Abstract
Background
The present work aimed to evaluate the antimycobacterial activity and cytotoxicity of Microcystis aeruginosa toxins, the MC-LR variant and purified extract of [D-Leu1] microcystin-LR.
Methods
The antimicrobial activity of M. aeruginosa extract and microcystin was evaluated by resazurin microtiter assay against Mycobacterium tuberculosis, M. terrae, M. chelonae and M. kansasii. The cytotoxicity assay was performed by trypan blue exclusion against the HTC cell line.
Results
Antimicrobial activity was observed in the hexanic extract of M. aeruginosa (RST 9501 strain) against M. tuberculosis, including sensitive and resistant strains with minimal inhibitory concentrations (MIC) between 1.93 μM and 0.06 μM. The high activity of M. aeruginosa hexanic extract could be attributed to the major presence of the toxins MC-LR and [D-Leu1] MC-LR that showed activity at MIC between 53 and 0.42 μM against tested mycobacterial strains. Even at the highest concentration tested, no toxicity of M. aeruginosa extracts was identified against HTC cells.
Conclusions
These preliminary results suggest that [D-Leu1] MC-LR is a promising candidate for the development of a new antimycobacterial agent.
Mycobacteria; Antimycobacterial agents; Cytotoxic activity; Microcystins
Background
“Nontuberculous mycobacteria” is a general expression applied for different species
of the genusMycobacterium that do not belong to the
Mycobacterium tuberculosis complex [11 Falkinham JO: The changing pattern of nontuberculous
mycobacterial disease. Can J Infect Dis 2003,
14(5):281-286.]. They are also recognized as causative agents of
opportunistic infections in humans that affect mainly patients with preexisting
pulmonary diseases – such as chronic obstructive pulmonary disease or tuberculosis
(TB) – or those with impaired systemic immunity [22 van Ingen J, Boeree MJ, van Soolingen D, Mouton JW: Resistance
mechanisms and drug susceptibility testing of nontuberculous mycobacteria.
Drug Resist Updat 2012,
15(3):149-161.]-[44 Dias-Baptista IMF, Uso SMRS, Marcondes-Machado J: Trends in
multidrug-resistant tuberculosis. J Venom Anim Toxins incl
Trop Dis 2008, 14(2):203-223. [
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1678–91992008000200003&lng=en&nrm=iso&tlng=en
]
http://www.scielo.br/scielo.php?script=s...
]. The latter group includes
patients with HIV infection, leukemia and under immunosuppressive therapy [55 Al-Mahruqi SH, van Ingen J, Al-Busaidy S, Boeree MJ, Al-Zadjali S,
Patel A, et al.: Clinical relevance of nontuberculous mycobacteria, Oman.
Emerg Infect Dis 2009,
15(2):292-294.],[66 Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C,
Gordin F, et al.: An official ATS/IDSA statement: diagnosis, treatment,
and prevention of nontuberculous mycobacterial diseases. Am J
Respir Crit Care Med 2007,
175(4):367-416.].
For most nontuberculous mycobacterial infections, treatment is based on drugs that may differ according to the causal agent, in particular between slow- (e.g. M. avium, M. kansasii) and fast-growing species (e.g. M. abscessus, M. fortuitum) [11 Falkinham JO: The changing pattern of nontuberculous mycobacterial disease. Can J Infect Dis 2003, 14(5):281-286.]. In general, drug therapy is long, costly, and often associated with toxic side effects. In addition, high rates of natural antibiotic resistance are common among nontuberculous mycobacteria, which increases the challenges for new drug discovery [11 Falkinham JO: The changing pattern of nontuberculous mycobacterial disease. Can J Infect Dis 2003, 14(5):281-286.],[77 Brown-Elliott BA, Nash KA, Wallace RJ Jr: Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 2012, 25(3):545-582.].
TB remains a major global health problem reaching millions of people every year and ranking as the second leading cause of death among infectious diseases worldwide [88 World Health Organization: Global Tuberculosis Report 2012. World Health Organization, Geneva, Switzerland; 2012. Mitchison DA: Prevention of drug resistance by combined drug treatment of tuberculosis. Handb Exp Pharmacol 2012, 211:87-98.]. The current treatment available against TB establishes a multidrug regimen that lasts a minimum of six months and does not guarantee a complete eradication of the infection [99 Raviglione MC, Smith IM: XDR tuberculosis–implications for global public health. N Engl J Med 2007, 356(7):656-659.].
Furthermore, the increased number of TB cases due to multidrug resistant and extensively drug resistant strains (MDR and XDR) and HIV co-infection have pointed out the urgent need for alternative treatment. In recent years, research on the development of new anti-TB therapies has focused on novel agents from both synthetic and natural sources [1010 Demain AL, Sanchez S: Microbial drug discovery: 80 years of progress. J Antibiot (Tokyo) 2009, 62(1):5-16.]. For thousands of years, plant-derived drugs have been empirically used in the treatment of numerous human disorders. Many conventional drugs originate from plant sources, such as aspirin (from willow bark), digoxin (from foxglove), quinine (from cinchona bark), and morphine (from the opium poppy) [1111 Peña S, Scarone L, Manta E, Stewart L, Yardley V, Croft S, et al.: Synthesis of aMicrocystis aeruginosa predicted metabolite with antimalarial activity. Bioorg Med Chem Lett 2012, 22(15):4994-4997.].
Marine natural products play an important role in drug development particularly in anticancer, antibiotic and antiparasitic therapies. It is well known that macrocyclic peptides may demonstrate drug-like physicochemical and pharmacokinetic properties such as good metabolic stability, solubility, lipophilicity and bioavailability [1212 Kehr JC, Picchi DG, Dittmann E: Natural product biosyntheses in cyanobacteria: a treasure trove of unique enzymes. Beilstein J Org Chem 2011, 7:1622-1635.]. More than 800 secondary metabolites belonging to several classes of substances have been isolated and identified, which includes enzyme inhibitors; photosynthesis inhibitors; antimicrobial, antimitotic, immunosuppressive and antitumor peptides[1313 Tan LT: Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68(7):954-979.]-[1515 Luukkainen R, Namikoshi M, Sivonen K, Rinehart KL, Niemelä SI: Isolation and identification of 12 microcystins from four strains and two bloom samples of Microcystis spp.: structure of a new hepatotoxin. Toxicon 1994, 32(1):133-139.].
Microcystins, more than 65 structural variants are currently known, are cyclic heptapeptides, composed of seven amino acids, namely, five non-protein and two protein amino acids. These two protein amino acids distinguish microcystins from one another, while the other amino acids are more or less constant among the variants [1616 Matthiensen A, Beattie KA, Yunes JS, Kaya K, Codd GA: [D-Leu1] Microcystin-LR, from the cyanobacterium Microcystis RST 9501 and from a Microcystis bloom in the Patos Lagoon estuary, Brazil. Phytochemistry 2000, 55(5):383-387.]-[1818 Prasanna R, Sood A, Jaiswal P, Nayak S, Gupta V, Chaudhary V, et al.: Rediscovering cyanobacteria as valuable sources of bioactive compounds. Prikl Biokhim Mikrobiol 2010, 46(2):133-147.].
Structural variations have been identified at all seven positions of the heptapeptide ring. Microcystin-LR (MC-LR) (Figure 1) is the most commonly identified cyanotoxin in environmental samples, which presents a leucine (L) and an arginine (R) respectively in X and Y positions of the cyclic heptapeptide [1919 Funari E, Testai E: Human health risk assessment related to cyanotoxins exposure. Crit Rev Toxicol 2008, 38(2):97-125.],[2020 Sivonen K, Jones G: Cyanobacterial toxins. In Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. Edited by Chorus L, Bartram J. World Health Organization and E&FN Spon, London; 1999:41-111.]. The combined presence of the two L-amino acids is used in the nomenclature of the variants while the position 1, which contains D-Ala, is relatively conserved[2121 Chong MW, Gu KD, Lam PK, Yang M, Fong WF: Study on the cytotoxicity of microcystin-LR on cultured cells. Chemosphere 2000, 41(1–2):143-147.].
General structure of microcystins (LR) with leucine (L) in the amino acid position 2 and arginine (R) in the amino acid position 4 and the structural differences in the position 1 of MC-LR (left) and [D-Leu1] MC-LR (right).
Another variant of microcystin is [D-Leu1] MC-LR, which contains D-Leu in position 1. This toxin was detected in cells of Microcystis aeruginosa (RST 9501 strain) isolated from Patos Lagoon (southern Brazil). This major waterbody has a history of extensive nuisance blooms and scums of hepatotoxic Microcystis[2222 Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G: Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 1971, 35(2):171-205.].
The present study evaluated the antimycobacterial activity and cytotoxicity of Microcystis aeruginosa toxins, the variant MC-LR and purified extract of [D-Leu1] microcystin-LR againstMycobacterium tuberculosis, M. chelonae, M. terrae and M. kansasii.
Methods
Microcystis culture conditions
Microcystis RST 9501 (UPC Culture Collection, Federal University of Rio Grande) isolated from the estuary of Patos Lagoon is the [D-Leu1] MC-LR producing variant and was grown in BG-11 medium with nitrate as previously described [2323 Beattie KA, Kaya K, Sano T, Codd GA: Three dehydrobutyrine-containing microcystins from Nostoc. Phytochemistry 1998, 47(7):1289-1292.],[2424 Matthiensen A, Yunes JS, Codd GA: Ocorrência, distribuição e toxicidade de cianobactérias no estuário da Lagoa dos Patos. RS Rev Bras Biol 1999, 59(3):361-376.].
Preparation of microcystis aeruginosa extracts
The extract was prepared using lyophilized cells of Microcystis aeruginosa according to the protocol described by Beattie et al.[2424 Matthiensen A, Yunes JS, Codd GA: Ocorrência, distribuição e toxicidade de cianobactérias no estuário da Lagoa dos Patos. RS Rev Bras Biol 1999, 59(3):361-376.]. Briefly, the cells were dissolved in absolute methanol (Sigma, USA), sonicated three times and centrifuged (10,000 × g) at 4°C, for ten minutes. Extracts were evaporated at 40°C and then redissolved in ultrapure water (Direct Q3, Millipore, USA). The other extract preparations, presented in Table 1, replaced methanol with hexane, chloroform or water. Finally, samples were centrifuged and the supernatant was collected and stored at-20°C. Microcystin content was determined using a commercial enzyme-linked immunosorbent assay (ELISA) with polyclonal antibodies (EnviroLogix Inc., USA). Different concentrations of microcystin were prepared after appropriate dilutions with phosphate buffered saline (PBS – Ca+2 and Mg+2free). Characterization of microcystins produced by the strain RST 9501 was previously reported by Matthiensen et al.[1717 Lemes GAF, Kist LW, Bogo MR, Yunes JS: Biodegradation of [D-Leu1] microcystin-LR by a bacterium isolated from the sediments of the Patos Lagoon estuary, Brazil. J Venom Anim Toxins incl Trop Dis 2015, 21:4. doi:10.1186/s40409–015–0001–3],[2525 Lawton LA, Edwards C, Codd GA: Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst 1994, 119(7):1525-1530.]. For the extraction of microcystin from cells of the strain RST 9501, the toxin [D-Leu1] microcystin-LR was purified from cell extracts, following Lawton et al.[2626 Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, et al.: Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol 1998, 36(2):362-366.]. The chemical compound microcystin-LR was purchased from Sigma (USA).
Finally, both toxins were resuspended in water and then analyzed by high performance liquid chromatography (HPLC – Shimadzu SCL-10Avp, Japan) to determine the concentration of microcystins prior to tests.
Microcystin analysis
Analysis of microcystin obtained from Microcystis RST 9501 was performed using the HPLC equipment Shimadzu SCL-10AVP (Japan). The analysis was carried out using a C18 Luna (4.6 × 250 mm, 5 μm particle size; Phenomenex, USA) reversed-phase column at 40°C with UV detection at 238 nm. The mobile phase was Milli-Q water/CH3CN (J. T. Baker, USA), both containing 0.05 % (v/v) trifluoroacetic acid (Merck, Germany), initially at 65:35 and using a linear gradient over 20 minutes of 100 % CH3CN at 1 mL.min-1.
Isolates and mycobacterium spp. preparation
The antimicrobial activity of Microcystis aeruginosa extract and microcystin were evaluated againstMycobacterium tuberculosis H37Rv (ATCC 27294) pan-susceptible strain and against two clinical isolate mono-resistant to isoniazid and rifampicin with katG S315T and rpoB S531L respectively. Furthermore, M. aeruginosa extract, [D-Leu1] MC-LR, and microcystin-LR (Sigma, USA) toxins were tested against the nontuberculous mycobacteria: M. terrae (ATCC15755), M. chelonae (ATCC 946) and M. kansasii (ATCC12478).
The bacterial suspensions obtained of culture in Ogawa Kudoh medium for about 14 days were prepared in sterile water containing pearls of glass of 3 mm. The suspension was homogenized by vortex agitation and the turbidity was adjusted in agreement with tube one of the scale of McFarland (3.2 × 107 cfu/mL). The inoculum was prepared diluting the bacterial suspension in the proportion of 1:25 in medium 7H9 broth [4.7 g of Middlebrook 7H9 broth base (BD Difco, USA) 2 mL of glycerol (Vetec, Brazil) in 900 mL of water] enriched with 10 % oleic acid-albumin-dextrose-catalase (OADC – BBL, Media Additives, USA) [2727 Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F: Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance inMycobacterium tuberculosis. Antimicrob Agents Chemother 2002, 46(8):2720-2722.].
Evaluation of antimycobacterial activity
The method used for the determination of the antimycobacterial activity was the resazurin microtiter assay [2828 Silva NC, Sarmento B, Pintado M: The importance of antimicrobial peptides and their potential for therapeutic use in ophthalmology. Int J Antimicrob Agents 2013, 41(1):5-10.]. In brief, the assay is accomplished on microplates (96 wells) using resazurin as indicator of cellular viability. Medium 7H9 enriched with 10 % OADC was employed. The extracts and pure microcystin were weighed, dissolved in DMSO and the determination of minimal inhibitory concentration (MIC) was carried out starting from 53 to 0.06 μM in serial dilutions of 1:2.
Cytotoxicity assay
The HTC cell line was obtained from the Cell Bank of Rio de Janeiro, Brazil. HTC cells were grown in RPMI 1640 medium (Gibco, USA) supplemented with sodium bicarbonate (0.2 g/L) (Vetec, Brazil), L-glutamine (0.3 g/L) (Vetec, Brazil), Hepes (25 mM) (Acros, USA) and b-mercaptoethanol (5 × 10–5 M) (Sigma, Germany), with 10 % fetal bovine serum (Gibco, Brazil), 1 % of antibiotic and antimycotic (penicillin – 100 U/mL, streptomycin-100 mg/mL and amphotericin B - 0.25 mg/mL), in disposable plastic flasks, at 37°C.
The cytotoxicity assay was performed by trypan blue exclusion after 24 hours of incubation with microcystins. Three independent experiments were carried out using triplicates in each experiment. Data are expressed as mean + standard error and analyzed using ANOVA followed by Tukey’s multiple range test. Significance level was fixed in 0.05.
Results
M. aeruginosa RST 9501 extracts were evaluated against M. tuberculosis pan-susceptible (H37Rv), rifampicin- (RIFr) and isoniazid-resistant strains (INHr). The MIC for these four extracts ranged from 1.93 μM to 0.06 μM. The aqueous and chloroformic extracts did not present antimycobacterial activity within these concentrations.
The methanolic extract had a MIC of 1.93 μM against H37Rv and of 0.96 μM against the tested resistant strains. The hexanic extract showed the highest activity, with a MIC of 0.12 μM against INHr, ≤ 0.60 μM against RIFr and ≤ 0.06 μM against H37Rv (Table 1). This high activity of the hexanic extract could be attributed to the possible greater concentration of lipophilic compounds. Therefore, the molecules of microcystins (MC-LR and [D-Leu1] MC-LR) were evaluated against M. tuberculosis H37Rv, RIFr and INHr strains.
The cyanotoxin MC-LR did not present any inhibitory activity on the three strains at the concentration of 53 μM. On the other hand, [D-Leu1] MC-LR was active with a similar MIC (13.2 μM) for susceptible and resistant M. tuberculosis strains (Table 2). Therefore, the isolated cyanotoxin showed a MIC up to 220 times higher than that of the hexanic extract.
The two microcystins were evaluated against three nontuberculous mycobacteria showing high activity for all species tested. M. terrae was the most resistant to nontuberculous mycobacteria, it showed antimycobacterial activity against the two tested toxins with MIC of 6.74 μM and 1.08 μM for [D-Leu1] MC-LR and MC-LR, respectively (Table 3).
A further comparison between both toxins effects suggested that microcystin MC-LR showed lower activity than [D-Leu1] MC-LR against M. chelonae and M. kansasii, with a minimum inhibitory concentration of 2.15 μM for the two strains, while the other variant showed minimum inhibitory concentration of 0.84 μM and 0.42 μM for M. chelonae and M. kansasii, respectively.
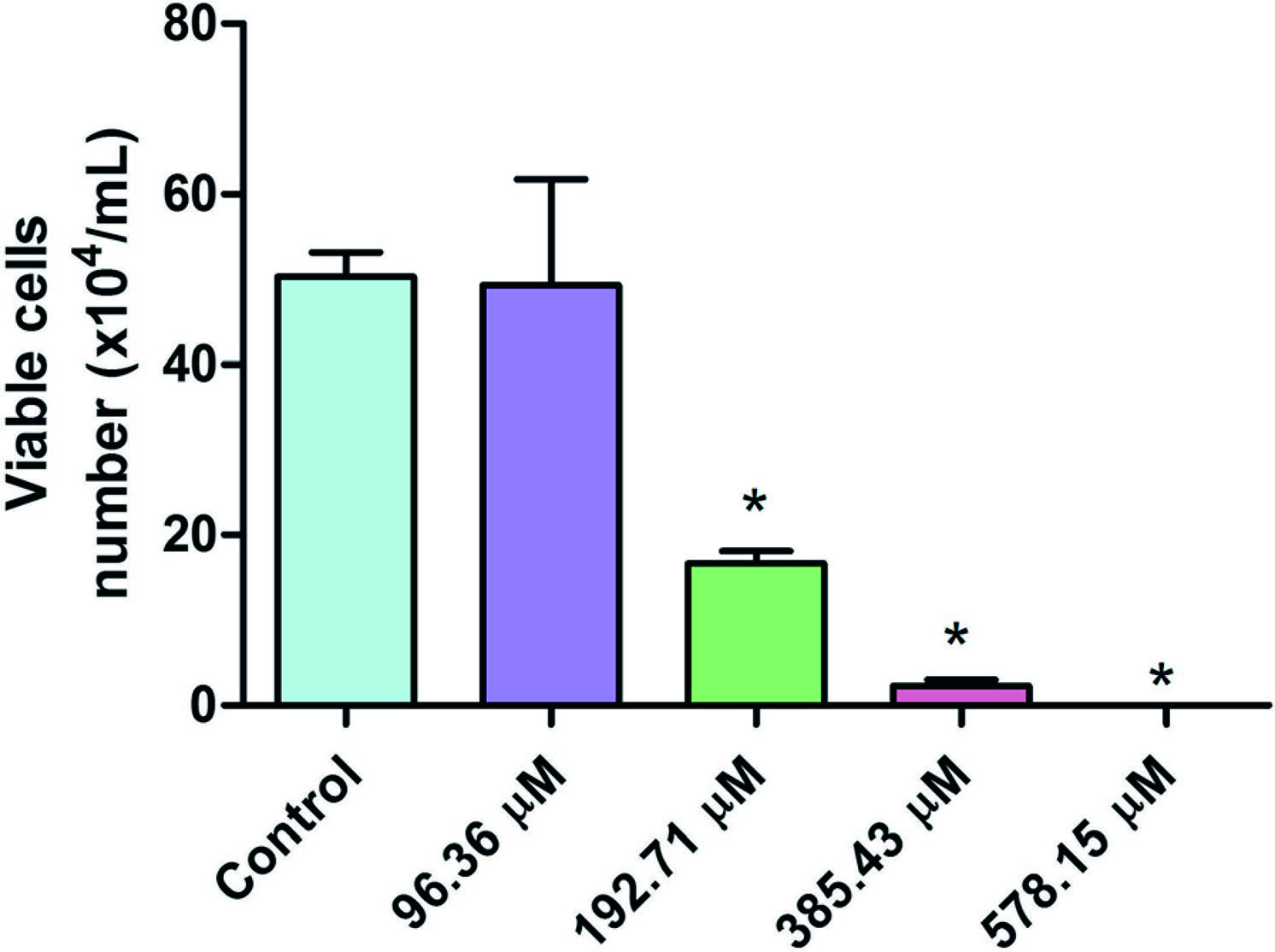
Exposure of HTC cells to Microcystis aeruginosa strain 9501 which produces [D-Leu1] MC-LR induces a decrease in viable cell number, as determined by trypan blue exclusion, in a concentration dependent manner, 24 hours after exposure (Figure 2). There were no differences in the number of viable cells (p > 0.05) between control and treated cells in the lowest concentration (96.36 μM). However, from the concentration of 192.71 μM on, there was significant difference (p < 0.05) in the number of viable cells, which indicates cytotoxic effect. Note that at the highest concentration (578.15 μM) no viable cell was found.
Number of HTC viable cell by trypan blue exclusion test 24 hours after exposure to different concentrations of Microcystis aeruginosa strain 9501 (578.15 to 93.36 μM) which produces [D-Leu1] MC-LR. Data are expressed as mean + standard error. *Indicates significant difference from the control (p < 0.05).
Discussion
In recent years, there has been a growing interest in the study of natural peptide molecules, whose mode of action promises both low susceptibility to multidrug resistance mechanisms and high activity against a vast range of microorganisms [2929 Rotem S, Mor A: Antimicrobial peptide mimics for improved therapeutic properties. Biochim Biophys Acta 2009, 1788(8):1582-1592.],[3030 Jamieson AG, Boutard N, Sabatino D, Lubell WD: Peptide scanning for studying structure-activity relationships in drug discovery. Chem Biol Drug Des 2013, 81(1):148-165.]. The application of peptides for drug discovery is merited because of their ease of synthesis, large structural diversity and commonly high potency [3131 Madhumathi V, Deepa P, Jeyachandran S, Manoharan C, Vijayakumar S: Antimicrobial activity of cyanobacteria isolated from freshwater lake. Int J Microbiol Res 2011, 2(3):213-216.].
The present study identified in extracts from M. aeruginosa RST 9501 active compounds with antimycobacterial activity against M. tuberculosis growth, including sensitive and resistant strains. Several metabolites produced by cyanobacteria have been identified in the literature. There is a special interest, because these secondary metabolites are not only disease causing agents but also bioactive molecules applied to further studies [1919 Funari E, Testai E: Human health risk assessment related to cyanotoxins exposure. Crit Rev Toxicol 2008, 38(2):97-125.],[3232 Jaiswal P, Prasanna R, Singh PK: Characterization of the biocidal spectrum of extracellular filtrates of Microcystis aeruginosa. Indian J Microbiol 2011, 51(4):509-514.]-[3434 Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC: Marine cyanobacteria – a prolific source of natural products. Tetrahedron 2001, 57(46):9347-9377.]. Some works have identified a range of cyanobacteria compounds isolated from toxic blooms of Microcystis, Anabaena and Nostocknown to produce a diverse array of bioactive compounds exhibiting a broad spectrum of activity, including anticancer, antiviral, antibacterial, antifungal and anti-inflammatory activity, besides cytotoxic activities [1919 Funari E, Testai E: Human health risk assessment related to cyanotoxins exposure. Crit Rev Toxicol 2008, 38(2):97-125.],[3232 Jaiswal P, Prasanna R, Singh PK: Characterization of the biocidal spectrum of extracellular filtrates of Microcystis aeruginosa. Indian J Microbiol 2011, 51(4):509-514.],[3434 Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC: Marine cyanobacteria – a prolific source of natural products. Tetrahedron 2001, 57(46):9347-9377.],[3535 Pauli GF, Case RJ, Inui T, Wang Y, Cho S, Fischer NH, et al.: New perspectives on natural products in TB drug research. Life Sci 2005, 78(5):485-494.].
In this study, four M. aeruginosa extracts were tested, the methanolic extract was more active against resistant strains (RIFr and INHr) than sensitive M. tuberculosis strains. Microcystins are a group of chemically related cyclic peptides [1313 Tan LT: Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68(7):954-979.] and most commonly studied group of cyanotoxins,. Therefore, in order to identify the possible active compounds derived from the hexane extraction that was more active among the extracts from M. aeruginosa cells evaluated, the two known cyanotoxins ([D-Leu1] MC-LR and MC-LR) were evaluated against M. tuberuculosis and three nontuberculous mycobacteria species.
Between the two variants derived from the same cyanobacterial toxin, just [D-Leu1] MC-LR showed antimicrobial activity against three different strains of M. tuberculosis. However, the MIC of this variant was higher than that of the methanolic and hexane extracts. According to Pauli et al.[3636 Katoch VM: Infections due to non-tuberculous mycobacteria (NTM). Indian J Med Res 2004, 120(4):290-304.], the differences between the extract and pure cyanotoxin activity can be attributable to the MIC of the crude extract, which may not be a reliable antimycobacterial activity indicator, since it, the activity may be to antagonist between the substances, or otherwise, synergism between them, that decreasing or increasing effects on the MIC. Moreover, an extract with high activity (lower MIC) could have several compounds with moderate antimycobacterial activity; becoming it the most active extract with a pure and isolated substance.
The activity of [D-Leu1] MC-LR was unchanged against strains of M. tuberculosis resistant to rifampicin and isoniazid compared to pan-susceptible strains. This is an important finding since the resistance to isoniazid and rifampicin comprise a major drawback of tuberculosis control programs.
Interestingly, the small antimicrobial activity of the two studied microcystins variants observed against different strains of M. tuberculosis was not observed for nontuberculous mycobacteria. MC-LR was less active than [D-Leu1] MC-LR against M. chelonae and M. kansasii. A few studies have shown that drug susceptibility of nontuberculous mycobacteria are distinct from that observed in M. tuberculosis because, in general, the resistance of nontuberculous mycobacteria is related to cell wall permeability and efflux pumps, specially in the presence of a specific mutation [3737 Falkinham JO 3rd: Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev 1996, 9(2):177-215.]. In this study, however, M. terrae was less susceptible against cyanotoxins. In addition, the MIC against M. tuberculosis strains was higher than that used against all nontuberculous mycobacteria.
The activity of these molecules against M. chelonae is significant, since it indicates that the low permeability limits the activity of this kind of hydrophilic molecules, which impairs treatment [3838 MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA: Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 1990, 264(2):187-192.]. Regarding the mechanism of action proposed for microcystins is the inhibiting serine/threonine phosphatases 1 and 2A, which leads to protein phosphorylation and the consequence is cytoskeletal damage, liver necrosis and hemorrhage in the liver which is directly related to their cytotoxicity and tumor promoting activity [3939 Zegura B, Sedmak B, Filipic M: Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 2003, 41(1):41-48.]. However, there is only a few data on tissue concentrations of microcystins in exposed humans or animals, which were obtained after exposure to high toxic doses of microcystins [4040 Mandal SM, Barbosa AEAD, Franco OL: Lipopeptides in microbial infection control: scope and reality for industry. Biotechnol Adv 2013, 31(2):338-345.].
In our study, a single difference in the chemical constitution of the heptapeptide was significantly important in increasing the antimycobacterial activity. In addition, considering that leucine is a hydrophobic amino acid, this characteristic may interfere in the activity of the peptide that was enhanced by presence.
According to Mandal et al.[4141 Gerard J, Lloyd R, Barsby T, Haden P, Kelly MT, Andersen RJ: Massetolides A-H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J Nat Prod 1997, 60(3):223-229.], variations of amino acid residues in peptides have received considerable attention since they alter the activity against pathogenic microorganisms, which has a significant impact on antibacterial activity [4141 Gerard J, Lloyd R, Barsby T, Haden P, Kelly MT, Andersen RJ: Massetolides A-H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J Nat Prod 1997, 60(3):223-229.]. A study showed a difference in their activity againstM. tuberculosis and Mycobacterium avium-intracellulare by the change of only one amino acid residue in their peptide moiety [4242 De Souza Votto AP, Renon VP, Yunes JS, Rumjanek VM, Marques Capella MA, Neto VM, et al.: Sensitivity to microcystins: a comparative study in human cell lines with and without multidrug resistance phenotype. Cell Biol Int 2007, 31(11):1359-1366.].
Peptides may adopt secondary structures, which are responsible for their receptor affinity and biological activity. The rational design can be sufficient to endow antibacterial efficacy and to circumvent drawback effects in this potential therapeutic agent [3030 Jamieson AG, Boutard N, Sabatino D, Lubell WD: Peptide scanning for studying structure-activity relationships in drug discovery. Chem Biol Drug Des 2013, 81(1):148-165.],[3131 Madhumathi V, Deepa P, Jeyachandran S, Manoharan C, Vijayakumar S: Antimicrobial activity of cyanobacteria isolated from freshwater lake. Int J Microbiol Res 2011, 2(3):213-216.].
According to Votto et al.[43] and considering that microcystins may provoke oxidative stress, the difference in sensitivity of MDR and non-MDR cells can be associated with dissimilar antioxidant defenses. In this context, the higher catalase activity observed in the same work may help to explain, at least partially, the resistance of MDR cells to microcystin exposure. This MDR cell line with higher catalase activity also showed lower DNA damage than its parental cell line, suggesting the involvement of reactive oxygen species (ROS) in the toxicity exerted by this cyanotoxin. Therefore, the significant increase in ROS production observed in non-MDR cells, in contrast with MDR cells when both cell lines were exposed to microcystins, suggest that MDR cells, at least in part, were more resistant to microcystins due to a higher antioxidant competence. These authors also present other factors which may have contributed for the resistance to microcystin in MDR cells [43].
The choice of the cell line used in this study was supported by the fact that it is in the liver that microcystins are metabolized. The results show a significant sensitivity of tumoral liver cells to this substance in only 24 hours of exposure.
Conclusions
In the present study, the antimicrobial activity of the hexanic extract from M. aeruginosa RST 9501 against M. tuberculosis – including sensitive and resistance strains, and nontuberculous mycobacteria – was observed and possibly associated with the presence of cyanotoxins. When the activity of these toxins was assessed, the variant [D-Leu1] MC-LR was the most active against tested mycobacterial strains. Moreover, was not identified cytotoxic activity at concentrations whose antimicrobial activity. Also, was not identified cytotoxic activity at concentrations which antimicrobial activity was observed. These results showed the importance of detailed studies on the activity of extracts and toxins derived from M. aeruginosa strains as promising bioactive molecules in the treatment of mycobacterial diseases.
Ethics committee approval
The present study was approved by the Ethics Committee of the Federal University of Rio Grande.
Acknowledgments
The authors would like to thank CNPq and FAURG for the funding of this research.
References
-
1Falkinham JO: The changing pattern of nontuberculous mycobacterial disease. Can J Infect Dis 2003, 14(5):281-286.
-
2van Ingen J, Boeree MJ, van Soolingen D, Mouton JW: Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat 2012, 15(3):149-161.
-
3Oliva VM, Cezário GAG, Cocato RA, Marcondes-Machado J: Pulmonary tuberculosis: hematology, serum biochemistry and the relation with the disease duration. J Venom Anim Toxins incl Trop Dis 2008, 14(1):71-81. [ http://www.scielo.br/scielo.php?pid=S1678–91992008000100006&script=sci_arttext ]
» http://www.scielo.br/scielo.php?pid=S1678–91992008000100006&script=sci_arttext -
4Dias-Baptista IMF, Uso SMRS, Marcondes-Machado J: Trends in multidrug-resistant tuberculosis. J Venom Anim Toxins incl Trop Dis 2008, 14(2):203-223. [ http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1678–91992008000200003&lng=en&nrm=iso&tlng=en ]
» http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1678–91992008000200003&lng=en&nrm=iso&tlng=en -
5Al-Mahruqi SH, van Ingen J, Al-Busaidy S, Boeree MJ, Al-Zadjali S, Patel A, et al.: Clinical relevance of nontuberculous mycobacteria, Oman. Emerg Infect Dis 2009, 15(2):292-294.
-
6Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al.: An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007, 175(4):367-416.
-
7Brown-Elliott BA, Nash KA, Wallace RJ Jr: Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 2012, 25(3):545-582.
-
8World Health Organization: Global Tuberculosis Report 2012. World Health Organization, Geneva, Switzerland; 2012. Mitchison DA: Prevention of drug resistance by combined drug treatment of tuberculosis. Handb Exp Pharmacol 2012, 211:87-98.
-
9Raviglione MC, Smith IM: XDR tuberculosis–implications for global public health. N Engl J Med 2007, 356(7):656-659.
-
10Demain AL, Sanchez S: Microbial drug discovery: 80 years of progress. J Antibiot (Tokyo) 2009, 62(1):5-16.
-
11Peña S, Scarone L, Manta E, Stewart L, Yardley V, Croft S, et al.: Synthesis of aMicrocystis aeruginosa predicted metabolite with antimalarial activity. Bioorg Med Chem Lett 2012, 22(15):4994-4997.
-
12Kehr JC, Picchi DG, Dittmann E: Natural product biosyntheses in cyanobacteria: a treasure trove of unique enzymes. Beilstein J Org Chem 2011, 7:1622-1635.
-
13Tan LT: Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68(7):954-979.
-
14Volk RB, Furkert FH: Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol Res 2006, 161(2):180-186.
-
15Luukkainen R, Namikoshi M, Sivonen K, Rinehart KL, Niemelä SI: Isolation and identification of 12 microcystins from four strains and two bloom samples of Microcystis spp.: structure of a new hepatotoxin. Toxicon 1994, 32(1):133-139.
-
16Matthiensen A, Beattie KA, Yunes JS, Kaya K, Codd GA: [D-Leu1] Microcystin-LR, from the cyanobacterium Microcystis RST 9501 and from a Microcystis bloom in the Patos Lagoon estuary, Brazil. Phytochemistry 2000, 55(5):383-387.
-
17Lemes GAF, Kist LW, Bogo MR, Yunes JS: Biodegradation of [D-Leu1] microcystin-LR by a bacterium isolated from the sediments of the Patos Lagoon estuary, Brazil. J Venom Anim Toxins incl Trop Dis 2015, 21:4. doi:10.1186/s40409–015–0001–3
-
18Prasanna R, Sood A, Jaiswal P, Nayak S, Gupta V, Chaudhary V, et al.: Rediscovering cyanobacteria as valuable sources of bioactive compounds. Prikl Biokhim Mikrobiol 2010, 46(2):133-147.
-
19Funari E, Testai E: Human health risk assessment related to cyanotoxins exposure. Crit Rev Toxicol 2008, 38(2):97-125.
-
20Sivonen K, Jones G: Cyanobacterial toxins. In Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management Edited by Chorus L, Bartram J. World Health Organization and E&FN Spon, London; 1999:41-111.
-
21Chong MW, Gu KD, Lam PK, Yang M, Fong WF: Study on the cytotoxicity of microcystin-LR on cultured cells. Chemosphere 2000, 41(1–2):143-147.
-
22Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G: Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 1971, 35(2):171-205.
-
23Beattie KA, Kaya K, Sano T, Codd GA: Three dehydrobutyrine-containing microcystins from Nostoc. Phytochemistry 1998, 47(7):1289-1292.
-
24Matthiensen A, Yunes JS, Codd GA: Ocorrência, distribuição e toxicidade de cianobactérias no estuário da Lagoa dos Patos. RS Rev Bras Biol 1999, 59(3):361-376.
-
25Lawton LA, Edwards C, Codd GA: Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst 1994, 119(7):1525-1530.
-
26Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, et al.: Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol 1998, 36(2):362-366.
-
27Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F: Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance inMycobacterium tuberculosis. Antimicrob Agents Chemother 2002, 46(8):2720-2722.
-
28Silva NC, Sarmento B, Pintado M: The importance of antimicrobial peptides and their potential for therapeutic use in ophthalmology. Int J Antimicrob Agents 2013, 41(1):5-10.
-
29Rotem S, Mor A: Antimicrobial peptide mimics for improved therapeutic properties. Biochim Biophys Acta 2009, 1788(8):1582-1592.
-
30Jamieson AG, Boutard N, Sabatino D, Lubell WD: Peptide scanning for studying structure-activity relationships in drug discovery. Chem Biol Drug Des 2013, 81(1):148-165.
-
31Madhumathi V, Deepa P, Jeyachandran S, Manoharan C, Vijayakumar S: Antimicrobial activity of cyanobacteria isolated from freshwater lake. Int J Microbiol Res 2011, 2(3):213-216.
-
32Jaiswal P, Prasanna R, Singh PK: Characterization of the biocidal spectrum of extracellular filtrates of Microcystis aeruginosa. Indian J Microbiol 2011, 51(4):509-514.
-
33Jaiswal P, Singh PK, Prasanna R: Cyanobacterial bioactive molecules – an overview of their toxic properties. Can J Microbiol 2008, 54(9):701-717.
-
34Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC: Marine cyanobacteria – a prolific source of natural products. Tetrahedron 2001, 57(46):9347-9377.
-
35Pauli GF, Case RJ, Inui T, Wang Y, Cho S, Fischer NH, et al.: New perspectives on natural products in TB drug research. Life Sci 2005, 78(5):485-494.
-
36Katoch VM: Infections due to non-tuberculous mycobacteria (NTM). Indian J Med Res 2004, 120(4):290-304.
-
37Falkinham JO 3rd: Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev 1996, 9(2):177-215.
-
38MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA: Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 1990, 264(2):187-192.
-
39Zegura B, Sedmak B, Filipic M: Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 2003, 41(1):41-48.
-
40Mandal SM, Barbosa AEAD, Franco OL: Lipopeptides in microbial infection control: scope and reality for industry. Biotechnol Adv 2013, 31(2):338-345.
-
41Gerard J, Lloyd R, Barsby T, Haden P, Kelly MT, Andersen RJ: Massetolides A-H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J Nat Prod 1997, 60(3):223-229.
-
42De Souza Votto AP, Renon VP, Yunes JS, Rumjanek VM, Marques Capella MA, Neto VM, et al.: Sensitivity to microcystins: a comparative study in human cell lines with and without multidrug resistance phenotype. Cell Biol Int 2007, 31(11):1359-1366.
Publication Dates
-
Publication in this collection
2015
History
-
Received
25 Aug 2014 -
Accepted
6 Mar 2015 -
Accepted
20 Mar 2015