Abstract
Background:
Propolis exhibits huge potential in the pharmaceutical industry. In the present study, its effects were investigated on dendritic cells (DCs) stimulated with a tumor antigen (MAGE-1) and retinoic acid (RA) and on T lymphocytes to observe a possible differential activation of T lymphocytes, driving preferentially to Th1 or Treg cells.
Methods:
Cell viability, lymphocyte proliferation, gene expression (T-bet and FoxP3), and cytokine production by DCs (TNF-α, IL-10, IL-6 and IL-1β) and lymphocytes (IFN-γ and TGF-β) were analyzed.
Results:
MAGE-1 and RA alone or in combination with propolis inhibited TNF-α production and induced a higher lymphoproliferation compared to control, while MAGE-1 + propolis induced IL-6 production. Propolis in combination with RA induced FoxP3 expression. MAGE-1 induced IFN-γ production while propolis inhibited it, returning to basal levels. RA inhibited TGF-β production, what was counteracted by propolis.
Conclusion:
Propolis affected immunological parameters inhibiting pro-inflammatory cytokines and favoring the regulatory profile, opening perspectives for the control of inflammatory conditions.
Keywords:
Dendritic cells; T CD4+ cells; MAGE-1; Retinoic acid; Propolis; Immunomodulation
Background
Propolis is produced by honeybees from different parts of plants and presents possible applications in the pharmaceutical and food industry [11. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011 Jan 27;133(2):253-60. -55. Ripari N, Sartori AA, Honorio MS, Conte FL, Tasca KI, Santiago KB, Sforcin JM. Propolis antiviral and immunomodulatory activity: a review and perspectives for COVID-19 treatment. J Pharm. Pharmacol. 2021 Feb 8;73(3):281-99.]. It has been used in folk medicine for centuries due to its medicinal properties. Incas used propolis as an antipyretic agent; Romans and Greeks used it for treating wounds [66. Weis WA, Ripari N, Conte FL, Honorio MS, Sartori AA, Matucci RH, Sforcin JM. An overview about apitherapy and its clinical applications. Phytomed Plus. 2022 May;2(2):100239.]. There are different types of propolis in Brazil such as green, red and brown and their pharmacological properties may vary according to their chemical composition, which is complex and depends on the botanical source and geographical location where they were produced. Propolis composition may include aromatic aldehydes, amino acids, fatty acids, diterpenes, sesquiterpenes, esters, lignans, alcohols, vitamins, and minerals [22. Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res. 2016 Jun;30(6):894-905., 66. Weis WA, Ripari N, Conte FL, Honorio MS, Sartori AA, Matucci RH, Sforcin JM. An overview about apitherapy and its clinical applications. Phytomed Plus. 2022 May;2(2):100239.].
Propolis anti-inflammatory action has been investigated both in vitro and in vivo [77. Tanaka M, Okamoto Y, Fukui T, Masuzawa T. Suppression of interleukin 17 production by Brazilian propolis in mice with collagen-induced arthritis. Inflammopharmacology. 2012 Feb;20(1):19-26., 88. Piñeros AR, de Lima MHF, Rodrigues T, Gembre AF, Bertolini TB, Fonseca MD, Berretta AA, Ramalho LNZ, Cunha FQ, Hori JI, Bonato VLD. Green propolis increases myeloid suppressor cells and CD4+ Foxp3+ cells and reduces Th2 inflammation in the lungs after allergen exposure. J Ethnopharmacol. 2020 Apr 24;252:112496.]. Propolis may exert pro- or anti-inflammatory activity depending on concentration, intake period and experimental conditions, affecting mechanisms involved in the inflammatory/immune response such as neutrophil adhesion and transmigration, cytokines, chemokines, prostaglandin E2, C-reactive protein, and signaling pathways [99. Hori JI, Zamboni DS, Carrão DB, Goldman GH, Berretta AA. The inhibition of inflammasome by Brazilian propolis (EPP-AF). Evid Based Complement Alternat Med. 2013;2013:1-11.-1212. Franchin M, Freires IA, Lazarini JG, Nani BD, Cunha MG, Colon DF, Alencar SM, Rosalen PL. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur J Med Chem. 2018;153:49-55.].
Innate immunity is involved in the recognition of pathogens, leading to inflammatory responses. The sensing of microbes by receptors expressed in antigen presenting cells (APCs) such as dendritic cells (DCs) induces the activation of adaptive immunity [1313. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343-53.].
The acquired immune response is regulated by cytokines that determine the lymphocyte profile generated after T cell activation and differentiation: Th1 cells are characterized by differentiation of T naïve cells in the presence of IL-12, with activation of T-bet, STAT-1 and STAT-4 transcription factors and IFN-γ production. These cells enhance the microbicide activity of macrophages, the migration of leucocytes and the production of pro-inflammatory cytokines, promoting protection against tumor cells and intracellular microorganisms. Th2 cells, characterized by STAT-3, GATA-3 and IL-4, promote an immune response against extracellular parasites. Th17 cells are involved in eliminating extracellular bacterial and fungal pathogens and are classified by RORc and IL-17. T regulatory (Treg) cells control the immune response against self and non-self-antigens, inflammation, autoimmune diseases, allergy, asthma and pathogen-induced immunopathology, and feto-maternal tolerance. The main markers of Treg cells are CD25, CTLA-4, GITR, LAG-3, CD127, FoxP3, TGF-β and IL-10 [1414. Corthay A. How do regulatory T cells work? Scand. J Immunol. 2009;70:326-36.-1616. Cantrell D. Signaling in lymphocyte activation. Cold Spring Harb Perspect Biol. 2015;7:a018788.].
The modulation of the immune response has been an approach for treating several diseases, and natural products have been investigated for their immunomodulatory action [1717. Yao S, Zhu Y, Chen L. Advances in targeting cell surface signaling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130-46.-1919. Di Sotto A, Vitalone A, Di Giacomo S. Plant-derived nutraceuticals and immune system modulation: an evidence-based overview. Vaccines. 2020;8:468.]. Our research group has been studying propolis for almost 30 years [11. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011 Jan 27;133(2):253-60. , 22. Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res. 2016 Jun;30(6):894-905., 66. Weis WA, Ripari N, Conte FL, Honorio MS, Sartori AA, Matucci RH, Sforcin JM. An overview about apitherapy and its clinical applications. Phytomed Plus. 2022 May;2(2):100239., 2020. Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol. 2007;113(1):1-14.].
Propolis effects on APCs and other cells involved in the immune response have been documented [1111. Santiago KB, Conti BJ, Cardoso EO, Golim MA, Sforcin JM. Immunomodulatory/anti-inflammatory effects of a propolis-containing mouthwash on human monocytes. Pathog Dis. 2016;74:ftw081., 2121. Simões LM, Gregório LE, da Silva Filho AA, de Souza ML, Azzolini AE, Bastos JK, Lucisano-Valim YM. Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. J Ethnopharmacol. 2004 Sep;94(1):59-65.-2323. Conti BJ, Santiago KB, Cardoso EO, Freire PP, Carvalho RF, Golim MA, Sforcin JM. Propolis modulates miRNAs involved in TLR-4 pathway, NF-κB activation, cytokine production and in the bactericidal activity of human dendritic cells. J Pharm Pharmacol. 2016 Dec;68(12):1604-12.]. Here, we sought to advance in the knowledge about propolis immunomodulatory effects on DCs and T cells, assuming that it may modulate antigen presentation and T lymphocytes activation. Melanoma-associated antigen 1 (MAGE-1) present in melanoma and other tumors [2424. Büeler H, Mulligan RC. Induction of antigen-specific tumor immunity by genetic and cellular vaccines against MAGE: enhanced tumor protection by coexpression of granulocyte-macrophage colony-stimulating factor and B7-1. Mol Med. 1996 Sep;2(5):545-55.] was used as an antigen, leading to Th1 cells activation. Retinoic acid (RA), a vitamin A metabolite, promotes expansion of human Tregs in vitro and prevents them from converting to Th1 or Th17 cells, sustaining Foxp3 and other Treg-related markers and their suppressive action [2525. Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, Brand DD, Ryffel B, Horwitz DA, Quismorio FP, Liu Z, Li B, Olsen NJ, Zheng SG. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2014 Aug 19;111(33):E3432-40.]. Lymphocyte proliferation, transcription factors activation (T-bet and FoxP3) and cytokine production by DCs (TNF-α, IL-10, IL-6 and IL-1β) and T lymphocytes (IFN-γ and TGF-β) were analyzed, in order to investigate whether propolis could drive preferentially to a differential activation profile such as Th1 or Treg.
Methods
Propolis, MAGE-1, retinoic acid, and combinations
Green propolis was produced by Africanized honeybees (Apis mellifera L.) in the Beekeeping Section (UNESP, Campus Botucatu, Brazil) and kept at -20°C. The same sample has been used in all assays performed by our group, preparing fresh extracts. Its composition was analyzed by gas chromatography-mass spectrometry (GC-MS) [2626. Bankova V, Boudourova-Krasteva G, Popov S, Sforcin JM, Funari SRC. Seasonal variations of the chemical composition of Brazilian propolis. Apidologie 1998;29(4):361-7.]; in addition, a new chromatographic analysis of the same frozen sample was performed years later, demonstrating no effect of time and freezing on its chemical composition [2727. Conti BJ, Bankova V, Sforcin JM. Chemical composition of the same Brazilian propolis sample analysed in 1997 and in 2012: no freezing effect. Nat Prod Commun. 2015 Jul;10(7):1279-80.].
Propolis was ground and 30% ethanolic extracts were prepared using 70% ethanol [2828. Sforcin JM, Orsi RO, Bankova V. Effect of propolis, some isolated compounds and its source plant on antibody production. J Ethnopharmacol. 2005 Apr 26;98(3):301-5.]. Its dry weight was calculated (110 mg/mL). Propolis was diluted in RPMI 1640 (Cultilab, Brazil) supplemented with 10% fetal bovine serum (FBS) to obtain 5 μg/mL.
Human MAGE-1 (Enzo Life Science, USA) was diluted in RPMI 1640 to obtain 10 μg/mL. RA (Cayman Chemical, USA) was diluted in dimethyl sulfoxide (DMSO) and then in RPMI to obtain 10-7 M.
The combinations of propolis with MAGE-1 and RA were prepared according to previous standardization in our laboratory.
Healthy blood donors and monocyte isolation
Venous blood was obtained from five healthy volunteers’ donors (aged between 20 and 40 years, both genders, non-smokers, not sick or using any type of medication) and centrifuged using Ficoll-Paque (GE Healthcare Bio-Sciences, Sweden) to obtain the peripheral blood mononuclear cells (PBMC). All subjects signed an informed consent for the study, which was approved by the Ethics Committee of Botucatu Medical School, UNESP (CAAE: 42600915.0.0000.5411).
Monocytes and lymphocytes were isolated by the negative magnetic selection technique “MACS: magnetic-activated cell sorting” (Miltenyi Biotec Inc., USA). Monocytes were used immediately for DCs differentiation and lymphocytes were cryopreserved in RPMI containing 10% FBS + 10% DMSO and stored in liquid nitrogen.
CD14+ and CD4+ T cells phenotyping
CD14+ and CD4+ cells were transferred to cytometry tubes (BD Becton Dickinson and Company, USA) and centrifuged at 650 g for 10 min. After discarding the supernatant, cells were incubated with monoclonal antibodies (mAbs - Biolegend, USA) anti-CD14 conjugated with PerCP-CY5.5 and anti-CD4 conjugated with PerCP-CY5.5 (0.3 μL) for 30 min. A control tube (autofluorescence) with no labeled cells and an isotypic control tube were included in each test. Cells were analyzed in a flow cytometer model FACS CaliburTM (BD Becton Dickinson and Company, USA), acquiring 50.000 events.
DC generation and phenotyping
DCs were generated from monocytes isolated from PBMC. Purified monocytes (1 × 106 cells/mL) were resuspended in RPMI 1640 containing human recombinant IL-4 (80 ng/mL) and GM-CSF (80 ng/mL) (R&D Systems, USA) for 7 days at 37°C and 5% CO2 [2929. Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003 Jan 6;197(1):7-17., 3030. Landi A, Babiuk LA, Hurk SVDLVD. Dendritic cells matured by a prostaglandin E2-containing cocktail can produce high levels of IL-12p70 and are more mature and Th1-biased than dendritic cells treated with TNF-α or LPS. Immunobiology. 2011 Jun;216(6):649-62.]. Then, cells were incubated with mAbs (Biolegend, USA) anti-CD14-PerCP-Cy 5.5 (0.3 μL), anti-CD1a-FITC (1 μL), anti-CD83-PE (1 μL) and anti-CD11c-APC (1 μL) for 30 min. A Fluorescence Minus One (FMO) control was included.
This phenotyping protocol was performed to assure the cell differentiation and analyzed in a flow cytometer model FACS CaliburTM (BD Becton Dickinson and Company, USA). A total of 50.000 events were acquired and the expression of following cell surface markers was analyzed: CD14low/CD1ahigh/CD11chigh/CD83low [3131. Polancec DS, Kos VM, Banjanac M, Vrancic M, Cuzic S, Belamaric D, Parnham MJ, Polancec D, Haber VE. Azithromycin drives in vitro GM-CSF/IL-4-induced differentiation of human blood monocytes toward dendritic-like cells with regulatory properties. J Leukoc Biol. 2012 Feb;91(2):229-43.].
DCs were incubated with propolis alone or in combination with MAGE-1 and RA for 48 h.
Cell viability
Cell viability was performed using the 3-(4,5-dimethyl-thiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT - Sigma-Aldrich, USA) colorimetric assay.
DCs were incubated with the stimuli in a final volume of 100 μL. Supernatants were removed and 100 μL of MTT (1 mg/mL) were added to the culture cells. After 3 h, MTT was removed and 100 μL of DMSO (Sigma-Aldrich, USA) was added to dissolve the formazan salt. The absorbance was recorded at 540 nm and the percentage of cell viability was calculated using the formula: [(OD test/OD control) x 100].
Cytokine production by DCs
In an attempt to investigate propolis modulatory effects, the production of pro- and anti-inflammatory cytokines was analyzed after DCs incubation with the stimuli. The supernatants were harvested from the cell cultures for TNF-α, IL-6, IL-1β and IL-10 quantitation by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instruction (R&D Systems, USA). Lipopolysaccharide 1 µg/mL (isolated from Escherichia coli O26:B6 - Sigma-Aldrich, USA) was used as a positive control. The absorbance was determined at 450 nm using a microplate reader (ELx800, BioTek, Germany).
T CD4+ cell proliferation
Isolated CD4+ cells were labeled with carboxy-fluorescein succinimidyl ester (CFSE) (Cell-Trace CFSE Proliferation Kit, Molecular Probes, Invitrogen, USA) to monitor lymphoproliferation. For the co-culture assays, DCs incubated with MAGE-1 or RA simultaneously or not with propolis for 48 h were incubated with CFSE-labeled autologous CD4+ T lymphocytes (ratio DCs/lymphocytes = 1/10) for 120 h. Phytohemagglutinin (PHA - 2.5 μg/mL) was used as a positive control for cell proliferation and cells without any marking (autofluorescence) were used as a negative control, in addition to FMO control under the same conditions. After incubation, the lymphocyte proliferation was evaluated in a flow cytometer model FACS CaliburTM (BD Becton Dickinson and Company, USA), and a total of 50.000 events were acquired.
Transcription factor gene expression
T-bet and FoxP3 expression by T CD4+ cells was evaluated by real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR), using the 7300 Real Time PCR System (Applied Biosystems, USA).
After treating DCs with propolis alone or in combination with the stimuli by 48 h, cells were incubated with lymphocytes by 120 h. The total RNA was extracted from lymphocytes using the RNeasy Mini Kit (Qiagen, The Netherlands) and treated with RQ1 RNase-Free DNase (Promega, USA). cDNA synthesis was performed using the ProtoScript II Reverse Transcriptase kit (BioLabs, USA). The GoTaq-qPCR Master Mix (Promega, USA) was used and Table 1 presents the primers sequence. Each reaction was performed in triplicate and the conditions were: 50°C/2 min, 95°C/10 min for initial denaturation, 40 cycles at 95°C/15s and 60°C/60s followed by the melting curve.
The expression values of the transcripts were normalized using the glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The differential expression of the selected genes was performed using a standard-curve [3232. Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005 Mar 21;6:62.]. All samples were standardized in relation to an RNA sample using a relative value of 100.
Intracytoplasmic cytokine analysis
Six hours before ending the incubation of the co-cultures, cells were treated with brefeldin A (Biolegend, USA) in order to prevent the release of cytokines from the cell cytoplasm.
Cells were labeled with anti-CD4 conjugated to PerCP/Cy5.5 (OKT4 clone - Biolegend, USA) which allowed the selection of the gate of only the CD4+ lymphocytes and, for Treg cells, with anti-CD25 conjugated with APC (clone M-A251 - Biolegend). Cells were incubated for 30 min in the dark at 4°C and then centrifuged for 10 min at 650 g. After, the supernatant was discarded and cells were incubated for 15 min with 100 μL of the solution A of Fix & Perm Cell Fixation and Permeabilization kit (Nordic MUbio, The Netherlands). After washing with ISOTON, cells were centrifuged at 605 g for 10 min, the supernatant was discarded and 100 μL of solution B of the kit Fix & Perm containing anti-IFN-γ conjugated with PE (clone B27 - Biolegend) and anti-TGF-β1 conjugated with PE (clone TW4-2F8 - Biolegend). After incubation, the cells were analyzed by flow cytometry and, for each test, an isotypic control with the respective test fluorochromes, an autofluorescent control and FMO controls were included. The analyses were performed using the flow cytometer model FACS CaliburTM (BD Becton Dickinson and Company, USA) and the FlowJo software vX.0.7. 50.000 acquisition events were standardized per sample and the population of interest was optimized by establishing a gate based on size (FSC) and granularity (SSC) parameters. The results were expressed as the percentage of CD4 positive cells expressing IFN-γ or TGF-β1.
Statistical analysis
Data were analyzed using the Graph Pad statistical software (Graph Pad Prisma, USA). Analysis of variance (ANOVA) and Dunnett’s test were employed (p < 0.05). Data were expressed as the mean ± standard deviation of 5 individuals. A p value of less than 0.05 was considered significant.
Results
DC phenotyping and viability
DCs were properly generated from monocytes, presenting the typical cell markers CD11chigh, CD1ahigh, CD83low and CD14low (Figure 1).
To verify a possible cytotoxic effect, DCs were incubated with propolis and the stimuli (MAGE-1 and RA) simultaneously or not and cell viability was assessed (Figures 2A and 2B). Neither the treatments nor the solvents (propolis: 70% ethanol - 0.013%; RA - DMSO 0.0002%) affected cell viability (data not shown).
Dendritic cell phenotype after monocyte incubation with IL-4 and GM-CSF. (A) Dot plot related to size (FSC-H) x granularity (SSC-H). Histograms represent cell surface markers: (B) CD11chigh, (C) CD1ahigh, (D) CD83low and (E) CD14low.
Viability (%) of dendritic cells (1 × 106cells/mL) after 48 h incubation with RPMI 1640 (control - C), propolis (P - 5 μg/mL), (A) MAGE-1 (M - 10 μg/mL), (B) retinoic acid (RA - 10-7M) and their combination. Data represent mean and standard deviation of five subjects (p > 0.05).
Cytokine production by DCs
MAGE-1 and RA alone or in combination with propolis inhibited TNF-α production by DCs compared to control (Figures 3A and 3B, respectively).
MAGE-1 + propolis seemed to induce slightly IL-10 production, although not significantly (Figure 3C). RA alone or in combination with propolis exerted no effect on IL-10 (Figure 3D).
MAGE-1 alone or in combination with propolis induced IL-6 production, while RA did not affect it (Figures 3E and 3F).
No differences were seen in IL-1β production; however, MAGE-1 showed a tendency to increase it, whereas the combination with propolis maintained IL-1β levels similar to control (Figures 3G and 3H).
Cytokine production (pg/mL) by dendritic cells (1 × 106cells/mL) after 48 h incubation with RPMI 1640 (control - C), propolis (P - 5 μg/mL), (A, C, E, G) MAGE-1 (M - 10 μg/mL), (B, D, F, H) retinoic acid (RA - 10-7M), their combination, and LPS (1 µg/mL). Data represent mean and standard deviation of five subjects. Significantly different from control: *(p < 0.05); **(p < 0.01); ***(p < 0.001). Significantly different from the respective combination: #(p < 0.05); ##(p < 0.01); ###(p < 0.001).
T lymphocyte proliferation
A possible influence of propolis and stimuli on lymphoproliferation was assessed. Representative Dot Plots of lymphocyte proliferation are shown in Figure 4 (panels A, B, C and D). A higher percentage of proliferation was seen after co-culture of lymphocytes with DCs treated with propolis, MAGE-1 and RA, alone or in combination compared to untreated DCs (Figures 4E and 4F).
Representative dot plots of lymphocyte proliferation after 120 h of co-culture with autologous dendritic cells. (A) Gate of lymphocytes by size (FSC-H) x granularity (SSC-H). (B) Gate of CD4+ cells. (C) Proliferation of control lymphocytes (cells incubated with RPMI). (D) Proliferation of lymphocytes incubated with the positive control (PHA - 2.5 μg/mL). Percentage (%) of lymphocytes (1 × 106cells/mL) proliferation after 120 h of co-culture with autologous dendritic cells treated only with RPMI 1640 (control - C), PHA (2.5 μg/mL), propolis (P - 5 μg/mL), (E) MAGE-1 (M - 10 μg/mL), (F) retinoic acid (RA - 10-7M) and their combination for 48 h. Data represent mean and standard-deviation (n = 5). Significantly different from control: *(p < 0.05); **(p < 0.01); ***(p < 0.001).
Transcription factor expression and cytokine production
Since transcription factors and cytokines are signatures of T cell subsets, we analyzed T-bet mRNA levels and the percentage of lymphocytes expressing IFN-γ, to observe the effects of propolis and stimuli on the differentiation of Th1 cells. Propolis and MAGE-1 did not affect T-bet expression (p > 0.05) (Figure 5A). MAGE-1 induced IFN-γ production by T CD4+ cells (p < 0.01), while propolis led it to basal levels (Figure 5B).
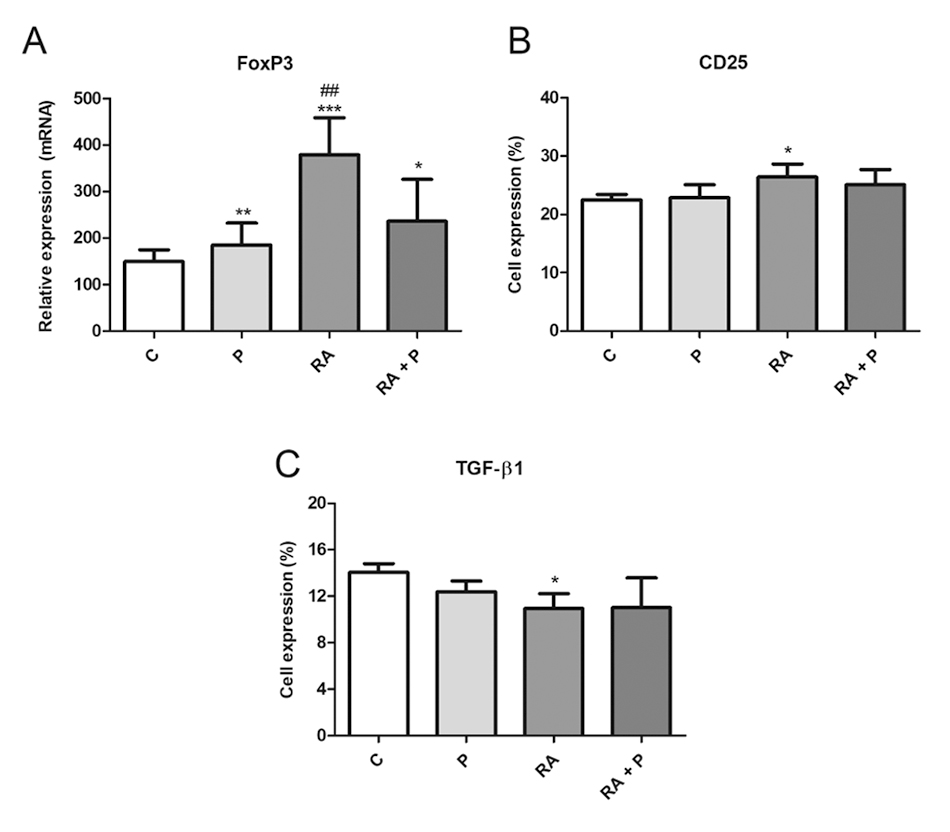
To evaluate the activation status of Treg cells, CD25 and FoxP3 were examined. TGF-β1 was analyzed to verify the functionality of the cell population in our culture. RA induced FoxP3 and CD25 expression (Figures 6A and 6B, respectively) and inhibited TGF-β1 production (Figure 6C). Propolis alone or in combination with RA stimulated FoxP3 expression (Figure 6A).
(A) T-bet relative expression and (B) percentage (%) of lymphocytes expressing IFN-γ after 120 h of co-culture with autologous dendritic cells treated with RPMI 1640 (control - C), MAGE-1 (M - 10 μg/mL), propolis (P - 5 μg/mL) or their combination. Data represent mean and standard deviation (n = 5). Significantly different from control: **(p < 0.01). Significantly different from M + P: #(p < 0.05).
(A) FoxP3 relative expression and percentage (%) of lymphocytes expressing (B) CD25 and (C) TGF-β1 after 120 h of co-culture with autologous dendritic cells treated with RPMI 1640 (control - C), retinoic acid (A - 10-7 M), propolis (P - 5 μg/mL) or their combination. Data represent mean and standard deviation (n = 5). Significantly different from control: *(p < 0.05), **(p < 0.01), ***(p < 0.001). Significantly different from RA + P: ##(p < 0.01).
Discussion
DCs are professional APCs, linking innate and adaptive immunity with the additional activation of naïve T lymphocytes and determining the balance between Th1, Th2, Th17 and Treg cells [3333. De Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Sem Immunopathol. 2005 Jan;26(3):289-307.]. Monocyte-derived DCs are an interesting model to investigate the function of DCs in vitro [3434. O’Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci. 2015 Nov;72(22):4309-25.]. GM-CSF and IL-4 lead to the differentiation of monocytes in DCs with immature phenotype expressing high levels of CD11c and CD1a, and declining the levels of adhesion molecules as LFA-1, ICAM-1 and LFA-3, class II major histocompatibility complex (HLA-DR), co-stimulatory molecules (CD40, B7-1/CD80, B7-2/CD86) and CD14 [3535. Liu XZ, Zhan L, Xu F, Ma D, Li Z, Guo N, Li X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J Immunol. 2010 Dec 15;185(12):7244-51.].
Monocytes were properly differentiated into immature DCs and treated with propolis, MAGE-1 and RA, which did not affect cell viability. Likewise, other studies have shown that propolis or its compounds exert no cytotoxic action on DCs [2323. Conti BJ, Santiago KB, Cardoso EO, Freire PP, Carvalho RF, Golim MA, Sforcin JM. Propolis modulates miRNAs involved in TLR-4 pathway, NF-κB activation, cytokine production and in the bactericidal activity of human dendritic cells. J Pharm Pharmacol. 2016 Dec;68(12):1604-12., 3636. Kim SI, Jeong YI, Jung ID, Lee JS, Lee CM, Yoon MS, Seong EY, Kim JI, Lee JD, Park IM. p-Coumaric acid inhibits indoleamine 2, 3-dioxygenase expression in murine dendritic cells. Int Immunopharmacol. 2007 Jun;7(6):805-15., 3737. Huang RY, Yu YL, Cheng WC, Ouyang CN, Fu E, Chu CL. Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol. 2010 Jun 15;184(12):6815-21.].
Regarding the innate immunity and the production of pro-inflammatory cytokines by DCs, MAGE-1 and RA alone or in combination with propolis inhibited its production. MAGE-1 showed a tendency to increase IL-1β production, while the combination with propolis maintained basal levels. Propolis and the stimuli did not affect IL-6 production. The combination propolis + MAGE-1 showed a tendency to increase the production of the anti-inflammatory cytokine IL-10. In agreement with these findings, propolis exerted an anti-inflammatory action in the production of cytokines by human monocytes, decreasing TNF-α and IL-6 levels in combination with MAGE-1 and RA, and increasing IL-10 production in combination with MAGE-1, what indicated that propolis potentially affected innate immunity by downmodulating the pro-inflammatory activity of monocytes [3838. Conte FL, Santiago KB, Conti BJ, Cardoso EO, Oliveira LPG, Feltran GS, Zambuzzi WF, Golim MA, Cruz MT, Sforcin JM. Propolis from southeastern Brazil produced by Apis mellifera affects innate immunity by modulating cell marker expression, cytokine production and intracellular pathways in human monocytes. J Pharm Pharmacol. 2021 Mar 4;73(2):135-44.].
Besides the specific nature of each antigen, the role of DCs driving the immune response is essential to define the signals that will be communicated to naïve T lymphocytes, inducing apoptosis, anergy, tolerance or activation of Th1, Th2, Th17 or a Treg profile [3939. De Lastic AL, Rodi M, Mouzaki A. Effect of dendritic cell state and antigen-presentation conditions on resulting T-cell phenotypes and Th cytokine profiles. Immunobiology. 2016 Aug;221(8):862-70.]. Regarding adaptive immunity, our findings are in agreement with these authors, who evaluated MAGE-3 and the activation of Th1, Th2 or Th17 profiles. After DCs incubation with this antigen, a strong polarization was seen towards the Th1 profile.
There was a higher proliferation of lymphocytes after coculture with DCs treated with propolis, MAGE-1 and RA than control. In contrast, 9-cis RA (a RA derivative) inhibited the lymphoproliferation induced by DCs, associated to reduced IFN-y levels [4040. Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, de Madariaga A, Domingo JC. 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator-activated receptor γ blocks some of the 9cRA activities, and precludes them to mature phenotype development. J Immunol. 2007 May 15;178(10):6130-9.].
MAGE-1 and MAGE-3 are clinically relevant antigens expressed in human melanomas and other tumors, but not in normal tissues except testis [2424. Büeler H, Mulligan RC. Induction of antigen-specific tumor immunity by genetic and cellular vaccines against MAGE: enhanced tumor protection by coexpression of granulocyte-macrophage colony-stimulating factor and B7-1. Mol Med. 1996 Sep;2(5):545-55.]. Here, MAGE-1 increased IFN-y production but this effect was prevented by propolis, suggesting that it may inhibit the Th1 profile. In fact, Okamoto et al. [4141. Okamoto Y, Hara T, Ebato T, Fukui T, Masuzawa T. Brazilian propolis ameliorates trinitrobenzene sulfonic acid-induced colitis in mice by inhibiting Th1 differentiation. Int Immunopharmacol. 2013 Jun;16(2):178-83.] demonstrated that murine spleen cells treated with Brazilian propolis inhibited the generation of Th1 cells, reducing T-bet expression and IFN-y production. Additionally, BALB/c mice fed with propolis after induction of colitis had a lower Th1 cell-mediated inflammatory response and low IFN-y levels.
Inhibition of Th1 profile may be associated with an impaired antitumoral immune response. Nonetheless, other cells may attack tumor cells, such as natural killer cells, macrophages and T CD8+ lymphocytes. Mice with metastasis treated with propolis exhibited activation of T CD8+ cells, suggesting its effect on the antitumoral immune response. The antitumor activity of propolis in vivo may be associated to its immunomodulatory effect and the activation of macrophage and T CD8+ cells [4242. Orsolic N, Basic I. Immunomodulation by water-soluble derivative of propolis: a factor of antitumor reactivity. J Ethnopharmacol. 2003 Feb;84(2-3):265-73., 4343. Orsolic N, Saranovic AB, Basic I. Direct and indirect mechanism(s) of antitumour activity of propolis and its polyphenolic compounds. Planta Med. 2006 Jan;72(1):20-7.].
On the other hand, Th1 cells may exert an inflammatory response causing a pathologic condition as observed in autoimmune diseases. Our findings and those of the literature highlight the potential of propolis in controlling inflammatory processes.
Treg cells exert a critical role in inducing and maintaining the peripheral tolerance and antigen-induced inflammation. These cells are typically immunosuppressive due to the production of TGF-β and IL-10, blocking T cell activation and function. TGF-β suppresses target cells while IL-10 inhibits the activation of APCs and the effects of IFN-y, controlling inflammatory responses [1515. Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harb. Perspect Biol. 2012;4:a007021., 4444. Bono MR, Tejon G, Flores-Santibañez F, Fernandez D, Rosemblatt M, Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients. 2016 Jun;8(6):E349., 4545. Namdari H, Izad M, Rezaei F, Amirghofran Z. Differential regulation of CD4+ T cell subsets by silymarin in vitro and in ovalbumin immunized mice. Daru. 2018 Dec;26(2):215-27.]. RA is a vitamin A metabolite that impairs the conversion of Treg cells into a Th1 or Th17 profile, maintaining FoxP3 expression. Propolis induced Foxp3 expression without affecting CD25 expression and TGF-β production. Propolis + RA induced the expression of Foxp3 and slightly that of CD25 nonsignificantly. RA inhibited TGF-β production, which was counteracted by propolis. This indicates that propolis leads to the activation of a regulatory profile, which has been observed both in vitro and in vivo [77. Tanaka M, Okamoto Y, Fukui T, Masuzawa T. Suppression of interleukin 17 production by Brazilian propolis in mice with collagen-induced arthritis. Inflammopharmacology. 2012 Feb;20(1):19-26., 88. Piñeros AR, de Lima MHF, Rodrigues T, Gembre AF, Bertolini TB, Fonseca MD, Berretta AA, Ramalho LNZ, Cunha FQ, Hori JI, Bonato VLD. Green propolis increases myeloid suppressor cells and CD4+ Foxp3+ cells and reduces Th2 inflammation in the lungs after allergen exposure. J Ethnopharmacol. 2020 Apr 24;252:112496., 4141. Okamoto Y, Hara T, Ebato T, Fukui T, Masuzawa T. Brazilian propolis ameliorates trinitrobenzene sulfonic acid-induced colitis in mice by inhibiting Th1 differentiation. Int Immunopharmacol. 2013 Jun;16(2):178-83.].
Treg cells play an important role in infectious diseases, tumors and periodontitis. In HIV-infected patients, disease progression is directly related to immune hyperactivation, and in these cases there is a reduction in the number and function of Treg cells. On the other hand, studies with Treg cells in malignant neoplasms suggested that the increased activity of these cells is associated with an impaired antitumor immune response. Thus, inhibition of Treg cell function could have positive results as a therapeutic strategy for the treatment of cancer [4646. Melo KM, Carvalho BTC. Células T regulatórias: mecanismos de ação e função nas doenças humanas. Rev Bras Alergia Imunopatol. 2009 Set-Out;32(5):184-8.]. Regarding periodontitis, Cafferata et al. [4747. Cafferata EA, Jerez A, Vernal R, Monasterio G, Pandis N, Faggion Jr CM. The therapeutic potential of regulatory T lymphocytes in periodontitis: a systematic review. J Periodontal Res. 2018 Nov 25;54(3):207-17.] reported that an approach for treating periodontitis would be an increase in the number of Treg cells or in the levels of anti-inflammatory cytokines such as IL-10 and TGF-β1 produced in part by these cells.
The interest in the therapeutic applications of propolis is expressive [22. Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res. 2016 Jun;30(6):894-905., 44. Conte FL, Tasca KI, Santiago KB, Cardoso EO, Romagnoli GG, Golim MA, Braz AMM, Berretta AA, Souza LR, Sforcin JM. Propolis increases Foxp3 expression and lymphocyte proliferation in HIV-infected people: a randomized, double blind, parallel-group and placebo-controlled study. Biomed Pharmacother. 2021 Oct;142:111984., 4848. Zabaiou N, Fouache A, Trousson A, Baron S, Zellagui A, Lahouel M, Lobaccaro JA. Biological properties of propolis extracts: something new from an ancient product. Chem Phys Lipids. 2017 Oct;207(Pt B):214-22., 4949. Cardoso EO, Santiago KB, Conti BJ, Conte FL, Tasca KI, Romagnoli GG, Golim MA, Rainho CA, Bastos JK, Sforcin JM. Brazilian green propolis: a novel tool to improve the cytotoxic and immunomodulatory action of docetaxel on MCF-7 breast cancer cells and on women monocyte. Phytother Res. 2022;36(1):448-61.] and research has advanced considerably to discover its main mechanisms of action. Although it is still difficult to obtain a universal standardization, the analysis of its chemical composition has revealed interesting molecules with immunomodulatory action [11. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011 Jan 27;133(2):253-60. ]. Propolis samples produced in the south of Brazil under organic conditions were grouped in seven types according to chromatographic methods, which seemed to be a source of bioactive compounds with antioxidant, antibacterial and anti-inflammatory action [5050. Tiveron AP, Rosalen PL, Franchin M, Lacerda RC, Bueno-Silva B, Benso B, Denny C, Ikegaki M, Alencar SM. Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of south Brazilian organic propolis. PLoS One. 2016 Nov 1;11(11):e0165588.]. Here, a properly characterized propolis sample was used and its main compounds were flavonoids (kaempferid, 5,6,7-trihydroxy-3,4’-dimethoxyflavone, aromadendrine-4’-methyl ether); essential oils (spathulenol, (2Z,6E)-farnesol, benzyl benzoate and prenylated acetophenones); aromatic acids (dihydrocinnamic acid, p-coumaric acid, ferulic acid, caffeic acid, 3,5-diprenyl-p-coumaric acid, 2,2-dimethyl-6-carboxy-ethenyl-8-prenyl-2H-1-benzo-pyran); a prenylated p-coumaric acid and two benzopyranes: E and Z 2,2-dimethyl-6-carboxyethenyl-8-prenyl-2H-benzopyranes); di- and triterpenes, among others. Furthermore, investigating the same propolis sample in our research allows us to propose mechanisms of action displayed by this sample.
Previous findings of our group revealed that propolis induced TLR-4 expression, NF-kB pathway, TNF-α, IL-6 and IL-10 production, increasing DCs bactericidal activity [2323. Conti BJ, Santiago KB, Cardoso EO, Freire PP, Carvalho RF, Golim MA, Sforcin JM. Propolis modulates miRNAs involved in TLR-4 pathway, NF-κB activation, cytokine production and in the bactericidal activity of human dendritic cells. J Pharm Pharmacol. 2016 Dec;68(12):1604-12.]. Here, propolis-treated DCs stimulated lymphocyte proliferation and led to Th1 and Treg profiles. Although it is difficult to precisely indicate which constituents of propolis may be involved in our findings, it is likely that phenolic acids (caffeic, dihydrocinnamic and p-coumaric acids) stimulated DCs, as they participated in the stimulating action of propolis in monocytes [5151. Cardoso EO, Conti BJ, Santiago KB, Conte FL, Oliveira LPG, Hernandes RT, Golim MA, Sforcin JM. Phenolic compounds alone or in combination may be involved in propolis effects on human monocytes. J Pharm Pharmacol. 2017 Jan;69(1):99-108.]. In addition, previous findings of our group revealed that propolis constituents act by binding to TLR-2 and TLR-4, since some biological activities displayed by monocytes were affected by blocking such receptors [5252. Búfalo MC, Bordon-Graciani AP, Conti BJ, Golim MA, Sforcin JM. The immunomodulatory effect of propolis on receptors expression, cytokine production and fungicidal activity of human monocytes. J Pharm Pharmacol. 2014 Oct;66(10):1497-504.].
Evidence points to the potential of propolis and its constituents for the development of new anti-inflammatory drugs, inhibiting cytokines, intracellular signaling pathways, cell adhesion and migration [1212. Franchin M, Freires IA, Lazarini JG, Nani BD, Cunha MG, Colon DF, Alencar SM, Rosalen PL. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur J Med Chem. 2018;153:49-55.]. Constituents from Brazilian green propolis such as baccharin exerted an anti-inflammatory action by inhibiting the production of cytokines and eicosanoids in mice, while p-coumaric acid also stimulated IL-10 production [5353. Ferreira JC, Reis MB, Coelho GDP, Gastaldello GH, Peti APF, Rodrigues DM, Bastos JK, Campo VL, Sorgi CA, Faccioli LH, Gardinassi LG, Tefé-Silva C, Zoccal KF. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J Ethnopharmacol. 2021 Oct 5;278:114255.]. In a clinical trial, propolis increased Foxp3 expression by lymphocytes in HIV-infected people exhibiting a previous inflammatory status [44. Conte FL, Tasca KI, Santiago KB, Cardoso EO, Romagnoli GG, Golim MA, Braz AMM, Berretta AA, Souza LR, Sforcin JM. Propolis increases Foxp3 expression and lymphocyte proliferation in HIV-infected people: a randomized, double blind, parallel-group and placebo-controlled study. Biomed Pharmacother. 2021 Oct;142:111984.]. Our findings have practical applications and indicate that propolis should be further investigated in vivo to control inflammatory and autoimmune diseases, and pathogen-induced immunopathology. Propolis isolated compounds should be evaluated in clinical trials as well.
Conclusions
Together, our data revealed that propolis modulates DC and T cell functions, indicating that the in vitro model using MAGE-1 and RA-treated DCs seemed to be feasible to affect Th1 and Treg cells subsets. These findings are unprecedented and relevant, revealing propolis potential to treat inflammatory conditions such as autoimmune diseases and pathogen-induced immunopathology.
References
- 1. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011 Jan 27;133(2):253-60.
- 2. Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res. 2016 Jun;30(6):894-905.
- 3. Bankova V, Popova M, Trusheva B. The phytochemistry of the honeybee. Phytochemistry. 2018 Nov;155:1-11.
- 4. Conte FL, Tasca KI, Santiago KB, Cardoso EO, Romagnoli GG, Golim MA, Braz AMM, Berretta AA, Souza LR, Sforcin JM. Propolis increases Foxp3 expression and lymphocyte proliferation in HIV-infected people: a randomized, double blind, parallel-group and placebo-controlled study. Biomed Pharmacother. 2021 Oct;142:111984.
- 5. Ripari N, Sartori AA, Honorio MS, Conte FL, Tasca KI, Santiago KB, Sforcin JM. Propolis antiviral and immunomodulatory activity: a review and perspectives for COVID-19 treatment. J Pharm. Pharmacol. 2021 Feb 8;73(3):281-99.
- 6. Weis WA, Ripari N, Conte FL, Honorio MS, Sartori AA, Matucci RH, Sforcin JM. An overview about apitherapy and its clinical applications. Phytomed Plus. 2022 May;2(2):100239.
- 7. Tanaka M, Okamoto Y, Fukui T, Masuzawa T. Suppression of interleukin 17 production by Brazilian propolis in mice with collagen-induced arthritis. Inflammopharmacology. 2012 Feb;20(1):19-26.
- 8. Piñeros AR, de Lima MHF, Rodrigues T, Gembre AF, Bertolini TB, Fonseca MD, Berretta AA, Ramalho LNZ, Cunha FQ, Hori JI, Bonato VLD. Green propolis increases myeloid suppressor cells and CD4+ Foxp3+ cells and reduces Th2 inflammation in the lungs after allergen exposure. J Ethnopharmacol. 2020 Apr 24;252:112496.
- 9. Hori JI, Zamboni DS, Carrão DB, Goldman GH, Berretta AA. The inhibition of inflammasome by Brazilian propolis (EPP-AF). Evid Based Complement Alternat Med. 2013;2013:1-11.
- 10. Bueno-Silva B, Kawamoto D, Ando-Suguimoto ES, Alencar SM, Rosalen PL, Mayer MPA. Brazilian red propolis attenuates inflammatory signaling cascade in LPS-activated macrophages. PLoS One. 2015:10:1-14.
- 11. Santiago KB, Conti BJ, Cardoso EO, Golim MA, Sforcin JM. Immunomodulatory/anti-inflammatory effects of a propolis-containing mouthwash on human monocytes. Pathog Dis. 2016;74:ftw081.
- 12. Franchin M, Freires IA, Lazarini JG, Nani BD, Cunha MG, Colon DF, Alencar SM, Rosalen PL. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur J Med Chem. 2018;153:49-55.
- 13. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343-53.
- 14. Corthay A. How do regulatory T cells work? Scand. J Immunol. 2009;70:326-36.
- 15. Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harb. Perspect Biol. 2012;4:a007021.
- 16. Cantrell D. Signaling in lymphocyte activation. Cold Spring Harb Perspect Biol. 2015;7:a018788.
- 17. Yao S, Zhu Y, Chen L. Advances in targeting cell surface signaling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130-46.
- 18. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273-90.
- 19. Di Sotto A, Vitalone A, Di Giacomo S. Plant-derived nutraceuticals and immune system modulation: an evidence-based overview. Vaccines. 2020;8:468.
- 20. Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol. 2007;113(1):1-14.
- 21. Simões LM, Gregório LE, da Silva Filho AA, de Souza ML, Azzolini AE, Bastos JK, Lucisano-Valim YM. Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. J Ethnopharmacol. 2004 Sep;94(1):59-65.
- 22. Orsatti CL, Sforcin JM. Propolis immunomodulatory activity on TLR-2 and TLR-4 expression by chronically stressed mice. Nat Prod Res. 2012;26(5):446-53.
- 23. Conti BJ, Santiago KB, Cardoso EO, Freire PP, Carvalho RF, Golim MA, Sforcin JM. Propolis modulates miRNAs involved in TLR-4 pathway, NF-κB activation, cytokine production and in the bactericidal activity of human dendritic cells. J Pharm Pharmacol. 2016 Dec;68(12):1604-12.
- 24. Büeler H, Mulligan RC. Induction of antigen-specific tumor immunity by genetic and cellular vaccines against MAGE: enhanced tumor protection by coexpression of granulocyte-macrophage colony-stimulating factor and B7-1. Mol Med. 1996 Sep;2(5):545-55.
- 25. Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, Brand DD, Ryffel B, Horwitz DA, Quismorio FP, Liu Z, Li B, Olsen NJ, Zheng SG. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2014 Aug 19;111(33):E3432-40.
- 26. Bankova V, Boudourova-Krasteva G, Popov S, Sforcin JM, Funari SRC. Seasonal variations of the chemical composition of Brazilian propolis. Apidologie 1998;29(4):361-7.
- 27. Conti BJ, Bankova V, Sforcin JM. Chemical composition of the same Brazilian propolis sample analysed in 1997 and in 2012: no freezing effect. Nat Prod Commun. 2015 Jul;10(7):1279-80.
- 28. Sforcin JM, Orsi RO, Bankova V. Effect of propolis, some isolated compounds and its source plant on antibody production. J Ethnopharmacol. 2005 Apr 26;98(3):301-5.
- 29. Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003 Jan 6;197(1):7-17.
- 30. Landi A, Babiuk LA, Hurk SVDLVD. Dendritic cells matured by a prostaglandin E2-containing cocktail can produce high levels of IL-12p70 and are more mature and Th1-biased than dendritic cells treated with TNF-α or LPS. Immunobiology. 2011 Jun;216(6):649-62.
- 31. Polancec DS, Kos VM, Banjanac M, Vrancic M, Cuzic S, Belamaric D, Parnham MJ, Polancec D, Haber VE. Azithromycin drives in vitro GM-CSF/IL-4-induced differentiation of human blood monocytes toward dendritic-like cells with regulatory properties. J Leukoc Biol. 2012 Feb;91(2):229-43.
- 32. Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005 Mar 21;6:62.
- 33. De Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Sem Immunopathol. 2005 Jan;26(3):289-307.
- 34. O’Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci. 2015 Nov;72(22):4309-25.
- 35. Liu XZ, Zhan L, Xu F, Ma D, Li Z, Guo N, Li X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J Immunol. 2010 Dec 15;185(12):7244-51.
- 36. Kim SI, Jeong YI, Jung ID, Lee JS, Lee CM, Yoon MS, Seong EY, Kim JI, Lee JD, Park IM. p-Coumaric acid inhibits indoleamine 2, 3-dioxygenase expression in murine dendritic cells. Int Immunopharmacol. 2007 Jun;7(6):805-15.
- 37. Huang RY, Yu YL, Cheng WC, Ouyang CN, Fu E, Chu CL. Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol. 2010 Jun 15;184(12):6815-21.
- 38. Conte FL, Santiago KB, Conti BJ, Cardoso EO, Oliveira LPG, Feltran GS, Zambuzzi WF, Golim MA, Cruz MT, Sforcin JM. Propolis from southeastern Brazil produced by Apis mellifera affects innate immunity by modulating cell marker expression, cytokine production and intracellular pathways in human monocytes. J Pharm Pharmacol. 2021 Mar 4;73(2):135-44.
- 39. De Lastic AL, Rodi M, Mouzaki A. Effect of dendritic cell state and antigen-presentation conditions on resulting T-cell phenotypes and Th cytokine profiles. Immunobiology. 2016 Aug;221(8):862-70.
- 40. Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, de Madariaga A, Domingo JC. 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator-activated receptor γ blocks some of the 9cRA activities, and precludes them to mature phenotype development. J Immunol. 2007 May 15;178(10):6130-9.
- 41. Okamoto Y, Hara T, Ebato T, Fukui T, Masuzawa T. Brazilian propolis ameliorates trinitrobenzene sulfonic acid-induced colitis in mice by inhibiting Th1 differentiation. Int Immunopharmacol. 2013 Jun;16(2):178-83.
- 42. Orsolic N, Basic I. Immunomodulation by water-soluble derivative of propolis: a factor of antitumor reactivity. J Ethnopharmacol. 2003 Feb;84(2-3):265-73.
- 43. Orsolic N, Saranovic AB, Basic I. Direct and indirect mechanism(s) of antitumour activity of propolis and its polyphenolic compounds. Planta Med. 2006 Jan;72(1):20-7.
- 44. Bono MR, Tejon G, Flores-Santibañez F, Fernandez D, Rosemblatt M, Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients. 2016 Jun;8(6):E349.
- 45. Namdari H, Izad M, Rezaei F, Amirghofran Z. Differential regulation of CD4+ T cell subsets by silymarin in vitro and in ovalbumin immunized mice. Daru. 2018 Dec;26(2):215-27.
- 46. Melo KM, Carvalho BTC. Células T regulatórias: mecanismos de ação e função nas doenças humanas. Rev Bras Alergia Imunopatol. 2009 Set-Out;32(5):184-8.
- 47. Cafferata EA, Jerez A, Vernal R, Monasterio G, Pandis N, Faggion Jr CM. The therapeutic potential of regulatory T lymphocytes in periodontitis: a systematic review. J Periodontal Res. 2018 Nov 25;54(3):207-17.
- 48. Zabaiou N, Fouache A, Trousson A, Baron S, Zellagui A, Lahouel M, Lobaccaro JA. Biological properties of propolis extracts: something new from an ancient product. Chem Phys Lipids. 2017 Oct;207(Pt B):214-22.
- 49. Cardoso EO, Santiago KB, Conti BJ, Conte FL, Tasca KI, Romagnoli GG, Golim MA, Rainho CA, Bastos JK, Sforcin JM. Brazilian green propolis: a novel tool to improve the cytotoxic and immunomodulatory action of docetaxel on MCF-7 breast cancer cells and on women monocyte. Phytother Res. 2022;36(1):448-61.
- 50. Tiveron AP, Rosalen PL, Franchin M, Lacerda RC, Bueno-Silva B, Benso B, Denny C, Ikegaki M, Alencar SM. Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of south Brazilian organic propolis. PLoS One. 2016 Nov 1;11(11):e0165588.
- 51. Cardoso EO, Conti BJ, Santiago KB, Conte FL, Oliveira LPG, Hernandes RT, Golim MA, Sforcin JM. Phenolic compounds alone or in combination may be involved in propolis effects on human monocytes. J Pharm Pharmacol. 2017 Jan;69(1):99-108.
- 52. Búfalo MC, Bordon-Graciani AP, Conti BJ, Golim MA, Sforcin JM. The immunomodulatory effect of propolis on receptors expression, cytokine production and fungicidal activity of human monocytes. J Pharm Pharmacol. 2014 Oct;66(10):1497-504.
- 53. Ferreira JC, Reis MB, Coelho GDP, Gastaldello GH, Peti APF, Rodrigues DM, Bastos JK, Campo VL, Sorgi CA, Faccioli LH, Gardinassi LG, Tefé-Silva C, Zoccal KF. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J Ethnopharmacol. 2021 Oct 5;278:114255.
-
Availability of data and materials
All data generated or analyzed during this study are included in this article. -
Funding
This study was supported by the São Paulo Research Foundation (FAPESP - grant n. 2015/03493-3 and 2015/02596-3) and in part by the Coordination for the Improvement of Higher Education Personnel (CAPES - Finance Code 001). -
Ethical approval
This study was approved on February 13, 2019 by the Ethics Committee of Botucatu Medical School, UNESP (CAAE: 42600915.0.0000.5411). -
Consent for publication
Not applicable.
Publication Dates
-
Publication in this collection
13 Jan 2023 -
Date of issue
2023
History
-
Received
14 July 2022 -
Accepted
13 Dec 2022