Abstract
The aim of this paper was to evaluate the immune reconstitution of HIV-1 patients subjected to highly active antiretroviral therapy (HAART) for two years or more according to CD45RA and CD45RO cell count; determination of IL-2, IFN-gamma, IL-4, IL-10 and TNF-alpha serum levels; CD4+ T and CD8+ T lymphocyte count; and plasma viral load (VL) determination. For this purpose, a cross sectional study was carried out in the Tropical Diseases Area, Botucatu School of Medicine, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil. Between June 2001 and April 2002, 37 HIV-1 infected patients were evaluated, 13 with treatment indication but untreated (G1), 9 subjected to HAART for 5-7 months (G2), and 15 treated for two years or more (G3); both treated groups used medication regularly and without failure. Forty-nine normal individuals were studied as controls (GC-1 and GC-2). There was a tendency (p<0.10) for the predominance of two nucleoside reverse transcriptase inhibitors (NRTI) associated with one non-nucleoside reverse transcriptase inhibitor (NNRTI) regimen in G2; and two NRTI associated with a protease inhibitor (PI) in G3. Statistical differences between groups were seen for CD45RA (G1<[G3=GC-2]; p<0.05) and CD45RO (G1<GC-2<G3; p<0.01) cells, and CD4+ T lymphocyte count (G1<G3; G2-intermediate; p<0.05), VL determination (G1>[G2=G3]; p<0.001), TNF-alpha serum determination ([G1>G3; G2=intermediate]>GC-1; p<0.001), IL-2 (G1<[G2=G3=GC-1]; p<0.01), IFN-gamma ([G1=GC-1]<[GC-2=G3]; p<0.001), IL-4 and IL-10 ([G1=G2=G3]>GC-1; p<0.001), serum cytokine profiles, with a higher proportion of subtype 2 in G1 and mature subtype 0 in G2 and G3 (p<0.005). There was no statistical difference for CD8+ T lymphocyte counts (G1=G2=G3; p<0.50). Consistency was seen between positive correlations of profile 1 definer cytokines (IL-2 and IFN-gamma), CD45RA and CD45RO cells, and CD4+ T lymphocyte counts and between positive correlations of profile 2 definer cytokines (IL-4 and IL-10) with TNF-alpha, and VL. The negative correlations were also consistent as they expressed the inverse of the positives. The variables with the highest number of correlations were IL-2, IFN-gamma, and VL, followed by CD45RA and CD45RO cells, and IL-10. The variables with the lowest number of correlations were CD4+ T and CD8+ T lymphocytes. The results express the partial but important immune reconstitution in HIV-1 infected individuals with the interference of HAART and the importance of cytokines especially IL-2 and IFN-gamma, and CD45RA and CD45RO cells as surrogate markers of this reconstitution.
HIV; immune reconstitution; antiretroviral; HAART; cytokines; CD45RA; CD45RO
ORIGINAL PAPER
Immune reconstitution in HIV-1 infected patients treated for two years with highly active antiretroviral therapy
Almeida R. A. M. B.I; Souza L. R.I; Calvi S. A.II; Ikoma M. R. V.III; Silva V. A.III; Curi P. R.IV; Meira D. A.I
IDepartment of Tropical Diseases and Imaging Diagnosis, Botucatu School of Medicine, São Paulo State University, Unesp, Botucatu, São Paulo, Brazil
IIApplied Immunology Laboratory of Tropical Diseases, Botucatu School of Medicine, São Paulo State University, Unesp, Botucatu, São Paulo, Brazil
IIIBotucatu Hemocenter, Botucatu, São Paulo, Brazil
IVBotucatu School of Veterinary Medicine and Animal Husbandry, São Paulo State University, Unesp, Botucatu, São Paulo, Brazil
Correspondence to Correspondence to: Ricardo Augusto Monteiro de Barros Almeida Departamento de Doenças Tropicais e Diagnóstico por Imagem Faculdade de Medicina de Botucatu, Unesp Distrito de Rubião Jr., S/N 18618-000, Botucatu, SP, Brasil Email: ralmeida@fmb.unesp.br
ABSTRACT
The aim of this paper was to evaluate the immune reconstitution of HIV-1 patients subjected to highly active antiretroviral therapy (HAART) for two years or more according to CD45RA and CD45RO cell count; determination of IL-2, IFN-g, IL-4, IL-10 and TNF-a serum levels; CD4+ T and CD8+ T lymphocyte count; and plasma viral load (VL) determination. For this purpose, a cross sectional study was carried out in the Tropical Diseases Area, Botucatu School of Medicine, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil. Between June 2001 and April 2002, 37 HIV-1 infected patients were evaluated, 13 with treatment indication but untreated (G1), 9 subjected to HAART for 5-7 months (G2), and 15 treated for two years or more (G3); both treated groups used medication regularly and without failure. Forty-nine normal individuals were studied as controls (GC-1 and GC-2). There was a tendency (p<0.10) for the predominance of two nucleoside reverse transcriptase inhibitors (NRTI) associated with one non-nucleoside reverse transcriptase inhibitor (NNRTI) regimen in G2; and two NRTI associated with a protease inhibitor (PI) in G3. Statistical differences between groups were seen for CD45RA (G1<[G3=GC-2]; p<0.05) and CD45RO (G1<GC-2<G3; p<0.01) cells, and CD4+ T lymphocyte count (G1<G3; G2-intermediate; p<0.05), VL determination (G1>[G2=G3]; p<0.001), TNF-a serum determination ([G1>G3; G2=intermediate]>GC-1; p<0.001), IL-2 (G1<[G2=G3=GC-1]; p<0.01), IFN-g ([G1=GC-1]<[GC-2=G3]; p<0.001), IL-4 and IL-10 ([G1=G2=G3]>GC-1; p<0.001), serum cytokine profiles, with a higher proportion of subtype 2 in G1 and mature subtype 0 in G2 and G3 (p<0.005). There was no statistical difference for CD8+ T lymphocyte counts (G1=G2=G3; p<0.50). Consistency was seen between positive correlations of profile 1 definer cytokines (IL-2 and IFN-g), CD45RA and CD45RO cells, and CD4+ T lymphocyte counts and between positive correlations of profile 2 definer cytokines (IL-4 and IL-10) with TNF-a, and VL. The negative correlations were also consistent as they expressed the inverse of the positives. The variables with the highest number of correlations were IL-2, IFN-g, and VL, followed by CD45RA and CD45RO cells, and IL-10. The variables with the lowest number of correlations were CD4+ T and CD8+ T lymphocytes. The results express the partial but important immune reconstitution in HIV-1 infected individuals with the interference of HAART and the importance of cytokines especially IL-2 and IFN-g, and CD45RA and CD45RO cells as surrogate markers of this reconstitution.
KEY WORDS: HIV, immune reconstitution, antiretroviral, HAART, cytokines, CD45RA, CD45RO.
INTRODUCTION
The introduction of highly active antiretroviral therapy (HAART) was a major milestone in the attempt to recuperate HIV-1 infected individuals' immune systems. Despite this recuperation being slow, partial, and variable, the suppression of HIV-1 replication has permitted a higher level of control over associated diseases with a consequent decrease in mortality and improvement in quality of life. (10)
The main surrogate markers of natural history and therapeutic efficacy currently used in HIV infected patient follow-up are clinical condition, CD4+ T lymphocyte count, and plasma viral load (VL) determination (21, 23). However, after VL drops below detectable levels, it does not allow us to consistently express the behavior of immune response reconstitution. Also, CD4+ T lymphocyte count can show irregular behavior without significant elevation even in individuals with major clinical improvement (4) and not demonstrate whether this elevation expresses the real immune system reconstitution through the production of naive cells, which have CD45RA surface molecules, or just the recirculation of memory cells, also known as CD45RO (10, 13, 20). In addition, CD4+ T lymphocyte count does not characterize the quality of the immunomodulatory substances they produce.
Other indices might be useful for a better evaluation of these patients' immune reconstitution, considering both qualitative and quantitative aspects. From these we can highlight serum cytokine profile (1, 5, 6, 7, 9, 17, 22, 26, 28) and CD45RA and CD45RO cell count. (10, 13, 20)
PARTICIPANTS
Between June 2001 and April 2002, 37 HIV-1 patients on or not on HAART were studied. They were treated at the Special Outpatient Clinic and Infirmary of Tropical Diseases, Botucatu School of Medicine, UNESP. Twenty-five were male and twelve female, aged between 18 and 62 years (X=37.6 years).
There were also 49 normal individuals, controls, blood donors from the Botucatu Hemocenter. Out of these, 36 were male and 13 female, aged between 18 and 57 years (X=33.2 years).
METHODS
Groups were formed as follows: Group G1, with 13 HIV-1 infected individuals, untreated but indicated for HAART; Group G2, with nine HIV-1 infected individuals on HAART for 5 to 7 months; Group G3, with 15 HIV-1 infected individuals on HAART for more than 24 months; and GC-1 with 30 normal individuals and GC-2 with 19 normal individuals, both control groups without clinical complaints and negative for anti-HIV-1/2, HTLV-1/2, hepatitis B and C, syphilis and Chagas' disease antibodies.
Exclusion criteria were: other causes of immunosuppression such as neoplasias, transplants, treatment with immunosuppressive substances, or autoimmune diseases, those using immunostimulants, those in any stage of pregnancy, those irregularly using antiretrovirals and/or with therapeutic failure, and those who did not give informed consent.
The antiretroviral (ARV) regimens used in each group were: without treatment; two nucleoside reverse transcriptase inhibitors (NRTI) associated with one non-nucleoside reverse transcriptase inhibitor (NNRTI); and two NRTI associated with a protease inhibitor (PI).
All HIV-1 infected individuals were subjected to VL, and serum TNF-a, IFN-g, IL-2, IL-4 and IL-10 determination; and CD4+ T and CD8+ T lymphocyte count. CD45RA and CD45RO cell counts were performed in six G1 and eight G3 individuals. Other routine laboratory examinations such as hemogram and blood biochemistry were also carried out.
All 30 GC-1 individuals were subjected to serum TNF-a, IFN-g, IL-2, IL-4, and IL-10 determination; normal values were obtained by mean + 2SD (Table 1). Serum cytokine profiles were determined as per subtypes described by Spellberg & Edwards-Jr. (27) (Table 2). All 19 GC-2 individuals were subjected to CD45RA and CD45RO cell count.
Exams were performed by the Clinical Analysis Laboratory of Botucatu University Hospital and the Applied Immunology Laboratory of Tropical Diseases Area, both of Botucatu School of Medicine, UNESP, and the Botucatu Hemocenter.
Quantitative determination of T lymphocytes with CD4+ and CD8+ markers was performed by flow cytometry using FACSCount reagent kits (Becton Dickinson). CD45RA and CD45RO cell counts were performed by flow cytometry using specific CD45RA monoclonal antibody kits, clone ALB11, mouse isotype IgG1 conjugated with fluorescein isothiocyanate (FITC), and CD45RO, clone UCHL1, mouse isotype IgG2a conjugated with phycoerythrin (PE). To obtain the absolute number of counted cells, their relative number was multiplied by the total number of lymphocytes from the hemogram. The following controls were used: mouse IgG, subclasses IgG1-FITC and IgG2a-PE, all manufactured by Beckman Coulter.
Sera aliquots were stored at -70°C for determination of TNF-a, IFN-g, IL-2, IL-4, and IL-10 cytokines; levels were determined by ELISA using commercial kits (R&D Systems). The assay detection limit varied from 3 to 5 pg/ml, depending on the cytokine.
HIV-1 VL was determined using NucliSens HIV-1 QT (Organon Teknika). The assay detection limit was 80 copies of plasma RNA/ml.
Statistical Analysis
Group comparisons were made with the non-parametric Kruskal-Wallis test and analysis of variance for entirely randomized experiments (ANOVA-ERE). The c2 test was used for serum cytokine profile proportion comparisons in the three HIV-1 groups, and for PI proportion comparisons in the two HIV-1 groups under treatment. When the proportion was zero, Yates correction was applied. For the c2 test, statistical significance was when p<0.05 and the value equal or higher than 3.84 for one degree of freedom, and equal or higher than 5.99 for two degrees of freedom. In all other analyses, statistical significance was p<0.05. For the correlations between pairs of variables, Spearman coefficient of rank correlation was calculated with significance a=0.05 and a=0.01, (30).
This study was approved by the Research Ethics Committee of Botucatu School of Medicine - UNESP.
RESULTS
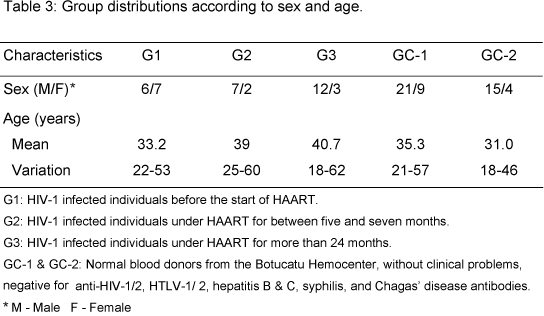
Table 3 shows groups distribution according to sex and age. Males predominated in all groups, except G1. Mean age between groups was from 31 to 41 years. The highest mean age in HIV-1 infected groups was in G3. The mean age of HIV-1 individuals was slightly higher than that of controls. The prevailing mode of transmission in all infected groups was heterosexual.
Groups were not homogeneous to ARVtreatment regimens as they were grouped using this parameter. G1 patients had not yet been treated; six G2 patients received two NRTI associated with one NNRTI, and three G2 patients, two NRTI associated with one PI; 11 G3 patients received two NRTI associated with one PI, and four G3 received two NRTI and one NNRTI. Although there was no significant difference between groups under treatment, PI tended to predominate in G3 (c21=3.70; p<0.10).
At exams collection, G1 had the highest number of individuals (six) with AIDS defining diseases; there were also 2 oligosymptomatic and 5 asymptomatic patients. In G2, asymptomatic was predominant; there were no oligosymptomatic, and only one individual with AIDS, who showed paradoxical reaction to tuberculosis. All G3 were asymptomatic.
Analyzing data from Table 4, statistical difference was seen between G1, and G3 and GC-2 for CD45RA cell count, with patients in G1 showing lower counts than those in G3 and GC-2 which were similar, and between G1, G3, and GC-2 for CD45RO cell count, with G1 showing lower counts than GC-2, and GC-2 patients showing lower counts than G3.
Table 4 shows patient characterization in relation to serum cytokine level determination. There was a progressive TNF-a serum level decrease from G1 to G3, and all showed higher TNF-a than GC-1. Statistical difference was seen between G1 and G2, G3, and GC-1 for IL-2, with serum levels in G1 being less elevated than in groups G2, G3, and GC-1, which had no statistical difference between them. There was no difference in IFN-g serum levels between G1 and GC-1, but they were less elevated than in G2 and G3, which were not statistically different either. There was no statistical difference between G1, G2 and G3 for IL-10 and IL-4 serum levels, however these were higher than in GC-1 individuals. Statistical difference was seen for serum cytokine profile with predominance of profile 2 in G1, and pronounced predominance of mature profile 0 in G2 and G3 (c22=13.65; p<0.005). In G3, only mature profile 0 was seen. No patient in the HIV-1 groups showed profile 1.
According to data in Table 4, there was statistical difference between groups for CD4+ T lymphocyte count with progressive increase from G1 to G3; this did not occur with CD8+ T lymphocyte count.
In G1, all individuals had detectable VL and in G2 there were seven undetectable and two detectable VL patients; in G3 there were nine undetectable and six were greater than 80 copies/ml; statistical difference was seen between groups, with G1 being statistically greater than both G2 and G3 which had no difference between them (Table 4).
Table 5 shows a linear correlation between the pairs of variables studied. There was a significantly positive correlation (a=0.05) between TNF-a and IL-4; IL-2 and CD4+ T lymphocytes; IFN-g and CD45RA cells; IFN-g and CD45RO cells; IL-10 and VL; and CD8+ T lymphocytes and CD45RA cells. There was a significantly strong positive correlation (a=0.01) between TNF-a and IL-10; TNF-a and VL; IL-2 and IFN-g; IL-2 and CD45RA cells; IL-10 and IL-4; CD4+ T lymphocytes and CD45RA cells; CD4+ T lymphocytes and CD45RO cells; and CD45RA and CD45RO cells. Analysis of the table also shows that there was a significantly negative correlation (a=0.05) between IL-10 and CD45RA cells; IL-4 and CD45RO cells; VL and CD45RA cells; and VL and CD45RO cells. A significantly strong negative correlation was seen (a=0.01) between TNF-a and IL-2; TNF-a and IFN-g; IL-2 and IL-10; IL-2 and IL-4; IL-2 and VL; IFN-g and IL-10; IFN-g and IL-4; IFN-g and CV; and CD4+ T lymphocytes and VL.
The highest number of significant correlations was for IL-2, IFN-g, and VL, with seven correlations each; this was followed by IL-10, CD45RA and CD45RO cells with six, TNF-a and IL-4 with five, CD4+ T lymphocytes with four, and CD8+ T with only one significant correlation.
DISCUSSION
For a better evaluation of the qualitative aspects of the immune system behavior in patients on HAART, this paper used three other markers including serum cytokine determination and CD45RA and CD45RO cell count.
In relation to patient homogeneity, the sex behavior, age and transmission mechanism are in agreement with the most recent epidemiological condition of HIV-1 infected individuals in Brazil (18).
Predominance of infected individuals with AIDS defining diseases among untreated patients is strong evidence for the benefit of the treatment (11).
CD45RA cell count increased significantly with the treatment in patients treated for at least 2 years, with predominance of PI; levels reached the same values as in control group individuals, which did not occur with CD4+ cells. According to literature, as treatment goes on, there is a progressive increase in the number of naive CD4+ T lymphocytes (10). These findings suggest that CD45RA was more significant than CD4+ and VL in the evaluation of immune reconstitution. They perhaps suggest the partial preserved capacity of thymus regeneration (4,10). There was a significant increase in CD45RO cells in this study for patients treated for over two years. It should be mentioned that the mean of values from this group was higher than that from controls. This is in agreement with Mezzaroma et al. (20), Haase (10), and Powderly et al. (24). This count, similar to that of CD45RA, was more sensitive than CD4+ count and VL values as indicators of immune reconstitution of patients on HAART. Another result of this study, which in a way reinforces these statements, was the strongly positive correlation between CD4+ T lymphocytes and CD45RA and CD45RO cells, at the same time that there was a weak negative correlation between both CD45RA and CD45RO and VL.
There were few references in literature where serum cytokines (2) have been determined as surrogate markers for HAART. Meira et al. (19) showed the importance of serum cytokine determination in HIV-1 infected patients classified into groups according to CD4+ T lymphocyte count. These authors (19) emphasized the use of such markers, together with CD45RA and CD45RO cell counts, as qualitative parameters in the evaluation of immune reconstitution of patients on HAART.
Serum TNF-a values decreased as treatment went on with lower levels in the over-two-years-treatment group, with PI predominance. This decrease, even though at levels about four times higher than in controls, seems to suggest a compatibility with clinical improvement since all patients in this group were asymptomatic. Ledru et al. (15) have reported that the decrease in TNF-a levels is compatible with the decrease of viral replication and apoptosis once this cytokine induces these phenomena (1, 15). Lew et al. (16) have reported a decrease in the number of T cells producers of TNF-a in the early weeks of HAART. Kaufmann et al. (13) showed a decrease in TNF-a secretion with the same treatment. Aukrust et al. (2) have reported data agreeing with this study, showing that plasma and in vitro TNF-a levels fall with treatment but not to normal levels. These authors (2) associate the persistent activation of TNF-a system components to therapeutic, virological, and immunological failure.
Serum IL-2 increased in the two under-treatment groups as treatment went on, reaching levels statistically equal to those of normal individuals. In absolute values, the G3 mean was higher than that of control group. This increase indicates a recuperation of the infected individual's immune system. Kaufmann et al. (13) report an increase in IL-2 secretion and Imami et al. (12), an elevation in specific RNA expression by RT-PCR in the first weeks of treatment. Weiss et al. (29) found increased levels of CD4+ T cells, producers of IL-2; in most studied patients, IL-2 production capacity under stimulation was similar to that of seronegative control individuals.
At pretreatment phase, IFN-g levels were statistically equal to those of normal individuals, increasing significantly with treatment, which is in agreement with Imami et al. (12). This increase may be explained by the maintenance of large quantities of CD8+ T lymphocytes, which are the main IFN-g producers. (8)
IL-4 and IL-10 were not statistically different between infected groups as treatment went on, but were always higher than in controls. However, in absolute values, in the pretreatment group, they were higher than in the other infected groups; this is in agreement with Imami et al. (12).
Characterization of serum cytokine profiles in patients from the three study groups showed none with profile 1 (Th-1). Most studied patients were considered as having mature 0 profile (Th-0), or 2 (Th-2). Meira et al. (19) called attention to the predominance of Th-2 (53.1%) and Th-0 (38.7%) profiles in their patients. Although the study group formation was different, results agreed. Other authors (14, 25) have also reported a very small number of profile 1 patients, agreeing with this study. These authors (14, 25), however, did not mention profile 0 in their findings.
When only patients under treatment are considered, absolute serum cytokine median values show patients treated for over 2 years with higher IFN-g and lower IL-4. These variations, although small, are important because they may be interpreted as an evolution of the mature 0 related to the duration of treatment and predominance of PI.
There was a progressive increase in CD4+ T lymphocyte counts in patients on HAART for over 2 years, with PI predominance; the median passed 300 cells/mm3 by a small margin. Therefore, according to Haase (10), the increase in CD4+ T lymphocyte count with treatment was progressive but partial.
There was no difference in CD8+ T lymphocyte count behavior with treatment. This therefore did not offer any support for the evaluation of immune recuperation. Autran et al. (3) and Kaufmann et al. (13) found similar results.
HIV-1 VL showed a marked decrease with treatment duration, with some individuals remaining with VL at detectable levels (>80 copies/ml). These results agree with literature (3, 20).
The relationships of pairs of variables showed coherence of positive correlations between profile 1 defining cytokines (IL-2 and IFN-g), CD45RA and CD45RO cells, and CD4+ T lymphocyte count, as well as for the association between them. These correlations also show coherence of correlations between profile 2 defining cytokines (IL-4 and IL-10) and TNF-a, and VL, as well as for the association between them. The negative correlations are also coherent as they express the reverse of positive associations. The variables with the highest number of positive or negative correlations were IL-2, IFN-g, and VL, followed by CD45RA and CD45RO cells, and IL-10. The variables with the lowest number of correlations were CD4+ T and CD8+ T lymphocytes.
These results show the importance of cytokines especially IL-2 and IFN-g, VL, and CD45RA and CD45RO cells as surrogate markers of HIV-1 infection with HAART interference. In literature (21), however, most authors consider CD4+ T lymphocyte count and VL are the markers of natural history, biological activity, and therapeutic efficacy in HIV-1 individuals.
The best performance was in the group of patients treated for over 2 years, and the analysis of all parameters used perhaps suggests that their immune reconstitution may be attributed to longer treatment time and participation of PIs. Hence there is still the need to perform further studies with these variables with more patients and longer treatment duration.
ACKNOWLEDGMENTS
To Dr. Fábio Cardoso Iuan and Botucatu Hemocenter for control group organization and performing of hemogram, flow cytometry, and VL. To the Applied Immunology Laboratory of Tropical Diseases Area, for serum cytokine determination. To Botucatu University Hospital, Botucatu School of Medicine for follow-up structure and other routine examinations. To CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the Masters scholarship of one author, and funds to purchase the kits for serum cytokine determination and CD45RA and CD45RO cells. And finally we thank the patients and blood donors for participating in this study.
Received: March 28, 2005
Accepted: September 26, 2005
Published online: February 24, 2006
- 1 ALFANO M., POLI G. Multiple roles of cytokines in HIV infection, replication, and therapy. In: COSSARIZZA A., KAPLAN D. Eds. Cellular aspects of HIV infection. New York: Wiley-Liss, 2001: 293-311.
- 2 AUKRUST P., MULLER F., LIEN E., NORDOY I., LIABAKK NB., KVALE D., ESPEVIK T., FROLAND SS. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J. Infect. Dis., 1999, 179, 74-82.
- 3 AUTRAN B., CARCELAIN G., LI TS., BLANC C., MATHEZ D., TUBIANA R., KATLAMA C., DEBRE P., LEIBOWITCH J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science, 1997, 277, 112-6.
- 4 CARCELAIN G., LI T., RENAUD M., DEBRÉ P., AUTRAN B. Immune reconstitution of the CD4 T-cell compartment in HIV infection. In: COSSARIZZA A., KAPLAN D. Eds. Cellular aspects of HIV infection. New York: Wiley-Liss, 2001: 399-422.
- 5 CLERICI M., FUSI ML., RUZZANTE S., PICONI S., BIASIN M., ARIENTI D., TRABATTONI D., VILLA ML. Type 1 and Type 2 cytokines in HIV infection a possible role in apoptosis and disease progression. Ann. Med., 1997, 29, 185-8.
- 6 CLERICI M., SHEARER GM. A Th1 >Th2 switch is a critical step in the etiology of HIV infection. Immunol. Today, 1993, 14, 107-11.
- 7 CLERICI M., SHEARER GM. The Th1 Th2 hypothesis of HIV infection: new insights. Immunol. Today, 1994, 15, 575-81.
- 8 GOUGEON ML., LECOEUR H., PINTO LMO., LEDRU E. Homeostasis and restoration of the immune system in HAART-treated HIV-infected patients: implications of apoptosis. In: COSSARIZZA A., KAPLAN D. Eds. Cellular aspects of HIV infection. New York: Wiley-Liss, 2001: 249-68.
- 9 GRAZIOSI C., PANTALEO G., GANTT KR., FORTIN JP., DEMAREST JF., COHEN OJ., SEKALY RP., FAUCI AS. Lack of evidence for the dichotomy of Th1 and Th2 predominance in HIV-infected individuals. Science, 1994, 265, 248-52.
- 10 HAASE AT. Population biology of HIV-1 infection: Viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol., 1999, 17, 625-56.
- 11 HAMMER SM., SQUIRES KE., HUGHES MD., GRIMES JM., DEMETER LM., CURRIER JS., ERON Jr JJ., FEINBERG JE., BALFOUR Jr HH., DEYTON LR., CHODAKEWITZ JA., FISCHL MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trial Group 320 study team. N. Engl. J. Med., 1997, 337, 725-33.
- 12 IMAMI N., ANTONOPOULOS C., HARDY GA., GAZZARD B., GOTCH FM. Assessment of type 1 and type 2 cytokines in HIV type 1-infected individuals: impact of highly active antiretroviral therapy. AIDS Res. Hum. Retroviruses, 1999, 15, 1499-508.
- 13 KAUFMANN GR., ZAUNDERS J., COOPER DA. Immune reconstitution in HIV-1 infected subjects treated with potent antiretroviral therapy. Sex. Transm. Infect., 1999, 75, 218-24.
- 14 KLEIN SA., DOBMEYER JM., DOBMEYER TS., PAPE M., OTTMANN OG., HELM EB., HOELZER D., ROSSOL R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS, 1997, 11, 1111-8.
- 15 LEDRU E., LECOEUR H., GARCIA S., DEBORD T., GOUGEON ML. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J. Immunol., 1998, 160, 3194-206.
- 16 LEW E., GALLAGHER L., KUEHNERT M., RIMLAND D., HUBBARD M., PAREKH B., ZELL E., JARVIS W., JASON J. Intracellular cytokines in the acute response to highly active antiretroviral therapy. AIDS, 2001, 15, 1665-70.
- 17 MAGGI E., MAZZETTI M., RAVINA A., ANNUNZIATO F., DE CARLI M., PICCINNI MP., MANETTI R., CARBONARI M., PESCE AM., DEL PRETE G., ROMAGNANI S. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in Th2 and Th0 cells. Science, 1994, 265, 244-8.
- 18 MEIRA DA. Acquired Immunodeficiency Syndrome in Brazil. Croat. Med. J., 2002, 43, 475-9.
- 19 MEIRA DA., ANTUNES MC., SOUZA LR., MARCONDES-MACHADO J., CALVI SA., LIMA CRG., HENRIQUES RMS., PARDINI MI., SILVA VA., IUAN FC., CURI PR. Nível sérico de citocinas como indicadores da fase evolutiva em indivíduos com infecção pelo HIV-1, doentes ou não. J. Bras. AIDS, 2000, 1, 17-27.
- 20 MEZZAROMA I., CARLESIMO M., PINTER E., ALARIO C., SACCO G., MURATORI DS., BERNARDI ML., PAGANELLI R., AIUTI F. Long-term evaluation of T-cell subsets and T-cell function after HAART in advanced stage HIV-1 disease. AIDS, 1999, 13, 1187-93.
- 21 MILDVAN D., LANDAY A., DE GRUTOLLA V., MACHADO SG., KAGAN J. An approach to the validation of markers for use in AIDS clinical trials. Clin. Infect. Dis., 1997, 24, 764-74.
- 22 MOSMANN TR., COFFMAN RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol., 1989, 7, 145-73.
- 23 PANTALEO G., COHEN O., GRAZIOSI C., VACCAREZZA M., PAOLUCCI S., DEMAREST JF., FAUCI AS. Immunopathogenesis of human immunodeficiency virus infection. In: DE VITA Jr VT., HELLMAN S., ROSENBERG SA., CURRAN J., ESSEX M., FAUCI AS. Eds. AIDS: etiology, diagnosis, treatment, and prevention. 4.ed. Philadelphia: Lippincott-Raven, 1997: 75-88.
- 24 POWDERLY WG., LANDAY A., LEDERMAN MM. Recovery of the immune system with antiretroviral therapy: the end of opportunism? J. Am. Med. Assoc., 1998, 280, 72-7.
- 25 SCOTT-ALGARA D., VUILLIER F., MARASESCU M., DE SAINT MARTIN J., DIGHIERO G. Serum levels of IL-2, IL-1 alpha, TNF-alpha, and soluble receptor of IL-2 in HIV-1-infected patients. AIDS Res. Hum. Retroviruses, 1991, 7, 381-6.
- 26 SHEARER GM., CLERICI M. Cytokine profiles in HIV type 1 disease and protection. AIDS Res. Hum. Retroviruses, 1998, 14 (suppl 2), 149-52.
- 27 SPELLBERG B., EDWARDS Jr JE. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis., 2001, 32, 76-102.
- 28 TANAKA M., HIRABAYASHI Y., GATANAGA H., AIZAWA S., HACHIYA A., TAKAHASHI Y., TASHIRO E., KOHSAKA T., OYAMADA M., IDA S., OKA S. Reduction in interleukin-2-producing cells but not Th1 to Th2 shift in moderate and advanced stages of human immunodeficiency virus type 1-infection: direct analysis of intracellular cytokine concentrations in CD4+ CD8- T cells. Scand. J. Immunol., 1999, 50, 550-4.
- 29 WEISS L., ANCUTA P., GIRARD PM., BOUHLAL H., ROUX A., CAVAILLON NH., KAZATCHKINE MD. Restoration of normal interleukin-2 production by CD4+ T cells of human immunodeficiency virus-infected patients after 9 months of highly active antiretroviral therapy. J. Infect. Dis., 1999, 180, 1057-63.
- 30 ZAR JH. Biostatistical analysis. 3.ed. New Jersey: Prentice Hall, 1996. 662p.
Publication Dates
-
Publication in this collection
22 Mar 2006 -
Date of issue
2006
History
-
Accepted
26 Sept 2005 -
Received
28 Mar 2005