Abstract
Snake venom metalloproteases (SVMPs) comprise a family of snake venom toxins responsible for most of local and systemic effects observed during envenomation by snakes from the Viperidae family. The vascular system and more specifically the endothelium seem to be the preferential targets of these proteins. This work describes the effects of rACLF, a recombinant SVMP from Agkistrodon contortrix laticinctus on human umbilical vein endothelial cells (HUVECs) in vitro. Our results showed that rACLF activates HUVECs by the release of mediators involved in inflammation and hemostasis such as prostacyclin and interleukin-8. We also demonstrated that rACLF increased the expression of ICAM-I and decay accelerating factor (DAF). Moreover, rACLF protects the HUVECs against apoptosis induced by serum deprivation. These results suggest that the endothelial cell activation induced by SVMPs may have a significant role in the development of the local inflammatory lesion observed in Viperidae envenomation.
rACLF; snake venom metalloprotease; endothelial cells; inflammation
ORIGINAL PAPERS
rACLF, a recombinant snake venom metalloprotease, activates endothelial cells in vitro
De Moraes C. K.I; Fritzen M.II; Chudzinski-Tavassi A. M.II; Selistre-de-Araújo H. S.I
IDepartment of Physiological Sciences, Federal University of São Carlos, São Carlos, São Paulo State, Brazil
IILaboratory of Biochemistry and Biophysics, Butantan Institute, São Paulo, São Paulo State, Brazil
Correspondence to Correspondence to: Heloísa S. Selistre-de-Araújo Departamento de Ciências Fisiológicas Universidade Federal de São Carlos, São Carlos, SP Brasil. Fax: + 55-16-3351-83-27 Email: hsaraujo@power.ufscar.br
ABSTRACT
Snake venom metalloproteases (SVMPs) comprise a family of snake venom toxins responsible for most of local and systemic effects observed during envenomation by snakes from the Viperidae family. The vascular system and more specifically the endothelium seem to be the preferential targets of these proteins. This work describes the effects of rACLF, a recombinant SVMP from Agkistrodon contortrix laticinctus on human umbilical vein endothelial cells (HUVECs) in vitro. Our results showed that rACLF activates HUVECs by the release of mediators involved in inflammation and hemostasis such as prostacyclin and interleukin-8. We also demonstrated that rACLF increased the expression of ICAM-I and decay accelerating factor (DAF). Moreover, rACLF protects the HUVECs against apoptosis induced by serum deprivation. These results suggest that the endothelial cell activation induced by SVMPs may have a significant role in the development of the local inflammatory lesion observed in Viperidae envenomation.
Key words: rACLF, snake venom metalloprotease, endothelial cells, inflammation.
INTRODUCTION
The vascular endothelium plays a critical role in the regulation of vascular tone, homeostasis, and in the immune and inflammatory systems. Endothelium has also a central function in controlling leukocyte adhesion and migration by close interactions between circulating cells and endothelium. Leukocyte rolling and migration across the endothelium barrier is controlled by expression of adhesion molecules and chemokines on the luminal surface of the endothelium (6, 8, 15).
Snake venom metalloproteinases comprise a subfamily of zinc-dependent enzymes of varying molecular mass found in large quantities in Viperidae snake venom. SVMPs are responsible for most of the local and systemic effects observed during envenomation such as hemorrhage, skin lesions, necrosis, inflammation and cytokine-dependent inflammatory cells influx (4, 11, 13, 14). SVMPs are divided into four groups (PI, II, III and IV) and subgroups that differ for the presence of additional domains on the carboxyl side of the metalloproteinase domain (4, 12, 17). SVMPs display several activities such as degradation of blood coagulating factors (7, 30), extracellular matrix (ECM) components (4, 14, 23, 26) and cell receptors such as the cell-surface b1-integrin receptor (18). Besides these activities, SVMPs were reported to induce apoptosis in endothelial cells (2, 32).
ACLF is a 23,000-Da non-hemorrhagic metalloprotease from the venom of the snake Agkistrodon contortrix laticinctus, (28) with fibrinolytic and fibrinogenolytic activities (25, 29). It belongs to the PI class of SVMPs, since it possesses only the metalloproteinase domain with no additional C-terminal domains. Recently, we have demonstrated that rACLF degrades laminin, fibronectin, thrombospondin and collagen IV in vitro (23) and strongly decreases the viability of HeLa tumor cells but not of human fibroblasts. Also, this enzyme significantly increases the expression of growth-related oncogene (GRO) and monocyte chemoattractant protein 1 (MCP-1) chemokines by fibroblasts (23).
In order to achieve a better understanding on the role of metalloprotease activity in envenomation, we analyzed the effect of rACLF (recombinant ACLF) on the release and expression of mediators involved in homeostasis and/or inflammation by HUVECs.
MATERIALS AND METHODS
Materials
His-Bind metal chelation resin was from Qiagen (Valencia, CA, USA). RPMI 1640 culture medium, HAM F12 culture medium, fetal bovine serum (FBS), trypsin-EDTA, penicillin and streptomycin were purchased from Cultilab (Campinas, SP, Brazil). Collagenase was obtained from Worthington Biochemical Corporation (New Jersey, USA) and gelatin was purchased from ICN Biomedicals Inc. (Ohio, USA). Endothelial cell growth supplement (ECGS) from bovine neural tissue, heparin, L-glutamine, 2-mercaptoethanol, sodium pyruvate, vanadium chloride, sodium nitrate, polymyxin B, isopropyl thiol-b-D-galactopyranoside (IPTG), imidazole and urea were from Sigma Chemical Co. (St Louis, MO, USA). Tumor necrosis factor-alpha (TNF-a) and PE-conjugated mouse anti-human monoclonal antibodies (MoAbs; IgG1) against ICAM-1 (CD 54), DAF (CD 55) or control IgG1 were purchased from BD Biosciences Pharmingen (San Diego, CA, USA). Enzyme immunoassay kits (ELISA) for prostacyclin (PGI2) determinations were from Cayman Chemical Company (Ann Arbor, MI, USA) and interleukin (IL)-8 was from Oncogene Research Products (San Diego, CA, USA).
Expression, Purification and Refolding of rACLF
The open reading frame (ORF) coding for pre-ACLF was isolated from a venom gland cDNA library of Agkistrodon contortrix laticinctus and subcloned on the pET28a vector (25, 29). Expression, purification and refolding of the recombinant protein were performed as previously described (25). Briefly, transformed cells of Escherichia coli strain BL21 (DE3) were grown at 37ºC in Luria-Bertani medium, supplemented with kanamycin (30µg/ml) to a cell density of A660nm=0.40.6. After induction of protein expression with 1mM IPTG for 2h, cells were disrupted by sonication and inclusion bodies were solubilized in 20mM Tris-HCl buffer, pH7.9, with 5mM imidazole plus 500mM NaCl with 6M urea. The lysate was centrifuged (10,000Xg) and the supernatant was applied onto a Ni-NTA resin equilibrated with the same buffer. After washing with buffer with 20mM imidazole, the recombinant protein was eluted with the buffer containing 1M imidazole.
The purified protein was diluted with reducing solution (10mM DTT in 50mM Tris-HCl, pH8.5, with 6M urea) and then diluted with oxidation buffer (50mM Tris-HCl, pH8.5, 5mM cysteine, 1mM cystine, 5mM CaCl2, 100mM ZnCl2 and 6M urea). After dialysis with decreasing concentrations of urea, the solution was concentrated by high-pressure filtration in Amicon (MWCO 10kDa) and the protein concentration was determined by the Bradford method (5). In vitro activation of pro-enzyme was carried out by incubating the zymogen at 37ºC for 1h and analyzing by SDS-PAGE.
Endothelial Cell Culture
HUVECs were obtained by collagenase digestion of umbilical veins according to the method of Jaffe et al. (16). Cells were seeded on 2% gelatin-coated 25cm2 tissue culture flasks and identified by its cobblestone morphology and von Willebrand factor (vWF) staining. Initially, cells were grown in RPMI 1640 medium, supplemented with 10% FBS, heparin (45µg/ml), ECGS (25µg/ml), sodium pyruvate (1mM), L-glutamine (2mM), penicillin (100U/ml), streptomycin (100µg/ml) and 2-mercaptoethanol (50µM), at 37°C, in a humidified 5% CO2 incubator. Having reached confluence, cells were detached by mild treatment with trypsin-EDTA (0.05% and 0.1mM, respectively) and washed with 10% FBS-supplemented PBS (Ca2+/Mg2+-free; PBS/FBS). HUVECs were used between the first and third passages. Some experiments were performed in the presence of polymyxin B (7µg/ml) to rule out lipopolysaccharide (LPS) interference. In order to exclude interference of cellular debris, supernatants from HUVECs exposed for 1h to rACLF were centrifuged for 10min, 400Xg, at 4°C.
Cellular Apoptosis Detection
HUVECs were incubated in RPMI containing 10% FBS and were stimulated during 48h with or without rACLF. Adherent and floating cells were pooled, washed and fixed in cold 70% ethanol. After centrifugation, cellular DNA was stained with a propidium iodide solution (100µg/ml) in 0.1% Triton-X100, 1mM EDTA. Nuclear endothelial cell changes and necrosis were evaluated by fluorescence microscopy using acridine orange and ethidium bromide solution (100µg/ml of each one).
IL-8 and PGI2 Assays
To determine the effect of the protease on the release of Weibel Palade bodies, IL-8 levels were measured by ELISA (Oncogene Research Products) in the supernatants of HUVECs that were treated or not with rACLF for 1h. Then, cells were washed and after addition of fresh medium, the constitutive synthesis of IL-8 was measured 24h later. IL-8 levels are expressed as pg/ml. Production of 6-keto-protaglandin F1a (PGI2 stable metabolite) was measured by ELISA (Cayman Chemical Company, MI, USA).
ICAM-1 and DAF Expression
After 1h HUVEC, treatment with rACLF, cells were washed and further cultured at 37°C in RPMI containing FBS (10%) during 12 or 2448h for ICAM-1 and DAF measurement, respectively. Then, cells were harvested by treatment with trypsin-EDTA solution and, after washing with PBS/FBS, they were centrifuged (10min, 200Xg, at 4°C), resuspended in PBS/FBS and incubated during 30min at 4°C with saturating concentrations of PE-conjugated anti-CD54, anti-CD55 or equivalent concentrations of an isotypic control. Cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry in a FACScan cytometer (Becton Dickinson, Mountain View, CA). Appropriate settings of forward and side scatter gates were used to examine 5,000 cells per experiment. The percentage of positive cells was determined by thresholds set using isotypic controls. The number of fluorescent molecules per cell was inferred by assessing the mean intensity of fluorescence expressed as arbitrary units (AUF).
Statistical Analysis
All results are expressed as means ± standard error (S.E.). We used one-way analysis of variance (ANOVA) followed by Dunnett test to analyze data and values of p lower than 0.05 were considered statistically significant.
RESULTS
rACLF Protects HUVECs from Apoptosis
The percentage of apoptotic cells in rACLF-treated cultures was similar to that of control, indicating that this enzyme did not induce HUVEC apoptosis at the dose tested after 48h incubation (Table 1). On the other hand, when cell death was induced by serum reduction (1%), rACLF induced significant protection compared to the control. This protective effect was also shown to be concentration-dependent in cells after 24h of serum deprivation (Figure 1). The best effect (about 60%) was observed with 1,000nM rACLF.
Interleukin-8 (IL-8) Release by HUVECs
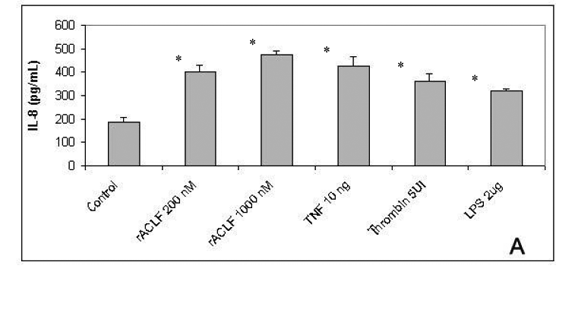
IL-8 belongs to CXC chemokines family and is an important chemoattractant for PNM, mainly for neutrophils. It also stimulates neutrophil degranulation and adherence to endothelial cells by CD 11b/CD18. Moreover, IL-8 is able to induce angiogenesis and enhanced endothelial cell survival and proliferation (19). To examine the role of rACLF on endothelial chemotatic properties, the release of IL-8 was analyzed. As demonstrated in Figure 2A, the treatment of HUVECs for 1h with rACLF (200 and 1,000nM) significantly triggered the release of IL-8, similar to the effect of TNF, thrombin or LPS.
Prostacyclin (PGI2) Release by HUVECs
Since PGI2 and nitric oxide (NO) are the main platelet inhibitors derived from endothelial cells, the release of PGI2 by rACLF was evaluated. Figure 2B shows that rACLF significantly increased the release of PGI2 from HUVECs.
ICAM-1 and DAF Expression
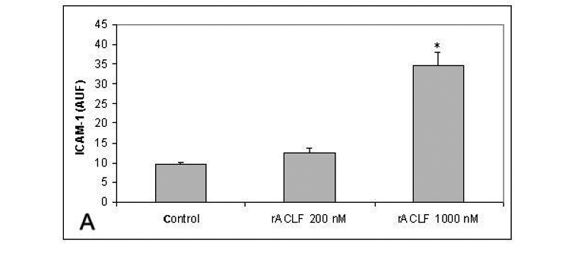
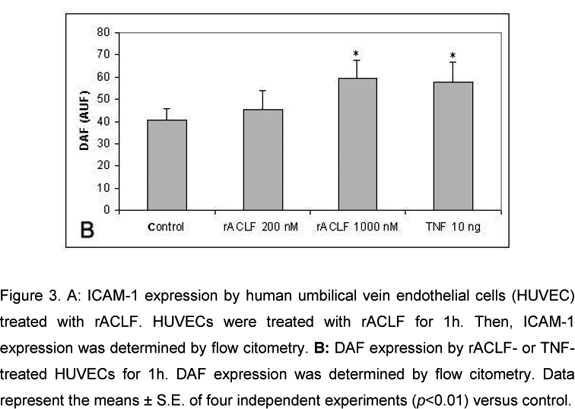
The effect of rACLF on the expression of ICAM-1 of HUVECs was examined by flow citometry. Non-treated cells expressed very low levels of ICAM-1. When the cells were treated with 1,000nM rACLF for 1h, the expression of ICAM-1 was significantly increased (Figure 3A). We also examined the effect of rACLF on the decay-accelerating factor (DAF, CD 55) expression. DAF is a cell-surface protein that prevents the formation and accelerates the decay of C3 and C5 convertases, the central amplification enzymes of the complement cascade (20). DAF can be up-regulated in vivo during inflammation or in vitro by TNF-a (1). A significant increase in DAF expression was observed following treatment with 1,000nM rACLF (Figure 3B).
DISCUSSION
We have previously demonstrated that rACLF, a recombinant PI SVMP from A. c. laticinctus, induces the release of chemokines by human fibroblasts in vitro (23). This observation suggests a role for this type of enzyme in the inflammatory reaction observed in snake bites. To contribute to a better understanding of the mechanisms involved in the pathogenesis of such local lesions, we have studied the effects of rACLF on endothelial cells. Our results showed that rACLF was not cytotoxic to endothelial cells, since it was not able to induce either apoptosis or detachment of HUVECs. We also observed that rACLF protects HUVECs against cell death in a concentration-dependent manner. Control cells cultured in a medium with low FBS died after 24h. However, the addition of rACLF to these cultures significantly enhanced cell survival. Schattner and coworkers also reported similar effect for Berythractivase, a PIII SVMP non-hemorrhagic from Bothrops erythromelas that did not modify HUVEC morphology or either induce apoptosis (27). However, Berythractivase had no effect on cell survival. Insularinase A, a PI SVMP from B. insularis venom is procoagulant and is not able to induce detachment of endothelial cells. (22)
There are several reports on the ability of SVMPs to induce apoptosis in endothelial cells (9, 21, 32, 34, 35). BaP1, a PI SVMP from B. asper with weak hemorrhagic activity induced detachment of endothelial cells by proteolysis of matrix components, resulting in anoikis in endothelial cells (9). Moreover, apoptosis was dependent on BaP1 catalytic activity. Jarharagin, a PIII SVMP from B. jararaca also induced anoikis in endothelial cells, but had no effect in fibroblasts or murine peritoneal adherent cells (MPAC) (32).
It has been demonstrated that SVMPs are involved in the pathogenesis of local inflammation by inducing edema and releasing matrix metalloproteases and inflammatory cytokines (14, 24). Our data demonstrate increased levels of IL-8 in the supernatant of rACLF-treated cells. IL-8 is the prototype member of the CXC subfamily of chemokines and can be induced by diverse inflammatory stimuli in many cells (31, 33). Most biological activities attributed to IL-8 are due to its potent chemoattractant activity for neutrophils and its ability to stimulate endothelial cells matrix metallopeptidade expression (19). During the inflammatory reaction, not only occurs an increase in chemokine levels but also an up-regulation of endothelial cell adhesion molecules, which promotes leukocyte recruitment and migration. Leukocyte migration into tissues is a multistep process mediated by three major classes of adhesion molecules: integrins, selectins and immunoglobulins that are expressed by both leucocytes and endothelial cells (3). rACLF up-regulated ICAM-1 expression levels in HUVECs. Similar effect was observed in HUVECs treated with Beythractivase (27, 30).
Fernandes and coworkers reported that BaP1 induced a marked leukocyte infiltration into the mouse peritoneal cavity and this effect was related to the ability of BaP1 to up-regulate the expression of leukocyte adhesion molecules LECAM-1, LFA-1 and CD 18 (11).
Since it was previously demonstrated that SVMPs activate the complement system (10), we further investigated the ability of rACLF to induce the expression of complement inhibitor proteins (CIPs) on the HUVEC surface. Our results showed that rACLF up-regulated DAF expression, which in turn can help in cell protection against complement-mediated cell lysis, reinforcing the response against endothelial injury.
In addition, we demonstrated that rACLF increased the PGI2 production, a molecule involved in the regulation of vascular tone and potent platelet activation inhibitor. However, more experiments are required to verify the effects of rACLF on platelet function/aggregation.
In conclusion, our results showed that rACLF activates HUVECs by modulating mediators involved in homeostasis and inflammation. Besides that, rACLF is not cytotoxic to endothelial cells and inhibits cell death. Since rACLF belongs to the PI class of SVMPs, our results strongly suggest that the catalytic activity is important for the effects observed in this study. However, the substrate for rACLF and its mechanism of action remains to be elucidated. To our knowledge, this is the first report of a recombinant SVMP activating endothelial cells, therefore modulating inflammation and survival mechanisms.
ACKNOWLEDGEMENTS
This work was supported by FAPESP (The State of São Paulo Research Foundation, Grant no. 02/08043-6).
Received: June 18, 2007
Accepted: November 9, 2007
Abstract published online: November 13, 2007
Full paper published online: March 8, 2008
Conflicts of interest: There is no conflict
- 1 AHMAD SR., LIDINGTON EA., OHTA R., OKADA N., ROBSON MG., DAVIES KA., LEITGES M., HARRIS C., HASKARD DO., MASON JC. Decay-accelerating factor induction by tumor necrosis factor-a, through a phosphatidylinositol-3 kinase and protein C-dependent pathway, protects murine vascular endothelial cells against complement deposition. Immunology, 2003, 110, 258-68.
- 2 ARAKI S., MASUDA S., MAEDA H., YING MJ., HAYASHI H. Involvement of specific integrins in apoptosis induced by vascular apoptosis-inducing protein-1. Toxicon, 2002, 40, 535-42.
- 3 BEVILACQUA MP., NELSON RM. Endothelial-leukocyte adhesion molecules in inflammation and metastasis. Thromb. Haemost, 1993, 70,152-4.
- 4 BJARNASON JB., FOX JW. Haemorrhagic metalloproteinase from snake venom. Pharmac. Ther, 1994, 62, 325-72.
- 5 BRADFORD MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem, 1976, 72, 248-54.
- 6 CARLOS T., HARLAN J. Leukocyte-endothelial adhesion molecule. Blood, 1994, 84, 2068-101.
- 7 CHEN RQ., JIN Y., WU JB, ZHOU XD., LI DS., LU QM., WANG WY., XIONG YL. A novel high-molecular-weight metalloproteinase cleaves fragment F1 of activated human prothrombin. Toxicon, 2004, 44, 281-7.
- 8 CINES DB., POLLAK ES., BUCK CA., LOSCALZO J., ZIMMERMAN GA., MCEVER RP., POBER JS., WICK TM., KONKLE BA., SCHWARTZ BS., BARNATHAN ES., MCCRAE KR., HUG BA., SCHMIDT AM., STERN DM. Endothelial cells in physiology and in the pathophysiology of vascular disorder. Blood, 1998, 91, 3527-61.
- 9 DIAZ C., VALVERDE L., BRENES O., RUCAVADO A., GUTIERREZ JM. Characterization of events associated with apoptosis/anoikis induced by snake venom metalloproteinase BaP1 on endothelial cells. J. Cell Biochem, 2005, 94, 520-8.
- 10 FARSKY SH., GONÇALVES LR., GUTIERREZ JM., CORREA AP., RUCAVADO A., GASQUE P., TAMBOURGI DV. Bothrops asper snake venom and its metalloproteinase BaP-1 activate the complement system. Role in leukocyte recruitment. Mediators Inflamm, 2000, 9, 213-21.
- 11 FERNANDES MC., ZAMUNER SR., ZULIANI JP., RUCAVADO A., GUTIÉRREZ JM., TEIXEIRA CFP. Inflammatory effects of BaP1, a metalloproteinase isolated from Bothrops asper snake venom: Leukocyte recruitment and release of cytokines. Toxicon, 2006, 47, 549-59.
- 12 FOX JW., SERRANO SMT. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon, 2005, 45, 969-85.
- 13 FRANCESCHI A., RUCAVADO A., MORA N., GUTIERREZ JM. Purification and characterization of BaH4, a hemorrhagic metalloproteinase from the venom of the snake Bothrops asper Toxicon, 2000, 38, 63-77.
- 14 GUTIÉRREZ JM., RUCAVADO A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie, 2000, 82, 841-50.
- 15 HILLYER P., MORDELET E., FLYNN G., MALE D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leukocyte migration. Clin. Exp. Immunol, 2003, 134, 431-41.
- 16 JAFFE EA., NACHMAN RL., BECKER CG., MINICK CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest., 1973, 52, 2745-56.
- 17 JEON OH., KIM DS. Molecular cloning and functional characterization of a snake venom metalloprotease. Eur. J. Biochem, 1999, 263, 526-33.
- 18 KAMIGUTI AS., HAY CRM., ZUZEL M. Inhibition of collagen-induced platelet aggregation as the result of cleavage of alpha 2 beta 1-integrin by the snake venom metalloproteinase jararhagin. Biochem. J., 1996, 320, 635-41.
- 19 LI A., DUBEY S., VARNEY ML., DAVE BJ., SINGH RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol, 2003, 170, 3369-76.
- 20 LUBLIN DM., ATKINSON JP. Decay-accelerating factor: biochemistry, molecular biology and function. Annu. Rev. Immunol., 1989, 7, 35-58.
- 21 MASUDA S., HAYASHI H., ATODA H., MORITA T., ARAKI S. Purification, cDNA cloning and characterization of vascular apoptosis-inducing protein, HV1, from Trimeresurus flavoviridis Eur. J. Biochem, 2001, 268, 1-7.
- 22 MODESTO JC., JUNQUEIRA-DE-AZEVEDO IL., NEVES-FERREIRA AG., FRITZEN M., OLIVA ML., HO PL., PERALES J., CHUDZINSKI-TAVASSI AM. Insularinase A, a prothrombin-activator from Bothrops insularis venom, is a metalloproteinase derived from a gene encoding protease and disintegrin domains. Biol. Chem, 2005, 386, 589-600.
- 23 MORAES CK., SELISTRE-DE-ARAUJO HS. Effect of rACLF, a recombinant snake venom metalloprotease on cell viability, chemokine expression and degradation of extracellular matrix proteins. Toxicon, 2006, 48, 6418.
- 24 PETRICEVICH VL., TEIXEIRA CFT., TAMBOURGI DV., GUTIÉRREZ JM. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon, 2000, 38, 1253-66.
- 25 RAMOS OHP., CARMONA AK., SELISTRE-DE-ARAUJO HS. Expression, refolding, and in vitro activation of a recombinant snake venom pro-metalloprotease. Protein Expr. Purif, 2003, 28, 34-41.
- 26 RUCAVADO A., LOMONTE B., OVADIA M., GUTIERREZ JM. Local tissue damage induced by BaP1, a metalloproteinase isolated from Bothrops asper (terciopelo) snake venom. Exp. Mol. Pathol 1995, 63, 186-99.
- 27 SCHATTNER M., FRITZEN M., VENTURA JS., MODESTO JCA., POZNER RG., MOURA-DA-SILVA AM., CHUDZINSKI-TAVASSI AM. The snake venom metalloproteases berythractivase and jararhagin activate endothelial cells. Biol. Chem., 2005, 386, 369-74.
- 28 SELISTRE-DE-ARAUJO HS., OWNBY CL. Molecular cloning and sequence analysis of cDNAs for metalloproteinases from broad-copperhead Agkistrodon contortrix laticintus Arch. Biochem. Biophys ., 1995, 320, 141-8.
- 29 SELISTRE-DE-ARAUJO HS., SOUZA EL., BELTRAMINI LM., OWNBY CL., SOUZA DHF. Expression, refolding, and activity of a recombinant nonhemorrhagic snake venom metalloprotease. Protein Expr. Purif, 2000, 19, 41-7.
- 30 SILVA MB., SCHATTNER M., RAMOS CR., JUNQUEIRA-DE-AZEVEDO IL., GUARNIERI MC., LAZZARI MA., SAMPAIO CA., POZNER RG., VENTURA JS., HO PL., CHUDZINSKI-TAVASSI AM. A prothrombin activator from Bothrops erythromelas (jararaca-da-seca) snake venom: characterization and molecular cloning. Biochem. J, 2003, 369,129-39.
- 31 STRIETER RM., BURDICK MD., GOMPERTS BN., BELPERIO JA., KEANE MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev., 2005, 16, 593-609.
- 32 TANJONI I., WEINLICH R., DELLA-CASA MS., CLISSA PB., SALDANHA-GAMA RF., FREITAS MS., BARJA-FIDALGO C., AMARANTE-MENDES GP., MOURA-DA-SILVA AM. Jarharagin, a snake venom metalloproteinase, induces a specialized form of apoptosis (anoikis) selective to endotelial cells. Apoptosis, 2005, 10, 851-61.
- 33 WOLFF B., BURNS AR., MIDDLETON J., ROT A. Endothelial cell "memory" of inflammation stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J. Exp. Med, 1998, 188, 1757-62.
- 34 WU WB., HUANG TF. Activation of MMP-2, cleavage of matrix proteins, and adherens junctions during a snake venom metalloproteinase-induced endothelial cell apoptosis. Exp. Cell Res., 2003, 288, 143-57.
- 35 YOU WK., SEO HJ., CHUNG KH, KIM DS. A novel metalloprotease from Gloydius halys venom induces endothelial cell apoptosis through its protease and disintegrin-like domains. J. Biochem., 2003, 134, 739-49.
Publication Dates
-
Publication in this collection
11 Sept 2009 -
Date of issue
2008
History
-
Received
18 June 2007 -
Accepted
09 Nov 2007