Abstract
Peptide toxins are usually highly bridged proteins with multipairs of intrachain disulfide bonds. Analysis of disulfide connectivity is an important facet of protein structure determination. In this paper, we successfully assigned the disulfide linkage of two novel peptide toxins, called HNTX-III and HNTX-IV, isolated from the venom of Ornithoctonus hainana spider. Both peptides are useful inhibitors of TTX-sensitive voltage-gated sodium channels and are composed of six cysteine residues that form three disulfide bonds, respectively. Firstly, the peptides were partially reduced by tris(2-carboxyethyl)-phosphine (TCEP) in 0.1 M citrate buffer containing 6 M guanidine-HCl at 40° C for ten minutes. Subsequently, the partially reduced intermediates containing free thiols were separated by reversed-phase high-performance liquid chromatography (RP-HPLC) and alkylated by rapid carboxamidomethylation. Then, the disulfide bonds of the intermediates were analyzed by Edman degradation. By using the strategy above, disulfide linkages of HNTX-III and HNTX-IV were determined as I-IV, II-V and III-VI pattern. In addition, this study also showed that this method may have a great potential for determining the disulfide bonds of spider peptide toxins.
disulfide bonds; TCEP; partial reduction; HNTX-III; HNTX-IV
ORIGINAL PAPER
Determination of disulfide bridges of two spider toxins: hainantoxin-III and hainantoxin-IV
Wang WI; Liu ZII; Qian WIII; Fang YI; Liang SII
ICollege of Pharmaceutical Sciences, Central South University, Changsha, Hunan, China
IICollege of Life Sciences, Hunan Normal University, Changsha, Hunan, China
IIIAdministrative Center for Basic Research, Ministry of Science and Technology, China
Correspondence to Correspondence to: Songping Liang College of Life Sciences, Hunan Normal University Changsha, Hunan, 410081, China Phone: +86 731 8872556. Fax: +86 731 8861304 Email: liangsp@hunnu.edu.cn
ABSTRACT
Peptide toxins are usually highly bridged proteins with multipairs of intrachain disulfide bonds. Analysis of disulfide connectivity is an important facet of protein structure determination. In this paper, we successfully assigned the disulfide linkage of two novel peptide toxins, called HNTX-III and HNTX-IV, isolated from the venom of Ornithoctonus hainana spider. Both peptides are useful inhibitors of TTX-sensitive voltage-gated sodium channels and are composed of six cysteine residues that form three disulfide bonds, respectively. Firstly, the peptides were partially reduced by tris(2-carboxyethyl)-phosphine (TCEP) in 0.1 M citrate buffer containing 6 M guanidine-HCl at 40° C for ten minutes. Subsequently, the partially reduced intermediates containing free thiols were separated by reversed-phase high-performance liquid chromatography (RP-HPLC) and alkylated by rapid carboxamidomethylation. Then, the disulfide bonds of the intermediates were analyzed by Edman degradation. By using the strategy above, disulfide linkages of HNTX-III and HNTX-IV were determined as I-IV, II-V and III-VI pattern. In addition, this study also showed that this method may have a great potential for determining the disulfide bonds of spider peptide toxins.
Key words: disulfide bonds, TCEP, partial reduction, HNTX-III, HNTX-IV.
INTRODUCTION
Recently, venom peptides have been attracting attention from pharmacologists and biochemists. These peptides isolated from venoms of spiders, snails, scorpions, snakes and other animals are produced in a combinational manner that leads to a great diversity (1-4). Most venom peptides are highly bridged proteins with multipairs of intrachain disulfide bonds. It is well known that disulfide bonds confer conformational stability to folded proteins. Therefore, the understanding of disulfide linkage patterns is necessary for further studies relating structure to function of venom peptides (5-7).
The traditional method for disulfide bond assignment consists of breaking the peptide chain with proteases and isolating bridged fragments. This method would work appropriately if cysteine residues were well dispersed in the peptide chain. However, it is not suitable for determining disulfide linkage patterns of venom peptides, where cysteine residues are tightly clustered; thus, enzymes rarely yield diagnostic fragments. In addition, peptide cleavage is usually produced in basic solutions, which would result in ambiguities due to disulfide scrambling problems.
Partial reduction to create a series of intermediates containing both disulfides and thiols has been widely used in assignment of disulfide bonds. Water-soluble tris(2-carboxyethyl)-phosphine (TCEP) has proven to be a better reducing reagent than dithiothreitol and 2-mercaptoethanol for this purpose. Among the advantages of TCEP are that it can selectively reduce the disulfide bonds depending on their accessibility and, more importantly, the process can be performed at acid pH to suppress the scrambling among the disulfide bonds (8, 9). Therefore, the method presented in Figure 1 can be employed to identify the disulfide linkage pattern of venom peptides. First, the peptide was partially reduced by TCEP at pH 3.0 and separated by RP-HPLC, which yielded intermediates with intact disulfide bond/bonds and free thiols. The intermediates were then alkylated with iodoacetamide (IAA) and labeled cysteine residues are determined by sequence analysis. In the current study, we utilized this method to successfully determine the disulfide bonds of HNTX-III and HNTX-IV. Both toxins are novel peptides isolated from Ornithoctonus hainana spider venom and are useful inhibitors of neuronal TTX-sensitive voltage-gated sodium channels. They have six cysteine residues that constitute three disulfide bonds as revealed by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) and sequence analysis (10, 11). The determination of disulfide linkages of both toxins may help to interpret their structure and function. Moreover, this study may also confirm that strategies employed herein are very consistent to assign disulfide bonds of venom peptides.
MATERIALS AND METHODS
Peptides and Reagents
HNTX-III and HNTX-IV were purified by ion-exchange and RP-HPLC according to our previous work (10). TCEP, IAA, trifluoroacetic acid, guanidine-HCl were purchased from Sigma (USA). Sequencing reagents were acquired from Applied Biosystems (USA) while the others consisted of analytical grades.
Partial Reduction of Peptides
TCEP stock solution (0.1 M) was prepared in 0.1 mol/L citrate buffer (pH 3). HNTX-III or HNTX-IV (0.1 mg) were dissolved in 10 µL of 0.1 mol/L citrate buffer (pH 3) containing 6 mol/L guanidine-HCl. Partial reduction of disulfide bonds was carried out by adding 10 µL of 0.1 mol/L TCEP and incubated at 40° C for 10 minutes at pH 3. The reaction was finished by submitting the solution to RP-HPLC column.
Intermediate Separation by RP-HPLC
The partially reduced intermediates were isolated by RP-HPLC (Vydac® column, C18, Grace Vydac, USA, 300 Å, 4.6 mm x 250 mm) with linear gradient elution in Waters Alliance System (USA). The gradient was 20~30% acetonitrile/0.1% trifluoroacetic acid (TFA) in 40 minutes for HNTX-III, or 18~40% acetonitrile/0.1% TFA in 40 minutes for HNTX-IV. The masses of collected peptide isomers were determined by MALDI-TOF MS. Before alkylation of partially reduced intermediates, collected solutions were concentrated to roughly 20 µL.
Rapid Carboxamidomethylation of Partially Reduced Intermediates
Appropriate isomers containing free thiols were alkylated by adding 100 µL of 0.5 mol/L iodoacetamide (pH 8.3). Approximately after 30 seconds of reaction time in darkness, 100 µL 2% TFA was added to acidify the mixture and extinguish the reaction; subsequently, alkylated peptides were desalted by RP-HPLC. The predominant fractions were collected and identified by MALDI-TOF MS and amino acid sequence analysis.
Mass Spectrometry
The molecular masses of peptides were determined by MALDI-TOF MS (Voyager-DE® STR Biospectometry workstation, Applied Biosystems, USA). Ionization was achieved through irradiation with nitrogen laser (337 nm), with 20 kV acceleration voltage whereas α -cyano-4-hydroxy-cimamic acid (CCA) was employed as matrix.
Amino Acid Sequence Analysis
The carboxymethylated peptides were submitted to N-terminal sequencing on a gas-phase automatic sequencer (model 491, Applied Biosystems, USA). The Edman degradation was performed with a normal automatic cycle program.
RESULTS AND DISCUSSION
Partial Reduction of HNTX-III and HNTX-IV
Native HNTX-III and HNTX-IV consist of 33 and 35 amino acid residues, respectively. Six cysteine residues that form three disulfide bonds are clustered together in small peptides. Besides, two cysteine residues among them are adjacent to each other (10, 11). Therefore, it is difficult to assign the disulfide connectivity of both peptides by means of traditional strategy.
TCEP has proven to be an excellent reagent that can partially reduce disulfide bonds. However, the reduction reaction may be affected by many conditions, including buffer pH, temperature, TCEP concentration, time of incubation, etc. In the beginning, the reduction of both peptides was investigated at pH ranging from 2 to 5. We found that increased acidity slowed the reaction, but it could also suppress the disulfide scrambling as much as possible. Citrate buffer (pH 3.0) was chosen for reducing both peptides. Since the two peptides presented tight structures with disulfide bonds buried in the core of molecules, high doses of guanidine-HCl (6 M) were employed to facilitate the access to buried bonds. Moreover, higher temperature and higher doses of TCEP accelerated the reaction rate, because they increased yields of partially reduced intermediates from the peptides.
Therefore, both native peptides (0.1 mg) were first dissolved in 10 µL citrate buffer (pH 3) containing 6 M guanidine-HCl and incubated at 40° C for 30 minutes. Then, the partial reduction was carried out by adding 10 µL TCEP stock solution (0.1 M) at 40° C for 10 minutes.
Characterization of Partially Reduced Intermediates of HNTX-III and HNTX-IV
Subsequently, the partially reduced intermediates were fractionated by RP-HPLC. As shown in Figure 2 A, the partial reduction of HNTX-III yielded four HPLC peaks. The first one and the last one were the native intact peptide and fully reduced isomer, respectively, whereas the two peaks between them were partially reduced intermediates, as determined by MALDI-TOF analysis. Those with mass increases of 0, 4 and 6 Da over native peptide mass corresponded to the 3-disulfide (native, N), 1-disulfide (Ia and Ib) and 0-disulfide (completely, R) species, respectively. The partial reduction of HNTX-IV also yielded high levels of partially reduced isomers and unlike that of HNTX-III, five HPLC peaks were obtained (Figure 2 B). These peaks were composed of intact peptides and partially or fully reduced ones. Those with 0-Da, 2-Da, 4-Da and 6-Da increases over native peptide mass corresponded to the 3-disulfide (native, N), 2-disulfide (II), 1-disulfide (Ia and Ib) and 0-disulfide (completely, R) species, respectively. The 2-disulfide intermediate of HNTX-III was absent in the HPLC profile, but the one from HNTX-IV was evident. This may be due to the instability of 2-disulfide intermediates of HNTX-III or because its amount was small to be detected by HPLC. The distinct structures of both peptides may explain this difference.
Sequence Analysis of Partially Reduced Intermediates
The peaks (called Ia and Ib in Figure 2 A, and named II, Ia and Ib in Figure 2 B) were collected and concentrated (about 20 µL) by lyophilization. The partially reduced peptides containing free thiols were alkylated by adding 100 µL of 0.5 mol/L iodoacetamide (pH 8.3). Reaction was quenched after 30 seconds through addition of 100 µL 2% TFA, then the mixture was immediately purified by analytical RP-HPLC. Since favorably positioned thiols would also undergo reoxidation to disulfide bonds, the rapid alkylation with iodoacetamide minimized these problems. There was a 58-Da change from original molecular weight after the alkyl group was conjugated to the single free thiol upon alkylation. The masses of the five alkylated isomers (two for HNTX-III and three for HNTX-IV) determined by MALDI-TOF corresponded to the above mass results.
Next, the alkylated cysteine residues from partially reduced intermediates were determined by sequence analysis. The HPLC profiles of cysteine cycles of partially reduced intermediates of HNTX-III are shown in Figure 3. After Edman degradation of the alkylated peak Ia in Figure 2 A, Pth-CM-Cys signals were observed in chromatograms at the 2nd, 9th, 17th and 22th cycles, while no Pth-CM-Cys signals were present at the 16th and 29th cycles (Figure 3 A). This means that the disulfide bond Cys16-Cys29 was kept intact upon reduction. In peak Ib of Figure 2 A, Pth-CM-Cys signals were only observed in chromatograms at the 2nd, 17th, 16th and 29th cycles in the HPLC cysteine profiles (Figure 3 B), indicating that Cys9 was still linked to Cys22 by a disulfide bond. By process of elimination, then, the third disulfide bond must be between Cys2 and Cys17.
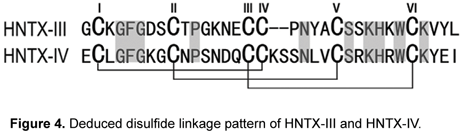
Similar results were found in the intermediate sequence analysis of HNTX-IV. As shown in Figure 4, sequence analysis of the alkylated peak II indicated that the only reduced disulfide bridge was Cys2-Cys17 (Figure 4 C), while peak Ia revealed that Cys16 was still connected to Cys31 by a disulfide bridge (Figure 4 D). Therefore, the third disulfide bridge may be between Cys9 and Cys24, which agrees with results of sequencing alkylated peak Ib (data not shown).
Disulfide Linkage Pattern of HNTX-III and HNTX-IV
The present results indicate that the disulfide linkage of HNTX-III is Cys2-Cys17, Cys9-Cys22 and Cys16-Cys29; whereas the one of HNTX-IV is Cys2-Cys17, Cys9-Cys24, and Cys16-Cys31 (Figure 4). This type of bond was named I-IV, II-V, III-VI disulfide pattern, which is common in most venom peptides that adopt inhibitor cystine knot motif.
ACKNOWLEDGEMENTS
This work was supported by the National Science Foundation (projects 30430170 and 30700127).
Received: July 14, 2008
Accepted: February 12, 2009
Abstract published online: March 5, 2009
Full paper published online: May 30, 2009
CONFLICTS OF INTEREST: There is no conflict
FINANCIAL SOURCE: National Science Foundation
- 1. Escoubas P. Molecular diversification in spider venoms: a web of combinatorial peptide libraries. Mol Divers. 2006;10(4):545-54.
- 2. Escoubas P, Rash L. Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon. 2004;43(5):555-74.
- 3. Sollod BL, Wilson D, Zhaxybayeva O, Gogarten JP, Drinkwater R, King G.F. Were arachnids the first to use combinatorial peptide libraries? Peptides. 2005;26(1):131-9.
- 4. Tedford HW, Sollod BL, Maggio F, King GF. Australian funnel-web spiders: master insecticide chemists. Toxicon. 2004;43(5):601-18.
- 5. Craik DJ, Daly NL, Waine C. The cystine knot motif in toxins and implications for drug design. Toxicon. 2001;39(1):43-60.
- 6. Norton RS, Pallaghy PK. The cystine knot structure of ion channel toxins and related polypeptides. Toxicon. 1998;36(11):1573-83.
- 7. Zhang DY, Liang SP. Assignment of the three disulfide bridges of huwentoxin-I, a neurotoxin from spider Selenocosmia huwena J Protein Chem. 1993;12(6):735-40.
- 8. Gray WR. Disulfide structures of highly bridged peptides: a new strategy for analysis. Protein Sci. 1993;2(10):1732-48.
- 9. Gray WR. Echistatin disulfide bridges: selective reduction and linkage assignment. Protein Sci. 1993;2(10):1749-55.
- 10. Liu Z, Dai J, Chen Z, Hu W, Xiao Y, Liang S. Isolation and characterization of hainantoxin-IV, a novel antagonist of tetrodotoxin-sensitive sodium channels from the Chinese bird spider Selenocosmia hainana Cell Mol Life Sci. 2003;60(5): 972-8.
- 11. Xiao Y, Liang S. Inhibition of neuronal tetrodotoxin-sensitive Na+ channels by two spider toxins: hainantoxin-III and hainantoxin-IV. Eur J Pharmacol. 2003; 477(1):1-7.
Correspondence to:
Publication Dates
-
Publication in this collection
09 June 2009 -
Date of issue
2009
History
-
Received
14 July 2008 -
Accepted
12 Feb 2009