Abstract
Snake venoms are rich sources of active proteins that have been employed in the diagnosis and treatment of health disorders and antivenom therapy. Developing countries demand fast economical downstream processes for the purification of this biomolecule type without requiring sophisticated equipment. We developed an alternative, simple and easy to scale-up method, able to purify simultaneously protease and phospholipase A2 toxins from Bothrops alternatus venom. It comprises a multiple-step partition procedure with polyethylene-glycol/phosphate aqueous two-phase systems followed by a gel filtration chromatographic step. Two single bands in SDS-polyacrylamide gel electrophoresis and increased proteolytic and phospholipase A2 specific activities evidence the homogeneity of the isolated proteins.
partition; snake toxins; isolation; phospholipase A2; proteases
ORIGINAL PAPER
An alternative method to isolate protease and phospholipase A2 toxins from snake venoms based on partitioning of aqueous two-phase systems
Gómez GNI; Nerli BBII; Acosta OCIII; Picó GAII; Leiva LCAI
IProtein Research Laboratory (LabInPro), Biochemistry Department, School of Natural and Exact Sciences, Northeast National University (UNNE), Corrientes, Argentina
IILaboratory of Physical Chemistry Applied to Bioseparation Processes, Department of Physical Chemistry, School of Biochemical and Pharmaceutical Sciences, National University of Rosario, Rosario, Argentina
IIISchool of Veterinary Science, Northeast National University (UNNE), Corrientes, Argentina
Correspondence to Correspondence to: Laura C. A. Leiva Departamento de Bioquímica Facultad de Ciencias Exactas y Naturales y Agrimensura (FaCENA) Universidad Nacional del Nordeste (UNNE) Av. Libertad 5470, (3400) Corrientes, Argentina Phone: +54 0379 4457996 ext. 112 Email: lcleiva@exa.unne.edu.ar
ABSTRACT
Snake venoms are rich sources of active proteins that have been employed in the diagnosis and treatment of health disorders and antivenom therapy. Developing countries demand fast economical downstream processes for the purification of this biomolecule type without requiring sophisticated equipment. We developed an alternative, simple and easy to scale-up method, able to purify simultaneously protease and phospholipase A2 toxins from Bothrops alternatus venom. It comprises a multiple-step partition procedure with polyethylene-glycol/phosphate aqueous two-phase systems followed by a gel filtration chromatographic step. Two single bands in SDS-polyacrylamide gel electrophoresis and increased proteolytic and phospholipase A2 specific activities evidence the homogeneity of the isolated proteins.
Key words: partition, snake toxins, isolation, phospholipase A2, proteases.
INTRODUCTION
Snake venoms are rich sources of bioactive polypeptides and proteins. Some of these components that exhibit enzymatic activities include proteinases, phospholipases A2, nucleotidases, phosphodiesterases and L-amino acid oxidases. Other snake venom proteins and polypeptides do not exhibit any enzymatic activity and are described as "non-enzymatic proteins" (1-3).
Studies on snake venoms have yielded extensive important information on biological systems and insights into medical problems (4). Currently, interest has grown due to the pharmacological potential of venom components as antibiotics, antitumor agents, hemostasis disorder treatments, as well as analytical reagents (5-11).
As one would expect, numerous methods have been developed in order to both isolate and characterize the different toxins. But a single method, even one with a high resolving power, usually does not yield a pure protein. Conventional isolation of venom toxins involves a complex sequence of chromatographic steps. Thus, gel filtration, ion-exchange and reversed-phase high-pressure liquid chromatography (RP-HPLC) are widely used to purify individual venom proteins (1, 12-14). This methodology is expensive, requires technologically advanced equipment columns, pumps and matrix and demands long periods of time.
In recent years, partitioning in aqueous two-phase systems (ATPSs) has been shown to provide a powerful method for the starting steps of the macromolecule downstream process (15, 16). This is due to several advantages with regard to the traditional techniques such as its ease scaling-up, low-material cost, rapid phase disengagement and low interfacial tension, the latter of which minimizes the biomolecular degradation (17). Besides, no sophisticated equipment is needed. ATPSs are comprised of aqueous solutions either of two water-soluble polymers, usually polyethylene glycol (PEG) and dextran, or of a polymer and a salt, usually PEG and phosphate or sulfate (18). At present, no model that allows the a priori calculation of protein partitioning in ATPS has been developed, therefore, the application of this technique requires the utilization of previous experimental work. ATPSs have been successfully used to purify different biomolecules and organelles, for example, plasmid DNA, alpha-1-antitrypsin from human plasma, polysaccharide-coated liposomes and other products (19-22). However, there is no report documenting an isolation of toxins from venoms by partitioning in aqueous biphasic systems.
Bothrops alternatus, a pit viper widespread in South America countries, is responsible for a predominance of deadly snakebite cases. The goal of the present work was to develop an easily accessible methodology, based on partitioning in PEG/phosphate ATPSs, able to purify simultaneously protease and phospholipase A2 toxins. This will contribute both to obtaining proteins for biotechnological and/or pharmacological studies and to facilitating the production of more specific antivenoms suitable for antivenom therapy.
MATERIALS AND METHODS
Chemicals
Pooled venom was obtained from several adult specimens of Bothrops alternatus captured in northeastern Argentina, and then maintained at the serpentarium of the local zoo in Corrientes, Argentina. Crude venom was lyophilized; after that, it was kept frozen at 20ºC. When required, the venom was diluted with phosphate buffered saline solution (PBS), pH 7.2. The small amount of insoluble material was centrifuged and the clear supernatant was applied for studies. Sephadex G-75 superfine, polyethyleneglycol of molecular weight 3,350 (PEG3350) (used without further purification) and Coomasie brillant blue (G) were purchased from Sigma Chem Co. (USA). The other reagents were of analytical grade.
Total Protein Concentration Determination
The protein concentration in both phases of the systems was determined according to the Bradford (23) method with a sample of pooled freeze-dried venom as the standard. Blank systems without protein were used as reference and no interference from phase components was observed. Absorbance measurements were carried out on a Boeco S-22 UV/visible Spectrophotometer (Germany).
Enzyme Assays
Proteolytic activity was evaluated using casein from bovine milk as substrate, according to Friedrich and Tu (24) with the Lomonte and Gutiérrez (25) modification. One milliliter of enzyme solution, ranging from 0.014 to 1.800 mg/mL, was incubated with 2.0 mL of a 1% casein solution for 30 minutes at 37ºC. Then, 4.0 mL of 5 % trichloroacetic acid was added and the sample was left at room temperature for 30 minutes. The tubes were centrifuged and the absorbance of the clear supernatant was determined by spectrophotometric measurement at 280 nm. Control solution was applied, in which the enzyme solution was omitted. The caseinolytic activity, expressed as units/mg, is defined as the percentual change in absorbance yielded by 1 mg of enzyme incubated with casein at 37ºC for 30 minutes.
An alternative colorimetric method, described by Wang and Huang (26) with minor modifications, was also used to test proteolytic activity in phases and in fractions eluted from the chromatography column due to its lower sample volume requirement. The reaction mixture, consisting of 255 µL of azocasein (5 mg/mL) in phosphate buffer saline (pH 7.2) and 45 µL of the phase for testing, was incubated at 37ºC for 90 minutes. The reaction was stopped by the addition of 600 µL of 5% trichloroacetic acid, and the mixture was maintained for 30 minutes at room temperature. After centrifugation at 3000 rpm for 5 minutes, 400 µL of the supernatant was mixed with an equal volume of 0.5 M NaOH and absorbance was determined at 450 nm. Proteolytic activity was expressed as the change in absorbance at 450 nm/90 minutes of incubation (Δ450/90 min).
Phospholipase A2 activity was evidenced by the formation of hemolytic halos in agarose-erythrocyte egg yolk gels caused by enzymatic hydrolysis of lecithins to lysolecithins, with the latter being able to lyse red blood cell membranes. This indirect hemolytic activity was assayed as described by Gutiérrez et al. (27). Three hundred microliters of packed sheep erythrocytes was washed four times with saline solution; 300 µL of 1:3 egg yolk solution in saline solution and 250 µL of 0.01 M CaCl2 solution were added to 25 mL of 1% (w/v) of agar (at 50ºC) and dissolved in PBS pH 7.2. The mixture was applied to plastic plates (135 x 80 mm) and allowed to gel. Then, 3 mm-diameter wells were filled with 15 µL of the phase for testing. After 20 hours of incubation at 37ΊC, the diameters of the hemolytic halos were measured. In order to determine the minimum indirect hemolytic dose (MiHD) of isolated enzyme, 15 µL of solutions containing different amounts of isolated phospholipase A2 (from 0.031 to 2.00 mg/mL) was applied into the wells. After 18 hours of incubation at 37ºC, the hemolytic halo diameters were measured and the dose-response curves were plotted. The minimum indirect hemolytic dose (MiHD) is defined as the amount of enzyme that induces a hemolytic halo of 15 mm in diameter. PBS was used as a control.
Preparation of the Aqueous Two-Phase System
Stock solution of potassium phosphate (Pi), pH 7.0 (30% w/w), solid PEG 3350 and water were mixed in order to prepare phase systems. The final composition of the system was 12.3 13.7 74% w/w of Pi, PEG and water, respectively. After a gentle mixing, low-speed centrifugation was used in order to speed up phase separation of the system components; and then 5 mL of each phase was mixed to reconstitute the two-phase system in which the partitioning of venom proteins was assayed.
The system composition used in this work was chosen according to the binodial diagrams from the literature (16). The biphasic system composition was chosen with a low PEG concentration sufficient to produce an adequate phase separation at the assayed temperature.
Multiple-Step Partition Procedure
The partitioning of venom proteins was analyzed by dissolving 500 µL of venom solution (50 mg/mL) in the two-phase preformed system containing 5 mL of each equilibrated phase, with the change of the total volume of each phase being negligible. After mixing by gentle inversion for 10 minutes and leaving it to settle for at least 60 minutes, the system was centrifuged at low speed for the two-phase separation. Visual estimates of the top/bottom volumes were carried out in graduated centrifuge tubes, with no volume change being observed during the partitioning. A second extraction step was carried out as follows. The main portion (5 mL) of bottom phase from the first was transferred into a tube containing 5 mL of a pure top phase (from a previously prepared phase system obtained under identical conditions but without venom solution), mixed gently and left to reach the phase equilibrium again. The third and fourth extraction steps were carried out under similar conditions but using 4.5 and 4 mL of bottom phase and equal volume of pure top phase. The temperature was maintained constant at 25ºC and controlled to within ± 0.1ºC by immersing the tubes in a thermostatic bath. Total protein concentration, proteolytic and phospholipase A2 activity in each phase were determined in order to evaluate the ATP system performance in venom protein purification. All the measurements were carried out in triplicate. Protein purity in starting, intermediate and final samples was analyzed by SDS-polyacrylamide gel electrophoresis.
Gel Filtration Chromatography
Three milliliters of bottom phase (Pi-enriched) was filtered using Amicon Ultra-10 membrane (Millipore, Bedford, MA). Two hundred and fifty microliters of this concentrated bottom phase was loaded onto Sephadex G-75 column (1 cm x 95 cm) equilibrated with PBS buffer, pH 7.2. Proteins were eluted with the same buffer. The flow rate was 7.5 mL/h while fractions of 0.75 mL were collected for absorbance at 280 nm and enzymatic measurements (proteolytic and phospholipase A2 activities).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
In order both to analyze the protein partition in the ATPS and to evaluate the purity of enzymes assayed, electrophoresis was performed on 12% polyacrylamide slab gels following the method of Laemmli (28). Protein concentration of the samples was close to 1 mg/mL. Gels were either stained with Coomassie brilliant blue R-250 or with silver nitrate (29). Standards molecular mass markers were run in parallel to determine the molecular mass of the enzymes isolated.
RESULTS AND DISCUSSION
Aqueous Two-Phase Partitioning
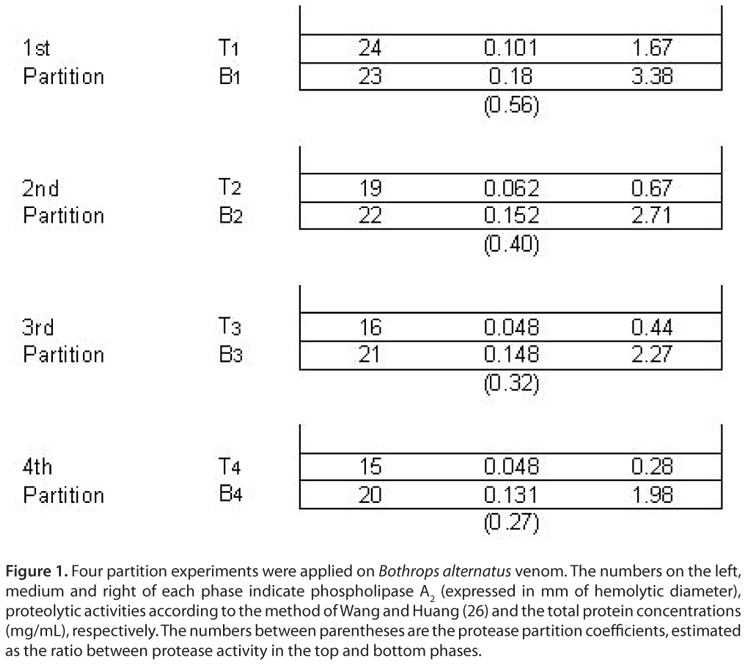
Figure 1 shows the partitioning pattern produced by proteins from B. alternatus venom with protease activity corresponding to the overall extraction process. It is clear that proteolytic activity was retained mainly in the phosphate-enriched phase in all the extractions steps, thus indicating an uneven protein distribution between phases. The partition coefficient was observed to change at each step. At starting extraction steps it decreased markedly (from 0.56 to 0.40) and then, it decreased slightly (from 0.32 to 0.27).
On the other hand, venom proteins with phospholipase A2 activity exhibited an even distribution among phases (similar hemolytic halo diameters) at the first extraction step; however, in the subsequent stages its partitioning equilibrium was displaced to the bottom phase. No significant changes were observed between the hemolytic halo diameters corresponding to the 3rd and 4th extractions, thus suggesting only slight differences between the partition coefficients corresponding to these steps. Partition coefficients were not calculated in this case since the diameter of the hemolytic halo increased with phospholipase A2 activity in a non-proportional manner.
Fluctuations observed in partitioning coefficients may be attributed to the heterogeneity of venom protein fractions. Based on literature findings, we are able to assume that B. alternatus venom exhibits a variety of phospholipase A2 and protease enzymes in its composition (30-38). At initial extraction steps all protein fractions are present in the system and each enzyme isoform is distributed between the phases, according to its structural properties. As the extraction process progresses (last stages) an aqueous two-phase selectivity is evidenced. The remaining bottom phase presents the depletion of both proteins that were partitioned in the top phase and the enrichment of those proteins that preferred the bottom phase in the former steps. Under these experimental conditions partition coefficients behave as true thermodynamic constants and converge to singular values that only depend on temperature. Besides, this convergence suggests that more homogeneous enzymes are being partitioned in the later steps, a fact that is relevant for both biochemical studies and improvement of anti-venom specificity.
The SDS-PAGE (Figure 2), carried out under non-reducing conditions, shows B. alternatus venom proteins and their distribution between phases as measured by the PEG3350/Pi system, after each partitioning step. The whole venom shows three peak bands, a wide band that comprises proteins of molecular mass 50-60 kDa compatible with proteinases (59 kDa) and two closely placed bands of intermediate molecular mass (25 and 28 kDa respectivey) assignable to phospholipases A2 and serine proteases (30, 31).
After four extraction steps the bottom phase (B4) remained was expected to contain mainly proteinases (according to data shown in Figure 1), and was analyzed by SDS-PAGE. Only two bands corresponding to high (~59 kDa) and low (25 kDa) molecular mass proteins were observed in a bottom phase (Figure 2, line 2). Those with molecular masses close to 28 kDa are partitioned into top phase (Figure 2 and Figure 3.) being compatible with serine proteases. Under reducing conditions (Figure 3, line 2) the 25 kDa band is absent and replaced by a single band at 14 kDa. Similar features were reported for an acidic phospholipase A2 previously isolated in our laboratory by traditional chromatographic methods, thus suggesting that this enzyme is present in venom as a homo-dimer (30).
Phospholipase A2 and Protease Separation
According to the previous results, the bottom phase obtained after four partitioning steps contains only two main protein fractions with molecular weights that differ significantly from each other. Therefore, either gel filtration chromatography or another size-based separation technique appears to provide an easy alternative for separating the proteins for further studies or uses. As expected, fractionation by Sephadex G-75 presented two protein peaks, the first showing proteolytic activity and the second (II), phospholipase A2 activity (Figure 4). The homogeneity of these isolated proteins was demonstrated by SDS-PAGE (Figures 5 and 6) showing a single band under reducing conditions for each protein.
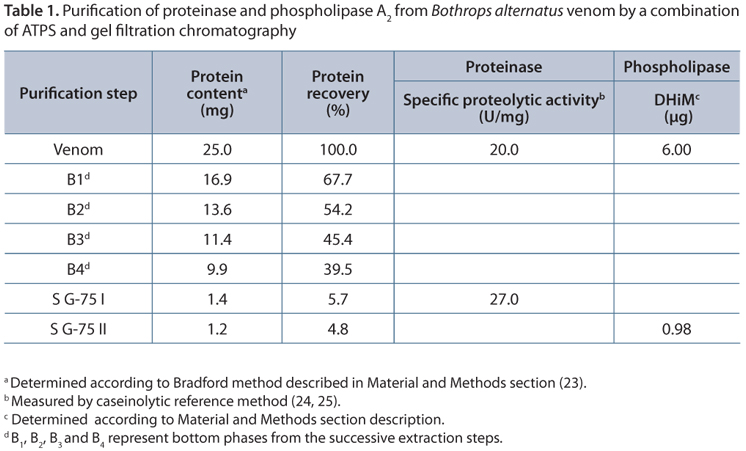
The overall performance of the proposed method is shown in Table 1. Recoveries values close to 5% for both phospholipase A2 and proteinase proteins are comparable to those obtained by traditional purification methods. Besides, values of both specific proteolytic activity (20.0 U/mg) and MiHD (6.00 µg), determined on starting venom, agree with those previously obtained in our laboratory (30, 39). A slight increase in the proteolytic specific activity is observed in the final product with regard to the whole venom. A rough analysis of these results leads inevitably to wrong conclusions. According to the Venomics analysis of Bothrops alternatus reported by Ohler et al. (32), phospholipases represent the 7.8% of the total venom components while proteinases represent the 43.1%. Consequently, ATPS extraction combined with gel filtration shows, a priori, a poor performance since only small fractions of starting phospholipase A2 (5.7%) and proteinase proteins (4.8%) are recovered in the final product. However, taking into account the heterogeneity of venom toxins, our results might be due to the elimination of enzyme isoforms with different activity during the purification process. The drastic decrease observed in the minimum indirect hemolytic dose (MiHD) to estimate phospholipase A2 activity must be interpreted in a similar manner. A further biochemical and physiological characterization of these proteins is required to clarify this point
In summary, a simple method, based on PEG/Pi aqueous two-phase systems capable of obtaining both protease and phospholipase A2 toxins from B. alternatus venom, was proposed. The technique of four sequential extractions enables the elimination of several venom components and yields a bottom phase that contains mainly phospholipase A2 and protease toxins. This mixture exhibits values of specific proteolytic activity and MiHD sufficient for use as a sensitizing agent for antiserum manufacturing. This will contribute to the obtainment of more specific anti-sera and therefore the avoidance of adverse effects (40). The presence of a low PEG concentration (close to 3 % w/w) in the bottom phase does not represent a disadvantage since this polymer possesses a low immunogenic capability (41).
Additionally, the phospholipase A2 and protease present in the bottom extract can be separated from each other by incorporating a gel filtration chromatography procedure. In this case, our overall results show purification degrees and recoveries comparable to those reported in the literature. However, in contrast to conventional methods, our process does not require either initial centrifugation or ionic exchange/reverse phase chromatographic steps (42-44). This both shortens the long processing times which may affect protein stability and reduces operating costs, rendering this technique potentially suitable for different purposes.
Finally, the toxic composition of snake venom varies among different snake species or even within the same snake secreted in different regions or seasons. Therefore, the production of more specific antisera for each region is desirable since it will contribute to reducing the dose of antibodies infused intravenously during the treatment of patients with snakebites and therefore, to avoiding anaphylactic reactions (45). Research efforts have focused on developing techniques to obtain a more specific antivenom. Frequently, they are expensive or too complicated and cannot be adopted by developing countries, which present the highest index of snakebite accidents. At this point, the method proposed in the present work offers a remarkable advantage, since it is extremely simple and feasible for being accomplished in a laboratory whose instrument standards preclude high costs.
ACKNOWLEDGMENTS
The authors would like to thank Laura Rey for supplying Bothrops alternatus venom (Serpentarium of the local zoo, Corrientes, Argentina). This work was financially supported by General Secretariat of Sciences and Technology, Northeast National University, Argentina (Projects n. PI F009/08 and PI F 018/10). G. N. Gómez is the recipient of a fellowship from General Secretariat of Sciences and Technology, Northeast National University, Argentina.
Received: April 4, 2012.
Accepted: June 28, 2012.
Abstract published online: June 29, 2012.
Full paper published online: August 31, 2012.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
FINANCIAL SOURCE: General Secretariat of Sciences and Technology UNNE (PI F009/08 and PI F 018/10) provided the financial grants.
- 1. Harvey AL. Snake toxins. New York: Pergamon Press. 1991.
- 2. Tu AT. Tissue damaging effects of snake venoms. In: Dekker M, editor. Handbook of natural toxins: reptile venoms and toxins. New York; 1991. p. 827.
- 3. Barbosa PS, Martins AM, Toyama MH, Joazeiro PP, Beriam LO, Fonteles MC. et al. Purification and biological effects of a C-type lectin isolated from Bothrops moojeni J Venom Anim Toxins incl Trop Dis. 2010;16(3):493-504.
- 4. Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2 Annu Rev Pharmacol. Toxicol. 1999;39:175-89.
- 5. Santamaría C, Lario S, Angulo Y, Pizarro-Cerda J, Gorvel JP, Moreno E, et al. Antimicrobial activity of myotoxic phospholipases A2 from crotalid snake venoms and synthetic peptide variants derived from their C-terminal region. Toxicon. 2005;45(7):807-15.
- 6. Rodriguez VM, Marcussi S, Cambraia RS, de Araújo AL, Malta-Neto NR, Hamaguchi A, et al. Bactericidal and neurotoxic activities of two myotoxic phospholipases A2 from Bothrops neuwiedi pauloensis snake venom. Toxicon. 2004;44(3):305-14.
- 7. Roberto PC, Kashima S, Marcussi S, Pereira JO, Astolfi-Filho S, Nomizo A, et al. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A2 from Bothrops jararacussu venom. Protein Journal. 2004;23(4):273-85.
- 8. Yang RS, Tang CH, Chuang WJ, Huang TH, Peng HC, Fu WM. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45(5):661-9.
- 9. Rádis-Baptista G. Integrins, cancer and snake toxins (mini-review). J Venom Anim Toxins incl Trop Dis. 2005;11(3):217-41.
- 10. von Segesser LK, Mueller X, Marty B, Horisberger J, Corno A. Alternatives to unfractionated heparin for anticoagulation in cardiopulmonary bypass. Perfusion. 2001;16(5):411-6.
- 11. Klein JD, Walker FJ. Purification of a protein C activator from the venom of the southern copperhead (Agkistrodon contortrix contortrix). Biochemistry. 1986;25(15):4175-9.
- 12. Bonfim VL, Ponce-Soto LA, Martins de Souza D, Souza GH, Baldasso PA, Eberlin MN, et al. Structural and functional characterization of myotoxin, Cr-IV 1, a phospholipase A2 D49 from the venom of the snake Calloselasma rhodostoma Biologicals. 2008;36(3):168-76.
- 13. Ghorbanpur M, Zare Mirakabadi A, Zokaee F, Zolfagarrian H, Rabiei H. Purification and partial characterization of a coagulant serine protease from the venom of the Iranian snake Agkistrodon halys J Venom Anim Toxins incl Trop Dis. 2009;15(3):411-23.
- 14. Galbiatti C, Rocha T, Randazzo-Moura P, Ponce-Soto LA, Marangoni S, Cruz-Höfling MA, et al. Pharmacological and partial biochemical characterization of a new non-myotoxic neurotoxic PLA2 (Bmaj-9) isolated from Bothrops marajoensis snake venom. J Venom Anim Toxins incl Trop Dis. 2012;18(1):62-72.
- 15. Zaslavsky BY. Aqueous two-phase partitioning. Physical Chemistry and Bioanalytical Applications. New York: Marcel Dekker. 1994. p. 509-87.
- 16. Albertsson PA. Partition of Cell Particles and Macromolecules. 2nd ed. New York: John Wiley & Sons. 1971.
- 17. Asenjo JA, Andrews, B. Aqueous two-phase systems for protein separation: phase separation and applications. J Chromatogr A. 2012;1238:1-10.
- 18. Grünfelder T, Pessoa Filho PA, Maurer G. Liquid-liquid equilibrium of aqueous two-phase systems containing some synthetic polyelectrolytes and polyethylene glycol. J Chem Eng. 2009;54(2)198-207.
- 19. Johansson HO, Matos T, Luz JS, Feitosa E, Oliveira CC, Pessoa AJr, et al. Plasmid DNA partitioning and separation using poly(ethylene glycol)/poly(acrylate)/salt aqueous two-phase systems. J Chromatogr A. 2012;1233:30-5.
- 20. Reh G, Nerli B, Picó G. Isolation of alpha-1-antitrypsin from human plasma by partitioning in aqueous biphasic systems of polyethyleneglycol-phosphate. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780(2):389-96.
- 21. Kang EC, Akiyoshi K, Sunamoto J. Partition of polysaccharide-coated liposomes in aqueous two-phase systems. Int J Biol Macromol. 1994;16(6):348-53.
- 22. Rito Palomares M. The practical application of aqueous two-phase processes for the recovery of biological products. J Chromatogr B Analyt Technol Biomed Life Sci. 1976;73:248-54.
- 23. Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding, Analys. Biochem. 1976;7(72):248-54.
- 24. Friedrich C, Tu AT. Role of metals in snake venoms for hemorrhagic, esterase and proteolytic activities. Bioch Pharmacol. 1971;20(7):1549-56.
- 25. Lomonte B, Gutiérrez JM. La actividad proteolítica de los venenos de serpientes de Costa Rica sobre la caseína. Rev Biol Trop. 1983;31(1):37-40.
- 26. Wang WJ, Huang TF. Purification and characterization of a novel metalloproteinase, acurhagin, from Agkistrodon acutus venom. Thromb Haemost. 2002;87(4):641-50.
- 27. Gutiérrez JM, Avila C, Rojas E, Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26(4):411-3.
- 28. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227(5259):680-5.
- 29. Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins. RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8(2):93-9.
- 30. Garcia Denegri ME, Acosta OC, Huancahuire-Vega S, Martins-de-Souza D, Marangoni S, Maruñak SL, et al. Isolation and functional characterization of a new acidic PLA(2) Ba SpII RP4 of the Bothrops alternatus snake venom from Argentina. Toxicon. 2010;56(1):64-74.
- 31. Gay CC, Leiva LC, Maruñak S, Teibler P, Acosta de Pérez O. Proteolytic, edematogenic and miotoxic activities of a hemorrhagic metalloprotease isolated from Bothrops alternatus venom. Toxicon. 2005;46(5):546-54.
- 32. Ohler M, Georgieva D, Seifert J, von Bergen M, Arni RK, Genov N, et al. The venomics of Bothrops alternatus is a pool of acidic proteins with predominant hemorrhagic and coagulopathic activities. J Proteome Res. 2010;9(5):2422-37.
- 33. Nisenbom HE, Seki C, Vidal JC. Phospholipase A2 from Bothrops alternatus (víbora de la cruz) venom. Purification and some characteristic properties. Toxicon. 1986;24(3):259-72.
- 34. Souza DH, Iemma MR, Ferreira LL, Faria JP, Oliva ML, Zingali RB, et al. The disintegrin-like domain of the snake venom metalloprotease alternagin inhibits alpha2beta1integrin-mediated cell adhesion. Arch Biochem Biophys. 2000;384(2):341-50.
- 35. Cominetti MR, Ribeiro JU, Fox JW, Selistre-de-Araujo HS. BaG, a new dimeric metalloproteinase/disintegrin from the Bothrops alternatus snake venom that interacts with alpha5beta1 integrin. Arch Biochem Biophys. 2003;416(2):171-9.
- 36. Costa JO, Petri CB, Hamaguchi A, Homsi-Brandeburgo MI, Oliveira CZ, Soares AM, et al. Purification and functional characterization of two fibrinogenolytic enzymes from Bothrops alternatus venom. J Venom Anim Toxins incl Trop Dis. 2007;13(3):640-54.
- 37. Smolka MB, Marangoni S, Oliveira B, Novello JC. Purification and partial characterization of a thrombin-like enzyme, balterobin, from the venom of Bothrops alternatus Toxicon. 1998;36(7):1059-63.
- 38. Costa J de O, Fonseca KC, Mamede CC, Beletti ME, Santos-Filho NA, Soares AM, et al. Bhalternin: functional and structural characterization of a new thrombin-like enzyme from Bothrops alternatus snake venom. Toxicon. 2010;55(7):1365-77.
- 39. Ruiz de Torrent RM, Leiva LC, Acosta de Pérez OC. Efecto proteolítico de venenos de serpientes del género Bothrops (yarará) de la Argentina y su neutralización por un antiveneno bivalente. Acta Toxicol Argent. 2001;9(1):9-12.
- 40. Rodríguez JP, Gay CC, Fusco LS, Gauna MC, Acosta OC, Leiva LC. Cross-neutralization of the coagulant activity of Crotalus durissus terrificus venom from the northeast of Argentina by bivalent bothropic antivenom. J Venom Anim Toxins incl Trop Dis. 2012;18(1):116-23.
- 41. Picó G, Bassani G, Farruggia B, Nerli B. Calorimetric investigation of the protein-flexible chain polymer interactions and its relationship with a protein partition in aqueous two-phase systems. Int J Biol Macromol. 2007;40(3):268-75.
- 42. Fernández J, Gutiérrez JM, Angulo Y, Sanz L, Juárez P, Calvete JJ, et al. Isolation of an acidic phospholipase A2 from the venom of the snake Bothrops asper of Costa Rica: Biochemical and toxicological characterization. Biochimie. 2010;92(3):273-83.
- 43. Fuly AL, Calil-Elias S, Martinez AM, Melo PA, Guimarães JA. Myotoxicity induced by an acidic Asp-49 phospholipase A(2) isolated from Lachesis muta snake venom: Comparison with lysophosphatidylcholine. Int J Biochem Cell B. 2003;35(10):1470-81.
- 44. Ponce-Soto LA, Lomonte B, Gutiérrez JM, Rodrigues-Simioni L, Novello JC, Marangoni S. Structural and functional properties of BaTX, a new Lys49 phospholipase A2 homologue isolated from the venom of the snake Bothrops alternatus. Biochim Biophys Acta. 2007;1770(4):585-93.
- 45. Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standartization and control of antivenoms. Toxicon. 2003;41(5):541-57.
Publication Dates
-
Publication in this collection
14 Sept 2012 -
Date of issue
2012
History
-
Received
04 Apr 2012 -
Accepted
28 June 2012