kDa were only produced by normally parasitized larvae. Afterwards, using in vivo pulse labeling experiments with (S32) methionine to clarify the polydnavirus action, these authors detected that a 33 kDa polypeptide was produced in larvae that received an injection of calyx cell extract (where polydnaviruses are located). The authors concluded that this polypeptide results from a viral gene expression or is a protein induced by the virus presence.
In the S. littoralis - C. inanitus system the analysis of haemolymph proteins, Grossniklaus-Bürgin et al. (12) showed that the host not only contained a 212 kDa protein - absent in non-parasitized larvae - but also a large protein band of 70 kDa appeared one instar earlier in parasitized larvae. The protein of 212 kDa was induced by C. inanitus larvae in all instars investigated (1st, 2nd, and 3rd) and the authors also found it in the corresponding instar of their host: from the 3rd to the 5th instar when the parasitoid leaves the host.
In this study, small changes or biochemical alterations were observed on the haemolymph protein profile of Ascia larvae parasitized by Cotesia glomerata. The main changes were produced on the 3rd and 5th days as well as the one day extension in the host larval stage. In this 3rd to 5th day period after parasitism, the parasitoid larvae are in the 2nd instar and feed themselves intensely with the haemolymph nutrients, which could explain the low protein levels observed on the 3rd day of parasitized host larvae. Another hypothesis could be the quantity and size increase in the released parasitoid teratocytes, which are associated with host protein degradation to parasitoid nourishment. According to Dover et al. (7) parasitism efficiency is related to host immune system suppression by polydnavirus, possibly in the up and down regulation of the protein synthesis in parasitized larvae. However, the high protein levels observed in parasitized larvae haemolymph of the 4th and 5th days could be explained by the following: a) the host keeps its own normal development because the parasitoid C. glomerata is koinobiont; and b) host immune response suppression through polydnavirus action on protein synthesis up and down regulation respectively, on the 5th and 3rd days of parasitized larvae.
These results showed that C. glomerata is potentially able to modify the environment where its descendents should grow up; these changes being advantageous to its development. However, the regulatory mechanisms involved in physiological and biochemical changes of Ascia monuste orseis - C. glometrata system need to be more deeply investigated.
ACKNOWLEDGEMENTS
We thank A. Rodrigues and Marcelo A. Harada Penna for their important technical assistance. M. Scaglia received support from CNPq (National Council of Research), Process-142081/96-5.
Received December 17, 2001
Accepted March 25, 2002
- 1 BECKAGE NE., RIDDFORD LM. Incomplete host developmental arrest induced by parasitism of horworm Manduca sexta larvae by Apanteles smerinthi Ann. Entomol. Soc. Am, 1982, 75, 24-7.
- 2 BECKAGE NE., TEMPLETON TJ., NIELSEN BD., COOK DI., STOLTZ DB. Parasitism-induced haemolymph polypeptides in Manduca sexta (L) larvae parasitized by the Braconidae wasp Cotesia congregata (Say). Insect Biochem, 1987, 17, 439-55.
- 3 CAMPOS-FARINHA AE., CHAUD-NETTO J., GOBBI N. Biologia reprodutiva de Cotesia flavipes (Cam.) VI. Discriminação de lagartas parasitadas e não parasitadas de Diatraea saccharalis Fabr. (Lep.Pyralidae), tempo de desenvolvimento e razão sexual dos parasitóides. Arq. Inst. Biol., 2000, 67, 229-34.
- 4 CONTE H. Morfologia do corpo gorduroso em larvas de Diatraea saccharalis (Lepidoptera: Pyralidae) não parasitadas e parasitadas pelo Cotesia flavipes (Hymenoptera: Braconidae) Rio Claro: Universidade Estadual Paulista, Instituto de Biociências, 1994. 160p. (PhD. Thesis).
- 5 DAHLMAN DL., VINSON SB. Teratocytes developmental and biochemical characteristics. In: BECKAGE NE., THOMPSON SN., FREDRICI BA. Eds. Parasites and pathogens of insects San Diego: Academic Press, 1993: 145-65.
- 6 DOETEZER AK., FOERTER L. Efeito do parasitismo por Gliptapanteles muesebecki (Blanchard) no consumo e utilização do alimento por Pseudaletia sequax Franclemont. Ann. Soc. Entomol. (Brasil), 1998, 27, 255-64.
- 7 DOVER BA., MENON A., BROWN RC., STRAND MR. Suppression of juvenile hormone esterase in Heliothis virescens by Microplitis demolitor. J. Insect Physiol., 1995, 41, 817-20.
- 8 DUSHAY MS., BECKAGE N. Dose-dependent separation of Cotesia congregata associated polydnavirus. Effects on Manduca sexta larval development and immunity. J. Insect Physiol., 1993, 39, 1029-40.
- 9 FLEMING JGW. Polydnavirus: mutualist and pathogens. Annu. Rev. Entomol, 1992, 37, 401-25.
- 10 GOBBI N., CUNHA MCA. Observações preliminares referentes ao relacionamento entre lagartas de Ascia monuste orseis (Godart, 1818) (Lepidoptera - Pieridae) e seu parasita Apanteles ayerzay (Brethes, 1920) (Hymenoptera - Braconidae). Naturalia, 1983, 8, 193-6.
- 11 GOBBI N., JANINI AE., TAUK SM., FOWLER HG., SILVA OA. Efeito do parasitismo de Cotesia glomerata (Linnaeus,1758) (Hymenoptera-Braconidae) no consumo alimentar de Ascia monuste orseis (Lepidoptera- Pieridae). Ann. Soc. Entomol. (Brasil), 1989, 18, 169-75.
- 12 GROSSNIKLAUS-BÜRGIN C., PFISTER-WLHELEM R., MEYER V., TREIBLMAYR K., LANZERIN B. Physiological and endocrine changes associated with polydnavirus/venom in the parasitoid-host system Chelonus inanitus - Spodoptera littoralis. J. Insect Physiol,. 1998, 44, 305-21.
- 13 HAMES BD., RICKWOOD D. One dimensional polyacrylamide gel electrophoresis. In: BY HAMES BD., RICKWOOD D. Eds. Gel electrophoresis of proteins.: a practical approach. New York: IRL Press, 1990: 1-147.
- 14 HARVEY JA., JERVIS MA., GOLS R., JIANG N., VET LEM. Development of the parasitoid, Cotesia rubecula (Hymenoptera: Braconidae) in Pieris rapae and Pieris brassicae (Lep.: Pieridae): evidence for host regulation. J. Insect Physiol, 1999, 45, 173-82.
- 15 KRAMER KJ., CORPUZ L., CHOI HK., MUTHUKRISHNAN S. Sequence of a cDNA and expression of the gene encoding epidermal and Gut Chitinases of Manduca sexta. Insect Biochem. Mol. Biol., 1993, 23, 691-701.
- 16 SATO Y. Experimental studies on parasitization by Apanteles glomeratus L. (Hymenoptera: Braconidae) I. Parasitization of different species of genus Pieris. Appl. Entomol. Zool., 1976, 11, 165-75.
- 17 SATO Y. Experimental studies on parasitization by Apanteles glomeratus L (Hymenoptera: Braconidae ) II. Parasitization by Apanteles glomeratus L. Reared in Pieris melete Ménétriès. Appl. Entomol. Zool., 1977, 12, 330-3.
- 18 SATO Y. Experimental studies on parasitization by Apanteles glomeratus IV. Factors leading a female to the host. Physiol. Entomol, 1979, 4, 63-70.
- 19 SEDMAK JJ., GROSSBERG SE. A rapid, sensitive and versatile assay for protein using Coomassie Blue G-250. Anal. Biochem, 1977, 79, 544-52.
- 20 SHELBY KS., WEBB BA. Polydnavirus injection inhibits translation of specific growth-associated host proteins. Insect Biochem. Mol. Biol., 1997, 27, 262-70.
- 21 SLANSKY F. Utilization of energy and nitrogen by larvae of the imported cabbageworm. Pieris rapae, as affected by parasitism by Apanteles glomeratus Environ. Entomol, 1978, 7, 179-85.
- 22 SUMMERS MD., DIB-HAJJ S. Polydnavirus-facilitated endoparasite protection against host immune defense. Proc. Nat. Acad. Sci., 1995, 92, 29-36.
- 23 TANAKA T. Physiological interactions between a host, Pseudaletia (= Leucena) separata and its parasitoid, Microplitis mediator and Apanteles kariyai. Mem. Fac. Sci. Kyoto Univ, 1986, 11, 1-27.
- 24 VALAITIS AP. Characterization of haemolymph juvenile hormone esterase from Limantria dispar. Insect Biochem, 1991, 21, 583-96.
- 25 VINSON SB. Host selection by insect parasitoids. Annu. Rev. Entomol., 1976, 21, 109-33.
- 26 ZHANG DQ., DAHLMAN DL., JARLFORORS UE. Effects of Microplitis croceipes teratocytes on host haemolymph protein content and fat body proliferation. J. Insect Physiol, 1997, 43, 577-87.
Publication Dates
-
Publication in this collection
09 Dec 2003 -
Date of issue
2003
History
-
Accepted
25 Mar 2002 -
Received
17 Dec 2001