Abstract
This investigation was performed in order to assess the inflammatory response induced by Naja haje arabica venom (NhaV) in rat hind paw. The inflammatory response was estimated by measuring the edema with a Plethysmometer. The venom (0.625-10mug/paw) produced a dose and time-dependent increase in non-hemorrhagic paw edema. The response to NhaV was maximal within 15 min and disappeared in 24 h. Five mug/paw of NhaV was chosen to test the effect of various drugs on the edema induced by this venom. Quinacrine (QNC), a phospholipase A2 (PLA2) inhibitor, and dipyridamole (DPM), an adenosine transport inhibitor, attenuated venom-induced edema in rat paw (P<0.001). Commercially available antivenom was ineffective when administered intravenously, whereas its local administration with NhaV attenuated the edema formation (P<0.001). In conclusion, NhaV-induced edema in rat paw involves PLA2 and adenosine mechanisms. Additionally, the use of polyspecific antivenom, intravenously, was ineffective in preventing NhaV-induced edema.
rat paw edema; Naja haje arabica venom; inflammatory mediators; inhibitor; antivenom
ORIGINAL PAPERS
Pharmacological characterization of rat paw edema induced by Naja haje arabica venom
Al-Asmari A. K.
Research Center, Armed Forces Hospital, Riyadh, Saudi Arabia
Correspondence Correspondence to A. K. AL-ASMARI PO Box: 7897(775S), Riyadh, 11159 Saudi Arabia Phone: + 996 1 4777714 ext. 3811. Fax: +996 1 4777714 ext. 6896 Email: akasmari@medu.net.sa or drasmari@hotmail.com
ABSTRACT
This investigation was performed in order to assess the inflammatory response induced by Naja haje arabica venom (NhaV) in rat hind paw. The inflammatory response was estimated by measuring the edema with a Plethysmometer. The venom (0.625-10mg/paw) produced a dose and time-dependent increase in non-hemorrhagic paw edema. The response to NhaV was maximal within 15 min and disappeared in 24 h. Five mg/paw of NhaV was chosen to test the effect of various drugs on the edema induced by this venom. Quinacrine (QNC), a phospholipase A2 (PLA2) inhibitor, and dipyridamole (DPM), an adenosine transport inhibitor, attenuated venom-induced edema in rat paw (P<0.001). Commercially available antivenom was ineffective when administered intravenously, whereas its local administration with NhaV attenuated the edema formation (P<0.001). In conclusion, NhaV-induced edema in rat paw involves PLA2 and adenosine mechanisms. Additionally, the use of polyspecific antivenom, intravenously, was ineffective in preventing NhaV-induced edema.
Key words: rat paw edema, Naja haje arabica venom, inflammatory mediators, inhibitor, antivenom.
INTRODUCTION
Snakebites are significant medical problems in many parts of the major continents, especially Africa and Asia. In West Africa, the annual bite incidence is 40-120 bites per 100,000 people (50). Cobra is the common name for some members of the Elapidae family; known for their intimidating looks and deadly bites. Updated information about cobras may be found at the professional website Cobra Information Site (http://www.cobras.org/).
Many venomous snakes of medical importance inhabit the Kingdom of Saudi Arabia and surrounding areas, where several of the most lethal snakes are found (24, 25). Naja haje arabica (the Arabian cobra) and Walterinnesia aegyptia (the Egyptian black cobra; the desert cobra) are representatives of the Elapidae family. Researchers have reported their habits, habitats, distribution (8-10, 24, 25), clinical manifestations caused by their bites, and their venom characterization (2, 3, 20, 34, 39, 52, 63, 66, 70-72). Highly complex pathophysiological features of local and systemic nature characterize the cases of snake envenoming (70). Local manifestations usually include edema, pain, hemorrhage, and necrosis (6, 35-37, 47, 55, 70, 71). Systemic complications are characterized by hypofibrinogenemia, thrombocytopenia, and a decline in the coagulation factors V and VIII:C (40, 41, 57, 74). Edema is a common feature of the cutaneous inflammatory response and is dependent on a synergism between the mediators of vascular permeability and the blood flow (17, 75, 77, 78). An important consequence of altered capillary permeability in local inflammation is the extravasation of leucocytes (29). The degree of accumulation of these cells at inflammatory sites in the skin is related to local blood flow (18, 31, 32).
Several researchers have studied the biochemical and pharmacological effects of venoms from different species of snakes from different localities, but the inflammatory effect of the Arabian cobra (Naja haje arabica) venom has not been studied so far. This work shows dose-related and time-course inflammatory effects of NhaV by using the rat paw edema model. The effects of quinacrine (QNC), dipyridamole (DPM), and commercial antivenom were examined. The objectives of this study were to examine the ability of NhaV in inducing paw edema in rats, and to identify the underlying pharmacological mechanisms of this effect.
MATERIAL AND METHODS
Venom and antivenom
Naja haje arabica venom was obtained from the National Serpentarium, Riyadh, Saudi Arabia. Professional hunters collected the snakes from the wild throughout the Kingdom Saudi Arabia. The animals were kept in the serpentarium facility at the previously mentioned place. A specialized team of the Venomology Unit was responsible for scientific classification, milking of specimens, lyophilization and storage of the venom.
The venom was dissolved in saline (final concentration: 10mg/ml) and immediately stored at -20 °C until used. The antivenom utilized in this study was obtained from Al-Hayatt Company, Riyadh, Saudi Arabia. It was produced in hyperimmunized horses by using a mixture of the venoms from Echis carinatus, Echis coloratus, Bitis arietans, Cerastes cerastes, Naja haje, and Walterinnesia aegyptia. The antivenom was dialyzed to remove the preservatives.
Drugs and solutions
Dipyridamole (DPM) was obtained from Sigma, USA, and quinacrine (QNC) was obtained from I.C.N. Biochemicals, Aurora, Ohio, USA.
Animals
Male Wistar rats weighing 145-155g (mean = 150g) were used in all the experiments. These animals were provided by the Armed Forces Hospital, Research Center (Animal House Services). All experiments were carried out according to the methods described by Faria et al. (22) and modified by Al-Asmari (4). Animals were injected subcutaneously in their subplantar region of the right hind paw, with 0.1 ml NhaV (0.625-10mg/paw). The left paws were used as control, and received the same volume (0.1ml) of sterile saline. Edema was measured after 0.25, 0.5, 2, 4, 6, and 24 h by using a Plethysmometer (Model 7150, Ugo Basile, Italy). The results were expressed as mean differences between the final and initial volumes of the injected paws. We could not inject a dose higher than 10mg/paw into the rats because it was lethal.
Influence of three substances on the NhaV-induced edema
Three groups of rats (n = 6) were pretreated with three different classes of substances: 1) dipyridamole (40mg/kg, 50mg/kg, 60mg/kg and 70mg/kg), was administered intraperitonially (i.p.), 30 min before the injection of NhaV (5mg/paw). 2) quinacrine (40mg/kg, 50mg/kg, 60mg/kg and 70mg/kg), was administered i.p., 30 min before the injection of NhaV (5mg/paw). The venom dose of 5<mg/paw was equivalent to 0.033mg/kg body weight of the animals. 3) the commercial antivenom was locally injected (2, 4, 6 and 8mg/kg), together with the same dose of venom, after 30 min incubation at 37°C.
In a separate group of experiments, the commercial antivenom was intravenously administered (up to 6mg/kg) immediately before the subplantar injection of NhaV.
Statistical analysis
Data were presented as the mean ± SEM, evaluated by analysis of variance (ANOVA) and followed by a Bonferroni test (SPSS Program). A p-value of less than 0.05 was considered significant.
RESULTS
Effects of NhaV on rat paw edema
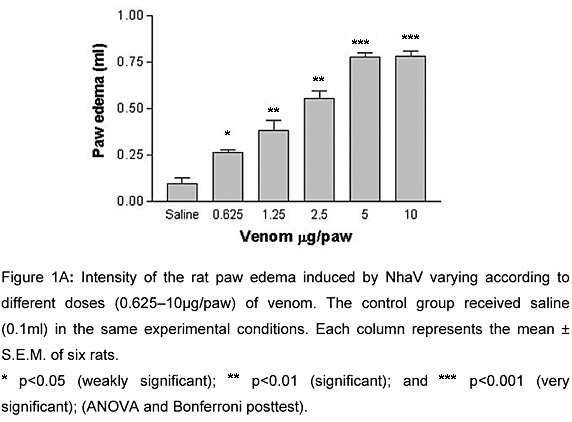
Five mg/paw of NhaV caused significant increase (n = 6; p<0.001) in paw edema, compared to the saline-injected controls.
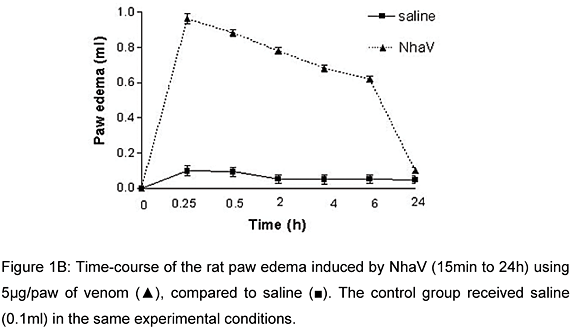
Subplantar injection of NhaV (0.625-10mg/paw) produced a dose and time-dependent non-hemorrhagic edema (Figure 1A). The maximal response was observed 15 min after the venom injection, gradually decreasing in 24 h (Figure 1B). For additional experiments, 5mg/paw of NhaV was used.
Modulation of venom-induced edema with PLA2 and adenosine transport inhibitors
Higher doses of QNC (60 and 70mg/kg) significantly attenuated the venom-induced edema (p<0.001). At 40mg/kg, the effect on edema was insignificant (Figure 2). Treating the animals with DPM (60 and 70mg/kg) also caused a significant (p<0.001) reduction in the venom-induced edema (Figure 3).
Local injection of antivenom (6 and 8 mg/kg) caused a significant (p<0.001) reduction in the NhaV-induced paw edema in a dose-dependent manner. Two mg/kg failed to reduce the edema (Figure 4). The commercial antivenom (up to 6mg/kg), intravenously administered, failed to modify the edema induced by injecting NhaV into the paws of the animals.
DISCUSSION
Previous studies on cobra (Elapidae family) venoms were mainly directed to their neurocardiotoxic effects on experimental animals, due to the nature of the major components present in these venoms. Different toxic effects produced by cobra venoms were due to their contents of neurotoxins, cardiotoxins, activated complement factors, and enzyme toxins (64). Common initial envenoming signs were reported as hypoglycemia (1), general metabolic disturbances (44), muscular dystrophy (49), nephrotoxicity (30), and induction of cytotoxicity (15). Although there are some studies (16, 27) on the venoms local effects, such as myonecrosis and hemorrhage (mainly caused by viper venoms), none of them investigated the edematogenic effect of NhaV, which is well-known only for other Elapids.
The present study shows that subplantar injection of NhaV can cause a significant paw edema in rats. Envenoming also produced edema and altered vascular permeability in mouse hind paw (42, 69). Following these responses, there was an abundant leukocyte infiltration and consequent hemorrhage due to the high doses of venom (42). Since hemorrhage appeared right after the venom injection and interfered in the development of the inflammatory edema (13, 22, 45), most of the previous works were performed with pre-heated viper venoms. The components of cobra venom differ from those of viper venom, usually in the main causative factors like PLA2.
Further works on the NhaV enzymatic (phospholipase A2 [PLA2, E.C.3.1.1.4], hyaluronidase [E.C.4.2.2.1], L-amino-acid-oxidase [LAAO, E.C.1.4.3.2], phosphodiesterase [PDE, E.C.3.1.4.1], and other proteolytic enymes) and biological activity (lethality, neuro and myotoxicity) were tested and speculated by Al-Asmari (2). Increased vascular permeability and blood flow play an important role in the edema formation (17, 75, 78). The capacity of potentiating inflammatory edema in response to different inflammatory mediators is well-known for some exogenously applied vasodilators, such as calcitonin gene-related peptide (CGRP), prostaglandin E2, and prostacyclin (76). Inverse comparisons, based on the use of edema inhibitors, showed that the drugs tested in this work could reverse the edema induced by NhaV. This venom, alone, induced rat paw edema without causing hemorrhage, since it is free of metalloproteinase activity. Furthermore, NhaV had no proteolytic activity when L-BAPNA and DMCasein were used (2). This can refer to a direct or indirect relationship between the local blood flow and the intensity of the edema, compared to other workers (12, 22, 42).
Dipyridamole is a potent inhibitor of platelet aggregation. It suppresses adenosine cellular uptake into erythrocytes, endothelial cells, and platelets, increasing the levels of circulating adenosine and intraplatelet cAMP (19, 48, 79). It also inhibits PDE, an enzyme responsible for the breakdown of cGMP, which has anti-aggregating effect. The edema reduction might be due to DPM effect on PDE and other mediators responsible for inducing inflammation.
Quinacrine is a direct inhibitor of PLA2 action (7, 23, 65). It has been shown to act as a noncompetitive inhibitor of the nicotinic acetylcholine receptor (nAChR), though this mechanism is still controversial (60). Phospholipase A2 leads to an increase in capillary permeability and to myonecrosis by the disruption of lipid membranes (in vitro and in vivo) that end in hemolysis, as an indirect effect (38, 54). It is also involved in membrane signal transduction, cellular function, and inflammatory responses (7). Lipid membrane hydrolysis by PLA2 results in membrane perturbation, cell degranulation, and stripping of receptors in the cell surface (46, 56). The enzymatic effect on arachidonic acid provides a number of inflammatory mediators via cyclooxygenase or lipoxygenase action (22). The complement system is activated by the cobra venom factor (CVF) infusion (34) through an alternative pathway. Cobra venom factor and zymosan-activated plasma (ZAP) increased the extravascular accumulation of radiolabeled albumin in rats, mice (61, 68) and rabbits (73). Moreover, the CVF used in some previous studies contained PLA2, which may affect the hemodynamic and permeability responses (58) by the generation of arachidonic acid metabolites (51).
It is important to refer to the other uses of QNC (also known as mepacrine) as antimalarial agent (53), cell membrane stabilizer, scavenger of oxygen-derived free radicals (ODFR) and inhibitor of tumor necrotic factor (TNF) (59, 62), and potent PLA2 inhibitor (20). Though the elapidic venom usually shows low hyaluronidase activity, Naja haje venom showed a considerable activity of this enzyme (2). Corticosteroids also acted directly on leukocytes and other types of cells, inhibiting the release of cytokines and other inflammatory mediators (11). Gutierrez et al. (28) and Lomonte et al. (43) had a similar report on the kinetics and cell composition of the inflammatory infiltrate observed in rat footpad, where many proteases such as kallikrein (which produces bradykinin and kallidin), are found as active factors (21, 26). These kinins are potent vasodilators that increase capillary permeability and are easily produced in the tissues after injury, being the most important agents in edema formation (26,33). The primary difference between our findings and those of other researchers, like Faria et al. (22), was that NhaV induced edema without potentiators. Secondly, some NhaV components decreased the muscle contraction by blocking the nerve conduction postsynaptically.
Finally, we attempted to examine the ability of polyspecific antivenom in neutralizing the edematogenic activity of NhaV. The use of antivenom, intravenoulsly, proved to be ineffective against the rat paw edema induced by NhaV. When a mixture of venom and antivenom was injected into the rat paws, an inhibitory effect was observed. This situation could raise questions about using antivenom in the treatment of victims. Benbassat and Shalev (14) investigated this fact by reviewing reported data on the effect of other snake antivenoms in vitro, on laboratory animals, and on humans. These authors also re-examined alternative methods of treatment in order to assess the efficacy of using antivenom. Tilbury et al. (67), reporting about a different species, also speculated and reviewed this situation.
In regard to the edema, it could be suggested that the amount of antibodies produced against the edematogenic component(s) of NhaV was very low (highly diluted antiserum) to neutralize them. This finding is in agreement with other works (4, 5, 22). Further studies, attempting to purify the edematogenic component(s) present in this venom, are necessary to elucidate this aspect.
ACKNOWLEDGEMENTS
The author would like to thank Riyadh Military Hospital, for supporting this work; Nasreddin M. Abdo, for his help in this study; and Mr. Abbas Manthiri, for his assistance in the experimental study.
Received: December 2, 2003
Accepted: June 18, 2004
Published online: February 11, 2005.
- 1 ABU-SINNA G., AL-ZAHABY AS., ABD EL-AAL A., ABD EL-BASIT A., SOLIMAN NA. The effect of the viper Cerastes cerastes cerastes venom and venom fractions on carbohydrate metabolism. Toxicon, 31, 791-801.
- 2 AL-ASMARI AK. Production and assessment of ovine antivenoms for the treatment of snake envenoming in Saudi Arabia London: University of London, Faculty of Medicine, 1996. 205p. (Doctor of Philosophy)
- 3 AL-ASMARI AK. Assessment of an ovine antivenom raised against venom from the desert black cobra (Walterinnesia aegyptia). Toxicon, 1997, 35, 141-5.
- 4 AL-ASMARI AK. Pharmacological characterization of the rat's paw oedema induced by Echis coloratus venom. On line J. Biol. Sci., 2003a, 3, 309-19.
- 5 AL-ASMARI AK. Pharmacological characterizations of the rat paw edema induced by Echis pyramidum venom. On line J. Biol. Sci., 2003a, 3, 824-33.
- 6 AL-JAMMAZ I., AL-SADOON MK., FAHEEM A. Effect of LD50 Dose of Echis coloratus venom on serum and tissue metabolites and some enzymes of male albino rats. J. King Saud. Univ. Sci., 1999, 2, 61-8.
- 7 AL-MOUTAERY A., TARIQ M. Effect of quinacrine, a phospholipase A2 inhibitor on stress and chemically induced gastroduodenal ulcers. Digestion, 1997, 58, 129-37.
- 8 AL-SADOON MK. Survey of the reptilian fauna of the Kingdom of Saudi Arabia. 1. The snake fauna of the Central Region. J. King Saud. Univ. Sci., 1989, 2, 53-69.
- 9 AL-SADOON MK., AL-FARRAJ SA. Venomous snakes of Saudi Arabia. Species, distribution and precautionary methods Riyadh: Al-Resalah Press, 1992. 125p.
- 10 AL-SADOON MK., AL-FARRAJ SA., ABDO NM. Survey of the reptilian fauna of the Kingdom of Saudi Arabia. III. An ecological survey of the lizard, amphisbaenian and snake fauna of Al-Zulfi Area. Bull. Maryland Herpetol. Soc., 1991, 27, 1-22.
- 11 ANGELI A., MASERA RG., SARTORI ML., FORTUNATI N., RACCA S., DOVIO A., SATAURENGHI A., FRAIRIA R. Modulation by cytokines of glucocorticoid action. Ann. N.Y. Acad. Sci., 1999, 876, 210-20.
- 12 ANTUNES E., MARANGONI RA., BRAIN SD., DE NUCCI G. Phoneutria nigriventer (armed spider) venom induces increased vascular permeability in rat and rabbit skin in vivo. Toxicon, 1992, 30, 1011-6.
- 13 ASSAKURA MT., REICHL AP., MANDELBAUM FR. Comparison of immunological biochemical and biophysical properties of three hemorrhagic factors isolated from the venom of Bothrops jararaca (jararaca). Toxicon, 1986, 24, 943-6.
- 14 BENBASSAT J., SHALEV O. Envenomation by Echis coloratus (Mid-East saw- scaled viper): a review of the literature and indication for treatment. Isr. J. Med. Sci., 1993, 29, 239-50.
- 15 BERTKE EM., ATKINS JH. Effect of Centruroides sculpturatus venom upon rat tissues: a histopathological study. Toxicon, 1961, 2, 205-18.
- 16 BJARNASON JB., FOX JW. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther, 1994, 62, 325-72.
- 17 BRAIN SD., WILLIAMS TJ. Inflammatory mechanism of inflamed-tissue factor. Agents Actions, 1985, 3, 348-56.
- 18 BUCKLEY TL., BRAIN SD., COLLINS PD., WILLIAM TJ. Inflammation edema induced by interactions between interleukin-1 and neuropeptide calcitonin gene-related peptide. J. Immunol, 1991, 146, 3424-30.
- 19 CARNEIRO AS., RIBEIRO OG., DE FRANCO M., CABRERA WHK., VORRARO F., SIQUEIRA M., IBANEZ OM., STAROBINAS N. Local inflammatory reaction induced by Bothrops jararaca venom differs in mice selected for acute inflammatory response. Toxicon, 2002, 40, 1571-9.
- 20 CHIARIELLO M., AMBROSIO G., CAPPELLI-BIGAZZI M., PERONE-FILARDI P., TRITTO I., NEVOLA E., GOLINA P. Reduction in infarct size by the phospholipase inhibitor quinacrine in dogs with coronary artery occlusion. Am. Heart J., 1990, 120, 801-7.
- 21 ERDOS EG. Structure and function of biologically active peptides: bradykinin, kallidin and congeners. Ann. N.Y. Acad. Sci, 1963, 104, 1.
- 22 FARIA L., DE ANTUNES E., BON C., LOBO., DE A. Pharmacological characterization of the rat paw edema induced by Bothrops lanceolatus (Fer de Lance) venom. Toxicon, 2001, 39, 825-30.
- 23 FLOWER RJ. Glucocorticoids and the inhibition of phospholipase A2 In: SCHLELMER RP., CLAMOR HN., ORONSKY AL. Eds. Anti-inflammatory steroid action. Basic and Clinic Aspects New York: Academic Press, 1989: 48-66.
- 24 GASPERETTI J. Walterinnesia aegyptia Lataste a rare black snake. J. Saudi Arab. Nat. Hist. Soc., 1976, 1, 2-8.
- 25 GASPERETTI J. Snakes of Arabia. Fauna Saudi Arabia, 1988, 9, 169-450.
- 26 GOTH A. Medical pharmacology St. Louis, Missouri: Mosby Company, 1978. 766p.
- 27 GUTIERREZ JM., LOMONTE B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon, 1995, 33, 1405-24.
- 28 GUTIERREZ JM., CHAVES F., CERDAS L. Inflammatory infiltrate in skeletal muscle injected with Bothrops asper venom. Rev. Biol. Trop., 1986, 34, 209-19.
- 29 HAMBLIN AS. Cytokines. In: DALE M., FOREMAN JC., FAN TAI-PING D. Eds. Textbook of immunopharmacology 3.ed. London: Blackwell Scientific Publications, 1994: 179-92.
- 30 ICKOWIZ M., SHULOV A., NAOR D. The effect of Vipera palestina venom on the thymus, lymph nodes and kidneys. Toxicon, 1966, 3, 305-6.
- 31 ISSEKUTZ AC. Effect of vasoactive agents on polymorphonuclear leukocyte emigration in vivo. Lab. Invest, 1981, 45, 234-40.
- 32 ISSEKUTZ AC., MOVAT HZ. The effect of vasodilator prostaglandins on polymorphonuclear leukocyte infiltration and vascular injury. Am. J. Pathol, 1979, 107, 300-9.
- 33 JOHNSON AR., ERDOS EG. Release of histamine from mast cells by vasoactive peptides. Proc. Soc. Exp. Biol. Med., 1973, 142, 12-52.
- 34 JOHNSON R., COOPER JA., MALIK AB. Effect of complement activation with cobra venom factor on pulmonary vascular permeability. Am. Physiol. Soc, 1986, 86, 2202-9.
- 35 JORGE MT., RIBEIRO LA., O'CONNELL JL. Prognostic factors for amputation in the case of envenoming by snakes of the Bothrops genus (Viperidae). Ann. Trop. Med. Parasitol., 1999, 93, 401-8.
- 36 KAMIGUTI AS., CARDOSO JL., THEAKSTON RD., SANO-MARTINS IS., HUTTON RA., RUGMAN P., WARRELL DA., HAY CR. Coagulopathy and haemorrhage in human victims of Bothrops jararaca envenoming in Brasil. Toxicon, 1991, 9, 961-72.
- 37 KINGSTON ME. Management of snakebite in Saudi Arabia. King Faisal Special. Hosp. Med. J., 1981, 1, 87-94.
- 38 KINI RM., EVANS HJ. A model to explain the pharmacological effects of snake venom phospholipase A2 Toxicon, 1989, 27, 613-35.
- 39 LEE CY. Mode of action of cobra venom and its purified toxins. In: SIMPSON LL. Ed. Neuropoisons New York: Plenum Press, 1971: 21-70.
- 40 LOBO DE ARAUJO A., DONATO JL., Bon C. Purification from Bothrops lanceolatus (Fer de lance) venom of a fibrino(geno)lytic enzyme with esterolytic activity. Toxicon, 1998, 36, 745-58.
- 41 LOBO DE ARAUJO A., RADVANYI F., BON C. Acidic phospholipase A2 from Bothrops lanceolatus venom: purification and molecular, enzymatic and pharmacological properties. Toxicon, 1994, 32, 1069-81.
- 42 LOBO DE ARAUJO A., OLIVIA DE SOUZA A., ALICE DA., CRUZ-HOFLING M., FLORES CA., BON C. Bothrops lanceolatus (Fer de lance) venom induces edema formation and increases vascular permeability in the mouse hind paw. Toxicon, 2000, 38, 209-21.
- 43 LOMONTE B., TARKOWSKI A., HANSON LA. Host response to Bothrops asper snake venom. Analysis of oedema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation, 1993, 17, 93-105.
- 44 MAHMOUD I. Biochemical and physiological studies on the action of viper venom Cerastes vipera on mice Mus musculus. Egypt: Cairo University, Faculty of Science, 1983. 139p. [Masters Thesis].
- 45 MANDELBAUN FR., REICHL AP., ASSAKURA MT. Some physical and biochemical characteristics of HF2, one of the hemorrhagic factors in the venom of Bothrops jararaca In: OHSAKA A., HAYASHI K., SAWAI Y. Eds. INTERNATIONAL SYMPOSIUM ON ANIMAL, PLANT AND MICROBIAL TOXINS, 4, Tokyo, 1974. Proceedings of the Fourth International Symposium on Animal, Plant and Microbial Toxins. New York: Plenum Press, 1975, 111-21.
- 46 MANSBACH GM. Phospholipases: old enzymes with new meaning. Gastroenterology, 1990, 98, 1369-82.
- 47 MILANI-JUNIOR R., JORGE MT., DE CAMPOS FP., MARTINS FP., BOUSSO A., CARDOSO JL., RIBEIRO LA., FAN HW., FRANCA FO., SANO-MARTINS IS., CARDOSO D., IDE FERNANDEZ C., FERNANDES JC., ALDRED VL., SANDOVAL MP., PUORTO G., THEAKSTON RD., WARRELL DA. Snakebites by jararacusu (Bothrops jararacussu): clinicopathological studies of 29 proven cases in São Paulo State, Brasil. Q. J. Med., 1997, 90, 323-34.
- 48 MOCANDA S., VANE JR. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N. Engl. J. Med., 1979, 300, 1142-7.
- 49 MOHAMED AH.., KHALED LZ. Effect of the venom of Cerastes cerastes on nerve tissue and skeletal muscle. Toxicon, 1966, 3, 223-4.
- 50 NORRIS R., MINTON S. Snake envenomations, cobra. E. Med., 2002, 3, 1-12. (http://www.emedicine.com/emerg/)
- 51 PIPER PJ. Formation and actions of leukotrienes. Physiol. Rev, 1984, 64, 744-61.
- 52 REID HA., THEAKSTON RDG. The management of snakebites. Bull. World Health Organ., 1983, 61, 885-95.
- 53 ROLLO IM. Drugs used in chemotherapy of malaria. In: GOODMAN LS., GILMAN A. Eds. The pharmacological basis of therapeutics New York: MacMillan, 1980: 1038-78.
- 54 ROSENBERG P. Pharmacology of phospholipase A2 from snake venoms. In: LEE CY. Ed. Handbook of experimental pharmacology New York: Springer Verlag, 1979, 52: 403-47.
- 55 ROSENFELD G. Symptomatology, pathology and treatment of snake bites in South America. In: BUCHERL W., BUCKLEY EE. Eds. Venomous animals and their venoms. New York: Academic Press, 1971, 345-403.
- 56 SAKATA T., NAKAMURA E., TSURUTA Y., TAMAKI M., TERAOKA H., TOJO H., ONO T., OKAMOTO M. Presence of pancreatic-type phospholipase A2 mRNA in rat gastric mucosa and lung. Biochim. Biophys. Acta, 1989, 1007, 124-6.
- 57 SCHAEFFER RC., BRISTON C., CHILTON SM., CARLSON RW. Disseminated intravascular coagulation following Echis carinatus venom in dogs: effects of a synthetic thrombin inhibitor. J. Lab. Clin. Med, 1986, 107, 488-97.
- 58 SHAW JO., ROBERTS MF., ULEVITCH RJ., HENSON P., DENNIS EA. Phospholipase A2 contamination of cobra venom factor preparations. Am. J. Pathol, 1978, 91, 517-30.
- 59 SHEN C., WANG D., CHANG M., HSU K. Protective effect of mepacrine on hypoxia-reoxygenation induced acute lung injury in rats. J. Appl. Physiol, 1995, 78, 225-31.
- 60 SPITZMAUL G., DILGER JP., BOUZAT C. The noncompetitive inhibitor quinacrine modifies the desensitization kinetics of muscle acetylcholine receptors. Mol. Pharmacol, 2001, 60, 235-43.
- 61 STIMLER PN., HUGLI TE., BLOOR CM. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am. J. Pathol, 1980, 100, 327-48.
- 62 STRUHAR D., KIVITZ S., TOPILSKY M. Quinacrine inhibits oxygen radicals release from human alveolar macrophages. Int. J. Immunopharmacol, 1992, 14, 257-77.
- 63 SUTHERLAND SK., COULTER AR., HARRIS RD., LOVERING KE., ROBERTS ID. A study of the major Australian snake venoms in the monkey (Macaca fascicularis). I. The movement of injected venom, methods which retard this movement, and the response to this antivenom. Pathology, 1981, 13, 13-27.
- 64 TAN NH., PONNUDURAI GA. comparative study of the biological properties of venoms from snakes of the genus Vipera (true adders). Comp. Biochem. Physiol, 1990, 96, 683-8.
- 65 TARIQ M., KHAN HA., AL-MOUTAERY K., AL-DEEB S. Dipyridamole potentiates 1 - methyl - 4 phenyl - 1, 2, 3, 6 tetrahydropyridine (MPTP) induced experimental parkinsonism in mice. Parkinsonism Relat. Disord., 1998, 4, 43-50.
- 66 THEAKSTON RDG., REID HA., IDDON D. Standardization tests for estimation of defibrinating, coagulant, hemorrhagic and necrotizing effects of snake venom. Toxicon, 1982, 20, 363.
- 67 TILBURY CR., MADKOUR MM., SALTISSI D., SULEIMAN M. Acute renal failure following the bite of Burton's carpet viper Echis coloratus Gunther in Saudi Arabia: case report and review. Saudi Med. J., 1987, 8, 87-95.
- 68 TILL GO., JOHNSON KG., KUNKEL R., WARD PA. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J. Clin. Invest, 1982, 69, 1126-35.
- 69 TREBIEN HA., CALIXTO JB. Pharmacological evaluation of rat paw edema induced by Bothrops jararaca venom. Agents Actions, 1989, 26, 292-300.
- 70 WARRELL DA. Venomous bites and stings in Saudi Arabia. Saudi Med. J., 1993, 14, 196-202.
- 71 WARRELL DA. Clinical toxicology of snake bites in Africa and the Middle East/Arabian Peninsula. In: MEIER J., WHITE J. Eds. Handbook of clinical toxicology of animal venoms and poisons 5.ed. Boca Raton, Florida: CRC Press, 1995: 433-92.
- 72 WARRELL DA., ARNETT C. The importance of bites by the saw-scaled or carpet viper (Echis carinatus). Epidemiological studies in Nigeria and a review of the world literature. Acta Trop. (Basel), 1976, 33: 307-41.
- 73 WEBSTER RO., LARSON GL., MITCHEL BC., GOINS AJ., HENSON PM. Absence of inflammatory lung injury in rabbits challenged intravascularly with complement-derived chemotactic factors. Am. Rev. Respir. Dis, 1982, 125, 335-40.
- 74 WEISS HJ., PHILLIPS LL., HOPEWELL WS., PHILLIPS G., CHRISTY NP., NITTI RF. Heparin therapy in a patient bitten by a saw scaled viper (Echis carinatus), a snake whose venom activates prothrombin. Am. J. Med, 1973, 54, 653-62.
- 75 WILLIAMS TJ. Prostaglandin E2, prostaglandin I2 and the vascular changes in inflammation. Br. J. Pharmacol, 1979, 65, 517-24.
- 76 WILLIAMS TJ. Interactions between prostaglandins, leukotrienes and other mediators of inflammation. Br. Med. Bull, 1983, 39, 239-42.
- 77 WILLIAMS TJ., MORLEY J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature, 1973, 246, 215-7.
- 78 WILLIAMS TJ., PECK MJ. Role of prostaglandin-mediated vasodilataion in inflammation. Nature, 1977, 270, 530-2.
- 79 ZAHAVI M., ZAHAVI J., KAKKAR VV. Effect of adenyl-cyclase activators, phosphodiesterase inhibitors and pyridoxal-5-phosphate on platelet aggregation and adenosine -3'-5'-cyclic monophosphate accumulation. Thromb. Haemost, 1984, 52, 205-9.
Publication Dates
-
Publication in this collection
03 May 2005 -
Date of issue
Mar 2005
History
-
Reviewed
18 June 2004 -
Received
02 Dec 2003 -
Accepted
11 Feb 2005