Abstract
Components with phospholipase A2 activity were isolated by gel filtration and cationic exchange chromatography from the venom of Bothrops asper snakes from Chiriguaná, Colombia (9°22´N; 73°37´W). Five fractions were obtained by the gel filtration, and PLA2 activity was found in fraction 3 (F3). In the cationic exchange chromatography, F3 showed eight components with PLA2 activity. Six of these components appeared as one band in polyacrylamide gel electrophoresis (SDS-PAGE). Fractions II and VII exhibited an optimal activity at pH 9 and 52ºC. The optimum calcium concentration for fraction II was 48 mM and for fraction VII, 384 mM. Both fractions showed thermal stability. Fraction II was stable at pH values between 2.5 and 9, and fraction VII, between 2.5 and 8. The Michaelis Menten constant (K M) was 3.5x10-3 M for fraction II and 1.6x10-3 M for fraction VII. The molecular weight was 16,000 Dalton for fraction II and 17,000 Dalton for fraction VII. Both isoenzymes did not show any toxic activity (DL50) at 5.3 and 4 µg/g. The two fractions showed different kinetic constant (K M), calcium requirement, and substrate specificity for haemolytic activity.
phospholipase; isoenzymes; snake; venom; Bothrops asper; Colombia
ORIGINAL PAPER
Purification and partial characterization of phospholipases A2 from Bothrops asper (barba amarilla) snake venom from Chiriguaná (Cesar, Colombia)
Ramírez-Avila J.I; Quevedo B. E.II; López E.III; Renjifo J. M.I
ISerum Group, National Institute of Health (INS), Bogotá, Colombia, South America
IIFaculty of Engineering, National University of Colombia, Bogotá, Colombia, South America
IIIDepartment of Chemistry, Faculty of Sciences, National University of Colombia, Bogotá, Colombia, South America
Correspondence Correspondence to J. Ramírez-Avila Avenida calle 26, N° 51-60, Bogotá, D. C., Colombia South America Phone: (571) 220-77-00, Ext. 485. Fax: (571) 222-34-68 Email: jramirezavila61@yahoo.com
ABSTRACT
Components with phospholipase A2 activity were isolated by gel filtration and cationic exchange chromatography from the venom of Bothrops asper snakes from Chiriguaná, Colombia (9°22´N; 73°37´W). Five fractions were obtained by the gel filtration, and PLA2 activity was found in fraction 3 (F3). In the cationic exchange chromatography, F3 showed eight components with PLA2 activity. Six of these components appeared as one band in polyacrylamide gel electrophoresis (SDS-PAGE). Fractions II and VII exhibited an optimal activity at pH 9 and 52ºC. The optimum calcium concentration for fraction II was 48 mM and for fraction VII, 384 mM. Both fractions showed thermal stability. Fraction II was stable at pH values between 2.5 and 9, and fraction VII, between 2.5 and 8. The Michaelis Menten constant (KM) was 3.5x10-3 M for fraction II and 1.6x10-3 M for fraction VII. The molecular weight was 16,000 Dalton for fraction II and 17,000 Dalton for fraction VII. Both isoenzymes did not show any toxic activity (DL50) at 5.3 and 4 µg/g. The two fractions showed different kinetic constant (KM), calcium requirement, and substrate specificity for haemolytic activity.
Key words: phospholipase, isoenzymes, snake, venom, Bothrops asper, Colombia
INTRODUCTION
Phospholipases A2 (PLA2s) (EC.3.1.1.4) catalyzes the hydrolysis of the 2-acyl ester linkage of 1,2-diacyl-3-sn-phosphoglycerides, with Ca2+ requirement (20,25). These enzymes play an important role in the lipid metabolism, and have been widely used to study the structure of lipoproteins (1) among other applications.
Snake venoms are particularly rich in PLA2s (5,12,15,22) and some of them contain more than one isoform of this enzyme. Many of these isoenzymes have similar molecular weights but can be differentiated in a small number of amino acids (9,20) by ion-exchange chromatography (14,15,26) or isoelectric focusing (23). Snake venom PLA2s are able to induce several biological effects, such as pre-synaptic or post-synaptic neurotoxicity, cardiotoxicity, myotoxicity, platelet aggregation, oedema formation, haemolysis, anticoagulation, convulsion, and hypotension (26). This diversity of physiological functions of PLA2s isoenzymes from snake venoms is very important for the production of antivenoms and to understand the accelerated evolution of this enzyme in the Viperidae venom (19,20). Therefore, it is also very important to identify the diversity of isoforms present in the same species and in different populations.
In Colombia, snakes of the Bothrops genus have medical importance since they are responsible for more than 90% of the total snakebites (unpublished information from INS). Bothrops asper (Family Viperidae, Subfamily Crotalinae) is the only species of that genus found in northern Colombia. It is poorly differentiated from Bothrops atrox, and there is a great controversy upon the taxonomic status of these two species (2). Snakes of the Bothrops genus from northern Colombia are classified as B. asper, based on the data published by Campbell and Lamar (2).
In the present study, we report the isolation and partial characterization of snake venom fractions with phospholipases A2 activity from B. asper snakes from Chiriguaná (Cesar), northern Colombia.
MATERIALS AND METHODS
Venom
B. asper venom was obtained from several specimens collected in Chiriguaná (Cesar, Colombia). Venom was filtered, lyophilized and stored at -20°C in the venom bank of the Instituto Nacional de Salud, Bogotá, D. C., Colombia.
Isolation of phospholipase A2
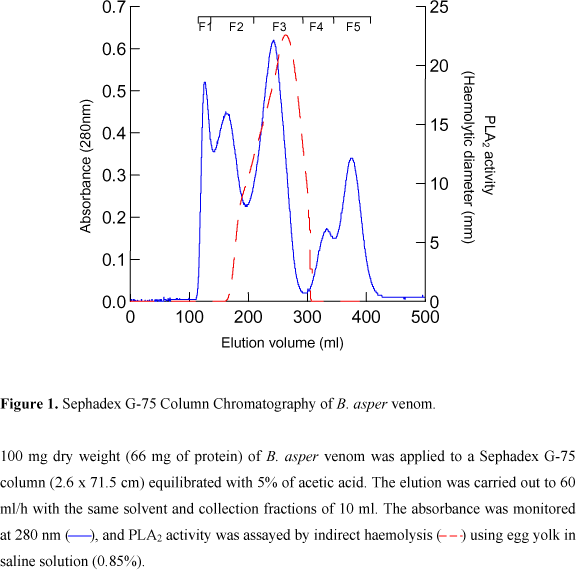
Crude venom (100 mg) was dissolved in 1 ml of 5% acetic acid, cleared by centrifugation for 30 minutes at 1,800 g, submitted to gel filtration on Sephadex G-75 (Pharmacia LKB, Sweden) column (2.6 x 71.5 cm), and then, eluted with the same solvent. Fractions of 10 ml were collected at a flow rate of 60 ml/h using a Frac-200 fraction collector (Pharmacia LKB). F3 with PLA2 activity was taken up from the gel filtration and applied to a Sephadex G-25 (Pharmacia LKB) column (1.6 x 14.5 cm) previously equilibrated, and then, eluted with 10 mM of acetic acid, pH 5.
F3 in 10 mM of acetic acid, pH 5 was applied to ion-exchange chromatography on a MONO S (HR 5/5) column (Pharmacia LKB), which had been previously equilibrated with a similar solvent. The elution used a constant concentration gradient, from 0 to 1 M of NaCl with the same solvent. Fractions of 1 ml were collected at 60 ml/h. The separation was done on FPLC (Pharmacia LKB); the absorbances at 280 nm, conductivities and collected fractions were monitored. The resulting eight peaks were collected, pooled, and dialyzed using membranes (Spectra/Por 1) with a molecular weight of 6,000-8,000 Da, and later lyophilized. An aliquot of dialyzed fractions was used for the determination of protein concentration by the Lowry method (13).
Polyacrylamide gel electrophoresis (SDS-PAGE)
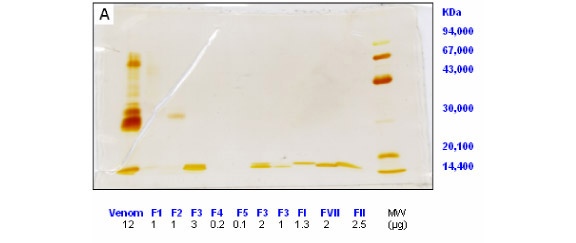
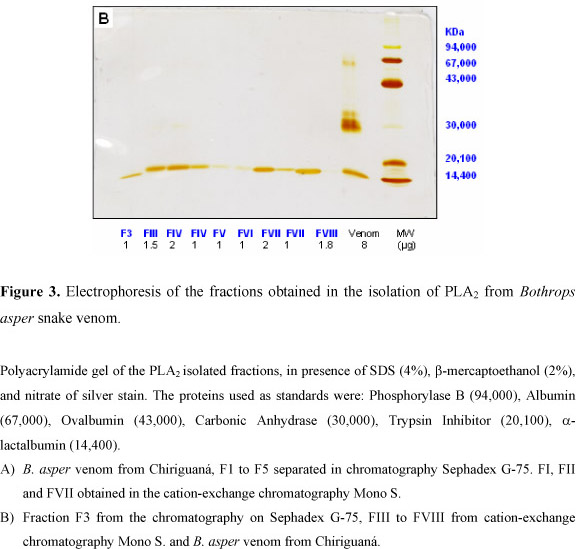
Gel for SDS-PAGE was prepared and run using a Hoeffer minigel electrophoresis system. Electrophoresis was performed in presence of sodium dodecyl sulfate (SDS) in 0.75 mm thick slab gels, according to the Laemmli method (10). The gels were silver stained (17). The mobility of standard proteins (14.4-94 KDa) and molecular weights of the samples were analysed using the Kodak Digital Science 1D Image Analysis Software (4).
Lethality assays
Lethality assays were performed with ICR male mice (16-18 g) by intraperitoneal (i.p). injection with 0.2 ml of the tested solution. The LD50 was evaluated 48 hours after the injection (21).
Phospholipase A2 activity
Phospholipase A2 activity was assayed by three procedures. The first one was the indirect haemolytic method, an adaptation of the procedures of Habermann and Hardt (8), and Gene et al. (6); the second was the potentiometric titration (16), and the third, the colorimetric assay with phenol red (11).
The substrate for the indirect haemolysis was prepared with 0.68 g of agarose A dissolved in 66.5 ml of 50 mM phosphate buffer, pH 7.5, in boiling water bath. The solution was cooled to 52°C, mixed with 1.2 ml of 10 mM CaCl2, 16.5 ml of 3.5 mM egg lecithin, and 1.2 ml of lamb erythrocytes, and poured into a glass plate (15.4 X 17.6 cm) previously heated to 52°C. After layer consolidation, cylindrical holes (2.5 mm) were punched. Each hole was filled with 10 µ l of enzyme solution and incubated for 20 hours at 37°C. During incubation, the enzyme diffused into the gel and cleared the erythrocyte by haemolysis, forming a halo. The diameter of these areas was measured in millimetres (mm).
Indirect haemolysis assay was used to identify the presence of phospholipase A2 in venom fractionation and determine its thermal and pH stability. Thermal stability was studied from 6 to 92°C; the enzymatic solution was incubated for 30 minutes, cooled in an ice bath for 5 minutes, and the residual activity was assayed.
The assays for pH stability were performed at pH values ranging from 2.5 to 11.3; the enzymatic solution was placed in a different buffer for 19 hours at room temperature (18°C), and the residual activity was assayed.
The potentiometric titration method was used to quantify PLA2 activity in the enzyme isolation process and in the determination of optimal pH and temperature, using an egg yolk suspension. The same procedure was used for the determination of the optimal concentration of Ca2+ and the Michaelis Menten constant (KM), but this time, phosphatidylcholine (Merck) was used instead of egg yolk. One unit of enzymatic activity was defined as the release of 1.0 meq of fatty acid per min. Specific activity corresponds to the number of meq of fatty acid liberated per min per mg of protein.
Colorimetric assay was used in order to determine substrate specificity (phosphatidylcholine, PC; phosphatidylethanolamine, PE; phosphatidylinositol, PD; sphingomyelin, SG; and cardiolipin, CP). The different substrates were prepared, as described by Lobo de Araujo and Radvanyi (11), to a final concentration of 0.27% (W/V). One unit of enzymatic activity was defined as absorbance decrease of 0.01 per min.
RESULTS
Eight fractions of B. asper venom with phospholipase A2 activity were isolated in a two-step purification procedure. First, the venom was separated by molecular weight on Sephadex G-75 into five fractions (Figure 1); then, the fraction containing phospholipase activity (F3) was chromatographied on MONO S (HR5/5) resulting in eight fractions with phospholipase activity (Figure 2). Table 1 presents the recovery of protein and activity of all fractions obtained from the two-step purification procedure. F3, resulting from the first step, accounted for 33% of the venom protein, and the isoenzymes FII and FVII accounted for 10.8% and 8.0% of the protein bounded to the column. PAGE of F3 from the first step purification procedure showed two bands of 16.2 and 17.2 KDa (Figure 3A); the isoenzymes resulting from the cation-exchange chromatography migrated as a single band in SDS-PAGE, and their molecular weights were from 16 to 17 KDa (Figure 3B). These two fractions were the most interesting since they had more PLA2 activity and protein.
The optimal pH for the FI and FII isoenzymes was 9, and for FVII, 9-10 (Figure 4A), and the optimal temperature was 52ºC for the three fractions (Figure 4B). The optimal concentration of Ca2+ for the FII isoenzyme was 48 mM, and for the FVII isoenzyme, it was 384 mM. FII and FVII showed thermal stability at all the temperatures studied for 30 minutes, and at 92°C for 60 minutes. The FII isoenzyme was stable at pH values ranging from 2.5 to 9, and FVII isoenzyme, from 2.5 to 8.
The Michaelis Menten constant (KM) for the FII isoenzyme was 3.5x10-3 M, and for the FVII isoenzyme, it was 1.6x10-3 M (Figure 5). Kinetic data were analysed by a non-linear model using the SYSTAT program. The assay of substrate specificity showed the following order: PC>PD>CP>SG>PE for the FII and FVII isoenzymes. Evaluation of substrate specificity by the potentiometer method showed no reaction with lysolecithin, confirming that the isolated enzymes were phospholipases A2 (PLA2s). The FII and FVII isoenzymes did not show any lethal activity in a dose of 96 and 71.4 µg per 16-18 g mouse.
DISCUSSION
The specific activity of PLA2 found in the venom of B. asper from Chiriguaná was 0.05 U/mg of protein, similar to other venoms of the Bothrops genus (0.02 mM of fatty acid/min/mg) and to N. naja venom, which is 0.08 mM of fatty acid/min/mg (12).
The procedure described above for isolation of different fractions with PLA2 activity from the B. asper venom is simple, quick, and efficient. The first step of purification on gel-filtration chromatography using 5% of acetic acid facilitated the elution of PLA2 into one fraction (F3). The total activity of this fraction was 6.12 U, which presented a twofold increase in the activity in crude venom (3.52 U), as showed in PLA2 from Bothrops insularis (3). F3 represents 33% of the crude venom protein, and has a specific activity of 0.28 U/mg protein, with a purity factor of 5.28. In other venoms from Bothrops snakes, such as B. insularis (3), it was found that 6% of the venom protein was PLA2, and for Bothrops lanceolatus alone, 2% was PLA2 (12). The results showed that in B. asper venom, the proportion of PLA2 is high.
The step of cationic-exchange chromatography using 10 mM of acetic acid facilitated the elution of the eight fractions with PLA2 from B. asper venom, and showed the multiple forms of PLA2s present in snake venoms (12,15,23). The isoenzymes found in B. asper were not dimeric forms, as in other venoms (20,30); however, the same bands are observed in electrophoresis under non-reducing conditions. PLA2s isolated from B. atrox (15) and Bothrops n. nummifer (30) were found in the monomeric state, suggested to be the common state of PLA2 present in Bothrops venoms.
All isoenzymes showed one band in electrophoresis with a molecular weight of 17 KDa, except for FVII and FVIII (16 KDa). For B. atrox (15) and B. n. nummifer (30), the PLA2s had a molecular weight of 14 KDa, and for B. lanceolatus (12), this weight was 15 KDa. Cogo et al. (3) found 4 polypeptide chains between 14 and 17 KDa in the PLA2 fraction from B. insularis venom. Gutiérrez and Lomonte (7) summarized the molecular weights found for many myotoxic PLA2s from 10.7 to 16 KDa, in which differences can be observed depending on the procedure used for the determination. In SDS-PAGE, the molecular weights of some myotoxic PLA2s were found between 15 and 16 KDa, very close to the values determined for all the PLA2 fractions isolated in this study.
The optimal pH value for the FI, FII and FVII isoenzymes was 9, at 25°C, very close to those related as generic for the PLA2 enzymes isolated from other snake venoms (7.5 and 8.5) (9). The activity is completely inhibited at pH 6 and 12. Salach et al. (24) found 6 isoenzymes in N. naja venom with optimum pH (7.9 to 8.0) at 25°C, and no significant difference was observed, except for IIC. This difference may be related to the enzyme conformational change by pH, as shown by Viljoen et al. (28).
The optimal temperature was different for 3 isoenzymes (FI, FII and FVII), and the maximum temperature was 52°C. The activities of these three isoenzymes decreased between 10°C and 22°C, and were completely eliminated at 72°C. Nair et al. (18) studied the optimal PLA2 temperatures for different total snake venoms, and found that the venom of several Naja species (Elapidae) showed their maximum activity at 65°C. In the Viperidae family, Agkistrodon piscivorus, Bitis gabonica, and Echis carinatus have their maximum activity at 65°C, 55°C, and 50°C, respectively. Besides, several species of Crotalus have their maximum activity at 45°C. For Trimeresurus flavoviridis venom, it was found that the optimal temperatures for the three isoenzymes were 40°C, 45°C, and >75°C (29).
Determination of the optimal concentration of Ca2+ showed an absolute dependence of this ion for two of the isoenzymes studied (FII and FVII). Using 4 mM of phosphatidylcholine as substrate at pH 9 and 52°C, the FII isoenzyme presented maximum activity with 48 mM of Ca2+, while FVII required 348 mM. These differences in the concentration of Ca2+ are probably related to the enzyme conformational change produced by this divalent ion (5).
FVII isoenzyme showed high activity with phosphatidylcholine from egg yolk, in contrast to FI, FII and FVIII assayed by the haemolytic method (results not shown). When egg yolk was used as substrate in the same method, the maximum activity corresponded to the FI and FII isoenzymes, progressively decreasing in the other fractions (Figure 2). These results suggest that one of the differences between the 8 fractions is their substrate specificity, being FVII highly specific to egg yolk phosphatidylcholine. However, the specific substrate for the FII and FVII isoforms from B. asper was: PC>PD>CP>SG>PE, being this order different in PLA2s isolated from other venoms. In T. flavoviridis, the order was PC>PE>Pserina>PD=0 for three of the isolated isoenzymes (29). The FII and FVII activity on sphingomyelin (SG) was interesting since that substrate is normally resistant to the action of Phospholipase A2 from several fountains (24,27).
The kinetics constant (KM) showed different responses for the FII and FVII isoenzymes. FII presented a typical behavior (5), but needed a critical micelle concentration of the substrate (cmc=1 mM) to initiate its catalytic activity. On the other hand, FVII could act on monomeric substrates, what suggests that there is not a universal kinetic model for phospholipases or enzymes with the same origin (6).
The Michaelis Menten constant was 3.5x10-3 and 1.6x10-3 mM for the FII and FVII isoenzymes, respectively. These KM values are different in PLA2s isolated from other snake venoms, and were determined using phosphatidylcholine from egg yolk as substrate in the watery media. The KM value for the PLA2 isolated from C. atrox venom was 7x10-3 M, using phosphatidylcholine as substrate in the media with diethyl ether (9). For three PLA2 isoenzymes from T. flavoviridis venom, KM values were 2.8x10-6 M, 4.3x10-6 M, and 2.2x10-6 M, using phosphatidylcholine as substrate in the watery media (29).
The two isoenzymes studied did not present any lethal activity for 90 and 71 µg doses when injected intraperitonially into mice between 16 and 18 g (5.3 µg/g and 4 µg/g), and the crude venom presented lethal activity for 58 µg. An acid phospholipase A2, isolated from B. lanceolatus venom, was not toxic when 300 µg were injected into mice (12).
ACKNOWLEDGEMENTS
The authors would like to thank the personnel of Grupo de Sueros: José Arquímedes González, Luz Losada, and Dioselina Cañón for their contribution; María Cristina Forero, María Carlina Castillo, and Francisco Ruiz for their support and suggestions. Vladimir Corredor and Carlos Arturo Hernandez by the revision and comments on the manuscript. This work was supported by Instituto Nacional de Salud (INS) and Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología "Francisco José de Caldas" (COLCIENCIAS), research grant CT. 217/95.
Received: April 3, 2003
Accepted: October 10, 2003
Published online: September 10, 2004
- 1 AGGERBECK LP., KEZDY FJ.,SCANU AM. Enzymatic probes of lipoprotein structure; hydrolysis of human serum low density lipoprotein-2 by phospholipase A2 J. Biol. Chem., 1976, 251, 3823-30.
- 2 CAMPBELL JA., LAMAR WW. The venomous reptiles of Latin America New York: University Press, 1989. 425p.
- 3 COGO JC., PRADO-FRANCESCHI J., GIGLIO JR., CORRADO AP., CRUZ-HÖFLING MA., DONATO JL., LEITE GB., RODRIGUES-SIMIONI L. An unusual presynaptic action of Bothrops insularis snake venom mediated by phospholipase A2 fraction. Toxicon, 1998, 36, 1323-32.
- 4 EASTMAN KODAK COMPANY. Kodak digital science: 1D image analysis software. New York: Rochester, 1996.
- 5 FUJII S., IKEDA K., HAYASHI K. Catalytic and toxicity mechanisms of secretory phospholipases A2 J. Toxicol. Toxin Rev., 1998, 17, 279-313.
- 6 GENÉ JA., GÓMEZ M., GUTIÉRREZ JM., CERDAS L. Neutralization of hyaluronidase and indirect hemolytic activities of Costa Rica snake venoms by a polyvalent antivenom. Toxicon, 1985, 23, 1015-8.
- 7 GUTIÉRREZ JM., LOMONTE B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon, 1995, 33, 1405-24.
- 8 HABERMANN E., HARDT K LA Sensitive and specific plate test for the quantitation of phospholipases. Anal. Biochem., 1972, 50, 163-73.
- 9 IWANAGA S., SUZUKI T. Enzymes in snake venom. In: LEE CY. Ed. Snake venom handbook of experimental pharmacology. New York: Editorial Board, Springer-Verlag Berlin Heidelberg, 1979: 61-158.
- 10 LAEMMLI UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1970, 227, 680-5.
- 11 LOBO DE ARAUJO A., RADVANYI F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon, 1987, 25, 1181-8.
- 12 LOBO DE ARAUJO A., RADVANYI F., BON C. Purification of an acidic phospholipase A2 from Bothrops lanceolatus (Fer de Lance) venom: molecular and enzymatic properties. Toxicon, 1994, 32, 1069-81.
- 13 LOWRY OH., ROSEBROUGH NJ., FARR AL., RANDALL RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem., 1951, 193, 265-75.
- 14 MANCUSO LC., CORREA MM., VIEIRA CA., CUNHA OAB., LACHAT JJ., SELISTRE DE ARAUJO HS., OWNBY CL., GIGLIO JR. Fractionation of Bothrops pirajai snake venom: Isolation and characterization of piratoxin-I, a new myotoxic protein. Toxicon, 1995, 33, 615-26.
- 15 MARAGANORE JM., MERUTKA G., CHO W., WELCHES W., KÉZDY FJ., HEINRIKSON RL. A new class of phospholipases A2 with lysine in place of aspartate 49. Functional consequences for calcium and substrate binding. J. Biol. Chem., 1984, 259, 13839-43.
- 16 MARTIN-MOUTOT N., ROCHAT H. Isolation and characterization of a toxic phospholipase A2 in the spitting cobra (Naja mossambica mossambica) venom. Toxicon, 1979, 17, 127-36
- 17 MERRIL CR., GOLDMAN D., SEDMAN SA., EBERT MH. Ultrasensitive stain for proteins in polyaxrylamide gels shows regional variation in cerebrospinal fluid proteins. Science, 1981, 221, 1437-8.
- 18 NAIR BC., NAIR C., ELLIOTT WB. Temperature stability of phospholipase A2 activity. -II. Variations in optimum temperature of phospholipase A2 from various snake venom. Toxicon, 1976, 14, 43-7.
- 19 OGAWA T., NAKASHIMA KI., NOBIHISA I., DESHIMARU M., SHIMAHIGASHI Y., FUKUMAKI Y., SAKAKI Y., HATTORI S., OHON M. Accelerated evolution of snake venom phospholipase A2 isozymes for acquisition of diverse physiological functions. Toxicon, 1996, 34, 1229-36.
- 20 OHNO M., MÉNEZ R., OGAWA T., DANSE JM., SHIMOHIGASHI Y., FROMEN C., DUCANCEL F., ZINN-JUSTIN S., LE DU MH., BOULAIN JC., TAMIYA T., MÉNEZ A. Molecular evolution of snake toxins: Is the functional diversity of snake toxins associated with a mechanism of accelerated evolution? In: MOLDAVE K. Ed. Progress in nucleic acid research and molecular biology. San Diego: Academic Press, 1998: 307-64.
-
21ORGANIZAÇÃO PANAMERICANA DA SAÚDE. Manual de procedimientos: producción y pruebas de control en la preparación de antisueros diftérico, tetánico, botulínico, antivenenos y de la gangrena gaseosa 1977. 104p.
- 22 ROSENBERG P. Pharmacology of phospholipase A2 from snake venoms. In: LEE CY. Ed. Snake venom: handbook of experimental pharmacology. New York: Editorial Board, Springer-Verlag Berlin Heidelberg, 1979: 403-47.
- 23 SALACH JI., TURINI P., SENG R., HAUBER J., SINGER TP. Phospholipase A of snake venoms: I- Isolation and molecular properties of isoenzymes from Naja naja and Vipera russellii venoms. J. Biol. Chem., 1971a, 246, 331-9.
- 24 SALACH JI., SENG R., TISDALE H., SINGER T. Phospholipase A of snake venoms: II- Catalytic properties of the enzyme from Naja naja J. Biol. Chem., 1971b, 246, 340-7.
- 25 SLOTBOOM AJ., VERHEIJ HM., DE HASS GH. On the mechanism of phospholipases A2 In: HAWTHORNE JN., ANSELL GB. Eds. Phospholipids, new biochemistry. Amsterdam: Elsevier Biomedical Press/ North Holland Press, 1982: 359-434.
- 26 SOARES AM., RODRIGUES VM., HOMSI-BRANDEBURGO MI., TAYOMA MH., LOMBARDI FR., ARNI RK., GIGLIO JR. A rapid procedure for the isolation of the Lys-49 myotoxin II from Bothrops moojeni (Caissaca) venom: biochemical characterization, crystallization, myotoxic and edematogenic activity. Toxicon, 1998, 36, 503-14.
- 27 VAN DEENEN LLM., DE HAAS GH. The substrate specificity of phospholipase A. Biochem. Physiol. Acta, 1963, 70, 538-53.
- 28 VILJOEN CC., BOTES DP., SCHABORT JC. Spectral properties of Bitis gabonica venom phospholipase A2 in the presence of divalent metal ion, substrate and hydrolysis products. Toxicon, 1975, 13, 343-51.
- 29 VISHWANATH BS., KINI RM., GOWDA TV. Characterization of three edema-inducing phospholipase A2 enzymes from Habu (Trimeresurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon, 1987, 25, 501-15.
- 30 WELCHES W., FELSHER D., LANDSHULZ W., MARAGANORE JM. A rapid method for the purification of monomer and/or dimer phospholipases A2 in crotalid snake venoms. Toxicon, 1985, 23, 747-54.
Correspondence to
Publication Dates
-
Publication in this collection
24 Sept 2004 -
Date of issue
2004
History
-
Received
03 Apr 2003 -
Accepted
10 Oct 2003