Abstract
Antivenoms against snake and scorpion envenomations are usually equine in nature and composed mostly of F(ab')2; additionally, phenol and m-cresol are mainly employed for their preservation. Although there is no study on this subject, m-cresol is utilized by most manufacturers in a concentration that ranges from 0.15 to 0.35 g%. Decreasing the concentration of m-cresol to its minimal effective level may protect victims from its toxic effects and keep the antivenom stable during its shelf life without forming any aggregates. In the present work, different concentrations of m-cresol, ranging from 0.1 to 0.35 g%, were used with some selected batches of snake and scorpion antivenoms. A low concentration of 0.15 g% showed an acceptable preserving result that complies perfectly with antimicrobial specifications stated by the British Pharmacopoeia. Tested antivenoms (in 12 batches), when kept in a cold room for 39 months (more than their shelf life), retained their physical, chemical and microbiological activities according to the specifications of pharmacopeias. The present data demonstrated that reduction of m-cresol concentration to 0.15 g% in case of equine F(ab')2 antivenoms will improve safety of such preparations and preserve their stability during their shelf life.
antivenoms; preservative; m-cresol; stability
ORIGINAL PAPER
What is the optimum concentration of m-cresol in antivenoms?
Abd-Elsalam MA; Abdoon N; Al-Ahaidib MS
National Antivenom and Vaccine Production Center, King Abdulaziz Medical City, King Fahad National Guard Hospital, Riyadh, Kingdom of Saudi Arabia
Correspondence to Correspondence to: Mohammad A. Abd-Elsalam National Antivenom and Vaccine Production Center King Abdulaziz Medical City King Fahad National Guard Hospital P.O. Box 22490, Riyadh, 11426, Kingdom of Saudi Arabia. Email: atefabuzeid@yahoo.com or abuzeidm@hotmail.com.
ABSTRACT
Antivenoms against snake and scorpion envenomations are usually equine in nature and composed mostly of F(ab')2; additionally, phenol and m-cresol are mainly employed for their preservation. Although there is no study on this subject, m-cresol is utilized by most manufacturers in a concentration that ranges from 0.15 to 0.35 g%. Decreasing the concentration of m-cresol to its minimal effective level may protect victims from its toxic effects and keep the antivenom stable during its shelf life without forming any aggregates. In the present work, different concentrations of m-cresol, ranging from 0.1 to 0.35 g%, were used with some selected batches of snake and scorpion antivenoms. A low concentration of 0.15 g% showed an acceptable preserving result that complies perfectly with antimicrobial specifications stated by the British Pharmacopoeia. Tested antivenoms (in 12 batches), when kept in a cold room for 39 months (more than their shelf life), retained their physical, chemical and microbiological activities according to the specifications of pharmacopeias. The present data demonstrated that reduction of m-cresol concentration to 0.15 g% in case of equine F(ab')2 antivenoms will improve safety of such preparations and preserve their stability during their shelf life.
Key words: antivenoms, preservative, m-cresol, stability.
INTRODUCTION
Antivenoms are refined and concentrated preparations of equine serum globulins - mostly F(ab')2 - obtained by fractionating blood from healthy horses that were previously immunized with different type of venoms (1, 2). For more than a century, antivenoms have been used effectively as the only treatment for snakebites and envenomations caused by other poisonous animals including spiders and scorpions (3). Most antivenoms are produced in liquid form to lower their cost and ease their use. Currently, there are eight antimicrobial preservatives commonly used in licensed parenteral products of which m-cresol and phenol are habitually employed by manufacturers as preservatives in antivenom formulations (4-6). Usually, phenol concentrations in antivenoms range from 0.15 to 0.5 g% (7-10). Combinations of phenol and thimerosal in different ratios have also been effectively used in some antivenom preparations (11, 12). M-cresol, a phenol derivative, is frequently utilized as preservative in numerous antivenom preparations in concentrations that range between 0.15 and 0.35 g% (4, 7, 13).
Despite their efficacy as additive, the use of these agents involves the possibility of some adverse effects. It is well known that phenol and cresol are toxic to humans in certain amounts (5, 14-19). Repeated exposure may cause harmful effects on the liver and kidney. Additionally, potential development of multiple organ failure with persistence of organ dysfunction associated with the overdose of injectable phenol was already reported (20). Turbidity, a signal of physical instability in antivenom preparations, increases proportionally to elevation of phenol concentration (6). Turbidity in equine antivenoms depends, at least, in one way, on the interaction between initial protein concentration in the serum, and addition of phenol during fractionation of serum (10).
Protein aggregation - besides loss of activity and safety of antivenom - is another important indicator of product instability caused by phenols, which are hydrophobic substances that may induce an increase in protein denaturation (3, 12, 21). García et al. (12) found differences in the augmentation of aggregate levels and dimmers among antivenoms stored for three years that were devoid of phenol and contained different preservatives, meaning that these substances could accelerate the normal denaturation process.
It is quite curious that such a wide range of preservatives employed in different concentrations in antivenoms was not tested to find the minimum concentration of phenols that may be utilized to restore maximum preserving activity and to maintain stability over the shelf life. In the present study, an attempt was made to optimize the effective preservative concentration in antivenoms by performing a well designed study according to the British Pharmacopoeia (22). Different types of antivenoms with varying concentrations of m-cresol were used in order to determine the minimum concentration of the mentioned preservative in antivenoms that achieve the best antimicrobial activity. A complete potency and stability study over the real shelf life of antivenom products was carried out to ensure their stability and safety. Meanwhile, improvements in antivenom quality will be focused on the obtainment of a more stable product in compliance with good manufacturing practices and at an affordable quality.
MATERIALS AND METHODS
Materials
Seven batches of polyvalent scorpion antivenom with different m-cresol concentrations - namely 0.0, 0.1, 0.15, 0.2, 0.25, 0.3 and 0.35% - presenting potency of 55 and 60 LD50/ mL respectively to neutralize Androctonus crassicauda and Leiurus quinquestriatus scorpion venoms were prepared according to the method of Pope (23, 24) modified by Harms (13) and improved by the National Antivenom and Vaccine Production Center, Riyadh. Immunoglobulins were precipitated by (NH4)2SO4, desalted and digested by pepsin. F(ab')2 was subjected to a series of purification steps, sterilized by bacterial filtration and placed in vials under aseptic conditions. Moreover, other seven batches of polyvalent snake antivenom with the same m-cresol concentrations presenting potencies of 35 LD50, 30 LD50, 40 LD50, 40 LD50, 60 LD50 and 80 LD50,/mL to neutralize respectively Naja haje, Walterinessia aegyptia, Echis carinatus, Echis coloratus, Cerastes cerastes, and Bitis arietans snake venoms were prepared.
The strains of challenge organisms used for testing the antimicrobial efficacy of the preservative (m-cresol) were: Pseudomonas aeruginosa ATCC 9027, Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6633, Candida albicans ATCC 10231 and Aspergilus niger ATCC 16404.
Methods
Determination of Antimicrobial Activity
The method employed followed the one described in the British Pharmacopoeia XVI C 5.1.3 (22). The test was conducted in sterile capped bacteriological containers. In brief, a series of six containers for each concentration of m-cresol to be examined was inoculated, with a suspension of one of the test microorganisms to give an inoculum of 105 to 106 microorganisms per milliliter of preparation. The final solution was mixed thoroughly to ensure homogeneous distribution. The inoculated product was incubated at 22.5 ± 2.5°C protected from light. One milliliter of the inoculated sample was removed, at zero, 6 and 24 hours and then at an interval of 7, 14 and 28 days. The number of viable microorganisms was determined by the plate count method. Any residual antimicrobial activity was eliminated by dilution. The initial concentration of viable microorganism in each preparation was estimated based on the concentration of microorganism in each of the standard inoculums as determined by the plate count method.
The antimicrobial activity of each concentration was evaluated in terms of the log reduction in the number of viable microorganisms using as baseline the value obtained for the inoculums.
Product stability over the real shelf lives
One hundred vials from each antivenom batch were kept in cold room at 5 ± 3°C for 39 months protected from light. The quality control tests including pH, sterility, pyrogen, abnormal toxicity, protein concentration, as well as neutralizing potency against lethal activity of different venoms were performed according to the methods described in the British Pharmacopoeia (22). Determination of m-cresol concentration was carried out according to method of Bose and Gupta (25). All tests were performed at zero time and then 39 months after production.
RESULTS
Antimicrobial Activity
In time-matched control experiments, negative control tests were conducted utilizing microbiological media used for cultivation of different strains of microorganisms. No growth was detected after incubating the media for recommended time and under the recommended conditions.
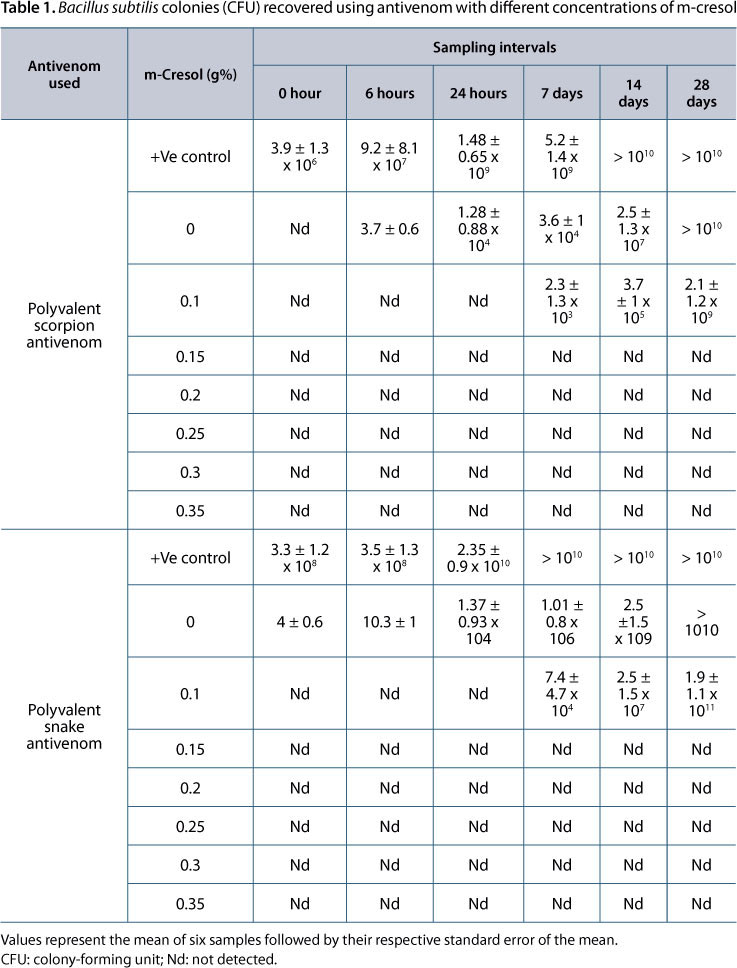
In general, m-cresol in a concentration as low as 0.15 g% provoked complete inhibition of growth in all tested bacterial strains, with both polyvalent scorpion and snake antivenom. This effect was observed during the whole period of the study. The same result was obtained when higher concentrations of the preservative were used with the two types of antivenom (Tables 1, 2 and 3).
Similar pattern was observed when fungi were used as challenge microorganisms. Low concentrations of m-cresol, (0.15 g% and more) provoked complete growth inhibition of both Aspergilus niger and Candida albicans. The inhibition started 24 hours after incubation and was maintained up to 28 days of the experiment when concentrations of 0.15 and 0.2 g% were used, and at zero time and afterwards when higher m-cresol concentrations were tested (Tables 4 and 5).
Stability over the Real Shelf Lives
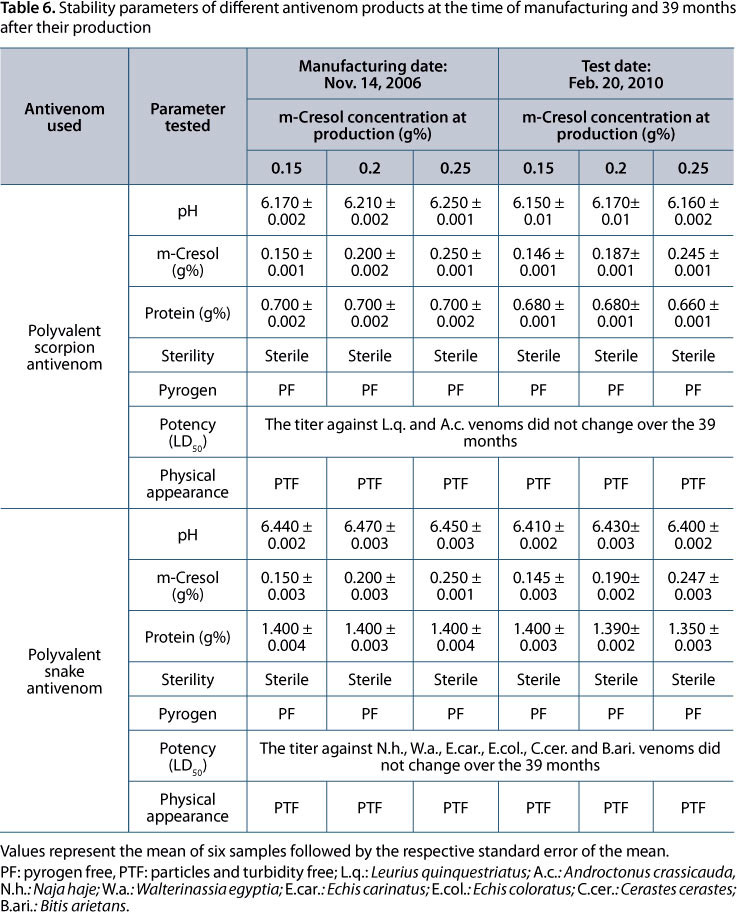
Vials of polyvalent scorpion and snake antivenom, containing concentrations of m-cresol ranging from 0.15 to 0.25 g% and kept in recommended conditions of storage for 39 months showed no change in their physicochemical and pharmacological characteristics from those at the time of production. No variations occurred in potency, protein and m-cresol concentrations, pH, sterility or safety, during the study period. Products remained sterile and pyrogen free for the entire period of the experiment (Table 6).
DISCUSSION
Although m-cresol is employed by many antivenom manufacturers as preservative in their preparations, no clear recommendation was found in North American or British pharmacopoeias regarding the actual concentration that should be used to keep the product sterile and stable over the period of its shelf life. This situation gave a good chance for formulators to employ different amounts of the same preservative, sometimes even to a level that may harm the patient. This discrepancy in concentration is clearly observed in most antivenom preparations (9, 10, 12, 26).
In this study, both polyvalent snake and scorpion antivenoms - containing m-cresol in a concentration of 0.15 g% and more - completely prevented the growth and proliferation of tested microorganisms. This result observes the requirements for the antimicrobial activity stated by the British Pharmacopoeia 2002, which states that the preservative properties of the preparation are adequate if, in the test conditions, there is a significant drop or no increase in the number of microorganisms in the preparation after the evaluation period in adequate conditions. In addition, all antivenom preparations - containing m-cresol in a concentration from 0.15 to 0.25 g% and kept in cold room (at 5 ± 3°C) for more than their real shelf life - did not show any aggregation or other physical instability.
Segura et al. (6) observed that the reduction of phenol to a concentration of 0.15 g% maintains the physical stability of antivenoms and decreases their liability of forming aggregates (6). Other researchers corroborate such finding, especially if the protein content is elevated, which may lead to loss of antivenom activity and to adverse reactions due to activation of the immune response (4, 21, 26-30). At the same time, the decrease of m-cresol concentrations may diminish antivenom toxicity to humans, particularly in certain circumstances in which high doses are required, such as in the two cases mentioned by Buntain (31) and Ganthavorn (32) (750 to 1150 mL of antivenom). Likewise, all quality control tests performed with antivenoms, according to the pharmacopoeial requirements, indicated that their chemical and pharmacological activities were maintained during the whole period of the experiment. Present results also support the conclusion that for maximum protection of patients, the preservative concentration in the final product should be below a level that may be toxic to humans while retaining the product efficiency (33).
CONCLUSIONS
According to the present study, it is recommended for antivenom producers to reduce m-cresol or phenol to their minimal concentrations to ensure sterility and stability over the real shelf life of the product. At the same time, it is necessary to decrease the toxic load of preservatives, especially if large volumes of antivenoms are required.
ACKNOWLEDGEMENTS
The authors thank professor Mohammad Shohayeb, University of Tanta, Egypt, for his valuable suggestions and comments on this work.
Submission status
Received: April 24, 2010.
Accepted: October 26, 2010.
Abstract published online: November 5, 2010.
Full paper published online: February 28, 2011.
Conflicts of interest
There is no conflict.
Ethics committee approval
The tests performed in this research are under the guidelines of the Ethics Committee for Safe Animal Handling of the Kingdom of Saudi Arabia.
-
1World Health Organization (WHO). Progress in the characterization of venoms and standardization of antivenoms. Geneva, 1981. WHO Offset Publication n. 58.
- 2. Theakston RDG, Warrell DA, Griffiths E. Reporter of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41(5):541-57.
- 3. Rodrigues-Silva R, Antunes GFC, Velarde DT, Santoro MM. Thermal stability studies of hyperimmune horse antivenoms. Toxicon. 1999;37(1):33-45.
- 4. Christensen PA. The stability of refined antivenin. Toxicon. 1975;13(1):75-7.
- 5. Meyer BK, Ni A, Hu B, Shi L. Antimicrobial preservative use in parenteral products: past and present. J Pharm Sci. 2007;96(12):3155-67.
- 6. Segura A, Herrera M, González E, Vargas M, Solano G, Gutiérrez JM, et al. Stability of equine IgG antivenom obtained by caprylic precipitation: towards a liquid formulation stable at tropical room temperature. Toxicon. 2009;53(6):609-15.
- 7. Delsal JL, Chamsy H Mir. Purification et concentration des plasmas antitoxiques 1- Historique, techniques industrielles et choix de la methode adoptee en Iran. Immunol. 1953;17:110-34.
- 8. Latifi M. Immunological studies on antiscorpion serum and antivenin. In: Rosenberg P, editor. Toxins, animal, plants and microbial. Oxford: Pergamon Press; 1978. p. 619-28.
- 9. Raw I, Guildon R, Higashi HG, Kelen EM. Antivenins in Brazil: Preparation. In: Tu AT, editor. Handbook of natural toxins. Reptile venoms and toxins. New York: Marcel Dekker; 1991. V. 5. p. 557-81.
- 10. Rojas G, Jimenez JM, Gutiérrez M. Caprylic acid fractionation of hyperimmune horse plasma: description of a simple procedure for antivenom production. Toxicon. 1994;32(3):351-63.
- 11. Bolaños R, Cerdas L, Taylor R. The production and characterization of a coral snake (Micrurus mipartitus hertwigi) antivenin. Toxicon. 1975;13(2):139-42.
- 12. García M, Monge M, León G, Lizano S, Segura E, Solano G, et al. Effect of preservatives on IgG aggregation, complement-activating effect and hypotensive activity of horse polyvalent antivenom used in snakebite envenomation. Biological. 2002;30(2):143-51.
- 13. Harms AJ. The purification of antitoxic plasmas by enzyme treatment and heat denaturation. Biochem J. 1948;42(3):390-7.
- 14. Truppman ES, Ellenby JD. Major electrocardiographic changes during chemical face peeling. Plast Reconstr Surg. 1979;63(1):44-8.
- 15. Cheng M, Kligerman AD. Evaluation of genotoxicity of cresols using siste-chromatid exchange (SCE). Mutat Res. 1984;137(1):51-5.
- 16. Wexler MR, Halon DA, Teitelpaum A, Tadjer G, Peled IJ. Prevention of cardiac arrhythmias produced in an animal model by the topical application of a phenol preparation in common use for face peeling. Plast Reconstr Surg. 1984;73(4):595-8.
- 17. Harvey SC. Antisepticos y desinfectantes; fungicidas; ectoparasiticidas. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. Las bases farmacológicas de la terapeútica. Buenos Aires: Editorial Medica Panamericana; 1988. p. 914-33.
- 18. Budavari S, editor. The Merk Index. 20th ed. New Jersey: Merck Research Laboratories Division of Merck and Co. Inc.; 1996. p. 437.
- 19. Zychar BC, Castro Jr NC, Marcelino JR, Gonçalves LRC. Phenol used as a preservative in Bothorps antivenom induces impairment in leukocyte-endothelial interactions. Toxicon. 2008;51(7):1151-7.
- 20. Gupta S, Ashrith G, Chandra D, Gupta AK, Finkel KW, Guntupalli JS. Acute phenol poisoning: a life-threatening hazard of chronic pain relief. Clin Toxicol (Phila). 2008;46(3):250-3.
- 21. Morais VM, Massaldi H. Snake antivenoms: adverse reactions and production technology. J Venom Anim Toxins incl Trop Dis. 2009;15(1):2-18.
-
22British Pharmacopoeia. Version 6. Efficacy of antimicrobial preservation. Appendix XVI C. London: The Stationary Office; 2002. p. A322-4.
- 23. Pope CG. The action of proteolytic enzymes as antitoxins and proteins of immune sera. I. True digestion of the protein. B J Exp Pathol. 1939a;20:132-49.
- 24. Pope CG. The action of proteolytic enzymes antitoxins and proteins of immune sera. II. Heat denaturation after partial enzyme action. B J Exp Pathol. 1939b; 20:201-2.
- 25. Bose D, Gupta VK. Simple spectrophotometric method for determination of m-cresol. Chem Anal. 1994;39:53-9.
- 26. Atemnkenq MA, De Cock K, Plaizier-Vercammesn J. Post-marketing assessment of content and efficacy of preservatives in artimisinin derived antimalarial suspensions for paediatric use. Malar J. 2007;6:12.
- 27. Rojas G, Espinoza M, Lomonte B, Gutiérrez JM. Effect of storage temperature on the stability of the liquid polyvalent antivenom produced in Costa Rica. Toxicon. 1994; 28(1):101-5.
- 28. Otero-Pantino R, Cardozo J, Higash H, Nunez V, Diaz A, Toro M, et al. A randomized, blinded, comparative trail of one pepsin-digested and two whole IgG antivenoms for Bothrops snake bites in Uraba, Colombia. The Regional Group of Antivenom Therapy Research (REGATHER). Am J Trop Med Hyg. 1998;58(2):183-9.
- 29. Dart RC, Mcnally J. Efficacy, safety, and use of snake antivenom in the United States. Ann Emerg Med. 2001;37(2):181-8.
- 30. Clark RF, McKinney PF, Chase PB, Walter FG. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (Ovine) antivenom. Ann Emerg Med. 2002;39(6):671-7.
- 31. Buntain WL. Successful venomous snakebite neutralization with massive antivenom infusion in a child. J Trauma. 1983;23(11):1012-3.
- 32. Ganthavorn S. A case of king cobra bite. Toxicon. 1971;9(3):293-4.
-
33The United States Pharmacopoeial Convention, Inc. The United States Pharmacopoeia. Antimicrobial effectiveness testing. Microbiological tests, general requirements for tests and assays. Rockville; 2003. p 2085.
Correspondence to:
Publication Dates
-
Publication in this collection
01 Mar 2011 -
Date of issue
2011
History
-
Received
24 Apr 2010 -
Accepted
26 Oct 2010