Abstract
Ionizing radiation has been successfully employed to modify the immunological properties of biomolecules. Very promising results were obtained when crude animal venoms, as well as isolated toxins, were treated with 60Co gamma rays, yielding toxoids with good immunogenicity. The achievement of modified antigens with lower toxicity and preserved or improved immunogenicity can be very useful. Ionizing radiation has already been proven to be a powerful tool to attenuate snake venom toxicity without affecting, and even increasing, their immunogenic properties. However, little is known about the modifications that irradiated molecules undergo and even less about the immunological response that such antigens elicit. In the present work, we investigated the immunological behavior of bothropstoxin-1, a K49 phospholipase, before and after irradiation. Structural modifications of the toxin were analyzed by SDS-PAGE. Isogenic mice were immunized with either the native or the irradiated toxin. The circulating antibodies were isotyped and titrated by ELISA. According to our data, irradiation promoted structural modifications in the toxin characterized by higher molecular weight forms of proteins (aggregates and oligomers). The results also indicated that irradiated toxins were immunogenic and antibodies elicited by them were able to recognize the native toxin in ELISA. These findings suggest that irradiation of toxic proteins can promote significant modifications in their structures; however they still retain many of the original antigenic and immunological properties of native proteins. Also, our data indicate that irradiated proteins induce higher titers of IgG2a and IgG2b, suggesting that Th1 cells are predominantly involved in the immune response.
Immunology; ionizing radiation; gamma rays; bothropstoxin-1
ORIGINAL PAPER
Study of irradiated bothropstoxin-1 with 60Co gamma rays: immune system behavior
Caproni PI; Baptista JAI; Almeida TL deI; Passos LACII; Nascimento NI
IBiotechnology Center, Nuclear and Energy Research Institute, IPEN, National Council of Nuclear Energy, CNEN, University of São Paulo, USP, São Paulo, São Paulo State, Brazil
IILaboratory of Genetics and Embryo Cryopreservation, State University of Campinas, UNICAMP, Campinas, São Paulo State, Brazil
Correspondence to Correspondence to: Priscila Caproni Centro de Biotecnologia Instituto de Pesquisas Energéticas e Nucleares, IPEN, USP Av. Professor Lineu Prestes, 2242 São Paulo, SP, 05508-000, Brasil Phone: +55 11 3133 9690 Email: pricaproni@hotmail.com
ABSTRACT
Ionizing radiation has been successfully employed to modify the immunological properties of biomolecules. Very promising results were obtained when crude animal venoms, as well as isolated toxins, were treated with 60Co gamma rays, yielding toxoids with good immunogenicity. The achievement of modified antigens with lower toxicity and preserved or improved immunogenicity can be very useful. Ionizing radiation has already been proven to be a powerful tool to attenuate snake venom toxicity without affecting, and even increasing, their immunogenic properties. However, little is known about the modifications that irradiated molecules undergo and even less about the immunological response that such antigens elicit. In the present work, we investigated the immunological behavior of bothropstoxin-1, a K49 phospholipase, before and after irradiation. Structural modifications of the toxin were analyzed by SDS-PAGE. Isogenic mice were immunized with either the native or the irradiated toxin. The circulating antibodies were isotyped and titrated by ELISA. According to our data, irradiation promoted structural modifications in the toxin characterized by higher molecular weight forms of proteins (aggregates and oligomers). The results also indicated that irradiated toxins were immunogenic and antibodies elicited by them were able to recognize the native toxin in ELISA. These findings suggest that irradiation of toxic proteins can promote significant modifications in their structures; however they still retain many of the original antigenic and immunological properties of native proteins. Also, our data indicate that irradiated proteins induce higher titers of IgG2a and IgG2b, suggesting that Th1 cells are predominantly involved in the immune response.
Key words: Immunology, ionizing radiation, gamma rays, bothropstoxin-1.
INTRODUCTION
Annually, 20,000 cases of snakebites are reported in Brazil and serotherapy is the only effective method applicable, since administered in time, with adequate dose and route (1, 2). The effectiveness of serotherapy in preventing local tissue damage caused by snake venoms is limited, at least in part, by the rapid action of toxins compared with the slow distribution of antibodies (3).
Bothrops snake venoms cause pronounced local effects including hemorrhage, edema, pain and myonecrosis and are responsible for the majority of human deaths (about 70%) among other snake venoms (4, 5). The bothropstoxin-1(BTHX-1), the main myotoxic component of Bothrops jararacussu venom, is devoid of phospholipase A2 (PLA2) activity, but capable of blocking neuromuscular transmition (6).
Some snake species present venoms with low immunogenicity and high toxicity, what requires the development of techniques that increase the immune response and reduce the toxicity of venoms. To improve antisera production, much effort has been focused on decreasing venom toxicity. Several procedures have been employed to detoxify venoms, including ionizing radiation (5).
Ionizing radiation consists of electromagnetic waves that result from nuclear transitions. It can interact with biomolecules in two ways: directly, when the radiation hits the molecule, or indirectly, when free radicals are generated to react with the target molecule. Radiation promotes changes in enzymatic, pharmacological and immunological properties of proteins; the two latter being more radioresistant (7-9).
Radiation has been successfully employed to modify biomolecules, by reducing or abolishing their biological activity without affecting their immunogenic properties (10). Thus, this methodology could be used to produce toxoids and vaccines.
The immune system is constituted by cells and molecules highly specialized on the combat of infectious agents and presents two types of immune response, innate and adaptative (11, 12).
Once the bothropic antiserum is not effective in neutralizing the effects of Bothrops jararacussu venom, the use of irradiated BTHX-1 to produce antibodies can be helpful in reducing the venom effects. In the present work, we evaluated the impact on the immune system of irradiated bothropstoxin-1 (BTHX-1), a K49 phospholipase, as a model to further characterize the immune response against irradiated proteins.
MATERIALS AND METHODS
Bothropstoxin-1 Purification
Crude venom of Bothrops jararacussu (from the Butantan Institute, USP) was purified by cation exchange column. The venom sample (60 mg) was dissolved in 1 mL of 50 mM sodium acetate, pH 5. After centrifugation, the supernatant was injected into a 1 mL Resource-S® cation exchange column (Pharmacia Biotech, Sweden) connected to a dual pump fast protein liquid chromatography (FPLC) system. Buffers A and B consisted of 25 mM sodium phosphate buffer, pH 7.8, and 25 mM sodium phosphate buffer, pH 7.8, containing 2 M NaCl, pH 7.8, respectively. Flow rate was 2.5 mL/min. After an initial 10 mL wash with 7.5% B buffer (0.15 M NaCl), elution of bound fractions was performed using a linear gradient (slope = 1%/mL) for 25 mL. The column was subsequently washed with 10 mL of B buffer, followed by 10 mL of A buffer to wash NaCl out of the column (13).
Animals
B10.PL isogenic mice were obtained from the animal housing facility of IPEN/CNEN and maintained in sterilized isolators and absorbent media, with food and water ad libitum. The manipulation of these animals before or during the experiments was in accordance with the "Principles of Laboratory Animal Care" (NIH publ. N. 86-23, revised in 1985) and "Principles of Ethics in Animal Experimentation" (Brazilian College of Animal Experimentation COBEA) and Project n. 26/Animal Research Ethics Committee (CEPA-IPEN/SP).
Proteins Irradiation
Bothropstoxin-1 was dissolved in 0.15 M NaCl to a final concentration of 2 mg/mL. This solution was irradiated with a 2,000 Gy dose using gamma rays derived from a 60Co source (Gamma Cell®, Atomic Agency of Canada Ltd) at room temperature and in the presence of atmospheric O2, in a 5,170 Gy/h dose rate.
SDS-PAGE
Purified bothropstoxin-1, native or irradiated, was submitted to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and non-reducing conditions.
Antibody Production
Anti-native or anti-irradiated bothropstoxin-1 specific antibodies were obtained by immunization of B10.PL mice following a classical immunization protocol (14). Subsequently, blood samples were collected and after centrifugation plasma was separated and frozen until the moment of use. The presence of specific antibodies in plasma samples was investigated by enzyme-linked immunosorbent assay (ELISA).
ELISA
Ninety-six-well microplates were coated with native bothropstoxin-1 (1.0 µg/well/100 µL) overnight. The plates were then blocked with 5% skim milk in phosphate buffered saline (PBS). The plasma samples were subsequently incubated for one hour after a 1/800, 1/20,000 or 1/40,000 dilution in PBS. Afterwards, peroxidase-labeled antibodies specific for mouse IgG1, IgG2a or IgG2b (Southern Biotechnology Associates Inc., USA) reacted individually with the bound antibodies. Finally, the reaction proceeded by addition of a chromogenic solution containing 0.5 mg/mL ortho phenyl diamine in 20 mL distilled water in the presence of 10 µL/mL hydrogen peroxide. After 20 minutes of incubation, the reaction was interrupted by the addition of 50 µL 2 M citric acid and the plates were analyzed on a microplate reader at 450 nm.
Statistical Analysis
The statistical analysis consisted of arithmetic mean (am) determination with 95% of trust interval (95% TI) of ELISA reactivity index (RI) and the differences between the averages were analyzed by the Student's t-test. Differences of prevalence between the diverse conjugates were determined by statistical column test. The reactivity index was calculated as follows: RI = [Optical density (OD) test sample / OD cutoff] x 100, where cutoff was calculated as the mean value of OD obtained from negative control sera (15).
RESULTS
Structural Modifications on SDS-PAGE
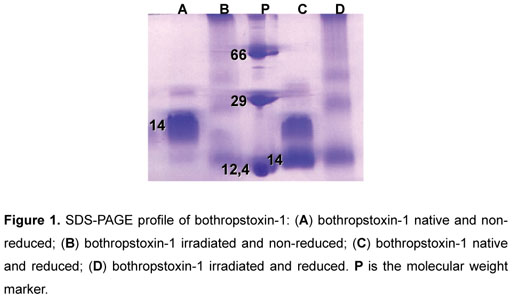
According to our data, SDS-PAGE protein profiles revealed that gamma irradiation causes breakdown of polypeptide chains, resulting, in turn, in high molecular weight aggregates (Figure 1).
Antibody Production
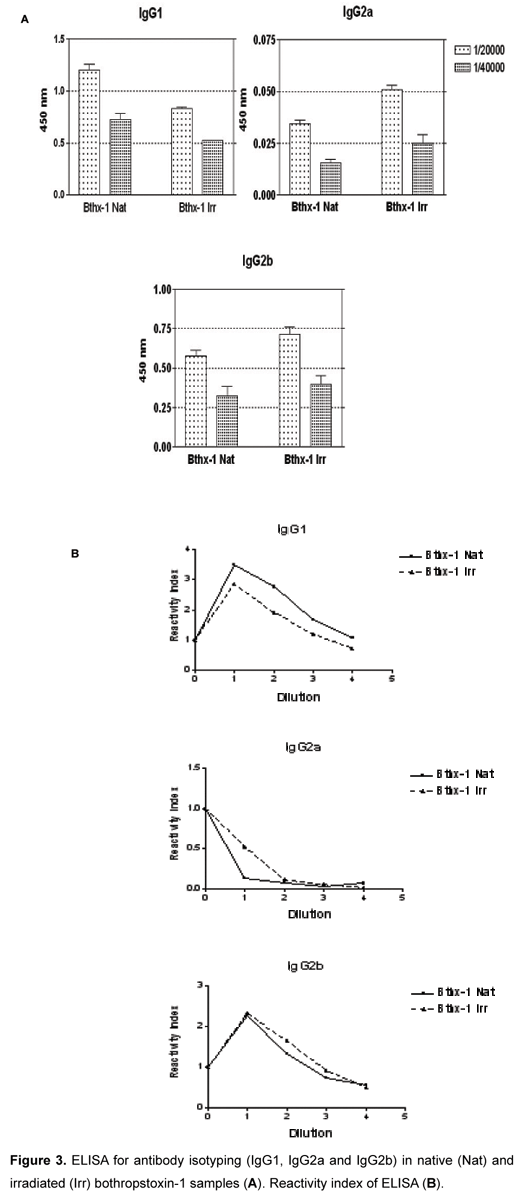
Sera from animals immunized with native or irradiated proteins were analyzed in order to evaluate levels of IgG (Figures 2 e 3), as well as to quantify specific isotypes (Figure 3).
In Figure 2 we can observe that antibody titers obtained from both toxin types were very similar. In the same assay, we can also note that RI presents no significant statistical difference.
DISCUSSION
SDS-PAGE allowed the identification of structural modifications, after the irradiation process (60Co), which can be explained by the breakdown of polypeptide chains in consequence of covalent bond rupture (16). Irradiated BTHX-1 did not present dissociation of subunits that form the dimer, even in the presence of the reducer agent (β -mercaptoethanol), suggesting that the irradiation resulted in the formation of intermolecular bonds resistant to the reducer agent.
As observed by another study, proteins can be converted into high molecular aggregates due to the production of inter-protein cross-linking reactions; hydrophobic and electrostatic interactions; and formation of disulfide bonds (16).
BTHX-1 is a very compact molecule that consists of a dimer (two subunits) formed by seven disulfide bonds. When a buffer sample is employed without the reducer agent, the disulfide bonds do not separate, indicating that the protein profile is more condensed. In the case of native BTHX-1 in the presence of the reducer agent β -mercaptoethanol disulfide bonds are dissociated, what does not occur to the dimer, and, because of this, there are two bands that correspond to the dimer itself.
The analysis of data obtained from antibodies, produced by immunization against irradiated toxin, suggests that they were capable of recognizing the native form of the toxin. The same phenomenon was observed by Baptista et al. (17), who also studied BTHX-1. While the native protein induced a predominance of polarization to Th2 response, the irradiated molecules apparently promoted a switch towards a Th1 pattern.
The native BTHX-1 presented higher titers of IgG1, which indicates an immune response of type Th2 predominance (humoral immunity). Brewer et al. (18) observed this relation in a macrophage depletion assay; they verified an IgG1 increase in serum levels, which has its production influenced by Th2 cells that participate in humoral immunity, helping the antibody production by B lymphocytes (19). However, the irradiated BTHX-1 produces higher titers of IgG2a and IgG2b, indicating a predominance of Th1 cell response, involved in cellular immunity (11). Th1 cells influence on IgG2a production, as observed by Brewer et al. (18), and are involved in cellular immunity, more specifically, in macrophage activation (11).
Thus, toxin irradiation appears to be a powerful detoxification tool that also improves immunogenicity. Besides, venoms are weakly immunogenic and very toxic, causing difficulties for serotherapy, specially regarding antiserum availability and the welfare of the immunized animal. Therefore, the use of irradiated toxins can be very helpful in many aspects.
CONCLUSIONS
These results indicate that native BTHX-1 presents high titers of IgG 1 while the irradiated toxin produces high titers of IgG2a and IgG2b, suggesting that the irradiation process promotes a switch from Th2 to Th1 immune response
ACKNOWLEDGMENTS
We thank CNPq for the financial support.
Received: June 6, 2008
Accepted: July 4, 2008
Abstract published online: July 14, 2008
Full paper published online: May 30, 2009
CONFLICTS OF INTEREST: There is no conflict
FINANCIAL SOURCE: CNPq
-
1Brasil. Ministério da Saúde. Secretaria Nacional de Ações Básicas de Saúde: Acidentes Ofídicos Contribuição ao Estudo da Morbidade. Brasília: Ministério da Saúde; 1998. 2 vols.
- 2. Cupo P, Azevedo-Marques MM, Menezes JB, Hering SE. Reações de hipersensibilidade imediata após uso intravenoso de soros antivenenos: valor prognóstico dos testes de sensibilidade. Rev Inst Med Trop São Paulo. 1991;33(2):115-22.
- 3. Abib H, Laraba-Djebari F. Effects of 60Co gamma radiation on toxicity and hemorrhagic, myonecrotic, and edema-forming activities of Cerastes cerastes venom. Can J Physiol Pharmacol. 2003;81(12):1125-30.
- 4. Zamuner SR, Cruz-Höfling MA, Corrado AP, Hyslop S, Rodrigues-Simioni L. Comparison of the neurotoxic and myotoxic effects of Brazilian Bothrops venoms and their neutralization by commercial antivenom. Toxicon. 2004;44(3):259-71.
- 5. Camey KU, Velarde DT, Sanchez EF. Pharmacological characterization and neutralization of venoms used in the production of bothropic antivenom in Brazil. Toxicon. 2002;40(5):501-9.
- 6. Oshima-Franco Y, Leite GB, Silva GH, Cardoso DF, Hyslop S, Giglio JR, Cruz-Höfling Ma, Rodrigues-Simioni L. Neutralization of the pharmacological effects of bothropstoxin-1 from Bothrops jararacussu (jararacuçu) venom by crotoxin antiserum and heparin. Toxicon. 2001;39(10):1477-85.
- 7. Butler J, Hoey BM, Swallow AJ. Radiation chemistry. Annu Rep Prog Chem. 1987;83:129-75.
- 8. Garrison WM. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem Rev. 1987;87(2):381-98.
- 9. Grosh DS, Hoopywood LE. Biological effects of radiation. 2nd ed. New York: Academic Press; 1979.
- 10. Nascimento N, Seebart CS, Francis B, Rogero JR, Kaiser II. Influence of ionizing radiation on crotoxin: biochemical and immunological aspects. Toxicon. 1996;34(1):123-31.
- 11. Delves PJ, Roitt Im. The immune system first of two parts. Adv Immunol. 2000;343(1):37-49.
- 12. Janeway CA, Travers P, Walport M, Shlomchik MJ. Imunobiologia: o sistema imune na saúde e na doença. Porto Alegre: Editora ArtMed; 2002. 767 p.
- 13. Spencer PJ, Aird SD, Boni-Mitake M, Nascimento N, Rogero JR. A single-step purification of bothropstoxin-1. Braz J Med Biol Res. 1998;31(9):1125-7.
- 14. Harlow E, Lane D. Antibodies. A laboratory manual. New York: Cold Sprig Harbor Laboratory; 1988. 726 p.
- 15. Vitaliano SN, Silva DAO, Ferreira RA, Bevilacqua E, Mineo JR. Avaliação dos níveis de anticorpos IgG anti-Toxoplasma gondii em lobos-guará (Chrysocyon brachyurus) por meio de ensaios imunoenzimáticos utilizando conjugados homólogos, heterólogos e de afinidade. Horizonte Cient. 2005;1:1-18.
- 16. Moon S, Song KB. Effect of gamma-irradiation on the molecular properties of ovalbumin and ovomucoid and protection by ascorbic acid. Food Chem. 2001;74(4):479-83.
- 17. Baptista JA, Spencer PJ, Oliveira JE, Casare MS, Nascimento N. Immune response against antigens irradiated with 60Co gamma rays. J Radioanal Nuclear Chem. 2006;269(3):565-9.
- 18. Brewer JM, Richmond J, Alexander J. The demonstration of an essential role for macrophages in the in vivo generation of IgG2a antibodies. Clin Exp Immunol. 1994; 97(1):164-71.
- 19. Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551-79.
Correspondence to:
Publication Dates
-
Publication in this collection
10 June 2009 -
Date of issue
2009
History
-
Accepted
04 July 2008 -
Received
06 June 2008