Abstract
Scorpion envenomation remains a real health problem in many countries. In scorpionism cases, it is often recommended that patients be treated with species-specific antivenom. Androctonus crassicauda venom has been used as antigen for antivenom production in Turkey, where this antivenom, called Turkish antivenom, has also been effective in the treatment of envenomation caused by species other than A. crassicauda. The present study aimed at determining the paraspecific effects and potency of the Turkish antivenom against Mesobuthus gibbosus (Brullé, 1832) venom. To assess the venom toxicity and the antivenom efficacy, we determined the Minimum Lethal Dose (MLD) and the Minimum Effective Dose (MED) instead of LD50 and ED50, respectively. Androctonus crassicauda antivenom was capable of neutralizing M. gibbosus venom (20 MLD). This was the first study indicating that A. crassicauda antivenom can be used for the treatment of Mesobuthus gibbosus stings, especially in Aegean Region, Turkey.
scorpion; antivenom; Androctonus crassicauda; Mesobuthus gibbosus; paraspecific activity
ORIGINAL PAPER
Determination of potency and paraspecific effects of Androctonus crassicauda (Olivier, 1807) antivenom against Mesobuthus gibbosus (Brullé, 1832) venom (Scorpiones: Buthidae)
Ozkan O.I, II; Adiguzel S.I; Kar S.II; Yakistiran S.I; Cesaretli Y.I; Karaer K. Z.II
IRefik Saydam Hygiene Center, Ankara, Turkey
IIDepartment of Entomology and Protozoology, Faculty of Veterinary Medicine, Ankara University, Ankara, Turkey
Correspondence to Correspondence to: Ozcan Ozkan Refik Saydam Hygiene Center Ankara, Turkey Phone: +90 312 458 2372 Fax: +90 312 458 2395 Email: ozcanozkan_62@hotmail.com
ABSTRACT
Scorpion envenomation remains a real health problem in many countries. In scorpionism cases, it is often recommended that patients be treated with species-specific antivenom. Androctonus crassicauda venom has been used as antigen for antivenom production in Turkey, where this antivenom, called Turkish antivenom, has also been effective in the treatment of envenomation caused by species other than A. crassicauda. The present study aimed at determining the paraspecific effects and potency of the Turkish antivenom against Mesobuthus gibbosus (Brullé, 1832) venom. To assess the venom toxicity and the antivenom efficacy, we determined the Minimum Lethal Dose (MLD) and the Minimum Effective Dose (MED) instead of LD50 and ED50, respectively. Androctonus crassicauda antivenom was capable of neutralizing M. gibbosus venom (20 MLD). This was the first study indicating that A. crassicauda antivenom can be used for the treatment of Mesobuthus gibbosus stings, especially in Aegean Region, Turkey.
Key words: scorpion, antivenom, Androctonus crassicauda, Mesobuthus gibbosus, paraspecific activity.
INTRODUCTION
Envenomation by arachnids causes significant injuries all over the world. Scorpion sting is the most important type of arachnid envenomation resulting in adult morbidity and pediatric mortality. Scorpionism remains a real health problem in developing countries, especially in tropical and subtropical regions including urban areas (3, 5, 11, 13).
Scorpion venom contains short polypeptide neurotoxins consisting of simple, low-molecular-weight proteins of lethal and paralytic effects (4, 14, 20). Lethal scorpions mostly belong to the family Buthidae (9, 12, 14), among which, species of the genera Androctonus, Leiurus, and Mesobuthus are the main responsible for envenomation in Turkey (10, 14).
It is recommended that patients envenomed by scorpion stings be treated with species-specific antivenom (1). The scorpion-antivenom treatment introduced in 1909 is still the only method of therapy (3, 16). In Turkey, scorpion antivenom has been produced by the Refik Saydam Hygiene Center (RSHC) since 1942 (4, 14, 16, 17). Venom obtained through maceration of telsons of A. crassicauda scorpions has been used as antigen for antivenom. This antivenom, called Turkish antivenom, has also been used against other scorpion species (1, 10, 17, 19).
The present study aimed at determining the paraspecific effects and potency of A. crassicauda antivenom against Mesobuthus gibbosus venom.
MATERIALS AND METHODS
Animals
Scorpions: Androctonus crassicauda specimens were collected from Southeastern Anatolia region (Sanliurfa) and M. gibbosus (Figure1) specimens were collected from Aegean region (Mugla), Turkey.
Experimental animals: Healthy female Swiss albino mice, weighed 25±1g, were used for determination of the Minimum Lethal Dose (MLD) and Minimum Effective Dose (MED). They were bred at the Animal Facility Center, Faculty of Medicine, Ankara University, Ankara, Turkey. Animals were kept in an experimental room under room temperature (22±2°C) and 60±10% humidity and were fed with commercial rodent pellets ad libitum throughout the experiment.
Venom
Venom was obtained from mature A. crassicauda and M. gibbosus scorpions through electrical stimulation of their telson. The venom was mixed with sterile double distilled water and centrifuged at 15,000rpm for 15min at 4°C. The supernatant was immediately lyophilized and stored at -20°C until use.
Antivenom
Antivenom was prepared from a pool of hyperimmune sera obtained from horses immunized with A. crassicauda venom.
Determination of Minimum Lethal Doses
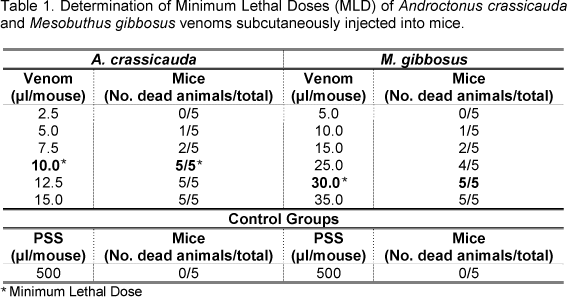
The Minimum Lethal Dose (MLD) was determined to assess the lethal toxicity of A. crasssicauda and M. gibbosus venoms. Groups of five mice were subcutaneously injected with venom diluted in physiologic saline solution (PSS; 0.9% sodium chloride solution) at doses ranging from 5.0 to 35.0µl/mouse (M. gibbosus venom) and from 2.5 to 15.0µl/mouse (A. crasssicauda venom). The volume of venom injected was kept constant at 500µl/mouse. Control group was injected with PSS only. Following treatment with venom solution, animals were monitored for 48 hours and the number of dead animals was recorded at the end of the experiment. The dose that killed 100 percent animals was considered the MLD.
Serum Neutralization Tests
A solution of A. crassicauda venom (40 MLD) and a solution of M. gibbosus venom (20 MLD), both diluted in PSS to a 2500µl volume, were prepared. Then, each solution was mixed with hyperimmune horse sera and incubated for one hour at 37°C for antigen-antibody reaction. Groups of six mice were subcutaneously injected with the solutions. The injection volume was kept constant at 500µl/mouse. Control groups were injected with PSS only. The number of living mice was recorded after 48h.
Determination of Minimum Effective Doses
Androctonus crassicauda antivenom was prepared at doses ranging from 100µl to 1000µl. The final volume was made up to 5000µl with PSS and the solutions were incubated at 37ºC for 60 min. Then, 500µl of each solution was subcutaneously injected into groups of sixmice previously injected with 20 MLD of M. gibbosus venom or 40 MLD of A. crassicauda venom. Positive control groups were only injected with 20 MLD of M. gibbosus venom and 40 MLD of A. crassicauda venom diluted in PSS. After administration, animals were monitored for 48 hours and the number of living animals was recorded. The antivenom doses that prevented 100% deaths in the groups were considered the minimum effective doses (MED).
RESULTS
The MLD of A. crassicauda and M. gibbosus venoms were 10µl/mouse and 30µl/mouse, respectively (Table 1).
To assess the antivenom potency, increasing doses of hyperimmune sera were used (100, 400, 700, 1000µl) while the amounts of A. crassicauda and M. gibbosus venoms were kept constant (40 and 20 MLD, respectively). Changes in the neutralization capacity of the hyperimmune horse sera against A. crassicauda and M. gibbosus venoms were shown in Table 2. The minimum effective dose of the antivenom against both A. crassicauda and M. gibbosus venoms was 400µl. All control mice died. The antivenom dose needed to neutralize M. gibbosus venom was 20 times higher than the MLD of a mouse.
DISCUSSION
Out of the approximately 1500 scorpion species described so far, 50 present venoms that are dangerous for humans. Most of these species belong to the genera Buthus, Parabuthus, Mesobuthus, Tityus, Leiurus, Androctonus, and Centruroides of the Buthidae family (5, 8, 12, 14, 16). The most common and lethal species are: Tityus serrulatus and T. bahiensis in South America, especially in Brazil; Centruroides suffusus, C. limpidus, C. sculpturatus in Mexico; Leiurus quinquestriatus, Androctonus crassicauda, A. mauretanicus, A. australis, A. amoreuxi, and Buthus occitanus in Middle East and North Africa; Parabuthus granulatus and P. transvaalicus in South Africa; Mesobuthus tamulus and Palamneus swammerdami in India (3, 6, 16).
Scorpions as well as human envenomation cases are common in Turkey due to its geographical location, climate and socioeconomic structure (11, 14). Therefore, scorpion envenomation is an important health problem in several regions of the country, especially in South-Eastern Anatolia (2, 11, 15, 17). Important scorpions threatening public health in Turkey are A. crassicauda, L. quinquestriatus, M. gibbosus and M. eupeus (2, 10, 12, 14, 15).
The toxicity of scorpion venoms depends on several factors including the genus, species, age, structure, physiological and feeding states of the animal; the amount of venom injected; the climate of the region; and the time of animal collection (8, 11, 14).
Krifi et al. (7) reported that the difficulties in standardizing the venom quality and the LD50 determination are in part related to: the species used; the animals' geographical origin, age and body weight; the season and procedures of venom extraction; the number of specimens milked; the breeding conditions; the venom injection route; and the LD50 determination method.
The above-mentioned parameters must always be specified for the determination of any venom LD50 or antivenom potency. As the subcutaneous route provides the highest LD50 value and is the most frequent route of accidental scorpion envenomation, it should be used to estimate the antivenom potency (7). The venom variability must also be considered during the production of antivenom, which has to be capable of neutralizing the toxic effects of all kinds of venoms from a specific scorpion species (7, 16).
Theakston et al. (16) carried out a review of the potency assays used in several countries and noticed that the most acceptable test is the standard murine lethality assay (determination of LD50 of venom and ED50 of antivenom). However, considerable variations were found such as the way the test was carried out, the volumes injected, the routes used for injection of venom/antivenom mixtures, the LD50 value, the mouse strain used and the animals' weight range.
The antivenom activities should be expressed according to national or regional standards and a toxin neutralization unit must be used.
In recent years, in vivo tests other than mouse lethality assay have been vastly used (16). In the present study, MLD and MED were determined instead of LD50 and ED50.
The role of antivenom on the treatment of scorpion stings remains controversial (16). Bücherl (4) emphasized that the antivenom must be administered to the patient at an early stage since it can be much more effective if given within two hours after the sting. Alexander (1) recommended that scorpion-stung patients be treated with species-specific antivenom. In case the latter is not available, antivenom prepared against A. crassicauda venom could be used to treat envenomation caused by Old World scorpions and antivenom prepared against Centruroides sculturatus venom could be used to treat envenomation by New World scorpion species (1).
Whittemore et al. (19) reported that the Turkish antivenom (antivenom produced against A. crassicauda venom at Refik Saydam Hygiene Center) was more effective than homologous antivenom in neutralizing A. australis venom in Algeria and had the same neutralization capacity as homologous antivenoms against B. occinatus venom in South Europe and North Africa, T. serrulatus and T. bahiensis venoms in South America, and L. quinquestriatus venom in Anatolia (19).
Tulga (18) observed that antivenom prepared against the Turkish A. crassicauda venom was capable of neutralizing L. quinquestriatus venom from Israel. However, Kasapo lu (6) stated that further studies are needed about the potency and paraspecific effects of the Turkish antivenom on the treatment of envenomation by M. gibbosus.
lu (6) stated that further studies are needed about the potency and paraspecific effects of the Turkish antivenom on the treatment of envenomation by M. gibbosus.
Altinkaynak et al. (2) recommended that the Turkish antivenom be used to treat M. gibbosus envenomation cases since it was effective in 91.6% of the cases studied. The antivenom was not effective in only two cases (9 and 13-month-old infants) because the patients had received late first aid and hospitalization and the sting site was on the neck (2).
Soker and Haspolat (15) verified that the Turkish antivenom was effective in 90.3% of all scorpionism cases in South-Eastern Anatolia.
In the present investigation, A. crassicauda antivenom neutralized both A. crassicauda and M. gibbosus venoms. The Turkish antivenom was effective against M. gibbosus venom. Therefore, antivenom produced against A. crassicauda venom can be used in the treatment of envenomation by M. gibbosus, especially in Aegean region of Turkey.
ACKNOWLEDGMENTS
We wish to thank Karina Luiz Chamma for her valuable comments on this manuscript. The present study was dedicated to all members of JVATiTD staff.
Received: March 15, 2006
Accepted: May 16, 2006
Abstract published online: May 17, 2006
Full paper published online: May 31, 2007
Conflicts of interest: There is no conflict.
- 1 ALEXANDER JO. Scorpion sting. In: Arthropods and human skin. Berlin: Springer-Verlag, 1984, 199-207.
- 2 ALTINKAYNAK S., ERTEKIN V., ALP H. Scorpion envenomation in children. Turk. Arch. Pediatr., 2002, 37, 48-54.
- 3 BALOZET L. Scorpionism in the Old World. In: BÜCHERL W., BUCKLEY E. Eds., Venomous animals and their venoms. Venomous invertebrates. New York: Academic Press. 1971, 56, 349-71.
- 4 BÜCHERL W. Classification, biology and venom extraction of scorpion In: BÜCHERL W., BUCKLEY E. Eds., Venomous Animals and their Venoms. Venomous Invertebrates. New York: Academic Express. 1971, 3, 317-47.
- 5 ISBISTER GK., GRAUDINS A., WHITE J., WARRELL D. Antivenom treatment in Arachnidism. J. Toxicol. Clin. Toxicol., 2003, 41, 291-300.
- 6 KASAPOGLU ND. What is the importance of using antivenom in scorpionism cases in the Region of Turkey? 0zmir Devlet Hastanesi T1p Dergisi, 1991, 29, 121-26.
- 7 KRIFI MN., MARRAKCHI N., EL AYEB M., DELLAGI K. Effect of some variables on the in vivo determination of scorpion and viper venom toxicities. Biologicals, 1998, 26, 277-88.
- 8 MULLEN GR., STOCKWELL SA. Scorpions (Scorpiones) In: Mullen G, Durden G (eds.), Medical and Veterinary Entomology, 2002, 20, 411-23.
- 9 MOHAMED AH., DARWISH MA., HANI-AYOBE M. Immunological studies on scorpion (B. quinquestriatus) antivenin. Toxicon, 1975, 13, 67-8.
- 10 OYTUN S. Tibbi Entomoloji. A.U Faculty of Medicine, 3. Press, Ankara. 1969, 36-45.
- 11 OZKAN O., FILAZI A. The determination of acute lethal dose-50 (LD50) levels of venom in mice, obtained by different methods from scorpions, Androctonus crassicauda (Oliver 1807). Acta Parasitol. Turcica, 2004, 28, 50-3.
- 12 ZKAN O., KARAER Z. Scorpions in Turkey. Turk. Hij. Tecr. Biyol. Derg., 2004, 61, 50-3.
- 13 OZKAN O., KARAER Z. Body structures of scorpions. Acta Parasitol. Turcica. 2004, 28, 54-8.
- 14 OZKAN O., KAT I. Mesobuthus eupeus scorpionism in Sanliurfa region of Turkey. J. Venom. Anim. Toxins incl. Trop. Dis., 2005, 11, 479-91.
- 15 SOKER M., HASPOLAT K. Scorpion sting in children. Çocuk Sagligi ve HastalklarDergisi, 2000, 43, 43-51.
- 16 THEAKSTON RD., WARRELL DA., GRIFFITHS E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon, 2003, 41, 541-57.
- 17 TULGA T. Cross-reactions between anti-scorpion (Buthus quinquestriatus) and anti-scorpion (Prionurus crassicauda) sera. Turk. Hij. Tecr. Biyol. Derg., 1960, 20, 191-203.
- 18 TULGA T. Scorpions found in Turkey and paraspecific action of an antivenin produced with the venom of the species Androctonus crassicauda Turk. Hij. Tecr. Biyol. Derg., 1964, 24, 146-55.
- 19 WHITTEMORE FW., KEEGAN HL., BOROWITZ JL. Studies of scorpion antivenins. 1. Paraspecificity. Bull. World Health Organ., 1961, 25, 185-8.
- 20 ZLOTKIN E., MIRANDA F., ROCHAT H. Chemistry and pharmacology of Buthinae scorpion venoms. In: BETTINI, S. Ed. Handbook of experimental pharmacology-arthropod venoms. Berlin: Spinger-Verlag, 1978, 48, 317-29.
Publication Dates
-
Publication in this collection
13 June 2007 -
Date of issue
2007
History
-
Accepted
16 May 2006 -
Received
15 Mar 2006