Abstract
Eighty-nine of 144 isolates of Clostridium perfringens obtained from 187 samples of 71 bovine in several Brazilian states were submitted to esterase electrophoresis for typing. Mobilites electrophoresis, as parameter, were settled down by isolates from ATCC pattern of types A, B, C, and D. Of the 89 isolates, 43 (48.3%) were characterized as electrophoretic type A, 20 (22.5%) as D, 18 (20.2%) as C, and 3 (3.4%) as B. Five (5.6%) isolates did not identity with any type. Similarly, esterase electrophoresis enabled the typing of 94.4 % of the isolates, demonstrating to be an appropriate method for animal sample analyses.
Clostridium perfringens; bovine; electrophoresis; esterase
ORIGINAL PAPER
Esterase electrophoresis of Clostridium perfringens bovine strains
L. BaldassiI; M.L. BarbosaII; E.E. BachIII; S.T. IariaIV
ILaboratório de Bacteriologia Geral e Micobacterioses, Instituto Biológico
IIInstituto Adolfo Lutz
IIIInstituto Biológico/UNINOVE
IVDepartamento de Microbiologia, Instituto de Ciências Biomédicas, Universidade de São Paulo, USP, São Paulo, Brasil
Correspondence Correspondence to L. Baldassi Laboratório de Bacteriologia Geral e Micobacterioses, Instituto Biológico, Instituto Biológico Avenida Conselheiro Rodrigues Alves, 1252 04014-002, São Paulo, SP, Brasil baldassi@biologico.sp.gov.br
ABSTRACT
Eighty-nine of 144 isolates of Clostridium perfringens obtained from 187 samples of 71 bovine in several Brazilian states were submitted to esterase electrophoresis for typing. Mobilites electrophoresis, as parameter, were settled down by isolates from ATCC pattern of types A, B, C, and D. Of the 89 isolates, 43 (48.3%) were characterized as electrophoretic type A, 20 (22.5%) as D, 18 (20.2%) as C, and 3 (3.4%) as B. Five (5.6%) isolates did not identity with any type. Similarly, esterase electrophoresis enabled the typing of 94.4 % of the isolates, demonstrating to be an appropriate method for animal sample analyses.
Keywords:Clostridium perfringens, bovine, electrophoresis, esterase.
INTRODUCTION
Identification of anaerobic microorganisms from the Clostridium genus allowed the characterization of diseases with different clinical signs, such as: tetanus, botulism, gaseous gangrene, and enterotoxaemia. From the pathogenic microorganisms, C. perfringens is the most widely spread and a prevalent agent in soil, sewer water, and the intestinal tract of man and animals (10,13,19,29).
Clostridium perfringens and its toxins are involved in several diseases: food toxinosis in man (16); septicemia after parturition and abortion; wound infection (19); pneumonia and empyema (5); meningitis (18); corneal wounds (35); mionecrosis (23); and cystitis (21). In animals, enterotoxaemia was observed (32,33).
Bacteria belonging to the C. perfringens species, a member of the Clostridium genus, are characterized as straight, Gram-positive bacilli, 0.9-1.3 µm wide by 3.0-9.0 µm long, with rounded edges and well-defined capsule. Different from the rest of the pathogenic clostridia, they are immobile and rarely produce spores both in tissues and usual laboratory media (8,14).
Classification of C. perfringens into types (A-E) is based on the production of one or more toxins - alpha, beta, epsilon, and iota (Table 1) (36). These toxins have several different biological properties including lethal activity (34). Therefore, the classification into different types is restricted to the use of laboratory animals (30). In addition, only the biological properties of the toxins are shown.
Immunodiffusion serological assays (9), contraimmuno-electrophoresis (11,15), radioimmunoassay, ELISA (4), and typing by bacteriocines (20) have resulted in advances in the detection and study of toxins. However, the above tests are not adequate for routine studies. The limitation of antisera techniques is that antiserum needs to be produced in animal, usually rabbit, but this is time consuming and expensive. Bacteriocin method does not use antiserum, however, this method is limited for typing. The use of polyacrilamide gel electrophoresis in the presence of sodium dodecil sulfate (SDS-PAGE) for the characterization of microorganisms has increased lately (17). In bacteriology, these studies have started with Escherichia coli (22) and then with some other bacteria, including a few anaerobic ones (28,37,38).
Electrophoretic analysis of bacterial enzymes has been used in studies of taxonomy, epidemiology, and population genetics (31,12). Electrophoretic studies of esterases may differentiate bacteria of the same species according to motility and isoesterase type; they may also help in epidemiological research (12,27).
In the last decade, Clostridium perfringens has been increasingly isolated in bovine samples representative of populations from all over the country, which were decimated by the disease (33). These losses represent serious problems for animal health, impacting on the economy and public health. Control measures should be established. Method standardization is required for isolate typing. These methods should be carried out by commercial laboratories, should not use laboratory animals, and should not depend on imported products. Based on these facts, the objective of this study was to classify and typify strains identified as Clostridium perfringens by biochemical methods isolated in the post-mortem examination of bovines, using the esterase electrophoretic polymorphism technique.
MATERIALS AND METHODS
Clostridium isolation
Samples obtained in the post-mortem examination of 71 bovines, clinically suspected of clostridiosis, were analyzed for the presence of anaerobic microorganisms. This led to the isolation of 187 strains of Clostridium spp, from which 144 were biochemically classified as C. perfringens according to Baldassi et al. (3). From this, 89 strains were selected; these samples came from the states of São Paulo (65), Bahia (5), Minas Gerais (3), Mato Grosso (10), Mato Grosso do Sul (1), Distrito Federal (1), Goiás (1), Piauí (1), Pará (1) and Pernambuco (1) (Table 2). Four C. perfringens strains from the American Type Culture Collection (ATCC), ATCC 3624 (type A), ATCC 3626 (type B), ATCC 3628 (type C) and ATCC 3629 (type D) were used as positive controls.
Both the standard and isolated strains were cultured in cooked meat broth (CMB) at 37°C for 18-24 hours and kept at 4°C. From this culture, a 3.0 µL aliquot was streaked in a plate containing Brain Heart Infusion (BHI) agar with 5% sheep blood and incubated in anaerobiosis in McIntosh & Fields jars at 37°C for 18 to 24 hours.
After incubation, colonies were observed in relation to aspect, color, presence, and type of hemolysis, and bacterial morphology, which was microscopically assessed in smears stained by Gram (20). Five colonies that were shiny Gram-positive bacilli presented double hemolysis, and straight edges were cultured in 10 mL of Tryptose-Yeast Extract broth (TYE) and incubated at 37°C for 18 to 24 hours.
Protein extraction and electrophoretic analysis
All cultures classified as Clostridium perfringens and the standards were submitted to protein extraction and electrophoresis analysis. All the volume in TYE was transferred to 90 mL of the same medium and incubated in the same conditions described (27). This culture was then centrifuged at 7,500xg, at 4°C for 15 minutes; the sediment was resuspended in 10.0 mL of tris-glycine buffer 60 mM, pH 8.7. The suspension was centrifuged and washed twice with the same buffer in order to remove the exopolysacharide present in the bacterial capsule. The sediment was then resuspended in 1.0 mL of the same buffer and ground in a porcelain mortar containing liquid nitrogen (1). The extract was centrifuged again at 7,500xg, at 4°C for 30 minutes for cell debris removal and the final sediment was submitted to protein concentration determination (6).
Aliquots equal to 18 µl of all extracts containing the equivalent to 250 µg of Bovine Serum Albumin (BSA) were applied to 7% polyacrilamide gel. After running the procedure using tris-glycine buffer 0.1M, pH 8.2, in horizontal equipment, gels were stained for the enzyme esterase according to Pons et al. (27).
All gels were dried in a BIO-RAD model 583 and were then analyzed by a densitometer (BIO-RAD, model GS-700) using Molecular Analyst software.
RESULTS
Analysis and interpretation of polyacrilamide gel was performed by three different procedures: naked eye, counting, and electrophoretic mobility measurement of the bands. This allowed the comparison of the results between the isolates with the standards.
Standard strains A, B, and D presented bands with characteristic electrophoretic mobility (Rm) (Figure 1) and correspondent absorbance x area were: strain A from 1.0 to 1.2 cm; strain B from 1.5 to 1.7 cm; strain D from 0.6 to 1.4 cm. Strain C, however, produced two bands: one 1.5 to 1.7 cm and the other 2.0 to 2.4 cm. These results were also confirmed by specific computer software and analyzed by electrophoretic migration graphs with absorbance x area (Figure 2).
Polyacrilamide gel stained for esterase - electrophoretic analysis of ATCC C. perfringens strains 3624 type A, 3626 type B, 3628 type C, and 3629 type D.
Mobility (cm) correlated with absorbance and area of ATCC C. perfringens strains 3624 type A, 3626 type B, 3628 type C, and 3629 type D.
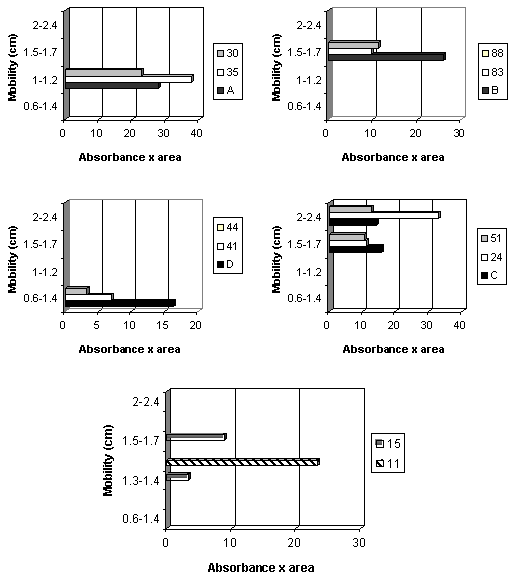
The 89 isolates and selected strains were classified according to the Rm parameters established for the standard strains. Figure 3 shows graphs of two isolates classified for each of the four C. perfringens types, and two without identification.
Mobility (cm) correlated with absorbance and area from electrophoretic analysis of 2 C. perfringens strains isolated and identified as types: A (isolates 35 and 30), B (isolates 83 and 88), C (isolates 24 and 51), D (isolates 41 and 44); and without type identification (isolates 11 and 15).
Gel analysis of all isolates identified as C. perfringens in a densitometer associated with computer resulted in 43 (48.3% of the total) strains classified as type A; 3 (3.4%) type B; 18 (20.2%) type C; 20 (22.5%) type D; and 5 (5.6%) that could not be placed in one group only.
DISCUSSION
Diseases caused by Clostridia are still a major economic problem in Brazil. Despite the fact that they may be well controlled when vaccination is performed rigorously, deaths in herds - mainly in bovines - caused by C. perfringens are responsible for serious economic losses, decrease in meat export, and in the amount protein available for the population. The cause of many of these deaths has not been determined as diagnosis is restricted to the genus and species of the bacterial agent whose type is not identified.
In this study, Clostridium strains isolated from samples obtained in post-mortem examination of bovine and biochemically identified as perfringens were studied in an attempt to determine their types.
The 89 strains biochemically classified as C. perfringens were initially elected by two basic characteristics: presence of double hemolysis in blood agar, although ICMSF (16) also refers to the existence of non-hemolytic colonies or present only one hemolysis halo; and lecithinase production in egg yolk agar even though Pinegar and Stringer (26) reported the occurrence of strains that are weakly producers of lecithinase or that do not produce it at all.
Electrophoretic studies may differentiate bacteria based on their motility and isoesterase types. The technique is useful in the differentiating between species, and the advantages are that it does not require specific and difficult-to-find reagents, in addition to allowing the analysis of several extracts in the same assay, which lasts only three hours (12,27). The number of extracts may vary according to the quantity of wells present in the gel. Another advantage is that samples from different sources, human or animal, may be analyzed. Thus, C. perfringens typing using esterase electrophoresis may be a milestone in the analysis applied to epidemiology and ecology (27).
Different authors studying free extracts from bacterial cells observed that esterases allowed the differentiation of serotypes of B. cereus (2), B. thurigiensis (24,25), and Mycobacterium species (7).
Esterase electrophoresis for C. perfringens was performed by Pons et al. (27) to identify the electrophoretic patterns in different animal species. In this study, these values were defined for one animal species (bovine) and the standards were the ATCC serological types A, B, C, and D. Analysis of esterase isoenzymes presented in the electrophoresis study allowed the separation between C. perfringens types; definition of the results was 94.4%.
The presence of characteristic bands for each ATCC standard was observed in the electrophoretic staining esterase (Figure 1). These bands presented different Rm values and correspondent absorbance x area, which were considered as markers for each type, thus allowing the comparison with those presented by each of the isolated strains tested (Figure 2). Further studies are necessary to compare toxin producer and electrophoretic type strains. From the 89 strains isolated and tested, 43 (48.3%) were characterized as belonging to electrophoretic type A; 20 (22.5%) as D; 18 (20.2%) as C; and 3 (3.4%) as B. Results obtained for the five strains (5.6%) that were classified into two types suggest the presence of more than one type of C. perfringens, which would not be detected by Gram staining or biochemical tests, because both would react in the same manner in these tests, as they belong to the same genus and species.
Based on the results of this study, we may conclude that the polymorphism of esterases presented a 94.4% definition in the classification of C. perfringens in its different types. From the 89 C. perfringens strains studied, type A was predominant (48.3%), followed by type D (22.5%), regardless of region or organ of origin. This technique also detects the circulating types and may greatly help in measuring the control and prevention of diseases caused by C. perfringens. As a consequence, it may increase the amount of bovine meat available due to the decrease of both lethality and risk of food poisoning related to this etiology.
ACKNOWLEDGMENTS
The authors would like to thank all the veterinarians that provided samples.
Received September 23, 2002
Accepted November 6, 2002
- 1 BACH EE., GUZZO SD. Serological relationship and chemical composition of exopolysaccharides (EPS) from different pathotype of Xanthomonas campestris pv. citri and X. campestris pv. manihotis. J. Phytopathol, 1994, 141, 1-9.
- 2 BAILLIE A., NORRIS JR. Studies of enzyme changes during sporulation in Bacillus cereus using starch gel electrophoresis. J. Appl. Bacteriol, 1963, 26, 102-6.
- 3 BALDASSI L., CALIL BEM., PORTUGAL MASC., MOULIN AAP., MOURÃO, MAF. Morte súbita de caprinos por enterotoxemia. Braz. J. Vet. Res. Anim. Sci., 1995, 32, 109-13.
- 4 BARTHOLOMEW BA., STRINGER MF. Observations on the purification of Clostridium perfringens type A enterotoxin and the production of a specific antiserum. FEMS Microbiol. Lett., 1983, 18, 43-8.
- 5 BAYER AS., NELSON SC., GALPIN JE., CHOW AW., GUZE LB. Necrotizing pneumonia and empyema due to Clostridium perfringens Report and review of the literature. Am. J. Med., 1975, 59, 851-9.
- 6 BRADFORD MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 1976, 72, 248-54.
- 7 CANN DC., WILLOX ME. Analysis of multimolecular enzymes as an aid to the identification of certain rapidly growing Mycobacteria, using starch gel electrophoresis. J. Appl. Bacteriol., 1965, 28, 165.
- 8 CATO EP., GEORGE WL., FINEGOLD SM. Genus Clostridium Prazmowski 1880. In: BERGEYS manual of systematic Bacteriology Baltimore: Williams & Wilkins, 1986, 1141-200.
- 9 ELLNER VR., BOHAN CD. Serology of the soluble antigens of Clostridium perfringens types A-F by agar-gel diffusion. J. Bacteriol, 1962, 83, 284-96.
- 10 ESTY JR. The biology of Clostridium welchii J. Bacteriol, 1920, 5, 375-429.
- 11 GIUGLIANO LG., STRINGER MF., DRASAR BS. Detection of Clostridium perfringens enterotoxin by tissue culture and double-gel diffusion methods. J. Med. Microbiol., 1983, 16, 233-7.
- 12 GOULLET PH., PICARD B. Characterization of enterobacteria by esterase specific-activity profiles. J. Gen. Microbiol, 1990, 136, 431-40.
- 13 HATHEWAY CL. Toxigenic Clostridia. Clin. Microbiol. Rev., 1990, 3, 66-98.
- 14 HOLLIDAY MG. Rapid identification of Clostridium perfringens by counter-immunoelectrophoresis. Med. Lab. Sci., 1985, 42, 322-5.
- 15 HOLT JG., KRIEG NR., SNEATH PHA., STALEY JT., WILLIAMS ST. Bergeys manual of determinative Bacteriology 9.ed. Baltimore: Williams & Wilkins, 1994, 559-60.
- 16 INTERNATIONAL COMISSION ON MICROBIOLOGICAL SPECIFICATIONS FOR FOOD OF THE INTERNATIONAL UNION OF BIOLOGICAL SOCIETIES FOR FOODS. Clostridium perfringens In: JAMES & JAMES. Micro-organisms in foods 5: microbiological specifications of food pathogens London: Chapman & Hall, 1996: 112-5.
- 17 LAEMMLI UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature, 1970, 227, 680-5.
- 18 MACKAY NNS., GRUNEBERG RN., HARRIES BJ., THOMAS PK. Primary Clostridium welchii meningitis. Br. Med. J., 1971, 1, 591-2.
- 19 MACLENNAN JD. Histotoxic clostridial infection of man. Bacteriol. Rev., 1962, 26, 177-276.
- 20 MAHONY DE. Bacteriocin susceptibility of Clostridium perfringens: a provisional typing schema. Appl. Microbiol., 1974, 28, 172-6.
- 21 MALIWAN N. Emphysematous cystitis associated with Clostridium perfringens bacteremia. J Urol., 1979, 121, 819-20.
- 22 MILKMAN R. Electrophoretic variation in Escherichia coli from natural sources. Science, 1973, 182, 1024-6.
- 23 MOHR JA., GRIFFITHS W., HOLM R., GARCIA-MORAL C., FLOURNOY DJ. Clostridial myonecrosis (gas gangrene) during cephalosporin prophylaxis. J. Am. Med. Assoc., 1978, 239, 847-9.
- 24 NORRIS JR. Electrophoretic analysis of bacterial esterase systems-an aid to taxonomy. J. Gen. Microbiol, 1962, 28, 7.
- 25 NORRIS JR. Classification of Bacillus thuringiensis J. Appl. Bacteriol, 1964, 27, 439-47.
- 26 PINEGAR JA., STRINGER MF. Outbreaks of food poisoning attributed to lecithinase-negative Clostridium welchii J. Clin. Pathol., 1977, 30, 491-2.
- 27 PONS JL., PICARD B., NIEL P., LELUAN G., GOULLET P. Esterase electrophoretic polymorphism of human and animal strains of Clostridium perfringens Appl. Environm. Microbiol., 1993, 59, 496-501.
- 28 POXTON IR., BROWN R. Sodium dodecyl sulphate-polyacrilamide gel electrophoresis of cell-surface proteins as an aid to the identification of the Bacteroides fragilis group. J. Gen. Microbiol, 1979, 112, 211-7.
- 29 ROOD JI., COLE ST. Molecular genetics and pathogenesis of C. perfringens Microbiol. Rev., 1991, 55, 621-48.
- 30 ROSS HE., WARREN ME., BARNES JM. Clostridium welchii iota toxin: its activation by trypsin. J. Gen. Microbiol, 1949, 3, 148-52.
- 31 SELANDER RK., CAUGANT DA., OCHMAN H., MUSSER JM., GILMOUR MN., WHITTAM TS. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl. Environm. Microbiol., 1986, 51, 873-84.
- 32 SIGURDARSON S., THORSTEINSSON T. Sudden death of Icelandic dairy cattle. Vet. Rec., 1990, 127, 410.
- 33 SILVEIRA D., SOUZA AM., MESQUITA AJ. Enterotoxemia em bovinos: uma enfermidade de importância emergente. Bol. Tec. Inf. Rhodia-Mérieux, 1995, 2, 1-4.
- 34 SMITH LD., HOBBS G. Genus Clostridium Prazmowski, 1880. In: BUCHANAN RE., GIBBONS NE. Eds. Bergeys manual of determinative bacteriology 8 ed. Baltimore: Williams & Wilkins, 1974, 551-72.
- 35 STERN GA., HODES BL., STOCK EL. Clostridium perfringens corneal ulcer. Arch. Ophtalmol., 1979, 97, 661-3.
- 36 STERN M., WARRACK GH. The types of Clostridium perfringens J. Pathol. Bacteriol., 1964, 88, 2079-83.
- 37 STROM A., DYER JK., MARSH C., TRIBBLE JL. Identification and characterization of species of the family Bacterioidaceae by polyacrylamide gel electrophoresis. J. Dental Res., 1976, 35, 252-6.
- 38 SWINDLEHURST CA., SHAH HN., PARR CW., WILLIAMS RA. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of polipeptides from Bacteroides melaninogenicus J. Appl. Bacteriol, 1977, 43, 319-24.
Publication Dates
-
Publication in this collection
09 Dec 2003 -
Date of issue
2003
History
-
Received
23 Sept 2002 -
Accepted
06 Nov 2002