Abstract
Red scorpions Mesobuthus tamulus (Coconsis, Pocock) were obtained from different regions of West and South India (Ratnagiri, Chiplun and Ahmednagar from Maharashtra and Chennai from Tamil Nadu, respectively). Their venoms composition was analyzed using gel electrophoresis (SDS-PAGE). All venom samples shared six bands of 170, 80, 60, 57, 43, and 38 kDa molecular weights. Bands of 115 kDa and 51.5 kDa were characteristic of venoms obtained from red scorpions of Chiplun region, and the 26kDa band was absent in scorpion venom from Tamil Nadu. The separated protein band patterns suggest that the venoms from Ratnagiri, Ahmednagar and Tamil Nadu had high similarities in their biochemical composition but differed from that of Chiplun region. These data were also supported by the Jaccard (J) index. The J value was 0.33 for venom obtained from Ratnagiri-Ahmednagar, 0.31 for venom from Ratnagiri-Tamil Nadu, and 0.3 for venom from Ratnagiri-Chiplun region. This suggests the existence of genetic variation among the different strains of red scorpion in western and southern India. The antiserum produced by Haffkine Biopharmaceuticals Corporation Ltd. completely neutralized proteins of venoms from all the regions studied.
red scorpion; SDS-PAGE; Mesobuthus tamulus
ORIGINAL PAPER
Intraspecific variation in protein pattern of red scorpion (Mesobuthus tamulus, coconsis, pocock) venoms from Western and Southern India
Badhe R. V.I; Thomas A. B.I; Harer S. L.I; Deshpande A. D.I; Salvi N.II; Waghmare A.II
IDepartment of Pharmaceutical Chemistry, Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune, Maharashtra, India
IIHaffkine Biopharmaceutical Corporation Ltd, Pimpri, Pune, Maharashtra, India
Correspondence to Correspondence to: Ravindra V. Badhe Department of Pharmaceutical Chemistry Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research Pimpri, Pune, 411018, Maharashtra, India Phone: +9194 2243 2038. Email: badheravi@yahoo.co.in
ABSTRACT
Red scorpions Mesobuthus tamulus (Coconsis, Pocock) were obtained from different regions of West and South India (Ratnagiri, Chiplun and Ahmednagar from Maharashtra and Chennai from Tamil Nadu, respectively). Their venoms composition was analyzed using gel electrophoresis (SDS-PAGE). All venom samples shared six bands of 170, 80, 60, 57, 43, and 38 kDa molecular weights. Bands of 115 kDa and 51.5 kDa were characteristic of venoms obtained from red scorpions of Chiplun region, and the 26kDa band was absent in scorpion venom from Tamil Nadu. The separated protein band patterns suggest that the venoms from Ratnagiri, Ahmednagar and Tamil Nadu had high similarities in their biochemical composition but differed from that of Chiplun region. These data were also supported by the Jaccard (J) index. The J value was 0.33 for venom obtained from Ratnagiri-Ahmednagar, 0.31 for venom from Ratnagiri-Tamil Nadu, and 0.3 for venom from Ratnagiri-Chiplun region. This suggests the existence of genetic variation among the different strains of red scorpion in western and southern India. The antiserum produced by Haffkine Biopharmaceuticals Corporation Ltd. completely neutralized proteins of venoms from all the regions studied.
Key Words: red scorpion, SDS-PAGE, Mesobuthus tamulus.
INTRODUCTION
Gel electrophoresis of proteins is widely used in insect molecular systematic. In this technique, identical proteins migrate to the same distance under electrical forces applied to an electrophoretic gel while non-identical proteins usually migrate to different distances (2). Studies on venoms, including insect venoms, have profoundly affected modern biochemistry, pharmacology and medicine. Venoms have provided an excellent source of highly concentrated active enzymes, cytotoxins and neurotoxins as research tools to study the sub-cellular functioning of mammalian nervous and cardiovascular systems. Insect venom previously had little direct use in modern medicine, but this situation is rapidly changing as more information is becoming available. As new techniques for isolating, identifying and especially producing individual venom components are developed, the use and role of venom in medicine will certainly increase.
The low-molecular-weight biogenic amines (histamine, dopamine, nor-adrenalin, etc.) found in venom samples are involved in local reactions and their release from a single sting can lead to systemic reactions. They act on blood vessels and nerve endings inducing swelling, redness, pain and itching. Major toxic effects of venom can be attributed to the presence of larger peptides such as melittin, dopamine and mostly cell degranulating factors. These peptides can cause damage to the cell membrane leading to release of enzymes from lysozymes and mast cell granules, resulting in cytolysis. Additionally, they can act as neurotoxins provoking hyper-excitability. High-molecular-weight enzymes, except for highly cytotoxic phospholipase A2, are regarded as less harmful. Hyluronidase has an indirect effect of increasing the absorption of active peptides (12). The various enzymes and vasoactive components induce local toxic inflammation at the sting region. If the sting occurs in a highly vascular area, or even intravascularly, the toxic components are rapidly spread and might give rise to systemic reactions. Several simultaneous stings will cause more reactions. A number of children and adults, especially pregnant women, succumb to the sting by the red scorpion Buthus tamulus in Konkan region, India, especially in the coastline (8).
The protein patterns of venoms from red scorpions Mesobuthus tamulus (Conconsis, Pocock) from Ratnagiri, Chiplun, Ahmednagar and Tamil Nadu were analyzed using polyacrylamide gel electrophoresis (PAGE). This analysis was used to shed light on the biological activities and correlation between the composition of venoms from different strains of red scorpion found in West and South India.
MATERIALS AND METHODS
Venom collection
We used pure lyophilized red scorpion (Mesobuthus tamulus) venoms from the following regions of West and South India: Ratnagiri, Chiplun, Ahmednagar and Tamil Nadu (supplied by Irula Snake Catchers Industrial Co-operative Society Ltd., ISCICS, Chennai, India). Lyophilized venom samples were resuspended in normal saline and 50% v/v solution of glycerin in distilled water, resulting in a 1% venom solution, which was stored at 2-4°C for further experiments.
Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out, according to the method of Lammeli (9), with 15% polyacrylamide concentration. Electrophoresis patterns of venom samples from Ratnagiri, Chiplun, Ahmednagar and Tamil Nadu were obtained. Molecular weight markers (3-205 kDa) were used as standards (Banglore Gennie). Proteins were visualized using Coomassie blue stain and silver staining. Tentative identification of the proteins in venom samples was made by comparing the gel with the descriptions existing in literature. Western blot was carried out to check the neutralizing capacity of the anti-scorpion venom sera produced by Haffkine Biopharmaceuticals Corporation Ltd, Pimpri, Pune, India.
Data analysis
Similarity between scorpion strains was measured using the Jaccard Index [J] (10), which was calculated as follows:
J = a/a+b+c
Where a = bands shared between two strains; b = total number of bands in strain 1; c = total number of bands in strain 2.
RESULTS
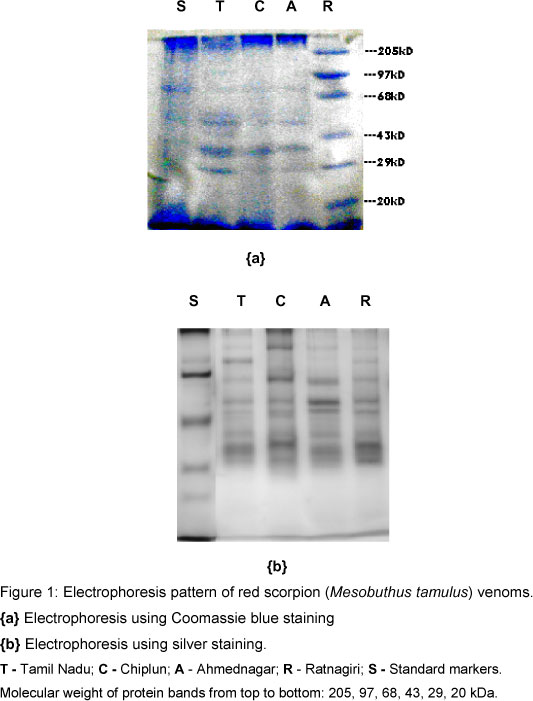
Ten protein bands (molecular weights: 170, 115, 79.6, 60, 57, 51.5, 43, 38, 29, and 26 kDa) were obtained for the venom of red scorpion from Chiplun region (Figure 1). The intensity of their color evidenced that the 57kDa band [band 5] had the highest protein concentration, followed by the 26kDa band [band 10].
Red scorpion venoms from both Ratnagiri and Ahmednagar showed bands with the following molecular weights: 170, 79.6, 60, 57, 43, 38, 29, and 26 kDa, from which band 4 [57 kDa] was prominent, followed by band 8 [26 kDa] (Figure 1 and Table 1).
The venom of red scorpion from Tamil Nadu presented seven bands with molecular weights of 170, 79.6, 60, 57, 43, and 38 kDa. Band 2 [79.6 kDa] was the most noticeable and the 26kDa band was absent.
Bands of 170, 79.6, 60, 57, 43, 38, 29, and 26 kDa molecular weights were characteristic of all venoms studied, except for that from Tamil Nadu, which did not present the 26kDa band (Table 1). Red scorpion venoms of Ratnagiri and Ahmednagar differed from that of Chiplun region by the absence of 115 and 51.5 kDa protein bands.
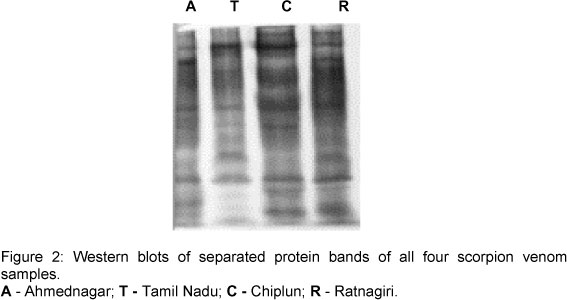
According to the Jaccard index equation, which was used to measure the similarity index between red scorpion venoms, the highest degree of similarity was observed between red scorpions from Ratnagiri and Ahmednagar [0.33], followed by those from Ratnagiri and Tamil Nadu [0.31]. The least similarity occurred between red scorpions from Ratnagiri and Chiplun [0.30]. Western blot showed complete neutralization of all the separated protein bands (Figure 2).
There is difference between the geographical conditions of Ahmednagar and those of Tamil Nadu, Ratnagiri, and Chiplun regions. The later are costal areas with an average annual temperature of 29°C and the former presents 38°C average temperature. There is also a difference in the average annual rainfall: around 700 mm in Ahmednagar, 2000 mm in Tamil Nadu, and 3500 mm in Ratnagiri and Chiplun. The later regions have relatively high humidity [70-100] (15), which may affect the scorpion food chain. These differences may lead to different critical lethal dose (CLD) values of scorpion venoms. For red scorpion venoms from Ratnagiri, Chiplun and Ahmednagar, CLD was 40 µg, and for those from Tamil Nadu it was 20 µg (8).
DISCUSSION
Ten bands of molecular weight ranging from 170 to 26 kDa were obtained for the red scorpion venom of Chiplun region. As bands of more than 100 kDa (i.e. 115 kDa and 170 kDa) are of very high molecular weight, there is no report about these components. However, they were reported to have neurotransmitter releasing property (6). The 51.5 kDa band has not been studied and its activity is unknown. Bands of 79.6 kDa and 60 kDa molecular weights, which were present in all four scorpion strains (from Ratnagiri, Ahmednagar, Tamil Nadu and Chiplun regions), characterize proteins with hyluronidase activity(5, 7). The 57 kDa protein was cited to be a NMDA (N-methyl-D-aspartic acid)-receptor activator (1). The 43 kDa protein has hyluronidase activity (13), and that of 38 kDa was reported to have chloride-ion blocking (4)and histamine releasing activities (3). Band of 29 kDa was characterized as protein with phospholipase A2 activity (14). The last protein band of 26 kDa, which is not present in Tamil Nadu scorpion venom, was cited to have hemorrhagic toxic and phosphodiesterase activities (11). Silver staining of bands revealed the same data.
Haffkine Biopharmaceutical Corporation Ltd., India, uses a mixture of venoms from different regions for immunization, and the antisera produced was capable of overcoming the effects of scorpion venoms from the Indian subcontinent. Western blot of the venom against the anti-scorpion venom serum corroborated these data (Figure 2).
Environmental conditions significantly affected venom lethality. According to the present test, it is clear that the venom sample of red scorpion from Tamil Nadu is more lethal at low concentrations. Out of the venom samples obtained from scorpions of Maharashtra region, that of Chiplun was more lethal (8).
Differences in the band patterns of separated proteins in all venom samples clearly suggest the existence of genetic variation among the scorpion strains of different regions in western and southern India.
Received: October 24, 2005
Accepted: January 23, 2006
Abstract published online: April 11, 2006
Full paper published online: November 30, 2006
- 1 BANKS BEC., SHIPOLINI AA. Chemistry and pharmacology of venom. In: VENOMS OF HYMENOPTERA, Piek T (Ed.). Academic Press, 1986, 329-416.
- 2 BERLOCHER SH. Insect molecular systematic. Ann. Rev. Entomol., 1984, 29, 40333.
- 3 CHATWAL GS., HABERMANN E. Neurotoxins, protease inhibitors and histamine releasers in the venom of Indian red scorpion (Buthus tamulus); isolation and partial characterization. Toxicon, 1981, 19, 807-23.
- 4 DHAWN R., JOSEPH S., LALA AK. Purification and characterization of a short insect toxin from venom of scorpion Buthos tamulus FEBS letters, 2002, 528, 261-6.
- 5 DIMITROV SD., NATCHAEV LA. Fractions of some bee venom components on a new type of modified cellulose. Toxicon, 1977, 15, 447-8.
- 6 EGYPT. Govt. of Egypt. A report on venoms collected from different insects and animals in Egypt. Egypt, 11/23/2000.
- 7 GAWADE SP. Excitatory effect of Buthus C-56 toxin on Drosophila larval neuromuscular junction. J. Venom. Anim. Toxins, 2003, 9, 57-9.
- 8 KANKONKAR RC., KULKARNI DG., HULIKAVI CB. Preparation of a potent anti-scorpion-venom-serum against the venom of red scorpion (Buthus tamulus), J. Postgrad Med., 1998, 4, 85-91.
- 9 LAMMELI UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1970, 227-80.
- 10 LUDWING JA., RAYOLDS JF. Statistical ecology. A primer on methods and computing, chapter 8, Diversity indices, John Willy and Sons, New York, 1988, 85-103.
- 11 MASTER RWP., RAO SS., SOMAN PD. Electrophoretic separation of biologically active constituents of scorpion venom. Biochem. Biophys. Acta, 1963, 71, 422-8.
- 12 MEIR J., WHITE J. Handbook of clinical toxicology of animal venom and poisons. C .R. Press, New York, 1995.
- 13 SCHMIDT JO. Biochemistry of insect venom. Ann. Rev. Ent., 1982, 27, 339-68.
- 14 SOBOTKA AK., FRANKLIN RM., ADKINSON NF., VALENTINE MD., BEAR H., LICHTENSTEIN LM. Allergy to insect sting. II: The major allergen in venom. J. Allergy Clin. Immunol., 1976, 57, 29-40.
- 15 VIDYAPEETH BSKK. A report on "Weather conditions in Maharashtra". Dapoli, Ratnagiri, M.S., 2004.
Publication Dates
-
Publication in this collection
11 Jan 2007 -
Date of issue
2006
History
-
Accepted
23 Jan 2006 -
Received
24 Dec 2005