Abstract
Leishmaniasis is a parasitic zoonosis caused by protozoans of the genus Leishmania transmitted by insects known as phlebotomines, which are found in wild or urban environments. It affects domestic and wild animals and transmission to man happens by accident. The disease occurs in tropical and sub-tropical areas, mainly in Asia, Europe, Africa, and the Americas. There are two forms that affect man: American cutaneous leishmaniasis (ACL) and American visceral leishmaniasis (AVL). The latter is caused by three species of Leishmania: Leishmania (Leishmania) donovani, Leishmania (Leishmania) infantum, and Leishmania (Leishmania) chagasi, which are grouped in the Leishmania (Leishmania) donovani complex. Wild reservoir hosts of L. chagasi known so far are foxes and marsupials. In domestic environment, dogs are the most important reservoir hosts and sources of infection to the vectors Lutzomyia longipalpis. Leishmaniasis is difficult to control, causing epidemic outbreaks, thus being an important public health problem. Due to lesions caused by the mucocutaneous type and the severity of those caused by the visceral type in humans, visceral leishmaniasis is one of the main public health concerns. This paper is part of the monograph presented at the end of the residency program in the field of Zoonosis and Public Health at the School of Veterinary Sciences and Animal Husbandry, São Paulo State University, UNESP, Botucatu, São Paulo State, Brazil, in 2005.
leishmaniasis; cutaneous leishmaniasis; visceral leishmaniasis; Leishmania
REVIEW ARTICLE
Impact of leishmaniasis on public health
Camargo L. B.I; Langoni H.I, II
IDepartment of Veterinary Hygiene and Public Health, School of Veterinary Medicine and Animal Husbandry, FMVZ, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil
IICenter for Zoonosis Research, NUPEZO, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil
Correspondence to Correspondence to: Hélio Langoni departamento de Higiene Veterinária e Saúde Pública Faculdade de Medicina Veterinária e Zootecnia, UNESP Distrito de Rubião Jr., s/n 18.618-000, Botucatu, SP, Brasil Phone: 55 14 3811 6270. Fax: 55 14 3811 6075 Email: hlangoni@fmvz.unesp.br
ABSTRACT
Leishmaniasis is a parasitic zoonosis caused by protozoans of the genus Leishmania transmitted by insects known as phlebotomines, which are found in wild or urban environments. It affects domestic and wild animals and transmission to man happens by accident. The disease occurs in tropical and sub-tropical areas, mainly in Asia, Europe, Africa, and the Americas. There are two forms that affect man: American cutaneous leishmaniasis (ACL) and American visceral leishmaniasis (AVL). The latter is caused by three species of Leishmania: Leishmania (Leishmania) donovani, Leishmania (Leishmania) infantum, and Leishmania (Leishmania) chagasi, which are grouped in the Leishmania (Leishmania) donovani complex. Wild reservoir hosts of L. chagasi known so far are foxes and marsupials. In domestic environment, dogs are the most important reservoir hosts and sources of infection to the vectors Lutzomyia longipalpis. Leishmaniasis is difficult to control, causing epidemic outbreaks, thus being an important public health problem. Due to lesions caused by the mucocutaneous type and the severity of those caused by the visceral type in humans, visceral leishmaniasis is one of the main public health concerns.
This paper is part of the monograph presented at the end of the residency program in the field of Zoonosis and Public Health at the School of Veterinary Sciences and Animal Husbandry, São Paulo State University, UNESP, Botucatu, São Paulo State, Brazil, in 2005.
Key words: leishmaniasis, cutaneous leishmaniasis, visceral leishmaniasis, Leishmania.
INTRODUCTION
Leishmaniasis is an important contagious parasitic zoonosis whose agent is transmitted by insects called phlebotomines, found in wild and urban environments. It affects domestic and wild animals and may be accidentally transmitted to humans (11).
The World Health Organization (WHO) considers Leishmaniasis one of the most important zoonosis, which occurs in 88 countries on four continents (12). In 1993, WHO considered it as the second most important disease caused by a protozoan (32). According to WHO, in 2003 new cases were due to environmental alterations such as intense human migration to urban areas as well as deforestation and to individual risk factors like AIDS, bad nutrition, genetic factors, among others (8).
It is a widespread disease found in Asia, Europe, Africa, and the Americas, where it has been reported since the colonial period (32). It is prevalent in tropical and subtropical areas, where vectors are mostly adapted.
There are two types of infection: cutaneous leishmaniasis and visceral leishmaniasis. The first mainly affects the structure of the skin and, in some cases, the cutaneous mucus. The visceral form, also called kala-azar, affects internal organs such as spleen, liver, lymph nodes, and bone marrow (28).
American cutaneous leishmaniasis (ACL) in man has been reported since antiquity; there are descriptions in literature from the 1st century B.C. (13). In Brazil, the cause of existent cutaneous and nasopharyngeal lesions was confirmed only in 1909 by Lindenberg, who found out that the agent forms present in cutaneous lesions of rural workers from cities of São Paulo State were identical to Leishmania tropica from the Old World (5). Six species of Leishmania of the subgenera Leishmania and Viannia were identified in Brazil as cause of human ACL; they are: Leishmania (Viannia) braziliensis, transmitted by Lutzomyia whitmani, Lutzomyia wellcomei and Lutzomyia intermedia (25); Leishmania (V.) amazonensis, whose main vectors are Lutzomyia flaviscutellata and Lutzomyia olmeca (34); and Leishmania (V.) guyanensis, which is transmitted by Lutzomyia umbratilis, Lutzomyia anduzei and Lutzomyia squamiventris (25).
American visceral leishmaniasis (AVL) was first reported in Brazil in 1934, when amastigote forms of Leishmania were found in histological sections of liver from patients who had died of suspected yellow fever. The disease was previously restricted to agricultural regions in Northeast Brazil, but today it also affects the periphery of great urban areas in São Paulo State (20). It can be caused by three species of Leishmania: Leishmania (Leishmania) donovani, Leishmania (Leishmania) infantum, and Leishmania (Leishmania) chagasi, which are grouped in the Leishmania (Leishmania) donovani complex. Wild hosts of L. chagasi are foxes and marsupials. In domestic environment, dogs are the most important reservoir hosts and sources of infection, being one of the targets of control strategies (20). The species of phlebotomines responsible for transmission is Lutzomyia longipalpis, which was first recorded in 1997 in the city of Araçatuba, São Paulo State, Brazil (14). It has been found in the urban area of 44 cities in the western region of São Paulo State, and until the present moment, it is the only species reported to be involved in AVL transmission (39).
Migration, occupation of hillsides and partially urbanized centers in the periphery of urban metropolises, deforestation, and the predatory process of settling are related to the increase of American cutaneous leishmaniasis cases (20).
Although it is required that the disease be notified, the available data are based on the passive detection of cases. In some areas, the number of people exposed to infection or asymptomatically infected is higher than the number of detected cases (20).
OBJECTIVE
The aim of the present study is to provide information on leishmaniasis, an emergent zoonosis leading to epidemics in different cities of São Paulo State, Brazil, with many human deaths and canine cases due to its visceral form.
LITERATURE REVIEW
In 1571, Pedro Pizarro reported that people who used to live in Vales Quentes, Peru, were decimated by a disease that disfigured the nose, later characterized as cutaneous leishmaniasis (19). Deformations caused by its agent were so significant that were painted in ceramic pieces by artists of the period (32). However, the agent identification only occurred at the end of the 19th century in India, when Cunninghan, in 1885, identified two amastigote forms in kala-azar cases. In 1903, Leishman and Donovan first described the protozoan responsible for the Indian kala-azar, which was later named Leishmania donovani. Also in 1903, Wright described the parasite found in oriental sore, which is presently known as Leishmania tropica (22).
Since 1904, kala-azar has been reported to occur not only in India, but also in China. In the American continent, the emergence of lesions in certain regions of the body was commonly called as "úlcera de Bauru" or "ferida brava". In 1909, Gaspar Vianna, at the Oswaldo Cruz Institute, related those lesions to a new species of Leishmania named Leishmania brazilienses (31).
Visceral leishmaniasis affects thousands of people every year and 90% of them are from five countries, including Brazil, where about 1980 cases are notified per year (31). The first case in Brazil was recorded in 1913, when Migone, in Paraguay, found the agent in autopsy material of a person from Boa Esperança, Mato Grosso State, Brazil (1).
Since then, transmission has been described in some cities in almost all the regions of Brazil, except for the southern region. The disease has shown significant changes in its transmission pattern; initially, it was predominant in agricultural environments and periphery of urban centers; recently, it also affects great urban centers. In the 1990's, approximately 90% of the notified cases of visceral leishmaniasis occurred in the northeast region of the country (11).
In São Paulo State, AVL was first recorded in 1998 in dogs from Araçatuba, and the causative agent was identified as Leishmania chagasi. In 1999, the first human case of AVL was recorded in the same city, and since then the disease has been reported in several regions, being transmission exclusively urban (15, 38).
VISCERAL LEISHMANIASIS
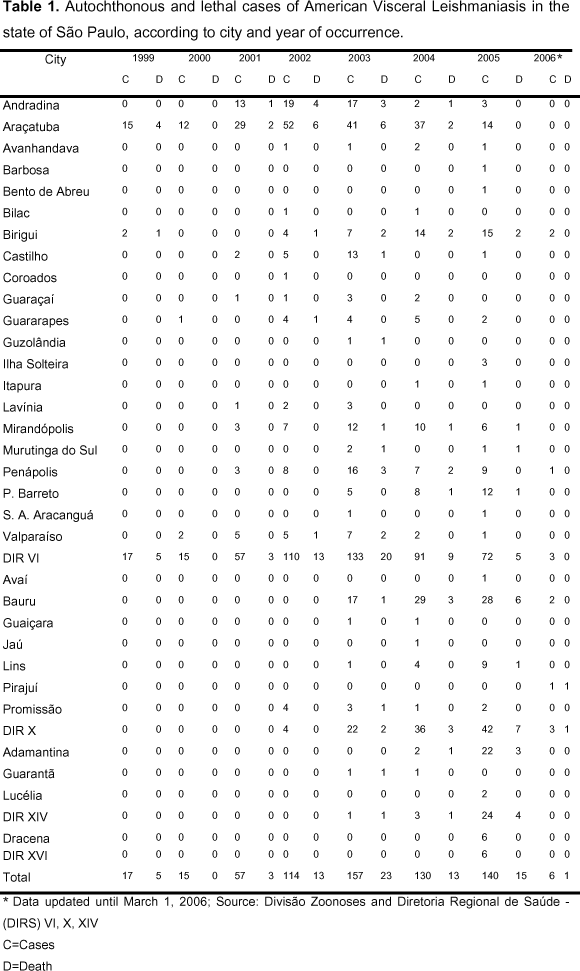
Visceral leishmaniasis is very important due to its clinical and epidemiological diversity. It may be fatal when not treated and epidemic with high lethality rate (11), as shown in Table 1.
American visceral leishmaniasis (AVL) or kala-azar is a serious and chronic disease, whose lethality can achieve 100% when adequate treatment is not instituted. It infects internal organs such as spleen, liver, lymph nodes, and bone marrow (24).
Epidemiology
It is caused by species of the Leishmania genus, belonging to the Leishmania (Leishmania) donovani complex (25). In Brazil, its etiological agent is L. chagasi (25).
Its occurrence depends basically on the presence of susceptible hosts and equally susceptible reservoirs (29).
The main form of transmission of the parasite to man and other mammal hosts is by the bite of the vector (females of Diptera belonging to the Psychodidae family, Phlebotomidae sub-family, generically known as phlebotomines). Lutzomyia (Lutzomyia) longipalpis is the main transmitter species of L. chagasi in Brazil (33).
The known wild reservoirs are foxes and marsupials. As these animals have synanthropic habits, the wild cycle of transmission can be linked to the domestic cycle. In domestic environment, dog is the most important reservoir, keeping the parasite endemic focus (30).
While feeding on blood, vectors ingest amastigote forms which, in their gastrointestinal tract, undergo a series of modifications until they are infective promastigote forms. When vectors will feed again on a new host, the promastigote forms are regurgitated with the saliva in the cutaneous tissue, completing the disease cycle (30).
In Brazil, studies on the AVL risk factors for dogs have not evidenced sexual, racial or age range related to the infection. However, it is believed that miniature breeds are less affected because they usually live inside the houses (21). Research carried out in Europe showed controversial results compared with these variables (23).
Clinical Aspects
In man, it can be asymptomatic when patients present positive serology or when parasites are found in the tissues without any manifestation of clinical symptoms. It can be oligosymptomatic with intermittent symptoms, low or no fever, and discrete hepatomegaly and splenomegaly, if detected.
The acute form of the disease can present abrupt or insidious onset. In the majority of cases, fever is the first symptom and can be high, continuous or intermittent, resolving in one or two weeks. Hepatosplenomegaly, weight loss and hemorrhages are observed as well as anemia or hyperglobulinemy.
The classic form presents long evolution of symptoms, nutritional impairment, hair loss, eyelash growth, lower limbs edema, fever, weight loss and cachexia. Severe hepatosplenomegaly, intense pallor of skin and mucosa due to severe anemia can also occur. Serious hemorrhage and, many times, decreased growth can be observed in children and young-adults.
There is also a refractory form, which does not respond or presents a delayed response to treatment with antimonial drugs. It is clinically more serious due to its rapid evolution without therapeutical response. In general, patients dye because of hemorrhages and secondary infections due to physical atony and immunosuppression caused by the agent (8). In dogs, it can also be asymptomatic, when animals are physically well but can be serologically positive.
An oligosymptomatic form with moderate weight loss, discrete skin lesions and lymphadenomegaly can occur.
The classic form of the disease in dogs with its evident symptoms can show weight loss (Figure 1), alopecia and ulcerative dermatosis (Figure 2), onychogryphosis (Figure 3), lymphadenopathy (Figure 4), nephropathy, anemia, thrombocytopenia, among others (18).
CUTANEOUS LEISHMANIASIS
American cutaneous leishmaniasis (ACL) is one of the six most important infecto-parasitic endemic diseases and represents one of the most common public health problems in the Americas.
In Brazil, it has been identified in almost all states. The number of cases has gradually increased in the last 20 years (7), and the country showed the highest occurrence, with more than thirty thousand annual cases (28), as shown in Table 2. Epidemic outbreaks have occurred in the southeastern, central western and northeast regions and, more recently, in the Amazon region, which is related to the predatory process of settling. Its importance is not only due to its high incidence and wide geographic distribution, but also because it assumes different forms with destructive, disfiguring and also incapacitating lesions that can affect the psychosocial development of men (27).
Epidemiology
American cutaneous leishmaniosis has been currently reported in all states of Brazil (10). It can be classified as purely wild cutaneous leishmaniasis, causing epidemic outbreaks due to deforestation or disordered exploration of forests; modified wild cutaneous leishmaniasis, which may cause seasonal epidemic outbreaks; periurban cutaneous leishmaniasis, which may be endemoepidemic; and interdomestic or peridomestic cutaneous leishmaniasis, which has the participation of domestic animals as reservoirs (35).
Several clinical-epidemiological forms have been reported to be related with different subgenera and species of Leishmania: Leishmania (Leishmania) amazonensis is spread to the Amazon primary and secondary forests and also to the northeast, southeastern and central western regions of Brazil. Its reservoirs are rodents and marsupials, and the main vectors are Lutzomyia flaviscutellata, Lutzomyia olmeca, and Lutzomyia reducta. Leishmania (Viannia) guyanensis is mainly found in forests and areas that are not flooded during rain periods, such as the north of the Amazon basin. Some reservoirs have been identified as its natural hosts: sloth, anteater, marsupials and rodents. Its vectors are Lutzomyia anduzei, Lutzomyia whitmani and Lutzomyia umbratilis. Leishmania (Viannia) braziliensis is widely spread from the south of Pará State to the northeast and central south of Brazil and some areas of Amazonia. Its vector is Psychodypes wellcomei. Currently, Leishmania (Viannia) braziliensis has been associated with Lutzomyia intermedia and Lutzomyia whitmani. Until the present moment, no wild animals were identified as its reservoirs; however, some domesticated species such as dogs, equines, mules and domestic or synanthropic rodents are frequently observed (10).
Clinical Aspects
In the cutaneous form, the disease can be identified as local, when generally painless ulcerated lesions with raised edges are more commonly observed; disseminated, causing less ulcerated lesions that affect all parts of the body; and diffused, which is considered chronic and rare and may compromise the patient's skin with frequent recurrence. The most affected areas are face and members, the mainly exposed parts of the body.
The mucous form is secondary to cutaneous lesions when dissemination by hematogenic or lymphatic route occurs and lesions are not correctly treated. After unnoticeable cutaneous infection caused by the bite of phlebotomines, lesions develop (also by dissemination) in the mucosa of aerial routes. They are most frequent in the nasal region (28). Compromised mucus covering the superior aerial routes leads to development of perforating or vegetating ulcers as well as necrosis of cartilaginous septum, nasal sinus, soft and hard palate, larynx and pharynx. It can also lead to deglutition, phonation and breathing problems, besides an extreme aesthetic alteration in the face, and may cause death due to chronicity and respiratory complications (26).
DIAGNOSIS OF CANINE AND HUMAN INFECTIONS
In man, clinical diagnosis is complex because lesihmaniasis can present symptoms that are common to other diseases such as Chagas' disease, malaria, schistosomiasis, typhoid fever, and tuberculosis (20).
In dogs, clinical diagnosis of visceral leishmaniasis is many times a problem for veterinarians since there is a wide spectrum of clinical signs from apparently healthy animals to advanced stages of the disease. It is systemic and chronic and can lead the animal to death.
Parasitological diagnosis with detection of the parasite amastigote forms can be made by biopsy of the lesion or exploratory puncture of spleen, liver, bone marrow and lymph nodes. The collected material is impressed in slides and Giemsa stained for cytological examination (Figure 5). Diagnosis can also be made by histologic examination, isolation in culture media or inoculation into laboratory animals. These methods show 100% specificity but their sensitivity is very changeable because parasites are not homogeneously distributed into the same tissue (37). They are invasive both for men and dogs, offering risks to the patients. Splenic exploratory puncture is a highly sensitive method to detect the parasite in men; however, it is inadvisable for the risks it presents. Biopsy can be performed by using a bistoury, and the biopsied region must be cleaned with water and soap before infiltration of 2% lidocaine (Xylocaine) as local anesthetic. Diagnosis is positive when the parasite is identified in tissues. It is not a highly sensitive method but sensitivity increases when infection is recent (10).
Culture is a confirmation method that allows later identification of the Leishmania species involved by using bimolecular techniques such as polymerase chain reaction (PCR). The parasite grows relatively well in culture media like NNN (McNeall, Novy & Nicolle) and LIT (Liver Infusion Tryptose) at 28°C from 24 to 26 days. After the fifth day, it is already possible to find its promastigote forms; however, the culture should be kept for one month before discarded as negative. Culture can be made with material from biopsies, lymph nodes and medular punctures and also from blood [Figure 6] (10).
For serological diagnosis, different techniques have been used. The tests differ in sensitivity, specificity, practical application, field conditions and availability of reagents. They present limitations since results can remain positive for a long time after treatment, not allowing evaluation of the therapy effect; also, cross-reactions with other diseases may occur. Nowadays, the most used tests are direct aglutination test (DAT), indirect fluorescent antibody test (IFAT) and enzyme-linked immunosorbent assay (ELISA), which use natural antigens and are limited in terms of specificity and reproducibility (37).
In several studies, DAT has shown highly changeable sensitivity, from 91% to 100%, and specificity from 72% to 100%. This test shows high intrinsic validity and is easy to carry out; however, it can present problems in the standardization and quality control of the antigen (6).
In Brazil, the most used tests for leishmaniasis diagnosis are IFAT and ELISA, which are considered tests of choice for population inquiries. ELISA is used for immunodiagnosis of visceral leishmaniasis. It is fast, easy to carry out and read, and more sensitive and less specific than IFAT. ELISA allows detection of low antibody titers and is accurate in the identification of subclinical or asymptomatic cases (16). It is also appropriate for visceral canine leishmaniasis diagnosis (17).
Indirect fluorescent antibody test (IFAT) presents low specificity, is recommended for confirmation and demands well-trained personnel. It is an expensive test and is not adapted for large-scale epidemiologic studies. One of its main limitations is the occurrence of cross-reactions with cutaneous leishmaniasis, Chagas' disease, malaria, schistosomiasis and pulmonary tuberculosis (37), which makes difficult the interpretation of epidemiological data.
For cutaneous leishmaniasis diagnosis, both tests (IFAT and ELISA) are useful, mainly in cases of multiple external lesions and mucous lesions; however, low titers cannot be detected (10). Montenegro's test can also be used to diagnose this type of leishmaniasis. It is an intradermal reaction that translates the delayed response of cellular hypersensitivity. The standardized antigen is inoculated into the epidermis and forms a small protuberance or papule when positive. Reading must be carried out within the following 48-72 hours. Result is positive when a nodule or papule equal or higher than 5mm in diameter, or ulceration, is formed. This test presents high sensitivity and has been positive in 90% cases of American cutaneous leishmaniasis (10).
Molecular tests, which besides the diagnosis allow the treatment monitoring and epidemiological studies, have also been carried out. Polimerase chain reaction (PCR) has been described as a sensitive test to detect the parasite, independent of the patient's immunocompetence or clinical history (41). Many research centers have evaluated the use of PCR for visceral leishmaniasis diagnosis using peripheral blood, since that splenic biopsy and bone marrow puncture are not considered appropriate techniques to be used outside the hospital environment (24). Although PCR is a sensitive test to detect Leishmania spp in a variety of clinical materials of both men and dogs, it is more widely used in epidemiological studies than in routine diagnosis (36). For large-scale use, PCR needs adjustments to become simpler and with lower operating costs. Today, kits can be used to diagnose leishmaniasis both in men and animals.
TREATMENT
Treatment of human cases
A pentavalent antimonial, which is sold under the name Glucantime® (N-methyl-glucamine antimoniate), can be used as first-choice drug; however, it presents many limitations: it cannot be administered to pregnant women and to patients with renal or hepatic failure, cardiac arrhythmia and Chagas' disease. Clinical follow-up and complementary examinations must be performed. The patient must be monitored due to side effects and risks.
When there is resistance to pentavalent antimonials, as a second choice we can use amphotericin B, which should not be mixed with other medicines or solutions that contain electrolytes. It must be infused protected from light, under orientation and medical follow-up in reference hospitals due to its high toxicity (8).
Treatment of canine cases
Studies have been carried out seeking cure, but although dogs showed to be clinically recovered, they still had amastigote forms in the skin, which keeps them as potential source of the parasite to phlebotomines (4). Frequent recurrences are still observed in changeable periods after treatment interruption (2, 3, 40). Considering these aspects, the Brazilian Ministry of Health, aiming at preserving the population health, indicated euthanasia of animals positive for serological tests like IFAT or ELISA (8).
PROPHYLAXIS AND CONTROL
The Brazilian program for prophylaxis and control of leishmaniasis launched more than 40 years ago combines three measures: free distribution of specific drugs, control of domestic reservoirs, and control of vectors. Control of reservoirs has been carried out by drawing a serological diagnosis of dogs in domestic environments, where transmission to man occurs, and positive animals have been euthanized (11).
The Brazilian Ministry of Health is responsible for establishing the actions of the "Program for Monitoring and Control of Visceral Leishmaniasis" in Brazil. This program has as its main objective the reduction of morbidity and mortality rates by means of diagnosis and early treatment of human cases, elimination of infected canine reservoirs, reduction of phlebotomines population, and informational and educational activities to the population (9).
Actions directed to vectors
Environmental management like yards, lands and public squares cleaning is indicated in order to modify the conditions that favor the establishment of breeding sites for immature vector forms. Simple measures such as keeping urban areas clean, properly wasting organic solid residues, eliminating humidity sources, not allowing domestic animals to stay inside the house, among others, certainly contribute to prevent or reduce the vector proliferation (11).
Chemical control by using insecticides of residual action is a vectorial control measure for collective protection. This measure is only against the adult insect and its objective is to prevent and/or reduce the contact between the transmitting insect and the human population, consequently reducing transmission risks. It should be applied to areas with records of autochthonous cases, areas of moderate and intense transmission, and areas of leishmaniasis outbreaks. The internal and external walls of the domicile, including the ceiling, and doghouses must be pulverized (11).
Actions directed to the canine population
Capture of stray dogs is essential, especially in urban areas, because besides being important sources of visceral leishmaniasis infection, they can transmit other zoonoses. It is a municipal duty and must be routinely carried out according to the norms of the Brazilian Sanitary Code.
It is mandatory to use fine-mesh screens on doghouses in residences and mainly in pet shops, veterinary clinics, animal shelters, and public veterinary hospitals, in order to prevent the access of phlebotomines and consequently reduce their contact with dogs. A number of works have demonstrated that, in experimental conditions, 4% Deltamethrin-impregnated dog collars were effective in protecting dogs against phlebotomine bites, with reduction of canine transmission in epidemic regions (11).
The Brazilian Ministry of Agriculture authorized in 2003 the use of a canine vaccine indicated for visceral leishmniasis, named Leishmune® and produced by Fort Dodge Saúde Animal Ltda. Experimental studies indicated it showed 76.0% efficacy against moderate and severe clinical signs of the disease in dogs. The vaccine could be used as a strategy for the control of human visceral leishmaniasis since it prevents infection in dogs and transmission of the parasites to vectors, and shows satisfactory cost-effectiveness and cost-benefit ratios (12).
Another relevant aspect to be considered about the vaccine introduction and related to the modification of the dog's immunological condition is that the tests available at the moment cannot differentiate between natural infection and that caused by vaccination, which makes vigilance and control actions difficult.
According to the information presented above, the Brazilian Ministry of Health, aiming at preserving the population's health, reinforces the non-indication of animal vaccine administration to humans and still indicates euthanasia of animals with positive serum samples, even vaccinated dogs that may be in the transmission areas (12). On the other hand, vaccine is authorized by the Brazilian Ministry of Agriculture, and there is the possibility of producing and using it in the future.
Health education activities
Information about the occurrence of leishmaniasis in the region, its clinical signs, and diagnosis services and treatment; adoption of preventive measures; development of health education activities for the community; and the establishment of associations for interinstitutional integration should be carried out (11). Health education activities are important for detection of the disease in man and in dogs at the earliest. According to the health politics in Brazil, the government is responsible for the identification of professionals and reference health care units to carry out laboratory exams and follow up patients, preventing the treatment ceasing and the disease complication; for the preparation of health education activities aiming at the active participation of the community in looking for assistance at the earliest; and for the adoption of control measures. All the suspect cases should be subjected to clinical and epidemiological analysis and auxiliary diagnosis methods. If confirmed, the patient must be treated and monthly evaluated (11).
CONCLUSION
The increasing spread of the disease to urban areas observed in the last 20 years led to the discussion of the control measures adopted until the present moment, which could not eliminate transmission and prevent new epidemics. Despite the innumerable studies on human and canine visceral leishmaniasis, many questions were not answered yet. Today, Brazil faces the leishmaniasis expansion, especially into urban regions. Its transmission cycle, which used to take place in wild and rural environments, nowadays also happens in urban centers. This geographic expansion of leishmaniasis led to the necessity of establishing more effective control measures. Since the methods used until the present moment have been partially effective in preventing and controlling the disease, new control strategies should be developed to produce effective immunogens to control canine and human leishmaniasis.
AKNOWLEDGEMENTS
We thank the graduate students Acácia Orieth Elias and Rodrigo Costa da Silva for English correction, and Juliana Giantomassi Machado and Juliano Leônidas Hoffmann for the help with illustrations.
Received: June 1, 2006
Accepted: June 1, 2006
Abstract published online: June 7, 2006
Full paper published online: November 30, 2006
- 1 ALENCAR JE., DIETZE R. Leishmaniose visceral (Calazar). In: VERONESI, R. Doenças Infecciosas e Parasitárias, 8 ed. Rio de Janeiro: Guanabara Koogan, 1991, 706-17.
- 2 ALVAR JA., MOLINA R., SAN ANDRES M. TESOURO M., NIETO J, VITUVIA M., GONZALEZ F., SAN ANDRES MD., BOGGIO J., RODRIGUEZ F. Canine leishmaniasis: clinical, parasitological, and entomological follow-up after chemotherapy. Ann. Trop. Med. Parasitol., 1994, 88, 1-8.
- 3 ALVAR JA. El pero como reservatório da la leishmaniosis. Med. Vet., 1995, 12, 7-8.
- 4 BANETH GE., SHAW S. Chemotherapy of canine leishmaniasis. Vet. Parasitol., 2002, 106, 315-24.
- 5 BASANO SA., CAMARGO LMA. Leishmaniose Tegumentar Americana: histórico, epidemiologia e perspectivas de controles. Rev. Bras. Epidemiol., 2004, 07, 3.
- 6 BOELAERT M., EL SAFI S., MOUSA H., GITHURE J., MBATI P., GURUBACHARYA V., SHRESTHA J., JACQUET D., DE MUYNCK A., LE RAY D., VAN DER STUYFT P. Multi-center evaluation of repeatability and reproducibility of the direct agglutination test for visceral leishmaniasis. Trop. Med. Int. Health, 1999, 4, 31-7.
- 7 BRASIL, Ministério da Saúde. Fundação Nacional de Epidemiologia. Guia de Vigilância Epidemiológica, Brasília, 1998.
- 8 BRASIL, Ministério da Saúde. Fundação Nacional de Epidemiologia. Guia de Vigilância Epidemiológica, Brasília-DF, 2002.
- 9 BRASIL, Ministério da Saúde. Fundação Nacional de Epidemiologia. Guia de Vigilância Epidemiológica, Brasília-DF, 2005.
- 10 BRASIL, Ministério da Saúde. Fundação Nacional de Epidemiologia. Manual de Controle da Leishmaniose Tegumentar Americana, Brasília-DF, 2000.
- 11 BRASIL, Ministério da Saúde. Fundação Nacional de Epidemiologia. Manual de Vigilância e Controle da Leishmaniose Visceral, Brasília-DF, 2003.
-
12BRASIL, Ministério da Saúde. Secretaria de Vigilância em Saúde. Nota Técnica. Vacina Anti-leishmaniose Visceral Canina – Leishmune®, Brasília-DF, 2005.
- 13 CAMARGO LMA, BARCINSKI MA. Leishmanioses, feridas bravas e calazar. Ciênc. Cultura, 2003, 1, 34-7.
- 14 CAMARGO-NEVES VLF. de, KATZ G. Leishmaniose visceral americana no Estado de São Paulo. Rev. Soc. Bras. Med. Trop., 1999, 32 (Suppl. 2), 63-4.
- 15 COSTA AIP. da, CASANOVA C., RODAS LAC., GALATI EAB. Atualização da distribuição geográfica e o primeiro encontro de Lutzomyia longipalpis em área urbana no Estado de São Paulo, Brasil, Notas e Informações. Rev. Saúde Pública, 1997, 31, 632-33.
- 16 EL-AMIN ER., WRIGHT EP., ABDEL RAHMAN AM., KOLK A., LAARMAN JJ., PONDMAN KW. Serodiagnosis of Sudanese visceral and mucosal leishmaniasis: comparison of ELISA-immunofluorescence and indirect haemagglutination. Trans. R. Soc. Trop. Med. Hyg., 1986, 80, 271-74.
- 17 EVANS TG., VASCONCELOS IA., LIMA JW., TEIXEIRA JM., MCAULLIFE IT., LOPES UG., PEARSON RD, VASCONCELOS AW. Canine visceral leishmaniasis in northeast Brazil: assessment of serodiagnosis methods. Am. J. Trop. Med. Hyg., 1990, 42, 118-23.
- 18 FERNADEZ-PEREZ FJ., GOMEZ-MUNOZ MT., MENDEZ S, ALUNDA JM. Leishmania specific lymphoproliferative responses and IgG1/IgG2 immunodetection patterns by Western blot in asymptomatic, symptomatic and treated dogs. Acta Tropica., 2003, 86, 83-91.
- 19 GOLDMAN L. Pre-Columbian Leishmaniasis. Arch. Dermatol, 1983, 119, 540.
- 20 GONTIJO CMF., MELO MN. Leishmaniose Visceral no Brasil: Quadro atual, desafios e perspectivas. Rev. Bras. Epidemiol., 2004, 7, 338-49.
- 21 GUERIN PJ., OLLIARO P., SUNDAR S., BOELAERT M., CROFT SL., DESJEUX P., WASSUNA MK., BRYCESON AD. Visceral leishmaniasis: current status of control, diagnosis and treatment and a proposed research and development agenda. Lancet. Infect. Dis., 2002, 2, 494-501.
- 22 HOARE CA. Early discoveries regarding the parasites of oriental sore. Trans. R. Soc. Trop. Med. Hyg., 1938, 32, 67-92.
- 23 KAR K. Serodiagnosis of Leishmaniasis. Crit. Rev. Microbiol., 1995, 21, 123-52.
- 24 LACHAUD L., CHABBERT E., DUBESSAY P., REYNES J., LAMOTHE J., BASTIEN P. Comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood. J. Clin. Microbiol., 2001, 39, 613-17.
- 25 LAINSON R., SHAW JJ. Evolution, classification and geographical distribution. In: Peters W., Killick-Kendrick R. The leishmaniasis in biology and medicine. Eds. London, Academic Press Inc. 1987, 1, 1-120.
- 26 LLANOS-CUENTAS A. Epidemiological studies on Andean Cutaneous Leishmaniasis and their significance for designing a control strategy. In: T. Goodman e C. Espinal. Int. Dev. Res. Center., 1991, 286-303.
- 27 MARZOCHI MC. A Leishmaniose no Brasil: as leishmanioses tegumentares. J. Bras. Med, 1992, 63, 82-104.
- 28 MARZOCHI MCA. Leishmaniose tegumentar americana. In: Benjamin Cimerman et al. (org.). Parasitologia humana e seus fundamentos gerais. São Paulo: Atheneu, 1999, 2, 39-64.
- 29 MAURICIO IL., STOHARD JR., MILES MA. The strange case of Leishmania chagasi, Parasitol. Today, 2000, 16, 188-9.
- 30 MORENO JE., ALVAR J. Canine leishmaniasis: epidemiological risk and the experimental model, Trends Parasitol., 2002, 18, 399-405.
- 31 PEDROSA CM., ROCHA EMM. Aspectos clínicos e epidemiológicos da leishmaniose visceral em menores de 15 anos procedentes de Alagoas, Brasil. Rev. Soc. Bras. Med. Trop., 2004, 37, 300-4.
- 32 RATH S., TRIVELIN LA., IMBRUNTO TR., TOMAZELA DM.; JESUS MN., MARZAL PC., ANDRADE JUNIOR AF., TEMPONE AG. Antimoniais empregados no tratamento da Leishmaniose: Estado da Arte. Quim. Nova, 2003, 26, 550-5.
- 33 SANTOS S. Incrimination of Lutzomyia cruzi as a vector of American visceral leishmaniasis. Med. Vet. Entomol. 1998, 12, 315-7.
- 34 SHAW J., LAINSON R. Pneumocystis and histoplasma infections in wild animals from the Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg., 1975, 69, 505-8.
- 35 SILVEIRA FT. Patogenia da leishmaniose tegumentar americana: caracterização clínica, histopatológica e imunológica da leishmaniose disseminada, com ênfase na Leishmania (Leishmania) amazonensis. Thesis (Doctorate). Faculdade de Medicina da Universidade de São Paulo, São Paulo, 2001.
- 36 SOLANO-GALLEGO L., MORELL P., ARBOIX M., ALBEROLA J., FERRER L. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J. Clin. Microbiol., 2001, 39, 560-3.
- 37 SUNDAR S., RAI M. Laboratory diagnosis of visceral Leishmaniasis. Clin. Diagn. Lab. Immunol., 2002, 951-8.
- 38 TOLEZANO JE., LUVIZOTTO MCR., ULIANA SRB., ARAUJO MFL., TANIGUCHI HH; BARBOSA JAR., BARBOSA JER., PINTO PLS., FLOETER WINTER L., SHAW JJ. Leishmaniose visceral americana (LVA) em Araçatuba, região Oeste do Estado de Paulo. Investigações laboratoriais e diagnóstico de uma doença emergente em terras paulistas. Rev. Soc. Bras. Med. Trop., 1999, 32 (Supl), 218.
- 39 TOLEZANO JE., RODRÍGUEZ E., BARBOSA JER., CUNHA E., TANIGUCHI HH., BARBOSA JAR. Expansão da Leishmaniose visceral por terras paulistas. Focos de transmissão de LV canina em municípios da região metropolitana de São Paulo. Rev. Soc. Bras. Med. Trop., 2003, 36(Supl 2), 360.
- 40 VEXENAT JA., OLLIARO PL., FONSECA DE CASTRO JA., CAVALCANTE R., FURTADO CAMPOS JH., TAVARES JP., MILES MA. Clinical recovery and limited cure in canine visceral leishmaniasis treated with aminosidine (paramomycin). Am. J. Trop. Med. Hyg., 1998, 58, 448-53.
- 41 WEISS JB. DNA probes and PCR for diagnosis of parasitic infections. Clin. Microbiol. Rev., 1995, 8, 113-30.
Publication Dates
-
Publication in this collection
11 Jan 2007 -
Date of issue
2006
History
-
Received
01 June 2006 -
Reviewed
01 June 2006 -
Accepted
07 June 2006