Abstract
Snake venoms are rich in enzymes such as phospholipase A2, proteolytic enzymes, hyaluronidases and phosphodiesterases, which are well characterized. However, L-amino acid oxidase (LAO EC.1.4.3.2) from snake venoms has not been extensively studied. A novel L-amino acid oxidase from Bungarus caeruleus venom was purified to homogeneity using a combination of ion-exchange by DEAE-cellulose chromatography and gel filtration on Sephadex® G-100 column. The purified monomer of LAO showed a molecular mass of 55 ±1 kDa estimated by SDS-PAGE. The specific activity of purified LAO was 6,230 ± 178 U/min/mg, versus 230 ± 3.0 U/min/mg for the whole desiccated venom, suggesting a 27-fold purification with a 25% yield. Optimal pH and temperature for maximum purified enzyme activity were 6.5 and 37ºC, respectively. Platelet aggregation studies show that purified LAO inhibited ADP-induced platelet aggregation dose-dependently at 0.01 to 0.1 µM with 50% inhibitory concentration (IC50) of 0.04 µM, whereas at a 0.08 µM concentration it did not induce appreciable aggregation on normal platelet-rich plasma (PRP). The purified protein catalyzed oxidative deamination of L-amino acids while the most specific substrate was L-leucine. The purified LAO oxidizes only L-forms, but not D-forms of amino acids, to produce H2O2. The enzyme is important for the purification and determination of certain amino acids and for the preparation of α-keto acids.
L-amino acid oxidase; Bungarus caeruleus; platelet aggregation
Purification of an L-amino acid oxidase from Bungarus caeruleus (Indian krait) venom
More SSI; Kiran KMII; Veena SMII; Gadag JRII
IDepartment of Biochemistry, Center for Postgraduate Studies, Jain University, Bangalore, Karnataka, India
IIDepartment of Biochemistry, Karnatak University, Dharwad, Karnataka, India
Correspondence to Correspondence to: Sunil S. More Department of Biochemistry, Jain University 18/3,9th Main Jayanagar 3rd Block, Bangalore 560011 Karnataka, India Phone: 08041210694. Fax: 080 41210692 Email: sunilacr@yahoo.co.in
ABSTRACT
Snake venoms are rich in enzymes such as phospholipase A2, proteolytic enzymes, hyaluronidases and phosphodiesterases, which are well characterized. However, L-amino acid oxidase (LAO EC.1.4.3.2) from snake venoms has not been extensively studied. A novel L-amino acid oxidase from Bungarus caeruleus venom was purified to homogeneity using a combination of ion-exchange by DEAE-cellulose chromatography and gel filtration on Sephadex® G-100 column. The purified monomer of LAO showed a molecular mass of 55 ±1 kDa estimated by SDS-PAGE. The specific activity of purified LAO was 6,230 ± 178 U/min/mg, versus 230 ± 3.0 U/min/mg for the whole desiccated venom, suggesting a 27-fold purification with a 25% yield. Optimal pH and temperature for maximum purified enzyme activity were 6.5 and 37ºC, respectively. Platelet aggregation studies show that purified LAO inhibited ADP-induced platelet aggregation dose-dependently at 0.01 to 0.1 µM with 50% inhibitory concentration (IC50) of 0.04 µM, whereas at a 0.08 µM concentration it did not induce appreciable aggregation on normal platelet-rich plasma (PRP). The purified protein catalyzed oxidative deamination of L-amino acids while the most specific substrate was L-leucine. The purified LAO oxidizes only L-forms, but not D-forms of amino acids, to produce H2O2. The enzyme is important for the purification and determination of certain amino acids and for the preparation of α-keto acids.
Key words: L-amino acid oxidase, Bungarus caeruleus, platelet aggregation.
INTRODUCTION
L-amino acid oxidase (LAO, EC1.4.3.2) is a flavoenzyme that catalyzes the stereospecific oxidative deamination of an L-amino acid and act as substrate to an α-keto acid along with the production of ammonia and hydrogen peroxide (H2O2). There was complete decomposition of L-amino acids like leucine, isoleucine, norleucine, α-amino butyric acid, phenylalanine, tyrosine, tryptophan norvaline, methionine, histidine citrulline, serine, threonine, aspartic acid, glutaric acid, lysine and ornithine by LAO.
The enzyme is widely distributed in many different organisms, including the venoms of a variety of snake species. Snake venom components have been widely used in medicine as diagnostic or therapeutic tools and also as models for studying processes in cell biology. The demonstrated presence of LAOs in mouse milk suggests a possible function of anti-bacterial role in the mammary gland (1). The enzyme has been isolated from different venoms and its effects on platelets, induction of apoptosis, hemorrhagic and antibacterial effects vary widely (2-9).
Venom LAOs are usually homodimeric FAD-binding glycoproteins with molecular mass from 110 to 150 kDa (10). They share sequence similarity with human monoamine oxidase and with bacterial and fungal LAOs only at the FAD-binding site. In addition, these proteins share significant similarity (more than 30% identity) with mouse interleukin 4-induced protein (1, 11). Recently, the LAO structure from Calloselasma rhodostoma has been determined by x-ray crystallography (12). The enzyme is structurally a dimer and each subunit consists of three domains: a FAD-binding domain, a substrate binding domain, and a helical domain.
Reported biological activities of venom LAOs include apoptosis-inducing activity on various human cell lines, inhibition or induction of platelet aggregation and antibacterial effect (2, 3, 7, 13,). These activities are mainly associated with the production of highly localized concentrations of H2O2, since catalase, a H2O2 scavenger, inhibits the biological effects of LAOs as well as those of H2O2. The present investigation reports the purification and characterization of a novel LAO, designated BCV-LAO, from the venom of Indian krait (Bungarus caeruleus) with respect to its molecular mass and biochemical properties.
MATERIALS AND METHODS
Reagents and Chemicals
Lyophilized Bungarus caeruleus venom was obtained from the Haffkine Institute, Parel, Mumbai, India. Chemicals and column materials, including Sephadex® G-100, were obtained from Pharmacia Biotech (Sweden). DEAE-cellulose, o-dianisidine hydrochloride and horseradish peroxidase were from Sigma Chemicals (USA). All other chemicals and reagents used were of analytic grade from commercial sources. The protein concentration of the final product was determined by a protein assay kit (Bangalore Genei Chemicals, India) with bovine serum albumin as standard.
Purification Procedures
Two-step purification of L-amino acid oxidase was carried out by the method of Wei et al. (14).
First step: DEAE-cellulose column chromatography
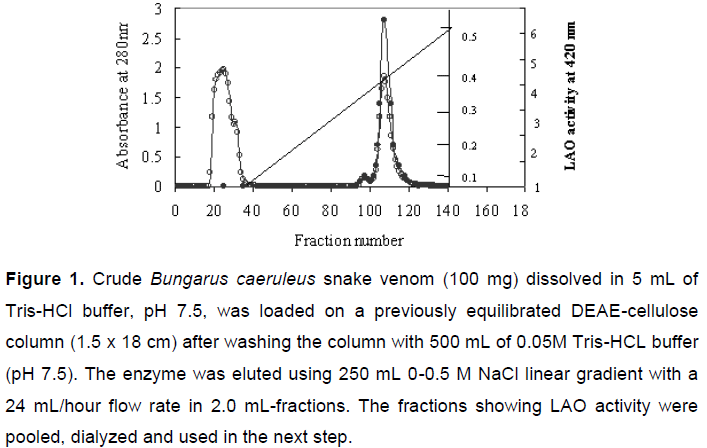
The crude Bungarus caeruleus snake venom (100 mg) dissolved in 5 mL of 0.05 M Tris-HCl buffer, pH 7.5, was loaded on a previously equilibrated DEAE-cellulose column (1.5 x 18 cm). After washing the column with 500 mL of 0.05 M Tris-HCL buffer, pH 7.5, the enzyme was eluted using 250 mL of 0-0.5 M NaCl linear gradient with a 24 mL/hour flow rate of 2.0 mL fractions. The fractions showing LAO activity were pooled, dialyzed and used in the next step.
Second step: Sephadex® G-100 column chromatography
The dialyzed fractions showing LAO activity were further subjected to gel filtration chromatography on a previously equilibrated Sephadex® G-100 column (1.5 x 60 cm). After washing the column with 500 mL of 0.05 M Tris-HCL buffer, pH 7.5, the protein fractions were eluted at a flow rate of 12 mL/hour in 1.2-mL fractions. The fractions showing LAO activity were pooled, dialyzed, lyophilized, stored at -20ºC and subsequently used for characterization and confirming their homogeneity.
LAO Activity
The LAO activity was assayed by measuring the initial rate of hydrogen peroxide production with a coupled peroxidase/dye assay (15). The dye formation was spectrophotometrically measured at 436 nm and 30ºC. The assay mixture, with pH 7.6, contained 10 mM L-leucine, 0.2 M Tris-HCl buffer, 0.2 mg/mL o-dianisidine hydrochloride, 100 U/mL horseradish peroxidase and LAO in limiting amounts. To test the enzymatic specificity of the purified LAO, L-leucine was replaced with other L-amino acids under identical assay conditions. The amount of purified LAO in the reaction mixture was 4.0 µg. One unit (U) is defined as the amount of enzyme that catalyzes the formation of 1 µmol H2O2 per minute. Protein concentration was determined by the dye binding method of Bradford (16) using bovine serum albumin as standard.
Stability Studies
To investigate the effects of the purified enzyme under freezing conditions in PBS (pH 7.6), it was stored for two months at -80ºC. The treated enzyme was incubated for 30 minutes at 30ºC. LAO activity was determined as described by Bergmeyer (17).
KM and Vmax Determinations
For the determination of KM and Vmax, the enzyme concentration was adjusted to catalyze 50% of 1 µmol H2O2 per minute with 10 mM L-leucine as substrate under optimum conditions (30ºC and pH 7.6). The activity was expressed as the formation of one micromole of H2O2 per minute and all the assays were performed in triplicate
Native Polyacrylamide Gel Electrophoresis (PAGE) of Purified L-amino Acid Oxidase
Homogeneity of the purified enzyme was tested by PAGE according to the method of Davis (18).
Purity Criterion and Molecular Mass Determination by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Molecular mass of the purified enzyme was determined by SDS-PAGE according to Laemmli (19), using standard molecular mass markers.
Molecular Mass Determination by Gel Filtration Chromatography
The molecular mass of the purified L-amino acid oxidase was estimated by gel filtration chromatography according to Andrews (20) on calibrated columns of Sephadex® G-75, using 0.05 M Tris-HCl buffer (pH 8.5).
Sephadex® G-75 was suspended in 0.05 M Tris-HCl buffer (pH 8.5) containing 100 mM NaCl and allowed to swell for 24 hours. The fine particles were removed by decanting the supernatant whereas the swollen gel was deaerated by leaving it overnight in a vacuum desiccator. The gel was packed into a column (1.5 x 60 cm) - equilibrated with the same buffer - and the flow rate was kept at 12 mL/hour using a peristaltic pump.
Void volume (Vo) of the column was determined through blue dextran (2 mg/mL in equilibration buffer containing 3% sucrose). The column was calibrated with standard molecular mass markers. Each standard protein (2 mg/mL) in the buffer containing 3% sucrose was layered on the gel. The elution was carried out with the same buffer at a constant flow rate (12 mL/hour). Three-milliliter fractions were collected on a fraction collector (FRAC-100®, Pharmacia, Sweden), while the protein elution was monitored by determining the absorbance at 280 nm using a Hitachi 150-20® spectrophotometer (Japan).
The total eluent volume up to the fraction presenting maximum absorbance was considered the eluent volume of the protein (Ve). Elution volumes of different standard proteins of known molecular mass were determined under similar conditions. The elution volume for the purified L-amino acid oxidase was also determined.
A calibration curve was obtained by plotting values of Ve/Vo against their respective logarithmic molecular masses. Lysozyme, chymotrypsinogen A, carbonic anhydrase, ovalbumin and bovine serum albumin were employed as standard proteins to obtain the calibration curve. From this curve, the molecular mass of purified L-amino acid oxidase was estimated
Platelet Aggregation Studies
Platelet aggregation was assessed according to Toyama et al. (21). Venous blood was collected, with informed consent, from healthy volunteers who denied taking any medication in the previous 14 days. Samples were collected by a two-syringe technique using sterile syringes, and immediately transferred into polypropylene
tubes containing 1/10th of the final volume of 3.8% trisodium citrate. Platelet-rich plasma (PRP) was prepared by centrifugation of citrated blood at 200 x g for ten minutes. Aggregation experiments were performed in triplicate at various concentrations of LAO.
Statistical Analysis
The results were expressed as the mean ± SEM. Data were evaluated by the analysis of variance (ANOVA) test. The significance level was set at p < 0.05.
RESULTS
A two-step protocol was standardized for LAO purification. The first step involved DEAE-cellulose ion exchange chromatography that had fractionated crude Bungarus caeruleus venom in three peaks with significant protein concentrations. Fractions 25-43 showed the highest LAO activity (Figure 1).
The second step involved gel filtration chromatography of lyophilized peak II obtained in step one, which was further resolved into three peaks with significant protein concentrations. LAO activity was observed at peak II (fractions 38-51) (Figure 2). The enzyme was purified 27-fold with a yield of 25.8%, having a specific activity of 6,230 ± 114 U/min/mg of protein (Table 1).
Purity Criterion and Molecular Mass Determination
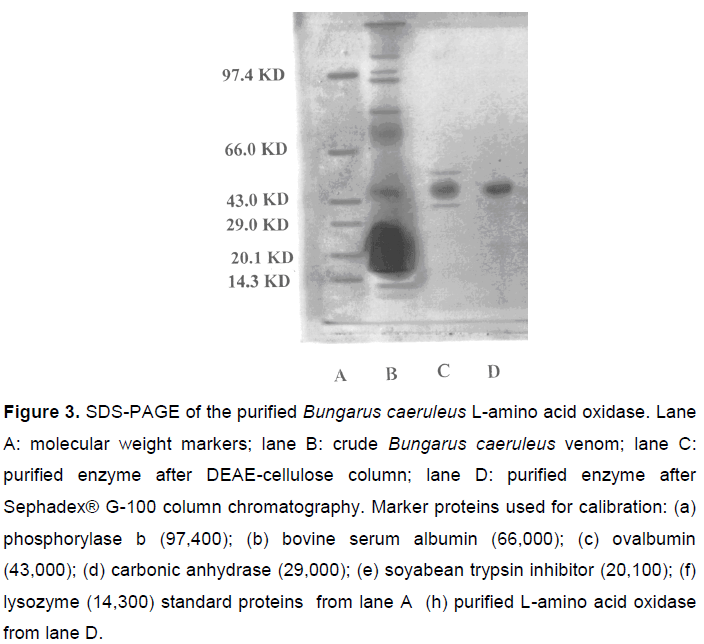
The purified L-amino acid oxidase enzyme, when subjected to native PAGE, showed a single band, indicating its homogeneity. The LAO molecular mass was estimated by SDS-PAGE (Figure 3) and by gel filtration on Sephadex® G-100 using standard protein molecular mass markers and was found to be 55.0 ± 1.0 kDa.
Enzyme Stability
The purified enzyme was found to be stable at -20 or -80ºC for two months and at 4ºC for one month, whereas its activity was enhanced by the addition of 10 µM FAD (data not shown). The enzyme activity remained unaffected between 30 and 40ºC. Freezing and thawing did not affect the enzyme activity as long as it was stored at a pH from 6.0 to 7.0.
LAO Substrate Specificity
The purified LAO presents broad substrate specificity when tested for a wide range of amino acids. It revealed high specificity for L-Glu, L-Leu, L-Met, L-Ile, L-Phe and L-Arg; whereas it presented moderate specific activity for L-His, L-Trp, L-Asn, L-Gln, L-Lys and L-Asp; it showed no specific activity for L-Thr, L-Ser, L-Pro, L-Val and L-Tyr (Table 2).
Temperature and pH Effects on Enzyme Activity
The optimum pH for L-amino acid oxidase activity is 4.5 while the best temperature is 37ºC.
Determination of Km and Vmax
The effect of varying concentrations of L-leucine at the initial LAO velocity showed a typical hyperbolic saturation curve. The Km value for L-amino acid oxidase, as determined in the Lineweaver-Burk plot, was found to be 48.61 µM/mL.
Platelet Aggregation
The LAO, preincubated with normal PRP for two minutes, inhibited ADP-induced platelet aggregation dose-dependently at 0.01 to 0.1 µM with IC50 of 0.04 µM. This inhibition was completely lost when PRP and LAO were incubated with catalase (0.3 mg/mL) before adding ADP (Figure 4). LAO at a concentration of 0.08 µM did not induce appreciable aggregation in normal PRP (Figure 5).
DISCUSSION
Snake venoms are commonly used as tools for the development of new therapeutic approaches since these substances present a broad range of pharmacological activities (22, 23)
In the present investigation, the Bungarus caeruleus venom L-amino acid oxidase was purified and its molecular mass characterized. The purified LAO from B. caeruleus Indian krait venom showed a single band of 55 ± 1 kDa. This corroborates a previous report by Tan and Swaminathan (24), in which the enzyme had a molecular mass of 57.4 kDa, as determined by SDS-PAGE. The crude venom was fractionated and the active component purified. The biochemical properties of the purified enzyme were consistent with snake L-amino acid oxidases found by other authors (25).
The molecular mass and structure of LAO, found in our experiments, were also described in numerous L-amino acid oxidases, ranging from 50 kDa in the monomeric form to 150 kDa as a dimer (26-28). The presence of multiple isoforms of this enzyme in some snake venoms was also reported (29, 30). The purified enzyme in the present study was called BCV-LAO. By the enzymatic assay, the LAO presented 28-fold more activity than crude venom, suggesting a significant degree of purification. LAO catalyzes the oxidative deamination of L-amino acids to produce the corresponding β-ketoacid, hydrogen peroxide and ammonia (31, 32).
The optimum pH and temperature for isolating LAO in B. caeruleus were, respectively, 6.5 and 37ºC, consistent with previous findings for other snake L-amino acid oxidases (33). Before the 1990s, snake venom LAOs were mainly utilized to identify optical isomers of amino acids and to prepare of α-keto acids (34, 35). Usually, the oxidizing activity is determined using L-Leu as substrate. In the case of viperid venoms, hydrophobic amino acids (including L-Leu) are the best substrates for LAO (36, 37). The substrate specificity of B. caeruleus LAO is in accordance with other studied snake venom LAOs. Hydrophobic amino acids (L-Phe, L-Met, L-Leu and L-Ile) are the best substrates, while L-Ser, Gly, L-Lys, LThr and L-Pro are not oxidized.
The catalytic differences may be explained by differences in side chain binding sites responsible for the enzyme substrate specificity. The presence of three to four hydrophobic subsites and two amino-binding subsites have been hypothesized for the enzymes isolated from the elapid venom of Naja naja kaouthia (28) as well as for the C. rhodostoma LAO (32).
Several LAOs inhibit whereas others induce platelet aggregation (in the same concentration range) by some supplementary mechanisms in addition to H2O2 release (38). One reason for such inhibition may be reduced ADP binding in platelets exposed to H2O2 (38). The inhibitory activity might also be explained by the interference of peroxide in the interaction between activated platelet integrin GPIIb/IIIa and fibrinogen. The induction of aggregation is connected with peroxide formation and subsequent tromboxane A2 synthesis requiring Ca2+, but is independent of ADP release (1). Snake venom LAOs are interesting multifunctional enzymes owing their effects, at least partly, to hydrogen peroxide release in the oxidation process. Ascertainment of the exact mechanisms of pharmacological activities require further studies.
Received: March 19, 2009
Accepted: May 12, 2009
Abstract published online: June 1, 2009
Conflicts of interest: There is no conflict.
- 1. Du XY, Clemetson KJ. Snake venom L-amino acid oxidases. Toxicon. 2002;40(2):659-65.
- 2. Suhr SM, Kim DS. Identification of the snake venom substance that induces apoptosis. Biochem Biophys Res Commun. 1996;224(1):134-9.
- 3. Stiles BG, Sexton FW, Weinstein SA. Antibacterial effects of different snake venoms: purification and characterization of antibacterial proteins from Pseudechis australis (Australian king brown or mulga snake) venom. Toxicon. 1991;29(9):1129-41.
- 4. Sun LK, Yoshii Y, Hyodo A, Tsurushima H, Saito A, Harakuni T, Li YP, Kariya K, Nozaki M, Morine N. Apoptotic effect in the glioma cells induced by specific protein extracted from Okinawa habu (Trimeresurus flavoviridis) venom in relation to oxidative stress. Toxicol In Vitro. 2003;17(2):169-77.
- 5. Stabeli RG, Marcussi S, Carlos GB, Pietro RCLR, Selistre-de-Araújo HS, Giglio JR, Oliveira EB, Soares AM. Platelet aggregation and antibacterial effects of an L-amino acid oxidase purified from Bothrops alternatus snake venom. Bioorg Med Chem. 2004;12(11):2881-6.
- 6. Tempone AG, Andrade HF Jr, Spencer PJ, Lourenço CO, Rogero JR, Nascimento N. Bothrops moojeni venom kills Leishmania spp. with hydrogen peroxide generated by its L-amino acid oxidase. Biochem Biophys Res Commun. 2001;280(3):620-4.
- 7. Takatsuka H, Sakurai Y, Yoshioka A, Kokubo T, Usami Y, Suzuki M, Matsui T, Titani K, Yagi H, Matsumoto M, Fujimura Y. Molecular characterization of L-amino acid oxidase from Agkistrodon halys blomhoffii with special reference to platelet aggregation. Biochim Biophys Acta. 2001;1544(1-2):267-77.
- 8. Zhang YI, Wang JH, Lee WH, Wang Q, Liu H, Zheng YT, Zhang Y. Molecular characterization of Trimeresurus stejnegeri venom L-amino acid oxidase with potential anti-HIV activity. Biochem Biophys Res Commun. 2003;309(3):598-604.
- 9. Ali SA, Stoeva S, Abbasi A, Alam JM, Kayed R, Faigle M, Neumeister B, Voelter W. Isolation, structural and functional characterization of an apoptosis-inducing L-amino acid oxidase from leaf-nosed viper (Eristocophis macmahoni) snake venom. Arch Biochem Biophys. 2000;384(2):216-26.
- 10. Sun Y, Nonobe E, Kobayashi Y, Kuraishi T, Aoki F, Yamamoto K, Sakai S. Characterization and expression of L-amino acid oxidase of mouse milk. J Biol Chem. 2002;277(21):19080-6.
- 11. Raibekas AA, Massey V. Primary structure of the snake venom L-amino acid oxidase shows high homology with the mouse B cell interleukin 4-induced Fig1 protein. Biochem Biophys Res Commun. 1998;248(3):476-8.
- 12. Pawelek PD, Cheah J, Coulombe R, Macheroux P, Ghisla S, Vrielink A. The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site. EMBO J.2000;19(16):4204-15.
- 13. Torri S, Naito M, Tsuruo T. Apoxin I, a novel apoptosis-inducing factor with L-amino acid oxidase activity purified from western diamondback rattlesnake venom. J Biol Chem. 1997;272(14):9539-42.
- 14. Wei JF, Wei Q, Lu QM, Tai H, Jin Y, Wang WY, Xiong YL . Purification, characterization and biological activity of an L-amino acid oxidase from Trimeresurus mucrosquamatus venom. Acta Biochim Biophys Sin. 2003;35(2):219-24.
- 15. Li ZY, Yu TF, Lian EC. Purification and characterization of L-amino acid oxidase from king cobra (Ophiophagus hannah) venom and its effects on human platelet aggregation. Toxicon. 1994;32(11):1349-58.
- 16. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-54.
- 17. Bergmeyer HU. L-amino acid oxidase. In: Bergmeyer HU, editor. Methods in enzymatic analysis. Weinheim: Verlag Chemie, GmbH; 1983. 2 vols. p. 149-50.
- 18. Davis BJ. Disc electrophoresis. II. Method and application to human serum proteins. Ann N Y Acad Sci. 1964;121(2):404-27
- 19. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680-5.
- 20. Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964;91(2):222-33.
- 21. Toyama MH, Toyama DO, Passero LF, Laurenti MD, Corbett CE, Tomokane TY, Fonseca FV, Antunes E, Joazeiro PP, Beriam LO, Martins MA, Monteiro HS, Fonteles MC. Isolation of a new L-amino acid oxidase from Crotalus durissus cascavella venom. Toxicon. 2006;47(1):47-57.
- 22. 15. Nathan I, Dvilansky A, Yirmiyahu T, Aharon M, Livne A. Impairment of platelet aggregation by Echis colorata venom mediated by L-amino acid oxidase or H2O2 Thromb Haemost. 1982;48(3):277-82.
- 23. Ahn MY, Lee BM, Kim YS. Characterization and cytotoxicity of L-amino acid oxidase from the venom of king cobra (Ophiophagus hannah). Int J Biochem Cell Biol. 1997;29(6):911-9.
- 24. Tan NH, Swaminathan S. Purification and properties of L-amino acid oxidase from monocellate cobra (Naja naja kaouthia) venom. Int J Biochem. 1992;24(6):967-73.
- 25. Suhr SM, Kim DS. Comparison of the apoptotic pathways induced by L-amino acid oxidase and hydrogen peroxide. J Biochem. 1999;125(2):305-9.
- 26. Kamata K, Arai Y, Sakamoto A, Kasuya Y, Samejima Y. Pharmacological action of the phospholipase A2 from the venom of Trimeresurus flavoviridis on the smooth muscle of the rat stomach. Life Sci. 1989;44(2):137-42.
- 27. Chen SZJ, Gopalakrishnakone P, Gwee MCE. Pharmacological effects and pathological changes induced by the venom of Pseudechis australis in isolated skeletal muscle preparations. Toxicon. 1994;32(3):303-15.
- 28. Tan NH, Saifuddin MN. Substrate specificity of king cobra (Ophiophagus hannah) venom L-amino acid oxidase. Int J Biochem. 1991;23(3):323-7.
- 29. Ponnudurai G, Chung MC, Tan NH. Purification and properties of the L-amino acid oxidase from Malayan pit viper (Calloselasma rhodostoma) venom. Arch Biochem Biophys. 1994;313(2):373-8.
- 30. Nakano K, Inamas Y, Hagihara S, Obo F. Isolation and properties of L-amino acid oxidase in habu snake (Trimeresurus flavoviridis) venom. Acta Med Univ Kagoshima. 1972;14:229-39.
- 31. Shaham N, Bdolah A. L-amino acid oxidase from Vipera palaestinae venom: purification and assay. Com Biochem Physiol. 1973;46(4):691-8.
- 32. Pessatti ML, Fontana JD, Furtado MFD, Guimarães MF, Zanette LRS, Costa WT, Baron M. Screening of Bothrops snake venoms for L-amino acid oxidase activity. Appl Biochem Biotechnol. 1995;51-52(1):197-210.
- 33. Wellner D, Meister A. Studies on the mechanism of action of L-amino acid oxidase . J Biol Chem . 1961;236:2357-64.
- 34. Wellner D, Meister A. Crystalline L-amino acid oxidase of Crotalus adamanteus . J Biol Chem . 1960;235:2013-8.
- 35. Tan NH. L-amino acid oxidases and lactate dehydrogenases. In: Bailey GS, editor. Enzymes from snake venom. Fort Collins: Alaken Inc.; 1998. p. 579-98.
- 36. Iwanaga S, Suzuki T. Enzymes in snake venoms. In: Lee CY, editor. Snake venoms. New York: Springer-Verlag, Berlin-Heidelberg; 1979. p. 61-158.
- 37. Souza DHF, Eugenio LM, Fletcher JE, Jiang MS, Garratt RC, Oliva G, Selistre-de-Araujo HS. Isolation and structural characterization of a cytotoxic L-amino acid oxidase from Agkistrodon contortrix laticinctus snake venom: preliminary crystallographic data. Arch Biochem Biophys. 1999;368(2):285-90.
- 38. Belisario MA, Tafuri S, Di Domenico C, Squillacioti C, Della Morte R, Lucisano A, Staiano N. H2O2 activity on platelet adhesion to fibrinogen and protein tyrosine phosphorylation. Biochim Biophys Acta. 2000;1495(2):183-93.
Publication Dates
-
Publication in this collection
05 Feb 2010 -
Date of issue
2010
History
-
Received
19 Mar 2009 -
Accepted
12 May 2009