Abstract
The immunohistochemical expression of neuron-specific enolase, NSE (a cytoplasmic glycolytic enzyme of the neurons), synaptophysin, SYN (a major membrane glycoprotein of synaptic vesicles), and Bcl-2 (anti-apoptotic protein) were determined in cerebral cortex of rats envenomed with neurotoxic venom from Egyptian cobra. Male rats were intramuscularly (IM) injected with a single injection of either physiological saline solution or ½ LD50 or LD50 of cobra venom and sacrificed 24, 48, or 72 hr after envenoming. Formalin-fixed paraffin sections were immunohistochemically studied by avidin-biotin-peroxidase complex method. Neuron histological structure and isolation of genomic DNA were also detected. The results showed a dose and time-dependent increase in NSE and SYN immunoreactivity in cerebral cortex of envenomed rats except in 72 hr high dose envenoming, where decreased SYN was observed. On the other hand, low dose venom induced high Bcl-2 expression 24 hr after envenoming, while the high dose decreased Bcl-2 protein expression. Temporal and spatial Bcl-2 expression was accompanied by DNA fragmentation in cerebral cortex of all envenomed rats, although no serious histological alterations were noticed. These results suggest that cobra venom may lead to neuronal injury and impairment of axonal transport as ascertained by alterations in NSE and SYN immunoreactivity. It could also indicate that venom alters the molecular machinery of apoptosis by inhibiting Bcl-2 expression; however, some vulnerable cells have the ability to overcome this by increasing Bcl-2 protein. These immunohistochemical investigations can be used as tools for detecting neuronal abnormalities even before the occurrence of any histological alterations in case of cerebral cortex neurotoxicity.
immunohistochemistry; neuron specific enolase; synaptophysin; Bcl-2; cerebral cortex; cobra snake venom
ORIGINAL PAPER
Immunohistochemical investigation of neuronal injury in cerebral cortex of cobra-envenomed rats
Rahmy T.R.I; Hassona I.A.II
IZoology Department, Faculty of Science, Suez Canal University, Ismailia, Egypt
IIBiology Department, Faculty of Science, United Arab Emirates University, UAE
Correspondence Correspondence to T. R. Rahmy Zoology Department, Faculty of Science Suez Canal University, Ismailia, Egypt E-mail: tarekrah@hotmail.com
ABSTRACT
The immunohistochemical expression of neuron-specific enolase, NSE (a cytoplasmic glycolytic enzyme of the neurons), synaptophysin, SYN (a major membrane glycoprotein of synaptic vesicles), and Bcl-2 (anti-apoptotic protein) were determined in cerebral cortex of rats envenomed with neurotoxic venom from Egyptian cobra. Male rats were intramuscularly (IM) injected with a single injection of either physiological saline solution or ½ LD50 or LD50 of cobra venom and sacrificed 24, 48, or 72 hr after envenoming. Formalin-fixed paraffin sections were immunohistochemically studied by avidin-biotin-peroxidase complex method. Neuron histological structure and isolation of genomic DNA were also detected. The results showed a dose and time-dependent increase in NSE and SYN immunoreactivity in cerebral cortex of envenomed rats except in 72 hr high dose envenoming, where decreased SYN was observed. On the other hand, low dose venom induced high Bcl-2 expression 24 hr after envenoming, while the high dose decreased Bcl-2 protein expression. Temporal and spatial Bcl-2 expression was accompanied by DNA fragmentation in cerebral cortex of all envenomed rats, although no serious histological alterations were noticed. These results suggest that cobra venom may lead to neuronal injury and impairment of axonal transport as ascertained by alterations in NSE and SYN immunoreactivity. It could also indicate that venom alters the molecular machinery of apoptosis by inhibiting Bcl-2 expression; however, some vulnerable cells have the ability to overcome this by increasing Bcl-2 protein. These immunohistochemical investigations can be used as tools for detecting neuronal abnormalities even before the occurrence of any histological alterations in case of cerebral cortex neurotoxicity.
Key words: immunohistochemistry, neuron specific enolase, synaptophysin, Bcl-2, cerebral cortex, cobra snake venom.
INTRODUCTION
Neurons are considered the most important components in the nervous system; astrocytes are supportive structure elements in the nervous system (2). The neuron-astrocyte interaction is important in the maintenance of brain homeostasis and is vital for neuronal survival following brain injury (34). Our previous study (11) indicated reactive astrocytosis in cerebral cortex of rats envenomed with cobra venom. Astrocytosis is considered to be related to neuronal abnormalities rather than solely a repair event following brain injury (4).
Detection of brain injury needs some immunohistochemistry techniques including NSE and other antibodies (35). Nogami et al. (28) revealed that anti-NSE immunostaining of neurons could be useful in evaluating neuronal damage in the brain injury region. Ding et al. (5) added that reduced NSE immunoreactivity reflects neuron loss. However, Kim and Suhr (18) found that tumor cells were strongly positive for NSE and SYN immunohistochemical expression. SYN is a major membrane glycoprotein of synaptic vesicles that is ubiquitously expressed in all neurons (21). It provides a useful tissue marker of synaptogenesis during normal conditions and may provide clues to cerebral pathogenesis (33). Most studies have assessed SYN as a measure of synaptic integrity (22).
Expression of the cell death regulatory protein, Bcl-2, has also been suggested to evaluate the mechanisms of neuronal cell death (9). Bcl-2 inhibits neuronal apoptosis during normal brain development as well as that induced by cytotoxic drugs or growth factor deprivation (29). Apoptotic neuronal cell death is accompanied by endonucleosomal DNA cleavage and differential expression of the anti-apoptotic protein Bcl-2 (36). Benjelloun et al. (3) added that cell survival was assessed by Bcl-2 expression and cell death was demonstrated by DNA fragmentation.
The purpose of this study was to investigate the established mechanisms of neuronal injury and apoptosis and their relevance to cell death induced by an environmental neurotoxin such as cobra venom. This will be achieved by detecting immunoexpression of selected neuron-biomarkers (NSE, SYN, and Bcl-2) in cerebral cortex of envenomed rats.
MATERIALS AND METHODS
Snake venom
Lyophilized venom of the Egyptian cobra (Naja haje) snake was reconstituted in saline solution. Two doses were used in this study:
1) ½ LD50 (0.0125 mg cobra venom/ g body weight); and
2) LD50 (0.025 mg cobra venom/ g body weight).
LD50 dose was used according to Rahmy and Hemmaid (30).
Experimental animals
Male healthy rats (Rattus rattus) weighing 200 ± 10 g were kept in laboratory conditions and provided with water and food. The rats were divided into 3 groups:
1. Control group. Eight normal rats intramuscularly (IM) injected with 0.1 ml saline solution, sacrificed 72 hr after injection.
2. Low dose envenomed group. Rats IM injected with 0.1 ml saline containing ½ LD50 dose of cobra venom. The rats were subdivided into three subgroups (10 rats each) sacrificed 24, 48, 72 hr after venom injection, respectively.
3. High dose envenomed group. Rats IM injected with 0.1 ml saline containing LD50 dose of cobra venom. The rats were subdivided into three subgroups (10 rats each) sacrificed 24, 48, 72 hr after venom injection, respectively.
Tissue sampling
Five rats from the control group and from each envenomed subgroup were anesthetized by chloroform. Each rat was transcardially perfused with 100 ml saline phosphate buffer (0.1 M, pH 7.6) followed by 400 ml of 10% neutral buffered (0.1 M phosphate buffer) formalin fixative solution. Brains were removed from each rat then fixed in the same fixative for 8 hr, followed by overnight washing in running tap water. The anterior part of the brain samples were dehydrated, cleared, and then infiltrated and embedded in paraffin wax. Paraffin blocks were serially sectioned to produce 5m-thick sections of cerebral cortex. Serial sections of cerebral cortex were used for histological and immunohistochemical studies.
The rest of non-perfused rats from control group and each envenomed subgroup were also anesthetized by chloroform. Brains were removed from each rat and stored at -80ºC to be used for DNA extraction.
Histological studies
Paraffin sections were stained with hematoxylin and eosin for regular histological investigation of control and envenomed tissues.
Immunohistochemical studies
Paraffin sections of perfused brains from control and envenomed groups were used for detecting NSE, SYN, and Bcl-2 immunoreactivity. The sections were de-waxed and incubated for 1hr at room temperature in 0.3% hydrogen peroxide in phosphate-buffered saline, pH 7.6 (PBS). The slides were washed 3 times (10 min. each) in the same buffer to quench endogenous peroxidase activity. They were incubated for 16 hr at 4 °C in PBS containing 2% normal goat serum (NGS) and 0.5% triton X-100, and then washed again in PBS at room temperature. This was followed by overnight incubation at 4°C with the primary monoclonal antibody (Mouse anti-EMA, ICN, Costa Mesa, CA, USA and Sigma Chem. Comp.) of anti-NSE, anti-SYN, or anti-Bcl-2 then washed 3 time in PBS-2% NGS. The primary antibodies were bounded by a rat adsorbed biotinylated anti-mouse secondary antibody (1,200, Vector Labs, Burlingame, CA, USA), in PBS for 1hr at room temperature. Slides were incubated in avidin-biotin complex linked to peroxidase (ABC Kit, Vector Labs, Burlingame, CA, USA). Peroxidase was seen with 0.03% diaminobenzidine hydrochloride and 0.005% hydrogen peroxide in 0.1 M Tris buffer. All sections were counter stained with hematoxylin dehydrated, cleared, and mounted in Canada balsam. Sections from all groups were simultaneously processed in the same tray.
Isolation of genomic DNA
DNA was extracted from 1g of brain tissue (from each group) frozen by liquid nitrogen and ground to a fine powder according to Sambrook et al. (31). Extracted DNA samples were fractionated by 1% Agarose gel electrophoresis. Twenty ml were loaded for each sample at a power supply of 60 V for 3 h. After electrophoresis run, the gel was photographed on a UV transilluminator at 312 nm wavelength using a Polaroid MP-4 land camera.
RESULTS
Histological neuron detection
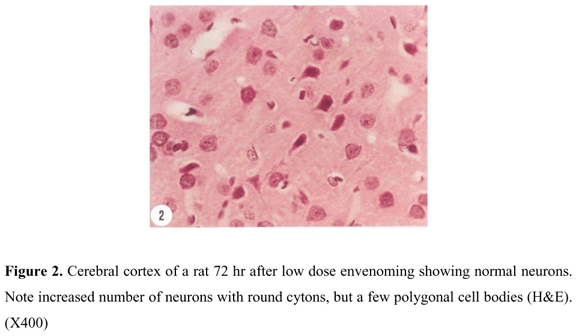
Cerebral cortex of control rats showed normal neurons with polygonal to round cell bodies. The nuclei were large with condensed chromatin in comparison with those of surrounding support cells. Control neurons also contained dense basophilic cytoplasm (Figure. 1). No serious histological alterations were recorded following envenoming with low or high dose venom except for the appearance of more rounded neurons characterized by dispersed chromatin and prominent nucleoli which reflect a high level of protein (enzyme) synthesis (Figure 2).
NSE immunohistochemical expression
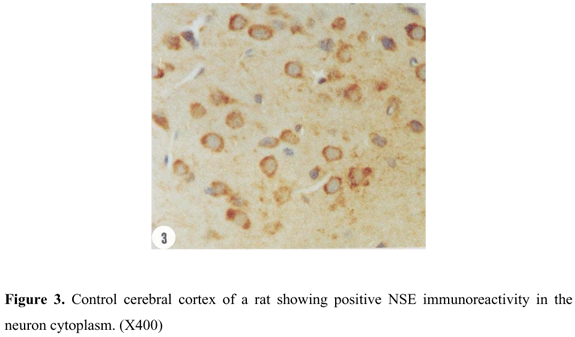
Cerebral cortex of control rats showed positive NSE reactivity represented by the neuron cytoplasm (Figure 3). Similar reactivity was recorded in cerebral cortex of rats envenomed in 24 hr low dose envenoming. A time dependent increase in neuron NSE reactivity was recorded 48 hr (Figure 4) and 72 hr (Figure 5) after envenoming. Another form of time-dependent increase in NSE reactivity was shown by neurons in cerebral cortex of high dose envenomed rats (Figures 6, 7, and 8 ).
SYN immunohistochemical expression
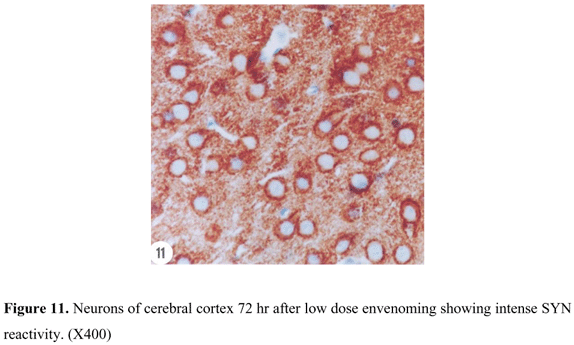
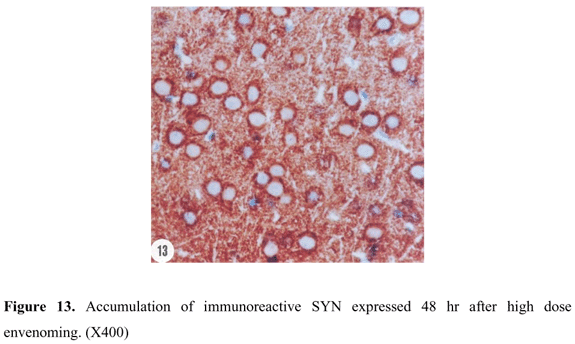
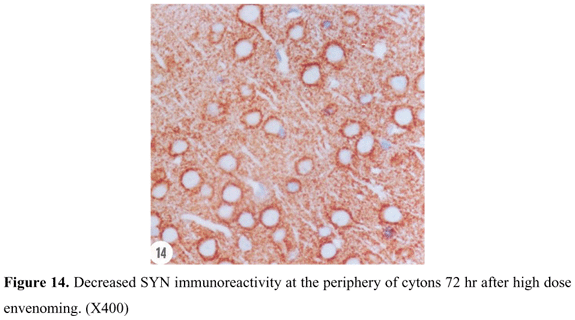
SYN reactivity was represented in a form of coarsely fine beaded reactivity at the neuron surface in cerebral cortex of control rats. Reactive granules were also scattered in the intercellular matrix between the neurons (Figure 9). Twenty-four hours after low dose envenoming, SYN reactivity was more or less similar to that of the control group. Forty-eight hours (Figure 10) and 72 hr (Figure 11) after envenoming with the same dose, a time-dependent increase in SYN immunoexpression was noticed in a form of dense bands at the periphery of the neurons. Twenty-four hr after high dose envenoming (Figure 12), SYN reactivity was increased in comparison to controls. Dense reactive bands were highly accumulated around the neurons and within the space between them 48 hr after high dose envenoming (Figure 13). However, SYN reactivity was decreased 72 hr after envenoming at this dose (Figure 14).
Bcl-2 immunohistochemical expression
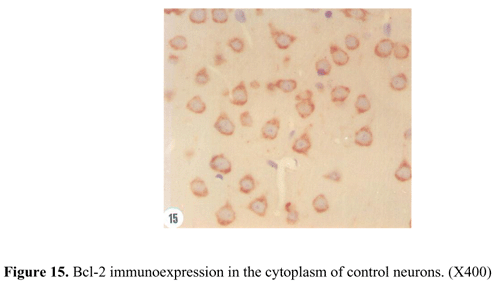
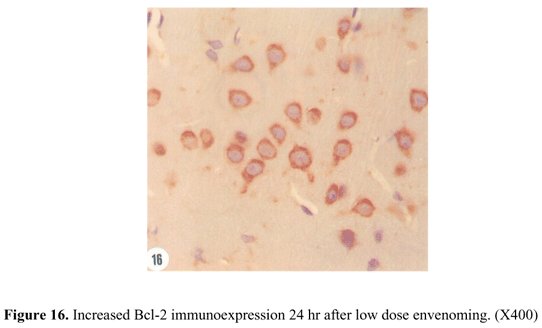
The control animals showed an evident expression of Bcl-2 protein located in all cell cytoplasmic components, but the nuclei were not immunostained (Figure 15). A rapid increase of Bcl-2 immunoreactivity was observed in cerebral cortex of low dose rats 24 hr after envenoming (Figure 16). This increase was reduced by 50% 48 hr after envenoming; however, the expression was still more pronounced than that of the control animals. Nevertheless, Bcl-2 immunoreactivity was progressively decreased 72 hr after envenoming to a level less than control animals. The diminished level of Bcl-2 expression included decreased number of immunostained cells and lower intensity of immunoreactivity (Figure 17).
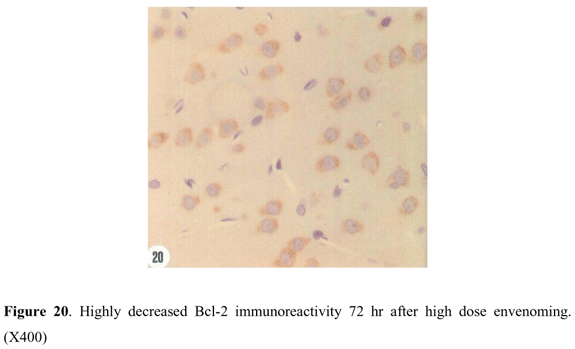
A loss of Bcl-2 immunoreactivity was also observed within the cerebral cortex 24 hr after high dose envenoming (Figure 18). Decline of Bcl-2 expression extended throughout the first 48 hr to a level lower than that of the control. However, only a few neuronal cells with intense Bcl-2 reactivity were noticed in certain areas of the cerebral cortex 24 hr and 48 hr after envenoming (Figure 19). This enhanced Bcl-2 immunoreactivity was not seen 72 hr after high dose envenoming. It seems likely that Bcl-2 protein expression is markedly inhibited after this time (Figure 20).
Genomic DNA isolation
Treatment with cobra venom induced DNA fragmentation in samples extracted from cerebral cortex of envenomed rats (Figure 21). This fragmentation was clearly evident when compared with DNA extracted from control animals and was represented by a single band in lane 1. Application of low dose induced a time-dependent DNA fragmentation. This was observed at 24 hr (lane 2), increased at 48 hr (lane 3), and markedly noticeable 72hr after envenoming (lane 4). A similar pattern of time-dependent DNA fragmentation was also seen in high dose envenoming (lanes 5, 6, and 7). In comparison, high dose was more effective in inducing DNA fragmentation than the low dose.
DISCUSSION
Snake venoms are not only limited to envenoming victims, but they are also proving to be valuable research tools and diagnostic agents. In this study, cobra venom, which is known to contain a variety of postsynaptic and presynaptic neurotoxins (20), was used to induce neurotoxic models to investigate NSE, NYS, and Bcl-2 immunoreactivity during neurotoxicity.
The results indicated a dose and time-dependent increase in NSE and SYN immunoexpression in cerebral cortex of envenomed rats. NSE is a biochemical diagnostic marker, which reflects pathogenic processes in the brain and is used to identify ongoing neuronal degeneration (38). The presence of NSE immunoreactivity indicates no evidence of neuronal cell loss (26). However, NSE overexpression could be correlated with overall histological evidence of damage (32). In this way, Woertgen et al. (40) proved a close relationship between NSE release and the severity of traumatic brain injury in a cortical impact model.
On the other hand, SYN is a synaptic protein involved in regulating transmitter release and synaptic plasticity (39). It is a very reliable presynaptic marker (24). SYN accumulation in a form of disclosed large coarse pericellular reactivity indicates abnormal axon transport (8). Increased SYN immnuoreactivity is usually associated with impaired synaptic function, resulting in cognitive deficits (19). This impairment could be due to a possible blockage of neuron receptors by common postsynaptic cobra toxins (41) so that synaptic vesicles are accumulated around neurons. This was confirmed by Apel et al. (1) who reported that the long chain neurotoxin alpha-cobratoxin do not affect the release of acetylcholine, but effect a block at the post-synaptic nicotinic receptors.
On the contrary, decreased SYN immunoreactivity 72 hours after high dose envenoming could be considered as a late-stage phenomenon, while its increase occurs at an early stage of injury (25). Decreased SYN is suggestive of synaptic pathology (7) and may occur as tissue degeneration progresses (37). It also reflects either post-transcriptional abnormalities of SYN or a diminished number of axonal projections of neurons of cerebral cortex (10).
This disturbance in NSE and SYN immunoreactivity could indicate possible neuronal abnormalities although this was not proved by routine histological techniques. However, neuronal abnormalities could be indicated by immunoexpression of the anti-apoptotic protein Bcl-2, which can determine whether a neuron survives or dies (23). In this way, Offen et al (29) explained that Bcl-2 overexpression in neurons provides protection against neurotoxins, while neurons deficient in Bcl-2 are more susceptible to neurotoxins. Jayanthi et al. (17) added that neuronal cells that overexpress Bcl-2 protein are protected against apoptosis. Thus, the increase in Bcl-2 24 hr after low dose envenoming is consistent with a protective role of neurons against apoptosis. However, the decreased Bcl-2 immunoexpression after 48 and 72 hr low dose envenoming and after high dose may signal neuronal vulnerability to pro-apoptotic stimuli and neuronal atrophy (16). Nevertheless, some vulnerable cells have the ability to overcome apoptosis by increasing Bcl-2 protein at certain stages of envenoming. Decrease in Bcl-2 protein is known to occur prior to apoptosis and could be considered as an important step in pro-apoptotic response to neurotoxicity (12). Therefore, it is likely that Bcl-2 reduction contributes to apoptosis and neuronal cell death (6). Niu et al. (27) explained that apoptosis is induced in neural cells by Bcl-2 down regulation and that the rate of apoptosis is inversely correlated with Bcl-2 expression.
Apoptotic changes of neuronal cells were also confirmed by the regional distribution of DNA fragmentation, which coincided with decreased Bcl-2 expression in cerebral cortex of envenomed rats. DNA fragmentation is considered as an early event in neuronal death following brain injury (42). The appearance of increased laddered DNA fragmentation is considered as hallmarks of apoptosis (13). Jan et al. (15) explained that cobra venom causes apoptosis in brain tumor cells by activating the pro-apoptotic c-Jun N-terminal kinase signal transduction pathway. It seems that these nuclear DNA changes and the disturbance in Bcl-2 immunoexpression occur earlier than cytoplasmic degeneration and involve a possible apoptotic neuron death mechanism (14).
These immunohistochemical studies could be considered as indicators of the disturbance in neuron functional activities and tools for detecting neuronal pathology even in the absence of obvious histological alterations. It is believed to be useful in cases of patients who survive for a short time after a fatal neurotoxic injury but without obvious structural alterations.
Received: July 29, 2002
Accepted: November 11, 2002
Published online: February 14, 2004
-
1APEL C., RICNY J., WAGNER G., WESSLER I. Alpha-Bungarotoxin, kappa-bungarotoxin, alpha-cobratoxin and erabutoxin-b do not affect [3H]acetylcholine release from the rat isolated left hemidiaphragm Naunyn Schmiedebergs. Arch. Pharmacol., 1995, 352, 646-52.
-
2BARRES BA. New roles for glia. J. Neurosci., 1991, 11, 3685-94.
-
3BENJELLOUN N., RENOLLEAU S., REPRESA A., BEN-ARI Y., CHARRIAUT-MARLANGUE C. Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal rat. Stroke, 1999, 30, 1916-23.
-
4CERUTTI SM., CHADI G. S100 immunoreactivity is increased in reactive astrocytes of the visual pathways following a mechanical lesion of the rat occipital cortex. Cell Biol. Int., 2000, 24, 35-49.
-
5DING M., HAGLID KG., HAMBERGER A. Quantitative immunochemistry on neuronal loss, reactive gliosis and BBB damage in cortex/striatum and hippocampus/amygdala after systemic kainic acid administration. Neurochem. Int., 2000, 36, 313-8.
-
6DONG GX., SINGH DK., DENDLE P., PRASAD RM. Regional expression of Bcl-2 mRNA and mitochondrial cytochrome c release after experimental brain injury in the rat. Brain Res., 2001, 903, 45-52.
-
7EASTWOOD SL., CAIRNS NJ., HARRISON PJ. Synaptophysin gene expression in schizophrenia. Investigation of synaptic pathology in the cerebral cortex. Br. J. Psychiatry, 2000, 176, 236-42.
-
8FERRER I., PUIG B., BLANCO R., MARTI E. Prion protein deposition and abnormal synaptic protein expression in the cerebellum in Creutzfeldt-Jakob disease. Neuroscience, 2000, 97, 715-26.
-
9GERBER J., BRUCK W., STADELMANN C., BUNKOWSKI S., LASSMANN H., NAU R. Expression of death-related proteins in dentate granule cells in human bacterial meningitis. Brain Pathol., 2001, 11, 422-31.
-
10GLANTZ LA., AUSTIN MC., LEWIS DA. Normal cellular levels of synaptophysin mRNA expression in the prefrontal cortex of subjects with schizophrenia. Biol. Psychiatry, 2000, 48, 389-97.
-
11HASSOUNA IA., RAHMY TR. Reactive astrocytic response and increased proliferating cell nuclear antigen expression in cerebral cortex of envenomed rats. J. Toxicol. Toxin Rev., 2001, 20, 245-59.
-
12HOSSAIN A., TSUCHIYA S., MINEGISHI M., OSADA M., IKAWA S., KIKUCHI H. The Ah receptor is not involved in 2,3,7,8-tetrachloridibenzo-p-dioxin-mediated apoptosis in human leukemic T cell lines. J. Biol. Chem., 1998, 237, 19853-8.
-
13HOU ST., CALLAGHAN D., FOURNIER MC., HILL I., KANG L., MASSIE B., MORLEY P., MURRAY C., RASQUINHA I., SLACK R., MACMANUS JP. The transcription factor E2F1 modulates apoptosis of neurons. J. Neurochem., 2000, 75, 91-100.
-
14ISUMI H., UCHIDA Y., HAYASHI T., FURUKAWA S., TAKASHIMA S. Neuron death and glial response in pontosubicular necrosis. The role of the growth inhibition factor. Clin. Neuropathol., 2000, 19, 77-84.
-
15JAN S., MAO C., VASSILEV A., NAVARA C., UCKUN F. COBRA-1, a rationally-designed epoxy-THF containing compound with potent tubulin depolymerizing activity as a novel anticancer agent. Bioorg. Med. Chem. Lett., 2000, 10, 1193-7.
-
16JARSKOG LF., GILMORE JH., SELINGER ES., LIEBERMAN JA. Cortical Bcl-2 protein expression and apoptotic regulation in schizophrenia. Biol. Psychiatry, 2000, 48, 641-50.
-
17JAYANTHI S., DENG X., BORDELON M., MCCOY MT., CADET JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J., 2001, 15, 1745-52.
-
18KIM DH., SUHR YL. Pseudopapillary neurocytoma of temporal lobe with glial differentiation. Acta Neuropathol. (Berl.), 1997, 94, 187-91.
-
19KING DL., ARENDASH GW. Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging, correlations with cognitive impairment. Brain Res., 2002, 926, 58-68.
-
20LACHUMANAN R., ARMUGAM A., DURAIRAJ P., GOPALAKRISHNAKONE P., TAN CH., JEYASEELAN K. In situ hybridization and immunohistochemical analysis of the expression of cardiotoxin and neurotoxin genes in Naja naja sputatrix. J. Histochem. Cytochem., 1999, 47, 551-60.
-
21MCMAHON H., BOLSHAKOV V., JANZ R., HAMMER R., SIEGELBAUM S., SÜDHOF T. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc. Natl. Acad. Sci. USA, 1996, 93, 4760-4.
-
22MINGER SL., HONER WG., ESIRI MM., MCDONALD B., KEENE J., NICOLL JÁ., CARTER J., HOPE T., FRANCIS PT. Synaptic pathology in prefrontal cortex is present only with severe dementia in Alzheimer disease. J. Neuropathol. Exp. Neurol., 2001, 60, 929-36.
-
23MOONEY SM., MILLER MW. Effects of prenatal exposure to ethanol on the expression of Bcl-2, bax and caspase 3 in the developing rat cerebral cortex and thalamus. Brain Res., 2001, 911, 71-81.
-
24MORI F., TANJI K., YOSHIMOTO M., TAKAHASHI H., WAKABAYASHI K. Immunohistochemical comparison of alpha- and beta-synuclein in adult rat central nervous system. Brain Res., 2002, 941, 118-26.
-
25MUKAETOVA-LADINSKA EB., GARCIA-SIERA F., HURT J., GERTZ HJ., XUEREB JH., HILLS R., BRAYNE C., HUPPERT FA., PAYKEL ES., MCGEE M., JAKES R., HONER WG., HARRINGTON CR., WISCHIK CM. Staging of cytoskeletal and beta-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer's disease. Am. J. Pathol., 2000, 157, 623-36.
-
26MULLER MB., LUCASSEN PJ., YASSOURIDIS A., HOOGENDIJK WJ., HOLSBOER F., SWAAB DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur. J. Neurosci., 2001, 14, 1603-12.
-
27NIU Y., ZHANG R., CHENG Y., SUN X., TIAN J. Effect of lead acetate on the apoptosis and the expression of Bcl-2 and bax genes in rat brain cells. Zhonghua Yu Fang Yi Xue Za Zhi, 2002, 36, 30-3.
-
28NOGAMI M., TAKATSU A., ISHIYAMA I. Immunohistochemical study of neuron-specific enolase in human brains from forensic autopsies. Forensic Sci. Int., 1998, 94, 97-109.
-
29OFFEN D., BEART PM., CHEUNG NS., PASCOE CJ., HOCHMAN A., GORODIN S., MELAMED E., BERNARD R., BERNARD O. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity. Proc. Natl. Acad. Sci. USA, 95, 5789-94.
-
30RAHMY TR., HEMMAID KZ. Prophylactic action of garlic on the histological and histochemical patterns of hepatic and gastric tissues in rats injected with a snake venom. J. Nat. Toxins, 2001, 10, 137-65.
-
31SAMBROOK J., FRITSCH E., MANIATIS T. Molecular cloning, a laboratory manual 2ed. New York: Cold Spring Harbor laboratory, 1989 3v.
-
32SANKAR R., SHIN DH., WASTERLAIN CG. Serum neuron-specific enolase is a marker for neuronal damage following status epilepticus in the rat. Epilepsy Res., 1997, 28, 129-33.
-
33SARNAT H., BORN D. Synaptophysin immunocytochemistry with thermal intensification, a marker of terminal axonal maturation in the human fetal nervous system. Brain Dev., 1999, 21, 41-50.
-
34SASAKI S., IWATA M. Immunocytochemical and ultrastructural study of the motor cortex in patients with lower motor neuron disease. Neurosci. Lett., 2000, 281, 45-8.
-
35SOETRISNO E., TJAHJADI G. Pathological aspects of brain tumors. Gan To Kagaku Ryoho, 2000, 27, 274-8.
-
36STUMM G., SCHLEGEL J., SCHAFER T., WURZ C., MENNEL HD., KRIEG JC., VEDDER H. Amphetamines induce apoptosis and regulation of Bcl-x splice variants in neocortical neurons. FASEB J., 1999, 13, 1065-72.
-
37TANAKA S., SAITO M., MORIMATSU M., OHAMA E. Immunohistochemical studies of the PrP(CJD) deposition in Creutzfeldt-Jakob disease. Neuropathology, 2000, 20, 124-33.
-
38WALLIN A., BLENNOW K., ROSENGREN L. Cerebrospinal fluid markers of pathogenetic processes in vascular dementia, with special reference to the subcortical subtype. Alzheimer Dis. Assoc. Disord., 1999, 13, S102-S7.
-
39WEBSTER MJ., SHANNON WEICKERT C., HERMAN MM., HYDE TM., KLEINMAN JE. Synaptophysin and GAP-43 mRNA levels in the hippocampus of subjects with schizophrenia. Schizophr. Res., 2001, 49, 89-98.
-
40WOERTGEN C., ROTHOERL RD., BRAWANSKI A. Neuron-specific enolase serum levels after controlled cortical impact injury in the rat. J. Neurotrauma, 2001, 18, 569-73.
-
41YANG CC. Cobratoxin, structure and function. J. Nat. Toxins, 1999, 8, 221-33.
-
42ZHANG ZG., BOWER L., ZHANG RL., CHEN S., WINDHAM JP., CHOPP MO. Three-dimensional measurement of cerebral microvascular plasma perfusion, glial fibrillary acidic protein and microtubule associated protein-2 immunoreactivity after embolic stroke in rats, a double fluorescent labeled laser-scanning confocal microscopic study. Brain Res., 1999, 844, 55-66.
Correspondence to
Publication Dates
-
Publication in this collection
02 Apr 2004 -
Date of issue
2004
History
-
Received
29 July 2002 -
Accepted
11 Nov 2002