Abstract
Background
Maintenance of scorpions under laboratory conditions is ideal for long-term venom collection to explore the therapeutic applications of scorpion venom. Collection of venom by electrical stimulation requires a reliable stimulator and effective restrainer. Thus, the present study was conducted to develop a convenient method to maintain scorpions and to extract their venom for toxicity studies via a modified restrainer and stimulator.
Methods
Four different scorpion species were collected, among which three species were maintained in the laboratory in containers that mimic their natural habitat. Venom was extracted from Hottentotta rugiscutis by electrical stimulation at 8 V for 18 months and LD50 was estimated by the graphic method of Miller and Tainter.
Results
A total of 373 scorpions including Hottentotta rugiscutis, Hottentotta tamulus, Lychas tricarinatus and Heterometrus swammerdami were collected, identified and maintained successfully, achieving a 97 % survival rate. Hottentotta rugiscutis yielded 6.0 mL of venom by electrical stimulation. The LD50 of H. rugiscutis venom was estimated to be 3.02 mg/kg of body weight in female Swiss albino mice.
Conclusions
Scorpions were successfully maintained for 18 months. Herein we have also documented a simple, cost-effective method of venom extraction by electrical stimulation using a modified restrainer. Furthermore, Hottentotta rugiscutis was reported for the first time in Karnataka.
Scorpion venom Restrainer; Hottentotta Heterometrus; Venom extraction LD50
Background
Scorpions are arthropods belonging to the class Arachnida and order Scorpiones. Globally, 1988 species are known, among which 113 species from 25 genera belonging to six families have been recorded in India [11. Bastawade DB, Jadhav SS, Sharma RM. Scorpionida. Zool Surv India. 2012;4(6):1–16.]. Scorpions of the family Buthidae are of prime importance due to the lethality of their venom to humans, especially to children in many tropical regions [22. Badhe RV, Thomas AB, Deshpande AD, Salvi N, Waghmare A. The action of red scorpion (Mesobuthus tamulus coconsis, Pocock) venom and its isolated protein fractions on blood sodium levels. J Venom Anim Toxins incl Trop Dis. 2007;13(1):82–93.]. A Buthidae family scorpion, Hottentotta tamulus (Fabricius, 1798), is widely distributed throughout the Deccan plateau of southern India [33. Kankonkar RC, Kulkurni DG, Hulikavi CB. Preparation of a potent anti-scorpion-venom serum against the venom of red scorpion (Buthus tamulus). J Postgrad Med. 1998;44(4):85–92., 44. Bawaskar HS, Bawaskar PH. Scorpion sting: Update. J Assoc Physicians India. 2012;60:46–55.].
As shown by an internal survey, Hottentotta rugiscutis (Pocock, 1897) scorpions are prevalent, live alongside the human habitat in the Chirathagundu village (Karnataka, India) and are known to cause considerable public health problems due to envenomations compared to three other regions – namely Hiriyuru, Nandihalli and Hindaskatte – where human activity is limited. As there is hardly any data on the lethality of H. rugiscutis venom, it is important to research these scorpions and to find out their effects on the local population.
Scorpion venom is a complex mixture of mucopolysaccharides, hyaluronidase, phospholipase, serotonin, histamine, enzyme inhibitors, and toxins, mainly neurotoxins that affect the function of Na+, K+ and other ion channels – and is used as investigatory tool in physiological and pharmacological research. The therapeutic properties of scorpion venom includes anticancer, antimicrobial, antiepileptic, analgesic, antimalarial, pesticidal and insecticidal activities and also may be used in modulating cardiovascular effects and autoimmune diseases [55. Joseph B, George J. Scorpion toxins and its applications. Int J Toxicol Pharmacol Res. 2012;4(3):57–61.].
To study the biological importance of venom and for the preparation of antivenom to treat human envenomations, a substantial amount of venom is needed and can be obtained by in-field venom collection or from scorpions maintained under laboratory conditions. In comparison with the former, the latter ensures that copious venom can be extracted at frequent intervals for a longer time. Long-term extraction or milking of venom from a pool of scorpions requires an efficient maintenance of scorpions, which otherwise would exhibit a difference in both the quantity and quality of venom obtained. Furthermore, to date, not much information is available on the effective maintenance of scorpions under laboratory conditions.
There are three main methods for the extraction of venom, namely maceration of the telson, manual stimulation and electrical stimulation of scorpions. Maceration involves the snipping and crushing of the telson to access the venom. It is a reliable method but has the obvious drawback of allowing only one extraction per individual animal. In the manual stimulation method, the animal is provoked manually to secrete the venom on a piece of parafilm [66. Zlotkin E, Sholov AS. A simple device for collecting scorpion venom. Toxicon. 1969;7(4):331–2.]. But disadvantages include difficulty in provoking the scorpions [77. Nisani Z, Dunbar SG, Hayes WK. Cost of venom regeneration in Parabuthus transvaalicus (Arachnida: Buthidae). Comp Biochem Physiol A Mol Integr Physiol. 2007;147(2):509–13.], low venom yield and less toxicity as compared to the venom obtained by the electrical stimulation method [7–9]. In the third method, stimulation via mild electric shock results in contraction of muscles around the telson causing the venom to squeeze out of the vesicle. However, extracting the venom by this method requires not only a cost-effective and reliable stimulator but also a restrainer.
Given this context, the present study was conducted to develop a convenient method for scorpion maintenance and venom extraction for toxicity studies with the aid of a modified restrainer and stimulator.
Methods
Animals
All the experiments were performed according to the guidelines approved by the Institutional Animal Ethics Committee (National College of Pharmacy, Shivamogga, Karnataka; no: NCP/IAEC/CL/206/01). Five groups of female Swiss albino mice aged two months (weighing 20–25 g), with each group containing five mice, were used for testing each dose of venom for toxicity and a sixth group was used as control. The mice were kept under room temperature where they had ad libitum access to rodent chow and tap water throughout the experiment.
Collection of scorpions
The scorpions were collected from four different locations namely, Hiriyuru, Hindaskatte, Nandihalli (Chitradurga district) and Chirathagundu (Bellary district) of Karnataka, India (Fig. 1). Brown colored scorpions (Hottentotta rugiscutis,Hottentotta tamulus and Lychas tricarinatus) were found underneath the stone and wood materials over damp soil. Black scorpions (Heterometrus swammerdami) were found burrowed in soil at depths of 5–10 inches. These were captured carefully using long forceps by holding the tip of their tail without harming the animal. Black and brown scorpions were then transferred into separate containers with soil of about two inches in depth upon which small stones were placed. A maximum of 15 scorpions were kept in each container, which contained tiny holes for adequate ventilation.
Map of Karnataka showing the locations of different scorpion species. At the bottom: a Hottentotta rugiscutis (Pocock, 1897); bHottentotta tamulus (Fabricius, 1798); c Lychas tricarinatus (Simon, 1884), d Heterometrus swammerdami (Simon, 1872)
Identification of scorpions
Based on the morphological characteristics such as body color, the shape and size of pedipalps, shape of sternum, color of telson and number of pectine teeth, the collected scorpions were identified to the species level [1010. Sureshan PM, Bastawade DB, Radhakrishnan C. Taxonomic studies on a collection of scorpions (Scorpiones: Arachnida) from Western Ghats in Kerala, India with two new distribution records. Zoos Print J. 2007;22(12):2903–8., 1111. Veronika K, Akilan K, Murugananthan A, Eswaramohan T. Diversity and identification key to the species of scorpions (Scorpiones: Arachnida) from Jaffna Peninsula, Sri Lanka. J Entomol Zool Stud. 2013;1(5):70–7.].
Maintenance of scorpions
In the laboratory, scorpions were placed in large plastic tubs (diameter: 54 cm; height: 22 cm) and cages [43 (l) × 27 (w) × 15 (h) cm] on top of a soil bed of about 3 inches in depth, above which proper size stones (~15 × 8 × 15 cm) were kept, mimicking their natural habitat. As a source of drinking water, a Petri dish lid containing tap water was kept at the center of the plastic tub/cage (Fig. 2). About 8–10 brown scorpions such as Hottentotta rugiscutis (Pocock, 1897), Hottentotta tamulus (Fabricius, 1798), and the black scorpion, Heterometrus swammerdami (Simon, 1872), were housed in cages and plastic tubs respectively, and then covered with a mosquito net of proper dimensions.
A scorpion habitat in the laboratory (left) and a black scorpion drinking the water (right). A plastic tub having soil substrate and stones mimicking scorpion habitat along with water-filled petri dish at the center as a water supplement. A black scorpion,Heterometrus swammerdami, drinking the water placed in the petri dish
Scorpions were fed once weekly such insects as grasshoppers, crickets and occasionally cockroaches [1212. de Roodt AR, Coronas FI, Lago N, Gonzalez ME, Laskowicz RD, Beltramino JC, et al. General biochemical and immunological characterization of the venom from the scorpion Tityus trivittatus of Argentina. Toxicon. 2010;55(2-3):307–19.]. The size of the insects fed to the scorpions was directly proportional to that of the scorpions themselves. At three-day intervals, the water content in the Petri dish was checked and, if necessary, refilled. Whenever necessary, soil was moistened with adequate water and unused dead insects and debris were removed. Scorpions found to be weak and mother scorpions with their young brood were housed in separate plastic containers to avoid any cannibalistic activity on the part of other scorpions. After 2 to 3 weeks, mother scorpions were placed in the common cage and scorplings were fed tiny insects. Molted scorpions were not fed insects for 3 to 4 days to avoid any physical damage to themselves, nor were subjected to venom extraction for about 20 days. The scorpions were manipulated only on the day of venom extraction and during the replacement of soil, which was carried out once every two months. The venom was not extracted on the day immediately after feeding and also no food was added until three days after the venom extraction [1313. Whittemore Jr FW, Keegan HL, Fitzgerald CM, Bryant HA, Flanigan JF. Studies of scorpion antivenins. 2. Venom collection and scorpion colony maintenance. Bull World Health Organ. 1963;28(4):505–11., 1414. Candido DM, Lucas S. Maintenance of scorpions of the genus Tityus Koch (scorpiones, buthidae) for venom obtention at Instituto Butantan, São Paulo, Brazil. J Venom Anim Toxins incl Trop Dis. 2004;10(1):86–97.].
Extraction of scorpion venom
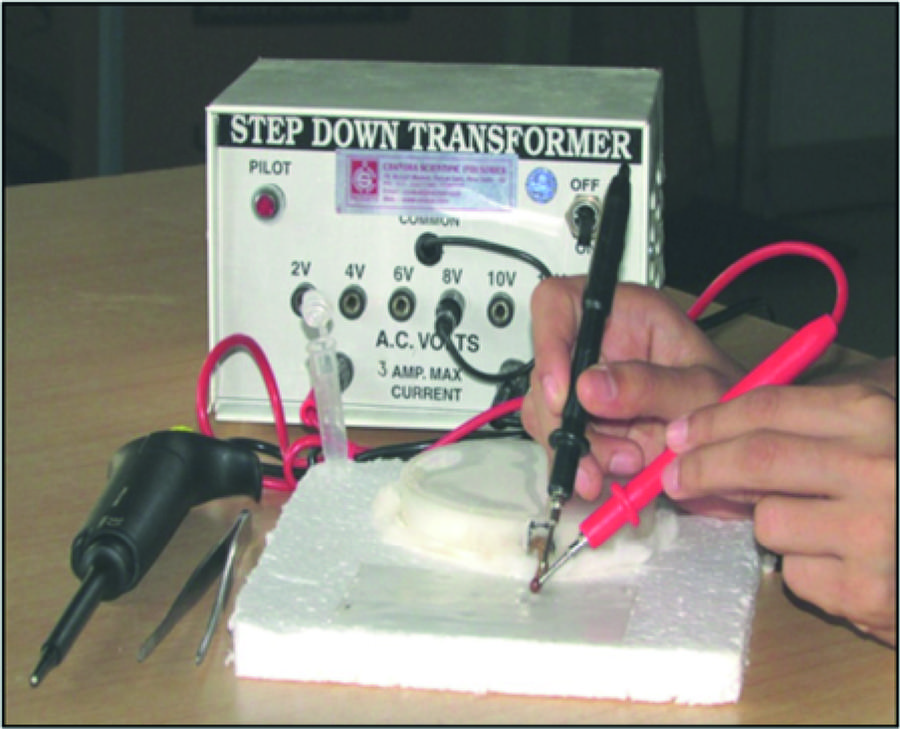
Extraction of venom was performed at monthly intervals by the electrical stimulation method using a restrainer developed by Deoras and Vad [1515. Deoras PJ, Vad NE. The milking of scorpions. Toxicon. 1962;1:41.] with slight modifications. Scorpions were restrained over a rectangular shaped thermocol board (20 × 16 cm) affixed at the center with a thick layer of cotton. Above this, a Petri dish lid with cotton inside and an opening on its side sufficiently large for protrusion of the scorpion tail, was placed in an inverted position. The tail was held firmly using forceps and electrically stimulated by pointing two electrodes connected to a step-down transformer (Chandra Scientific Industries, India) at junctions between the tail segments, one next to the telson and the other at the junction between IV and V metasomal segment (Fig. 3). Specimens of Hottentotta tamulus, Hottentotta rugiscutis and Heterometrus swammerdami were stimulated twice at 8 V and 10 V respectively, for 2 to 3 seconds, with a short interval between two stimulations. The venom was allowed to secrete over a piece of parafilm placed at the base of its tail and then transferred to a vial. The venom obtained in a single episode from many scorpions of a species were pooled, mixed with excess double-distilled water and centrifuged at 15,000 rpm for 20 minutes to remove the mucus; the supernatant was lyophilized and stored at –20 °C until use. For protein estimation and toxicity studies, venom was resuspended in phosphate buffered saline, pH 7.0 [1616. du Plessis JL. Collection of venom from southern African scorpions. Toxicon. 2005;45(5):681–2., 1717. Khoobdel M, Zahraei-Salehi T, Nayeri-Fasaei B, Khosravi M, Omidian Z, Motedayen MH, et al. Purification of the immunogenic fractions and determination of toxicity in Mesobuthus eupeus (Scorpionida: Buthidae) venom. J Arthropod Borne Dis. 2013;7(2):139–46.].
Extraction of venom by electrical stimulation method. Setup for milking of venom from Hottentotta rugiscutis by the electrical stimulation method using step-down transformer and restrainer
Lethality of scorpion venom
Toxicity of venom from Hottentotta rugiscutis of Chirathagundu region was studied by estimating the LD50 value under in vivo conditions. Five different doses covering the range of mortality from 0 to 100 % were tested. The test group mice were subcutaneously injected with one of five concentrations (2.60, 2.80, 3.02, 3.26, 3.52 mg/kg b.w.) of venom dissolved in 0.2 mL of phosphate buffered saline (PBS). Control group was injected with equivalent volume of PBS only. The animals were monitored for 24 hours and the LD50 was calculated by the graphical method of Miller and Tainter [1818. Randhawa MA. Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad. 2009;21(3):184–5.].
Results and discussion
A total of 351 brown scorpions and 22 black scorpions were collected at different time intervals from different regions of eastern Karnataka (Table 1). Transporting the scorpions in containers mimicking their natural habitat was apparently helpful in minimizing any sort of stress on them. Brown scorpions collected from the Hindaskatte and Chirathagundu regions were identified as Hottentotta rugiscutis, whereas the scorpions from Nandihalli and Hiriyuru were identified asHottentotta tamulus. The black scorpion from Chirathagundu was identified as Heterometrus swammerdami. Heretofore, the scorpion species, H. rugiscutis has not been reported from any part of Karnataka [1010. Sureshan PM, Bastawade DB, Radhakrishnan C. Taxonomic studies on a collection of scorpions (Scorpiones: Arachnida) from Western Ghats in Kerala, India with two new distribution records. Zoos Print J. 2007;22(12):2903–8.]. Although this study was focused on the collection of scorpions from the regions previously stated, we also collected another scorpion species haphazardly from the premises of Kuvempu University, Shivamogga district, with the aim of displaying the diversity of scorpions in Karnataka; it was identified as Lychas tricarinatus (Simon, 1884), but maintenance and venom collection were not carried out for this species (Fig. 1).
The scorpions maintained in cages and plastic tubs were active and healthy with a survival rate of 97 %. Employment of a Petri dish as a water reservoir makes water easily available to all scorpions (Fig. 2). This has proved to be a more efficient and easier method than the use of wet cotton balls placed in a container [1313. Whittemore Jr FW, Keegan HL, Fitzgerald CM, Bryant HA, Flanigan JF. Studies of scorpion antivenins. 2. Venom collection and scorpion colony maintenance. Bull World Health Organ. 1963;28(4):505–11., 1414. Candido DM, Lucas S. Maintenance of scorpions of the genus Tityus Koch (scorpiones, buthidae) for venom obtention at Instituto Butantan, São Paulo, Brazil. J Venom Anim Toxins incl Trop Dis. 2004;10(1):86–97.]. Adequate spraying of water restores the moisture content of the soil bed and is also helpful in the molting of scorpions [1919. Rubio M. Scorpions: Everything about purchase, care, feeding, and housing. Hauppauge: Barrons Educational Series; 2008.].
Feeding of scorpions once a week was noteworthy as it was observed that most of them did not consume insects when supplied at an interval of 3–4 days. It was crucial that insects be smaller than the scorpion to ensure that the latter can grab the prey easily with its pincers. All the insects were added to tubs/cages with their wings and forelimbs chopped so that they were easily accessible to the scorpions.
During the period of captivity, molting was observed at least twice in Heterometrus swammerdami, and only once in few Hottentotta rugiscutis and Hottentotta tamulus. Few scorpions gave birth to offspring, which may have been conceived after captivity, as we observed spermatophores (sperm containing tube-like structures) attached to mud particles or stones (Fig. 4) in many plastic tubs/cages, which is an indication of mating activity in laboratory conditions. Although it was not our motive to rear the scorplings, they were maintained on moral grounds, but ended up with only a 2 % survival rate. Rearing of scorplings in captivity is a difficult task with a much lower success rate as compared to the maintenance of adult scorpions [2020. Sissom WD, Polis GA, Watt DD. Field and laboratory methods. In: Polis GA, editor. The Biology of Scorpions. Stanford: Stanford University press; 1990. p. 449-450.], as also reported by Candido and Lucas and Gopalakrishnakone [1414. Candido DM, Lucas S. Maintenance of scorpions of the genus Tityus Koch (scorpiones, buthidae) for venom obtention at Instituto Butantan, São Paulo, Brazil. J Venom Anim Toxins incl Trop Dis. 2004;10(1):86–97., 2121. Gopalakrishnakone P, Cheah J, Gwee MC. Black scorpion (Heterometrus longimanus) as a laboratory animal: maintenance of a colony of scorpion for milking of venom for research, using a restraining device. Lab Anim. 1995;29(4):456–8.]. As it was observed, such factors as the size and type of feed, moisture content of soil and temperature of the environment may affect the survival of these scorplings.
Spermatophore and mother scorpion with young brood. A spermatophore attached on the surface of a soil clod found in the container (left) and mother scorpion with its young brood which were delivered during captivity (right)
Extraction of venom by electrical stimulation method
In total, 6.0 mL of venom was obtained from Hottentotta rugiscutis species at monthly intervals for the duration of 18 months (data not shown). The venom was also collected from other species for a short period in order to validate the stimulator and modified restrainer used. The H. rugiscutis and H. tamulus scorpions secreted a transparent watery pre-venom on initial stimulation, whereas on second stimulation, the venom obtained was viscous and whitish in color. But this secretion pattern was not consistently observed in all the scorpions of the particular species stimulated. However,Heterometrus swammerdami secreted relatively more viscous and whitish venom in all the stimulations. The quantity of venom procured from an individual scorpion varied with its size. The average amount of venom secreted from each individual scorpion in a single extraction procedure was ~ 8 μL during the period of first 12 months, but reduced to ~ 5 μL per scorpion for the next six months. Thus, maintaining the scorpions for more than 18 months may not be sufficiently productive in the context of venom yield, which was also noted by Candido and Lucas [1414. Candido DM, Lucas S. Maintenance of scorpions of the genus Tityus Koch (scorpiones, buthidae) for venom obtention at Instituto Butantan, São Paulo, Brazil. J Venom Anim Toxins incl Trop Dis. 2004;10(1):86–97.]. H. swammerdami secretes venom which is less toxic as they are relatively large in size and possess strong pedipalps, thus enabling them to attack the prey without the need of highly toxic venom. In contrast, H. rugiscutis and H. tamulus scorpions, which are small in size and possess relatively weak pedipalps, produce a more highly toxic venom [2222. Muruganathan A. Medicine update: Scorpion envenomation: What is new? Assoc Physicians India. 2013;23:421–3.]. H. rugiscutis is responsible for many envenomations in the region of Chirathagundu village. In an extensive survey of the literature, we found hardly any reports on the lethality of this species. In this context, further studies were carried out only on H. rugiscutis venom.
The stimulator used in the present study for the extraction of venom was more affordable than the one mentioned by Lowe and Farrell [88. Lowe RM, Farrell PM. A portable device for the electrical extraction of scorpion venom. Toxicon. 2011;57(2):244–7.] and can be employed in the field if connected to a small battery. The voltage required to stimulate the scorpions varies according to species and size. The venom from the species Androctonus mauretanicus and Buthus occitanus tunetanus were extracted by stimulating at 12 V, whereas Parabuthus sp., Tityus serrulatus and Heterometrus gravimanus were stimulated at 40 V, 12.5 V and 8 V, respectively [99. Oukkache N, Chgoury F, Lalaoui M, Cano AA, Ghalim N. Comparison between two methods of scorpion venom milking in Morocco. J Venom Anim Toxins incl Trop Dis. 2013;19(5). doi:10.1186/1678-9199-19-5., 1313. Whittemore Jr FW, Keegan HL, Fitzgerald CM, Bryant HA, Flanigan JF. Studies of scorpion antivenins. 2. Venom collection and scorpion colony maintenance. Bull World Health Organ. 1963;28(4):505–11., 1414. Candido DM, Lucas S. Maintenance of scorpions of the genus Tityus Koch (scorpiones, buthidae) for venom obtention at Instituto Butantan, São Paulo, Brazil. J Venom Anim Toxins incl Trop Dis. 2004;10(1):86–97., 1616. du Plessis JL. Collection of venom from southern African scorpions. Toxicon. 2005;45(5):681–2.]. The present study also revealed similar variations. H. rugiscutis and H. tamulus secreted the venom when stimulated at 8 V, while the H. swammerdami secreted the venom only at 10 or 12 V based on species size. The expression time reported in this study (2–3 s) was shorter than the finding of 15 s by Gopalakrishnakone et al. [2121. Gopalakrishnakone P, Cheah J, Gwee MC. Black scorpion (Heterometrus longimanus) as a laboratory animal: maintenance of a colony of scorpion for milking of venom for research, using a restraining device. Lab Anim. 1995;29(4):456–8.]. These variations can also be due to different stimulators and conditions used in those studies.
The restrainer used herein was simple, cheap and effective, which eliminated any possibility of injury to the animal or the handler. The spontaneous expulsion of venom is a concern that was avoided by anesthetizing the scorpion with 5 % CO2 or by placing it in a refrigerator (0 °C) for 3 to 5 minutes prior to venom collection [1313. Whittemore Jr FW, Keegan HL, Fitzgerald CM, Bryant HA, Flanigan JF. Studies of scorpion antivenins. 2. Venom collection and scorpion colony maintenance. Bull World Health Organ. 1963;28(4):505–11., 1616. du Plessis JL. Collection of venom from southern African scorpions. Toxicon. 2005;45(5):681–2.]. However, during this study, proper handling and use of the modified restrainer drastically reduced the problem of spontaneous expulsion of venom. Although the use of thermocol in the restrainer does not cause any problem, in the future it should be replaced with a wooden base to which a petri dish can be fixed with a spring holder to enable an easy restraining process.
Toxicity of scorpion venom
The protein content of venom from Hottentotta rugiscutis and H. tamulus were 0.79 mg and 0.70 mg/mg of lyophilized venom. The only report found until the present on the lethality of H. rugiscutis scorpion venom records an LD50 value of 4.2 mg/kg of body weight via intraperitoneal route [2323. Amir R, Alam JM, Khan JMA. Procoagulant property of venoms of some medically important scorpion species from Sindh Region, Pakistan. Pak J Zool. 1994;26(3):261–3.], but no report was found on the amount of protein present in that venom. In this study, the LD50 of this venom was estimated to be 3.02 mg/kg of body weight via subcutaneous route. Lethality of H. rugiscutis scorpion venom was determined using different doses after which the death or survival ratio was recorded over a 24-hour period (Table 2). The symptoms observed in the experimental animals after venom injection included agitation, hyper-excitability, sweating, paralysis, salivation, squeaking, hunched back movements, convulsions, weakness and death.
Conclusions
The method followed in this study for scorpion maintenance in the laboratory is valuable. The modified restrainer and stimulator are significantly efficient in venom extraction for both smaller and larger scorpions. This was the first report for the presence of H. rugiscutis in Karnataka. Furthermore, the elevated population of H. rugiscutis in the Chirathagundu region presents a threat to human life that requires the development of a therapeutic anti-venom.
Acknowledgments
The authors would like to thank to Dr. Deshabhushan Bastawade, Retired Scientist, Zoological Survey of India, Western Regional Station, Pune, for helping us in the identification of collected scorpions. We are also thankful to UGC, New Delhi, for their funding of this research [grant n.: 40-129/2011 (SR)].
References
-
1Bastawade DB, Jadhav SS, Sharma RM. Scorpionida. Zool Surv India. 2012;4(6):1–16.
-
2Badhe RV, Thomas AB, Deshpande AD, Salvi N, Waghmare A. The action of red scorpion (Mesobuthus tamulus coconsis, Pocock) venom and its isolated protein fractions on blood sodium levels. J Venom Anim Toxins incl Trop Dis. 2007;13(1):82–93.
-
3Kankonkar RC, Kulkurni DG, Hulikavi CB. Preparation of a potent anti-scorpion-venom serum against the venom of red scorpion (Buthus tamulus). J Postgrad Med. 1998;44(4):85–92.
-
4Bawaskar HS, Bawaskar PH. Scorpion sting: Update. J Assoc Physicians India. 2012;60:46–55.
-
5Joseph B, George J. Scorpion toxins and its applications. Int J Toxicol Pharmacol Res. 2012;4(3):57–61.
-
6Zlotkin E, Sholov AS. A simple device for collecting scorpion venom. Toxicon. 1969;7(4):331–2.
-
7Nisani Z, Dunbar SG, Hayes WK. Cost of venom regeneration in Parabuthus transvaalicus (Arachnida: Buthidae). Comp Biochem Physiol A Mol Integr Physiol. 2007;147(2):509–13.
-
8Lowe RM, Farrell PM. A portable device for the electrical extraction of scorpion venom. Toxicon. 2011;57(2):244–7.
-
9Oukkache N, Chgoury F, Lalaoui M, Cano AA, Ghalim N. Comparison between two methods of scorpion venom milking in Morocco. J Venom Anim Toxins incl Trop Dis. 2013;19(5). doi:10.1186/1678-9199-19-5.
-
10Sureshan PM, Bastawade DB, Radhakrishnan C. Taxonomic studies on a collection of scorpions (Scorpiones: Arachnida) from Western Ghats in Kerala, India with two new distribution records. Zoos Print J. 2007;22(12):2903–8.
-
11Veronika K, Akilan K, Murugananthan A, Eswaramohan T. Diversity and identification key to the species of scorpions (Scorpiones: Arachnida) from Jaffna Peninsula, Sri Lanka. J Entomol Zool Stud. 2013;1(5):70–7.
-
12de Roodt AR, Coronas FI, Lago N, Gonzalez ME, Laskowicz RD, Beltramino JC, et al. General biochemical and immunological characterization of the venom from the scorpion Tityus trivittatus of Argentina. Toxicon. 2010;55(2-3):307–19.
-
13Whittemore Jr FW, Keegan HL, Fitzgerald CM, Bryant HA, Flanigan JF. Studies of scorpion antivenins. 2. Venom collection and scorpion colony maintenance. Bull World Health Organ. 1963;28(4):505–11.
-
14Candido DM, Lucas S. Maintenance of scorpions of the genus Tityus Koch (scorpiones, buthidae) for venom obtention at Instituto Butantan, São Paulo, Brazil. J Venom Anim Toxins incl Trop Dis. 2004;10(1):86–97.
-
15Deoras PJ, Vad NE. The milking of scorpions. Toxicon. 1962;1:41.
-
16du Plessis JL. Collection of venom from southern African scorpions. Toxicon. 2005;45(5):681–2.
-
17Khoobdel M, Zahraei-Salehi T, Nayeri-Fasaei B, Khosravi M, Omidian Z, Motedayen MH, et al. Purification of the immunogenic fractions and determination of toxicity in Mesobuthus eupeus (Scorpionida: Buthidae) venom. J Arthropod Borne Dis. 2013;7(2):139–46.
-
18Randhawa MA. Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad. 2009;21(3):184–5.
-
19Rubio M. Scorpions: Everything about purchase, care, feeding, and housing. Hauppauge: Barrons Educational Series; 2008.
-
20Sissom WD, Polis GA, Watt DD. Field and laboratory methods. In: Polis GA, editor. The Biology of Scorpions. Stanford: Stanford University press; 1990. p. 449-450.
-
21Gopalakrishnakone P, Cheah J, Gwee MC. Black scorpion (Heterometrus longimanus) as a laboratory animal: maintenance of a colony of scorpion for milking of venom for research, using a restraining device. Lab Anim. 1995;29(4):456–8.
-
22Muruganathan A. Medicine update: Scorpion envenomation: What is new? Assoc Physicians India. 2013;23:421–3.
-
23Amir R, Alam JM, Khan JMA. Procoagulant property of venoms of some medically important scorpion species from Sindh Region, Pakistan. Pak J Zool. 1994;26(3):261–3.
-
Ethics committee approvalAll the experiments were performed according to the guidelines approved by the Institutional Animal Ethics Committee (National College of Pharmacy, Shivamogga, Karnataka; no: NCP/IAEC/CL/206/01).
Publication Dates
-
Publication in this collection
2015
History
-
Received
16 June 2015 -
Reviewed
30 Nov 2015 -
Accepted
4 Dec 2015