Abstract
A myotoxin phospholipase A2 homologue, BmooMtx, was isolated from the venom of Bothrops moojeni by a combination of ion-exchange chromatography on DEAE-Sephacel column and gel filtration on Sephadex G-75. SDS-PAGE showed the enzyme to be a monomer with a molecular weight of 16,500. BmooMtx induced release of creatine kinase and morphological analyses indicated that it provoked an intense myonecrosis, with visible leukocyte infiltrate and damaged muscle cells 24 hours after injection. Anti-BmooMTx antibodies partially neutralized the myotoxic activity of BmooMtx and crude B. moojeni venom, as judged by determination of plasma creatine kinase levels and histological evaluation of skeletal muscle in mice. Anti-BmooMTx antibodies were effective in reducing the plasma creatine kinase levels of crude B. alternatus and B. leucurus venoms, evidencing immunological cross-reactivity between BmooMTx and other bothropic venoms. Intraplantar (i.pl.) injection of BmooMtx (1 to 15 μg/animal) caused a dose- and time-dependent hyperalgesia and edematogenic responses. Dexamethasone (0.4 mg/kg), meloxicam (2 mg/kg) and promethazine (5 mg/kg) markedly reduced the hyperalgesia. Our data suggest that these drugs may likely serve as complementary therapies in cases of accidents with Bothrops moojeni, provided that such pharmacological treatments are administered immediately after the incident.
Bothrops moojeni; hyperalgesia; myotoxin; edema
ORIGINAL PAPER
Biological characterization of a myotoxin phosphoplipase A2 homologue purified from the venom of the snake Bothrops moojeni
Queiroz MRI, II; Mamede CCI; Fonseca KCI; Canabrava LCMNI; França LVIII; Silva MCI; Stanziola LI, II; Beletti MEI; Canabrava HANI, II; Oliveira FI, II
IInstitute of Biomedical Sciences, Federal University of Uberlândia, Uberlândia, Minas Gerais State, Brazil
IINational Institute of Science and Technology on Nano-Biopharmaceuticals (N-Biofar)
IIIDepartment of Biochemistry and Immunology, Medical School of Ribeirão Preto, University of São Paulo, USP, Ribeirão Preto, São Paulo State, Brazil
Correspondence to Correspondence to: Fábio de Oliveira Instituto de Ciências Biomédicas Universidade Federal de Uberlândia Av. Pará, 1720, Uberlandia, MG, Brasil. Phone: +55 34 32182200. Fax: +55 34 32182200. Email: foliveira@umuarama.ufu.br
ABSTRACT
A myotoxin phospholipase A2 homologue, BmooMtx, was isolated from the venom of Bothrops moojeni by a combination of ion-exchange chromatography on DEAE-Sephacel column and gel filtration on Sephadex G-75. SDS-PAGE showed the enzyme to be a monomer with a molecular weight of 16,500. BmooMtx induced release of creatine kinase and morphological analyses indicated that it provoked an intense myonecrosis, with visible leukocyte infiltrate and damaged muscle cells 24 hours after injection. Anti-BmooMTx antibodies partially neutralized the myotoxic activity of BmooMtx and crude B. moojeni venom, as judged by determination of plasma creatine kinase levels and histological evaluation of skeletal muscle in mice. Anti-BmooMTx antibodies were effective in reducing the plasma creatine kinase levels of crude B. alternatus and B. leucurus venoms, evidencing immunological cross-reactivity between BmooMTx and other bothropic venoms. Intraplantar (i.pl.) injection of BmooMtx (1 to 15 μg/animal) caused a dose- and time-dependent hyperalgesia and edematogenic responses. Dexamethasone (0.4 mg/kg), meloxicam (2 mg/kg) and promethazine (5 mg/kg) markedly reduced the hyperalgesia. Our data suggest that these drugs may likely serve as complementary therapies in cases of accidents with Bothrops moojeni, provided that such pharmacological treatments are administered immediately after the incident.
Key words:Bothrops moojeni, hyperalgesia, myotoxin, edema.
INTRODUCTION
Snakebite envenomations constitute a significant public health problem in Latin America (1-5). In Brazil, the genus Bothrops is responsible for the majority of envenomation cases (6). In a central region of Brazil (Triângulo Mineiro region, Minas Gerais state and in a restricted adjacent area in Goias state), snakes of the Bothrops, Crotalus and Micrurus genera were responsible for 74, 24 and 2% of the accidents, respectively (7).
Bothrops moojeni is a snake commonly known in Brazil as "caiçaca", "jararacão" or "jararaca" and is found in warm and dry regions from central Brazil to the southern state of Paraná (8). B. moojeni venom contains a variety of enzymes including acidic phospholipase A2, basic phospholipase A2, metalloproteinases, serine proteinases, l-amino acid oxidase and myotoxin phospholipase A2 which can contribute to the biological actions (edema, hemorrhage, necrosis, and anticoagulant and platelet-aggregating activities) of this venom (9-21).
Myotoxins are particularly abundant in snake venom and can promote myoglobinuria as well as acute renal failure (22, 23). They are classified into three groups: small myotoxins, cardiotoxins, and phospholipase A2 (PLA2) myotoxins (24). The phospholipase A2 group can be subdivided into neurotoxic and non-neurotoxic PLA2 (25). The latter group can be classified into aspartic acid 49 PLA2 myotoxins (Asp49), which have the ability to catalyze the hydrolysis of phospholipids, and lysine 49 PLA2 myotoxins (Lys49), which lack this activity (26). In this report, we describe the isolation and pharmacological characterization of BmooMtx, a Lys49 phospholipase A2 homologue purified from Bothrops moojeni venom.
MATERIALS AND METHODS
Materials
The venom pool from adult B. moojeni snakes from Uberlândia, Minas Gerais state was a contribution of Pentapharm do Brasil Ltda. It was dried in a vacuum desiccator at room temperature immediately after milking and then stored at -20°C. Experimental animals - male Swiss mice (18 to 22 g), Wistar rats (180 to 200 g) and rabbits (2.0 to 2.5 kg) - were obtained from the Federal University of Uberlândia, Uberlândia, Brazil. Animals were maintained under controlled light cycle (12/12 hours) and temperature (22 ± 2°C) with free access to food and water for two days prior to experiments. All experimental procedures followed the ethical parameters proposed by the International Society of Toxinology and by the Brazilian Society of Science in Laboratory Animals.
Acrylamide, ammonium persulfate, bromophenol blue, dexamethasone, indomethacin, meloxicam, β-mercaptoethanol, N,N'-methylene-bis-acrylamide, promethazine, sodium dodecyl sulphate (SDS) and N,N,N',N'-tetramethylethylenediame (TEMED) were purchased from Sigma Chemical Co. (USA). Glycine, Tris, molecular weight markers (LMW: low molecular weight) for electrophoresis and all chromatographic media were from Amersham Pharmacia Biotech (Sweden). All other reagents used were of analytical grade.
Isolation of BmooMtx
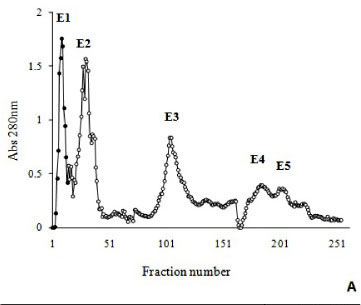
The crude venom from Bothrops moojeni (200 mg) was clarified by centrifugation at 10,000 x g for ten minutes. The supernatant solution was separated by chromatography on a DEAE-Sephacel column (1.7 x 15 cm), initially equilibrated with 50 mM ammonium bicarbonate, pH 7.8 and eluted with a concentration gradient (50 mM to 0.3 M) of the same buffer. Fractions of 3.0 mL/tube were collected, their absorbances at λ = 280 nm were read, and fractions corresponding to peak E1 were pooled, lyophilized, dissolved in 50 Mm ammonium bicarbonate, pH 7.8. The pooled fractions were applied to a 1 x 100 cm Sephadex G-75 column, equilibrated with the same buffer. The flow rate was 20 mL/hour and fractions of 3.0 mL were collected.
Estimation of Protein Concentration
Protein concentration was determined by the method of Itzhaki and Gill (27), using bovine serum albumin as standard.
Electrophoretic Analysis
Electrophoresis using polyacrylamide gels (SDS-PAGE) was performed as described by Laemmli (28) using 14% gels. It was carried out at 20 mA/gel in Tris-glycine buffer, pH 8.3, containing 0.01% SDS. The molecular weight standard proteins used were phosphorylase b (97,000), bovine serum albumin (66,000), ovoalbumin (45,000), carbonic anhydrase (30,000), soybean trypsin inhibitor (20,100) and α-lactalbumin (14,400). The slab gels were stained with Coomassie Blue R-250, 0.2% (w/v) in acetic acid:methanol:water (1:5:5, v/v). The relative molecular mass of the purified enzyme was estimated by Kodak 1D image analysis software (USA).
Production and Purification of Polyclonal Antibodies
Production and purification of polyclonal antibodies was performed as described by Rodrigues et al. (29). BmooMtx (0.6 to 1.2 mg) was emulsified with complete Freund's adjuvant and subcutaneously injected in male rabbits (2 to 2.5 kg). After 15 days, a booster dose with the same amount of protein, but emulsified with incomplete Freund's adjuvant, was injected. Rabbits were bled and polyclonal antibodies were obtained by affinity chromatography in Protein A Sepharose. Fractions containing IgG were then dialyzed against 0.05 M ammonium bicarbonate, pH 8.0, lyophilized and stored at -20°C.
Phospholipase A2 Activity
Phospholipase A2 activity of BmooMtx was performed as described by Dole (30). Briefly, crude egg yolk phospholipids (diluted 1:5 in 0.1 M Tris-HCl, 0.01 M CaCl2, pH 8.5) were used as substrate in the presence of 1% Triton X-100. The BmooMtx (5, 25 and 50 μg) was added to 10 mL of substrate, incubated at 37°C for 15 minutes, and then the free fatty acids were extracted and titrated with 0.018 M NaOH. Crude venom (10 μg) was utilized as a positive control.
Myotoxic Activity
Myotoxic activity was performed as described by Angulo et al. (31), with slight modifications. Briefly, BmooMtx or crude venom (20 μg), dissolved in 50 μL of 0.12 M NaCl, 0.04 M phosphate buffered saline (PBS), pH 7.2, was injected into groups of mice (18 to 22 g, n = 5), in their right gastrocnemius muscle. A control group received 50 μL of PBS. After 1, 2 or 3 hours, the animals were sacrificed by an overdose of ketamine/xylazine and blood samples were collected from cardiac puncture, and the plasma creatine kinase (CK; E.C. 2.7.3.2) activity was determined by a multi-channel analyzer apparatus Chem Well P2910 (Awareness Technology, USA). Activity was expressed as U/mL, with one unit corresponding to the phosphorylation of 1 nmoL of creatine per minute at 25°C.
Histological Examination of Myonecrosis
Myonecrotic activity was assayed on the basis of the morphological alterations induced by intramuscular injections of BmooMtx (20 μg/50mL) into the right gastrocnemius muscle of mice (18 to 22 g, n = 4). After 24 hours, the animals were sacrificed by an overdose of ketamine/xylazine and a small section of the central region of the muscle was excised and soaked in fixing solution (10% formaldehyde in PBS, v/v). The material was then dehydrated by increasing concentrations of ethanol and processed for inclusion in paraffin. The resulting blocks were sliced into 5-mm-thick sections, stained with 0.25% (w/v) hematoxylin-eosin and examined under a light microscope.
Neutralization Assays
Neutralization of myotoxic activities was determined by incubating crude venom or BmooMtx with anti-BmooMtx antibodies at a 1:50 ratio (w/w) for 120 minutes at 37°C. Then, the activities of the mixtures were tested as previously described.
Evaluation of Hyperalgesia
Groups of rats (180 to 200 g, n = 5) were injected with either 0.1 mL of sterile saline solution (0.15 M NaCl) (control group) or 0.1 mL of saline solution containing various concentrations of BmooMtx (1, 5 and 15 mg) into the subplantar surface of one hind paw. The pain threshold was measured at varying time points after BmooMtx or saline injection using an insight pressure apparatus (Ugo Basile, Italy), essentially as described by Randall and Selitto (32). Briefly, a force (in grams) with increasing magnitude was applied to the paw. When the animals reacted by withdrawing the paw, the force needed to induce such response was recorded and represented the pain threshold. To reduce stress, the rats were habituated to the apparatus two days before the experiments.
Evaluation of Edema Formation
BmooMtx was dissolved in sterile saline solution (0.15 M NaCl), and 0.1 mL of the final solution containing various concentrations of BmooMtx (1, 5 and 15 mg) was injected into the subplantar surface of each animal's right hind paw of the rat groups (180 to 200 g, n = 5). An equal volume of saline was injected into the contralateral paw (control). The volume increase (edema) of both paws was measured with the aid of a low-pressure pachymeter (Mitutoyo, Japan), at several intervals following injection (0, 1, 2, 3, 4, 5 and 24 hours). Results were calculated as the difference between the values obtained in the two paws and expressed as the percentage increase in paw volume.
The influence of Various Substances on BmooMtx-Induced Edema and Hyperalgesia
The rat groups (n = 5) were pretreated with different classes of drugs, as follows: (1) H1 receptor antagonist promethazine (5 mg/kg, i.pl., 30 minutes before); (2) the cyclooxygenase inhibitor indomethacin (4 mg/kg, i.pl., 30 minutes before); (3) the cyclooxygenase II inhibitor meloxicam (2 mg/kg, i.pl., 20 minutes before) and (4) the phospholipase A2 inhibitor dexamethasone (0.4 mg/kg, i.pl., 60 minutes before). Following the appropriate time intervals, the animals received an intraplantar injection of 5 mg BmooMtx to enable measurement of edema and hyperalgesia as described previously.
RESULTS
Isolation and Biochemical Characterization of BmooMtx
Myotoxin phospholipase A2 homologue from B. moojeni venom was purified by a two-step procedure involving ion exchange chromatography on DEAE Sephacel and gel filtration on Sephadex G-75. The fractionation of B. moojeni venom by ion exchange chromatography resulted in five main protein peaks (E1 to E5, Figure 1 - A). Myotoxic activity was concentrated in peak 1. The fraction corresponding to peak 1 (E1) was further analyzed using size-exclusion chromatography (Sephadex G-75), and four protein peaks were observed (Figure 1 - B). The third peak, which contains the myotoxin phospholipase A2 homologue designated BmooMtx, induced hyperalgesia and edema in the mouse paw and release of creatine kinase due to necrosis of muscle fibers. However, phospholipase A2 and anticoagulant activities were not detected.
BmooMtx represented ~5% (w/w) of the initial desiccated venom. Electrophoretic analysis (SDS-PAGE) (Figure 1 - C) under denaturing and reducing conditions indicated that the BmooMtx enzyme was highly purified, and had a molecular weight of about 16,500.
Myotoxic Activity
A slight increase in plasma CK activity was observed after intramuscular injection of 20 μg BmooMtx, B. moojeni, B. alternatus and B. leucurus venoms, with CK values reaching respective peaks of 1930.175 ± 273.628; 1943.125 ± 246.938; 503.367 ± 7.128 and 1441.667 ± 367.554 U/L three hours after injection (Figure 2 - A and B).
Neutralization of the Myotoxic Activity by Anti-BmooMTx Antibodies
BmooMtx was able to induce production of polyclonal antibodies in rabbits. When anti-BmooMtx antibodies were incubated with crude B. moojeni, B. alternatus and B. leucurus venoms, at a BmooMtx:antibody ratio of 1:50 (w:w), myotoxic activity (measured by release of creatine kinase) of the venoms was efficiently inhibited. Anti-BmooMtx antibodies neutralized the myotoxic activity of purified enzyme (74%), B. moojeni (68%), B. alternatus (100%) and B. leucurus (92%) three hours after injection (Figure 2 - A and B).
Histological Examination
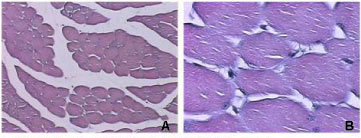
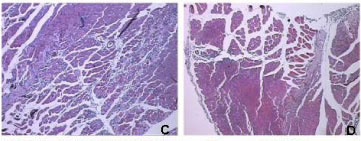
Like B. moojeni crude venom, BmooMtx induced myotoxicity. Histopathological analysis revealed a drastic myonecrosis and leukocyte infiltration induced by BmooMtx. When compared with control cells, gastrocnemius muscle cells of mice injected with BmooMtx showed extensive cellular destruction, displaying contracted and clumped fibers in different stages of degeneration. Inflammatory cells were observed surrounding the altered striated muscle cells. BmooMtx induced an intense infiltration of inflammatory cells (mainly polymorphonuclear) and a degeneration of striated muscle cells characterized by the loss of myofibrils. There was no evidence of vascular lesions or hemorrhage. Gastrocnemius muscles from controls injected with saline had normal morphology with evident peripheral nuclei and without signals of lesion (Figure 3 - A and B)
Anti-BmooMtx antibodies only partially reduced myotoxic activity of BmooMtx. Figures 3 - C, E and G show that degeneration of muscle fibers and infiltration of leukocytes are present on the periphery and in the central region of the gastrocnemius muscle. The central region of the gastrocnemius muscle treated with BmooMtx preincubated for 120 minutes at 37°C with anti-BmooMtx antibodies at the ratio 1:50 presented no lesion signals.
Pharmacological Characterization of BmooMtx-induced Hyperalgesia and Edema
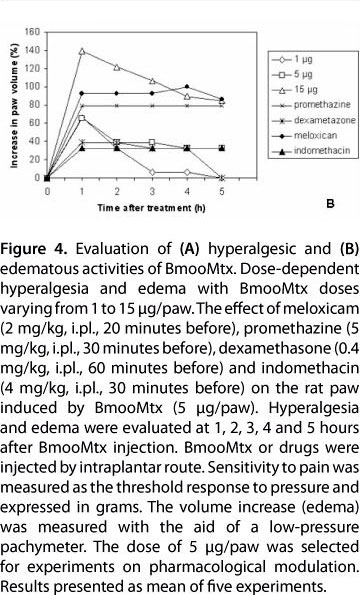
The intraplantar injection of BmooMtx caused a significant increase in sensitivity to pain. A conspicuous and similar hyperalgesic response was observed when a dose of 1, 5 or 15 μg of BmooMtx was administered (Figure 4 - A). The intensity of the hyperalgesia induced by 5 μg was similar to that produced by 15 μg BmooMtx/paw. The hyperalgesic response peaked 1 to 5 hours after enzyme injection. Previous treatment of the animals with the cyclooxygenase II inhibitor meloxicam (2 mg/kg, i.pl., 20 minutes before), the histamine H1 receptor antagonist promethazine (5 mg/kg, i.pl., 30 minutes before) and the phospholipase A2 inhibitor dexamethasone (0.4 mg/kg, i.pl., 60 minutes before) virtually abolished BmooMtx-induced hyperalgesia whereas the treatment of animals with the cyclooxygenase inhibitor indomethacin (4 mg/kg, i.p, 30 minutes before) had little effect on the BmooMtx-induced pain.
BmooMtx also induced footpad edema, evidencing local increase in vascular permeability. The intraplantar injection of BmooMtx (1, 5 and 15 μg/paw) in rats caused a dose-dependent edema. The maximum increase in hind-paw swelling occurred one hour after enzyme injection, gradually decreased over the next five hours (Figure 4 - B), and completely disappeared within 24 hours (data not shown). In the first hour, both indomethacin and dexamethasone partially reduced (36%) the BmooMtx-induced edema. Interestingly, at all times tested, treatment of the animals with meloxicam and promethazine markedly increased the formation of edema.
DISCUSSION
Snake venoms constitute a rich source of myotoxins, which show remarkable functional diversity through pre- or post-synaptic neurotoxicity, cardiotoxicity, myotoxicity, platelet aggregation inhibition, edema, pain, hemolysis, anticoagulation, convulsion and hypotension (9, 33-34).
Five isoforms of PLA2 myotoxin phospholipase A2 homologue have been isolated from the venom of Bothrops moojeni: M-VI (35), MjTX-I (21), MjTX-II (20), BmooTX-I (9) and BmTX-I (10). Myotoxins MjTX-I, MjTX-II and M-VI are Lys49 homologues, while myotoxins BmooTX-I and BmTX-I are Asp49 variants. In the present work, a myotoxin phospholipase A2 homologue from Bothrops moojeni venom, denominated BmooMtx, was purified by a combination of ion exchange and gel filtration chromatography (Figure 1 - A and B). The molecular weight observed at SDS-PAGE (16,500) was similar to those reported for MjTX-I (13,400), M-IV (14,000), BmTX-I (14,240) and BmooTX-I (15,000), from the same venom, a presynaptic phospholipase A2, neuwieditoxin-I (14,000), isolated from the venom of Bothrops neuwiedi pauloensis and an acidic phospholipase A2 (14,000) from the venom of Crotalus durissus cascavella (35, 36).
Soares et al. (20, 21 and 37), Santos-Filho et al. (9) and Calgarotto et al. (10) demonstrate that both Lys49 and Asp49 myotoxic PLA2s of B. moojeni cause significant myotoxicity as well as local edema and hyperalgesia in the rat hind paw after intraplantar injection. Results obtained from BmooMtx indicate that enzymatic activity is not required for induction of pharmacological effects (hyperalgesia and edema) induced by this enzyme since BmooMtx did not show catalytic activity on egg yolk (data not shown). In addition, this enzyme did not present anticoagulant activity. Several works suggest that catalytic activity in the myotoxic PLA2s is required to produce an in vitro anticoagulant effect. Although not sequenced, BmooMtx was not enzymatically active, thus indicating that it likely belongs to the Lys49 PLA2 family.
The CK level was increased by the injection of BmooMtx and the crude venoms from B. moojeni, B. alternatus and B. leucurus. The crude venom of B. moojeni presented the greatest rise in the CK level among the venoms, while B. alternatus crude venom produced the lowest increase. Results of neutralization experiments clearly evidenced that anti-BmooMtx was able to neutralize myotoxic activity of BmooMtx and crude venoms of B. alternatus (100%) and B. leucurus (92%), although the latter only partially reduced myotoxic activity of crude venom of B. moojeni (68%) (Figure 2 - A). These results show that the B. moojeni venom shares some common myotoxins that induce antibodies that cross-react with crude venoms of B. alternatus and B. leucurus.
Interestingly, anti-BmooMtx antibodies were more efficient at neutralizing the myotoxic activity of B. alternatus venom, which can be accounted for the fact that crude venom of B. alternatus presents a lower number of myotoxic components than the other venoms (Figure 2 - B).
Several studies show that proteolytic enzymes of bothropic venoms are also able to increase the plasma CK level (14-15). Results obtained in our laboratories showed that the crude venom of B. moojeni provoked a greater proteolytic activity than other venoms, whereas the crude venom of B. alternatus produced the lowest level of such activity (data not shown). These data help to explain the low neutralization of B. moojeni venom by anti-BmooMtx: the antibodies neutralized only the myotoxins but had no effect on the proteolytic enzymes of this venom.
The histopathological profile of BmooMtx is similar to those of other Lys49 myotoxic phospholipase A2 homologues from Bothrops venom, including BnSP-7 from B. neuwiedi, myotoxin I from B. atrox and BaTx from B. alternatus (38-40). Figures 3 - D, F and H reveal that anti-BmooMtx antibodies reduced myotoxic activity of BmooMtx since the central region of gastrocnemius muscles treated with BmooMtx preincubated for 120 minutes at 37°C with anti-BmooMtx antibodies at the ratio 1:50 presented no signals of injury.
Our results presented herein demonstrate that BmooMtx caused significant local hyperalgesia in the rat hind paw after intraplantar injection (1, 5 and 15 μg/paw). In this study, the paw withdrawal reflex threshold following plantar mechanical stimulus, utilized to measure the noniceptive response to BmooMtx in rats, was lower in the area of local tissue damage created by BmooMtx than in the contralateral paw injected with saline. The intraplantar injection of BmooMtx, therefore, caused a significant increase in sensitivity to pain. A conspicuous and similar hyperalgesic response was observed when doses of 5 and 15 μg of BmooMtx were administered (Figure 4 - A). The intensity of the hyperalgesia induced by 5 μg was similar to that produced by 15 μg of BmooMtx/paw. The peak of the hyperalgesic response occurred up to five hours after enzyme injection. The hyperalgesic intensity induced by 1 μg/paw was time-dependent and lower than that induced by 5 or 15 μg/paw. The injection of saline (control group) did not modify the pain threshold of the animals. The hyperalgesia was of a local nature, since the pain threshold measurements of non-injected, contralateral paws did not differ from preinjection values (data not shown).
BmooMtx also induced footpad edema, evidencing the local increase in vascular permeability. The intraplantar injection of BmooMtx (1, 5 and 15 μg/paw) in rats caused a dose-dependent edema. The hind-paw swelling peaked one hour after enzyme injection, gradually decreased over the next five hours and completely disappeared within 24 hours (data not shown).
Our present work has analyzed the role of several mediators in BmooMtx-induced hyperalgesia by using specific inhibitors or receptor antagonists. A dose of 5 μg/paw was selected for experiments on the pharmacological modulation. Pretreatment with meloxicam, a type 2 cyclooxygenase inhibitor, reduces BmooMtx-induced hyperalgesia. This finding suggests that prostaglandins generated by type 2 cyclooxygenase activity play a significant role in hyperplasia evoked by BmooMtx. On the other hand, indomethacin, a potent non-selective inhibitor of cyclooxygenase activity, which may also inhibit phospholipase A and C, did not block hyperalgesia induced by BmooMtx. The data presented herein also suggest the participation of prostaglandins in BmooMtx-induced hyperalgesia since this effect was markedly reduced by dexamethasone. In addition, our results suggest that histamine may play a role in the hyperalgesic response to BmooMtx since promethazine treatment was effective in reducing the BmooMtx-induced hyperalgesia. Thus, the present results stress the relevance of phospholipase A2, prostaglandins and histamine activity in the genesis of BmooMtx-induced hyperalgesia.
The pharmacological modulation of edema was also investigated in the present study. Our data show that meloxicam, promethazine and dexamethasone inhibit the hyperalgesic responses. However, our results show that promethazine and meloxicam are able to increase edematogenic responses and thus suggest that hyperalgesic and edematogenic responses induced by BmooMtx in the rat hind paw occur, at least partially, by a different pathway.
In conclusion, BmooMtx is an enzyme isolated from B. moojeni venom, belonging to the Lys49 PLA2 subgroup, which induces pain, paw edema, and also provokes myotoxic activity.
Submission status
Received: July 30, 2010.
Accepted: December 21, 2010.
Abstract published online: January 25, 2011.
Full paper published online: February 28, 2011.
Financial source
The State of Minas Gerais Research Foundation (FAPEMIG), the National Council for Scientific and Technological Development (CNPq) and the Ministry of Science and Technology (MCT) of Brazil.
Conflicts of interest
There is no conflict.
Ethics committee approval
The present study was approved by the Ethics Committee on Animal Research of the Federal University of Uberlândia (CEUA/UFU) under the protocol n. 028/09, and followed the protocols of the International Society of Toxinology and the Brazilian Society of Science in Laboratory Animals.
- 1. Fan HW, Cardoso JLC. Clinical toxicology of snake bites in South America. In: Meier J, White J, editors. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton: CRC Press; 1995. p. 667-88.
- 2. Gutiérrez JM. Clinical toxicology of snakebite in Central America. In: Meier J, White J, editors. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton: CRC Press; 1995. p. 645-65.
- 3. Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76(5):515-24.
- 4. Warrell D. Snakebites in Central and South America: epidemiology, clinical features, and clinical management. In: Campbell JA, Lamar WW, editors. The venomous reptiles of the Western Hemisphere. Ithaca: Comstock Publishing Associates; 2004. p. 709-61. 2 vol.
- 5. Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5(11):218.
- 6. Queiroz GP, Pessoa LA, Portaro FC, Furtado M de F, Tambourgi DV. Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon. 2008;52(8):842-51.
- 7. da Silva CJ, Jorge MT, Ribeiro LA. Epidemiology of snakebite in a central region of Brazil. Toxicon. 2003;41(1):251-5.
- 8. Hoge AR. Preliminary account on neotropical crotalinae (Serpentes:Viperidae). Mem Inst Butantan. 1965;32(1):109-84.
- 9. Santos-Filho NA, Silveira LB, Oliveira CZ, Bernardes CP, Menaldo DL, Fuly AL, et al. A new acidic myotoxic, anti-platelet and prostaglandin I2 inductor phospholipase A2 isolated from Bothrops moojeni snake venom. Toxicon. 2008;52(8):908-17.
- 10. Calgarotto AK, Damico DC, Ponce-Soto LA, Baldasso PA, Da Silva SL, Souza GH, et al. Biological and biochemical characterization of new basic phospholipase A2 BmTX-I isolated from Bothrops moojeni snake venom. Toxicon. 2008;51(8):1509-19.
- 11. Assakura MT, Reichl AP, Asperti MCA, Mandelbaum FR. Isolation of the major proteolytic enzyme from the venom of the snake Bothrops moojeni (Caissaca). Toxicon. 1985;23(4):691-706.
- 12. Serrano SMT, Matos NFC, Mandelbaum FR, Sampaio CAM. Basic proteinases from Bothrops moojeni (caissaca) venom - I. Isolation and activity of two serine proteinases MSPI and MSPII on synthetic substrates and on platelet aggregation. Toxicon. 1993;31(4):471-81.
- 13. Oliveira F, Rodrigues VM, Borges MH, Soares AM, Hamaguchi A, Giglio JR, et al. Purification and partial characterization of a new proteolytic enzyme from the venom of Bothrops moojeni (caissaca). Biochem Mol Biol Int. 1999;47(6):1069-77.
- 14. Bernardes CP, Santos-Filho NA, Costa TR, Gomes MS, Torres FS, Costa J, et al. Isolation and structural characterization of a new fibrinogenolytic metalloproteinase from Bothrops moojeni snake venom. Toxicon. 2008;51(4):574-84.
- 15. Gomes MS, Mendes MM, de Oliveira F, de Andrade RM, Bernardes CP, Hamaguchi A, et al. BthMP: a new weakly hemorrhagic metalloproteinase from Bothrops moojeni snake venom. Toxicon. 2009;53(1):24-32.
- 16. Stocker K, Barlow GH. The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol. 1976;45(1):214-23.
- 17. Serrano SM, Sampaio CA, Mandelbaum FR. Basic proteinases from Bothrops moojeni (caissaca) venom - II. Isolation of the metalloproteinase MPB. Comparison of the proteolytic activity on natural substrates by MPB, MSP 1 and MSP 2. Toxicon. 1993;31(4):483-92.
- 18. Stábeli RG, Sant'Ana CD, Ribeiro PH, Costa TR, Ticli FK, Pires MG, et al. Cytotoxic L-amino acid oxidase from Bothrops moojeni: biochemical and functional characterization. Int J Biol Macromol. 2007;41(2):132-40.
- 19. Lomonte B, Gutiérrez JM, Furtado MF, Otero R, Rosso JP, Vargas O, et al. Isolation of basic myotoxins from Bothrops moojeni and Bothrops atrox snake venoms. Toxicon. 1990;28(10):1137-46.
- 20. Soares AM, Rodrigues VM, Homsi-Brandeburgo MI, Toyama MH, Lombardi FR, Arni RK, et al. A rapid procedure for the isolation of the Lys-49 myotoxin II from Bothrops moojeni (caissaca) venom: biochemical characterization, crystallization, myotoxic and edematogenic activity. Toxicon. 1998;36(3):503-14.
- 21. Soares AM, Andrião-Escarso SH, Angulo Y, Lomonte B, Gutiérrez JM, Marangoni S, et al. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A2 homologue from Bothrops moojeni (caissaca) snake venom. Arch Biochem Biophys. 2000;373(1):7-15.
- 22. Lomonte B, Angulo Y, Calderón L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42(8):885-901.
- 23. Azevedo-Marques MM, Cupo P, Coimbra TM, Hering SE, Rossi MA, Laure CJ. Myonecrosis, myoglobinuria and acute renal failure induced by South American rattlesnake (Crotalus durissus terrificus) envenomation in Brazil. Toxicon. 1985;23(4):631-6.
- 24. Harris JB, Cullen MJ. Muscle necrosis caused by snake venoms and toxins. Electron Microsc Rev. 1990;3(2):183-211.
- 25. Mebs D, Ownby CL. Myotoxic components of snake venoms: their biochemical and biological activities. Pharmacol Ther. 1990;48(2):223-36.
- 26. Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488(1-2):1-19.
- 27. Itzhaki RF, Gill DM. A micro-biuret method for estimating proteins. Anal Biochem. 1964;9(1):401-10.
- 28. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680-5.
- 29. Rodrigues VM, Soares AM, Andrião-Escarso SH, Franceschi AM, Rucavado A, Gutiérrez JM, et al. Pathological alterations induced by neuwiedase, a metalloproteinase isolated from Bothrops neuwiedi snake venom. Biochimie. 2001;83(6):471-9.
- 30. Dole VP. Relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956;35(2):150-4.
- 31. Angulo Y, Olamendi-Portugal T, Possani LD, Lomonte B. Isolation and characterization of myotoxin II from Atropoides (Bothrops) nummifer snake venom, a new Lys49 phospholipase A2 homologue. Int J Biochem Cell Biol. 2000;32(1):63-71.
- 32. Randall LO, Selitto JJ. A method for measurement of analgesic activity of inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111(4):409-19.
- 33. Andrião-Escarso SH, Soares AM, Fontes MR, Fuly AL, Corrêa FM, Rosa JC. Structural and functional characterization of an acidic platelet aggregation inhibitor and hypotensive phospholipase A2 from Bothrops jararacussu snake venom. Biochem Pharmacol. 2002;64(4):723-32.
- 34. Rodrigues RS, Izidoro LF, Teixeira SS, Silveira LB, Hamaguchi A, Homsi-Brandeburgo MI, et al. Isolation and functional characterization of a new myotoxic acidic phospholipase A2 from Bothrops pauloensis snake venom. Toxicon. 2007;50(1):153-65.
- 35. Borja-Oliveira CR, Kassab BH, Soares AM, Toyama MH, Giglio JR, Marangoni S, et al. Purification and n-terminal sequencing of two presynaptic neurotoxic PLA2, neuwieditoxin-I and neuwieditoxin-II, from Bothrops neuwiedi pauloensis (jararaca pintada) venom. J Venom Anim Toxins incl Trop Dis. 2007;13(1):103-21.
- 36. Guarnieri MC, Melo ESL, Melo KMS, Albuquerque-Modesto JC, Prieto-da-Silva ARB, Rádis-Baptista G. Cloning of a novel acidic phospholipase A2 from the venom gland of Crotalus durissus cascavella (Brazilian northeastern rattlesnake). J Venom Anim Toxins incl Trop Dis. 2009;15(4):745-61.
- 37. Soares AM, Rodrigues VM, Homsi-Brandeburgo MI, Toyama MH, Lombardi FR, Arni RK, et al. Phospholipase A2 homolog 2 (myotoxin II) (mjtx-II) (M-VI), q9i834. 2007. (Swiss-Prot - international sequence bank database).
- 38. Oliveira Cde F, Lopes Dda S, Mendes MM, Homsi-Brandeburgo MI, Hamaguchi A, de Alcântara TM, et al. Insights of local tissue damage and regeneration induced by BnSP-7, a myotoxin isolated from Bothrops (neuwiedi) pauloensis snake venom. Toxicon. 2009;53(5): 560-9.
- 39. Núñez V, Arce V, Gutiérrez JM, Lomonte B. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops atrox. Toxicon. 2004;44(1):91-101.
- 40. Ponce-Soto LA, Lomonte B, Gutiérrez JM, Rodrigues-Simioni L, Novello JC, Marangoni S. Structural and functional properties of BaTX, a new Lys49 phospholipase A2 homologue isolated from the venom of the snake Bothrops alternatus Biochim Biophys Acta. 2007;1770(4):585-93.
Correspondence to:
Publication Dates
-
Publication in this collection
01 Mar 2011 -
Date of issue
2011
History
-
Received
30 July 2010 -
Accepted
21 Dec 2010