Abstract
In the present study, manganese (Mn2+), a neuromuscular blocker with pre and postsynaptic actions, was used to verify the neurotoxicity and myotoxicity induced by Crotalus durissus terrificus (Cdt) and Bothrops jararacussu (Bjssu) venoms in biventer cervicis preparations (BCp). Preparations pretreated with 0.66 and 1.6mM Mn2+ did not affect Cdt venom-induced blockage nor change KCl-induced contracture but partially reduced ACh-induced contracture. However, both Mn2+ concentrations partially hindered Bjssu venom-induced blockage after washing the preparations with Krebs solution, and only 1.6mM Mn2+ preparations significantly recovered ACh-induced contracture. The effect of Cdt venom myotoxicity on contractile responses was different from that of Bjssu venom myotoxicity. Pretreatment with 1.6mM Mn2+ partially reduced muscle damage percentage and creatine kinase (CK) activity (U/l) induced by both venoms. In conclusion, Mn2+ interfered in ACh-induced contracture of the nicotinic receptor; did not prevent Cdt venom neurotoxicity but partially reduced its myotoxicity in vitro due to the stabilizing action of this venom on the sarcolemmal membrane; and partially attenuated myotoxicity and neuromuscular blockage induced by Bjssu venom. The Mn2+ dual action (pre and postsynaptic) is useful to study snake venoms since most of them present one or both of these actions; besides, Mn2+ allowed recovering coherent interpretation of experimental versus clinical results.
contracture; divalent cation; Mn2+; myotoxicity; neuromuscular blockage; neurotoxicity
ORIGINAL PAPER
Effects of manganese (Mn2+) on neurotoxic and myotoxic activities induced by Crotalus durissus terrificus and Bothrops jararacussu venoms in chick biventer cervicis preparations
Bueno L. G. F.I; Leite G. B.I; Cruz-Höfling M. A.II; Rodrigues-Simioni L.I; Oshima-Franco Y.I
IDepartment of Pharmacology, Faculty of Medical Sciences, State University of Campinas, UNICAMP, Campinas, São Paulo State, Brazil
IIDepartment of Histology and Embryology, Institute of Biology, State University of Campinas, UNICAMP, Campinas, São Paulo State, Brazil
Correspondence to Correspondence to: Yoko Oshima-Franco Departamento de Farmacologia Faculdade de Ciências Médicas Universidade Estadual de Campinas (UNICAMP) Caixa Postal 6111, 13083-970, Campinas, SP, Brasil Fax: +55 19 3289 2968 Email: yofranco@terra.com.br
ABSTRACT
In the present study, manganese (Mn2+), a neuromuscular blocker with pre and postsynaptic actions, was used to verify the neurotoxicity and myotoxicity induced by Crotalus durissus terrificus (Cdt) and Bothrops jararacussu (Bjssu) venoms in biventer cervicis preparations (BCp). Preparations pretreated with 0.66 and 1.6mM Mn2+ did not affect Cdt venom-induced blockage nor change KCl-induced contracture but partially reduced ACh-induced contracture. However, both Mn2+ concentrations partially hindered Bjssu venom-induced blockage after washing the preparations with Krebs solution, and only 1.6mM Mn2+ preparations significantly recovered ACh-induced contracture. The effect of Cdt venom myotoxicity on contractile responses was different from that of Bjssu venom myotoxicity. Pretreatment with 1.6mM Mn2+ partially reduced muscle damage percentage and creatine kinase (CK) activity (U/l) induced by both venoms. In conclusion, Mn2+ interfered in ACh-induced contracture of the nicotinic receptor; did not prevent Cdt venom neurotoxicity but partially reduced its myotoxicity in vitro due to the stabilizing action of this venom on the sarcolemmal membrane; and partially attenuated myotoxicity and neuromuscular blockage induced by Bjssu venom. The Mn2+ dual action (pre and postsynaptic) is useful to study snake venoms since most of them present one or both of these actions; besides, Mn2+ allowed recovering coherent interpretation of experimental versus clinical results.
Key words: contracture, divalent cation, Mn2+, myotoxicity, neuromuscular blockage, neurotoxicity.
INTRODUCTION
Venoms from snakes of the genera Crotalus and Bothrops have been extensively investigated in Brazil because of their epidemiological importance regarding lethality (1.8%) and frequency (90%), respectively (1, 6).
Although the current trend is to study crude venom fractions (10, 14, 22, 35, 38, 41, 44) to better characterize the venom pathophysiological effects, it is extremely important to investigate the substance that attenuates or highlights the actions induced by the whole venom, which is actually inoculated by the snake in an accident. Crotalic venoms have neurotoxic, myotoxic and coagulant actions, and Bothropic venoms present proteolytic, coagulant and hemorrhagic activities (3, 7, 11, 25, 33, 43, 45, 46). However, under experimental conditions, both venoms induced twitch-responses blockage in neuromuscular preparations and, when microscopically analyzed, myonecrosis was observed (11, 16, 37, 39, 42). The in vitro effects of these venoms were quite similar regarding the parameters twitch responses and morphological aspects of the preparations. To elucidate such effects, manganese was chosen as a pharmacological tool since it is a reversible neuroblocking agent with pre and postsynaptic actions (28, 30, 35, 36, 44), ideal to study crotalic and bothropic venoms, which have pre and postsynaptic actions, respectively.
MATERIALS AND METHODS
Venoms and Reagents
Dried Cdt and Bjssu venoms were supplied by Butantan Institute (São Paulo, SP, Brazil). Manganese chloride was from Sigma-Aldrich Co. (St. Louis, MO, USA). The materials for histological analysis and creatine kinase (CK) test were from: Leika (Nublock/Heidelberg, Germany) and Merck (Rio de Janeiro, RJ, Brazil), and Randox® 335 (England, UK), respectively.
Animals
HY-Line W36 male chicks (48 days old) were supplied by Granja Globo Aves Agrovícola Ltda (Mogi Mirim, SP, Brazil). The chicks were housed at 25±3ºC under 12h light/dark cycle and had free access to food and water.
This work (protocol number 623-1) was approved by the Institutional Committee for Ethics in Animal Experimentation (CEEA/IB, UNICAMP) and was carried out within the guidelines of the Brazilian College for Animal Experimentation (COBEA).
Chick Biventer Cervicis Muscle Preparation
Chicks were killed by halothane inhalation and biventer cervicis muscles were removed (15) and mounted under a tension of 1g/0.5cm in a 5ml organ bath containing warmed (37ºC), aerated (95%O2+5%CO2), modified Krebs solution (28) of the following composition (mM, pH 7.5): NaCl, 118.1; KCl, 4.8; CaCl2, 2.5; MgSO4, 1.2; NaHCO3, 12.5; and glucose, 11.1. A bipolar platinum ring electrode was inserted around the tendon within which the nerve trunk supplying the muscle runs. Field stimulation was done using a Grass S48 stimulator (0.1Hz, 0.2ms, 68V). Muscle contractions and contractures were recorded isometrically via a force-displacement transducer (Load Cell BG- 10GM) coupled to a Gould model RS3400 physiograph. Contractures to exogenously applied acetylcholine (ACh, 110µM, for 60s) and potassium chloride (KCl, 20mM, for 180s) were recorded in the absence of field stimulation prior to treatment and at the end of the experiment, as a test for the presence of myotoxic and neurotoxic activities (18). The BCps were allowed to stabilize for at least 20min before the addition of ACh or KCl. Then, Mn2+ (0.66 or 1.6mM), Cdt venom (10µg/ml), Bjssu venom (200µg/ml) or Mn2+ (0.66 or 1.6mM) followed by Cdt venom (10µg/ml) or Bjssu venom (200µg/ml) were applied to the bath. These concentrations were chosen based on previous works (47). Control experiments were carried out using Krebs solution.
Creatine Kinase Activity
To determine CK activity, 100µl samples were withdrawn from the bathing solution 0, 15, 30, 60, 90 and 120min after each treatment, except for Cdt venom and Mn2+ treatments followed by Cdt venom (0, 15, 30 and 60min) (n=518 measurements per treatment every time interval). The withdrawn volume was replaced with an equal volume of Krebs solution. The collected samples were stored for 2h at 4ºC until CK activity (expressed in units/l), which was measured using a commercial kit (CK NAC, Randox® 335, England, UK).
Morphological and Morphometric Analysis
After 120min incubation with Krebs solution, Mn2+, Bjssu venom or Mn2+ + Bjssu venom, or after 70min incubation with Cdt venom or Mn2+ + Cdt venom, BCp were rapidly removed from the bath and fixed in Bouin's fluid for 2448h; then, tissues were washed three times with aqueous ammonia solution followed by dehydration in ethanol series and embedded in Historesin (Leica). Two-µm thick sections, obtained using a Leica RM 2035 microtome (Leica Instruments Gmbh, Nubloch/Heidelberg, Germany), were stained with 0.5% toluidine blue for examination under light microscopy using an Olympus microscope (Olympus Optical Co. Ltd, Tokyo, Japan). The muscle damage extent was qualitatively and quantitatively assessed by counting 70 fibers (normal and damaged) in four non-overlapping areas from a total of 280 fibers per preparation. The following fibers were considered damaged: dark cells; edematous cells, some of which showed sarcolemma rupture; and ghost cells. This procedure was used for all experiments (control and treated preparations, n=56 preparations/treatment).
Statistical Analysis
Each experimental protocol (twitch tension and contracture records for BCp, CK bath content and muscle fibers counting) was repeated at least five times. Results were reported as the mean ± S.E.M. and were used for statistical comparison of data from: Repeated Measures ANOVA followed by post-hoc of Tukey and Kruskal-Wallis, and Dunn's multiple test. Values of p<0.05 were considered significant.
RESULTS
Neuromuscular Effects of Mn2+, and Cdt and Bjssu Venoms on Chick Biventer Cervicis Muscle Preparation
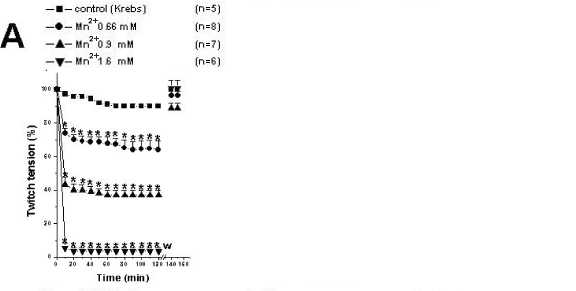
Figure 1A shows that Mn2+ (0.66, 0.9 and 1.6mM) caused a concentration-dependent neuromuscular blockage followed by twitch recovery after washing the preparations with fresh Krebs solution. Control preparations only received Krebs solution (n=5) for 120min. The Mn2+ concentrations 0.66 and 1.6mM produced partial and total neuromuscular blockages, respectively, and were chosen for the posterior tests including preparations pretreated with Mn2+ followed by addition of venoms. There was total recovery after washing the preparations, which indicated that Mn2+ effect is transitory.
Ten µg/ml Cdt venom (Fig. 1B) and 200µg/ml Bjssu venom (Fig. 1C) produced irreversible neuromuscular blockage. The time needed for 50% neuromuscular blockage induced by Cdt and Bjssu venoms was 29.2±3.0min and 48.8±4.5min, respectively. Note that differently from Mn2+ alone, twitch-tension blockage induced by both venoms was irreversible after washing.
In preparations pretreated with 0.66 and 1.6mM Mn2+, Cdt venom-induced blockage was not prevented. However, there was a decrease in time by 50% (after 10min) induced by this venom when the lowest concentration of Mn2+ was used; p<0.05 (Fig. 1B).
Preparations pretreated with 0.66mM Mn2+ partially hindered neuromuscular blockage induced by Bjssu venom after 70min and after washing the preparations with Krebs solution. However, when preparations were pretreated with 1.6Mm Mn2+, partial recovery could only be observed after washing. The recovery of the twitch-tension responses (p<0.05) was 42.6±6.9% (n=8) and 35.6±7.9% (n=5), compared with the initial amplitude for preparations pretreated with Mn2+ (0.66 and 1.6 mM), respectively (Fig 1C). Preparations pretreated with 1.6mM Mn2+ only showed manganese effect, since the effects of both venoms were masked by this ion (Figs. 1B and 1C).
ACh and KCl-induced Contractures in BCp
Independent of the concentration used, Mn2+ ions significantly affected ACh-induced contracture but did not alter KCl-induced contracture when compared with control (Fig. 2A).
Figure 2B shows that Cdt venom did not significantly affect ACh and KCl-induced contractures. In preparations pretreated with 0.66 and 1.6mM Mn2+ followed by Cdt venom addition, blockage of ACh-induced contracture (which was significantly different from that caused by the venom alone) was observed but there was no alteration of the KCl-induced contracture, when compared with that induced by the venom alone.
Figure 2C shows that Bjssu venom practically prevented ACh-induced contracture and significantly altered KCl-induced contracture, when compared with control. Pretreatment of preparations with 1.6mM Mn2+ only, followed by Bjssu venom addition, significantly prevented blockage of ACh-induced contracture, when compared with the venom alone. However, blockage of KCl-induced contracture was not significantly prevented by neither of the Mn2+ concentrations used, when compared with the venom alone.
In control preparations, contracture induced by ACh and KCl was stable after 120min indirect stimulation.
Creatine Kinase Activity
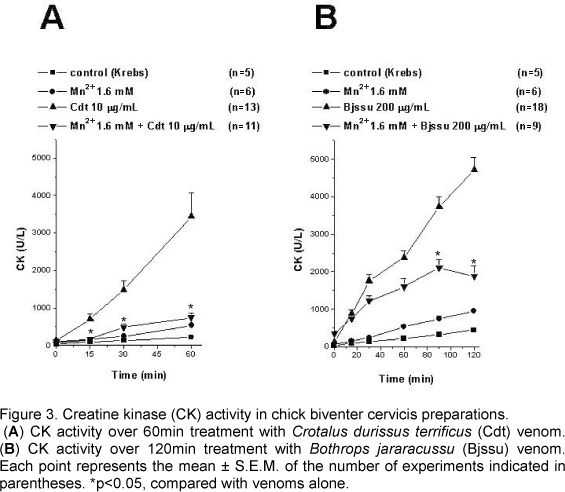
As shown in Figures 3A and 3B, Cdt and Bjssu whole venoms caused concentration and time-dependent increase in the CK release rate in isolated BCp, which reached 3449.7±619.1U/l (n=13) after 60min and 4717.6±322.8U/l (n=18) after 120 min, respectively.
When compared with Cdt venom alone, preincubation with Mn2+ (1.6mM) significantly decreased (p<0.05) CK activity after 15min. At 60min, CK release was 742±131U/l (n=11; Fig. 3A).
When compared with Bjssu venom alone, preincubation with Mn2+ (1.6 mM) significantly decreased (p<0.05) CK activity after 90min. At 120min, CK release was 1870.8±288.2U/l (n=9; Fig. 3B).
Morphological Changes
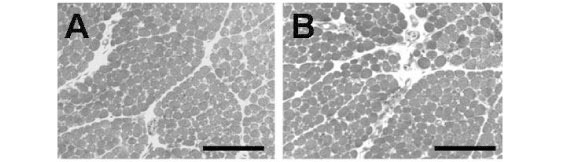
Figure 4A shows chick BCp incubated with Krebs solution alone (control) presenting normal muscle morphology, regular muscle fiber fascicles and uniform intensity of cells staining. The fibers cross-sections showed regular arrangement of the cross-sectional myofibril bundles. The muscles incubated with 1.6mM Mn2+ presented the same characteristic as those incubated with Krebs solution only (Fig. 4B). Changes were mainly related to fibers that were darker and therefore provided poor visualization of cross-sectioned myofibril bundles. The percentages of affected fibers in preparations incubated with Krebs solution (1.1±0.4%) and Mn2+ (2.7±0.8%) were not significantly different.
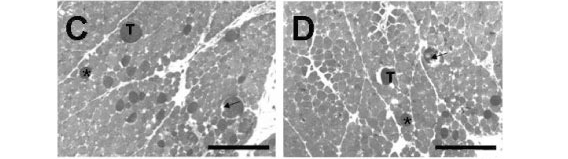
When preparations were incubated with Cdt venom (Fig. 4C), myonecrosis included fascicles disorganization and presence of a higher number of dark cells, several of which were edematous and showed ruptured sarcolemma. The percentage of damaged fibers was 16.4±1.5% (n=6). Pretreating the preparations with 1.6mM Mn2+ followed by Cdt venom led to a 60% decrease in the percentage of damaged fibers (9.9±2.1%, n=5, Fig. 4D), which significantly differed from that of Cdt venom preparations.
When BCp was incubated with Bjssu venom, damaged fibers showed not only higher percentage (31.9±2.4%, n=6), but also higher severity, including the presence of ghost cells (Fig. 4E). Pretreating the preparations with Mn2+ followed by Bjssu venom addition (Fig. 4F) significantly reduced (55%) damaged fibers percentage to 17.5±1.8% (n=6; p<0.05).
DISCUSSION
Snake venoms consist of numerous pharmacologically active components and predominantly act on the peripheral nervous system, where the neuromuscular junction is the favorite target (23, 27).
Neurotoxins from snake venoms that cause neuromuscular paralysis act either presynaptically by blocking acetylcholine (ACh) release or postsynaptically by blocking nicotinic receptors. These different mechanisms of action can be easily differentiated using chick biventer cervicis preparation (18), since a pure presynaptically active neurotoxin can prevent nerve-evoked twitches without affecting the responses to cholinoceptor agonists, KCl-induced depolarization or direct muscle stimulation. On the other hand, postsynaptically active neurotoxins can block the responses to cholinoceptor agonists as well as to indirect stimulation but cannot affect the responses to high K+ concentrations or direct muscle stimulation (18).
In the present work, Mn2+ was used as a pharmacological tool since it acts either presynaptically (4, 28, 30, 32, 35, 36) or postsynaptically, mainly on sarcolemmal membranes (28, 35, 36, 44), in an attempt to better understand the in vitro actions of Cdt and Bjssu venoms related to their neurotoxicity and myotoxicity.
Relevant Aspects considering Myographic Parameters
Manganese ions (Mn2+): Mn2+ acts as a presynaptic agent (4, 30, 32). Besides, it has a dual action on mouse neuromuscular preparations involving Ca2+ channels at the nervous terminal: as an antagonist (inducing neuromuscular blockage) and as an agonist (recovering twitch tension) (35, 36, 44). However, in BCp, twitch recovery was only observed after washing the preparations, which indicated a predominant antagonist action.
Mn2+ significantly blocked acetylcholine responses including those to indirect stimulation, but did not affect the responses to elevated K+ concentrations, similarly to pure postsynaptic neurotoxins (18). However, Mn2+ also acts on the sarcolemmal membrane (28), which was observed in twitch responses to direct stimulation that were partially inhibited by high concentrations of this cation (3mM). Mn2+ ions, like magnesium ions, may stabilize the postsynaptic membrane, thereby depressing its excitability (13). Although in a previous study using mouse preparation, potassium channels, ryanodine and nicotinic receptors were not considered possible targets for Mn2+ (36), the present results indicated that the nicotinic receptor can be a possible target, since BCp is multi-innervated and contains numerous nicotinic receptors throughout the muscle fiber responding with a contracture to cholinergic agonists. However, further studies using different techniques must be carried out to elucidate the actual involvement of Mn2+ with nicotinic receptors.
Cdt venom and manganese: In the present study, Cdt venom prevented nerve-evoked twitches without affecting the response to cholinoceptor agonists, which corroborates the findings about the interference of Cdt venom on ACh release (8, 21) through a presynaptic action.
Mn2+ did not affect the neurotoxicity induced by Cdt venom. Data from literature have shown that, in mouse diaphragm preparations, crotoxin (the major toxin from crude venom) had a triphasic action characterized by immediate transient contraction depression, which can only be observed when the safety factor for transmission is reduced, as in media containing low Ca2+ or high Mg2+ concentrations, followed by augmentation and then paralysis of the muscle (11, 12, 20). Since the first two responses, depression and facilitation, are evident without latency, they may not be due to the action of enzymatic phospholipases but may be consequences of the binding of the toxin to the terminal membrane of motor nerves (12). The facilitation of neuromuscular transmission is manifested as an increase in the quantal contents of end-plate potentials, in the frequency of miniature end-plate potentials (m.e.p.p.s) and in the muscle contractile responses. Electron microscopic examination showed that ultrastructural changes such as decreased synaptic vesicles and the presence of W-shaped indentations in the axolemma were only observed after complete diaphragm paralysis (12). These observations indicate that the immediate cause of toxin-induced blockage is not transmitter depletion but alteration of the excitation-secretion coupling system (12).
The use of BCp showed faster blockage when the preparation was pretreated with Mn2+ (0.66mM) and later with Cdt venom, which can be explained by the Mn2+ antagonizing action. Indeed, considering the recovery parameter, the fact that twitch recovery occurs after washing shows that manganese was incapable of decreasing the complex blockage induced by crotoxin, responsible for the in vivo neurotoxic effect.
In addition, pretreatment with both Mn2+concentrations studied reduced the ACh-induced contractures after complete blockage by Cdt venom. This phenomenon confirmed that the postsynaptic action observed is really caused by manganese, which may exert its action by affecting membrane Ca2+ permeability (28, 35, 36) or nicotinic receptors or both, and not caused by an action of Cdt venom.
Bjssu venom and Mn2+: Differently from Cdt venom, Bjssu venom does not have neurotoxic action in vivo, but induces muscular paralysis in vitro, like BthTX-I (22, 24, 34, 42), its major toxin. Mn2+ was able to hinder the Bjssu venom neurotoxin effect in mouse preparations, probably via Ca2+ channels, preventing the binding of the toxin to the cells (35, 44). The use of manganese and whole venom in BCp (9) was less efficient, probably due to the interference of other constituents present in crude venom, differently from when a pure toxin as BthTX-I was used.
Mn2+ prevented, although partially, Bjssu venom neurotoxicity, since pretreatment with a higher Mn2+ concentration (1.6mM) significantly reduced the blockage of ACh-induced contracture, and both concentrations (0.66 and 1.6mM) reduced twitch tension blockage caused by this venom. Since the blockage induced by Bjssu venom is caused by persistent depolarization on excitable membranes (42) and muscle damage is an important event, our results confirmed that substances that attenuate muscle depolarization and/or muscle damage, as manganese, are potential candidates to prevent muscular paralysis.
Relevant Aspects considering Histological Parameters
Necrosis following snakebite is a very complex phenomenon that may be produced by the action of specific factors and/or by general secondary tissue reactions (29). Therefore, a growing interest on venom components responsible for myonecrosis and their mode of action has arisen during the last decades (17). In the present study, myonecrosis induced by Cdt and Bjssu venoms was evaluated through biochemical and histological tests using BCp, and a correlation between CK activity and tissue damage was observed using pure toxins. However, crude venoms effects need a careful interpretation (29).
Cdt venom and Mn2+: The presence of myotoxic components in snake venoms is expected to reduce the contracture response by the skeletal muscle with the addition of a high concentration of potassium (18), whose response was not observed for Cdt venom in the present experiment (see Fig. 2B), considering the myographic parameter.
However, even for venoms that cause no local myotoxic effect during human accidents, like Cdt venom (2, 26), in vitro myotoxicity has been reported including Crotalus durissus cascavella venom (5). This lack of correlation between in vivo and in vitro effects can be attributed to some factors such as the venom constituents and the host defense response.
Historically, these differences in vivo and in vitro lead to some postulates on myotoxicity, as that by Gutierrez and Ownby (17) on local and systemic skeletal muscle degeneration, corroborating other findings (2, 16, 26). Briefly, Cdt venom has a systemic myotoxic activity characterized by myoglobin release from damaged skeletal muscle into serum and urine (2), although the local damage at the bite site has been minimal or absent (26). Gutierrez and Ownby (17) explained this systemic myotoxicity: "PLA2s that bind to muscle cells in a more selective way are not sequestered by non-specific interactions with other cells and, consequently, are systemically distributed and reach muscle cells in other locations". Therefore, the interpretation of myotoxicity in experiments in vitro is very important since the second factor (the host defense responses) is usually neglected. As experimentally the venom is added to bath containing the isolated preparation without a distribution mechanism, there is a local effect, in which the onset of myonecrosis induced by crotoxin probably results from the progressive loss of the sarcolemma integrity due to hydrolysis of constituent phospholipids (16).
The in vitro myotoxicity induced by Cdt venom, however, was reduced by manganese, both considering morphological change and CK release, probably due to the sarcolemmal action of this cation.
Bjssu venom and Mn2+: Bites by Bjssu snakes frequently produce severe local necrosis, which sometimes requires amputation of the affected member (31), as well as systemic necrosis, whose signal is renal failure (19, 31) sometimes leading to death. As myotoxicity is the main toxic action of this venom, it is interesting to investigate substances that avoid it. Manganese was able to partially counteract this effect and, considering CK parameter, the venom myotoxicity was also reproduced by BthTX-I (40).
The mechanism by which manganese exerts some protection against the extensive damage induced by Bjssu venoms can be related to its action on the sarcolemmal membrane.
CONCLUSIONS
The most important findings obtained in BCp in vitro studies using Mn2+, and Cdt and Bjssu venoms, considering neurotoxic and myotoxic parameters, were:
1) Mn2+ interfered in the contracture induced by ACh nicotinic receptors (Fig. 2A);
2) The main action of Cdt venom (neurotoxicity) was not influenced by Mn2+, which had a predominant but complex presynaptic action.
3) The main action of Bjssu venom (myotoxicity) was positively influenced by the postsynaptic action of Mn2+. As myotoxicity was prevented, more fibers were intact to guarantee the twitch-tension responses, as observed in the recovery of muscular contraction after washing the preparations.
4) This work could lead to coherent interpretation of experimental versus clinical results.
ACKNOWLEDGEMENTS
We thank Marta Beatriz Leonardo, Helymar da Costa Machado, Benedito de Freitas Bueno, and Daniela Carla da Silva Damico for technical assistance.
Received: June 9, 2006
Accepted: September 12, 2006
Abstract published online: October 17, 2006
Full paper published online: May 31, 2007
Conflicts of interest: There is no conflict
Financial source: CNPq and FAEP/UNICAMP.
- 1 ARAÚJO FAA., SANTALÚCIA M., CABRAL RF. Epidemiologia dos acidentes por animais peçonhentos. In: CARDOSO JLC., FRANÇA FOS., WEN FH., MÁLAQUE CMS., HADDAD JÚNIOR V. Eds. Animais peçonhentos no Brasil: biologia, clínica e terapêutica dos acidentes São Paulo: Sarvier, 2003, 6-12.
- 2 AZEVEDO-MARQUES MM., CUPO P., COIMBRA TM., HERING SE., ROSSI M. A., LAURE CJ. Myonecrosis, myoglobinuria and acute renal failure induced by South American rattlesnake (Crotalus durissus terrificus) envenomation in Brazil. Toxicon, 1985, 23, 631-6.
- 3 AZEVEDO-MARQUES MM., HERING SE., CUPO P. Evidence that Crotalus durissus terrificus (South American rattlesnake) envenomation in humans causes myolysis rather than hemolysis. Toxicon, 1987, 25, 1163-8.
- 4 BALNAVE RJ., GAGE PW. The inhibitory effect of manganese on transmitter release at the neuromuscular junction of the toad. Br. J. Pharmacol., 1973, 47, 339-52.
- 5 BEGHINI DG., RODRIGUES-SIMIONI L., TOYAMA MH., NOVELLO JC., CRUZ-HÖFLING MA., MARANGONI S. Neurotoxic and myotoxic actions of crotoxin-like and Crotalus durissus cascavella whole venom in the chick biventer cervicis preparation. Toxicon, 2004, 43, 255-61.
- 6 BRASIL. Ministério da Saúde. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos Brasília, 2001, 112.
- 7 BRAZIL V. La défense contre I' ophidisme São Paulo: Pocai & Weiss, 1911. 181p.
- 8 BRAZIL OV., EXCELL BJ. Action of crotoxin and crotactin from the venom of Crotalus durissus terrificus (South American rattlesnake) on the frog neuromuscular junction. J. Physiol, 1971, 212, 34-35.
- 9 BUENO LGF., LEITE GB., OSHIMA-FRANCO Y., RODRIGUES-SIMIONI L. Effects of manganese (Mn2+) on the neuromuscular blockade caused by Crotalus durissus terrificus and Bothrops jararacussu venoms in chick biventer cervicis preparations. J. Venom. Anim. Toxins. incl. Trop. Dis., 2004, 10, 551.
- 10 CAMILLO MA., ARRUDA PAES PC., TRONCONE LR., ROGERO JR. Gyroxin fails to modify in vitro release of labelled dopamine and acetylcholine from rat and mouse striatal tissue. Toxicon, 2001, 39, 843-53.
- 11 CHANG CC., LEE JD. Crotoxin, the neurotoxin of South American rattlesnake venom, is a presynaptic toxin acting like -bungarotoxin. Naunyn-Schmiedeberg's. Arch. Pharmacol., 1977, 296, 159-68.
- 12 CHANG CC., LEE JD., EAKER D., FOHLMAN J. The presynaptic neuromuscular blocking action of taipoxin. A comparison with beta-bungarotoxin and crotoxin. Toxicon, 1977, 15, 571-6.
- 13 DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J. Physiol., 1954, 124, 370-84.
- 14 FLETCHER JE., YANG CC., ROSENBERG P. Basic phospholipase A2 from Naja nigricollis snake venom: phospholipid hydrolysis and effects on electrical and contractile activity of the rat heart. Toxicol. Appl. Pharmacol., 1982, 66, 39-54.
- 15 GINSBORG BL., WARRINER J. Isolated chick biventer cervicis-nerve muscle preparation. Br. J. Pharmacol., 1960, 15, 410-11.
- 16 GOPALAKRISNAKONE P., HAWGOOD BJ. Morphological changes induced by crotoxin in murine nerve and neuromuscular junction. Toxicon, 1984, 22, 791-804.
- 17 GUTIERREZ JM., OWNBY CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon, 2003, 42, 915-31.
- 18 HARVEY AL., BARFARAZ A., THOMSON E., FAIZ A., PRESTON S., HARRIS JB. Screening of snake venoms for neurotoxic and myotoxic effects using simple in vitro preparations from rodents and chicks. Toxicon, 1994, 32, 257-65.
- 19 HAVT A., FONTELES MC., MONTEIRO HS. The renal effects of Bothrops jararacussu venom and the role of PLA(2) and PAF blockers. Toxicon, 2001, 39, 1841-6.
- 20 HAWGOOD BJ. How similar are the actions of crotoxin and beta-bungarotoxin? Acta. Physiol. Pharmacol. Latinoam., 1989, 39, 397-406.
- 21 HAWGOOD BJ., SMITH JW. The mode of action at the mouse neuromuscular junction of the phospholipase A-crotapotin complex isolated from venom of the South American rattlesnake. Br. J. Pharmacol., 1977, 61, 597-606.
- 22 HELUANY NF., HOMSI-BRANDEBURGO MI., GIGLIO JR., PRADO-FRANCESCHI J., RODRIGUES-SIMIONI L. Effects induced by bothropstoxin, a component from Bothrops jararacussu snake venom, on mouse and chick muscle preparations. Toxicon, 1992, 30, 1203-10.
- 23 HODGSON WC., WICKRAMARATNA JC. In vitro neuromuscular activity of snake venoms. Clin. Exp. Pharmacol. Physiol., 2002, 29, 807-14.
- 24 HOMSI-BRANDEBURGO MI., QUEIROZ LS., SANTO-NETO H., RODRIGUES-SIMIONI L., GIGLIO JR. Fractionation of Bothrops jararacussu snake venom: partial chemical characterization and biological activity of bothropstoxin. Toxicon, 1988, 26, 615-27.
- 25 JORGE MT., RIBEIRO LA. Incoagulabilidade sanguínea no acidente crotálico. Rev. Soc. Bras. Med. Trop., 1988, 21, 121.
- 26 JORGE MT., RIBEIRO LA. Acidentes por serpentes peçonhentas do Brasil. Rev. Ass. Med. Bras., 1990, 36, 66-77.
- 27 LEWIS RL., GUTMANN L. Snake venoms and the neuromuscular junction. Semin. Neurol., 2004, 24, 175-9.
- 28 LIN-SHIAU SY., FU W.-M. Effects of divalent cations on neuromuscular transmission in the chick. Eur. J. Pharmacol., 1980, 64, 259-69.
- 29 MEBS D., EHRENFELD M., SAMEJIMA Y. Local necrotizing effect of snake venoms on skin and muscle: relationship to serum creatine kinase. Toxicon, 1983, 21, 393-404.
- 30 MEIRI U., RAHAMIMOFF R. Neuromuscular transmission: inhibition by manganese ions. Science, 1972, 176, 308-9.
- 31 MILANI JR R., JORGE MT., FERRAZ DE CAMPOS FP., MARTINS FP., BOUSSO A., CARDOSO JLC., RIBEIRO LA., FAN HW., FRANÇA FOS., SANO-MARTINS IS., CARDOSO D., FERNANDEZ ICOF., FERNANDES JC., ALDRED VL., SANDOVAL MP., PUORTO G., THEAKSTON RDG., WARRELL DA. Snake bites by the jararacuçu (Bothrops jararacussu): clinicopathological studies of 29 proven cases in São Paulo State, Brazil. Q. J. Med., 1997, 90, 323-34.
- 32 NACHSHEN DA. Selectivity of the Ca++ binding site in synaptosome Ca++ channels: inhibition of Ca++ influx by multivalent metal cations. J. Gen. Physiol., 1984, 83, 941-67.
- 33 NAHAS L., KAMIGUTI AS., BARROS MAR. Thrombin-like and factor X-activator components of Bothrops snake venom. Thromb. Haemost., 1979, 41, 314-28.
- 34 OSHIMA-FRANCO Y., ALVES CMV., ANDRÉO FILHO N., GERENUTTI M., CINTRA ACO., LEITE GB., RODRIGUES-SIMIONI L., SILVA MG. Neutralization of the neuromuscular activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom, by a hidroalcoholic extract of Casearia sylvestris sw (Guaçatonga). J. Venom. Anim. Toxins. incl. Trop. Dis., 2005, 11, 465-78.
- 35 OSHIMA-FRANCO Y., LEITE GB., DAL BELO CA., HYSLOP S., PRADO-FRANCESCHI J., CINTRA ACO., GIGLIO JR., CRUZ-HÖFLING MA., RODRIGUES-SIMIONI L. The presynaptic activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom. Basic. Clin. Pharmacol. Toxicol., 2004, 95, 175-82.
- 36 OSHIMA-FRANCO Y., LEITE GB., DAL BELO CA., RODRIGUES-SIMIONI L. Effects of manganese íons in the neuromuscular junction. Braz. J. Toxicol., 2005, 18, 17-26.
- 37 OSHIMA-FRANCO Y., LEITE GB., VALÉRIO A., HYSLOP S., ANDRIÃO-ESCARSO S., GIGLIO JR., PRADO-FRANCESCHI J., CRUZ-HÖFLING MA., RODRIGUES-SIMIONI L. Rabbit antivenom efficacy against myotoxic and neurotoxic activities of Bothrops jararacussu venom and bothropstoxin-I. J. Venom. Anim. Toxins., 2002, 8, 226-43.
- 38 OSHIMA-FRANCO Y., PRADO-FRANCESCHI J., CRUZ-HÖFLING MA., RODRIGUES-SIMIONI L. The myotoxic action of crotoxin and bothropstoxin-I on the mouse extensor digitorum longus (edl) preparation. J. Venom. Anim. Toxins., 1997, 3, 162.
- 39 QUEIROZ LS., SANTO NETO H., RODRIGUES-SIMIONI L., PRADO-FRANCESCHI J. Muscle necrosis and regeneration after envenomation by Bothrops jararacusssu snake venom. Toxicon, 1984, 22, 339-46.
- 40 RANDAZZO-MOURA P., LEITE GB., SILVA GH., PAFFARO JUNIOR VA., CINTRA ACO., CRUZ-HÖFLING MA., RODRIGUES-SIMIONI L., OSHIMA-FRANCO Y. Study of myotoxicity of bothropstoxin-I (BthTX-I) using manganese (Mn2+) in mouse phrenic nerve-diaphragm (PND) and extensor digitorum longus (EDL) preparations. Braz. J. Morphol. Sci, 2006, 23, 171-84.
- 41 RODRIGUES VM., MARCUSSI S., CAMBRAIA RS., DE ARAÚJO AL., MALTA-NETO NR., HAMAGUCHI A., FERRO EA., HOMSI-BRANDEBURGO MI., GIGLIO JR., SOARES AM. Bactericidal and neurotoxic activities of two myotoxic phospholipases A2 from Bothrops neuwiedi pauloensis snake venom. Toxicon, 2004, 44, 305-14.
- 42 RODRIGUES-SIMIONI L., BORGESE N., CECCARELLI B. The effects of Bothrops jararacussu venom and its components on frog nerve-muscle preparation. Neuroscience, 1983, 10, 475-89.
- 43 SANCHES EF., FREITAS TV., FERREIRA-ALVES DL., VELARDE DT., DINIZ MR., CORDEIRO MN., AGOSTINI-COTTA G., DINIZ CR. Biological activities of venoms from South American snakes. Toxicon, 1992, 30, 95-103.
- 44 SOARES AM., OSHIMA-FRANCO Y., VIEIRA CA., LEITE GB., FLETCHER J E., JIANG MS., CINTRA ACO., GIGLIO JR., RODRIGUES-SIMIONI L. Mn+2 ions reduce the enzymatic and pharmacological activities of bothropstoxin-I, a myotoxic Lys 49 phospholipase A2 homologue from Bothrops jararacussu snake venom. Int. J. Biochem. Cell. Biol., 2002, 34, 668-77.
- 45 VITAL BRAZIL O. Pharmacology of crystalline crotoxin. II. Neuromuscular blocking action. Mem. Inst. Butantan Simp. Internac.,1966, 33, 981-92.
- 46 VITAL BRAZIL O. Peçonhas. In: CORBETT CE. Eds. Farmacodinâmica Rio de Janeiro: Guanabara Koogan, 1982: 1044-74.
- 47 ZAMUNÉR SR., CRUZ-HÖFLING MA., CORRADO AP., HYSLOP S., RODRIGUES-SIMIONI L. Comparison of the neurotoxic and myotoxic effects of Brazilian Bothrops venoms and their neutralization by commercial antivenom. Toxicon, 2004, 44, 259-71.
Publication Dates
-
Publication in this collection
13 June 2007 -
Date of issue
2007
History
-
Received
09 June 2006 -
Accepted
12 Sept 2006