Abstract
Scorpions are included in the order Scorpiones; class Arachnida. Lethal scorpions are mostly of the Buthidae family. Among these, species belonging to Androctonus, Leiurus and Mesobuthus genera cause most scorpion envenomations in Turkey. This study was performed aiming the production of antivenom by using Androctonus crassicauda telsons. Venom toxicity is related to telson weight, size, and storing condition (open or closed). Telsons of A. crassicauda were collected in Southeastern Anatolia (especially in Harran town, Sanliurfa), Turkey. They were separated according to weight, size, and storing condition - open (a) and closed (b). Venom solution was prepared by maceration of telsons. Swiss albino mice were used to determine the lethal dose 50% (LD50), which was as follows: Group 1a - 2.31mg; Group 1b - 2.66mg; Group 2a - 2.32mg; Group 2b - 2.66mg; Group 3a - 6.66mg; Group 3b - 6.88mg. Among the groups of telsons, the first and the second groups showed different characteristics. However, there were no differences between their toxicity. In the third group, a fourfold amount of telsons was used for toxicity. In other words, telsons weighting from 19.99 to 20mg (first group) and from 29.99 to 30mg (second group) presented similar LD50 values, and telsons weighting from 10 to 19.99mg (third group) showed a fourfold higher LD50 value. This difference was caused by the maturity of scorpions and venom toxicity was related to their size. The first and second groups were considered to be mature and the third group, not adult. Therefore, we can conclude that obtaining open telsons due to environmental factors was not effective for venom toxicity.
scorpion; Androctonus crassicauda (Oliver, 1807); telson size and weight; LD50, acute toxicity
ORIGINAL PAPER
Study of the relationship between Androctonus crassicauda (Oliver, 1807; scorpiones, buthidae) venom toxicity and telson size, weight and storing condition
Ozkan O.I; Adiguzel S.I; Yakistiran S.I; Filazi A.II
IRefik Saydam Hygiene Center, Ankara, Turkey
IIDepartment of Toxicology and Pharmacology, Faculty of Veterinary Medicine, Ankara University, Ankara, Turkey
Correspondence to Correspondence to: Ozcan Ozkan Poison Research Center Refik Saydam Hygiene Center, Ankara, Turkey Phone: 00 90 312 4337001 Fax: 00 90 312 4337000 Email: ozcanozkan_62@hotmail.com
ABSTRACT
Scorpions are included in the order Scorpiones; class Arachnida. Lethal scorpions are mostly of the Buthidae family. Among these, species belonging to Androctonus, Leiurus and Mesobuthus genera cause most scorpion envenomations in Turkey. This study was performed aiming the production of antivenom by using Androctonus crassicauda telsons. Venom toxicity is related to telson weight, size, and storing condition (open or closed). Telsons of A. crassicauda were collected in Southeastern Anatolia (especially in Harran town, Sanliurfa), Turkey. They were separated according to weight, size, and storing condition open (a) and closed (b). Venom solution was prepared by maceration of telsons. Swiss albino mice were used to determine the lethal dose 50% (LD50), which was as follows: Group 1a - 2.31mg; Group 1b - 2.66mg; Group 2a - 2.32mg; Group 2b - 2.66mg; Group 3a - 6.66mg; Group 3b - 6.88mg. Among the groups of telsons, the first and the second groups showed different characteristics. However, there were no differences between their toxicity. In the third group, a fourfold amount of telsons was used for toxicity. In other words, telsons weighting from 19.99 to 20mg (first group) and from 29.99 to 30mg (second group) presented similar LD50 values, and telsons weighting from 10 to 19.99mg (third group) showed a fourfold higher LD50 value. This difference was caused by the maturity of scorpions and venom toxicity was related to their size. The first and second groups were considered to be mature and the third group, not adult. Therefore, we can conclude that obtaining open telsons due to environmental factors was not effective for venom toxicity.
Key words: scorpion, Androctonus crassicauda (Oliver, 1807), telson size and weight, LD50, acute toxicity.
INTRODUCTION
Scorpion sting is the most important arachnidan envenomation that causes adult morbidity and child mortality. It remains a real health problem in many countries from tropical and subtropical regions (4, 13, 14, 15, 17, 20, 27).
Scorpion venom contains small neurotoxin polypeptides consisting of low-molecular-weight simple proteins with lethal and paralytic effects (1, 3, 15, 28). Several studies on scorpion stings emphasized that clinical pictures ranged from local to severe autonomic and central nervous system symptoms and also death due to cardio and respiratory failure, especially in children (5, 7, 10, 11, 12, 17, 18, 21, 22). Lethal scorpions are mostly of the Buthidae family (16, 18, 27).
In Turkey, scorpion envenomations are mostly caused by the genera Androctonus, Leiurus and Mesobuthus (18, 19). In the same country, A. crassicauda venom, obtained by maceration of telsons, is utilized as antigen for antivenom. This antigen has also been used against other scorpion species (23, 24). Therefore, the aim of this study was to verify the relationship between A. crassicauda venom toxicity and its telson size, weight, and storing condition.
MATERIALS AND METHODS
Telsons
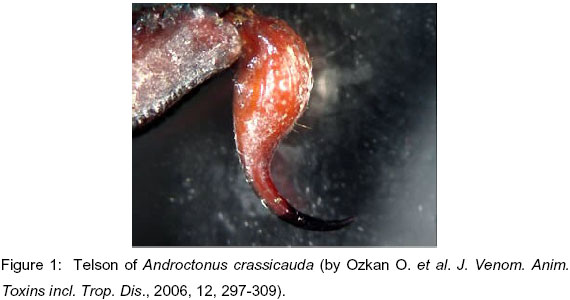
Androctonus crassicauda scorpions were collected in Southeastern Anatolia (especially in Harran town, Sanliurfa), Turkey. Telsons were separated according to their weight, size and storing condition (Figures 1, 2 and 3).
All telsons were weighted by using an electronic scale with sensitivity of 0.001mg. Telsons were randomly divided into three base groups. They were measured by using metal capillars, sensitivity of 0.1mm, considering the morphometric parameters (length, width, heigth) decribed by Vachon (25, 26).
Mice
Four hundred and sixty-four healthy female Swiss albino mice, weighing 23±1g, and 8 weeks old, were used to determine LD50. Throughout the experiment, the animals were kept in a laboratoryunder room temperature of 22±2°C and 60±10% humidity. They were fed with commercial mouse pellets ad libitum.
Preparation of Venom Solution
Venom solution was prepared by maceration (2, 14) of telsons from the six groups used (base groups 1st, 2nd, and 3rd groups and their subgroups open and closed telsons). Telsons were ground to a fine powder, which was dissolved in physiologic saline solution (PSS; chloride solution of 0.9%).
Lethal dose 50% (LD50) assay
A different concentration of venom solution was prepared for each group of mice. Then, 0.5ml was subcutaneously injected into each mouse. Control groups were treated with 0.5ml PSS, subcutaneously. After treatment, animals were monitored for 48 hours, and the number of dead animals was recorded at the end of the experiment. The dose that killed 50% of animals (LD50) was calculated by probit analysis (9).
Statistical analysis
Results were statistically evaluated by using probit analysis, Pearson's chi-square test and the probability value p. Telson size values were presented as mean ± standart derivation (SD). Differences were considered significant when p<0.005.
RESULTS
Telsons
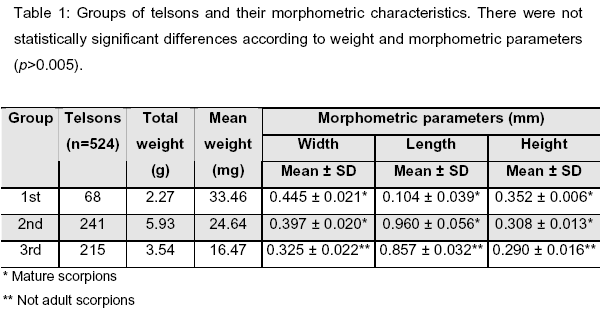
We used 524 telsons from A. crassicauda scorpions. According to weight, telsons were divided into three groups in the following manner: First group, 30-39.99mg; second group, 20-29.99mg; and third group, 10-19.99mg (Tables 2A, 2B and 2C). Within these base groups, there were two subgroups according to the storing condition: open (a) and closed (b). The mean weights of telsons were: First group, 33.46mg; second group, 24.64mg; third group, 16.47mg, as shown in Table 1.
Telsons were randomly divided into base groupsand the measurements of theirmorphometric parameters (length, width, heigth) were also shown in Table 1.
A stock venom solution at a concentration of 20mg/mlwas prepared through maceration of telsons.
Animals
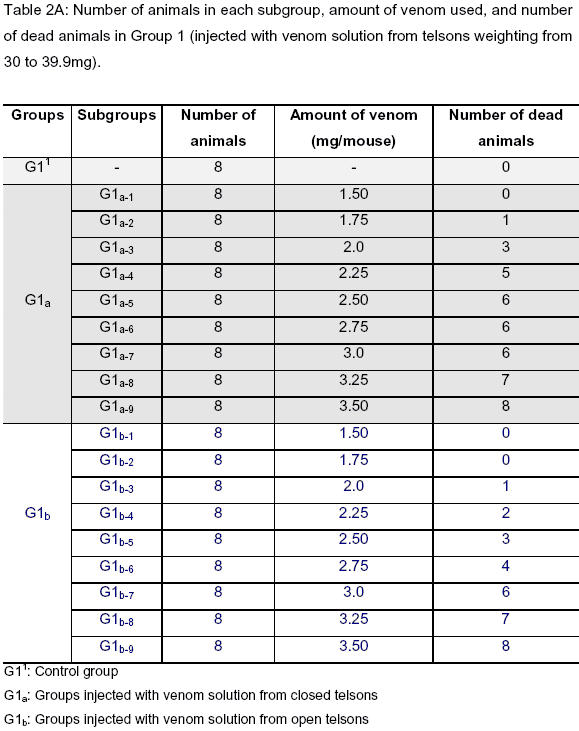
In the determination of LD50 values, Group 1 consisted of 64 mice in G1a, 72 mice inG1b, and 8 mice in the control group [G11]; Group 2 was comprised of 72 mice in G2a, 72 mice inG2b, and 8 mice in the control group [G21]; Group 3 presented 80 mice in G3a, 80 mice inG3b, and 8 mice in the control group [G31] (Tables 2A, 2B and 2C). The LD50 was found (95% confidence limit - CL) for each group and is presented in Table 3.
DISCUSSION
The aim of this study was to determine the structure of A. crassicauda telsons, the LD50 of its venom, and the relationship between its telson weight, size and storing condition.
To obtain venom, we used maceration, manual techniques, and electrical stimulation (18). Maceration is one of the oldest and simplest methods based on the principle of keeping telsons of dead scorpions in physiologic saline solution (1, 18, 23, 24).
We prepared three different venom solutions containing 20mg of grounded telson/ml. These solutions were subcutaneously injected into mice when the average of telsons used was evaluated as one telson; mean LD50 was considered as 7% of the first group, 9.4% of the second group, and 40.4% of the third group.Although average LD50 values of the first and second groups were different, there were no significant differences between their toxicity; in the third group, it was observed that an amount of venom fourfold higher, compared with the other groups, was used because it presented lower toxicity, which was probably due to the fact that the scorpions were not mature yet.Also, toxicity changed because scorpions may have used some of their venom for defense against being killed or caught or because they did not receive enough food during the experiment. In this study, similar results were seen in the first and second groups of telsons (20-40mg). Differently, Balozet declared the relationship between empty telson and full telson was 1:4 in order to kill mice (1). Although Balozet mentioned that factors such as transportation, drying method, storage conditions and usage period adopted could affect measurements (1), in this study, there were no differences between LD50 of telsons (open/closed), which became open due to environmental conditions.
In addition to Turkey, A. crassicauda has high prevalence in Azerbaijan, Iran, Iraq, Syria, Jordan, and Saudi Arabia (12, 18, 21, 27); and venom toxicity also changes according to regions and scorpion species (2). Total length of mature A. crassicauda varies from 90 to 100mm (6, 20). Measurements of telsons from the first and second groups (considered mature scorpions), and from the third group (considered not adult scorpions) are shown in Table 1.
According to lethality tests, different LD50 values were found for the same species. Ismail et al. found that LD50 of A. crassicauda venom was 0.64mg/kg, whereas venom obtained from Latoxan laboratory had an LD50 of 0.87mg/kg (12). Altinkurt and Altan (1) reported that LD50 of venom of A. crassicauda from Sanliurfa region was 11.5mg/kg. Therefore, envenomation due to scorpion stings as well as local or systemic clinical effects could be related to the scorpion species, its feeding state, the telson structure, the amount of venom injected, the number of stings, the patient's sensitivity, age and weight, and the climate of the region (1, 7, 8, 21).
In this study, the average amountofvenom obtained by maceration and subcutaneously injected into mice was 20mg/ml. The quantity that would kill a mouse (weighing approximately 23g) was calculated as 2.31mg [closed telsons] and 2.66mg [open telsons] (Table 3). Since a telson is about 20±1mg, 7%-8% of it corresponds to the LD50 of a mouse. This conclusion was similar to that of Ozkan and Filazi (18), who used the same species of scorpions. They stated that 3.6mg of grounded dry telson corresponds to the LD50 of a mouse (18). In our study, there were no differences between the subgroups and the base groups but the first and second groups of telsons were more toxic than the third group. However, the weight and size of telsons were not statistically significant (p>0.005) and did not correlate with toxicity. Tulga, using the same species of scorpions but different methodology, showed that 3.3%-5% of a telson corresponded to the LD50 of a mouse (23, 24).
Another study reported that differences between toxicities of scorpion venoms could be explained by the collection of scorpions as well as changes in their physiologic statuses and feeding patterns. Furthermore, during capturing and killing, the scorpions could have used some quantity of their venom to protect themselves. In addition, as already stated, factors such as transporting conditions, drying method, storage, and period of use of telsons could have influenced these differences (18).
Consequently, in our study, telsons weighting form 19.99 to 20mg (first group) and from 29.99 to 30mg (second group) had LD50 values similar to each other and telsons weighting from 10 to 19.99mg (third group) presented fourfold higher values. This difference was caused by the maturity of scorpions (biological status) and venom toxicity was related to their size. Therefore, it was seen that obtaining open telsons due to environmental factors was not effective for venom toxicity.
ACKNOWNLEDGMENTS
We wish to thank Karina Chamma for her valuable comments on the manuscript.
- 1 ALTINKURT O., ALTAN M. Pharmocological effects of the scorpion (Androctonus crassicauda) venom from Urfa environment on laboratory animals and the antagonistic effects of streptomycin to most of these effects. J. Fac. Pharm. Ankara. 1980, 10, 41-6.
- 2 AMARAL CF., REZENDE NA. Treatment of scorpion envenoming should include both a potent specific antivenom and support of vital functions. Toxicon, 2000, 38, 1005-7.
- 3 AY I., TUNCER M., ONUR R. Effects of Androctonus crassicauda scorpion venom on endothelium-dependent and independent vascular responses of rabbit aorta. Gen. Pharmacol., 1996, 27, 519-23.
- 4 BALOZET L. Scorpionism in the Old World. BÜCHERL W., BUCKLEY E. Eds. Venomous animals and their venoms. Venomous intervertebrates New York: Academic Express, 1971: 349-71.
- 5 BLUM A., JAWABREH S., GUMANOVSKY M., SOBOH S. Scorpion envenomation and myocardial damage. Isr. Med. Assoc. J., 2000, 2, 318-9.
- 6 DEMIRSOY A. Invertabrate (out of Insecta), Vol II/Part I, METEKSAN Published, (2th.ed.). 1998, 734-9.
- 7 DE ROODT AR., GARCIA SI., SALOMON OD., SEGRE L., DOLAB SA., FUNES RF., DE TITTO EH. Epidemiological and clinical aspects of scorpionism by Tityus trivittatus in Argentina. Toxicon, 2003, 41, 971-7.
- 8 DITTRICH K., POWER AP., SMITH NA., Scorpion sting syndrome: a ten year experience. Ann. Saudi Med., 1995, 15, 148-55.
- 9 FINNEY DJ. Probit analysis 3.ed. London: Cambridge University Press, 1971. 333p.
- 10 HISHAM MAHABA MA.Scorpion sting syndrome: epidemiology, clinical presentation and management of 2240 cases. East. Mediterr. Health J., 1997, 3, 82-9.
- 11 ISBISTER GK., GRAUDINS A., WHITE J., WARRELL D. Antivenom treatment in Arachnidism. J. Toxicol. Clin. Toxicol., 2003, 41, 291-300.
- 12 ISMAIL M., ABD-ELSALAM MA., AL-AHAIDIB MS. Androctonus crassicauda (Olivier), a dangerous and unduly neglected scorpion I. Pharmacological and clinical studies. Toxicon, 1994, 32, 1599-618.
- 13 KRIFI MN., MARRAKCHI N., EL AYEB M., DELLAGI K. Effect of some variables on the in vivo determination of scorpion and viper venom toxicities. Biologicals, 1998, 26, 277-88.
- 14 LOURENÇO WR., CUELLAR O. Scorpions, scorpionism, life history strategies and parthenogenesis. J. Venom. Anim. Toxins, 1995, 1, 51-62.
- 15 MAHADEVAN S. Scorpion sting. Indian Pediatr., 2000, 37, 504-14.
- 16 MULLEN G., STOCKWELL SA. Scorpions (Scorpiones) In: MULLEN G., DURDEN L. Eds. Medical and veterinary entomology. Amsterdam: Academic Press, 2002: 411-23.
- 17 OSNAYA-ROMERO N., DE JESUS MEDINA-HERNANDEZ T., FLORES- HERNANDEZ SS., LEON-ROJAS G. Clinical symptoms observed in children envenomated by scorpion stings, at the Children's Hospital from the state of Morelos, Mexico. Toxicon, 2001, 39, 781-5.
- 18 OZKAN O., FILAZI A. The determination of acute lethal dose-50 (LD50) levels of venom in mice, obtained by different methods from scorpions Androctonus crassicauda (Oliver 1807). Acta Parasitol. Turcica, 2004, 28, 50-3.
- 19 OZKAN O., KARAER Z. The scorpions in Turkey. Turk. Bull. Hyg. Exp. Biol., 2003, 60, 55-62.
- 20 OZKAN O., KARAER Z. Body structures of scorpions. Acta Parasitol. Turcica, 2004, 28, 54-8.
- 21 RADMANESH M. Androctonus crassicauda sting and its clinical study in Iran. J. Trop. Med. Hyg., 1990, 93, 323-6.
- 22 SEDDIK SS., WANAS S., SHEHATA A., FAWAZ S., HELMY MH. Development of an improved method for production of antiscorpion F(ab')2 fragment of IgG with high yield and potency. J. Nat. Toxins, 2002, 11, 123-32.
- 23 TULGA T. Cross-reactions between anti-scorpion (Buthus quinquestriatus) and anti-scorpion (Prionurus crassicauda) sera. Turk. Bull. Hyg. Exp. Biol., 1960, 20, 191-03.
- 24 TULGA T. Scorpions found in Turkey and paraspecific action of an antivenin produced with the venom of the species Androctonus crassicauda Turk. Bull. Hyg. Exp. Biol., 1964, 24, 146 155.
- 25 VACHON M. Études sur les scorpions (morphologie, bionomie et repartition mondiale). Inst. Pasteur d'Algérie, 1952a, 1-44.
- 26 VACHON M. Études sur les scorpions: caracteres servant a la classification. Inst. Pasteur d'Algérie, 1952b, 45-70.
- 27 VATANPOUR H. Effects of black scorpion Androctonus crassicauda venom on striated muscle preparation in vitro Iranian J. Pharmaceutical Res., 2003, 2, 17-22.
- 28 ZLOTKIN E., MIRANDA F., ROCHAT H. Chemistry and pharmacology of Buthinae scorpion venoms. In: BETTINI S. Ed. Handbook of experimental pharmacology-arthropod venoms Berlin: Spinger-Verlag, 1978: 317-69.
Publication Dates
-
Publication in this collection
26 June 2006 -
Date of issue
Apr 2006