Abstract
This paper describes a brief study on the crotoxin mechanism of action, regarding the transport of GABA and L-glutamate in rats cortico-cerebral synaptosomes and in heterologous systems, such as COS-7 cells expressing gabaergic transporters, and C6 glioma cells and Xenopus oocytes expressing glutamatergic transporters. Crotoxin concentrations over 1 µM caused an inhibitory effect of ³H-L-glutamate and ³H-GABA, and reversibly inhibited L-glutamate uptake by C6 glioma cells. When COS-7 cells were assayed, no inhibition of the ³H-GABA transport could be evidenced. Crotoxin kept its inhibitory effect on neurotransmitters uptake even when Ca2+ ions were removed from the medium, therefore, independently of its PLA2 activity. In addition, high concentrations (2 mM) of BPB did not avoid the action of crotoxin on the neurotransmitters uptake. Crotoxin also inhibited ³H-L-glutamate, independently on Na+ channel blockade by TTX. In addition, an evaluation of the lactic dehydrogenase activity indicated that uptake inhibition does not involve a hydrolytic action of crotoxin upon the membrane. We may also suggest that crotoxin acts, at least partially, altering the electrogenic equilibrium, as evidenced by confocal microscopy, when a fluorescent probe was used to verify cell permeability on C6 glioma cells in presence of crotoxin.
Crotoxin; L-glutamate; GABA; Crotalus durissus terrificus
ORIGINAL PAPER
Inhibition of L-glutamate and GABA synaptosome uptake by crotoxin, the major neurotoxin from Crotalus durissus terrificus venom
Cecchini, A.L.I,IV; Soares, A.M.II; Giglio, J.R.III; Amara, S.IV; Arantes, E.C.I
IDepartamento de Física e Química, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo (USP), Ribeirão Preto, São Paulo, Brasil
IIUnidade de Biotecnologia, Universidade de Ribeirão Preto (UNAERP), Ribeirão Preto, São Paulo, Brasil
IIIDepartamento de Bioquímica e Imunologia, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo (USP), Ribeirão Preto, São Paulo, Brasil
IVVollum Institute, Oregon Health Sciences University, Portland, Oregon, USA
Correspondence Correspondence to E. C. Arantes Departamento de Física e Química, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, USP; Avenida do Café, s/n, 14040-903, Ribeirão Preto, SP, Brazil Phone: 55 16 6024275, fax: 55 16 6332960 E-mail: ecabraga@fcfrp.usp.br
ABSTRACT
This paper describes a brief study on the crotoxin mechanism of action, regarding the transport of GABA and L-glutamate in rats cortico-cerebral synaptosomes and in heterologous systems, such as COS-7 cells expressing gabaergic transporters, and C6 glioma cells and Xenopus oocytes expressing glutamatergic transporters. Crotoxin concentrations over 1 µM caused an inhibitory effect of 3H-L-glutamate and 3H-GABA, and reversibly inhibited L-glutamate uptake by C6 glioma cells. When COS-7 cells were assayed, no inhibition of the 3H-GABA transport could be evidenced. Crotoxin kept its inhibitory effect on neurotransmitters uptake even when Ca2+ ions were removed from the medium, therefore, independently of its PLA2 activity. In addition, high concentrations (2 mM) of BPB did not avoid the action of crotoxin on the neurotransmitters uptake. Crotoxin also inhibited 3H-L-glutamate, independently on Na+ channel blockade by TTX. In addition, an evaluation of the lactic dehydrogenase activity indicated that uptake inhibition does not involve a hydrolytic action of crotoxin upon the membrane. We may also suggest that crotoxin acts, at least partially, altering the electrogenic equilibrium, as evidenced by confocal microscopy, when a fluorescent probe was used to verify cell permeability on C6 glioma cells in presence of crotoxin.
Key words: Crotoxin, L-glutamate, GABA, Crotalus durissus terrificus.
INTRODUCTION
Animal venoms are complex mixtures of bioactive components, predominantly polypeptides, which play offensive, defensive and digestive roles (15,36,62). Among those components are phospholipases A2 (PLA2, E.C. 3.1.1.4), which catalyze the hydrolysis of sn-2 ester bond of phospholipids, releasing free fatty acids, initially polyunsaturated, as arachidonic acid. PLA2s are also found in pancreatic secretions, platelets, neurons, mastocytes, inflammatory exudates, and snake and bee venoms.
Many of these phospholipases, although toxic, do not show detectable catalytic activity (6,53,56). Their Mr is usually about 14,000; they are monomeric, homomultimeric or heterodimeric enzymes, and may be obtained from a single snake, as many as 16 PLA2s, catalytically active or not (21,24,31).
Some phospholipases A2 are potent, mainly pre-synaptic, neurotoxins (34). Endogenous PLA2s are suggested to be involved with brain pathologies, inducing epilepsy, ischemia, trauma, and others (2). They may show neurotoxic, myotoxic, convulsant, inflammatory, and edema inducing activity (16,61). The convulsive activity induced by crotoxin (5) and its basic subunit CB (22) was reported. Crotoxin showed to be convulsive despite its low in vitro catalytic activity (34,46). Tzeng et al. (58) showed that crotoxin binds to specific sites in the synaptic membranes and that the highly conserved residues Tyr21 and Tyr24 of the basic PLA2 subunit are determinants of this binding. A 48kDa-membrane protein able to bind to crotoxin was reported by Krizaj et al. (33). In brain pathologies, it was already well established that several neurotransmitters (1,28), such as excitatory amino acids (27) are able to increase the release of free fatty acids, especially arachidonic acid.
Little is known about the participation of endogenous PLA2s in the glutamate uptake. However, it was shown that a toxic PLA2 paradoxin was able to significantly decrease this process in rat hippocampal mini-slices (9); and that transporter-mediated currents in Purkinje neurons are increased more than threefold by arachidonic acid, a second messenger that is liberated as a result of the activation of phospholipase A2 by Ca2+ (59).
Several papers have reported that toxins inhibit the uptake of many neurotransmitters, including GABA (g-aminobutiric acid), noradrenalin, serotonin, and choline (41,42,52,63), while studies on glutamate uptake are more limited (63). Data on neurotoxicity of PLA2s are scant and usually directed to b-bungarotoxin (20,23,40). This paper describes a study on the mechanism of action of crotoxin, a heterodimeric PLA2 from South American rattlesnake venom; regarding the transport of GABA and L-glutamate in rats cortico-cerebral synaptosomes, and in heterologous systems, such as COS-7 cells expressing gabaergic transporters, and C6 glioma cells and Xenopus oocytes, expressing glutamatergic transporters.
MATERIALS AND METHODS
3H-GABA, 3H-glutamate, ScintiVerse, sucrose, OptiPhase Supermix, 1900 TR Liquid Scintillation Analyzer, BPB (4-bromophenacyl bromide), DMEM (Dulbecco' s Modified Eagle Medium), and protein assay BCA kit were purchased from Perkinhelmer Life Science, Fisher Scientific, Wallac, Packard, Sigma-Aldrich, GIBCO and Pierce, respectively. All other reagents used were purchased from Sigma-Aldrich and Mallinckrodt.
Isolation of synaptosomes
Sprague Dawley male rats (200-250g) from Charles River Laboratory (USA) were sacrificed by decapitation and the brain cortex was separated and dept in 0.32 M sucrose. Brain tissue was homogenized at 130xg with the aid of a Potter-Elvehjen homogenizer and then centrifuged at 1,400xg, 4ºC for 10 min. Supernatant was collected and centrifuged at 1,400xg, 4ºC for 20 min. The sedimented material (mitochondria, myelin and synaptosomes) was gently dispersed in 0.32 M sucrose, homogenized at 3,200xg and centrifuged under a density gradient of 0.8 M-1,2 M sucrose at 60,000xg, 4ºC for 60 min. The upper phase (myelin) was discarded by suction, and the synaptosomal phase was collected and diluted to 0.4 M with Milli Q cold water. After a further centrifugation at 1,600xg, 4ºC for 20 min, the supernatant was discarded and the sediment resuspended in Tyrode buffer (136 mM NaCl; 5 mM KCl; 2.5 mM KH2PO4; 1 mM MgSO4; 25 mM Tris HCl; 2 mM CaCl2; 5 mM glucose; pH 7.4). For protein determination, the BCA protein assay kit was used. The amount of protein in each reaction well was 30-45 µg (18).
3H-GABA and 3H-L-Glutamate uptake
3H-GABA (36.2 Ci/mmol) and 3H-L-glutamate (22.5 Ci/mM) were used for the uptake experiments. In Rat Cortico-Cerebral Synaptosomes (RCCS), the final concentration of the radioactive component was 100 nM. The different crotoxin concentrations are shown in the corresponding figures. Different incubation times (5, 10, 15 and 20 min.) were assayed at room temperature. For the control, 60 mM of nipecotic acid (for GABA experiments) and 6.3 mM of L-trans-pyrrollidine - 2,4-dicarboxylic acid (PCD) (for L-glutamate experiments) were utilized. Assays were performed on 96 well plates, and after 3 min of incubation with the radioactive material, each well mixture was sucked and filtered through a Brandel System. Radioactivity pulses were counted with the aid of a WALLAC 1450 Microbeta scintillation analyzer using the Optiphase Supermix scintillation liquid. Data were expressed as rate of GABA or L-glutamate uptake in fmol/mg/min (7).
3H-GABA and 3H-L-Glutamate uptake in absence of Ca2+
Synaptosomes were prepared as above described. The Ca2+ from Tyrode buffer was now replaced by 7.6 mM of Sr2+ and 1 mM of EGTA.
3H-GABA and 3H-L-Glutamate uptake in presence of p-bromophenacyl bromide (BPB)
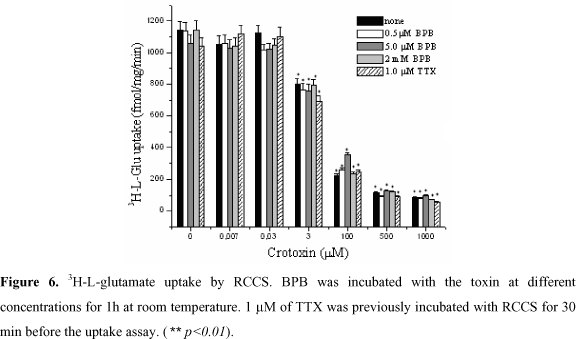
BPB (0.5, 5.0 and 2000 µM) was incubated with crotoxin for 1 hour at room temperature, using different toxin concentrations.
Tetrodotoxin (TTX)
TTX (1 µM) was incubated for 30 min with RCCS, followed by crotoxin at different concentrations.
C6 Glioma cells
These cells were originally cloned from a rat glioma (3), and then grown in 24 well plates supplemented with Dulbecco' s Modified Eagle Medium (DMEM) and 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 µg/ml). They were then kept at 37ºC under 5% (v/v) CO2. In experiments involving pre-incubation, the toxin was added in the cell medium kept at room temperature.
3H-L-Glutamate uptake in C6 glioma cells
C6 glioma cells are undifferentiated cells from kidney tumour, able to express the glutamatergic transporters EAAC1 (EAAT-3) endogenously (8,14,44). The cells were kept in Corning culture flasks containing DMEM, supplemented with FCS (10% final) plus penicillin (100 U/ml) and streptomycin (100 µg/ml), and plated every two days. For glutamate uptake assay, the following buffer solutions were used: 5 mM Tris base; 10 Mm HEPES; 2.5 mM KCl; 1.2 mM CaCl2; 1.2 mM MgCl2; 12 mM KH2PO4; 10 mM Dextrose; 140 mM NaCl; pH 7.2. As control, 140 mM choline chloride was used to substitute 140 mM NaCl. The treated cells were pre-incubated for 20 min with 0.015, 1.0 and 500 µM of crotoxin, and then 3H-glutamate (22.5 Ci/mM) was added up to 1 µM. After 5 min of incubation, the reaction was stopped by washing the cells twice with cold buffer containing Na+ or not, and then the cells were disrupted by 450 µl of 0.1% SDS/0.1 N NaOH. The plates were placed on an electronic shaker for 20 min, in order that all cells could get loose from the plate, and 400 µl was transferred into a vessel containing 4.4 ml of ScintiVerse scintillation liquid. Radioactive pulses were then counted in a 1900 TR Scintillation Analyzer.
C6 Glioma cells recovery
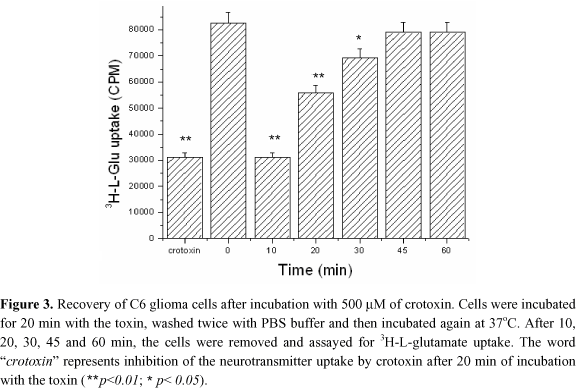
The cells were incubated with 500 mM crotoxin for 20 min and then washed with PBS four times. They were then incubated at 37ºC, and L-glutamate uptake assay was carried out 10, 20, 30, 45 and 60 min after incubation, in order to evaluate the cell recovery ability. In regard to the controls, Milli-Q water and incubation of cells with crotoxin for 20 min was followed by the uptake assay.
COS-7 cells
These cells were used for expression of GAT-1 and GAT-3 GABA transporter DNAs. They were kept in DMEM, in Corning culture flasks, transferred every 3 days using transference protocol, and washed with PBS buffer, which was then sucked out. 12 ml of DMEM plus penicillin (100 U/ml) and streptomycin (100 µg/ml) were then added as before, and 2 ml of the suspension was transferred to a new flask and kept at 37ºC for an additional 3-days period before the next transference, when cells were also transferred to 24 well plates. On the next day, transfixion of cells and gabaergic transporter DNA was carried out using Fugene protocol for experiment on GABA uptake.
Transformation of E. coli DH5a cells
GAT-1 and GAT-3 transporter DNAs were obtained from E. coli DH5a cells, and for the expressed DNA extraction, Maxi-Prep protocol was used (48).
3H-GABA uptake in COS-7 cells
These assays were carried out in 24 well plates. Two days before, the cells were transfected with GABA, GAT-1 and GAT-3 transporter DNAs using CMV vector. 3H-GABA was added, and 3H-GABA uptake data were obtained from a b counter. The treated cells were pre-incubated for 20 min with 0.015, 1 and 500 µM crotoxin and then, the radioactive material was added up to 1 µM. After 10 min of incubation, the reaction was stopped, the cells were washed twice with cold PBS, and then disrupted with 450 µl of 0.1% SDS/0.1 N NaOH. The plates were placed in a shaker for 20 min as above, and 400 µl were transferred to a vessel containing 4.5 ml of scintillation liquid. Counting was then performed during 1 min/vessel.
Statistics Analysis
Results are presented as the means ± S.E.M of values obtained. Statistical significance of differences between groups was evaluated using Student' s unpaired t-Test. p values <0.05 were considered significant.
RESULTS
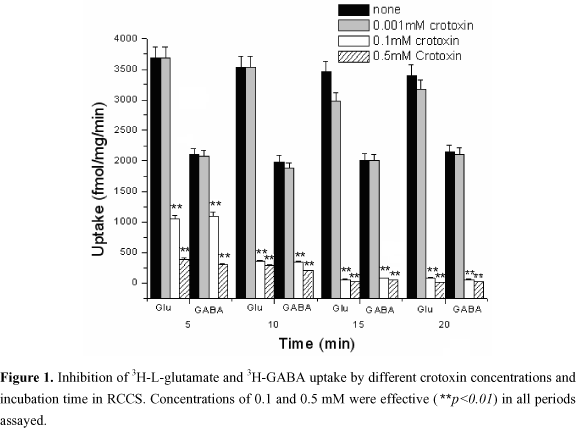
Figure 1 shows the action of crotoxin on the uptake of 3H-GABA and 3H-L-glutamate by RCCS, depending on pre-incubation time. Both 0.1 mM and 0.5 mM of crotoxin were able to significantly inhibit neurotransmitters uptake. Inhibition was time-dependent, since the uptake decreased as the incubation time of RCCS and crotoxin increased. The assay was repeated at least 3 times following the same conditions of temperature, incubation time, and dilutions, calculated by the crotoxin e .
Crotoxin concentrations higher than 1 µM caused an inhibitory effect of 3H-L-glutamate and 3H-GABA (Figure 2) for a chosen 20 min incubation period. Within this longer time, we could detect the uptake inhibition even at lower concentrations. This assay shows that crotoxin reversibly inhibited L-glutamate uptake by C6 glioma cells and did not disrupt cell membrane as cause of inhibition of the neurotransmitter uptake.
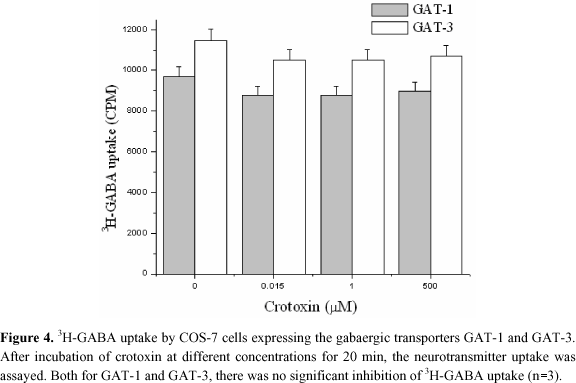
The word "crotoxin" in Figure 3 represents the assay in presence of this toxin after 20 min of incubation; and zero represents the assay in absence of crotoxin, which was replaced by water, washed twice with PBS, and incubated at 37ºC. We can see that cells were recovered with crotoxin after incubation, and that the toxin did not irreversibly injure them. When the heterologous system constituted by COS-7 cells was assayed, no inhibition of 3H-GABA transport by crotoxin could be evidenced (Figure 4).
Crotoxin keeps its inhibitory effect on neurotransmitters uptake even when Ca2+ ions are removed by EGTA and replaced by Sr2+ ions (Figure 5), therefore, independently of its PLA2 activity. In addition, high concentrations (2 mM) of BPB, which inhibits PLA2 activity, did not avoid the action of crotoxin on the neurotransmitters uptake, even after the incubation of BPB with crotoxin (Figure 6), showing that there is no heterodimer dissociation to evoke its activity. Figure 6 also shows that crotoxin inhibits 3H-L-glutamate, independently of Na+ channel blockade by TTX, demonstrating that this toxin does not potentiate crotoxin activity, and that its activity is not dependent on Na+ channel.
DISCUSSION
Crotoxin, the major neurotoxic component of Crotalus durissus terrificus venom (51) is a complex of two proteins: crotapotin, acidic component, catalytically inactive, ~9kDa, and PLA2, basic component, catalytically active, ~14kDa. Little is known about the crotoxin mode of action in the central nervous system. It was already shown that it acts initially on the peripheral nervous system blocking the Ach release on nerve terminals (29).
Ng and Howgood (42) showed that crotoxin (7 nM) inhibits 50% GABA uptake, and that isolated basic subunit presents half of this activity. Despite the available data regarding the action of crotoxin on the central nervous system are scant, mainly in experimental models as synaptosomes, it was already established by these authors that this toxin causes some alterations, inhibiting GABA transport (38), as well as the L-glutamate excitatory activity potentiation by some PLA2s (12,32,37).
Our results showed that increasing concentrations of crotoxin inhibit 3H-L-glutamate and 3H-GABA uptake (Figure 2). This inhibition is time-dependent, but independent on extracellular Ca2+ (Figures 5 and 6), since lower crotoxin concentrations (0.1 mM) result in a significant decrease of neurotransmitter uptake, mainly from 5 to 15 min (Figure 1), both in presence and absence of Ca2+. Neurons and glia accumulate neurotransmitters dependent on Na+ cotransport, through which the energy stored along transmembrane electrochemical transport may be used to move solute into the cell (17, 26, 57). This transporter is located in the neurons synaptic membrane and uses the same transmitter. This is probably the main mechanism to end synaptic transmission. When COS-7 heterologous cells or Xenopus oocytes (60) were used to express GABA or L-glutamate transporters, respectively, we could not reproduce the results obtained with synaptosomes (Figure 3). A suggested explanation for this fact is that crotoxin does not act directly upon transporters, but rather in their neighborhoods.
These cells, which express neuron components are not neurons and hence do not possess all components of a central nervous system cell. However, we cannot discard the possibility that the cloned transporters utilized are not able to express the binding site for crotoxin, as it was clearly evidenced in K+ channels, which were cloned and expressed in mammal cells (13) C6 glioma cells originated from a rat adrenal tumour, which display L-Glutamate transporters similar to those from neuron cells. Our assays showed that 1.2 mM of crotoxin inhibits up to 60% the uptake (Figure 3). After 45 min, the cells were already recovered with full ability to uptake 3H-L-glutamate.
Crotoxin induces convulsion when injected intracerebroventricularly. Recently, Dorandeu et al. (10) showed that intracerebralventricular (i.c.v.) injection of low doses (7 pmol) of crotoxin caused a prominent tonic-clonic convulsion. Increased L-Glu in the synaptic cleft has been one of the explanations for convulsive diseases (39). Our results show that extracellular Ca2+ does not significantly interfere in L-Glu (Figure 5) or GABA uptake (data not shown), in disagreement with Zhu et al. (64) and Mafra et al. data (35). We have to point out that L-Glu or GABA uptake indeed slightly decreases in the absence of external Ca2+, which was not significant. GABA is the main inhibitory neurotransmitter of mammal brain and is efficiently removed from the synaptic cleft and extracellular space by high affinity transporters of neurons and glial cells. At least three cDNAs of GABA transporters have already been isolated from rat brain, namely GAT-1 (19), GAT-2 (4) and GAT-3 (4,7). High levels of GAT-1 mRNA have been found along brain cortex and hippocampus, hence the choice of brain cortex for the synaptosomal preparations in this work.
An important aspect of neurotransmitter transport is that they are potentially electrogenic. For GABA and L-Glu, both Na+-dependent, a charge translocation through the membrane and the process itself, generate the current. When TTX (Figure 6), a Na+ channel blocking toxin, is added, no alteration in the neurotransmitter uptake is observed, since the blocked channel does not belong to the system that promotes the transport electrogenic equilibrium. Note, however, that crotoxin does not potentiate this blockade to compete with TTX since the inhibitory action of crotoxin on L-Glu uptake was kept. Norepinefrine (57), GABA (49,50), and L-glutamate (55) carriers can reversibly act, transporting substrates to the outside of cells when external K+ concentration increases and cell depolarization occurs. This reverse uptake may explain the non-vesicular Ca2+ independent release of GABA and L-Glu (43).
Irreversible inactivation of PLA2s by BPB is usually used to abolish catalytic, toxic, and consequently, epileptogenic activity (10). Addition of BPB, in order to inhibit any PLA2 activity resulting from the dissociation of the crotoxin complex, showed that crotoxin does not dissociate before exerting its activity (Figure 6). Consequently, no alteration of the crotoxin inhibitory activity on 3H-L-Glutamate could be detected, since the crotoxin complex is insensitive to the inhibitor (45,54). However, we cannot discard the possibility that crotoxin produces its effects indirectly. Our results show that crotoxin does not directly interfere in the neurotransmitters uptake mechanism inhibiting its carriers, but in neighboring targets, also present in the membranes of C6 glioma cells, where it causes GABA and L-glutamate uptake inhibition. Crotoxin does not dissociate into crotapotin and PLA2 before interacting with synaptosome membranes.
In addition, an evaluation of lactic dehydrogenase activity (data not shown) evidenced no statistical differences of values for synaptosomes in presence and absence of crotoxin, indicating that uptake inhibition does not involve a hydrolytic action of crotoxin upon the membrane. We may also suggest that crotoxin acts, at least partially, altering the electrogenic equilibrium, as evidenced by confocal microscopy, when a fluorescent probe DIBAC4 (36) {bis [1,3-dibutylbarbituric acid-(56)] trimethin-eoxolnol} was used to verify permeability on C6 glioma cells in presence of crotoxin (data not shown). While the precise mechanism of action of many venom components is still unknown, it is already established that it involves interactions with specific membrane components (25,30,47,48). Although it unclearly remains which role transporters play in presence of neurotoxins (in this case, crotoxin), the activation of a proton current could serve as another mechanism, maybe involving local pH changes, through which synaptic excitability could be modulated by substrate binding and transport (11). These interactions make these venom components attractive tools for development of therapeutic agents in the study of molecular targets on cell membranes.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), as well as the skillful technical assistance from Odete A. B. Cunha and Carlos A. Vieira.
Received: April 28, 2003
Accepted: September 29, 2003
Published online: September 10, 2004
- 1 BAZAN NG. Arachidonic acid in the modulation of excitable membrane function and at the onset of brain damage. Ann. NY Acad. Sci., 1989, 559, 1-16.
- 2 BAZAN NG., RODRIGUEZ DE TUCO EB., GEOFFREY A. Mediators of injury in neurotrauma: intracellular signal transduction and gene expression. J. Neurotrauma, 1995, 12, 791-814.
- 3 BENDA P., FAIVRE-BAUMAN A. Glutamate accumulation by a clone of glial cells. Brain, 1974, 76, 371-5.
- 4 BORDEN LA., SMITH KE., HARTIG PR., BRANCHEK TA., WEINSHANK RL. Molecular heterogeneity of the g-aminobutyric acid (GABA) transport system. J. Biol. Chem, 1992, 267, 21098-104.
- 5 BRAZIL OV. Neurotoxins from South American rattlesnake venom. J. Formos. Med. Assoc., 1972, 71, 394-400.
- 6 CINTRA AC., SAMPAIO SV., RAGHUVIR AK., GIGLIO JR. Assignment of the disulfide bridges in bothropstoxin-I, a myonecrotic Lys49 PLA2 homolog from Bothrops jararacussu snake venom. J. Protein Chem, 2001, 5, 377-82.
- 7 CLARK JA., DEUTCH AY., GALLIPOLI PZ., AMARA SG. Functional expression and CNS distribution of a b-alanine-sensitive neuronal GABA transporter. Neuron, 1992, 9, 337-48.
- 8 DAVIS KE., STRAFF DJ., WEINSTEIN EA., BANNERMAN PG., CORREALE DM., ROTHSTEIN JD., ROBINSON MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J. Neurosc, 1998, 18, 2475-85.
- 9 DORANDEU F., ANTIER D., PERNOT-MARINO I., LAPEYRE P., LALLEMENT G. Venom phospholipase A2-induced impairment of glutamate uptake: an indirect and non-selective effect related to phspholipid hydrolysis. J. Neurosci. Res, 1998, 51, 349-59.
- 10 DORANDEU F., PERNOT-MARINO I., VEYRET J., PERRICHON C., LALLEMENT G. Secreted phopholipase A2- induced neurotoxicity and epileptic seizures after intracerebral administration: an enxplained heterogeneity a emphasized with paradoxin and crotoxina. J. Neurosci. Res., 1998, 54, 848-62.
- 11 FAIRMAN WA., SONDERS MS., MURDOCH GH., AMARA, SG., Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nat. Neurosci, 1998, 1, 1105-13.
- 12 FAROOQUI AA., YANG HC., ROSENBENRG TA., HORROCKS LA. Phospholipase A(2) and its role in brain tissue. J. Neurochem., 1997, 69, 889-901.
- 13 FATHI-HB., ROWAN EG., HARVEY AL. The facilitatory actions of snake venom phospholipase A2 neurotoxins at the neuromuscular junction are not mediated through voltage-gated channels. Toxicon, 2001, 39, 1871-82.
- 14 FONTANA ACK., COUTINHO-NETTO J. Neuromodulação de sinapses glutamatérgicas por componentes purificados da peçonha da aranha social Parawixia bistriata Ribeirão Preto: Universidade de São Paulo - Faculdade de Medicina de Ribeirão Preto, 2001 167p. [Tese - Doutorado]
- 15 FRY BG. Structure-function properties of venom components from Australian elapids. Toxicon, 1999, 37, 11-32.
- 16 GANDOLFO G., LAMBEAU G., LAZDUNSKI M., GOTTESMANN C. Effects on behaviour and EEG of single chain phosholipase A(2) from snake and bee venoms injected into rat brain: Search for a functional antagonism. Pharmacol. Toxicol, 1996, 78, 341-7.
- 17 GEGELASHVILI G., SCHOUSBOE A. Cellular distribution and kinetic properties of high-affinity glutamate transporters. Brain Res. Bull, 1998, 45, 233-8.
- 18 GRAY EG., WHITTAKER VP. The isolation of nerver endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat, 1962, 96, 79-87.
- 19 GUASTELLA J., NELSON N., NELSON H., CZYZYK L., KEYNAN S., MIEDEL MC., DAVIDSON N., LESTER H., KANNER B. Clonning and expression of a rat brain GABA transporter. Science, 1990, 249, 1303-6.
- 20 GULYA K., BUDAI D., KASA P., RAKONCZAY Z. In vivo effects of b-bungarotoxin on the acetylcholine system in different brain areas of the rat. J. Neurochem., 1984, 43, 112-9.
- 21 GUTIÉRREZ JM., LOMONTE B. PHOSpholipase A2 myotoxins from Bothrops snake venoms. Toxicon, 1995, 33, 1405-24.
- 22 HABERMANN E., CHEN RAUDE D. Central neurotoxicity of apamin, crotamin, phospholipase A and alpha-amanitin. Toxicon, 1975, 13, 465-73.
- 23 HAREL M., KASHER R., NICOLAS A., GUSS JM., BALASS M., FRIDKIN M., SMIT AB., BREJC K., SIXMA TK., KATCHALSKI-KATZIR E., SUSSMAN JL., FUCHS S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron, 2001, 32, 265-75.
- 24 HAWGOOD B., BON C. Snake venom presynaptic toxins. In: TU A. Ed. Handbook of natural toxins New York: M. Dekker., 1995: 3-52.
- 25 HE P., CURRY FE. Measurement of membrane potential of endothelial cells in single perfused microvessels. Microvasc. Res., 1995, 50, 183-98.
- 26 KANNER BI., SCHULDINER S. Mechanism of transport and storage of neurotransmitters. CRC Crit. Rev. Biochem, 1987, 22, 1-38.
- 27 KATAYAMA Y., KAWAMATA T., MAEDA T., ISHIKAWA K., TSUBOKAWA T. Inhibition of the early phase of free fat acid liberation during cerebral ischemia by excitatory aminoacid antagonist administered by microdialysis. Brain Res, 1994, 635, 331-4.
- 28 KATSURA K., RODRIGUEZ DE TURCO EB., FOLBERGROVA J., BAZAN NG., SIESJO BK. Coupling among energy failure, loss of ion homeostasis, and phospholipase A2 and C activation during ischemia. J. Neurochem, 1993, 61, 1677-84.
- 29 KATTAH LS., SANTORO M M., DINIZ RC., DE LIMA ME. Crotoxin, the major toxin from the rattlesnake Crotalus durissus terrificus, inhibits 3H-choline uptake in guinea pig ileum. Braz. J. Med. Biol. Res, 2000, 33, 1093-7.
- 30 KEYNAN S., KANNER BI. g-aminobutyric acid transport in reconstitute preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry, 1988, 27, 12-7.
- 31 KINI RM. Phospholipase A2: a complex multifunctional protein puzzle. In: KINI RM. Ed. Venom phospholipase A2 enzymes: structure and mechanism Chichester: J. Wiley, 1997:1-28.
- 32 KOLKO M., BRUHN T., CHRISTENSEN T., LAZDUNSKI M., LAMBEAU G., BAZAN NG., DIEMER NH. Secretory phospholipase A2 potentiates glutamate-induced rat striatal neuronal death in vivo Neurosci. Lett., 1999, 274, 167-70.
- 33 KRIZAJ I., FAURE G., GUBENSEK F., BON C. Neurotoxic phospholipase A2 ammodytoxin and crotoxin bind to distinct high-affinity protein acceptors in Torpedo marmorata electric organ. Biochemistry, 1997, 36, 2779-87.
- 34 LAMBEAU G., BARHANIN J., SCHWEITZ H., QAR J., LAZDUNSKI M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the taipan venom. J. Biol. Chem., 1989, 264, 11503-10.
- 35 MAFRA RA., DE OLIVEIRA LC., FERREIRA CAG., DE LIMA ME., BEIRÃO PSL., CRUZ JS. REgulation of the glutamate uptake by extracellular calcium. Brain Res., 2002, 936, 21-6.
- 36 MANSBACH CMI., PIERONI G., VERGER R. Intestinal phospholipase, a novel enzyme. J. Clin. Invest., 1982, 69, 368-76.
- 37 MATSUZAWA A., MURAKAMI M., ATSUMI G., IMAI K., PRADOS P., INOUE K., KUDO I. Release of secretory phospholipase A2 from rat neuronal cells and its possible function in the regulation of catecholamine secretion. Biochem. J., 1996, 318, 701-9.
- 38 MELDRUM BS. Epilepsy and g-aminobutyric acid-mediated inhibition. Int. Rev. Neurobiol., 1975, 17, 1-36.
- 39 MILLER H.P., LEVEY AI., ROTHSTEIN J.D., TZINGOUNIS A.V., CONN P.J. Alterations in glutamate transporter protein levels in kindling-induced epilepsy. J. Neurochem, 1997, 68, 1564-70.
- 40 MOISE L., PISERCHIO A., BASUS VJ., HAWROT E. NMR. Structural analysis of alpha -bungarotoxin and its complex with the principal alpha-neurotoxin-binding sequence on the alpha 7 subunit of a neuronal nicotinic acetylcholine receptor. J. Biol. Chem, 2002, 277, 12406-17.
- 41 NG RH., HOWARD BD. Deenergization of nerve terminals by b-bungarotoxin. Biochemistry, 1978, 17, 4978-86.
- 42 NG RH., HOWARD BD. Inhibition by neurotoxic phospholipases A2 of synaptosomal uptake of g-aminobutyruc acid. J. Neurochem, 1981, 36, 310-2.
- 43 PIN JP., BOCKAERT J. Two distinct mechanisms, differentially affected by excitatory amino acids, trigger GABA release from fetal mouse striatal neurons in primary culture. J. Neurosci, 1989, 9, 648-56.
- 44 PINES G., DANBOLT NC., BJORAS M., ZHANG Y., BENDAHAN A., EIDE L., KOEPSELL H., STORM-MATHISEN J., SEEBERG E., KANNER BI. Cloning and expression of a rat L-glutamate transporter. Nature, 1992, 360, 464-7.
- 45 RADVANYI FR., BON, C. Catalytic activity and reactivity with p-bromophenacyl bromide of the phospholipase subunit of crotoxin: influence of dimerization and association with the non-catalytic subunit. J. Biol. Chem, 1982, 257, 12616-23.
- 46 RADVANYI F., JORDAN L., RUSSO MARIE F., BON C. A sensitive and continuous fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in the presence of serum albumin. Anal. Biochem, 1989, 177, 103-9.
- 47 ROBINSON MB., HUNTER-ENSOR M., SINOR JD. Pharmacologically distinct sodium-dependent L-(3H) glutamate transport process in rat rain. Brain Res, 1991, 544, 196-202.
- 48 ROBINSON MB., SINOR JD., DOWD LA., KERWIN JF Jr. Subtypes of sodium-dependent high-affinity L-(3H) glutamate transport activity: pharmacologic specificity and regulation by sodium and potassium. J. Neurochem, 1993, 60, 167-79.
- 49 SCHWARTZ E. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J. Physiol, 1982, 323, 211-27.
- 50 SCHWARTZ E. Depolarization without calcium can release GABA from a retinal neuron. Science, 1987, 238, 350-5.
- 51 SLOTTA KH., FRAENKEL-CONRAT HL. Schlangengiffe,III: Mitteilung reiningung und krystallization des klappershclangengiffes. Ber. Dtsch. Chem. Ges., 1938, 71, 1076-454.
- 52 SMITH CCT., BRADFORD H.F., THOMPSON EJ., MCDERMA J. Actions of b-bungarotoxin on aminoacid transmitter release. J. Neurochem, 1980, 34, 487-94.
- 53 SOARES AM., GUERRA-SA R., BORJA-OLIVEIRA CR., RODRIGUES VM., RODRIGUES-SIMIONI R., RODRIGUES V., FONTES MR., LOMONTE B., GUTIERREZ JM., GIGLIO JR. Structural and functional characterization of BnSP-7, a Lys49 myotoxic phospholipase A(2) homologue from Bothrops neuwiedi pauloensis venom. Arch. Biochem. Biophys, 2000, 378, 201-9.
- 54 SOARES AM., MANCIN AC., CECCHINI AL, ARANTES EC.; FRANÇA SC., GUTIÉRREZ JM., GIGLio JR. Effects of chemical modifications of crotoxin B, the phospholipase A2 subunit of crotoxin from Crotalus durissus terrificus snake venom, on its enzymatic and pharmacological activities. Int. J. Biochem. Cell Biol, 2001, 33, 877-88.
- 55 SZATKOWSKI M., BARBUR B., ATTWLL D. Non-vesicular release of glutamate from glial cells by reversed eletrogenic glutamate uptake. Nature, 1990, 348, 443-5.
- 56 TOYAMA MH., SOARES AM., WEN-HWA L., POLIKARPOV I., GIGLIO JR., Marangoni S. Amino acid sequence of piratoxin-II, a myotoxic lys49 phospholipase A(2) homologue from Bothrops pirajai venom. Biochimie, 2000, 3, 245-50.
- 57 TRENDELENBURG U. The TIPSA lecture: Functional aspects of the neuronal uptake of noradrenaline. Trends Pharmacol. Sci, 1991, 12, 334-7.
- 58 TZENG M-C., YEN C-H., HSEU M-J., TSENG C-C., TSAI M-D., DUPUREUR CM. Binding proteins on synaptic membranes for crotoxin and taipoxin, two phospholipase A2 with neurotoxicity. Toxicon, 1995, 33, 451-7.
- 59 TZINGOUNIA AV., LIN CL., ROTHSTEIN JD., KAVANAUGH MP. Arachidonic acid activates proton current in the rat glutamate transporter EAAT4. J. Biol. Chem, 1998, 273, 17315-7.
- 60 UHL GR., O´HARA B., SHIMADA S., ZACZEK R., DIGIORGIANNI J., NISHIMORI T. Dopamine transporter: expression in Xenopus oocytes. Mol. Brain Res, 1991, 9, 23-9.
- 61 VALENTIN E., LAMBEAU G. What can venom phospholipase A2 tell us about functional diversity of mammalian secreted phospholipase A2 Biochimie, 2000, 82, 815-31.
- 62 VERHEIJ HM., SLOTBOOM AJ., DE HAAS G. Structure and function of phospholipase A2 Rev. Physil. Biochem., 1981, 91, 91-203.
- 63 WERNICKE JF., OBERJAT T., HOWARD B.D. Neurotoxin-reduced neurotransmitter storage in brain synapses. J. Neurochem., 1974, 22, 781-8.
- 64 ZHU BG., CHEN YZ., XING BR. Effect of calcium on the uptake of glutamate by synaptosomes: possible involvement of two different mechanisms. J. Neural Transm, 1999, 106, 257-64.
Correspondence to
Publication Dates
-
Publication in this collection
24 Sept 2004 -
Date of issue
2004
History
-
Received
28 Apr 2003 -
Accepted
29 Sept 2003