Abstract
Venoms from snakes of the Bothrops genus are proteolytic, coagulant, hemorrhagic and nephrotoxic, causing edema, necrosis, hemorrhage and intense pain at the bite site, besides systemic alterations. Many adjuvants have been added to the venom used in the sensitization of antiserum-producer animals to increase antigenic induction and reduce the envenomation pathological effects. Gamma radiation from 60Co has been used as an attenuating agent of the venoms toxic properties. The main objective was to study, comparatively, clinical and laboratory aspects of goats inoculated with bothropic (Bothrops jararaca) venom, natural and irradiated from a 60Co source. Twelve goats were divided into two groups of six animals: GINV, inoculated with 0.5mg/kg of natural venom; and GIIV, inoculated with 0.5mg/kg of irradiated venom. Blood samples were collected immediately before and one, two, seven, and thirty days after venom injection. Local lesions were daily evaluated. The following exams were carried out: blood tests; biochemical tests of urea, creatinine, creatine kinase (CK), aspartate amino-transferase (AST) and alanine amino-transferase (ALT); clotting time; platelets count; and total serum immunoglobulin measurement. In the conditions of the present experiment, irradiated venom was less aggressive and more immunogenic than natural venom.
radiation; Bothrops jararaca; cobalt; goats
ORIGINAL PAPER
Biochemical and hematological study of goats envenomed with natural and 60Co-irradiated bothropic venom
Lucas de Oliveira P. C.I; Sakate M.II, III; Madruga R. A.I ; Barbosa N. P.U.I
IVeterinary Hospital, Uberaba School of Veterinary Medicine, UNIUBE, Uberaba, Minas Gerais State, Brazil
IIVeterinary Clinics, School of Veterinary Medicine and Animal Husbandry, UNESP, Botucatu, São Paulo State, Brazil
IIICenter for the Study of Venoms and Venomous Animals, CEVAP, UNESP, São Paulo State, Brazil
Correspondence to Correspondence to: Pedro Carlos Lucas de Oliveira Rua Bahia, 870 38050-130, Uberaba, Minas Gerais, Brasil Email: pedrolucaso@uol.com.br
ABSTRACT
Venoms from snakes of the Bothrops genus are proteolytic, coagulant, hemorrhagic and nephrotoxic, causing edema, necrosis, hemorrhage and intense pain at the bite site, besides systemic alterations. Many adjuvants have been added to the venom used in the sensitization of antiserum-producer animals to increase antigenic induction and reduce the envenomation pathological effects. Gamma radiation from 60Co has been used as an attenuating agent of the venoms toxic properties. The main objective was to study, comparatively, clinical and laboratory aspects of goats inoculated with bothropic (Bothrops jararaca) venom, natural and irradiated from a 60Co source. Twelve goats were divided into two groups of six animals: GINV, inoculated with 0.5mg/kg of natural venom; and GIIV, inoculated with 0.5mg/kg of irradiated venom. Blood samples were collected immediately before and one, two, seven, and thirty days after venom injection. Local lesions were daily evaluated. The following exams were carried out: blood tests; biochemical tests of urea, creatinine, creatine kinase (CK), aspartate amino-transferase (AST) and alanine amino-transferase (ALT); clotting time; platelets count; and total serum immunoglobulin measurement. In the conditions of the present experiment, irradiated venom was less aggressive and more immunogenic than natural venom.
Keys words: radiation, Bothrops jararaca, cobalt, goats.
INTRODUCTION
Envenomation by snakes, especially accidents caused by Bothrops jararaca, is common and of great importance for the Brazilian medicine and veterinary medicine. About 20 thousands ophidian accidents occur in Brazil every year, leading to 100 annual deaths (7, 30, 39, 42).
Venoms from snakes of the Bothrops genus present coagulant, proteolytic and hemorrhagic actions (39, 48) but many of their characteristics are not completely known yet.
The use of animals has been indispensable for anti-ophidic serum production; however, they may be injured by the venoms toxic effects (2). Therefore, there is the necessity to research into mechanisms that would reduce the venoms effects maintaining the capability to induce an adequate immune response for serum production. Many adjuvants have been added to venoms used in the sensitization of serum-producer animals, intending to increase the antigenic stimulus and reduce the envenomation undesirable effects (28).
Gamma radiation from 60Co has been used as an attenuating agent of the venoms toxic properties (14, 37, 38). Irradiation of biological materials from a 60Co source is the most used method because it does not require heating the venom which is susceptible to high temperatures (18).
Just few Brazilian governmental or private institutions dedicate to producing antivenom used in accidents by venomous animals. For such production, those institutions have to maintain large farms to raise hundreds of serum-producer horses. The raising of such animals is expensive because of their size and demanding physical characteristics (55). Goats are little-demanding animals that survive in wild environments such as the arid Brazilian northeast region (40) and thus can be inexpensively raised in areas not considered good enough for farming or even for bovine raising (40).
The objective of the present paper was to study, comparatively, clinical and laboratory aspects of goats inoculated with bothropic (Bothrops jararaca) venom, natural and irradiated from 60Co.
MATERIALS AND METHODS
Experimental Animals
Twelve goats (two males and ten females) of no specific breed from Miguelópolis region, São Paulo State, Brazil, were used. They were older than one year old, weighed between 16 and 23kg, and presented good clinical conditions, according to Radostits et al. apud Gay (16). Four weeks before the experiment, animals received 50ml 1% ivermectin (Merck-Sharp-Dohm, IVOMEC®), in a single 0.2mg/kg dose (44), and were kept isolated and under clinical observation. These animals had not received any vaccination and had not been kept isolated before.
Experimental Conditions
The animals were kept in collective rooms (six animals in each room) at the Uberaba Veterinary Hospital, Uberaba city, Minas Gerais State, Brazil. Rooms were about 16m2 of concrete pavement covered with sawdust (which was changed every day) and masonry walls painted with latex paint; they were naturally illuminated during the day and by electric light at night. Each animal received water ad libitum through a masonry water trough and was fed with triturated elephant grass from the farm of the Associated College of Uberaba (FAZU) and 200g of concentrated Purina, 25kg, CAPRINOTECH®, proper to goats, daily.
Venom Obtainment
Venom (200mg) was collected from B. jararaca specimens from the Center for the Study of Venoms and Venomous Animals, CEVAP-UNESP, Botucatu, São Paulo State, Brazil. After lyophilization, half of the venom collected (100mg) was conserved and another 100mg was used in the irradiation process.
Venom Irradiation
Venom (100mg) was diluted with saline solution (NaCl, 150mM, pH 3.0), subjected to irradiation from a 60Co source (2000Gy at a dose rate of 5.7KGy/hour) and kept under refrigeration, according to Rogero and Nascimento (45). Such procedures were carried out by the Institute of Energetic and Nuclear Research (IPEN).
Animals Envenomation
Goats were divided into two groups: one group inoculated with natural venom (GINV) and another group inoculated with irradiated venom (GIIV). Both groups received B. jararaca venom at the dose of 0.5mg/kg, as recommended by Araújo et al. (3). The venom was diluted in 2mg/ml saline solution and administered by deep intramuscular injection into the semimembranosus muscle.
Blood Collection
Blood samples were collected from the jugular vein in ethylenediamine tetraacetic acid (EDTA) potassium salt for blood analysis, and without anticoagulant for biochemical evaluation, immediately before and one, two, seven and thirty days after the venom injection.
Physical Examination
The animals were examined immediately before and daily after the venom injection, according to the protocol recommended by Radostits et al. apud Gay (16) . While persisting, lesions at the inoculation site were examined for the presence of edema, redness, wound, hemorrhage and necrosis; temperature and pain were assessed through palpation. To evaluate the intensity of local alterations, values from zero to four were attributed, where zero represented absence of lesion and four, the most intense lesion.
Laboratory Tests
For blood analysis, a CELM counter (CELM CC530®) was used for erythrocyte and total leukocyte counts and serum hemoglobin measurement; a centrifuge at 11000rpm during five minutes (Fanen, Centrimicro®) was used for microhematocrit measurement. For differential leukocyte count, one hundred cells were counted in glass slides stained with Romanowsky-type (23).
Serum urea was measured through ultraviolet enzymatic method using Urea Liquiform® kit (Labtest); creatinine through colorimetric method of final point using Creatinine Liquiform® kit (Labtest); CK through ultraviolet kinetic method using CK NaCl Liquiform® kit (Labtest); AST through ultraviolet kinetic method using AST/GOT Liquiform® kit (Labtest); ALT through ultraviolet kinetic method using ALT/GPT Liquiform® kit (Labtest); all of them were read under a semiautomatic spectrophotometer (Bioplus, Bio-2000®) (26).
Clotting time (minutes) was assessed by observing total blood at room temperature (26). Platelets were counted using Fônios indirect method (43). Total serum immunoglobulin was measured through electrophoresis using CELM equipment (CELM, FEA 250®) (43).
Results Analysis
The results were noted in individual cards, tabulated and analyzed to verify significant variations in the clinical signs and laboratory standards among animals of the same group and between groups.
Statistical Procedures
Comparison between groups was carried out through analysis of variance for entirely randomized design with multiple comparisons using the Tuckey method at 5% significance level (58).
RESULTS AND DISCUSSION
After the venoms injection, apathy and anorexia developed together with local alterations characterized as edema, pain, hotness and redness (Figures 1 and 2). Lesions that could be characterized as hemorrhage or necrosis were not observed, which disagreed with the statements by Rosenfeld (46), Rosenfeld et al. (47, 48), Araújo et al. (3), Murtaugh and Kaplan (35), Jorge et al. (24), and Oliveira (39), who reported that bothropic accidents were characterized by edema, necrosis, hemorrhage, blisters, bacterial contamination and intense pain at the bite site. Neurological signs were not observed, corroborating the statement that bothropic venom cannot reach the nervous system (48), but disagreeing with the reports by Pinho and Burdmann (41), Souza et al. (54), and Mosquera et al. (31).
The clinical signs presentation probably varied due to the dose of venom inoculated (0.5mg/kg), which was recommended by Araújo et al. (3) as enough to result in clinical signs of envenomation without causing death. The local and systemic changes observed were more intense in GINV than in GIIV, indicating that the irradiated venom was less aggressive.
Four weeks before the experiment, the animals of both groups were kept isolated under clinical observation and showed no changes. However, they were more active, ingested more food, gained weight and showed good hair characteristics, compared with their features thirty days after venom injection. Apparently, the venoms stimulated the goats organisms or combated some unknown aggressor agent already present before venom injection. Such hypothesis is possible considering the results of using venom or its components with therapeutic objectives reported by Fenard et al. (13), Bailey and Wilce (5), Correa et al. (11), Silva et al. (52, 53), and Gonçalves et al. (17).
The levels of erythrocytes and serum hemoglobin as well as globular volume gradually decreased until the seventh day after inoculation; however, on the thirtieth day, they were above the values observed before inoculation (Figure 3), corroborating data reported by Barraviera (7) an increase in the globular volume and hemoglobin levels but disagreeing with the results obtained by Sano-Martins et al. (51) no changes in red cells. The increase in erythrocytes and hemoglobin levels as well as in the globular volume on the thirtieth day suggested a probable stimulation or combat to some possible unknown aggressor agents by both venoms. Decreases in such values were more intense in GINV, and increases were predominant in GIIV, indicating natural venom caused more injury.
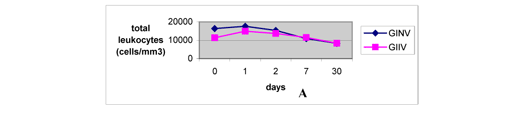
The levels of total leukocytes increased on the first day after inoculation, similarly to the reports by Barraviera (7) and Sano-Martins et al. (51), and decreased from the second day until the thirtieth day, when they were inferior to the levels observed before inoculation (Fig. 4A). This fact demonstrated the immunogenicity of both natural and irradiated venoms. The low number of leukocytes noticed on the thirtieth day may indicate that the animals were more resistant to unknown injuring agents or that such agents were destroyed or attenuated by the venoms. Increase in total leukocyte count was more significant and persistent in GIIV, demonstrating higher immune-system activation induced by the irradiated sample.
Band neutrophils were absent in leukocyte differential count. However, they were present in both groups on the first day after inoculation (Figure 4A), confirming the results obtained by Barraviera (7). This was more significant in GIIV than in GINV. Band neutrophils were not found in the leukograms done on the second and seventh days after inoculation, but on the thirtieth day they were present in the counts of both groups (Figure 4B). The presence of band neutrophils was an expected reaction to the local inflammatory processes installed in all the animals; however, GIIV animals showed larger increase, which indicated higher immune-system-activation capacity by the irradiated venom.
Venom injection stimulated the immune response in both groups, increasing blood concentrations of neutrophils, which corroborated the reports by Barraviera (7) and Sano-Martins et al. (51). On the first and second days after inoculation, the increases in neutrophils count were more significant in GINV animals, probably due to the intense inflammatory process in these animals. From the seventh day until the thirtieth day, neutrophils count was within normal standards and was similar between groups, reflecting the healing of the inflammatory process in both groups (Figure 4C).
Before venom injection, the number of lymphocytes was lower in GIIV animals; on the first day after inoculation, a proportional decrease was observed in GINV and GIIV (Figure 4D), corroborating data obtained by Barraviera (7). On the second day, the number of lymphocytes increased, mainly in GIIV. On the seventh day, the number of lymphocytes was similar between groups since it kept constant in GINV and decreased in GIIV. Such numbers decreased until the thirtieth day in both groups (Figure 4D).
The number of eosinophils was higher in GINV than in GIIV before venom injection but decreased in both groups on the first day after inoculation, mainly in GINV. From the second day, such number kept constant and similar between groups (Figure 4F). Data about the role of eosinophils in bothropic envenomation were not found in literature. The reduction in the number of eosinophils observed in both groups and the increase noticed in GINV were proportional to the intensity of the inflammatory process at the inoculation site; therefore, inflammation was more intense in GINV. As eosinophil values kept inferior to those observed before inoculation, the venoms could have eliminated or reduced the eosinophil-inductor factor.
The decreases in the lymphocyte and eosinophil levels, as well as the increases in neutrophil numbers reflected normal inflammatory responses (23).
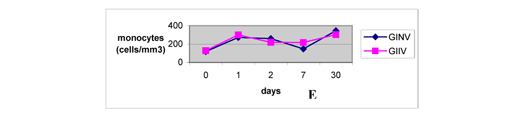
Monocytes were above the values obtained before the venom inoculation at all posterior moments evaluated (Figure 4E), confirming the report by Sano-Martins et al. (51). Although significant differences between groups were not observed, the increase in the number of monocytes was very significant since these cells are important antigen-presenting cells to lymphocytes B, which are immunoglobulin producers (23).
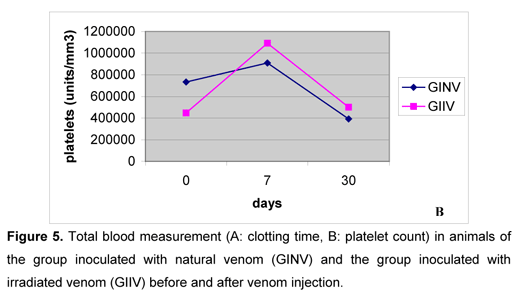
After the venoms inoculation, clotting time increased in both groups, gradually decreased until the seventh day, and again increased on the thirtieth day (Figure 5A). Such pattern corroborated the data obtained by Rosenfeld et al. (47, 48), Nahas et al. (36), Marlas et al. (29), Zingali et al., (59), Kamiguti and Cardoso (25), Barraviera and Pereira (8), Oliveira (39), Sano-Martins et al. (51), and Oliveira (39), who reported coagulant action by the bothropic venom, interfering in hemostasis. On the first and second days after inoculation, clotting times were higher in GINV and on the seventh and thirtieth days they were similar between groups. A little alteration in clotting time observed in GIIV animals demonstrated that the irradiated venom sample exerted little influence on clotting factors.
On the first and second days after inoculation, both groups showed marked platelet aggregation, making the counts impossible, similarly to the report by Usami et al. (56). They attributed this property to jararagin C, and Andrews et al. (1) and Usami et al. (57) indicated botrocetin as the responsible for such aggregation. On the seventh day, platelet numbers increased in both groups (Figure 5B), disagreeing with the reports by Sano-Martins et al. (51), who noticed low platelet numbers in bothropic envenomation cases. On the thirtieth day, platelet counts were similar between groups and to those values obtained before inoculation. The venom irradiation with 60Co source seemed to cause no influence in the platelets behavior.
On the first day after venom injection, serum levels of urea increased in GINV and did not change in GIIV. From the second day, such levels decreased, and on the thirtieth day, they were similar to those observed before inoculation in both groups. (Figure 6A), partially corroborating the reports by some authors (6, 9, 21, 22, 39), who affirmed that the nephrotoxic action of the bothropic venom leads to severe renal failure. The increase in the urea serum level may not be due to renal failure but just to an increase in pre-renal causes such as low renal pressure. GIIV animals showed lower urea levels, which indicated the little capacity of the irradiated venom sample to induce pre-renal uremia.
Serum levels of creatinine showed moderate alterations during the experiment in both groups (Figure 6B), demonstrating there was no or insufficient renal lesion to induce renal failure, disagreeing with the reports by Hudelson, Hudelson (22) and Oliveira (39).
The muscular enzymes evaluated were creatine kinase (CK), aspartate amino-transferase (AST) and alanine amino-transferase (ALT). They demonstrated similar behavior, increasing on the first day after inoculation, gradually decreasing, and showing levels similar to those observed before inoculation on the thirtieth day (Figure 6), which corroborated data obtained by Gutierrez et al. (19), Assakura et al. (4), Barraviera (7), Hudelson and Hudelson (22), Oliveira (39), and Calil-Elias et al. (10). These authors reported the existence of a proteolytic action due to the enzymes present in the bothropic venom, destroying the tissue and causing severe local necrosis. However, GINV animals exhibited serum levels of these enzymes higher than those observed in GIIV animals. Such local inflammatory signs indicated little proteolytic capacity by the irradiated venom. On the other hand, the possible existence of concomitant liver lesions should be considered, since these enzymes are not specific to muscular lesions and may indicate liver lesions.
Total immunoglobulin serum levels kept constant, without significant differences throughout the experiment in both groups. The absence of previous sensitization in the animals probably led to the constancy of the total immunoglobulin serum levels during the experimental period. There was no decrease in total immunoglobulin serum levels (Figure 7).
Bothrops jararaca venom, irradiated from a 60Co source, caused little systemic injuries to the goats of the present experiment, such as moderate local signs, and little alterations in clotting times, serum levels of urea and muscular enzymes, and in hematological parameters, confirming the reports by Salafranca (49, 50), Kankonkar (27), Gaitonde and Baride (15), Murata (32), Murata and Rogero (34), Costa and Rogero (12), Hati (20), Murata (33), Rogero and Nascimento (45), and Nascimento (37), who noticed a reduction in the toxic properties of venom irradiated by 60Co.
CONCLUSIONS
Under the conditions of the present experiment, intramuscular inoculation of natural or irradiated venom was similar to natural envenomation. Edema, hotness, pain and redness were observed at the inoculation site, but external hemorrhage and necrosis did not occur.
In goats inoculated with irradiated venom, decreases in the values of hemoglobin, erythrocyte and globular volume were less intense; increases in total leukocyte and band neutrophil counts were more intense and persistent than those observed in animals inoculated with natural venom. Therefore, the irradiated venom could be considered more antigenic. Little alterations in neutrophil, lymphocyte and eosinophil counts in goats that received irradiated venom were proportional to the moderate local inflammatory process in such animals.
Clotting time increased with the inoculation of both venoms, but such increase was inferior in animals that receive irradiated venom. Also, both venom samples led to platelet aggregation and increase in the platelet number.
The irradiated venom caused no changes in the serum level of urea and little changes in that of creatinine, indicating there was no renal failure. However, it increased the serum levels of muscular enzymes CK, AST, and ALT, although animals inoculated with natural venom exhibited higher serum levels of such enzymes.
The single inoculation in goats, without previous sensitization, did not lead to alterations in the serum levels of total immunoglobulins.
Irradiation of bothropic venom injected into goats reduced local and systemic injuries; making this type of venom more recommended than the natural venom for anti-bothropic serum production.
Received: August 24, 2006
Accepted: November 7, 2006
Abstract published online: November 27, 2006
Full paper published online: August 31, 2007
Conflicts of interest: There is no conflict.
Financial source: grants from University of Uberaba (UNIUBE), Brazil.
- 1 ANDREWS RK., BOOTH WJ., GORMAN JJ., CASTALDI PA., BERNDT MC. Purification of botrocetin from Bothrops jararaca venom. Analysis of the botrocetin-mediated interaction between von Willwbrand factor and the human platelet membrane glycoprotein Ib-IX complex. Biochemistry, 1989, 28, 8317-26.
- 2 ANGULO Y., ESTRADA R., GUTIERREZ JM. Clinical and laboratory alterations in horses during immunization with snake venoms for the production of polyvalent (Crotalinae) antivenom. Toxicon, 1997, 35, 81-90.
- 3 ARAÚJO P., ROSENFELD G., BELLUOMINI HE. Toxicidade dos venenos ofídicos doses mortais para bovinos. Arq. Inst. Biol, 1963, 30, 42-52.
- 4 ASSAKURA MT., REICHL AP., MANDELBAUM FR. Comparison of immunological, biochemical and biophysical properties of three hemorrhagic factors isolated from the venom of Bothrops jararaca (Jararaca). Toxicon, 1986, 24, 9, 946.
- 5 BAILEY P., WILCE J. Venom as a source of useful biologically active molecules. Emerg. Med., 2001, 13, 28-36.
- 6 BARBOSA PS., HAVT A., FACO PE., SOUSA TM., BEZERRA IS., FONTELES MC., TOYAMA MH., MARANGONI S., NOVELLO JC., MONTEIRO HS. Renal toxicity of Bothrops moojeni snake venom and its main myotoxins. Toxicon, 2002, 40, 1427-35.
- 7 BARRAVIERA B. Estudos clínicos dos acidentes ofídicos. J. Bras. Med., 1993, 65, 209-50.
- 8 BARRAVIERA B., PEREIRA PCM. Acidentes por serpentes do gênero Bothrops In: BARRAVIERA B. Venenos Animais: uma visão integrada. Rio de Janeiro: Editora de Publicações Científicas, 1994. 411p.
- 9 BOER-LIMA PA., GONTIJO JA., CRUZ-HOFLING MA. Bothrops moojeni snake venom-induced renal glomeruli changes in rat. Am. J. Trop. Med. Hyg., 2002, 67, 217-22.
- 10 CALIL-ELIAS S., THATTASSER YE., MARTINEZ AM., MELO PA. Effect of perimuscular injection of Bothrops jararacussu venom on plasma creatine kinase levels in mice: influence of dose and volume. Braz. J. Med. Biol. Res., 2002, 35, 1233-5.
- 11 CORREA MCJR., MARIA DA., MOURA-DA-SILVA AM., PIZZOCARO KF., RUIZ IR. Inhibition of melanoma cells tumorigenicity by the snake venom toxin jararhagin. Toxicon, 2002, 40, 739-48.
- 12 COSTA TA., ROGERO JR. Dano da radiação gama em crotamina (toxina de cascavel brasileira). Publ. IPEN, 1988, 151.
- 13 FENARD D., LAMBEAU G., VALENTIN E., LEFEBVRE J., LAZDUNSKI M., DOGLIO A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Invest., 1999, 104, 611-8.
- 14 FERREIRA JUNIOR RS., NASCIMENTO N., MARTINEZ JC., ALVES JB., MEIRA DA., BARRAVIERA B. Immunological assessment of mice hyperimmunized with native and Cobalt-60-irradiated Bothrops venoms. J. Venom. Anim. Toxins incl. Trop. Dis., 2005, 11, 447-64.
- 15 GAITONDE BB., BARIDE RM. Toxioidation of venoms of poisonous Indian snakes. Indian J. Med. Res, 1981, 73, 115-21.
- 16 GAY CC. Exame clínico de ovinos e caprinos. In: RADOSTITS M., MAYHEW IG., HOUSTON DM. Exame clínico e diagnóstico em Veterinária. Rio de Janeiro, 2002, 140-8.
- 17 GONÇALVES AR., SOARES MJ., SOUZA W., DAMATTA RA., ALVES EW. Ultrastructural alterations and growth inhibition of Trypanosoma cruzi and Leishmania major induced by Bothrops jararaca venom. Parasitol. Res, 2002, 88, 598-602.
- 18 GUIDOLIN R., CORREA A., CICARELLI RMB., PREVIDE E., MORAIS JF., HIGASHI HG. Esterilização de soros e vacinas por radiação gama de Cobalto. Rev. Saúde Públ, 1988, 22, 113-7.
- 19 GUTIÉRREZ JM., OWNBY CL., ODELL GV. Pathogenesis of myonecrosis induced by crude venom and a myotoxin of Bothrops asper. Exp. Mol. Pathol, 1984, 40, 367-79.
- 20 HATI AK. The effect of gamma irradiated detoxified viper venom as a toxoid against viper venom. The Snake, 1989, 21, 36-40.
- 21 HAVT A., FONTELES MC., MONTEIRO HS. The renal effects of Bothrops jararacussu venom and the role of PLA(2) and PAF blockers. Toxicon, 2001, 39, 1841-6.
- 22 HUDELSON S., HUDELSON P. Pathophysiology of snake envenomation and evaluation of treatments. Part 1. Compend. Contin. Educ. Pract. Vet, 1995, 17, 889-96.
- 23 JAIN NC. Essentials of veterinary hematology 5.ed. Philadelphia: Lea & Febiger, 1993. 417p.
- 24 JORGE MT., RIBEIRO LA., SILVA MLR., KUSANO EJU., MENDONÇA JS. Microbiological studies of abscesses complicating Bothrops snakebite in humans: a prospective study. Toxicon, 1993, 32, 743-8.
- 25 KAMIGUTI SA., CARDOSO JLC. Haemostatic changes caused by the venoms of South American snakes. Toxicon, 1989, 27, 955-63.
- 26 KANEKO JJ. Clinical biochemistry of domestic animals 5.ed. New York: Academic Press, 1997. 932 p.
- 27 KANKONKAR SR. Irradiated cobra (Naja naja) venom for biomedical applications. Int. Atomic En. Agency, 1974, 192, 253-62.
- 28 LI Q., OWNBY CL. Evaluation of four different immunogens for the production of snake antivenoms. Toxicon, 1992, 30, 1319-30.
- 29 MARLAS G., JOSEPH D., HUET C. Subunit structure of a potent platelet-activating glycoprotein isolated from the venom of Crotalus durissus cascavella Biochimic., 1983, 65, 619-28.
- 30 MORENO E., ANDRADE MQ., LIRA-DA-SILVA RM., TAVARES-NETO J. Características clínico-epidemiológicas dos acidentes ofídicos em Rio Branco, Acre. Rev. Soc. Bras. Med. Trop., 2005, 38, 15-21.
- 31 MOSQUERA A., IDROVO LA., TAFUR A., DEL B., OSCAR H. Stroke following Bothrops spp. snakebite. Neurology, 2003, 60, 1577-80.
- 32 MURATA Y. Effects of gamma radiation on Crotalus durissus terrificus venom. Arq. Biol. Tecnol, 1987, 30, 196.
- 33 MURATA Y. Gamma irradiation reduces the toxic activities of Crotalus durissus terrificus venom but does not affect their immunogenic activities. Toxicon, 1990, 28, 617-8.
- 34 MURATA Y., ROGERO JR. Análise cromatográfica por exclusão de amostras de veneno de cascavel irradiadas com Co60. Publ. IPEN, 1988, 154, 1-7.
- 35 MURTAUGH RJ., KAPLAN PM. Veterinary emergency and critical care medicine. Missouri: Mosby, 1992. 685 p.
- 36 NAHAS L., KAMIGUTI AS., BARROS MAR. Thrombin-like and factor X-activator components of Bothrops snake venoms. Thromb. Haemost, 1979, 41, 314-28.
- 37 NASCIMENTO N. Influence of ionizing radiation on crotoxin: biochemical and immunological aspects. Toxicon, 1996, 34, 123-31.
- 38 NETTO DP., CHIACCHIO SB., BICUDO PL. Humoral response and neutralization capacity of sheep serum inoculated with natural and Cobalt 60-irradiated Crotalus durissus terrificus venom (Laurenti, 1768). J. Venom. Anim. Toxins, 2002, 8, 297-314.
- 39 OLIVEIRA MMV. Serpentes venenosas, diagnóstico e tratamento dos acidentes ofídicos. Cad. Téc. Esc. Vet. Univ. Fed. Minas Gerais, 1999, 28, 1-66.
- 40 PINHEIRO JUNIOR, GC. Caprinos no Brasil Belo Horizonte: Itatiaia, 1973. 201p.
- 41 PINHO FM., BURDMANN EA. Fatal cerebral hemorrhage and acute renal failure after young Bothrops jararacussu snakebite. Ren. Fail, 2001, 23, 269-77.
- 42 PINHO FMO., OLIVEIRA ES., FALEIROS F. Acidente ofídico no estado de Goiás. Rev. Assoc. Med. Bras., 2004, 50, 93-6.
- 43 PRATT PW. Laboratory procedures for veterinary technicians Missouri: Mosby, 1997, 660 p.
- 44 ROBERSON EL. Drogas usadas contra nematódeos. In: BOOTH NH., McDONALD LE. Farmacologia e terapêutica em veterinária 6.ed. Rio de Janeiro: Guanabara Koogan, 1992, p.741.
- 45 ROGERO JR., NASCIMENTO N. Detoxification of snake venom using ionizing radiation. J. Venom Anim. Toxins, 1995, 1, 7-10.
- 46 ROSENFELD G. Acidentes por animais peçonhentos. In: VERONESI R. Doenças infecciosas e parasitárias 8.ed. Rio de Janeiro: Guanabara Koogan, 1991: 951-62.
- 47 ROSENFELD G., HAMPE OG., KELEN EMA. Coagulant and fibrinolytic activity of animal venoms: determination of coagulant and fibrinolytic index of different species. Mem. Inst. Butantan, 1959, 29, 143-63.
- 48 ROSENFELD G., NAHAS L., FLEURY CT. Envenenamentos por serpentes, aranhas e escorpiões. In: PRADO FC., RAMOS JA., VALLE JR. Atualização terapêutica 8.ed. São Paulo: Artes Médicas, 1970: 984-92.
- 49 SALAFRANCA ES. Detoxification of cobra venom and bacterial toxins for biological production. Int. Atomic En. Agency, 1972, 334, 87-94.
- 50 SALAFRANCA ES. Irradiated cobra (Naja naja philippinensis) venom. Int. J. Appl. Radiat. Isot, 1973, 24, 60.
- 51 SANO-MARTINS IS., SANTORO ML., MORENA P., SOUZA E SILVA MCC., TOMY SC., ANTONIO LC., NISHIKAWA AK., GONÇALVES ILC., LARSSON MHA., HAGIWARA MK., KAMIGUTI AS. Hematological changes induced by Bothrops jararaca venom in dogs. Braz. J. Med. Biol. Res., 1995, 28, 303-12.
- 52 SILVA RJ., SILVA MG., VILELA LC., FECCHIO D. Antitumor effect of Bothrops jararaca venom. Mediators Inflamm., 2002, 11, 99-104.
- 53 SILVA RJ., SILVA MG., VILELA LC., FECCHIO D. Cytokine profile of Ehrlich ascites tumor treated with Bothrops jararaca venom. Mediators Inflamm., 2002, 11, 197-201.
- 54 SOUZA FA., SPENCER PJ., ROGERO JR., NASCIMENTO N., DAL PAI-SILVA M., GALLACCI M. 60Co gamma irradiation prevents Bothrops jararacussu venom neurotoxicity and myotoxicity in isolated mouse neuromuscular junction. Toxicon, 2002, 40, 1101-6.
- 55 TORRES AP., JARDIM WR. Criação de cavalos e de outros eqüinos 3.ed. São Paulo: Nobel, 1987, 457p.
- 56 USAMI Y., FUGIMURA Y., MIURA S., SHIMA H., YOSHIDA A., YOSHIOKA A., HIRANO K., SUZUKI M., TITANI K. A 28kDA-protein with disintegrin-like structure (jararhagin-C) purified from Bothrops jararaca venom inhibits collagen and ADP-induced platelet aggregation. Biochem. Biophys. Res. Commun., 1994, 201, 331-9.
- 57 USAMI Y., FUJIMURA Y., SUZUKI M., OZEKI Y., NISHIO K., FUKUI H., TITANI K. Primary structure of two-chain botrocetin, a von Willebrand factor modulator purified from the venom of Bothrops jararaca. Proc. Natl. Acad. Sci. U.S.A, 1993, 90, 928-32.
- 58 ZAR JH. Biostatistical analysis 2.ed. Englewood Cliffs: Prentice Hall, 1984, 718 p.
- 59 ZINGALI RB., FRANCISCHETTI IM., CARLINI CR., GUIMARÃES JA. Biochemical and pharmacological screening of snake (Bothrops) venoms: characterization of components acting on blood coagulation and platelet aggregation. Braz. J. Med. Biol. Res., 1988, 21, 763-5.
Publication Dates
-
Publication in this collection
14 Sept 2007 -
Date of issue
2007
History
-
Accepted
07 Nov 2006 -
Received
24 Aug 2006