Abstract
Inflammatory response induced by the venom of the Arabian sand viper Cerastes gasperettii was studied by measuring rat hind-paw edema. Cerastes gasperettii venom (CgV, 3.75-240 µg/paw), heated for 30s at 97°C, caused a marked dose and time-dependent edema in rat paw. Response was maximal 2h after venom administration and ceased within 24h. Heated CgV was routinely used in our experiments at the dose of 120 µg/paw. Among all the drugs and antivenoms tested, cyproheptadine and 5-nitroindazole were the most effective in inhibiting edema formation. Aprotinin, mepyramine, dexamethasone, diclofenac, dipyridamole, Nomega-nitro-L-arginine, quinacrine, and nordihydroguaiaretic acid showed statistically (p<0.001) significant inhibitory effect, but with variations in their inhibition degree. Equine polyspecific and rabbit monospecific antivenoms significantly (p<0.001) reduced edema when locally administered (subplantar) but were ineffective when intravenously injected. We can conclude that the principal inflammatory mediators were serotonin, histamine, adenosine transport factors, phosphodiesterase (PDE), cyclooxygenase, lipoxygenase and phospholipase A2 (PLA2), in addition to other prostaglandins and cytokines.
inflammatory mediators; Cerastes gasperettii venom; edema; antagonist; antivenom
ORIGINAL PAPER
Pharmacological characterization of rat paw edema induced by Cerastes gasperettii (cerastes) venom
Al-Asmari A. K.; Abdo N. M.
Research Center, Armed Forces Hospital, Riyadh, Saudi Arabia
Correspondence to Correspondence to: Abdulrahman Khazim Al-Asmari P.O. BOX: 7897(775S), Riyadh, 11159, Saudi Arabia Phone: + 996-1-4777714 ext. 3811. Fax: +996-1-4777714 ext. 6896 Email: akasmari@medu.net.sa or drasmari@hotmail.com
ABSTRACT
Inflammatory response induced by the venom of the Arabian sand viper Cerastes gasperettii was studied by measuring rat hind-paw edema. Cerastes gasperettii venom (CgV, 3.75-240 µg/paw), heated for 30s at 97°C, caused a marked dose and time-dependent edema in rat paw. Response was maximal 2h after venom administration and ceased within 24h. Heated CgV was routinely used in our experiments at the dose of 120 µg/paw. Among all the drugs and antivenoms tested, cyproheptadine and 5-nitroindazole were the most effective in inhibiting edema formation. Aprotinin, mepyramine, dexamethasone, diclofenac, dipyridamole, Nw-nitro-L-arginine, quinacrine, and nordihydroguaiaretic acid showed statistically (p<0.001) significant inhibitory effect, but with variations in their inhibition degree. Equine polyspecific and rabbit monospecific antivenoms significantly (p<0.001) reduced edema when locally administered (subplantar) but were ineffective when intravenously injected. We can conclude that the principal inflammatory mediators were serotonin, histamine, adenosine transport factors, phosphodiesterase (PDE), cyclooxygenase, lipoxygenase and phospholipase A2 (PLA2), in addition to other prostaglandins and cytokines.
Key words: inflammatory mediators, Cerastes gasperettii venom, edema, antagonist, antivenom.
INTRODUCTION
The Kingdom of Saudi Arabia and surrounding areas are inhabited by several venomous snakes of medical importance. Among them are the families Elapidae, Hydrophiidae, Viperidae and Atractaspididae, in addition to the innocuous family Colubridae, which includes weakly venomous, back-fanged representatives (19). From the medical point of view, vipers, with their highly sophisticated venom delivery system, are the most important snakes in Arabia since they are responsible for most of the snakebites affecting humans, and the sand viper (Cerastes cerastes gasperettii) is the most frequent of all (20).
Vipers are widely distributed throughout Africa and Eurasia, including Senegal in West Africa, North Africa, South Africa, Kenya in the East-Central Africa, and also Turkmenistan, Uzbekistan, Aral Sea, and Sri Lanka in the Indian subcontinent (15, 20, 54). Cerastes cerastes mainly inhabits the North Africa (Algeria, Sudan, Chad and Sinai), and also Asia (Palestine, Jordan, Iraq and Iran). It is largely distributed in Arabia, except for areas of more than 1,500m in height (20). According to the records of the Ministry of Health, the incidence of snakebites in Saudi Arabia is approximately 2,494 cases per year.
Following envenoming, spread of the various venom components depends to a large extent on their molecular weights. Thus, Reid and Theakston (44) showed that most constituents of viper venoms have high molecular weights and are slowly absorbed by the lymphatic system to produce maximum serum levels within 6-24 hours. Pathophysiological picture after viper envenomation includes systemic and local effects. Local manifestations are mainly represented by edema, pain, hemorrhage and necrosis, which may result in prolonged or permanent disability (54).
True vipers of the Old World and pit vipers of the New World present venoms rich in proteolytic enzymes. Many coagulant and anticoagulant factors like ancrod, crotalase, batroxobin, and a and b-fibrinogenases have been isolated and purified from their venoms (14, 25, 36, 42, 47, 57). Knowledge on modulators of the coagulation factors of venoms from the Cerastes genus is scarce; however, Mohamed et al. (40), studying Cerastes cerastes, which is homologous to the recently classified species Cerastes gasperettii from the Arabian Peninsula, referred to the existence of a factor with a thrombin-like coagulant effect. Later, Mohamed and El-Damarawy (39) showed that the venoms of two species (Cerastes and Vipera) of the genus Cerastes do possess fibrinogenolytic and fibrinolytic activities. Labib et al. (31) found out that some venom fractions of these two species were procoagulant at low concentrations and anticoagulant at high concentrations.
Several researchers have studied the biochemical and pharmacological effects of venoms collected from various vipers (38, 49, 50, 51). However, to the best of our knowledge, the inflammatory effects of the venom from Cerastes gasperettii, especially those inhabiting the Arabian Peninsula, have not been investigated so far.
The present study reflected the dose and time-related inflammatory effects of CgV using the rat paw edema model and aimed to examine the capability of CgV to induce paw edema in rats and the employment of various drugs that could neutralize it and unveil the pharmacological mechanisms underlying these effects.
MATERIALS AND METHODS
Reagents
Cyproheptadine and dexamethasone were purchased from Merck & Co., Inc. (USA), and aprotinin from Buyer AG (Germany). Mepyramine, diclofenac, dipyridamole, and Nw-nitro-L-arginine were obtained from Sigma Chemical Co. (USA). Quinacrine was obtained from ICN Biochemicals (USA), 5-Nitroindazole from Aldrich Chemical Company, Inc. (USA), and Nordihydroguaiaretic acid [NDGA] from Fluka Chemie AG (Switzerland).
Venom and antivenom
Cerastes gasperettii venom (CgV) was obtained from the National Serpentarium, Riyadh, Saudi Arabia. It was dissolved in saline and stored at -20°C until used.
Two types of antivenoms [rabbit monospecific IgG and equine polyspecific F(ab)2] were used in this study. Equine antivenom, a national product, was obtained from Al-Hayatt Company, Riyadh, Saudi Arabia. It was raised in horses using a mixture of venoms from Echis carinatus, Echis coloratus, Bitis arietans, Cerastes cerastes, Naja haje, and Walterinnesia aegyptia and was dialyzed to remove preservatives. Monospecific antivenom was raised in rabbits and was produced in our laboratory.
Measurement of rat paw edema
Male Wistar rats weighing 170-190g (mean weight = 180 g) were used for all the experiments and were provided by the Armed Forces Hospital, Research Center (Animal House Services), Riyadh, Saudi Arabia. All experiments were carried out according to the methods described by Faria et al. (14) and Al Asmari (3, 4). Animals were divided into seven groups; each rat was injected into the subplantar region of its right hind paw with serial doses (3.75-240 µg/paw) of 0.1 ml heated (30 s, 97°C) CgV. The contralateral paw received the same volume (0.1 ml) of sterile saline for control. Paw volume was measured with a Plethysmometer (Model 7141, Ugo, Basile, Italy). Results of the dose-dependent edema measurements were expressed as mean differences between the volumes of pre and post injected paws. Such measurements were carried out in order to determine the minimum edematous CgV dose that could produce the highest edema level (plateau). To determine the time the edema took to achieve its peak and consequently subside, measurements were performed at 0.25, 1, 2, 4, 6, and 24 h after venom injection.
Influence of various substances on CgV-induced edema
In another set of experiment, drugs were titrated to determine their minimim (optimum) edematous effect. Groups of six rats each were pretreated with serial doses of a drug or antivenom as follows: Group A mepyramine (3-24 mg/kg), 15 min before venom; Group B diclofenac (5-80 mg/kg), 30 min before venom; Group C cyproheptadine (1.5-24 mg/kg), 15 min before venom; Group D dexamethasone (0.25-2 mg/kg), 2h before venom; Group E aprotinin (1X103-4X103 KIU/kg), 30 min before venom; Group F dipyridamole (30-120 mg/kg), 30 min before venom; Group G Nw-nitro-L-arginine (25-200 mg/kg), 30 min before venom; Group H quinacrine (25-200 mg/kg), 30 min before venom; Group I 5-Nitroindazole (20-80 mg/kg), 30 min before venom; Group J nordihydroguaiaretic acid (NDGA, 20-80 mg/kg), 30 min before venom. All drugs were intraperitoneally injected into rats.
Different doses (0.5-16 mg/kg) of equine (commercial) and rabbit antivenoms (mixed with 120 µg CgV and incubated for 30 min at 37°C) were also intravenously or locally administered to animals.
Then, the animals received subplantar injection of 120 µg/paw CgV (0.49 mg/kg) and an extra positive control group received heated 120 µg/paw (0.49 mg/kg) CgV alone in the same experimental conditions; edema measurements were carried out accordingly.
Statistical analysis
Data were presented as means ± SEM and were analyzed by ANOVA (GraphPad Instat and Prism Programs) followed by the Dunnett's Multiple Comparison Tests. Values of p<0.05 were considered significant (99% confidence interval).
RESULTS
Effect of Cerastes gasperettii venom on rat paw edema
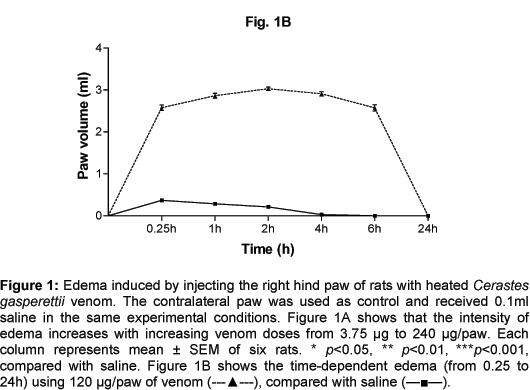
Heated CgV (3.75-240 µg/paw) induced dose (Figure 1A) and time-dependent (Figure 1B) non-hemorrhagic edema. The minimum edematous CgV dose that induced the highest edema level was 120 µg/paw (Figure 1A). Maximal time-dependent response was observed 2h after venom injection, gradually decreasing from 4 to 6h and subsiding after 24h (Figure 1B). According to these results, the dose of 120 µg/paw was routinely used for all further experiments.
Pharmacological modulation of heated Cerastes gasperettii venom on rat paw induced edema
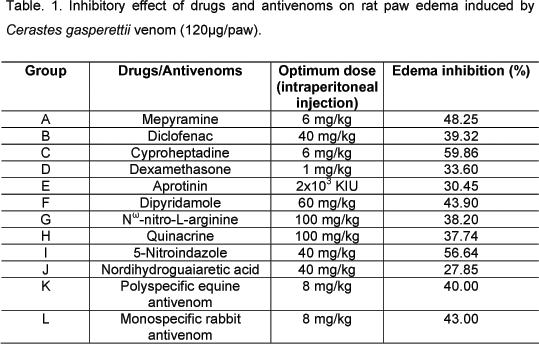
All drugs used were titrated against 120µg/paw heated CgV and the minimum effective dose that induced the maximum edema decrease (% inhibition) was determined (Table 1).
Comparative analysis of drugs and antivenoms inhibitory effect
The minimum effective dose of all the tested drugs and antivenoms was highly (p<0.001) significant in inhibiting CgV-induced edema compared with the positive control (Table 1). Drugs were classified into groups according to their percentage of edema inhibition. The most effective group of drugs included cyproheptadine (a histamine H1 and serotonin 5-HT2 receptor antagonist) with 59.86% edema inhibition, and 5-Nitroindazole (a neuronal nitric oxide synthase [nNOs] inhibitor) with 56.64% (Table 1). The second most effective group was represented by mepyramine (a histamine H1 receptor antagonist) with 48.25% inhibition, dipyridamole (an adenosine transport inhibitor) with 43.90%, and the two types of antivenoms [rabbit monospecific IgG and the equine polyspecific F(ab)2] with 43.00% and 44.00% edema inhibition, respectively. Diclofenac (a cyclooxygenase inhibitor) with 39.32%, Nw-nitro-L-arginine (a nitric oxide synthase inhibitor) with 38.20%, and quinacrine (a PLA2 inhibitor) with 37.74% edema inhibition represented the third most effective group. The fourth group included dexamethasone (an indirect PLA2 inhibitor) with 33.60%, aprotinin (a proteinase [kallikrein] inhibitor) with 30.45%, and NDGA (a cyclooxygenase and lipoxygenase inhibitor) with 27.85% edema inhibition.
Local administration of mixtures of CgV and the antivenoms significantly (p<0.001) reduced edema, as reported above (second group), compared with their intravenous administration which failed to cause any significant neutralization.
DISCUSSION
Snake venom components, especially those of viper venoms, activate, inhibit or liberate enzymes by destroying cellular organelles (1, 37). The different toxic effects of viper venoms are due to their proteolytic and lipolytic enzymes (48). Common initial signs of envenoming are hypoglycemia (2), general metabolic disturbance (35), muscular dystrophy (41), nephrotoxicity (28) and cytotoxicity (10).
The present study shows that subplantar injection of heated CgV in rats caused a significant paw edema similar to that induced by other viper venoms, which altered the vascular permeability in mice hind paws (13, 53), causing abundant leukocyte infiltration, and hemorrhage with higher venom doses (13). Since hemorrhage has been reported to appear soon after venom injection and to interfere with the inflammatory edema development (8, 14), in our study we employed heated venom in all the tests.
Development of edema induced by CgV was different from that observed with other vipers (including Echis coloratus and Echis pyramidum) venoms; CgV-edema volume achieved its peak in a longer time (3, 4).
Our results agreed with those of Lomonte (33), who reported that in his study edema lasted for more than 12 h.
Edema induced by CgV diminished after 2 hours while that induced by other viper or crotalid venoms like Bothrops jararaca venom (BjV) was still significant even after 6 hours (52). Also, mice injected with low doses of BjV (1 µg) presented edemas lasting for more than 8 hours. Similar results were observed in rats (53). Long-acting edema was also reported by Theakston (49), who attributed the slow absorption (maximum level, 6-12h) of viper venoms to their high molecular weights. Therefore, it could be assumed that venoms of different viper species trigger and maintain edema by different mechanisms, depending on their components' molecular weights.
Similarly to what has been observed with BjV (53), CgV-induced edema might be caused by the release of pharmacologically active substances at the injection site.
Increased vascular permeability and increased blood flow have been shown to play an important role in edema formation (11, 55, 56). Our results showed that CgV induced rat paw edema, which might indicate a direct or an indirect relationship between local blood flow and edema intensity, compared with the studies of Faria et al. (14), De Araujo et al. (13), and Antunes et al. (6).
To further understand the pharmacological mechanisms involved in the CgV-induced rat paw edema, we used different treatments.
Although all the tested drugs and antivenoms were statistically significant and effective, there were variations in their efficiency. The drugs cyproheptadine and 5-nitroindazole were the most effective in reducing edema, confirming their important roles in in vivo mast cell degranulation (12, 14) and in the increase of cytokine production (27).
Cyproheptadine leads to blockade of serotonin receptors; similarly methysergide was reported to decrease the edematogenic effects of many phlogistic agents such as sephadex (18) and collagenase (46). Ishii et al. (29) indicated that edema induced by arachidonic acid was slightly reduced by methysergide in mice. However, it is necessary to recall that edema induced by other crotalid venoms in rats (53) and mice (43) was not reversed by methysergide. The present results indicate that serotonin and histamine had an important role in decreasing CgV-induced edema, since cyproheptadine, which is a histamine (H1) and serotonin (5-HT) receptor antagonist,significantly reduced rat paw volume. Histamine seems to be an important autocoid in the formation of edema induced by many phlogistic agents. However, it has been reported that histamine might not be involved in edema induced by BjV in rats (53) and mice (43) since the use of antihistamine did not cause any significant change, which does not agree with the present results since mepyramine, an antagonist of H1 receptors, significantly reduced CgV-induced edema. Therefore, differences between the edematogenic effects induced by CgV and those caused by other viper and crotalid venoms like BjV and Echis coloratus and Echis pyramidum venoms (3, 4) could be attributed to the release of other autocoids.
Nitric oxide synthase (NOs) inhibitors have been used as tools to establish the role of nitric oxide (NO) in edema induced by different phlogistic agents (7). Many experimental data demonstrated that NO has an important role in inflammation, however, mechanisms of the NO contribution to such process are not completely elucidated yet. It might participate in the edema pathophysiology by several mechanisms such as increasing prostaglandin biosynthesis at the inflammatory site (45) and cytokine production (27). Nitric oxide has been shown to play a very important role in edema induced by carrageenin in rats (26, 32, 45) and mice (27). In rats, NO was involved in the acute inflammatory response following subplantar injection of bradykinin and serotonin (21). It was also involved in neurogenic inflammation induced by topical application of mustard oil on rat skin (32).
The present results also demonstrated that NO might play a role in reducing CgV-edema, since antagonists (5-Nitroindazole and Nw-nitro-L-arginine) significantly inhibited its formation. Knowledge about histamine and serotonin release due to CgV, in addition to activation of NO synthases, could clarify important steps in the edema formation.
Mepyramine, dipyridamole, rabbit IgG and equine F(ab)2 antivenoms were the second most effective in inhibiting edema, although the antivenoms failed to reverse venom-induced edema when intravenously administered, which may indicate the antivenom local action in countering myonecrosis. Cerastes gasperettii venom local effects (e.g. myonecrosis) and edematogenic effects have not been sufficiently studied so far, therefore employing viper antivenoms in the treatment of systemic symptoms could be doubtful. Benbassat and Shalev (9) investigated these situations by reviewing data on the in vitro and in vivo effects of viper venoms (on laboratory animals and humans) and reexamined alternative treatment methods in order to assess the efficacy of using antivenoms. Tilbury et al. (52) also speculated and reviewed this argument.
Mepyramine action is a second confirmation of the histamine release due to CgV. Dipyridamole is a potent inhibitor of platelet aggregation and suppresses adenosine cellular uptake by erythrocytes. It is also responsible for the inhibition of phosphodiesterase (PDE), which, together with several other effects on other mediators responsible for inflammation induction, may reduce edema (58).
The third most effective drugs in inhibiting edema were diclofenac, N-nitro-L-arginine and quinacrine, another NOs inhibitor that confirmed the specificity of CgV in inducing edema. Inhibition of PLA2 by quinacrine is a significant marker of induction of this mediator. Antagonists of the arachidonic acid metabolism such as cyclooxygenase and corticosteroids were shown to reduce edema induced by toxic proteins isolated from some insects (16, 24). The present results demonstrated the possible involvement of NOs, PLA2 and arachidonate metabolites in the edema formation, since antagonistic drugs (the third most effective group) also reduced rat paw volume.
The fourth most effective drugsincluded aprotinin, dexamethasone and NDGA. Significant reduction of venom-induced paw edema by dexamethasone was expected since corticosteroids were known to indirectly inhibit PLA2 action (17). Furthermore, corticosteroids directly acted on leucocytes and other cell types inhibiting the release of cytokines and other inflammatory mediators (5). Similar actions were observed in the kinetics and cell composition of the inflammatory infiltrate in rat paw (23, 34). Aprotinin inhibits many proteases such as kallikrein, whose products, bradykinin and kallidin, are active inflammatory inducers (16, 22). These kinins are potent vasodilators that increase the capillary permeability and are easily produced in tissues after injury; thus, being cardinal agents in the edema formation (22, 30). The primary difference between the present findings and those of De Faria et al. (14) was that CgV induced significant edema without the need of potentiators. Secondly, NDGA and dexamethasone reduced CgV-induced edema, which indicated that these drugs might be involved in blocking or reducing mast cell degranulation and activation by CgV. Furthermore, H1 and serotonin 5-HT2 receptor antagonists significantly reduced CgV-induced rat paw edema. Contradictory results were obtained with other venoms (13, 43, 53).
In conclusion, CgV induced edema 15 min after venom injection; peak volume was achieved after 2 h, which diminished and ceased within 24 h. There is release of active amines such as serotonin, histamine, arachidonic acid metabolites and NO at the injection site, since antagonistic drugs like cyproheptadine, mepyramine, NDGA, dexamethasone and NOs inhibitors reduced the edematogenic response at various doses. As cyproheptadine and 5-nitroindazole were the most active inhibitors of paw edema formation, we could suggest that at least serotonin, histamine and nNOs are important mediators and of high priority in edema induced by CgV.
Received: June 20, 2005
Accepted: October 27, 2005
Abstract published online: December 14, 2005
Full paper published online: August 31, 2006
- 1 ABDEL-NABI I., AWADALLA R., EL-SHAMY I. Biochemical effects of intraperitoneal injection of rats with the venom of the snake Echis carinatus Egypt. J. Zool., 1997, 29, 195-205.
- 2 ABU-SINNA G., AL-ZAHABY AS., ABD EL-AAL A., ABD EL-BASET A., SOLIMAN NA. The effect of the viper Cerastes cerastes cerastes venom and venom fractions on carbohydrate metabolism. Toxicon, 1993, 31, 791-801.
- 3 AL-ASMARI AK. Pharmacological characterization of the rat's paw oedema induced by Echis coloratus venom. On line J. Biol. Sci., 2003a, 3, 309-19.
- 4 AL-ASMARI AK. Pharmacological characterizations of the rat paw edema induced by Echis pyramidum venom. On line J. Biol. Sci., 2003b, 3, 824-33.
- 5 ANGELI A., MASERA RG., SARTORI ML., FORTUNATI N., RACCA S., DOVIO A., STAURENGHI A., FRAIRIA R. Modulation by cytokines of glucocorticoid action. Ann. N. Y. Acad. Sci., 1999, 876, 210-20.
- 6 ANTUNES E., GIRALDELO C., CIRINO G., DE NUCCI G. Effects of NG-monomethyl-L-arginine and its D-enantiomer on rat hind-paw oedema. In: MONCADA S., MARLETTA MA., HIBBS Jr. JB., HIGGS EA. Eds. The biology of nitric oxide. Colchester: Portland Press, 1992: 264-6.
- 7 ANTUNES E., MARANGONI RA., BRAIN SD., DE NUCCI G. Phoneutria nigriventer (armed spider) venom induces increased vascular permeability in rat and rabbit skin in vivo Toxicon, 1992, 30, 1011-6.
- 8 ASSAKURA MT., REICHL AP., MANDELBAUM FR. Comparison of immunological, biochemical and biophysical properties of three hemorrhagic factors isolated from the venom of Bothrops jararaca (jararaca). Toxicon, 1986, 24, 943-6.
- 9 BENBASSAT J., SHALEV O. Envenomation by Echis coloratus (Mid-East saw-scaled viper): a review of the literature and indication for treatment. Isr. J. Med. Sci., 1993, 29, 239-50.
- 10 BERTKE EM., ATKINS JH. Effect of Centruroides sculpturatus venom upon rat tissues: a histopathological study. Toxicon, 1961, 2, 205-18.
- 11 BRAIN SD., WILLIAMS TJ. Inflammatory mechanism of inflamed-tissue factor. Agents Actions, 1985, 3, 348-56.
- 12 CHERLIN VA., BORKIN LJ. Taxonomic revision of the snake genus Echis (Viperidae). I. An analysis of the history of study and synonymy. Proc. Zool. Inst. USSR Acad. Sci. Leningrad, 1990, 207, 175-92.
- 13 DE ARAUJO AL., DE SOUZA AO., DA CRUZ-HOFLING MA., FLORES CA., BON C. Bothrops lanceolatus (Fer de lance) venom induces oedema formation and increases vascular permeability in the mouse hind paw. Toxicon, 2000, 38, 209-21.
- 14 DE FARIA L., DE ANTUNES E., BON C., DE ARAUJO AL. Pharmacological characterization of the rat paw edema induced by Bothrops lanceolatus (Fer de lance) venom. Toxicon, 2001, 39, 825-30.
- 15 ERDO F., TOROK K., ARANYI P., SZEKELY JI. A new assay for antiphlogistic activity: zymosan-induced mouse ear inflammation. Agents Actions, 1993, 39, 137-42.
- 16 ERDOS EG. Structure and function of biologically active peptides: bradykinin, kallidin and congeners. Ann. N. Y. Acad. Sci., 1963, 104, 1.
- 17 FLOWER RJ. Glucocorticoids and the inhibition of phospholipase A2 In: SCHLELMER RP., CLAMOR HN., ORONSKY AL. Eds. Anti-inflammatory steroid action. Basic and clinic aspects. New York: Academic Press, 1989: 48-66.
- 18 FRANCISCHI JN., DIAS MF., ROCHA OA., DE ABREU CASTRO MS., KIYOMI FUNAYAMA TATSUO MA., FARINELLI P., PACHECO CM., FERREIRA-ALVES DL., SIROIS P. Pharmacological characterization of sephadex-induced oedema in rat paws: predominant role of serotonin and platelet-activating factor. Int. Arch. Allergy Immunol., 1996, 109, 398-406.
- 19 GASPERETTI J. Snakes in Arabia. J. Saudi Nat. Hist. Soc, 1977, 19, 3-16.
- 20 GASPERETTI J. Snakes of Arabia. Fauna of Saudi Arabia, 1988, 9, 169-450.
- 21 GIRALDELO CM., ZAPPELLINI A., MUSCARA MN., DE LUCA IM., HYSLOP S., CIRINO G., ZATZ R., DE NUCCI G., ANTUNES E. Effect of arginine analogues on rat hind paw oedema and mast cell activation in vitro. Eur. J. Pharmacol., 1994, 257, 87-93.
- 22 GOTH A. Medical pharmacology: principles & concepts. 9.ed. Missouri: The C. V. Mosby Company, 1978. 766p.
- 23 GUTIERREZ JM., CHAVES F., CERDAS L. Inflammatory infiltrate in skeletal muscle injected with Bothrops asper venom. Rev. Biol. Trop., 1986, 34, 209-14.
- 24 HO CL., HWANG LL., CHEN CT. Edema-inducing activity of lethal protein with phospholipase A1 activity isolated from the black-bellied hornet (Vespa basalis) venom. Toxicon, 1993, 31, 605-13.
- 25 HOLLEMAN WH., WEISS LJ. The thrombin-like enzyme from Bothrops atrox snake venom. Properties of the enzyme purified by affinity chromatography on p-aminobenzamidine substituted agarose. J. Biol. Chem., 1976, 251, 1663-9.
- 26 IALENTI A., IANARO A., MONCADA S., DI ROSA M. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol., 1992, 211, 177-82.
- 27 IANARO A., O'DONNEL CA., DI ROSA M., LHEW FY. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology, 1994, 82, 370-5.
- 28 ICKOWICZ M., SHULOV A., NAOR D. The effect of Vipera palestina venom on the thymus, lymph nodes and kidneys. Toxicon, 1966, 3, 305-6.
- 29 ISHII K., MOTOYOSHI S., KAWATA J., NAKAGAWA H., TAKEYAMA K. A useful method for differential evaluation of anti-inflammatory effects due to cyclooxygenase and 5-lipoxygenase inhibitions in mice. Jpn. J. Pharmacol., 1994, 65, 297-303.
- 30 JOHNSON AR., ERDOS EG. Release of histamine from mast cells by vasoactive peptides. Proc. Soc. Exp. Biol. Med., 1973, 142, 1252-6.
- 31 LABIB RS., AZAB MH., FARAG NW. Effects of Cerastes cerastes (Egyptian sand viper) and Cerastes vipera (Sahara sand viper) snake venoms on blood coagulation: separation of coagulant and anticoagulant factors and their correlation with arginine esterase and protease activities. Toxicon, 1981, 19, 85-94.
- 32 LIPPE IT., STABENTHEINER A. HOLZER P. Participation of nitric oxide in the mustard oil-induced neurogenic inflammation of the rat paw skin. Eur. J. Pharmacol., 1993, 232,113-20.
- 33 LOMONTE B. Edema-forming activity of bushmaster (Lachesis muta stenophrys) and Central America rattlesnake (Crotalus durissus durissus) venoms and neutralization by a polyvalent antivenom. Toxicon, 1989, 23, 173-6.
- 34 LOMONTE B., TARKOWSKI A., HANSON LA. Host response to Bothrops asper snake venom. Analysis of edema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation, 1993, 17, 93-105.
- 35 MAHMOUD I. Biochemical and physiological studies on the action of viper venom Cerastes vipera on mice Mus musculus. Egypt: Cairo University, Faculty of Science, 1983. 139p. [Masters - Thesis]
- 36 MARKLAND FS., DAMUS PS. Purification and properties of thrombin-like enzyme from the venom of Crotalus adamanteus (Eastern diamondback rattlesnake). J. Biol. Chem., 1971, 246, 6460-73.
- 37 MARSH N., GATTULLO D., PAGLIARO P., LOSANO G. The Gaboon viper, Bitis gabonica: haemorrhagic, metabolic, cardiovascular and clinical effects of the venom. Life Sci., 1997, 61, 763-9.
- 38 MOAV B., MOROZ CH., DE VRIES A. Activation of the fibrinolytic system of the guinea pig following inoculation of Echis colorata venom. Toxicon, 1963, 1, 109-12.
- 39 MOHAMED A., DAMARAWY NA. The role of the fibrinolytic enzyme system in the haemostatic defects following snake envenomation. Toxicon, 1974, 12, 467-75.
- 40 MOHAMED AH., EL-SEROUGI M., KHALED LZ. Effect of Cerastes cerastes venom on blood coagulation mechanism. Toxicon, 1969, 7, 181-4.
- 41 MOHAMED AH., KHALED LZ. Effect of the venom of Cerastes cerastes on nerve tissue and skeletal muscle. Toxicon, 1966, 3, 223-4.
- 42 OUYANG C., TENG CM., CHEN YC. Physicochemical properties of alpha and beta fibrinogenases of Trimeresurus mucrosquamatus venom. Biochim. Biophys. Acta, 1977, 481, 622-30.
- 43 PERALES J., AMORIM CZ., ROCHA SLG., DOMONT G., MOUSSATCHE H. Neutralization of the oedematogenic activity of Bothrops jararaca venom on the mouse paw by an antibothropic fraction isolated from opossum (Didelphis marsupialis) serum. Agents Actions, 1992, 37, 250-9.
- 44 REID HA., THEAKSTON RDG. The management of snakebites. Bull. World Health Organ., 1983, 61, 885-95.
- 45 SAUTEBIN L., IALENTI A., IANARO A., DI ROSA M. Endogenous nitric oxide increases prostaglandin biosynthesis in carrageenin rat paw oedema. Eur. J. Pharmacol., 1995, 286, 219-22.
- 46 SOUZA PINTO JC., REMACLE-VOLON G., SAMPAIO CA., DAMAS J. Collagenase-induced oedema in the rat paw and the kinin system. Eur. J. Pharmacol., 1995, 274, 101-7.
- 47 STOCKER K., BARLOW GH. The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol., 1976, 45, 214-23.
- 48 TAN NH., PONNUDURAI G. A comparative study of the biological properties of venoms from snakes of the genus Vipera (true adders). Comp. Biochem. Physiol. B, 1990, 96, 683-8.
- 49 THEAKSTON RD. The application of immunoassay techniques, including enzyme-linked immunosorbent assay (ELISA), to snake venom research. Toxicon, 1983, 21, 341-52.
- 50 THEAKSTON RDG., REID HA. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Organ., 1983, 61, 949-56.
- 51 THEAKSTON RDG., REID HA., IDDON D. Standardization tests for estimation of defibrinating, coagulant, hemorrhagic and necrotizing effects of snake venom. Toxicon, 1982, 20, 363.
- 52 TILBURY CR., MADKOUR MM., SALTISSI D., SULEIMAN M. Acute renal failure following the bite of Burton's carpet viper Echis coloratus Gunther in Saudi Arabia: case report and review. Saudi Med. J., 1987, 8, 87-95.
- 53 TREBIEN HA., CALIXTO JB. Pharmacological evaluation of rat paw oedema induced by Bothrops jararaca venom. Agents Actions, 1989, 26, 292-300.
- 54 WARRELL DA. Clinical toxicology of snake bites in Africa and the Middle East/Arabian Peninsula. In: MEIER J., WHITE J. Eds. Handbook of clinical toxicology of animal venoms and poisons 5.ed. Boca Raton: CRC Press, 1995: 433-92.
- 55 WILLIAMS TJ. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br. J. Pharmacol, 1979, 65, 517-24.
- 56 WILLIAMS TJ., PECK MJ. Role of prostaglandin-mediated vasodilatation in inflammation. Nature, 1977, 270, 530-2.
- 57 WILLIAMS WJ., ESNOUF MP. The fractionation of Russell's viper (Vipera russellii) venom with special reference to the coagulant protein. Biochem. J., 1962, 84, 52-62.
- 58 ZAHAVI M., ZAHAVI J., KAKKAR VV. Effect of adenyl-cyclase activators, phosphodiesterase inhibitors and pyridoxal-5-phosphate on platelet aggregation and adenosine-3'-5'-cyclic monophosphate accumulation. Thromb. Haemost, 1984, 52, 205-9.
Correspondence to:
Publication Dates
-
Publication in this collection
19 Sept 2006 -
Date of issue
2006
History
-
Accepted
27 Oct 2005 -
Received
20 June 2005