Abstract
With a view toward investigating the feeding behavior of Culicidae mosquitoes from an area of epizootic yellow fever transmission in the municipalities of Garruchos and Santo Antônio das Missões, Rio Grande do Sul State, Brazil, specimens were collected by aspiration from September 2005 to April 2007. The engorged females were submitted to blood meal identification by enzyme-linked immunosorbent assay (ELISA). A total of 142 blood-engorged samples were examined for human or monkey blood through species-specific IgG. Additional tests for specificity utilizing isotypes IgG1 and IgG4 of human monoclonal antibodies showed that only anti-human IgG1 was effective in recognizing blood meals of human origin. The results indicated a significant difference (p = 0.027) in detection patterns in samples of Haemagogus leucocelaenus recorded from human blood meals at Santo Antônio das Missões, which suggests some degree of exposure, since it was an area where epizootic outbreaks have been reported.
Culicidae; ELISA; feeding habits; isotypes; yellow fever
Biotin-avidin sandwich elisa with specific human isotypes IgG1 and IgG4 for Culicidae mosquito blood meal identification from an epizootic yellow fever area in Brazil
Marassá AMI; Paula MBII; Gomes ACII; Consales CAIII
IAdolfo Lutz Institute, São Paulo, São Paulo State, Brazil
IIDepartment of Epidemiology, School of Public Health, University of São Paulo, USP, São Paulo, São Paulo State, Brazil
IIIPasteur Institute, São Paulo, São Paulo State, Brazil
Correspondence to Correspondence to: Ana Maria Marassá Laboratório de Parasitoses Sistêmicas Instituto Adolfo Lutz, São Paulo, SP, Brasil. Phone: +55 11 3068-2891. Email: anamarassa@usp.br.
ABSTRACT
With a view toward investigating the feeding behavior of Culicidae mosquitoes from an area of epizootic yellow fever transmission in the municipalities of Garruchos and Santo Antônio das Missões, Rio Grande do Sul State, Brazil, specimens were collected by aspiration from September 2005 to April 2007. The engorged females were submitted to blood meal identification by enzyme-linked immunosorbent assay (ELISA). A total of 142 blood-engorged samples were examined for human or monkey blood through species-specific IgG. Additional tests for specificity utilizing isotypes IgG1 and IgG4 of human monoclonal antibodies showed that only anti-human IgG1 was effective in recognizing blood meals of human origin. The results indicated a significant difference (p = 0.027) in detection patterns in samples of Haemagogus leucocelaenus recorded from human blood meals at Santo Antônio das Missões, which suggests some degree of exposure, since it was an area where epizootic outbreaks have been reported.
Keywords: Culicidae, ELISA, feeding habits, isotypes, yellow fever.
INTRODUCTION
Forest canopy mosquitoes of the genera Haemagogus and Sabethes are known to transmit yellow fever and have recently been involved in major outbreaks of the disease in Brazil (1). Understanding their blood-feeding habits is thus of the utmost importance and permits the estimation of the degree of the human-vector contact significant to epidemiological studies.
In recent years, several investigators have adapted the enzyme-linked immunosorbent assay (ELISA) to identify hosts from which mosquitoes had taken a blood meal (2-6). In addition, the biotin-avidin sandwich ELISA assay has also been included in analyses of mosquito feeding habits and other arthropod-borne diseases (7-10).
This study sought to evaluate species-specific immunoglobulin IgG and human isotypes IgG1 and IgG4 through the use of monoclonal antibodies and exploit the specificity attainable for to identify human blood meals consumed by mosquitoes in an area of epizootic yellow fever transmission in the state of Rio Grande do Sul, Brazil.
MATERIALS AND METHODS
Study Area and Culicidae Sampling
Field studies were conducted at two localities in Rio Grande do Sul state, namely Santo Antônio das Missões (55º21'W, 28º29'S) and Garruchos (55º45'W, 28º17'S), from September 2005 to April 2007. Garruchos, situated on the western side of the Uruguay River, is a rural municipality 13 km from Santo Antônio das Missões.
A large-bore aspirator was utilized to collect resting mosquitoes from vegetation at two sites in Garruchos one of them located on Cachoeirinha farm and the other in the São José Velho area while in Santo Antônio das Missões, only one site was selected for investigation (11). Engorged female samples were placed individually in microtubes, transported to the laboratory and stored at 20ºC until the ELISA test could be performed. Mosquitoes were identified to the species level in accordance with Consoli and Oliveira (12) and Forattini (13).
Blood Meal Analyses
Blood-fed mosquitoes were identified using the biotin-avidin ELISA by the methodology of Marassá et al. (9).
The behavior of feeding on humans or monkeys was investigated using host-specific anti-human IgG (6284-00, Zymed, USA); anti-monkey IgG (617-101-012, Rockland, USA); biotin-conjugated anti-human IgG (6284-40, Zymed, USA); and biotin-conjugated anti-monkey IgG (617-106-012, Rockland, USA). All the tests carried out with extracts that were positive for human or monkey blood, by ELISA, included human IgG1 and IgG4 monoclonal antibodies.
ELISA Assay Optimization
Reagent concentrations were optimized by checkerboard titrations to determine the highest sensitivity and lowest background of mosquito suspensions. Ninety-six-well microplates (Nunc®, Maxisorp, Denmark) were coated with 50 µL/well of anti-human IgG1 (05-3300, Zymed, USA) and anti-human IgG4 (05-3800, Zymed, USA) diluted in PBS at concentrations of 20, 10, 5, 2.5, 1.25 and 0.625 µg/mL and incubated overnight at 4ºC. The plates were blocked with PBS/1% gelatin (Difco, Laboratory Inc., USA) and kept covered at room temperature for three hours.
Each plate was washed five times with PBS 0.05% Tween-20 (P-1379, Sigma, USA); then the competitive reaction was carried out in two consecutive wells by the addition of 50 µL/well of PBS/0.1% gelatin and 50 µL/well of positive control pool on each plate. The pool of 14 females that had fed on a human for artificial xenodiagnosis was diluted from 1:2 to 1:64. After 18 hours at 4ºC, biotin-conjugated anti-human IgG1 (05-3340, Zymed, USA) and biotin-conjugated anti-human IgG4 (05-3840, Zymed, USA) were added. After one hour at room temperature, 50 µL/well of avidin-alkaline phosphatase conjugate (A7294, Sigma, USA) in PBS/0.1% gelatin was added in each well. After another hour, the enzymatic reaction was obtained by adding p-nitrophenyl phosphatase (Sigma Chemical, USA) to diethanolamine buffer. Absorbance was measured by spectrophotometry (Multiskan® EX, Thermo Scientific, USA) at a wavelength of 405 nm.
Data Analyses
The chi-squared (χ2) test for proportions was calculated with Excel® (Microsoft, USA) for Windows XP to ascertain whether frequencies of human and monkey samples differed significantly (p < 0.05).
RESULTS
The ELISA system for human and monkey blood meals was evaluated for specificity by testing a total of 142 blood-engorged specimens of Culicidae mosquitoes collected at the two municipalities. According to Tables 1, 2 and 3, polyclonal anti-human IgG and anti-monkey IgG cross-reacted and could not provide the higher specificity needed for the detection of human or monkey blood meals.
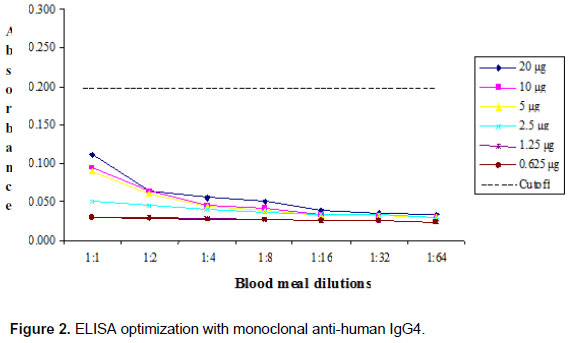
Additional tests for specificity were assayed with monoclonal antibodies, anti-human IgG1 and IgG4. Checkerboard titrations were undertaken to determine the highest specificity and lowest background of mosquito suspensions and the results are shown in Figures 1 and 2.
The absorbance value that determined the cutoff point to optimize the immunoenzymatic capture technique against IgG1 mAb was 0.207 (Figure 1). By contrast, absorbance values detected by ELISA against IgG4 mAb did not exceed the cutoff value of 0.200 to identify human blood meal samples (Figure 2).
The optimum concentration of anti-human IgG1 mAb for coating the plates was 5 µg/mL and according to what had been previously established for blood-fed specimens of Culex quinquefasciatus, dilution of biotin-anti-human IgG1 mAb and avidin was incorporated (8).
Serum dilutions from the primates Cebus spp. and Alouatta spp. were tested against IgG1 mAb, and were not detected by ELISA. The absorbance values for both Cebus spp. and Alouatta spp. were less than 0.030 while the mean absorbance cutoff for human blood meal samples was 0.207.
During processing of field-collected mosquitoes, samples investigated for human or monkey blood meal sources comprised specimens of: Aedes crinifer, Aedes serratus, Aedes scapularis, Culex (Culex) spp., Culex (Melanoconion) spp., Haemagogus leucocelaenus, Psorophora ferox, Psorophora albigenu, Sabethes albiprivus, Sabethes chloropterus and Sabethes quasicyaneus.
At Santo Antônio das Missões, Haemagogus leucocelaenus, Aedes scapularis and Sabethes albiprivus were the species that most frequently fed on humans (Table 1). At Cachoeirinha, Garruchos, the results indicated that Aedes scapularis and Haemagogus leucocelaenus had also consumed human blood (Table 2) whereas in the area of São José (Table 3) the frequency was not so evident and no difference was observed between these two sites (p < 0.05).
The present data reveal a significant difference only among samples of Haemagogus leucocelaenus that had fed on human and monkey blood at Santo Antônio das Missões (p = 0.027).
DISCUSSION
Knowledge of mosquito host-feeding patterns is an important indicator for the estimation of species vector potential in disease transmission cycles, and is of great value in the establishment of control measures.
Currently, methods for the identification of insect blood meals have included ELISA, which has been employed to determine host preference and has proven useful for field investigations (2-6). Among these techniques, the use of avidin-biotin system in ELISA has also been applied to determine sand fly and mosquito blood meal sources (7-10).
Further, sensitivity and specificity are conditions that must be taken into consideration when detecting small amounts of blood consumed by these insects. Thus, monoclonal antibodies offer the advantage of accuracy and have been successfully used in numerous investigations (14-18).
In the present study, the limitations of anti-human IgG and anti-monkey IgG, which revealed cross-reaction with human and monkey blood meals employed in the identification process, were overcome when responses to mAb isotypes were observed.
This was the first report in which anti-human IgG1 and IgG4 were evaluated to identify mosquito blood meals; and our results indicated that only anti-human IgG1 was effective in recognizing blood meals of human origin. Since IgG1 levels were higher (60-70%) than those of IgG4 (2-6%) in human sera and given the small amounts of blood consumed by mosquitoes ascertained as being 3.46 mg for Anopheles quadrimaculatus and 2.75 mg for Aedes aegypti these factors could explain the non-detection of IgG4 (19, 20).
The fact that serum samples from the non-human primates Cebus sp. and Alouatta sp. presented negative absorbance values indicates that human blood meals can be accurately identified, which reinforces the concept that the number and properties of isotypes vary greatly among species, a fact that could be exploited by means of monoclonals in any application (21). Furthermore, the most important improvement of the technique was the ability to detect of human blood to estimate the exposure to Haemagogus leucocelaenus in areas where the yellow fever virus has been isolated.
Subsequent tests that incorporated IgG1 to evaluate field-collected mosquitoes showed a significant difference among samples of Haemagogus leucocelaenus, from Santo Antônio, that had fed on human blood. This behavior may suggest human-vector contact and since the yellow fever virus was isolated in monkeys from the two municipalities of Rio Grande do Sul, the detection patterns recorded for samples of Haemagogus leucocelaenus emphasize the risk of yellow fever transmission and highlight the need for careful monitoring in the area (1, 22).
ACKNOWLEDGEMENTS
We wish to thank the Secretary of Health Surveillance, Ministry of Health, for the research funds; Maria Dulce Bianchi Rosa and Aristides Fernandes for their assistance in the laboratory; and Rodrigo Sportello for his help with the figures.
Received: February 10, 2009
Accepted: July 6, 2009
Abstract published online: July 7, 2009
Full paper published online: November 30, 2009
Conflicts of interest: There is no conflict.
Financial source: Secretary of Health Surveillance, Brazilian Ministry of Health and Pan American Health Organization, Proc. N. 25000.022.005/2004-56.
- 1. Vasconcelos PFC, Sperb AF, Monteiro HAO, Torres MAN, Sousa MRS, Vasconcelos HB, et al. Isolations of yellow fever virus from Haemagogus leucocelaenus in Rio Grande do Sul State, Brazil. Trans R Soc Trop Med Hyg. 2003;97(1):60-2.
- 2. Chow E, Wirtz RA, Scott TW. Identification of blood meals in Aedes aegypti by antibody sandwich enzyme-linked immunosorbent assay. J Am Mosq Control Assoc. 1993;9(2):196-205.
- 3. Muturi EJ, Muriu S, Shililu J, Mwangangi JM, Jacob BG, Mbogo C, et al. Blood-feeding patterns of Culex quinquefasciatus and other culicines and implications for disease transmission in Mwea rice scheme, Kenya. Parasitol Res. 2008;102(6):1329-35.
- 4. Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of Central North Carolina. J Med Entomol. 2006;43(3):543-51.
- 5. Service MW, Voller A, Bidwell DE. The enzyme-linked immunosorbent assay (ELISA) test for the identification of blood-meals of haematophagous insects. Bull Ent Res. 1986;76:321-30.
- 6. Zinser M, Ramberg F, Willott E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: blood meal analysis indicates feeding on both humans and birds. J Insect Sci. 2004;4(20):1-3.
- 7. Marassá AM, Consales CA, Galati EAB. Padronizaçăo da técnica imunoenzimática do ELISA de captura, no sistema avidina-biotina para a identificaçăo de sangue ingerido por Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912). Rev Soc Bras Med Trop. 2004;37(6):441-6.
- 8. Marassá AM, Consales CA, Galati EAB, Nunes VLB. Identificaçăo do sangue ingerido por Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) e Lutzomyia (Lutzomyia) almerioi (Galati & Nunes, 1999) pela técnica imunoenzimática do ELISA de captura, no sistema avidina-biotina. Rev Soc Bras Med Trop. 2006;39(2):183-6.
- 9. Marassá AM, Rosa MDB, Gomes AC, Consales CA. Biotin/avidin sandwich enzyme-linked immunosorbent assay for Culicidae mosquito blood meal identification. J Venom Anim Toxins incl Trop Dis. 2008;14(2):303-12.
- 10. de Oliveira AG, Marassá AM, Consales CA, Dorval MEC, Fernandes CE, de Oliveira GR, et al. Observations on the feeding habits of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) in Campo Grande, an endemic area of visceral leishmaniasis in Mato Grosso do Sul, Brazil. Acta Trop. 2008;107(3): 238-41.
- 11. Nasci RS. A light weight battery-powered aspirator for collecting resting mosquitoes in the field. Mosq News. 1981;41:808-11.
- 12. Consoli RAGB, Oliveira RL. Principais mosquitos de importância sanitária no Brasil. Rio de Janeiro: Editora da Fundaçăo Oswaldo Cruz; 1994. 225 p.
- 13. Forattini OP. Culicidologia Médica. Săo Paulo: Edusp; 2002. 860 p. 2 vol.
- 14. Achkar SM, Sinhorini IL, Ribeiro OG, Carrieri ML, Ceretta RS, Consales CA. Immunopathology of rabies infection in mice selected for high or low acute inflammatory reaction. J Venom Anim Toxins incl Trop Dis. 2007;13(1):39-55.
- 15. Gould EA, Buckley A, Cammack N. Use of the biotin-streptavidin interaction to improve flavivirus detection by immunofluorescence and ELISA tests. J Virol Methods.1985;11(1):41-8.
- 16. Pazarbasi A, Alptekin D, Luleyap HU, Kasap M, Kasap H. Use of enzyme-linked immunosorbent assay for detection of natural Leishmania infections in phlebotomine sand flies from southeastern Turkey. J Med Entomol. 2006;43(2):248-51.
- 17. Rambozzi L, Menzano A, Lavin S, Rossi L. Biotin-avidin amplified ELISA for detection of antibodies to Sarcoptes sacabiei in chamois (Rupicapra sp). Vet Res. 2004;35(6):701-8.
- 18. Remoue F, Alix E, Cornelie S, Sokhna C, Cisse B, Doucoure S, et al. IgE and IgG4 antibody responses to Aedes saliva in African children. Acta Trop. 2007;104(2-3):108-15.
- 19. Parslow TG, Stites DP, Terr AI, Imboden JB. Imunologia Médica. 10th ed. Rio de Janeiro: Guanabara Koogan; 2004. 684 p.
- 20. Jeffery GM. Blood meal volume in Anopheles quadrimaculatus, A. albimanus and Aedes aegypti Exp Parasitol. 1956;5(4):371-5.
- 21. Tizard IR, Schubot RM. Veterinary immunology: an introduction. 6th ed. Philadelphia: W.B. Saunders; 2000. p. 139-48.
- 22. Sallis ES, de Barros VL, Garmatz SL, Fighera RA, Graça DL. A case of yellow fever in a brown howler (Alouatta fusca) in Southern Brazil. J Vet Diagn Invest. 2003;15(6):574-6.
Publication Dates
-
Publication in this collection
27 Nov 2009 -
Date of issue
2009
History
-
Reviewed
06 July 2009 -
Received
10 Feb 2009 -
Accepted
30 Nov 2009