Abstract
In the present study, the effectiveness of Mimosa pudica tannins (MPT) in neutralizing the lethality of Naja kaouthia venom was compared with commercially derived tannins. Preincubation of MPT with N. kaouthia venom maintained 100% survival of mice after 24 hours. The mouse group in which there was no preincubation, no protection against the effects of the venom was observed. M. pudica tannin was found to be more effective in neutralizing the lethality of N. kaouthia venom when compared to commercial tannic acid. Two protein spots were missing in the two-dimensional gel electrophoresis (2-DE) of the MPT treated mouse indicating the down-regulation of venom proteins. The results from this study indicated that tannins obtained from M. pudica are better than tannic acid in neutralizing the lethality of N. kaouthia venom in vitro. However, further investigations are required to establish that M. pudica has potential for treating N. kaouthia snakebites.
plant extract; snake venom; therapy; tannin; phospholipase A2
ORIGINAL PAPER
Efficacy of tannins from Mimosa pudica and tannic acid in neutralizing cobra (Naja kaouthia) venom
Sia FYI; Vejayan JII; Jamuna AIII; Ambu SI
IInternational Medical University, Kuala Lumpur, Malaysia
IISchool of Medicine and Health Sciences, Monash University, Bandar Sunway, Selangor, Malaysia
IIIInstitute of Biological Sciences, University of Malaya, Kuala Lumpur, Malaysia
Correspondence to Correspondence to: Stephen Ambu International Medical University 126, Jalan 19/155B, Bukit Jalil 57000 Kuala Lumpur, Malaysia. Phone: +603 27317291. Fax: +603 86567229. Email: Stephen_ambu@imu.edu.my.
ABSTRACT
In the present study, the effectiveness of Mimosa pudica tannins (MPT) in neutralizing the lethality of Naja kaouthia venom was compared with commercially derived tannins. Preincubation of MPT with N. kaouthia venom maintained 100% survival of mice after 24 hours. The mouse group in which there was no preincubation, no protection against the effects of the venom was observed. M. pudica tannin was found to be more effective in neutralizing the lethality of N. kaouthia venom when compared to commercial tannic acid. Two protein spots were missing in the two-dimensional gel electrophoresis (2-DE) of the MPT treated mouse indicating the down-regulation of venom proteins. The results from this study indicated that tannins obtained from M. pudica are better than tannic acid in neutralizing the lethality of N. kaouthia venom in vitro. However, further investigations are required to establish that M. pudica has potential for treating N. kaouthia snakebites.
Key words: plant extract, snake venom, therapy, tannin, phospholipase A2.
INTRODUCTION
Snakebites represent a public health problem in numerous countries (1). In Malaysia, the common cobra (Naja kaouthia) is responsible for most bites, which display varied clinical pictures, the most prominent one being neurotoxicity (2). In some cases, the bites produce significant local swelling and extensive tissue necrosis that may lead to the development of long term complications, such as chronic ulceration, infection and osteomyelitis (3).
Antivenom therapy is the only specific treatment for systemic snake envenomations. However, antivenom does not provide enough protection against some toxic enzyme effects induced by snake venoms (4). Antivenom treatment, in some cases, is not be able to prevent local tissue necrosis and may also predispose the victim to the risk of hypersensitivity reactions such as anaphylaxis and serum sickness due to equine and ovine allergens present in the serum (2, 5).
In several countries, the development of antivenoms is costly and the supply is limited (3). Nevertheless, in many regions numerous plants have been successfully used as snake venom antidotes (6, 7). A preliminary study screened 17 plants for anti-snake venom activity and showed that only Mimosa pudica can significantly neutralize the lethal effects of five common species of venomous snakes (8). A work by Mahanta and Mukherjee (9) has demonstrated the anti-snake venom potential of this plant. Tannins comprise a group of polyphenolic compounds widely distributed in the plant kingdom that present the unique ability to form complexes with macromolecules, particularly proteins (10). Since snake venoms consist mainly of proteins (70-90%) that are responsible for a wide range of clinical signs and symptoms, this study aims to analyze the biological properties of tannins regarding anti-snake venom activity.
MATERIALS AND METHODS
Plant Material
Mimosa pudica plants were collected from the field in Bagan Lalang beach, Malaysia. Only M. pudica roots were cut into small pieces and dried in a convection oven at 50°C. The dried roots were finely ground to powder using a blender. The crude extract was prepared using 4 g of powdered root in 200 mL of deionized water. Subsequently, the sample was concentrated using a rotary evaporator and then redissolved by the addition of 1.5 mL of water. This sample was then subjected to tannin isolation using Sephadex (LH-20) chromatography as described in our previous work (8). It was estimated that each 100 μL of isolate contained 0.7 mg of dry weight material. The commercial source of tannin was Fluka BioChemika (Germany).
Venom and Antivenom
Lyophilized Naja kaouthia venom obtained from the Snake and Reptile Farm, Perlis, was freeze dried and stored at -80°C until further use. The commercial source of snake antivenom was the Thai Red Cross, Bangkok, Thailand.
In Vitro and In Vivo Animal Studies
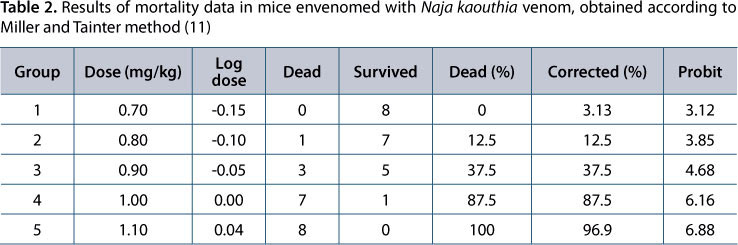
Male adult ICR mice (8-week-old) weighing between 20 and 25 g were used in the experiments. The LD50 of N. kaouthia was determined by using the graphical method of Miller and Tainter (11). Various dilutions of N. kaouthia venom were obtained by dissolving the lyophilized venom in saline (0.85% NaCl/water, w/v). An initial high dose of snake venom was administered to ensure 100% mortality in mice. From this, the dose was gradually adjusted by a decrease of 0.1 mg/kg until no death was observed. This was the dose range used for LD50 determination.
As for the anti-snake venom assay, four groups of mice (each group had eight mice) were employed (Table 1). All injections (200 μL) were given by the intraperitoneal (IP) route. Treated mice were observed over 24 hours for mortality.
In the in vivo experiment (rescue treatment), mice received treatment solutions and venom by separate routes. The IP injection of snake venom was immediately followed by IV injection into the tail vein of treatment solutions.
In the in vitro experiments, M. pudica tannin, tannic acid and monospecific N. kaouthia antivenom were given to mice. The aliquots of the treatment solution were mixed with equal volumes of snake venom (total volume was 200 μL) and IP injected into each mouse.
Two Dimensional Gel Electrophoresis (2-DE)
Mouse serum from two different treatment groups was utilized for comparison. One group was treated with a preincubated mixture of venom and tannin from M. pudica, while the other was treated with N. kaouthia venom only. M. pudica tannin was incubated (37°C) with N. kaouthia venom for ten minutes before the injection into each mouse. In both treatments, an injection (IP) of 200 μL was administered. Immediately after inoculation, mice were sacrificed with ether and blood was collected via cardiac puncture and stored at -80°C. Protein concentration was determined using the Bradford assay.
For the first dimension electrophoresis, IPG strips (Immobiline Dry Strip®, GE Healthcare-Bio-Sciences AB, Sweden, pH 3-10, 13 cm) were used. The first dimension isoelectric focusing (IEF) was run on IPGphor® (GE Healthcare-Bio-Sciences AB, Sweden) at 200 V for one hour, 1000 V for one hour and 8000 V for 3.5 hours. After the run, the strips were kept at -80°C. The proteins were separated during the second dimension by 15% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Fast staining of the gels was carried out using Coomassie Brilliant Blue. The gel images were scanned into a computer and the differences in protein expression between the M. pudica tannin-treated and venom-treated gels were analyzed by ImageMaster® 2D Platinum software (developed by the Swiss Institute of Bioinformatics in collaboration with GeneBio and GE Healthcare-Bio-Sciences AB, Sweden).
RESULTS
The crude water extract was separated into two fractions: MPMeOH eluted with 50% methanol (without any activity) and MPT eluted with 70% acetone (Figure 1).
Venom doses ranging from 0.7 to 1.1 mg/kg were injected into each group of mice. The data obtained is shown in Table 2. Using these data (probit analysis), the 2 LD50 of N. kaouthia venom were calculated to be 0.92 ± 0.10 mg/kg.
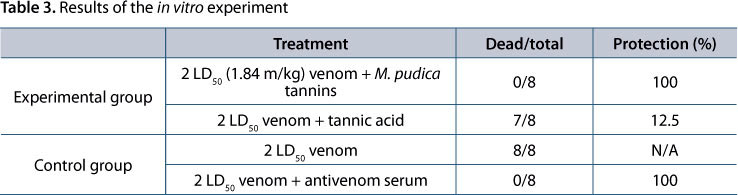
Preincubation of 2 LD50 (1.84 mg/kg) of N. kaouthia venom with M. pudica tannins was able to completely neutralize the snake venom since the survival rate of mice was 100% after 24 hours of observation (Table 3). Preincubation of the venom with commercial tannic acid showed only a mouse survival rate of 12.5%. Tannins obtained from M. pudica were eight times more effective in neutralizing the lethal effects of N. kaouthia venom compared to the tannic acid. As expected, all mice treated with the venom-antivenom mixture survived after 24 hours.
Results of the in vivo experiment (rescue treatment) showed that administration of N. kaouthia venom and M. pudica tannins by separate routes failed to inhibit the lethality in mice. The same result was observed in mice treated with tannic acid. Only the serum with antivenom was able to fully protect the mice against N. kaouthia venom-induced lethality after 24 hours.
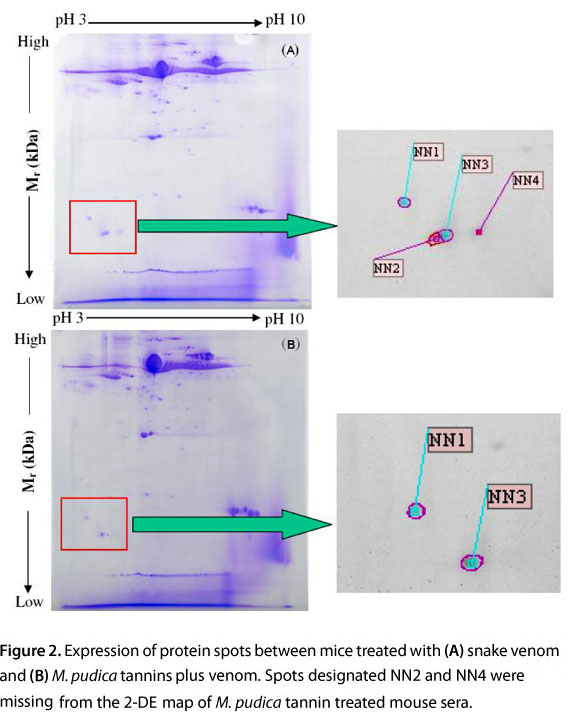
The analysis of scanned images of the 2-DE gels using the ImageMaster 2D® Platinum software, to compare the protein expression in sera from mice subjected to two different treatments, revealed two missing spots in the lower left region of the 2-DE serum map of mice treated with M. pudica tannins (Figure 2).
The labeled protein spots from each gel were then visualized in a three-dimensional perspective (Figure 3). Each identified protein spot on the gel showed up an individual peak. The analysis confirmed that two protein spots had been down-regulated due to the protein spots NN2 and NN4 that were missing from the 2-DE map of mice treated with M. pudica tannin and venom (no peaks).
DISCUSSION
The aim of the present study was to compare the efficacy of tannins obtained from M. pudica and commercial tannic acid on N. kaouthia venom neutralization. Both in vitro study (preincubation of plant extract with venom) and in vivo study (rescue treatment) were carried out to test the efficacy of both sources of tannins. Monovalent N. kaouthia antiserum was used as control. As tannins are known to precipitate proteins, it was expected that activities of some of the toxic enzymes of N. kaouthia would be inhibited (10).
The current results revealed that preincubation of M. pudica tannins with N. kaouthia venom (in vitro study) before their inoculation in mice was able to maintain 100% survival after 24 hours, which corroborates previous in vitro studies (8). On the other hand, the in vivo test of M. pudica tannins - administration of venom first followed immediately by the plant extract using separate injection routes - failed to rescue mice from the lethal effects of N. kaouthia venom.
The in vitro test of commercial tannic acid showed only 1/8 or 12.5% protection in mice. The tannic acid also failed to show any inhibition of lethality in the rescue experiment. Based on the study results, we found that M. pudica tannins are more effective than tannic acid in neutralizing 2 LD50 of N. kaouthia venom in vitro, but less efficient than antivenom.
In the 2-DE study, two spots representing venom proteins were missing in the 2-DE map of mice treated with M. pudica tannins. There are two types of plant tannins, namely hydrolysable and condensed tannins (10). Some plants, including Pentace burmanica and Areca catechu, that produce mainly condensed tannins are able to inhibit snake venom activities (12). In our study, the commercial tannic acid employed comprised the hydrolysable and gallotannin types, and it was established that M. pudica tannins were more effective in neutralizing the snake venom. We hypothesized that the roots of M. pudica contained a higher rate of condensed and hydrolysable tannins.
In our study, we also demonstrated that the rescue treatment failed to save mice from lethality induced by snake venom. This finding does not agree with several studies that found positive results by using in vitro neutralization methods of plant extracts for anti-snake venom assays. However, one work showed similar results regarding tannins of Musa paradisiaca extract that presented in vitro neutralization activities, but not in vivo (13). This can be attributed to the fact that tannins are able to non-specifically bind to N. kaouthia venom proteins and precipitate them, thus provoking the anti-lethal effects. Nevertheless, in in vivo studies tannins may interact with plasma proteins from the blood circulation. Therefore, there is a decrease in binding to N. kaouthia venom proteins and, hence, tannins become less efficient as anti-venom.
From the 2-DE results obtained, it was shown that tannin compounds of M. pudica root extract interacted with N. kaouthia venom proteins and produced down regulation of the two protein spots. We emphasize the importance of two missing spots in a particular region of the gel based on two previous studies, the protein spots on that region of the gel were identified as isoenzymes of phospholipase A2 when compared with the protein database (8, 14).
By comparing the results of the 2-DE gel with the results of the preliminary study by Vejayan et al. (8), it was demonstrated that the missing protein spots on the M. pudica treated gel were the isomers of PLA2 enzymes. Phospholipases A2 constitute major components of snake venoms and exhibit a wide range of pharmacological actions such as neurotoxicity; cardiotoxicity; myotoxicity; anticoagulant, hemorrhagic, edematous, hemolytic, convulsive and hypotensive activities (15, 16). By showing that PLA2 enzymes have been down-regulated, this explained why mice that received venom previously incubated with M. pudica tannins were fully protected from the lethal effects of N. kaouthia venom.
Based on the present observations, M. pudica demonstrated potential for an antagonist to N. kaouthia venom at in vitro tests.
ACKNOWLEDGEMENTS
We wish to thank the Dean, Research and Postgraduate Studies, IMU and the Dean, School of Medicine and Health Sciences, Monash University, Malaysia for their support in carrying out this study. Our appreciation is also extended to the technical staff of both universities for their assistance.
Submission status
Received: July 28, 2010.
Accepted: October 19, 2010.
Abstract published online: October 22, 2010.
Full paper published online: February 28, 2011.
Financial source
The International Medical University Research Fund BMS 102_2007 (13) and the Malaysian Ministry of Science and Technology (project number 02-02-10-SF0033) provided the financial grants.
Conflicts of interest
There is no conflict.
Ethics committee approval
The present study was approved by the Joint Committee of the IMU/RC and IMU/EC, International Medical University. The Ethics Committee/IRB reference number is: 4.6/02/2007
- 1. Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76(5):515-24.
- 2. Britt A, Burkhart K. Naja naja cobra bite. Am J Emerg Med. 1997;15(5):529-31.
- 3. Warrel DA. WHO/SEARO Guidelines for the clinical management of snake bites in the Southeast Asian region. Southeast Asian J Trop Med Public Health. 1999;(30 Suppl)1:1-85.
- 4. Sutherland SK. Antivenom use in Australia. Premedication, adverse reactions and the use of venom detection kits. Med J Aust. 1992;157(11-12):734-9.
- 5. Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41(5):541-57.
- 6. Mors WB, Do Nascimento MC, Pereira BM, Pereira NA. Plant natural products active against snake bite - the molecular approach. Phytochemistry. 2000;55(6):627-42.
- 7. Oshima-Franco Y, Alves CMV, Andreo Filho N, Gerenutti M, Cintra ACO, Leite GB, et al. Neutralization of the neuromuscular activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom, by a hydroalcoholic extract of Casearia sylvestris Sw. (Guaçatonga). J Venom Anim Toxins incl Trop Dis. 2005;11(4):465-78.
- 8. Vejayan J, Ibrahim H, Othman I. The potential of Mimosa pudica (Mimosaceae) against snake envenomation. J Trop Forest Science. 2007;19(4):189-97.
- 9. Mahanta M, Mukherjee AK. Neutralisation of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom by Mimosa pudica root extracts. J Ethanopharmacol. 2001;75(1):55-60.
- 10. Haslam E. Vegetable tannins - Lessons of a phytochemical lifetime. Phytochemistry. 2007;68(22-24):2713-21.
- 11. Miller LC, Tainter ML. Estimation of ED50 and its error by means of logarithmic probit graph paper. Proc Soc Exp Biol Med.1944;57(1):261-4.
- 12. Pithayanukul P, Ruenraroengsak P, Bavovada R, Pakmanee N, Suttisri R, Saen-oon S. Inhibition of Naja kaouthia venom activities by plant polyphenols. J Ethanopharmacol. 2005;97(3):527-33.
- 13. Borges MH, Alves DL, Raslan DS, Piló-Veloso D, Rodrigues VM, Homsi-Brandeburgo MI, et al. Neutralizing properties of Musa paradisiaca L. (Musaceae) juice on phospholipase A2, myotoxic, hemorrhagic and lethal activities of Crotalidae venoms. J Ethnopharmacol. 2005;98(1-2):21-9.
- 14. Kulkeaw K, Chaicumpa W, Sakolvaree Y, Tongtawe P, Tapchaisri P. Proteome and immunome of the venom of the Thai cobra, Naja kaouthia Toxicon. 2007; 49(7):1026-41.
- 15. Rosenberg P. Phospholipases. In: Shier WT, Mebs T, editors. Handbook of toxinology. New York: Marcel Dekker; 1990. p. 667-77.
- 16. Soares AM, Giglio JR. Chemical modifications of phospholipases A2 from snake venoms: effects on catalytic and pharmacological properties. Toxicon. 2003;42(8):855-68.
Correspondence to:
Publication Dates
-
Publication in this collection
01 Mar 2011 -
Date of issue
2011
History
-
Received
28 July 2010 -
Accepted
19 Oct 2010