Abstract
Schistosomes utilize proteinases to accomplish several activities such as tissue penetration, tissue digestion and evasion of host immune responses. Cathepsin L is a cysteine proteinase of the papain superfamily detected in their gut lumen which indicates that this enzyme contributes to the proteolysis of ingested hemoglobin. Due to the roles played in the schistosome biology, proteolytic enzymes are considered potential targets for developing and guiding antischistosomal therapies. In the present work, the cathepsin L1 cDNA coding of Schistosoma mansoni was cloned into the pAE vector that provides high-level expression of heterologous proteins in Escherichia coli. The recombinant protein was expressed as inclusion bodies, purified under denaturing conditions through nickel-charged chromatography and used for experimental animal vaccination. ELISA was performed with the pooled sera. Although this protein was shown to be immunogenic, mice immunized with three doses of recombinant protein plus aluminum hydroxide as adjuvant were not protected against S. mansoni infection.
Schistosoma mansoni; cysteine proteinase; cathepsin L1; vaccine; immunogenicity
ORIGINAL PAPER
Expression of schistosomal cathepsin l1 in Escherichia coli and evaluation of its protective capacity in an animal challenge
Miyasato PAI; Ramos CRRII; Abreu PAEIII; Dias WOII; Nascimento CI; Ho PLII; Kawano TI
IDepartment of Parasitology, Butantan Institute, São Paulo, São Paulo State, Brazil
IIBiotechnology Center, Butantan Institute, São Paulo, São Paulo State, Brazil
IIILaboratory of Bacteriology, Butantan Institute, São Paulo, São Paulo State, Brazil
Correspondence to Correspondence to: Paulo Lee Ho Centro de Biotecnologia, Instituto Butantan Av. Vital Brasil, 1500, São Paulo, SP, 05503-900, Brasil Phone: +55 11 3726 7222, ext. 2244. Fax: +55 11 3726 1505 Email: hoplee@butantan.gov.br.
ABSTRACT
Schistosomes utilize proteinases to accomplish several activities such as tissue penetration, tissue digestion and evasion of host immune responses. Cathepsin L is a cysteine proteinase of the papain superfamily detected in their gut lumen which indicates that this enzyme contributes to the proteolysis of ingested hemoglobin. Due to the roles played in the schistosome biology, proteolytic enzymes are considered potential targets for developing and guiding antischistosomal therapies. In the present work, the cathepsin L1 cDNA coding of Schistosoma mansoni was cloned into the pAE vector that provides high-level expression of heterologous proteins in Escherichia coli. The recombinant protein was expressed as inclusion bodies, purified under denaturing conditions through nickel-charged chromatography and used for experimental animal vaccination. ELISA was performed with the pooled sera. Although this protein was shown to be immunogenic, mice immunized with three doses of recombinant protein plus aluminum hydroxide as adjuvant were not protected against S. mansoni infection.
Key words: Schistosoma mansoni, cysteine proteinase, cathepsin L1, vaccine, immunogenicity.
INTRODUCTION
Schistosomiasis remains a major public health problem in developing countries. It is estimated that 200 million people are infected by it and six hundred million live in risk areas (1). Despite two decades of widespread chemotherapy with safe and effective drugs, the number of individuals with schistosomiasis remains high (2). The construction of water resources and agricultural requirements for human development have led to increasing transmission of schistosomiasis (3). Control of its transmission can be achieved by elimination of intermediary hosts, use of drugs like oxamniquine and praziquantel, besides providing adequate socioeconomic conditions and sanitary education for the population. However, the risk of reintroduction of the disease into a treated area, particularly where there are irrigation projects and population migration, must be taken into account (4, 5).
Reinfection demands repeated treatment and continued administration of medication, which can favor the emergence of drug-resistant parasites (6). Moreover, supplying efficient treatment that covers all parts of an endemic area can make chemotherapy an expensive and impractical alternative (7).Thus, vaccination would be a necessary approach to complement chemotherapy (8).
Several factors make schistosomiasis vaccine development an advantageous option:
protective mechanisms have been demonstrated in experimental animals,
rapid reinfection demands continuing treatment,
the risk of drug-resistance caused by expanded chemotherapy programs,
acquired partial immunity reduces the infection intensity as observed in adolescent and older age groups compared to children.
Control based on chemotherapy followed by vaccination would result in a short-term effect with long-term protection (2, 9). Thus, a definitive vaccine must be elaborated from diverse recombinant antigens and from different parasite stages to improve the possibilities for synergistic action by involving separate defense mechanisms (9).
Secreted or surface proteins are crucial to maintaining the pathway of parasites through their molecular interaction with host cells. Since these molecules are exposed to the host immune system, they become candidate targets for vaccine development and chemotherapy (10).
Many histochemical and immunolocalizational studies report that cathepsin L (EC 3.4.22.15) is packed into the lysosomal-like vesicles of gastrodermal cells of helminthes and plays an important role in nutrient acquisition (11). Its strong involvement in hemoglobin digestion indicates that this cysteine proteinase is important to Schistosoma mansoni (12). Cathepsin L1 was first isolated by Smith et al. (13) whereas its presence was detected in gastrodermal cells lining the gut and in excretory/secretory (ES) products (14), as later confirmed by immunolocalization studies (15). Michel et al. (16) constructed an expression plasmid in E. coli to produce recombinant schistosome cathepsin L. The utilization of purified cathepsins L1 and L2 from Fasciola hepatica for vaccination in rats, sheep and cattle has induced protection, ranging from 33 to 80%, in experimental challenge with F. hepatica metacercariae as well as provoked potent anti-embryonation/hatch rate effects (17, 18). However, there are still no studies on recombinant SmCL1 (cathepsin L1 from Schistosoma mansoni) as a vaccine antigen candidate against S. mansoni infection. Therefore, the general objective of the current work was to verify SmCL1 immunogenicity and feasibility as a vaccine antigen against this worm.
MATERIALS AND METHODS
Bacterial Strains and Plasmids
Escherichia coli DH5α and BL21-SI strains (Invitrogen, USA) were employed in all routine cloning and expression experiments. In the BL21-SI strain, the T7 RNA polymerase expression was controlled by the osmotically inducible promoter proU (19).
Parasites
Snail intermediate hosts (Biomphalaria glabrata) were infected with S. mansoni BH strain. Cercariae were allowed to shed from infected snails for two hours under a halogen lamp. Cercarial counts and viability were determined through stereomicroscopy (20).
Cloning Techniques
Total RNA was isolated from S. mansoni worms with Trizol® reagent (Invitrogen, USA). Two micrograms of total RNA was reverse-transcribed by SuperScript II® reverse transcriptase enzyme (Invitrogen, USA) using an oligo(dT)18 primer (GE Healthcare, USA). The first cDNA strand was utilized as a reference in the PCR reaction according to the following sense and antisense primers: forward SmCL1 5' ATGATATCCATATGCCTGTAAACCTCGAGTAC 3' and reverse SmCL1 5' GCACGCGTTAGTCGACGTAGATCATCGCTGACGTAGC 3'. Underlined sequences indicate NdeI restriction site in the forward primer and MluI and SalI restriction sites in the reverse primers, respectively. The PCR product was purified from agarose gels after electrophoretic separation using a commercial kit, cloned into pGEM®-T vector (Promega, USA), and sequenced with the T7 and SP6 promoter primers. The nucleotide sequence analysis of SmCL1 cDNA was performed on the ABI PRISM® 377 sequencer (Applied Biosystems, USA) using the ABI PRISM Big Dye® Terminator cycle sequencing kit. The cDNA sequence was subcloned into the expression vector based on pAE plasmid,modified to allow the expression of the recombinant protein with a His6 tag fusion in the C-terminal end at the NdeI and SalI sites, resulting in the pAE SmCL1 plasmid (21).
Expression and Purification of the Recombinant SmCL1 Protein
The pAE SmCL1 plasmid was employed to transform Escherichia coli BL21 SI strain. A half liter of LBON (Luria-Bertani medium without NaCl) was inoculated with 15 mL of the overnight bacterial culture from single colonies and grown until the optical density at 600 nm reached 0.8. The expression of recombinant protein was induced with 200 mM NaCl, overnight at 30ºC. The cells were harvested by centrifugation and the bacterial cell pellet was resuspended in a solution containing 20 mM Tris HCl (pH 8.0), 0.5 M NaCl and lysed in a French pressure cell.
The inclusion bodies containing the recombinant protein were isolated from bacterial lysates by centrifugation, and solubilized with 50 mL of denaturation buffer (8 M urea, 20 mM Tris HCl, pH 8.0, 0.5 M NaCl, 5 mM imidazole and 1 mM β-mercaptoethanol) and stirred for 16 hours at room temperature. The recombinant protein present in the suspension was purified by metal-affinity chromatography in Chelating-Sepharose® Fast Flow resin (GE Healthcare, USA), previously charged with 300 mM NiSO4 and equilibrated with denaturation buffer. The column was washed with ten volumes of denaturation buffer (8 M urea, 20 mM Tris HCl, pH 8.0, 0.5 M NaCl, 5 mM imidazole and 1 mM β-mercaptoethanol). This procedure was followed by three additional washings: ten volumes of a buffer (20 mM Tris HCl, 0.5 M NaCl, pH 8.0) containing 6 M urea and 20 mM imidazole in the first wash; ten volumes of the same buffer containing 4 M urea and 40 mM imidazole in the second wash; and ten volumes of the same buffer containing 3 M urea and 60 mM imidazole in the third wash.
The recombinant protein was eluted with five volumes of the elution buffer containing 20 mM Tris HCl, 0.5 M Na Cl (pH 8.0), 3 M urea and 1 M imidazole. Several steps of dialysis were also performed: first against a solution containing 20 mM Tris HCl, 0.5 M NaCl (pH 8.0), 2 M urea, 0.1% glycine, 10 mM EDTA and 0.5 M imidazole; a second step, against a solution containing 20 mM Tris HCl, 0.5 M NaCl, (pH 8.0), 2 M urea, 0.1% glycine and 10 mM EDTA. The last dialysis was against phosphate-buffered saline solution (PBS), 0.1% glycine and 2 M urea. Purified protein samples were analyzed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Immunization of Swiss Mice and Challenge with S. mansoni Cercariae
Two independent experiments were conducted in accordance with Brazilian animal protection and welfare laws and the research project was approved by the Ethics Committee on the Use of Animals (protocol number 160/2004), Butantan Institute.
Ten outbred female Swiss mice (4 to 6 weeks old, Butantan Institute) received footpad immunization with three doses of 20 μg of recombinant protein in PBS emulsified in aluminum hydroxide as adjuvant at intervals of seven days. The recombinant protein was adsorbed onto 10% (vol/vol) of Alhydrogel (2% Al(OH)3 Biosector, formerly Superfos Biosector, Denmark). At this concentration, the protein adsorption capacity of the adjuvant is 2.3 +/- 0.5 mg per mL, which is within the range of protein concentration used. The adjuvant was provided by the Vaccine Quality Control Section of Butantan Institute and we specifically followed the procedures routinely used in the section.
Control mice were injected with PBS in aluminum hydroxide. One day before each immunization, mice were bled from the retro-orbital plexus, and enzyme-linked immunosorbent assay (ELISA) was performed with pooled sera. Sixty days after the last immunization, animals were challenged by subcutaneous injection with 100 cercariae (each mouse) and perfused 45 days later. Protection was calculated by the equation [(C _ V)/C] x 100, where C is the average number of worms in control animals and V is the average number of worms in vaccinated animals, expressed as a percentage. Statistical analysis was done with Student's t-test (p < 0.05).
ELISA
ELISA was carried out according to Sato et al. (22). Plates were coated overnight at 4ºC with rSmCL1 (10 μg/mL), and blocked with 10% nonfat dry milk in PBS-T (PBS with 0.05% Tween-20) at 37ºC for one hour. Subsequently, plates were washed three times with PBS-T. After washes, serial dilutions with immunized animal sera were performed at 37ºC, for one hour each dilution. The reaction with goat anti-mouse total immunoglobulin G (IgG) conjugated to horseradish peroxidase (HRP) (dilution 1:15,000 - Sigma, USA) was performed in PBS-1% nonfat dry milk at 37ºC, for one hour and then washed with PBS-T. For HRP substrate, we added q-phenylenediamine (0.04%) in citrate phosphate buffer (pH 5.0) plus 0.01% H2O2 and the reaction was stopped by the addition of 50 mL of 4 M H2SO4. The absorbance was measured at 492 nm. The antibody titer was considered the last dilution of sera that registered an optical density of 0.1.
RESULTS
Cloning of SmCL1 cDNA
cDNA was obtained from RNA isolated from adult worms of the Brazilian endemic BH S.mansoni strain. The cDNA sequence of recombinant protein SmCL1 was amplified from two independent RT-PCRs. The amplified segments were cloned into pGEM-Tand five independent clones were sequenced. DNA sequence analysis indicated that SmCL1 cDNA sequences were the same found by Smith et al. (13).
Expression and Purification of Recombinant SmCL1
The expected band of recombinant SmCL1 protein (36.95 kDa) was observed, by SDS-12% PAGE, in bacterial cell lysates from induced cultures (Figure 1, lane 3). The recombinant SmCL1 was present in the pellet of bacterial cell lysates in an insoluble form as inclusion bodies, as revealed by 12% SDS-PAGE (Figure 1, lane 4). Recombinant proteins present in inclusion bodies were isolated, solubilized and purified (Figure 1, lane 6) under denaturing conditions through nickel charged chromatography, as previously described. The purified recombinant protein yield was approximately 50 mg per liter of total induced culture.
Antibody Response and Protection against Challenge with S. mansoni Cercariae in Mice Immunized with the Recombinant SmCL1
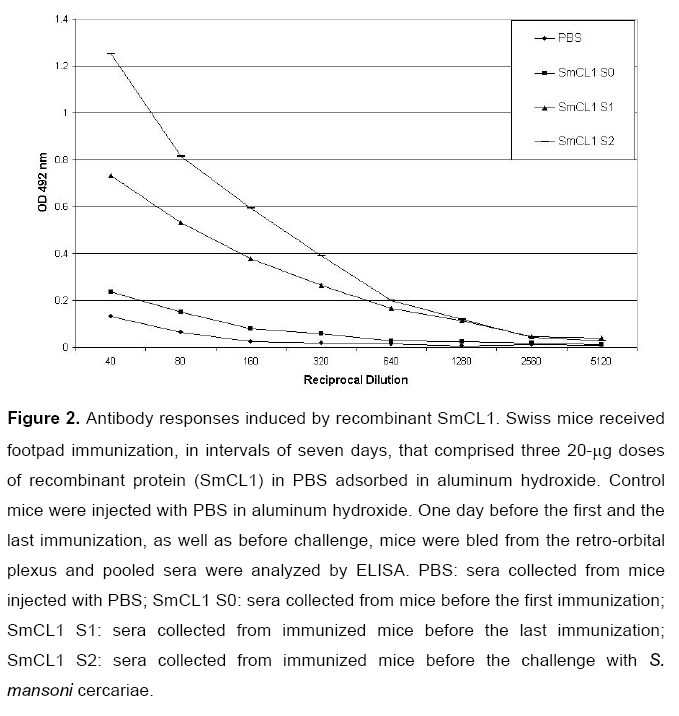
Swiss mice were immunized with three 20-μg doses of purified recombinant SmCL1 according to Materials and Methods. Negative control mice were injected with PBS in the presence of the adjuvant. Sera from the immunized animals were analyzed by ELISA using recombinant SmCL1 as coating antigen. Experimental group showed higher titer of anti-SmCL1 antibodies (1/1 280) than the control group (Figure 2). Animals were challenged with S. mansoni cercariae and the protection level was calculated by comparing the number of adult worms recovered from the experimental vaccinated group with the number from the control group. Mice immunized with recombinant SmCL1 showed a decrease of 21.8% in worm burden (Figure 3). However, the difference in the number of worms recovered from SmCL1 group and from the control group was not statistically significant. Recently, a SmCL1 related protein, called SmCL3, was described in databanks (unpublished results, accession n. EU022371) (Figure 4). We do not know if anti-SmCL1 also cross reacts with SmCL3.
DISCUSSION
The results presented herein showed the expression of the recombinant SmCL1 protein in Escherichia coli BL21 SI strain, with high final yield of purified protein. The expressed protein displayed six histidine residues at the carboxy terminus of the recombinant protein, which allowed its purification through affinity chromatography on nickel-charged beads. The recombinant protein was expressed as insoluble inclusion bodies, thus a solubilization step was necessary. In the final purification step, urea concentration in the buffer was maintained at 2 M to avoid protein precipitation, as observed in lower concentrations of urea.
The purified recombinant SmCL1 was not able to hydrolyze hemoglobin (results not shown), which indicates that the purified enzyme was inactive. However, Verity et al. (23) carried out immunization assays with two forms of recombinant cathepsin D of Schistosoma japonicum, one purified from Escherichia coli that was enzymatically inactive and the other, from insect cells, that was enzymatically active. The highest level of protection was achieved in animals immunized with the purified protein from E. coli, suggesting that even the inactive form of the protein can provide significant epitopes that contribute for protection. Therefore, immunizations were carried out with the inactive form of the recombinant SmCL1 expressed in E. coli to verify its immunoprophylactic potential.
The SmCL1 antigen showed to be immunogenic, being able to induce a more significant titer of anti-SmCL1 antibodies (1/1,280) than the control group. Nevertheless, mice immunized with recombinant SmCL1 showed only a 21.8% decrease in worm burden, below of the goal recommended by WHO (40%). In contrast, homologous cathepsin L1 of Fasciola hepatica revealed positive correlation between antibody titer and protection against challenge with Fasciola hepatica metacercariae (24). Moreover, in a recent study, Kesik et al. (18) obtained 78 to 80% of protection against challenge with metacercariae, using recombinant cathepsin L of Fasciola hepatica produced in E. coli.
At least three reasons could explain the lack of protection of the recombinant SmCL1 in our conditions. First, there is the possibility that this protein do not provide a complete protection. In this case, it would be necessary to co-administer this antigen with other antigens or adjuvants.
The second factor that could have influenced the protection response of recombinant SmCL1 was the lack of its correct folding, since it was not able to hydrolyze hemoglobin (results not shown). This consequence is distinct from that obtained for the recombinant inactive form of cathepsin D of Schistosoma japonicum, which showed significant protective activity, slightly higher than the recombinant active form produced in insect cells (23).
In our conditions, protective epitopes may not be presented to the immunized animals. In this regard, it would be interesting to express this antigen in another bacterial expression system, for instance in fusion with maltose binding protein in an attempt to express the protein in a soluble form as used for Schistosoma mansoni tegumental Sm13 antigen (25) or using a protocol able to produce functional and correctly folded F. hepatica cathepsin L in E. coli (18). On the other hand, expression systems like yeast or insect cells can also be explored in order to obtain functional SmCL1 and to evaluate if properly folded, the recombinant protein is able to improve the level of protection observed in unfolded proteins expressed in E. coli, as described herein. Interestingly, it was observed that even small differences in recombinant proteins may interfere in protection ability. For instance, vaccination and challenge experiments in rats with Fasciola hepatica procathepsin L3 protein, produced in yeasts or insect cells, showed that only the protein expressed in insect cells was able to induce protection, in contrast to the yeast derived antigen, although both elicited similar antibody responses (26).
The third reason that could elucidate the shortage of protection of the recombinant SmCL1 in the present study is the occurrence of positive diversification of cysteine proteinase genes in helminthes. The available data suggests that parasites possess an ample and complex panel of proteinases (11). Possibly, they take advantage of such multiple proteinases for immune evasion. The activity of several proteases _ other than SmCL1 _ that could participate in hemoglobin proteolysis, like cathepsin L2 or cathepsins B, C and D, could have contributed to the lack of protection of SmCL1. Each of these enzymes has an optimum pH for activity; consequently, the gradual decline in pH of the blood meal, from physiological to acidic pH in the schistosome gut, provides adequate conditions for each enzyme to degrade hemoglobins systematically. Probably, they act synergistically or possess activity redundancies to ensure the hemoglobin catabolism (27, 28). In this regard, it is expected that immunization with a combination of such proteases would improve the protection response. Moreover, the presence of paralogs in the same organisms would also assure this redundant effect in order to avoid function loss of SmCL1.
The analysis of Schistosoma mansoni transcriptome provides an important support for the comprehension of molecular mechanisms involved in the nutrition and metabolism of this parasite; the host-dependent development and maturation; the immune evasion and evolution mechanisms; as well as new potential vaccine candidates and drug targets (29). On this matter, S. mansoni transcriptome provided us an opportunity to search for potential SmCL1 paralogs (30). Some new sequences found in "Schistosoma mansoni EST Genome Project" _ assembled in S. mansoni EST sequences (SmAEs) and transcriptome analysis in Services Mirror _ IQ USP (http://verjo18.iq.usp.br/schisto/) _ presented similarity with other cathepsins L of the following organisms: Sarcophaga peregrina (SmSP), Fasciola gigantica (SmFG), Engraulis japonicus (SmEJ), Delia radicum (SmDR) and Tenebrio molitor (SmTM), showing that Schistosoma mansoni possesses more than the three full length cathepsins L-like proteins characterized so far (SmCL1, SmCL2 and SmCL3).
By alignment of the amino acid sequences from these cathepsins (Figure 4), we can observe variable identities of 17.4 to 37% among all the Schistosoma mansoni cathepsins L-like sequences with SmCL1. However, it was evident that the transcribed sequence uncovered by transcriptomic studies that present the best hit against Sarcophaga peregrina cathepsin L protein (SmSP) is, in fact, identical or close related to SmCL3 sequence recently deposited in the databanks (unpublished results, accession n. EU022371). The multiplicity of cathepsin L-like isoforms in Schistosoma mansoni may allow the parasite to escape from the immune response elicited by the vaccination with recombinant SmCL1. In this case, the use of associated paralogs may avoid the evasion mechanism and improve protection against infections. Indeed, immunization with F. hepatica cathepsin L1 combined with cathepsin L2 provides a higher level of protection than with the use of each antigen alone (31).
The development of an anti-schistosomiasis vaccine is an enormous challenge, due to the complexity of parasite-host interaction that results in immune response. It is possible that the lack of the protection observed in our experimental conditions are the combined results of all factors discussed before. Further studies must be carried out to clarify these points and demonstrate the feasibility of S. mansoni cathepsin L1 as a vaccine antigen.
The current Schistosoma vaccine candidates are not very effective. It is important to identify new antigens and to explore vaccination strategies to improve vaccine efficacy. Functional annotation of S. mansoni transcriptome and the public accessibility to its database (http://verjo18.iq.usp.br/schisto/), in combination with postgenomic technologies, provide the potential to identify a new generation of potential vaccine target molecules (7).
ACKNOWLEDGEMENTS
The present study was supported by the Butantan Foundation.
Received: July 23, 2008
Accepted: March 25, 2009
Abstract published online: March 31, 2009
Full paper published online: May 30, 2009
CONFLICTS OF INTEREST: There is no conflict
FINANCIAL SOURCE: Butantan Foundation
-
1World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. Report of the Joint WHO Expert Committees. WHO technical report series: 912. Geneva: World Health Organization. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_912.pdf
- 2. Bergquist, NR. Schistosomiasis vaccine development: progress and prospects. Mem Inst Oswaldo Cruz. 1998;93(Suppl I):95-101.
- 3. Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77(1):41-51.
- 4. Al-Sherbiny M, Osman A, Barakat R, El Morshedy H, Bergquist NR, Olds R. In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens. Acta Trop. 2003;88(2):117-30.
- 5. Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82(2):139-46.
- 6. Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123:S211-28.
- 7. McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21(1):225-42.
- 8. Bergquist NR, Al-Sherbiny M, Barakat R, Olds R. Blueprint for schistosomiasis vaccine development. Acta Trop. 2002;82(2):183-92.
- 9. Bergquist NR. Schistosomiasis vaccine development: approaches and prospects. Mem Inst Oswaldo Cruz. 1995;90(2):221-27.
- 10. Dalton JP, Brindley PJ, Knox DP, Brady CP, Hotez PJ, Donnelly S, O'Neill SM, Mulcahy G, Loukas A. Helminth vaccines: from mining genomic information for vaccine targets to systems used for protein expression. Int J Parasitol. 2003;33(5-6):621-40.
- 11. Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP. Proteinases and associated genes of parasitic helminths. Adv Parasitol. 1999;43:161-266.
- 12. Halton DW. Nutritional adaptations to parasitism within the platyhelminthes. Int J Parasitol. 1997;27(6):693-704.
- 13. Smith AM, Dalton JP, Clough KA, Kilbane CL, Harrop SA, Hole N, Brindley PJ. Adult Schistosoma mansoni express cathepsin L proteinase activity. Mol Biochem Parasitol. 1994;67(1):11-9.
- 14. Brady CP, Dowd AJ, Brindley PJ, Ryan T, Day SR, Dalton JP. Recombinant expression and localization of Schistosoma mansoni cathepsin L1 support its role in the degradation of host hemoglobin. Infect Immun. 1999;67(1):368-74.
- 15. Bogitsh BJ, Dalton JP, Brady CP, Brindley PJ. Gut-associated immunolocalization of the Schistosoma mansoni cysteine proteases, SmCL1 and SmCL2. J Parasitol. 2001;87(2):237-41.
- 16. Michel A, Ghoneim H, Resto M, Klinkert MG, Kunz W. Sequence, characterization and localization of a cysteine proteinase cathepsin L in Schistosoma mansoni Mol Biochem Parasitol. 1995;73(1-2):7-18.
- 17. Dalton JP, Neill SO, Stack C, Collins P, Walshe A, Sekiya M et al. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. Int J Parasitol. 2003;33(11): 1173-81.
- 18. Kesik M, Jedlina-Panasiuk L, Kozak-Cieszczyk M, Plucienniczak A, Wedrychowicz H. Enteral vaccination of rats against Fasciola hepatica using recombinant cysteine proteinase (cathepsin L1). Vaccine. 2007;25(18):3619-28.
- 19. Bhandari P, Gowrishankar J. An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J Bacteriol. 1997; 179(13):4403-6.
- 20. Tendler M, Brito CA, Vilar MM, Serra-Freire N, Diogo CM, Almeida MS, et al. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc Natl Acad Sci USA. 1996;93(1):269-73.
- 21. Ramos CRR, Abreu PAE, Nascimento ALTO, Ho PL. A high-copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz J Med Biol Res. 2004;37(8):1103-9.
- 22. Sato Y, Cowell JL, Sato H, Burstyn DG, Manclarck CR. Separation and purification of the hemagglutinins from Bordetella pertussis Infect Immun. 1983; 41(1):313-20.
- 23. Verity CK, McManus DP, Brindley PJ. Vaccine efficacy of recombinant cathepsin D aspartic protease from Schistosoma japonicum Parasite Immunol. 2001;23(3):153-62.
- 24. Mulcahy G, O'Connor F, McGonigle S, Dowd A, Clery DG, Andrews SJ, Dalton JP. Correlation of specific antibody titre and avidity with protection in cattle immunized against Fasciola hepatica Vaccine. 1998;16(9-10):932-9.
- 25. Chaves AC, Silveira E, Bezerra RP, Moreira KA, Lucena-Silva NLC, Abath FGC et al. Influence of partition parameters on a recombinant antigen of Schistosoma mansoni expressed on E. coli using poly(ethylene glycol)-hydroxypropyl starch aqueous two-phase system. World J Microbiol Biotechnol. 2002;18(7):645-8.
- 26. Reszka N, Cornelissen JB, Harmsen MM, Biénkowska-Szewczyk K, de Bree J, Boersma WJ, Rijsewijk FA. Fasciola hepatica procathepsin L3 protein expressed by a baculovirus recombinant can partly protect rats against fasciolosis. Vaccine. 2005;23(23):2987-93
- 27. Brindley PJ, Kalinna BH, Dalton JP, Day SR, Wong JY, Smythe ML, MacManus DP. Proteolytic degradation of host hemoglobin by schistosomes. Mol Biochem Parasitol.1997;89(1):1-9.
- 28. Dalton JP, Clough KA, Jones MK, Brindley PJ. Characterization of the cathepsin-like cysteine proteinases of Schistosoma mansoni Infect Immun. 1996;64(4):1328-34.
- 29. Hu W, Brindley PJ, McManus DP, Feng Z, Han ZG. Schistosome transcriptomes: new insights into the parasite and schistosomiasis. Trends Mol Med. 2004;10(5):217-25.
- 30. Verjovski-Almeida S, De Marco R, Martins EA, Guimarães PE, Ojopi EP, Paquola, AC et al. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni Nat Genet. 2003;35(2):148-57.
- 31. Piacenza L, Acosta D, Basmadjian I, Dalton JP, Carmona C. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Infect Immun. 1999;67(4):1954-61.
Correspondence to:
Publication Dates
-
Publication in this collection
02 July 2009 -
Date of issue
2009
History
-
Accepted
25 Mar 2009 -
Received
23 July 2008