Abstract
Snake venom proteins from the C-type lectin family have very distinct biological activities despite their highly conserved primary structure, which is homologous to the carbohydrate recognition region of true C-type lectins. We purified a lectin-like protein (BmLec) from Bothrops moojeni venom and investigated its effect on platelet aggregation, insulin secretion, antibacterial activity, and isolated kidney cells. The BmLec was purified using two chromatographic steps: affinity chromatography and reverse phase high performance liquid chromatography (HPLC). BmLec showed a dose-dependent platelet aggregation and significantly decreased the bacterial growth rate in approximately 15%. During scanning electron microscopy, the profile of Xanthomonas axonopodis pv. passiflorae treated with lectin disclosed a high vesiculation and membrane rupture. BmLec induced a strong and significant increase in insulin secretion at 2.8 and 16.7 mM glucose concentrations, and this effect was seen in the presence of EGTA in both experiments. BmLec (10 µg/mL) increased the perfusion pressure, renal vascular resistance and urinary flow. The glomerular filtration rate and percentages of sodium, potassium and chloride tubular transport were reduced at 60 minutes of perfusion. Renal alterations caused by BmLec were completely inhibited by indomethacin in all evaluated parameters. In conclusion, the C-type lectin isolated from Bothrops moojeni affected platelet aggregation, insulin secretion, antibacterial activity and isolated kidney function.
Bothrops moojeni; kidney; platelet aggregation; insulin; antibacterial activity
ORIGINAL PAPER
Purification and biological effects of a C-type lectin isolated from Bothrops moojeni
Barbosa PSFI; Martins AMCII; Toyama MHIII; Joazeiro PPIV; Beriam LOSV; Fonteles MCI; Monteiro HSAI
IDepartment of Physiology and Pharmacology, Federal University of Ceará, Fortaleza, Ceará State, Brazil
IIDepartment of Clinical and Toxicological Analyses, Federal University of Ceará, Fortaleza, Ceará State, Brazil
IIISão Paulo Experimental Coast Campus, São Paulo State University (UNESP Univ Estadual Paulista), São Vicente, São Paulo State, Brazil
IVDepartment of Histology, Institute of Biology, State University of Campinas, Campinas, São Paulo State, Brazil
VLaboratory of Plant Microbiology, Experimental Center, Biological Institute, Campinas, São Paulo State, Brazil
VIMackenzie Presbyterian University, São Paulo, São Paulo State, Brazil
Correspondence to Correspondence to: Helena Serra Azul Monteiro Departamento de Fisiologia e Farmacologia Faculdade de Medicina, Universidade Federal do Ceará Rua Cel. Nunes de Melo, 1127 Fortaleza, CE, 60.530-370, Brasil Email: martinsalice@gmail.com or hsazul@gmail.com.
ABSTRACT
Snake venom proteins from the C-type lectin family have very distinct biological activities despite their highly conserved primary structure, which is homologous to the carbohydrate recognition region of true C-type lectins. We purified a lectin-like protein (BmLec) from Bothrops moojeni venom and investigated its effect on platelet aggregation, insulin secretion, antibacterial activity, and isolated kidney cells. The BmLec was purified using two chromatographic steps: affinity chromatography and reverse phase high performance liquid chromatography (HPLC). BmLec showed a dose-dependent platelet aggregation and significantly decreased the bacterial growth rate in approximately 15%. During scanning electron microscopy, the profile of Xanthomonas axonopodis pv. passiflorae treated with lectin disclosed a high vesiculation and membrane rupture. BmLec induced a strong and significant increase in insulin secretion at 2.8 and 16.7 mM glucose concentrations, and this effect was seen in the presence of EGTA in both experiments. BmLec (10 µg/mL) increased the perfusion pressure, renal vascular resistance and urinary flow. The glomerular filtration rate and percentages of sodium, potassium and chloride tubular transport were reduced at 60 minutes of perfusion. Renal alterations caused by BmLec were completely inhibited by indomethacin in all evaluated parameters. In conclusion, the C-type lectin isolated from Bothrops moojeni affected platelet aggregation, insulin secretion, antibacterial activity and isolated kidney function.
Key words:Bothrops moojeni, kidney, platelet aggregation, insulin, antibacterial activity.
INTRODUCTION
Bothrops moojeni, a type of venomous pit viper, is found in warm dry regions of several Brazilian states (1). Snake venoms comprised a complex pool of organic and inorganic compounds. Furthermore, some venom contains toxins that act on integrins in the C-type lectin, disintegrin, and metalloprotease families (2, 3). Snake venoms with C-type lectin domains contain the conserved carbohydrate recognition domain (CRD) of other animal C-type lectins and share significant primary structure similarities with them; however, they do not necessarily bind to carbohydrate molecules nor require calcium ions for their activity (4-6).
Thus, lectins isolated from venom differ from other classical C-type lectins. These classical lectins are a type of carbohydrate-binding protein domain. The C-type designation refers to their requirement of calcium for binding to this carbohydrate domain. Proteins that contain C-type lectin domains have a diverse range of functions, including cell-to-cell adhesion, immune response to pathogens and apoptosis. Several C-type lectins bind to protein ligands, and only some of these binding interactions are Ca2+ dependent, such as the C-type lectins of coagulation factors IX/X, Von Willebrand factor (VWF) binding proteins, and natural killer cell receptors. The C-type lectins used to be classified into seven subgroups (I to VII) based on the order of the various protein domains in each protein (7, 8). This classification was subsequently updated in 2002 leading to an additional seven groups (VIII to XIV). Most recently, three more subgroups were added (XV to XVII) (6, 8).
The C-type lectins in Viperidae venoms have been described as disulfide-linked dimers of two homologous polypeptides of ~14 kDa. One such domain has been purified from Bothrops jararacussu, Bothrops jararaca and Crotalus atrox venom and characterized as a C-type galactoside-binding lectin (9, 10). They have erythrocyte-agglutinating activity and other properties, such as adhesion to plasmatic proteins that affect blood homeostasis by inhibiting or activating specific platelet membrane receptors and blood coagulation factors (11, 12). These proteins (i.e., alboaggregin-B, echicetin, botrocetin, bitiscetin, flavocetin-A, aggretin/rhodocytin, convulxin and agkistin) provide new insight into platelet function (13, 14). Other effects have also been reported for these proteins, including mitogenic activity on lymphocytes, release of cell calcium stores, inhibition of cell proliferation, renal effects and antimicrobial activity (15-18). Previously, it has been observed that crotacetin from the venom of C. durissus had a significant inhibitory activity on two different bacterial strains, Xanthomonas axonopodis pv passiflorae and Clavibacter michiganensis michiganensis (19). Previously, our group described the renal effects of C-type lectin isolated from Bothrops pirajai, such as the reduction of renal flow and glomerular filtration rate (20).
The C-type proteins have been described as an important component of these venoms, which are responsible for their biological effects. The study of the C-type galactoside-specific lectin isolated from Bothrops moojeni may contribute to the discovery of pharmacological tools.
Despite the biological importance of snake venom lectins, the aim of the present work was to study the effects of lectin isolated from Bothrops moojeni venom on platelet aggregation, insulin secretion, antibacterial activity and the functions of isolated kidneys.
MATERIAL AND METHODS
Purification of Lectin from Bothrops moojeni Venom
The lectin from B. moojeni venom (BmLec) was purified with two chromatographic steps; molecular exclusion chromatography and affinity chromatography. For the first fractionation, whole venom was purified following the protocols described by dos Santos et al. (21). In the second step, the whole venom (50 mg) was dissolved in 1 mL of calcium Tris-base saline (CTBS; Tris 20 mM, NaCl 150 mM, CaCl2 5 mM, pH 7.5). After complete dissolution, the venom was homogenized and clarified by ultracentrifugation at 4500 x g for five minutes. The affinity chromatography was carried out with lactose (0.8 x 5 cm) and equilibrated with the CTBS for 40 minutes, followed by injection of the venom solution. The lectin was eluted using a gradient of 0.1 to 0.3 M lactose in CTBS. The lectin-like fraction was pooled, extensively dialyzed against an ammonium bicarbonate buffer (0.1 M, pH 8.0) and lyophilized.
The purification homogeneity of the eluted protein was evaluated by reverse phase HPLC (0.1 x 30 cm column of µ-Bondapack C18®, Waters, USA) using a linear and discontinuous gradient (0-100%) of acetonitrile in 0.1% trifluoroacetic acid (v/v). One milligram of the lectin-like fraction from the affinity chromatography was dissolved in 250 mL of buffer A and centrifuged at 4,500 x g for two minutes. The supernatant was then applied to the analytical reverse phase HPLC and equilibrated with buffer A (0.1% trifluoroacetic acetic acid; TFA) for 15 minutes. Elution of the protein was subsequently conducted using a linear gradient of buffer B (66.6% acetonitrile in buffer A), and the chromatographic run was monitored at 214 nm of absorbance. After elution, the fraction was lyophilized and stored at 40ºC. The degree of purity of the lectin-like substance was assayed using two dimensional (2D) and mass spectrometry. SDS-PAGE using 12% acrylamide tricine gels confirmed the homogeneity of the fraction (22)
Characterization of Lectin Isolated from B. moojeni Venom
Amino acid analysis of BmLec was performed using approximately 1 nM of purified protein hydrolyzed with 6N HCl (200 mL) in the presence of 10 mL of phenol. Amino acid hydrolysis was carried out at 106ºC for 24 hours. After this time, the excess HCl was removed and the resulting hydrolyzed amino acids were re-dried with an aqueous solution of ethanol:water:triethylamine (2:2:1). The post-column derivatization was carried out with an aqueous solution of phenylisothiocyanate (ethanol:water:triethylamine:phenylisothiocyanate; 7:1:1:1 by volume). The samples and an amino acid standard were derivatized using a Pico-Tag® amino acid analyzer system (Waters, USA). After amino acid analysis, a new aliquot of BmLec was used for amino acid sequence determination. In this protocol, 10 mg of purified protein were dissolved in 200 mL of 6 M guanidine chloride (Merck, Germany) containing 0.4 mM Tris-HCl and 2 mM EDTA (pH 8.15). Nitrogen was flushed over the top of the protein solution for 15 minutes and then reduced with DTT (6 M, 200 mL) and carboxymethylated with 14C-iodoacetic acid and cold iodoacetic acid. Nitrogen was again flushed over the surface of the solution, and the reaction tube was sealed. This solution was incubated in the dark at 37ºC for one hour and desalting was carried out on a Sephadex G-25® column (0.7 x 12 cm) (Amersham Pharmacia, Sweden) in 1 mM acetic acid buffer. The eluted, reduced and carboxymethylated (RC) protein was then lyophilized and stored at 20ºC.
One sample of this RC-protein (4.5 mg) was then digested by Staphylococcus aureus protease V8 for 16 hours, 37ºC and pH 7.4, using an enzyme to substrate ratio of 1:30. The lectin-like peptide fragments obtained after the treatment of RC-protein with protease V8 were separated by reverse phase HPLC by means of analytical m-Bondapack C18® column (0.39 x 30 cm; Waters, USA), with 0.1% TFA as solvent A and acetonitrile containing 30% of solvent A (solvent B). The elution profile was monitored at 214 nm and the peptides were lyophilized. A second sample of the RC-protein was digested with Clostripain for eight hours at 37ºC and then lyophilized. The products were separated by reverse phase HPLC using a Waters PDA 991 system with a m-Bondapack C18® column.
The peptide peaks were isolated using a linear gradient (0-100% of acetonitrile in 0.1% TFA; v/v). The elution profile was monitored at 214 nm, and the peptides were lyophilized. Analysis of the amino acid sequence of the RC-protein, and that of the enzymatically or chemically digested fragments, was performed with an Applied Biosystems model Procise f gas-liquid protein sequencer® (USA). The phenylthiohydantoin (PTH) derivatives of the amino acids were identified with an Applied Biosystems model 450 microgradient PTH-analyser® (USA) (22, 23). The resulting primary structure of BmLec was aligned with homologous sequences obtained from the NCBI database (http://www.ncbi.nlm.nih.gov) using the algorithm BLAST. The structural analysis was maximized using Clustal X® (www.clustal.org).
Platelet Aggregation Studies
Venous blood was collected with informed consent from healthy volunteers who denied taking any medication in the previous 14 days. Blood was collected by a two-syringe technique using polypropylene syringes and 19-gauge needles and immediately transferred into polypropylene tubes containing 1/10th final volume of 3.8% trisodium citrate. Initially, whole blood was centrifuged to obtain the platelet-rich plasma (PRP). After removing the PRP, the remaining blood was centrifuged at 3,000 x g for five minutes to obtain washed platelet.
The platelet aggregation assays were conducted with a washed platelet preparation that was left for one hour at room temperature to recover its sensitivity to aggregation agents. Platelet counts were performed on a Coulter S Plus® (Coulter Electronics, USA). Platelet aggregation was measured turbidimetrically using a dual-channel whole blood Lumi-aggregometer (Chrono Log Corporation, USA). Platelets were suspended in a phosphate buffered saline buffer (400 µL) and pre-incubated with 1 mM CaCl2 (final concentration) at 37ºC for two minutes with stirring before being challenged with BmLec. The aggregation was recorded after seven minutes from the application of the lectin (19). As a positive control for platelet aggregation, we used 100 µM ADP and 10 µL thrombin (1 mg/1 mL).
Insulin Secretion Assay
Insulin secretion was measured in rat islets that were isolated by collagenase (EC 3.4.24.3) digestion. The pancreas was inflated with Hanks' balanced salt solution containing 0.7 mg collagenase/mL, excised and then maintained at 37ºC for 20 minutes. The digested tissue was washed four times and the islets were separated using a siliconized stretched Pasteur pipette. Groups of five islets were incubated for 30 minutes at 37ºC in 0.75 mL of Krebs-bicarbonate buffer (115 mM NaCl, 5 mM KCl, 2.56 mM CaCl2, 1 mM MgCl2, and 24 mM NaHCO3, and 5.6 mM glucose, pH 7.4) supplemented with 3 mg of BSA/mL and aerated with 95% O2/5% CO2. After this incubation, the medium was replaced with fresh buffer and the islets incubated for one hour in the presence of 2.8 or 16.7 mM glucose (control) or 16.7 mM glucose in the presence of 5 mg of BmLec. Other experiments were carried out in presence of BmLec with 10 mM EGTA 5H2O (21).
Antibacterial Activity against Xanthomonas axonopodis pv. passiflorae
The Xanthomonas axonopodis. pv. passiflorae (gram-negative) bacterial strain was collected from fresh agar plates and suspended in distilled sterilized water (A650 nm = 3 x 108 CFU/mL). Aliquots of the bacterial suspension were diluted to 103 CFU/mL and incubated with BmLec (150 mg/mL) for one hour at 37ºC. After incubation, survival was assayed on nutrient plates (Sigma-Aldrich, USA) (n = 5). We then evaluated the effect of BmLec on the bacterial membrane of Xanthomonas axonopodis pv passiflorae incubated with lectin using scanning electron microscopy. The bacterial suspension was centrifuged, and the pellets were fixed at 4ºC in 0.1 M cacodylate buffer (pH 7.4) containing 2.5% (v/v) glutaraldehyde for 12 hours. Bacterial samples were fixed a second time with 1% osmium tetraoxide for two hours at 4ºC. The samples were then dehydrated in increasing concentrations of ethanol. The specimens were coated with gold in vacuum using a sputter coater SCD 050® (BAL-TEC, Germany).
Electron micrographs were obtained using a JSM 5800 LV® scanning electron microscope (JEOL, Japan) (19).
Perfused Kidney Assay
Adult male Wistar rats (260-320 g) were fasted for 24 hours with unrestricted access to water. The rats were anesthetized with sodium pentobarbitone (50 mg/kg, intraperitoneally). After careful removal of the right kidney, the right renal artery was cannulated via the mesenteric artery without interrupting blood flow (24). The kidneys were perfused with a modified Krebs-Henseleit solution (MKHS; 118.0 mmol/L NaCl, 1.2 mmol/L KCl, 1.18mmol/L KH2PO4, 1.18mmol/L MgSO4.7H2O, 2.5 mmol/L CaCl2 and 25 mmol/L NaHCO3). Bovine serum albumin (BSA 6 g) was added to 100 mL of MKHS and dialyzed for 48 hours at 4ºC against ten volumes of MKHS. Immediately before the beginning of each perfusion protocol, 100 mg of urea, 50 mg of inulin and 50 mg of glucose were added to every 100 mL of perfusate, and the pH was adjusted to 7.4. In each experiment, 100 mL of MKHS were recirculated for 120 minutes.
Indomethacin (10 µg/mL) was added to the system at the beginning of each experiment. The BmLec (10 µg/mL) was added to the system 30 minutes after the beginning of each perfusion. The perfusion pressure (PP) was measured at the tip of the stainless steel cannula in the renal artery. Samples of urine and perfusate were collected at ten-minute intervals to analyze the sodium and potassium levels using flame photometry. Inulin was measured as previously described, and the osmolality was measured using a vapor pressure osmometer (Wescor 5100C®, USA) (25). The chloride analysis was carried out using a LabTest kit (Germany). The renal vascular resistance (RVR), urinary flow (UF) and glomerular filtration rate (GFR) were measured. The percentage of sodium (% TNa+), potassium (% TK+) and chloride tubular transport were also determined (26).
Statistical Analysis
Results are presented as the mean ± SEM from six experiments for each group. Differences between groups were compared using a Student's t-test (non-parametric test) or an analysis of variance (ANOVA) followed by a Bonferroni test with significance set at 5% (p < 0.05).
Committee of Ethics
The study protocol was approved by the Committee of Ethics from the Federal University of Ceará, in Fortaleza, Brazil.
RESULTS
The protein fraction was eluted after adding CTBS buffer to the column in the presence of 0.1 M lactose. The main activity was found in the signaled peak (Figure 1 a and b). This fraction was re-purified using the reverse phase HPLC and showed a main protein peak accounting for 96% of the whole lectin fraction (Figure 2 a). After dialysis against ammonium bicarbonate, the homogeneity of this fraction was verified using two dimensional electrophoresis, which showed a single main protein spot with an estimated molecular mass of 14-15 kDa, pI in 5.6 approximately (Figure 2 b). MALDI-TOFF mass spectrometer showed that the molecular mass of the lectin spot was 16307.36 Da (Figure 2 c). The amino acid analysis of BmLec showed a high amount of acid and basic amino acid residues, and the presence of eight half cysteines (Table 1).
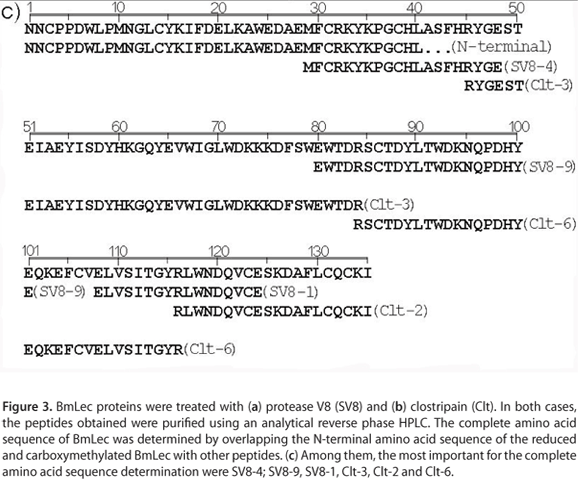
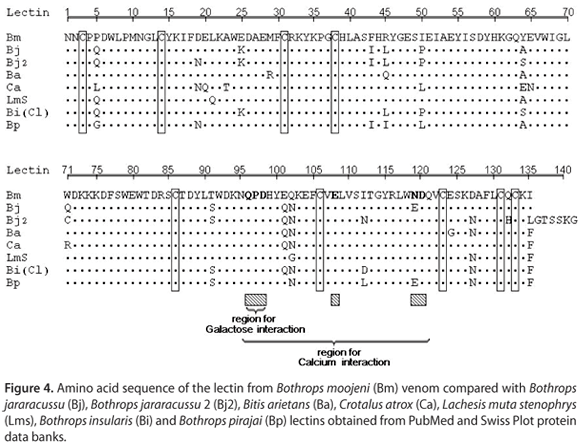
Two different BmLec proteins were subjected to treatment with protease V8 and clostripain. In both cases, the peptides obtained by incubation of BmLec with these enzymes were purified using an analytical reverse phase HPLC. The incubation of BmLec with protease V8 resulted in ten different peptides that were purified using a non-linear gradient concentration of aqueous acetonitrile (buffer B). The elution of the peptides was monitored at 214 nm (Figure 3 a). The aliquot of BmLec treated with clostripain resulted in the purification of seven different peptides (Figure 3 b). Complete amino acid sequences of BmLec were determined by overlapping the N-terminal amino acid sequence of reduced and carboxymethylated BmLec with other peptides. The amino acid sequences were SV8-4, SV8-9, SV8-1, Clt-3, Clt-2 and Clt-6 (Figure 3 c). The amino acid sequence of BmLec showed high similarity with sequences of other lectins, such as BjLec (lectin from Bothrops jararacussu), determined by automatic sequencing or molecular cloning and with those from Bitis arietans, Crotalus atrox, Lachesis muta stenophrys, Bothrops pirajai and Bothrops insularis, which were obtained solely by molecular cloning. The amino acid sequence of BmLec showed a conserved carbohydrate recognition domain (CRD) and other important amino acid residues for calcium binding (Figure 4).
In the present study, we observed that BmLec acted as a potent platelet aggregator at 10 mg (Figure 5 a). BmLec induced platelet aggregation in a dose-dependent fashion, with a minimal and maximum dose for aggregation activity of approximately 1.5 mg and 20 mg, respectively (Figure 5 b).
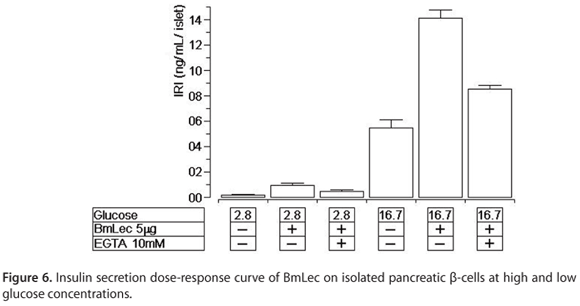
BmLec induced an increase in insulin secretion at low and high glucose concentrations, 2.8 and 16.7 mM, respectively (Figure 6). This effect was significantly affected by the presence of EGTA in both experiments.
In the antimicrobial activity assay, BmLec reduced the bacterial growth rate in approximately 15% (Figure 7 a). A scanning electron microscopy profile of Xanthomonas axonopodis pv. passiflorae was made in regard to the control group of bacteria without lectin (Figure 7 b). The ultrastructural alterations induced by BmLec on the bacteria (Xc) consisted mainly of high vesiculation in the bacterial membrane, whereas membrane rupture was only observed in some cases (Figure 7 c).
In the isolated kidney, the BmLec (10 mg/mL) increased the perfusion pressure, the renal vascular resistance and the urinary flow. The glomerular filtration rate and the percentages of sodium, potassium and chloride tubular transport were reduced at 60 minutes of perfusion. Indomethacin (10 mg/mL) reversed the renal alterations caused by BmLec (Table 2).
DISCUSSION
The purified protein from B. moojeni venom was obtained in two chromatographic steps, and its primary structure was determined, which showed common features of other C-type lectins, such as BjLec and others of this class. The conserved residues appear to form a general calcium-dependent and carbohydrate-binding framework. The amino acid sequence of BmLec showed a high structural homology with the galactose-binding lectin isolated from Bothrops jararacussu (BjLec), Bothrops insularis (BiLec) and Bothrops pirajai (BpLec), but its primary structure showed low homology with other C-type lectins, such as convulxin (Cvx). BmLec probably forms a dimeric structure with a molecular mass of 30 kDa, whereas Cvx-like C-type lectins form a tri-dimeric structure (20).
BmLec presents extra cystine residues that stabilize the three-dimensional structure by forming an interchain disulphide bond. Thus, this lectin forms a homodimmer similar to Bothrops jararacussu C-type lectin. These C-type lectins show a relatively conserved structure that is functionally distinct; however, some of the main properties were conserved and the amino acid changes appear to be crucial for the biological effects. The crystal structure of this type of lectin is inadequate for structural speculation at the three-dimensional level. Moreover, only a few successful structural studies for similar homodimeric C-type lectins were accomplished and did not show significant amino acid similarities with BmLec. It is generally agreed that the carbohydrate binding domain plays an important role in a possible interaction with cell membrane receptors and is, consequently, the mechanism through which this lectin is involved in the activation of cell signaling cascades.
Our data show that BmLec induced a strong and significant increase in insulin secretion at low and high glucose concentrations. These effects are similar to those induced by the venom obtained from Crotalus species. It has been shown that convulxin, other class of C-type lectin from Crotalus durissus ssp., induced similar insulin secretion at both sub- (2.8 mM) and supra-threshold (16.7 mM) glucose concentrations (21).
It has been shown that the binding of lectin to the bacterial membrane results in the formation of large bacterial clusters (aggregation), which, in some cases, can precipitate or remain as large agglutination structures (bacteria plus plant agglutinin). This agglutination might have an effect on the bacterial multiplication as observed in some bacterial strains treated with plant agglutinin. In addition, other proteins can inhibit bacterial multiplication and motility (18). Electron microscopy scanning showed a formation of large vesicles in the bacterial cell membrane and, in some cases, bacterial cell membrane rupture. This latter change might be a consequence of the interaction between lectin and the bacterial plasma membrane. It has been previously demonstrated that crotacetin induces platelet aggregation and inhibits antimicrobial activity in both gram-positive and gram-negative bacteria (19).
In the present work, BmLec increased the renal vascular resistance and the urinary flow; however, the glomerular filtration rate and the transport of electrolytes were reduced after infusion with venom. The venom effect on the RVR probably is involved in the decrease of the glomerular filtration rate by the reduction in the driving force that favors ultrafiltration.
It has been shown that plant and animal lectins may promote inflammatory mediator release and that prostaglandin might interfere in sodium, potassium and chloride tubular transportation (27, 28). In our experiments, indomethacin reversed the changes in the tubular transport of electrolytes induced by BmLec, suggesting the participation of eicosanoids in the process. In addition, the renal cortex produces prostaglandins that may inhibit tubular sodium reabsorption in the thick ascending limb of the loop of Henle and reduce chloride transport, thus exerting an influence on renal blood flow and glomerular filtration rate (28, 29).
In conclusion, the C-type galactoside specific lectin isolated from Bothrops moojeni venom had effects on platelet aggregation, insulin secretion, antibacterial activity and isolated kidney functions.
Submission status
Received: November 6, 2009.
Accepted: May 19, 2010.
Abstract published online: June 7, 2010.
Full paper published online: August 31, 2010.
Financial source
FAPESP and CNPq.
CONFLICTS OF INTEREST
There is no conflict.
ETHICS COMMITTEE APPROVAL
The present study was approved by the Ethics Committee from the Federal University of Ceará, in Fortaleza, Brazil.
- 1. Calgarotto AK, Damico DC, Ponce-Soto LA, Baldasso PA, Da Silva SL, Souza GH, et al. Biological and biochemical characterization of new basic phospholipase A(2) BmTX-I isolated from Bothrops moojeni snake venom. Toxicon. 2008;51(8):1509-19.
- 2. Morita T. C-type lectin-related proteins from snake venoms. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(4):357-73.
- 3. Marcinkiewicz C, Lobb RR, Marcinkiewicz MM, Daniel JL, Smith JB, Dangelmaier C, et al. Isolation and characterization of EMS16, a C-lectin type protein from Echis multisquamatus venom, a potent and selective inhibitor of the alpha 2-beta-1 integrin. Biochemistry. 2000;39(32):9859-67.
- 4. Rádis-Baptista G. Integrins, cancer and snake toxins (mini-review). J Venom Anim Toxins incl Trop Dis. 2005;11(3):217-41.
- 5. Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988;263(20):9557-60.
- 6. Drickamer K. C-type lectin-like domains. Curr Opin Struct Biol. 1999;9(5):585-90.
- 7. Drickamer K, Taylor ME. Glycan arrays for functional glycomics. Genome Biol. 2002;3(12): 1034.
- 8. Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005; 272(24):6179-217.
- 9. Castro HC, Lemos MG, Bon C, Zingali RB. Comparative evaluation of immunological and structural similarities of snake venom C-type lectin proteins. Toxicon. 2003;41(4):525-8.
- 10. de Carvalho DD, Marangoni S, Novello JC. Primary structure characterization of Bothrops jararacussu snake venom lectin. J Protein Chem. 2002;21(1):43-50.
- 11. Braud S, Bon C, Wisner A. Snake venom proteins acting on hemostasis. Biochimie. 2000;82(9-10):851-9.
- 12. Clemetson KJ, Navdaev A, Dormann D, Du XY, Clemetson JM. Multifunctional snake C-type lectins affecting platelets. Haemostasis. 2001;(3-6):148-54.
- 13. Ozeki Y, Matsui T, Hamako J, Suzuki M, Fujimura Y, Yoshida E, et al. C-type galactoside-binding lectin from Bothrops jararaca venom: comparison of its structure and function with those of botrocetin. Arch Biochem Biophys. 1994;308(1):306-10.
- 14. Lu Q, Navdaev A, Clemetson JM, Clemetson KJ. Snake venom C-type lectins interacting with platelet receptors. Structure-function relationships and effects on haemostasis. Toxicon. 2005;45(8):1089-98.
- 15. Hirabayashi J, Kasai K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J Biol Chem. 1991;266(35):23648-53.
- 16. Mastro AM, Hurley DJ, Winning RK, Filipowski R, Ogilvie ML, Gartner TK. Mitogenic activity of snake venom lectins. Cell Tissue Kinet. 1986;19(5):557-66.
- 17. Pereira-Bittencourt M, Carvalho DD, Gagliardi AR, Collins DC. The effect of a lectin from the venom of the snake Bothrops jararacussu on tumor cell proliferation. Anticancer Res. 1999;19(5B):4023-5.
- 18. Gaidamashvili M, van Staden J. Interaction of lectin-like proteins of South African medicinal plants with Staphylococcus aureus and Bacillus subtilis J Ethnopharmacol. 2002;80(2-3): 131-5.
- 19. Rádis-Baptista G, Moreno FB, de Lima Nogueira L, Martins AM, de Oliveira Toyama D, Toyama MH, et al. Crotacetin, a novel snake venom C-type lectin homolog of convulxin, exhibits an unpredictable antimicrobial activity. Cell Biochem Biophys. 2006;44(3):412-23.
- 20. Havt A, Toyama MH, do Nascimento NR, Toyama DO, Nobre AC, Martins AM, et al. A new C-type animal lectin isolated from Bothrops pirajai is responsible for the snake venom major effects in the isolated kidney. Int J Biochem Cell Biol. 2005;37(1):130-41
- 21. dos Santos ML, Fagundes FH, Teixeira BR, Toyama MH, Aparicio R. Purification and preliminary crystallographic analysis of a new Lys49-PLA2 from B. jararacussu. Int J Mol Sci. 2008;9(5):736-50.
- 22. Braga MD, Martins AM, Amora DN, de Menezes DB, Toyama MH, Toyama DO, et al. Purification and biological effects of C-type lectin isolated from Bothrops insularis venom. Toxicon. 2006;47(8):859-67.
- 23. Toyama MH, Carneiro EM, Marangoni S, Amaral MEC, Velloso LA, Boschero AC. Isolation and characterization of a convulxin-like protein from Crotalus durissus collilineatus venom. J Protein Chem. 2001;20(7):585-91.
- 24. Bowman RH. Gluconeogenesis in the isolated perfused rat kidney. J Biol Chem. 1970; 245(7):1604-12.
- 25. Walser M, Davidson DG, Orloff J. The renal clearance of alkali-stable inulin. J Clin Invest. 1955;34(10):1520-3.
- 26. Martinez-Maldonado M, Opava-Stitzer S. Free water clearance curves during saline, mannitol, glucose and urea diuresis in the rat. J Physiol. 1978;280(1):487-97.
- 27. Alencar NM, Teixeira EH, Assreuy AM, Cavada BS, Flores CA, Ribeiro RA. Leguminous lectins as tools for studying the role of sugar residues in leukocyte recruitment. Mediators Inflamm. 1999;8(2):107-13.
- 28. Havt A, Barbosa PS, Sousa TM, Martins AM, Nobre AC, Nascimento KS, et al. Renal alterations promoted by the lectins from Canavalia ensiformis (ConA) and Dioclea guianensis (DguiL) seeds. Protein Pept Lett. 2003;10(2):191-7.
- 29. Martins AM, Monteiro AM, Havt A, Barbosa PS, Soares TF, Evangelista JS, et al. Renal effects induced by lectin from Vaitarea macrocarpa seeds. J Pharm Pharmacol. 2005; 57(10):1329-33.
Publication Dates
-
Publication in this collection
30 Aug 2010 -
Date of issue
2010
History
-
Received
06 Nov 2009 -
Reviewed
19 May 2010