Abstract
In recent years, the search for natural plant products to fight viral diseases has been increasing. In this work, two Spondias species, namely S. mombin and S. tuberosa, found in Ceará state (Brazil), and their main phenolic components were evaluated against dengue virus. In vitro antiviral tests were performed against type-2 dengue virus by the MTT method and standard cytopathic effect reduction assay in C6/36 cells. Cytotoxicity was also evaluated by MTT. The presence of phenolic compounds quercetin, rutin, and ellagic acid in plant extracts was characterized by HPLC analysis. Both Spondias species extracts and components were nontoxic to the cells whereas rutin and quercetin displayed relevant antiviral activity with IC50 of 362.68 µg/mL and 500 µg/mL, respectively.
natural products; phenols; dengue; antiviral agents
ORIGINAL PAPERS
Antiviral activities of extracts and phenolic components of two Spondias species against dengue virus
Silva AraI; Morais SMI; Marques MMMI; Lima DMII; Santos SCCIII; Almeida RRIV; Vieira IGPIV; Guedes MIFI,III
IPostgraduate Program in Biotechnology, Ceará State University, Fortaleza, Ceará State, Brazil
IICenter for Health Sciences, University of Fortaleza, Fortaleza, Ceará State, Brazil
IIILaboratory of Biochemistry, Ceará State University, Fortaleza, Ceará State, Brazil
IVTechnological Development Park (PADETEC), Fortaleza, Ceará State, Brazil
Correspondence to Correspondence to: Selene Maia de Morais Laboratório de Química de Produtos Naturais Universidade Estadual do Ceará Fortaleza, CE, 60740-000, Brasil Phone: +55 85 3101 9789. Fax: +55 85 3101 9933 Email: selene.maia@pq.cnpq.br.
ABSTRACT
In recent years, the search for natural plant products to fight viral diseases has been increasing. In this work, two Spondias species, namely S. mombin and S. tuberosa, found in Ceará state (Brazil), and their main phenolic components were evaluated against dengue virus. In vitro antiviral tests were performed against type-2 dengue virus by the MTT method and standard cytopathic effect reduction assay in C6/36 cells. Cytotoxicity was also evaluated by MTT. The presence of phenolic compounds quercetin, rutin, and ellagic acid in plant extracts was characterized by HPLC analysis. Both Spondias species extracts and components were nontoxic to the cells whereas rutin and quercetin displayed relevant antiviral activity with IC50 of 362.68 µg/mL and 500 µg/mL, respectively.
Key words: natural products, phenols, dengue, antiviral agents.
INTRODUCTION
Dengue infection is caused by four serotypes of the dengue virus (DENV 1, 2, 3 and 4), a member of the Flaviviridae family; it is generally transmitted in a cycle involving humans and mosquito vectors. The global prevalence of dengue virus has grown dramatically in recent decades. Until now, there is no specific drug to combat infection and currently the most effective prevention measures lie in controlling the mosquito (1).
Efforts have been made to evaluate the antiviral activity of a wide range of natural products, including plants, in an attempt to isolate and characterize new compounds which can inhibit virus replication or treat viral infection (2). For this reason, plant extracts and components are becoming more important as potential sources for antiviral agents. Plant extract screening has led to the detection of some effective in vitro viral replication inhibitors (3, 4).
Hydroalcoholic extract of S. mombin inhibits replication of the herpes simplex and coxsakie B viruses, responsible for painful mouth ulcers. The active compounds against these viruses were identified as geraniin and galoilgeraniin at concentrations of 50 mg/L and two caffeoyl esters at concentrations of 100 mg/L (5, 6). No antiviral activity was reported for S. tuberosa.
In order to find new antiviral agents to combat dengue, Spondias mombin and S. tuberosa extracts and their main components were evaluated in vitro against DENV-2 in C6/36 cells.
MATERIALS AND METHODS
Cell Line
C6/36 (cloned cell line derived from larvae of Aedes albopictus) was provided by Dr. Fernanda Montenegro (Central Public Health Laboratory - LACEN, Fortaleza, Brazil) and grown at 28°C as monolayers in Leibovitz medium (L-15®, Cultilab, Brazil), supplemented with 10% tryptose phosphate broth, 1% penicillin/streptomycin (Gigco-BRL, USA) (50 U/mL), 1% amphotericin B (250 µg/mL) (Gibco-BRL, USA), and 10% fetal bovine serum (FBS, Sigma-Aldrich, USA).
Virus
Type-2 dengue virus (DENV-2), New Guinea strain, was provided by Dr. Benedito Antonio Lopes da Fonseca (Molecular Virology Laboratory, Ribeirão Preto Faculty of Medicine, São Paulo State, Brazil). The virus was replicated in C6/36 cells to generate working stocks for seven days at 28°C. Supernatant culture was collected and centrifuged at 2,000 rpm for 15 minutes. Supernatant was again collected and stored at -80°C as virus stock until use. Virus titer, expressed as plaque-formation units per milliliter (PFU/mL), was determined by standard plaque assay on Vero cells grown in 24-well plates.
Plant Material and Phenolic Components
Plant material was collected from their natural habitats in the state of Ceará (Northeast of Brazil). Voucher specimens (S. mombin-34.826 and S. tuberosa-34.887) were deposited in the Herbarium at the Department of Biology, Federal University of Ceará (UFC), Brazil. Quercetin, rutin, and ellagic acid were purchased from Sigma-Aldrich Co. (USA).
Preparation of Extracts
Leaves of S. mombin (3.59 kg) and S. tuberosa (3.23 kg) were soaked in methanol:water (80:20) at room temperature (25-28°C) for seven days. The solution was filtered through filter paper and evaporated in a rotary evaporator. The extracts were dried over a water bath with the temperature held below 60°C.
Phytochemical Analysis of Plant Extracts
The methanol:water (80:20 v/v) extract was submitted to silica gel column chromatography with elute mixtures of increasing polarity using hexane, chloroform, and ethyl acetate. Several 10-mL fractions were collected and after solvent evaporation, the resultant material was compared by silica gel thin layer chromatography (TLC) plates. Similar samples were joined, purified by crystallization and analyzed by infrared (IR) and nuclear magnetic resonance (NMR). The main compounds were also compared with standard compounds by TLC. IR spectra were recorded on a PerkinElmer FTIR Spectrum 100® spectrophotometer (Australia) and values expressed in cm_1. NMR spectra were recorded on a Brucker Avance DRX-500® spectrometer (Germany), in MeOD.
High-Pressure Liquid Chromatography (HPLC) Analysis
HPLC analyses of extracts from S. mombin and S. tuberosa were performed using a reversed-phase column (Hibar LiChrospher®, Germany, 100RP18 4.6 mm x 25 cm _ particle 5 mm) eluted at 1.25 mL/minute with a solvent mixture I:II [I: aqueous solution of H3PO4 (pH 2.8); II: acetonitrile] starting with 20% II/80% I until 12 minutes, then increasing II to reach 40% at 17 minutes, then with 40% II/60% I until 23 minutes, and again increasing II concentration to reach 80% at 25 minutes, with the detection wavelength set at 350 nm and 20 mL injection. A rutin standard was used to obtain calibration curves. This compound was dissolved in methanol at different concentrations (1.14 mg/mL, 0.57 mg/mL, 0.228 mg/mL, 0.114 mg/mL, 0.0456 mg/mL). Ellagic acid (standard) is also used to obtain calibration curves and it was dissolved in methanol at different concentrations (0.46 mg/mL, 0.92 mg/mL, 1.84 mg/mL).
Follow-up extractions and HPLC analysis were accomplished using the same procedure as for rutinw. Recovery was determined as follows: recovery (%) = (A _ B)/C x 100%, where "A" is the amount of detections, "B" is the amount of sample without added standard, and "C" is the amount of added standard. The relative standard deviations (RSD) of recoveries for the ellagic acid were 2.1 (n = 5; mean = 98). Identification of rutin, ellagic acid, and quercetin (Figure 1) in Spondias extracts was performed by HPLC-PDA, observing retention time (Rt) and UV-VIS spectra. The standards were prepared in methanol at 0.1 mg/mL.
Phytochemical Analysis of Plant Extracts
Chemical tests were performed following the protocols described by Matos (7), based on reactions with specific reagents for the main classes of natural products with precipitate formation or color change.
Viral Detection by RT-PCR
C6/36 cells (2 × 105 cells/well) were infected with DENV-2 (3.5 x 105 PFU) and infectivity was confirmed by RT-PCR. The purpose of this assay was to standardize days of C6/36 cell infection by DENV-2. Briefly, infected cells were removed each day of infection (1st to 8th days of infection) washed with phosphate buffered saline (PBS) and RNA was purified with Trizol® (Invitrogen, USA). The one-step kit (Qiagen, Germany) was used for cDNA synthesis and PCR following manufacturer's instructions. Primer oligonucleotide sequences were as follows: forward 5'-ACCATCGTGATAACA-3' reverse 3'-AGGCTGTGTCACCTA-5'. Amplification conditions were 50°C for 30 minutes and 95°C for 15 minutes, followed by 40 cycles of 94°C for one minute, 55°C for one minute, and 72°C for one minute, and a final extension at 70°C for ten minutes. The amplified product was then analyzed on 2% agarose gel.
Cytotoxicity Assay
Cell viability was evaluated by the MTT method (Sigma-Aldrich, USA) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (8, 9). Briefly, C6/36 cultures were prepared in 96-well plates (TPP, Switzerland), at 2 × 105 cells/well. After 24-hour incubation at 28°C, the culture medium was removed and cells exposed to different concentrations of the extracts, four wells per concentration prepared in 200-µL culture medium per well. Untreated controls had 200 µL of medium culture added. Cells were then incubated for seven days. The medium was removed and 50 µL of MTT solution (5 mg/mL) was added. The plates were reincubated for four hours and the MTT solution removed, 100 µL of DMSO was added to dissolve formazan crystals and the plates were gently shaken, whereby crystals were completely dissolved. The absorbance was read on ELISA equipment (Amersham Biosciences, USA) at 495 nm. The 50% cytotoxic concentration (CC50) was defined as the sample concentration that reduced cell viability 50% compared to untreated controls.
Antiviral Assay
Antiviral activity was also evaluated by the MTT method. In brief, to determine inhibitory potential of the sample for DENV-2 replication, 100 µL of virus suspension (3.5 x 105 PFU) was added to a cell shaker for one hour at 28°C. Thereafter, the viral suspension was removed and various concentrations of samples were added and incubated for seven days. This was followed by the same procedure as described in the previous paragraph. The 50% inhibitory concentration (IC50) was defined as the concentration that inhibited 50% of viral replication compared to virus controls. The selectivity index (SI), an important parameter for evaluating antiviral activity, was calculated from the CC50/IC50 ratio. Antiviral activity was determined a according to the following formula (10):
Where (ODt)DENV is the measured absorption of the various concentrations of Spondias species extracts and their phenolic components in DENV-infected cells, (ODc)DENV is the absorption of the untreated control DENV-infected cells, and (ODc)cells is the absorption of untreated control C6/36 cells. The compounds showing antiviral effect were submitted to standard cytopathic reduction assay.
Statistical Analysis
CC50 and IC50 values were obtained from linear regression analysis of concentration-effect curves and represent mean ± standard deviation values of three independent experiments. One way analysis of variance (ANOVA) was used to determine statistical differences followed by Tukey's multiple comparison. Statistical significance was set at p < 0.05.
RESULTS AND DISCUSSION
Chemical Composition of S. mombin and S. tuberosa
Leaves from the two Spondias species were soaked in methanol:water (80:20) for seven days at room temperature. The solutions obtained were then evaporated leaving the crude extracts. Extract yields of S. tuberosa and S. mombin were respectively 1.5 and 5.0%. Qualitative phytochemical analysis of S. mombin and S. tuberosa revealed the presence of phenols, hydrolysable tannins, flavones, flavonoids, leucoanthocyanidins, and saponins. Results showed both species presenting the same classes of compounds; this agrees with Corthout et al. (5) who reported the presence of ellagitannins in S. mombin.
Three main compounds were detected in Spondias species; structural characterization was performed by 1H and 13C-NMR spectroscopic analysis and comparison with ellagic acid, quercetin, and rutin data (11, 12). The relative percentage yields of these phenolic compounds in dried leaves for Spondias species were evaluated by HPLC-PDA, considering retention time (Rt) and UV-VIS spectral analysis. Identification of components was established by overlapping their peaks (retention time) and absorption spectra with those of rutin, ellagic acid, and quercetin standards. Chromatograms of S. mombin and S. tuberosa are shown in Figure 1. Rt for rutin, ellagic acid, and quercetin were respectively 5.11, 6.24, and 23.46 minutes.
The regression equation for rutin is Y = 25150017.88X + 203139.00 (R2 = 1.000); quantification limit 0.1 µg/mL; detection limit 0.04 µg/mL; relative standard deviations (RSD) less than 2.0%. The regression equation for ellagic acid is: Y = 25916407.4534X + 309482.00 (R2 = 0.9998); quantification limit 0.1 µg/mL; detection limit 0.04 µg/mL; RSD less than 2.0%. Each flavonoid peak was quantified using the rutin and ellagic acid linear regression equations. In these Spondias species extracts, rutin, ellagic acid, and quercetin were detected in different yields. Quercetin (2.36 ± 0.01 mg/g) and ellagic acid (41.56 ± 0.01 mg/g) were found in S. mombin and rutin (53.38 ± 1.71 mg/g), quercetin (24.46 ± 0.87 mg/g), and ellagic acid (169.76 ± 0.17 mg/g) in S. tuberosa (Table 1).
Cytotoxic Effect and Antiviral Activity
The cytotoxic effects of S. mombin and S. tuberosa extracts and related phenolic compounds were expressed by their CC50 values obtained by MTT method. No cytotoxicity was observed with any of these compounds at concentrations up to 1000 µg/mL, allowing us to estimate the CC50 for all compounds as greater than 1000 µg/mL. Extract cytotoxic levels were evaluated before carrying out the antiviral tests and they did not show toxicity towards C6/36 cells.
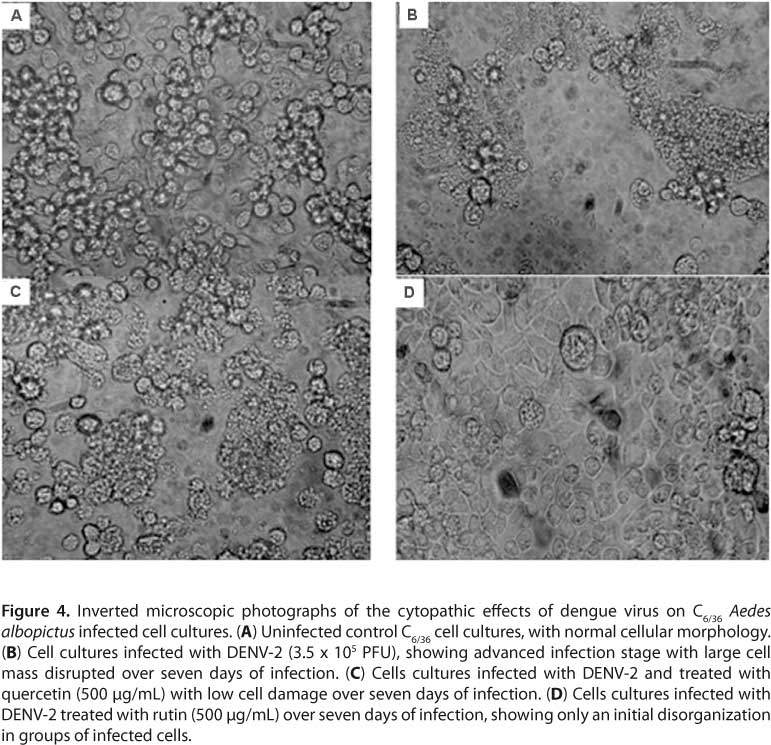
As per the RT-PCR results, virus replication could be detected on the fourth day. However, to optimize experiments, antiviral assays in this study were performed on the fifth day of infection (Figure 2). S. mombin and S. tuberosa extracts and components rutin, quercetin, and ellagic acid on DENV-2 in C6/36 cells inhibited virus replication respectively by 3.31, 17.98, 68.42, 50 and 25.02 (Figure 3) in a concentration of 500 µg/mL. Comparing IC50 values, rutin was more active against DENV-2 with an IC50 of 362.68 ± 0.04 µg/mL and SI of 2.75 than quercetin with an IC50 of 500.00 ± 0.01 µg/mL and SI of 2. Quercetin and rutin were also evaluated by standard cytopathic reduction assay inhibiting cell disruption (Figure 4).
There are few works studying natural products against the dengue virus; for example, Parida et al. (13) evaluated the antiviral action of neem (Azadirachta indica) extract and isolate azadirachtin against DENV-2 infected C6/36 cells. The extract showed an inhibitory effect against dengue virus at high concentrations (1.897 mg/mL), when evaluated by cytopathic effect inhibition. Azadirachtin did not show antiviral action. Talarico et al. (14) investigated the action of two sulfated polysaccharides from red algae against four dengue virus serotypes using different cell lines. The authors found differential susceptibility of vertebrate and invertebrate cells to the inhibitory action of compounds against DENV. With respect to host cell type, the polysaccharides were active inhibitors of DENV-2 and DENV-3 multiplication in Vero cells and in HepG2 and foreskin PH cells, but were inactive in mosquito C6/36 cells. There were no significant differences in DENV multiplication levels in these vertebrate and invertebrate cell lines, confirming that differential susceptibility of virus serotype or host cell is not due to virus growth ability. Sulfated galactomannans at 347 µg/mL reduced viral titer in immunofluorescence assays using DENV-1 in C6/36 cells (15).
Antiviral activity is one of the cited biological activities displayed by flavonoids. Quercetin inhibited HIV-1 integrase which mediates the insertion of viral DNA into host cellular DNA and is essential for viral replication and virion production (16). Quercetin and its glycoside (quercetin 7-rhamnoside) were tested against porcine epidemic diarrhea virus. Quercetin 7-rhamnoside was more active than quercetin with an IC50 of 0.014 mg/mL (17). Tao et al. (18) showed in vitro the anti-HIV and anti-HSV activities of sodium rutin sulfate, obtained synthetically by modifying natural flavonol glycoside rutin. Kang et al. (19) demonstrated the role of ellagic acid as a therapeutic agent against the hepatitis B virus.
In summary, the results of this study revealed the presence of quercetin, rutin, and ellagic acid in two Spondias species. This is the first study to report quercetin and rutin in these species. The evaluation of antiviral activity against DENV-2 in C6/36 cells suggests that rutin and quercetin have potential for the development of an anti-DENV agent. Further studies are required using other cell lines and in vivo assay to corroborate the effectiveness of these flavonoids against dengue virus.
ACKNOWLEDGEMENTS
The authors thank Brazilian government agencies Coordination for the Improvement of Higher Education Personnel (CAPES) and Ceará State Research Foundation (FUNCAP) for their financial support.
REFERENCES
1. Jain M, Ganju L, Katiyal A, Padwad Y, Mishra KP, Chanda S, et al. Effect of Hippophae rhamnoides leaf extract against dengue virus infection in human blood-derived macrophages. Phytomedicine 2008;15(10):793-9.
2. Simões CM, Amoros M, Girre L. Mechanism of antiviral activity of triterpenoid saponins. Phytother Res. 1999;13(4):323-8.
3. Mukhtar M, Arshad M, Ahmadb M, Pomerantz RJ, Wigdahl B, Parveen Z. Antiviral potentials of medicinal plants. Virus Res. 2008;131(2):111-20.
4. Yu C, Yan Y, Wu X, Zhang B, Wanga W, Wu Q. Anti-influenza virus effects of the aqueous extract from Mosla scabra. J Ethnopharmacol. 2010;127(2):280-5.
5. Corthout J, Pieters L, Claeys M, Geerts S, Vanden Berghe D, Vlietinck A. Antiviral ellagitannins from Spondias mombin. Phytochemistry. 1991;30(4):1129-30.
6. Corthout J, Pieters L, Claeys M, Geerts S, Vanden Berghe D, Vlietinck A. Antiviral caffeoyl esters from Spondias mombin. Phytochem. 1992;31(6):1979-81.
7. Matos FJA. Introdução à fotoquímica experimental. Fortaleza: Edições UFC; 1997.
8. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65(1-2):55-3.
9. Sieuwerts AM, Klijin JG, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: how to use this assay reliably to measure metabolic activity of cell cultures in vitro for assessment of growth characteristics, IC50-values and cell survival. Eur J Clin Chem Clin Biochem. 1995;33(11):813-23.
10. Cheng HY, Lin TC, Yang CM, Wang KC, Lin CC. Mechanism of action of the suppression of herpes simplex virus type 2 replication by pterocarnin A. Microbes Infect. 2004;6(8):738-44.
11. Rocha e Silva H, Silva CC, Caland Neto LJ, Citó AM, Chaves MH. Constituintes químicos das cascas do caule de Cenostigma macrophyllum: ocorrência de colesterol. Quím Nova. 2007;30(8):1877-81.
12. Lallemand JYM, Duteil M. 13C N.M.R. spectra of quercetin and rutin. Org Magn Reson. 1977;9:179-80.
13. Parida MM, Upadhyay C, Pandya G, Jana AM. Inhibitory potential of neem (Azadirachta indica Juss) leaves on dengue virus type-2 replication. J Ethnopharmacol. 2002;79(2):273-8.
14. Talarico LB, Pujol CA, Zibetti RG, Faría PC, Noseda MD, Duarte ME, et al. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res. 2005;66(2-3):103-10.
15. Ono L, Wollinger W, Rocco IM, Coimbra TL, Gorin PA, Sierakowski MR. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain). Antiviral Res. 2003;60(3):201-8.
16. Kim HJ, Woo ER, Shin CG, Park H. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J Nat Prod. 1998;61(1):145-8.
17. Choi HJ, Kim JH, Lee CH, Ahn YJ, Canção JH, Baek SH, et al. Antiviral activity of quercetin-7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81(1):77-81.
18. Tao J, Hu Q, Yang J, Li R, Li X, Lu C, et al. In vitro anti-HIV and -HSV activity and safety of sodium rutin sulfate as a microbicide candidate. Antiviral Res. 2007;75(3):227-33.
19. Kang EH, Kown TW, Oh GT, Park WF, Park S, Park SK, et al. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antiviral Res. 2006;72(2):100-6.
Submission status
Received: February 18, 2011.
Accepted: August 10, 2011.
Abstract: published online: August 16, 2011.
Full paper published online: November 30, 2011.
Conflicts of interest
There are no conflicts of interest.
Financial source
CAPES and FUNCAP provided the financial grants.
- 1. Jain M, Ganju L, Katiyal A, Padwad Y, Mishra KP, Chanda S, et al. Effect of Hippophae rhamnoides leaf extract against dengue virus infection in human blood-derived macrophages. Phytomedicine 2008;15(10):793-9.
- 2. Simões CM, Amoros M, Girre L. Mechanism of antiviral activity of triterpenoid saponins. Phytother Res. 1999;13(4):323-8.
- 3. Mukhtar M, Arshad M, Ahmadb M, Pomerantz RJ, Wigdahl B, Parveen Z. Antiviral potentials of medicinal plants. Virus Res. 2008;131(2):111-20.
- 4. Yu C, Yan Y, Wu X, Zhang B, Wanga W, Wu Q. Anti-influenza virus effects of the aqueous extract from Mosla scabra J Ethnopharmacol. 2010;127(2):280-5.
- 5. Corthout J, Pieters L, Claeys M, Geerts S, Vanden Berghe D, Vlietinck A. Antiviral ellagitannins from Spondias mombin Phytochemistry. 1991;30(4):1129-30.
- 6. Corthout J, Pieters L, Claeys M, Geerts S, Vanden Berghe D, Vlietinck A. Antiviral caffeoyl esters from Spondias mombin Phytochem. 1992;31(6):1979-81.
- 7. Matos FJA. Introdução à fotoquímica experimental. Fortaleza: Edições UFC; 1997.
- 8. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65(1-2):55-3.
- 9. Sieuwerts AM, Klijin JG, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: how to use this assay reliably to measure metabolic activity of cell cultures in vitro for assessment of growth characteristics, IC50-values and cell survival. Eur J Clin Chem Clin Biochem. 1995;33(11):813-23.
- 10. Cheng HY, Lin TC, Yang CM, Wang KC, Lin CC. Mechanism of action of the suppression of herpes simplex virus type 2 replication by pterocarnin A. Microbes Infect. 2004;6(8):738-44.
- 11. Rocha e Silva H, Silva CC, Caland Neto LJ, Citó AM, Chaves MH. Constituintes químicos das cascas do caule de Cenostigma macrophyllum: ocorrência de colesterol. Quím Nova. 2007;30(8):1877-81.
- 12. Lallemand JYM, Duteil M. 13C N.M.R. spectra of quercetin and rutin. Org Magn Reson. 1977;9:179-80.
- 13. Parida MM, Upadhyay C, Pandya G, Jana AM. Inhibitory potential of neem (Azadirachta indica Juss) leaves on dengue virus type-2 replication. J Ethnopharmacol. 2002;79(2):273-8.
- 14. Talarico LB, Pujol CA, Zibetti RG, Faría PC, Noseda MD, Duarte ME, et al. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res. 2005;66(2-3):103-10.
- 15. Ono L, Wollinger W, Rocco IM, Coimbra TL, Gorin PA, Sierakowski MR. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain). Antiviral Res. 2003;60(3):201-8.
- 16. Kim HJ, Woo ER, Shin CG, Park H. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J Nat Prod. 1998;61(1):145-8.
- 17. Choi HJ, Kim JH, Lee CH, Ahn YJ, Canção JH, Baek SH, et al. Antiviral activity of quercetin-7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81(1):77-81.
- 18. Tao J, Hu Q, Yang J, Li R, Li X, Lu C, et al. In vitro anti-HIV and -HSV activity and safety of sodium rutin sulfate as a microbicide candidate. Antiviral Res. 2007;75(3):227-33.
- 19. Kang EH, Kown TW, Oh GT, Park WF, Park S, Park SK, et al. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antiviral Res. 2006;72(2):100-6.
Publication Dates
-
Publication in this collection
05 Dec 2011 -
Date of issue
2011
History
-
Received
18 Feb 2011 -
Accepted
10 Aug 2011