Abstract
ELISA was used to evaluate, accompany, and compare the humoral immune response of Swiss mice during hyperimmunization with native and Cobalt-60-irradiated (60Co) venoms of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni. Potency and neutralization were evaluated by in vitro challenges. After hyperimmunization, immunity was observed by in vivo challenge, and the side effects were assessed. The animals immunization with one LD50 of each venom occurred on days 1, 15, 21, 30, and 45, when blood samples were collected; challenges happened on the 60th day. Results showed that ELISA was efficient in evaluating, accompanying and comparing mouse immune response during hyperimmunization. Serum titers produced with natural venom were similar to those produced with irradiated venom. Immunogenic capacity was maintained after 60Co-irradiation. The sera produced with native venom showed neutralizing potency and capacity similar to those of the sera produced with irradiated venom. All antibodies were able to neutralize five LD50 from these venoms. Clinical alterations were minimum during hyperimmunization with irradiated venom, however, necrosis and death occurred in animals inoculated with native venom.
venom; irradiation; Bothrops; ELISA; serum production; detoxification
ORIGINAL PAPER
Immunological assessment of mice hyperimmunized with native and Cobalt-60-irradiated Bothrops venoms
Ferreira Junior R. S.I,II; Nascimento N.III; Martinez J. C.IV; Alves J. B.III; Meira D. A.I; Barraviera B.I,II
IDepartment of Tropical Diseases, Botucatu School of Medicine, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil
IICenter for the Study of Venoms and Venomous Animals, CEVAP, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil
IIIRadiobiology Supervision - Nuclear Energy Research Institute (IPEN/CNEN/SP), São Paulo, Brazil
IVDepartment of Production Engineering, School of Engineering, São Paulo State University, UNESP, Bauru, São Paulo, Brazil.
Correspondence Correspondence to Rui Seabra Ferreira Junior Centro de Estudos de Venenos e Animais Peçonhentos - CEVAP, UNESP, Distrito Rubião Júnior, CP 577 18618-000, Botucatu, SP, Brasil Fone: 55-14-3815-3963, FAX: 55-14-3811-6054 Email: rseabra@cevap.org.br
ABSTRACT
ELISA was used to evaluate, accompany, and compare the humoral immune response of Swiss mice during hyperimmunization with native and Cobalt-60-irradiated (60Co) venoms of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni. Potency and neutralization were evaluated by in vitro challenges. After hyperimmunization, immunity was observed by in vivo challenge, and the side effects were assessed. The animals immunization with one LD50 of each venom occurred on days 1, 15, 21, 30, and 45, when blood samples were collected; challenges happened on the 60th day. Results showed that ELISA was efficient in evaluating, accompanying and comparing mouse immune response during hyperimmunization. Serum titers produced with natural venom were similar to those produced with irradiated venom. Immunogenic capacity was maintained after 60Co-irradiation. The sera produced with native venom showed neutralizing potency and capacity similar to those of the sera produced with irradiated venom. All antibodies were able to neutralize five LD50 from these venoms. Clinical alterations were minimum during hyperimmunization with irradiated venom, however, necrosis and death occurred in animals inoculated with native venom.
Key words: venom, irradiation, Bothrops, ELISA, serum production, detoxification
INTRODUCTION
Treatment of accidents caused by snakebites is made with heterologous serum obtained from the plasma of animals, generally equine (11, 29, 40) and ovine (18, 28, 36), hyperimmunized with snake venoms (45).
For obtaining hyperimmune anti-bothropic plasma, horses are inoculated with several doses of desiccated venoms together with adjuvants, which normally have toxic activity. Local and systemic reactions are frequently observed. These may also lead the animal to death (22).
Consequently to these observations, we have been searching for alternatives by preparing toxoids through venom biological detoxification but preserving immunogenicity (11, 45).
In regard to the techniques used in the toxoids preparation, there are innumerous works in which chemical and/or physical agents were utilized for this purpose. Among the chemical agents are: carboxymethyl cellulose (30), photooxidation in the presence of methylene blue (27), chelating agents (20), formalin (15), iodine (16), glutaraldehyde (22), and others. The principal physical agents are: x-ray radiation (19), ultraviolet radiation (46), heat (15), and gamma radiation (25). The latter, according to previous works (1, 7, 9, 12, 14, 17, 21, 31-38, 41-43), has proved to be an excellent tool of detoxification, since it is able to reduce toxicity without altering immunogenicity. In addition, this radiation does not add any new substance to the venom.
Researchers have been searching for alternatives to improve heterologous sera productivity by preparing less damaging toxoids, preserving their immunogenic capacity (11, 44). Therefore, the aims of this work were to evaluate and compare the humoral immune response; to verify the side effects; to determine and compare the serum neutralizing potency and capacity in mice inoculated with native and 60Co-irradiated venoms of B. jararaca, B. jararacussu and B. moojeni. For this purpose, we utilized the in vitro method and evaluated the immunogenicity acquired by the mice challenged in vivo with the respective native venoms after hyperimmunization, in order to employ these results in the serum production.
MATERIALS AND METHODS
Crude air-dried venom from a large number of Bothops snakes, Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni, was provided from The Center for the Study of Venoms and Venomous Animals CEVAP, UNESP. Mice were obtained from the colony housedat the same institute.
Venom irradiation
Whole Bothrops venoms were dissolved in saline solution (0.15M NaCl adjusted to pH 6.8), and their protein concentration adjusted to 2mg/ml as determined by the Bradford method (8). Samples were irradiated at a dose of 5.25KGy/h with 2000Gy using gamma rays derived from a 60Co source Gammacell 220 (Atomic Energy Agency of Canada Ltd) in the presence of O2 at room temperature. These experiments were performed at the Institute of Nuclear and Energetic Research IPEN/CNEM/SP.
Production of antibodies
Seven groups of eight Swiss male mice weighing 18-22g were immunized. Group I native B. jararaca venom; Group II irradiated B. jararaca venom; Group III native B. jararacussu venom; Group IV irradiated B. jararacussu venom; Group V native B. moojeni venom; Group VI irradiated B. moojeni venom; Group VII control. Each inoculum received had a LD50 of the respective venoms. The LD50 values of the native venoms utilized in this experiment were 2.4µg/g mice for B. jararaca, and 4.4µg/g mice for B. jararacussu and B. moojeni (33).
Inoculations were performed on day one, using a complete Freunds adjuvant intradermically; on day 15, using an incomplete Freunds adjuvant subcutaneously; and on days 21, 30 and 45, using PBS intraperitoneally.
The control group received inocula on the same days but only with the adjuvants.
All the animals were inoculated with 200µl of a solution containing 100µl of the excipient and 100µl of a mixture of the respective venom diluted in saline solution. The control group received 200µl of a solution containing 100µl of the excipient and 100µl of saline solution.
Before receiving each inoculum, all the animals were bled by the retro-orbital plexus, a 100-µl sample of blood was collected, and the serum was separated. From each group of animals, a serum pool was prepared, frozen and kept at -20oC.
Enzyme-linked immunoassay (ELISA)
This assay was performed in order to detect, in the animal sera, antibodies produced against the native and irradiated venom of Bothrops jararaca, B. jararacussu and B. moojeni. They were performed on days 15, 21, 30, 45 and 60, as described by Nascimento et al. (33). On the 60th day, the titration of the sera from the two groups studied was performed based on the dilution of 1:200 until 1:204,800.
Each well was read in an automatic microplate reader, using a 450nm wavelength filter.
All the serum samples were tested twice. The serum of non-immunized animals was used as control.
Potency evaluation "in vitro" of the native and irradiated antivenoms
For evaluation of the potency on the 60th day after the first inoculation, a 100µl aliquot of each serum pool was incubated with 100µl of a solution containing variable quantities of native venom. For this purpose, different dilutions in PBS, equivalent to 1, 3, 5, 10, and 15 LD50, were prepared. The incubation was performed in Eppendorf tubes kept in an incubator at 37oC for 30 minutes (28). Then, 200µl of each solution was individually inoculated into four mice intraperitoneally.
For control, eight mice were inoculated: four received 200µl of a solution containing five LD50 of the native venom diluted in PBS to confirm its toxicity; four received only 100µl of an aliquot of serum diluted in 100µl PBS to evaluate its innocuousness. After 48 hours, mortality rate was recorded.
Protective ability of the antivenom
To determine the antivenom neutralization capacity on the 60th day after the first inoculation, a constant amount of Bothrops jararaca venom (1.2mg/ml), Bothrops jararacussu venom (2.2µg/ml) or Bothrops moojeni venom (2.2µg/ml) was mixed with different dilution of Swiss mice antivenom raised against the non-irradiated (native venom) or irradiated venom. Following incubation at 37oC for 30 minutes, the mixtures were injected intraperitoneally into mice at a dose of 10µl/g body weight (28). Toxin neutralizing capacity (mg of toxin/ml of antivenom) was calculated as described by Kaiser et al., 1986 (26).
Evaluation of the "in vivo" neutralizing capacity
In order to verify the "in vivo" neutralizing capacity of the antibodies produced by the animals, at the end of the immunization process, four mice of each group studied were weighed and individually challenged with five LD50 of the native venom diluted in PBS. Each animal received 200µl of this solution intraperitoneally. After 48 hours, mortality rate was recorded.
Clinical assessment of the animals
The animals were observed throughout the hyperimmunization process. Presence of systemic alterations, local alterations in the skin and/or subcutaneously, as well as the number of dead animals, were recorded.
Statistical analysis
The significance of the differences between the means of two experimental groups was determined by the Snedecors F-test. Values of p<0.05 were considered statistically significant (13).
RESULTS
Results of the ELISA method for the serum produced with native and irradiated venom
Analyzing Figures 1, 2 and 3, we could observe that the optical density values in the ELISA test for the several dilutions of native and irradiated venoms of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni did not show statistical difference between the groups studied.
It was also verified that the antibodies could already be detected since the first inoculum.
Neutralizing capacity and potency
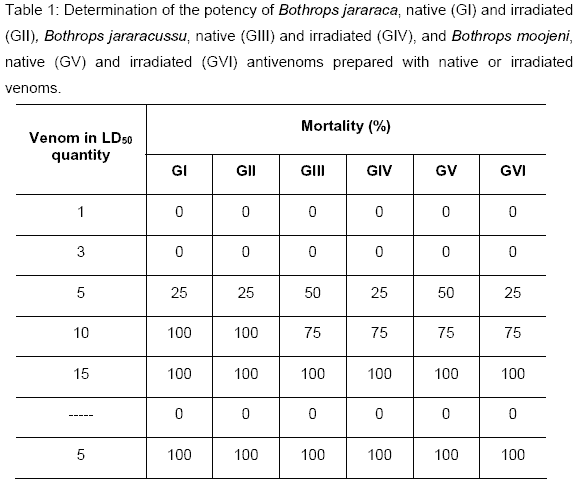
Analyzing Tables 1 and 2, we could observe that the sera pool, obtained from mice hyperimmunized with native and irradiated Bothrops jararaca venom, neutralized 4.8mg/ml. The pool of the sera obtained from mice hyperimmunized with native Bothrops jararacussu venom neutralized 35.2mg/ml, and mice hyperimmunized with irradiated Bothrops jararacussu venom neutralized 17.6mg/ml. The pool of the sera obtained from mice hyperimmunized with native and irradiated Bothrops moojeni venom neutralized 17.6mg/ml.
"In vivo" tests
From the groups studied, none of the animals died after 48 hours of observation.
Clinical assessment of the animals
Clinical evaluation permitted to observe that the animals of Groups I, III and V, inoculated with different Bothrops native venoms, showed necrosis at the inoculation site, as presented in Figure 4. Edema at the inoculation site was extensive in all the animals, and some deaths occurred during the hyperimmunization process.
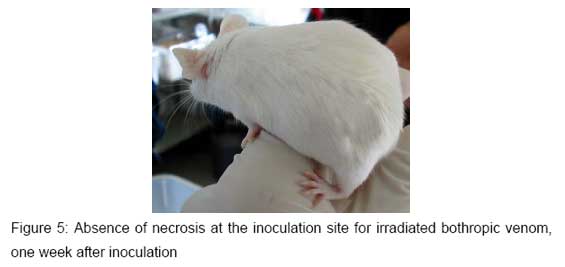
The animals of Groups II, IV and VI, inoculated with different Bothrops irradiated venoms, showed just a small edema at the inoculation site, as presented in Figure 5. This occurred only in the first inoculation during the hyperimmunization process. It must be pointed out that the same alterations were observed in the control group.
DISCUSSION
To date, the most efficient treatment known for accidents caused by venomous snakes is the specific heterologous serum (5, 45). Throughout history, antivenom has been produced in large animals, particularly Equidae in Brazil (11, 22, 40, 45).
Serum production in domesticated animals has some inconveniences, such as the undesirable effects caused by inoculation of crude venom. They are characterized by intense local reactions followed by necroses, fistulas, and hemorrhages (22), culminating in the removal of these animals from the production line. According to Angulo et al. (2), there are few works on clinical and physiological alterations in animals immunized for antivenom production.
In order to overcome these difficulties, several methods of venom detoxification have been studied to minimize the alterations caused by the venom (14-16, 19, 20, 22, 25, 27, 30, 46). In addition, researchers look for alternatives of antibodies production with high quality, low costs, the highest seric levels, and without side effects for the animals.
In this context, gamma radiation has showed to be an excellent tool for snake venom attenuation. Some authors (12, 32, 33) verified that, after irradiation, Crotalus durissus terrificus venom and its fractions antigenic and immunogenic properties were preserved and toxicity was reduced.
This way, 2000Gy irradiation is the ideal dose for snake venoms detoxification, since it preserves many immunological properties (31-33).
In the present study, we investigated the effectiveness of the technique of irradiation of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni venoms with 60Co in the production of antivenomous serum in mice.
The ELISA technique was used in the detection and accompaniment of the antibodiestiter during the hyperimmunization process. This method has showed to present the best specificity, sensitivity, fastness, simplicity, and low costs (3, 4, 6, 32, 35, 39, 44). Results showed that the optical density values obtained in the ELISA test for the several dilutions of the native and irradiated venoms of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni did not show statistical difference between the groups studied.
It was also verified that the antibodies could be detected since the first inoculum.
We can observe, from the titers of antibodies produced, that both the group hyperimmunized with native venom and the group hyperimmunized with irradiated venom were immunogenic and induced the production of antibodies able to recognize the respective native venoms of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni. These results confirm the data obtained by other authors (12, 32, 33).
Tests of the serum neutralizing potency and capacity were performed after the animals of the groups studied produced high titers of antibodies.
After the in vitro challenges with native venoms of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni, we could observe that those animals that received sera from animals immunized with irradiated venom showed the same lifetime as the animals that received sera from animals immunized with native venom.
These results, observed in the determination of the sera pool neutralizing capacity and potency, showed that, despite the titers of antibodies were similar according to the ELISA assay, the serum produced from the irradiated venom showed to be efficient, acting similarly to the serum obtained from the group inoculated with native venom.
Maria et al. (29) and Heneine et al. (24), verifying the correlation between the in vivo and in vitro methods for neutralization and potency of antibothropic sera, suggest that ELISA may be used as an in vitro technique to evaluate the Brazilian antibothropic sera potency during hyperimmunization. However, the classical in vivo test in mice must be used in the final tests of antivenom production. This way, the use of experimental animals in the final tests of serum neutralization and potency evaluation, as performed in the present work, cannot be disregarded.
For Hati et al. (23),detoxification may result in structural alterations of important epitopes and, in consequence of this, diminish the efficiency of antibodies produced against these toxins.
According to Cardi et al. (8), gamma irradiation proved to be the most successful method for crotoxin detoxification. These authors (8) suggest that toxicity reduction is due to a precocious crotoxin endocytosis by phagocytic cells, improving the antigen processing. This occurs because the irradiation promotes molecule oxidation, facilitating its phagocytosis due to the presence of scavenger receptors in the macrophages surface.
It is known that antigens, as they enter the organism, suffer an oxidation process by the defensive cells to facilitate phagocytosis (38). In irradiated samples, macrophages already find these molecules oxidized, and therefore, they eliminate this step of the process. A better processing, associated to a faster antigen presentation, makes the immune system produce more complete antibodies against a higher number of antigen epitopes (38).
Souza et al. (41) investigated the ability of the gamma radiation produced by 60Co (2000Gy) in the attenuation of the toxic effects caused by Bothrops jararacussu venom in neuromuscular junctions of mice. They concluded that the radiation is able to abolish paralysis and miotoxicity caused by the venom. According to the same authors, these facts support the hypothesis that gamma radiation may be an important tool to improve antiofidic sera production, since it reduces venoms toxicity, preserving immunogenicity.
Therefore, serum neutralization and potency tests showed that all the sera produced were able to neutralize the native homologous venoms. This agrees with the findings of other authors (7, 12, 21, 31-38, 42), which assured that ionizing radiation has the property to attenuate venoms toxicity without altering immunological properties.
In the tests performed in vivo, none of the animals of the groups studied died when challenged with five LD50 of the native venom after 48 hours of observation.
With regard to the side effects caused by the venoms, Carvalho et al. (10) cite the toxicity of the venoms and adjuvants, mainly the Freunds Complete Adjuvant, as the major problem in the production of commercial antivenoms. They cause inflammation and lesions at the inoculum site, reducing the longevity of animals that produce immunoglobulines.
In this experiment, all the mice were daily observed in order to detect clinical changes and lesions at the inoculation sites.
The alterations observed included edema at the inoculum site, abscesses and necroses in the animals inoculated with native venoms of Bothrops jararaca, Bothrops jararacussu and Bothrops moojeni. Some animals of these groups died during this process. None of the animals of the control group died or presented clinical alterations at the inoculation site.
The groups of animals that received irradiated venom showed only small edemas in the first inoculation, although they had also received the Freunds Complete Adjuvant.
Carvalho et al. (10) cite that the major problem in the production of commercial antivenoms is the venom and the Freunds Complete Adjuvant toxicity. The latter causes inflammation and lesions at the inoculum site, contributing to the reduction of longevity in horses used to produce immunoglobulines.
According to Angulo et al. (2), some moderate tissue alterations, such as edema, abscesses, fistulas, and fibroses, were observed in the animals during hyperimmunization with venoms of Bothrops genus snakes. Systemic alterations are generally not observed. Spencer (43) analyzed the immunological changes caused by 60Co-irradiated Bothrops jararacussu venom and concluded that the native antivenom serum does not neutralize the native venom myotoxic activity, whereas the irradiated antivenom serum is able to neutralize this activity.
Thus, our present results demonstrate that gamma radiation can be an important tool used in animal immunization schemes for anti-bothropic serum production. Despite the antibody titers obtained by the immunization schemes and the sera neutralizing capacity and potency were similar to those produced with native venom, a decrease in the venoms toxicity after their irradiation with 60Co was observed, and only minimal clinical changes occurred during hyperimmunization when irradiated venom was utilized.
ACKNOWLEDGEMENTS
The authors thank Dr. Silvia Regina Sartori Barraviera, Director of The Center for the Study of Venoms and Venomous Animals - CEVAP, São Paulo State University-UNESP and Karina Luiz Chamma for technical assistance.
Received: September 2, 2004
Accepted: January 12, 2005
Published online: October 30, 2005
- 1. ADRIANI EP. Irradiation of crotoxin in aqueous solution: the role of the main reactive species in structural and biological alterations. J. Venom. Anim. Toxins, 1996, 2, 168.
- 2. ÂNGULO Y., ESTRADA R., GUTIÉRREZ JM. Clinical and laboratory alterations in horses during immunization with snake venoms for the production of polyvalent (Crotalinae) antivenom. Toxicon, 1997, 35, 81-90.
- 3. BARBOSA CF., RODRIGUES RJ., OLORTEGUI CC., SANCHEZ EF., HENEINE LG. Determination of the neutralizing potency of horse antivenom against bothropic and crotalic venoms by indirect enzyme immunassay. Braz. J. Med. Biol. Res., 1995, 28, 1077-80.
- 4. BARRAL-NETO M., SCHRIEFER A., VINHAS V., ALMEIDA AR. Enzyme-linked immunosorbent assay for the detection of Bothrops jararaca venom. Toxicon, 1990, 28, 1053-61.
- 5. BARRAVIERA, B., LOMONTE B., TARKOWSKI A., HANSON LA., MEIRA DA. Acute-phase reactions, including cytokines, in patients bitten by Bothrops and Crotalus snakes in Brazil. J. Venom. Anim. Toxins, 1995, 1, 11-22.
- 6. BARRAVIERA B., SARTORI A., DA SILVA MFP., KANENO R., PERAÇOLI MTS. Use of an ELISA assay to evaluate venom, antivenom, IgG and IgM human antibody levels in serum and cerebrospinal fluid from patients bitten by Crotalus durissus terrificus in Brazil. J. Venom. Anim. Toxins, 1996, 2, 14-27.
- 7. BONI-MITAKE M., COSTA H., SPENCER PJ., VASSILIEFF VS., ROGERO JR. Effects of 60Co gamma radiation on crotamine. Braz. J. Med. Biol. Res., 2001, 34, 1531-38.
- 8. BRADFORD MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 1976, 72, 248-54.
- 9. CARDI BA., ANDRADE JUNIOR HF., ROGERO JR., NASCIMENTO N. Differential biodistribution of native and 2 kGy 60Co irradiated crotoxin in tissues of CBA/J mice. Nat. Toxins, 1998, 6, 19-25.
- 10. CARVALHO VT., GOMES RT., VIOTTI AP., FREITAS TV. Immunization with liposome-encapsulates Bothrops jararaca venom. Toxicon, 2000, 38, 881-86.
- 11. CHIPPAUX JP., GOYFFON M. Venoms, antivenoms and immunotherapy. Toxicon, 1998, 36, 823-46.
- 12. CLISSA PB., NASCIMENTO N., ROGERO JR. Toxicity and immunogenicity of Crotalus durissus terrificus venom treated with different doses of gamma rays. Toxicon, 1999, 37, 1131-41.
- 13. COSTA NETO PLO. Estatística. São Paulo: Edgard Blücher ,1999. 260 p.
- 14. COSTA H. 60Co gamma radiation effects on Apis mellifera venom: biochemical, pharmacological, and immunological aspects. J. Venom. Anim. Toxins, 2002, 8, 182.
- 15. COSTA LM. Estudo comparativo da resposta imune de cavalos ao veneno de Crotalus durissus terrificus, in natura, tratado com formaldeído e submetido à ação térmica. Vac. Soros, 1985, 1, 24-9.
- 16. DANIEL JP., HENEINE LG., TAVARES CA., NASCIMENTO MC., HENEINE IF. Generation of protective immune sera by Crotalus durissus terrificus detoxified by controlled iodination. Braz. J. Med. Biol. Res, 1987, 20, 713-20.
- 17. DE PAULA RA. Attainment and evaluation of antisera raised against irradiated whole crotalic venom or crotoxin in 60Co source. J. Venom. Anim. Toxins, 1996, 2, 166.
- 18. DOS SANTOS MC., DIMPERIO-LIMA R., DA SILVA WD. Influence of Crotalus venom on the response to sheep red blood cells. Braz. J. Med. Biol. Res., 1986, 19, 636.
- 19. FLOWERS HH. The effects of X-irradiation on the biological activity of Cottonmouth Mocacasin (Agkistrodon piscivorus) venom. Toxicon, 1963, 1, 131-36.
- 20. GIROX E., LACHMANN PJ. "In vivo" diminution by chelators of snake venom provoked hemorrhage and "in vitro" inhibition of proteolytic activity. Toxicon, 1981, 19, 481-92.
- 21. GUARNIERI-CRUZ MC., MURATA Y. Attenuation of Bothrops jararaca venom by ionizing radiation. Toxicon, 1990, 28, 92-8.
- 22. GUIDOLIN R., DA SILVA WD., HIGASHI HG., CARICATI CP., LIMA MLSR., MORAIS JF., PINTO JR., MARCELINO JR. Hiperimunização de cavalos soroprodutores com venenos botrópicos e crotálico tratados por glutaraldeido. Mem. Inst. Butantan, 1989, 51, 85-90.
- 23. HATI RN., MANDAL M., HATI AK. Active immunization of rabbits with gamma irradiated Russels viper venom toxoid. Toxicon, 1990, 28, 895-902.
- 24. HENEINE LG., CARVALHO ADJr., BARBOSA CF., ARAUJO DOS SANTOS MR. Development of an ELISA to assess the potency of horse therapeutic polyvalent antibothropic antivenom. Toxicon, 1998, 36, 1363-70.
- 25. HERRERA E., YARLEQUE A., CAMPOS S., ZAVALETA A. Efecto de la radiacion gamma sobre la actividad biologica y propriedades enzimaticas de los venenos de las serpientes Lachesis muta y Bothrops atrox.Inf. Nuclear, 1986, 3, 1-14.
- 26. KAISER II., MIDDLEBROOK JL., CRUMRINE MHM., STEVENSON WW. Cross-reactivity and neutralization by rabbit antisera raised against crotoxin, its subunits and two related toxins. Toxicon, 1986, 24, 669-78.
- 27. KOCHOLATY WF., GOETZ JC., ASHLEY BD., BILLING TA., LEDFORD EB. Immunogenic response of the venoms of Fer de Lance, Bothrops atrox asper, and La Cascabella, Crotalus durissus durissus, following photooxidative detoxification. Toxicon, 1968, 5, 153-8.
- 28. LEÓN G., VALVERDE JM., ROJAS G., LOMONTE B., GUTIÉRREZ JM. Comparative study on the ability of IgG and Fab sheep antivenoms to neutralize local hemorrhage, edema and myonecrosis induced by Bothrops asper (terciopelo) snake venom. Toxicon, 2000, 38, 233-44.
- 29. MARIA WS., PACHECO BG., BARBOSA CF., VELARDE D., CHÁVEZ-OLÓRTEGUI C. Determination of the neutralizing potency of horse antibothropic and anticrotalic antivenoms in blood samples colleted on filter paper. Toxicon, 2001, 39, 1607-9.
- 30. MOROZ C., GOLDBLUM N., DEVRIES A. Preparation of Vipera palestinae antineurotoxin using carboximethyl-cellulose-bound neurotoxin as antigen. Nature, 1963, 16, 697-8.
- 31. MURATA Y., NISHIKAWA AK., NASCIMENTO N., HIGASHI HG., DIAS DA SILVA W., ROGERO JR. Gamma irradiation reduces the toxic activities of Crotalus durissus terrificus venom but does not affect their immunogenic activities. Toxicon, 1992, 28, 617.
- 32. NASCIMENTO N. Biochemical and immunological characterization of the main products of crotoxin irradiation. J. Venom. Anim. Toxins, 1996, 2, 169.
- 33. NASCIMENTO N., SEEBART C., FRANCIS B., ROGERO JR., KAISER II. Influence of ionizing radiation on crotoxin: biochemical and immunological aspects. Toxicon, 1996, 34, 123-31.
- 34. NASCIMENTO N., SPENCER PJ., ANDRADE JR, H.F. Effects of gamma radiation on snake venoms. Radiat. Phys. Chem., 1998, 52, 665-9.
- 35. OGUIURA N., CAMARGO ME., DA SILVA ARP. HORTON DSPQ. Quantification of crotamine, a small basic myotoxin, in South American rattlesnake (Crotalus durissus terrificus) venom by enzyme-linked immunosorbent assay with parallel-lines analysis. Toxicon, 2000, 38, 443-8.
- 36. PONTES NETTO D., CHIACCHIO SB., BICUDO PL., ALFIERI AA., NASCIMENTO N. Humoral response and neutralization capacity of sheep serum inoculated with natural and Cobalt 60-irradiated Crotalus durissus terrificus venom (Laurenti, 1768). J. Venom. Anim. Toxins incl. Trop. Dis., 2002, 8, 297-314.
- 37. PONTES NETTO DP., CHIACCHIO SB., BICUDO PL., ALFIERI AA., NASCIMENTO N. Hematological changes in sheep inoculated with Crotalus durissus terrificus snake (Laurenti, 1768) venom irradiated with Cobalto60 and in the natural form. J.Venom. Anim. Toxins incl. Trop. Dis., 2004, 10, 34-52.
- 38. ROGERO JR., NASCIMENTO N. Detoxification of snake venom using ionizing radiation. J. Venom. Anim. Toxins, 1995, 1, 7-10.
- 39. SELVANAYAGAM ZE., GOPALAKRISHNAKONE P. Tests for detection of snakes venoms, toxins and venom antibodies: review on recent trends (1987-1997). Toxicon, 1999, 37, 565-86.
- 40. SJOSTROM L., AL-ABDULLA IH., RAWAT S., SMITH DC., LANDON J. A comparison of ovine and equine antivenoms. Toxicon, 1994, 32, 427-33.
- 41. SOUZA FAD., SPENCER PJ., ROGERO JR., NASCIMENTO N., DAL PAI-SILVA M., GALLACCI M. 60Co gamma irradiation prevents Bothrops jararacussu venom neurotoxicity and myotoxicity in isolated mouse neuromuscular junction. Toxicon, 2002, 40, 1101-6.
- 42. SOUZA-FILHO JN., GUARNIERI-CRUZ MC., MURATA Y., ROGERO JR. Detoxification of the crotoxin complex gamma radiation. Braz. J. Med. Res., 1992, 25, 103-13.
- 43. SPENCER PJ. Biochemical and immunological alterations of 60Co irradiated Bothrops jararacussu venom. J. Venom. Anim. Toxins, 1996, 2 , 165.
- 44. THEAKSTON RDG., LLOYD-JONES MJ., REID HA. Micro-ELISA for detecting and assaying snake venom and venom-antibody. Lancet, 1977, 24, 639-41.
- 45. THEAKSTON RDG., WARRELL DA., GRIFFITHS E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon, 2003, 41, 541-57.
- 46. TEJANSEN P., OTTOLENGHI A. The effect of ultra-violet light on the toxicity and enzymatic and antigenic activities of snake venom. Toxicon, 1970, 8, 225-33.
Publication Dates
-
Publication in this collection
21 Nov 2005 -
Date of issue
Dec 2005
History
-
Accepted
12 Jan 2005 -
Received
02 Sept 2004