ABSTRACT
This review aimed to analyze the scientific production on severity of oral mucositis as an adverse effect of chemotherapy. To this end, we performed a search at PubMed databases combining the keywords “oral mucositis” and “chemotherapy protocol”. To describe the investigation, the following variables were considered: journal, year/place, study design, sample, protocol used and incidence of oral mucositis. A total of 547 articles were retrieved, of which 26 were selected. Out of these 26, only 2 reported severity of oral mucositis; the others only reported the presence of the condition. Protocols for treating different types of carcinoma were evaluated in 16 (61.53%) studies, for hematological malignancies in 6 (23.07%), and for hematopoietic stem cell transplantation in 4 (15.4%). Protocols for hematopoietic stem cell transplantation entail a high risk for oral mucositis, just as chemotherapy with cytarabine and high-dose 5-fluorouracil, alkylating agents and platinumbased compounds. To provide the best prevention and treatment for oral mucositis, it is essential to know the chemotherapy protocols used and their effects on the oral cavity.
Keywords
Stomatitis/drug therapy; Stomatitis/chemically induced; Toxicity
RESUMO
Esta revisão teve como objetivo analisar a produção científica sobre a gravidade da mucosite oral como efeito adverso da quimioterapia. Para tal, nos bancos de dados do PubMed, foi realizada uma busca com a associação dos descritores “oral mucositis” com “chemotherapy protocol”. Para descrição da investigação, foram consideradas como variáveis: periódico, ano/local, delineamento da pesquisa, amostra, protocolo utilizado e incidência de mucosite oral. Foram analisados 547 artigos e, destes, 26 foram selecionados. Destes 26, apenas 2 tinham como objetivo avaliar a gravidade de mucosite oral; nos outros, a mucosite oral foi apenas relatada. Protocolos para tratamento de diferentes tipos de carcinoma foram avaliados em 16 (61,53%) estudos, para neoplasias hematológicas, em 6 (23,07%), e para transplante de células tronco hematopoiéticas em 4 (15,4%). Protocolos para transplante de células tronco hematopoiéticas são de alto risco para o desenvolvimento de mucosite oral, da mesma forma que os quimioterápicos citarabina e 5-fluorouracil em altas doses, agentes alquilantes e compostos derivados da platina. A fim de oferecer prevenção e tratamento mais adequados para mucosite oral, é imprescindível que se conheçam os protocolos quimioterápicos utilizados e seus efeitos sobre a cavidade oral.
Descritores
Estomatite/tratamento farmacológico; Estomatite/induzido quimicamente; Toxicidade
INTRODUCTION
The primary objective of oncological treatments, such as chemotherapy, is to destroy cancer cells. However, most chemotherapeutic agents do not act selectively, i.e particularly fast-growing cells, such as gastrointestinal, capillary, and immune cells.(11. Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S. Oral mucositis and outcomes of autologous hematopoietic stem-cell transplantation following high-dose melphalan conditioning for multiple myeloma. J Support Oncol. 2007;5(5):231-5.
2. Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stemcell transplantation. J Clin Oncol. 2001;19(8):2201-5.-33. Kwon Y. Mecanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016;9:2007-16. Review.) The toxicity of chemotherapeutic agents is known to be associated with the mode of action, dose and interaction between different agents in a given protocol.(44. Suresh AV, Varma PP, Sinha S, Deepika S, Raman R, Srinivasan M, et al. Risk-scoring system for predicting mucositis in patients of head and neck cancer receiving concurrent chemoradiotherapy [rssm-hm]. J Cancer Res Ther. 2010;6(4):448-51.,55. Pico JL, Avila-Garavito A, Naccache P. Mucositis: Its Occurrence, Consequences, and Treatment in Oncology Setting. Oncologist. 1998;3(6):446-51.)
Oral mucositis (OM) is an important adverse effect seen in cancer patients on chemotherapy and/or radiation therapy for the head and neck.(22. Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stemcell transplantation. J Clin Oncol. 2001;19(8):2201-5.,66. Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol. 2007;5(9 Suppl 4):3-11. Review.,77. Trucci VM, Veeck EB, Morosolli AR. Current strategies for the management of oral mucositis induced by radiotherapy or chemotherapy. Rev Odonto Cienc. 2009;24(3):309-14. Review.) This condition presents clinically as erosive and/or ulcerative lesions that can cause mild to severe pain.(11. Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S. Oral mucositis and outcomes of autologous hematopoietic stem-cell transplantation following high-dose melphalan conditioning for multiple myeloma. J Support Oncol. 2007;5(5):231-5.,22. Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stemcell transplantation. J Clin Oncol. 2001;19(8):2201-5.,88. Sonis ST. Oral mucositis. Anticancer Drugs. 2011;22(7):607-12. Review.) These lesions usually lead to a significant decrease in quality of life, since they can prolong hospital stay, affect the nutritional status of the patient, increase the risk of infections, and increase the prescription of opioids.(99. Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531-9.
10. Bezinelli LM, de Paula Eduardo F, da Graça Lopes RM, Biazevic MG, de Paula Eduardo C, Correa L, et al. Cost-effectiveness of the introduction of specialized oral care with laser therapy in hematopoietic stem cell transplantation. Hematol Oncol. 2014;32(1):31-9.-1111. Eduardo FP, Bezinelli LM, Orsi MC, Rodrigues M, Ribeiro MS, Hamerschlak N, et al. The influence of dental care associated with laser therapy on oral mucositis during allogeneic hematopoietic cell transplant: retrospective study. einstein (São Paulo). 2011;9(2):201-6.)
For these reasons, treatment of OM is extremely necessary, with the aim of relieving symptoms, accelerating tissue repair and controlling infections of oral origin. Currently, efforts are focused on preventing OM. The Mucositis Study Group of the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology (MASCC/ISOO) has published guidelines(1212. Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S; Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453-61. Review. Erratum in: Cancer. 2015;121(8):1339.) to orient the clinical practice for prevention/treatment of this condition. In these guidelines, preventive measures for OM are described without specification of the chemotherapy protocol used.
The known association between toxicity and the chemotherapy protocol used may guide selection of the right prevention methods to control OM in highrisk populations.
OBJECTIVE
To review the scientific production on severity of oral mucositis as an adverse effect of different chemotherapy protocols.
METHODS
An integrative review with data gathered from multiple articles to assist in understanding how different chemotherapy protocols can trigger mild or severe degrees of OM. In the field of health, the amount of information is ever growing, and evidencebased practice has become a must. In this way, the methodology of integrative reviews aims to synthesize knowledge to be incorporated in the practice by identifying independent studies on the same subject and analyzing their results.
This review was based on a search at PubMed databases. The search was started and completed in May 2016. Studies published in English, Spanish or Portuguese were selected for analysis. There were no restrictions regarding the year of publication and the studies retrieved were published before May 2016. After the search, the articles were selected according to their inclusion and exclusion criteria. The search strategy was based on a combination of the keywords “oral mucositis” and “chemotherapy protocols”.
The inclusion criteria were original articles with open access to the full text, whose study subjects were patients diagnosed with OM after chemotherapy. Case reports, clinical trials, and literature reviews were excluded, as well as articles that did not describe the outcomes of the chemotherapy-associated OM.
Study identification, selection and inclusion
Data collection was carried out by an independent researcher. After applying the search strategy with the keywords defined, the articles were selected. First the titles were read, and those that clearly did not meet the inclusion criteria for this review were readily excluded. Next, the abstracts of the selected articles were read and just like we did with the titles, the articles that clearly did not meet the predefined inclusion criteria of this review were excluded. Finally, all studies not excluded after these first two steps were read in full, and finally selected those to be included in this review.
Key data for each article were collected and entered into the database of the software Microsoft® Excel® for Mac 2011. The variables considered were journal, year/place, design, sample, protocol used and incidence of OM.
RESULTS
Using the keywords of choice, 547 articles were retrieved. Of those, 325 articles were excluded by the title, 173 by the abstract and 23 by the full text, which left us with 26 articles.
The 26 articles selected were in English, published between 1987 and 2015, and the largest number of studies was from the last 5 years (n=8). All articles were original, including 24 prospective and only two retrospective studies. Of the 26 articles, only two aimed to evaluate severity of OM triggered by a given chemotherapy protocol. The other 24 studies focused on treatment efficacy and safety, and just reported the incidence of OM.
Table 1 displays the sample of each study, the chemotherapy protocol used and the severity of OM developed in each protocol. The median number of subjects assessed in each article was 135 (minimum 16 and maximum 716). The mean age of participants was 43.54 years (minimum 5.2 years and maximum 65 years).
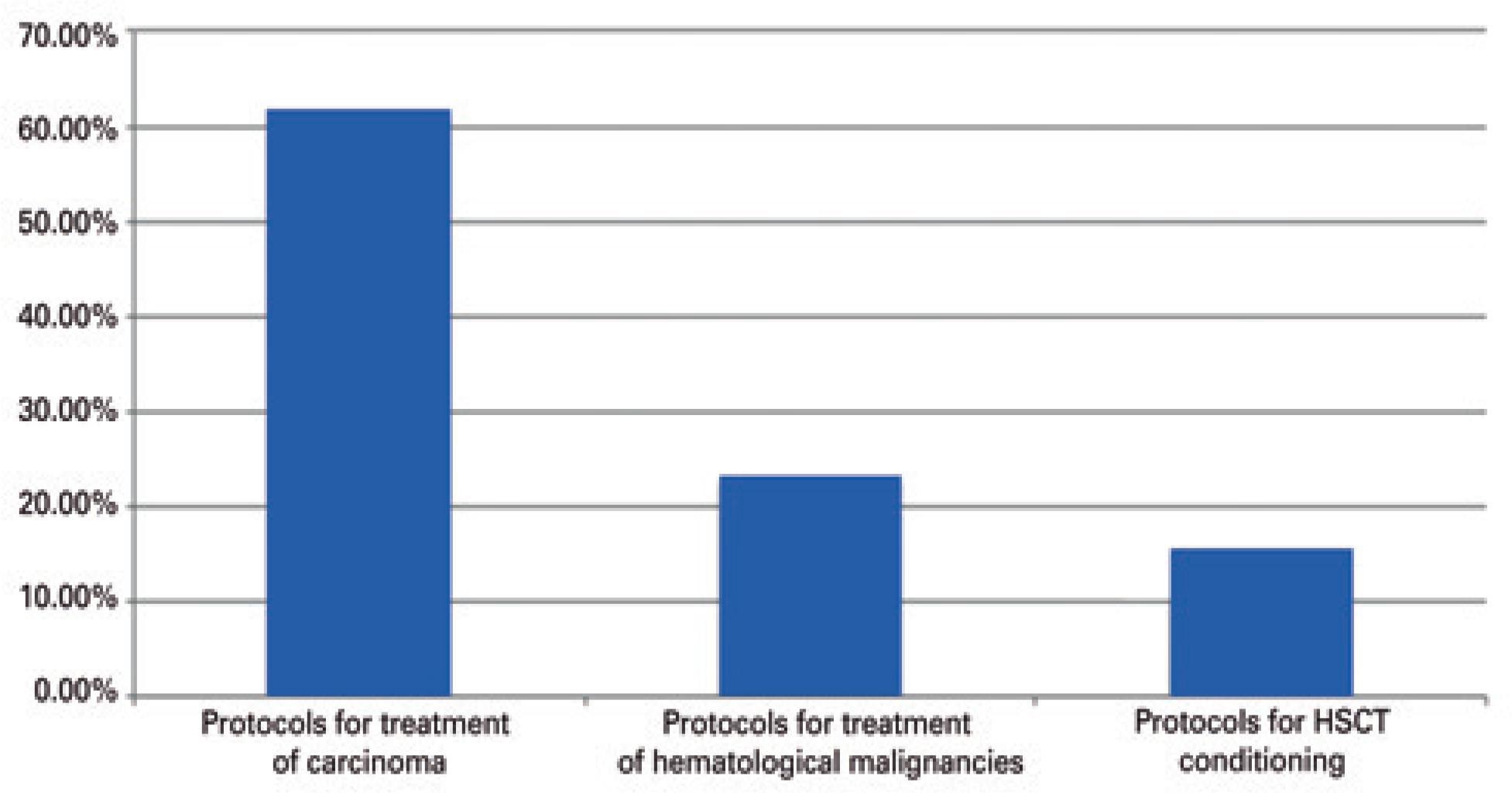
Most of the studies looked at protocols for treating carcinoma 16 (61.53%), 6 (23.07%) addressed therapy for hematological malignancies and 4 (15.4%) for hematopoietic stem cell transplantation (HSCT) (Figure 1).
Distribution of articles according to type of treatment
HSCT: hematopoietic stem cell transplantation.
In the carcinoma protocols, treatments with chemotherapeutic agents, such as 5-fluorouracil (5-FU) and platinum derivatives lead to more frequent and severe cases of OM between cycles – however, numbers varied. The use of 5-FU was strongly associated with the development of OM, according to Palappallil et al.(2020. Palappallil DS, Nair BL, Jayakumar KL, Puvathalil RT. Comparative study of the toxicity of 5-fluorouracil-adriamycin-cyclophosphamide versus adriamycincyclophosphamide followed by paclitaxel in carcinoma breast. Indian J Cancer. 2011;48(1):68-73.) The authors demonstrated that 90% of patients developed OM after using 5-FU. Bolus administration of said agent also showed higher toxicity (19.6% of patients with OM) than continuous infusion (3.6% of patients with OM). For chemotherapeutics, such as ifosfamide and doxorubicin, greater severity of OM (53% of patients) was observed in individuals receiving continuous infusion.
Four articles (15.4%) addressed conditioning regimens for HSCT. Two of them compared the use of melphalan with the BEAM protocol (carmustine, etoposide, cytarabine and melphalan). The incidence of severe OM with melphalan was 46% in one study(2525. Blijlevens N, Schwenkglenks M, Bacon P, D'Addio A, Einsele H, Maertens J, Niederwieser D, Rabitsch W, Roosaar A, Ruutu T, Schouten H, Stone R, Vokurka S, Quinn B, McCann S; European Blood and Marrow Transplantation Mucositis Advisory Group. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy-European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26(9):1519-25.) and 60% in another.(2727. Castagna L, Magagnoli M, Balzarotti M, Sarina B, Siracusano L, Nozza A, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in refractory/relapsed Hodgkin's lymphoma: a monocenter prospective study. Am J Hematol. 2007;82(2):122-7.) The BEAM protocol also showed high incidence of severe OM in both studies (42% and 50%). This same incidence of MO with the use of melphalan was also verified by Kremens et al.,(3131. Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, et al. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002;30(12):893-8.) (50%) and by Fadda et al.,(2626. Fadda G, Campus G, Lugliè P. Risk factors for oral mucositis in paediatric oncology patients receiving alkylant chemotherapy. BMC Oral Health. 2006;6:13.) (54.4%). These two authors also found a high incidence of OM among patients receiving busulfan (81.82%).
Of the 26 articles, 6 (23.07%) reported cases of MO after treatment of hematological malignancies. During the induction phase for leukemia, no patients had OM; in the consolidation phase, 6.1% had OM; and in the maintenance phase, the highest rate was 8.2%. When assessing the chemotherapeutic agents used, we observed that 26% of patients on daunorubicin and etoposide, and 37.5% of patients on high-dose cytarabine had severe OM. Another protocol studied was cyclophosphamide, doxorubicin, vincristine and prednisone CHOP, and of the 17 patients treated, 7 (41.16%) had some degree of OM.
DISCUSSION
Oral mucositis is an adverse effect of anticancer therapy that, in addition to causing discomfort/pain, may affect the nutritional health of patients. Thus, it is increasingly important to know how these lesions behave in order to provide effective prevention and treatment. Understanding the effects of chemotherapy protocols that lead to greater oral toxicity is therefore necessary.
Of the articles reviewed, it is interesting to note that only two focused on evaluating oral toxicity resulting from the anticancer agent of choice. Most of them aimed to show the response to oncological treatment using certain chemotherapy protocols, and reporting of OM was limited to the occurrence of the condition.
The mean age of subjects was 43.54 years. This mean age reflects the fact that most studies addressed treatments for carcinoma, which is more common in adults.
In this review, many articles described the effects of chemotherapy protocols for treatment of different types of carcinoma, particularly protocols based on the use of 5-FU and platinum derivatives (cisplatin and oxyplatin).(1313. Chibaudel B, Lacave R, Lefevre M, Soussan P, Antoine M, Périé S, et al. Induction therapy with cetuximab plus docetaxel, cisplatin, and 5-fluorouracil (ETPF) in patients with resectable nonmetastatic stage III or IV squamous cell carcinoma of the oropharynx. A GERCOR phase II ECHO-07 study. Cancer Med. 2015;4(5):721-31.
14. Bano N, Najam R, Qazi F, Mateen A. Gastrointestinal adverse effects in advanced colorectal carcinoma patients treated with different schedules of FOLFOX. Asian Pac J Cancer Prev. 2014;15(19):8089-93.
15. Wang HM, Hsu CL, Hsieh CH, Fan KH, Lin CY, Chang JT. Concurrent chemoradiotherapy using cisplatin, tegafur, and leucovorin for advanced squamous cell carcinoma of the hypopharynx and oropharynx. Biomed J. 2014;37(3):133-40.
16. Aapro M, Andre F, Blackwell K, Clavo E, Jahanzeb M, Papazisis K, et al. Adverse event management in patients with advanced cancer receiving oral everolimus: focus on breast cancer. Ann Oncol. 2014;25(4):763-73. Review.
17. Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, et al. Long-term outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin J Cancer. 2013;32(10):525-32.-1818. Lin HX, Hua YJ, Chen QY, Luo DH, Sun R, Qiu F, et al. Randomized study of sinusoidal chronomodulated versus flat intermittent induction chemotherapy with cisplatin and 5-fluorouracil followed by traditional radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. 2013;32(9):502-11.,2020. Palappallil DS, Nair BL, Jayakumar KL, Puvathalil RT. Comparative study of the toxicity of 5-fluorouracil-adriamycin-cyclophosphamide versus adriamycincyclophosphamide followed by paclitaxel in carcinoma breast. Indian J Cancer. 2011;48(1):68-73.
21. Baird R, Biondo A, Chhaya V, McLachlan J, Karpathakis A, Rahman S, et al. Toxicity associated with capecitabine plus oxaliplatin in colorectal cancer before and after an institutional policy of capecitabine dose reduction. Br J Cancer. 2011;104(1):43-50.-2222. Lee JO, Lee KW, Oh DY, Kim JH, Im SA, Kim TY, et al. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann Oncol. 2009;20(8):1402-7.,2828. Oh DY, Kim TY, Kwon JH, Lee JJ, Joh Y, Kim DW, et al. Docetaxel + 5-fluorouracil + cisplatin 3-day combination chemotherapy as a first-line treatment in patients with unresectable gastric cancer. Jpn J Clin Oncol. 2005;35(7):380-5.,3030. Saini A, Norman AR, Cunningham D, Chau I, Hill M, Tait D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer. 2003;88(12):1859-65.,3434. Lissoni A, Gabriele A, Gorga G, Tumolo S, Landoni F, Mangioni C, et al. Cisplatin, epirubicin and paclitaxel-containing chemotherapy in uterine adenocarcinoma. Ann Oncol. 1997;8(10):969-72.) In these articles, the incidence of OM varied a great deal, but we identified that patients who received cisplatin had more severe OM.(1515. Wang HM, Hsu CL, Hsieh CH, Fan KH, Lin CY, Chang JT. Concurrent chemoradiotherapy using cisplatin, tegafur, and leucovorin for advanced squamous cell carcinoma of the hypopharynx and oropharynx. Biomed J. 2014;37(3):133-40.
16. Aapro M, Andre F, Blackwell K, Clavo E, Jahanzeb M, Papazisis K, et al. Adverse event management in patients with advanced cancer receiving oral everolimus: focus on breast cancer. Ann Oncol. 2014;25(4):763-73. Review.-1717. Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, et al. Long-term outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin J Cancer. 2013;32(10):525-32.)
In the study by Lin et al.,(1818. Lin HX, Hua YJ, Chen QY, Luo DH, Sun R, Qiu F, et al. Randomized study of sinusoidal chronomodulated versus flat intermittent induction chemotherapy with cisplatin and 5-fluorouracil followed by traditional radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. 2013;32(9):502-11.) oral toxicity worsened with each subsequent cycle. Patients received three cycles of the same chemotherapy protocol, and in the first cycle, only 33.87% of patients developed OM - all cases were mild (grades 1 and 2). In the second cycle, 49.57% developed some degree of OM, and 12.18% were severe. In the third cycle, 100% of patients developed OM, of which 48.38% were grades 3 and 4. We found no evidence in the literature that OM has a cumulative effect with cycles, but in this review the increase in OM severity over the course of treatment was significant.
Palappallil et al.,(2020. Palappallil DS, Nair BL, Jayakumar KL, Puvathalil RT. Comparative study of the toxicity of 5-fluorouracil-adriamycin-cyclophosphamide versus adriamycincyclophosphamide followed by paclitaxel in carcinoma breast. Indian J Cancer. 2011;48(1):68-73.) proposed a comparison between two different protocols for treating breast carcinoma. In both protocols, patients received cyclophosphamide and doxorubicin but, in the first protocol, 5-FU was also administered. As much as 90% of patients who received 5-FU developed some degree of OM, against only 4% of the remaining patients, which corroborates the oral toxicity of 5-FU. Administration of 5-FU was studied by Saini et al.,(3030. Saini A, Norman AR, Cunningham D, Chau I, Hill M, Tait D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer. 2003;88(12):1859-65.) in 716 patients with colorectal carcinoma. Only 3.6% of those who received continuous infusions of the antineoplastic drug had severe OM, whereas 19.6% of patients who received bolus infusions of 5-FU had severe OM, which implies that oral toxicity is higher when the agent is administered as a bolus. Anderson et al.,(3636. Anderson H, Hopwood P, Prendiville J, Radford JA, Thatcher N, Ashcroft L. A randomized study of bolous vs continuous pump infusion of ifosfamide and doxorubicin with oral etoposide for small cell lung cancer. Br J Cancer. 1993;67(6):1385-90.) also observed the mode of administration of antineoplastic agents ifosfamide and doxorubicin. Differently from Saini et al.,(3030. Saini A, Norman AR, Cunningham D, Chau I, Hill M, Tait D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer. 2003;88(12):1859-65.) they observed a higher incidence of OM in patients who received continuous administration (53%). Only 31% of patients who received doxorubicin and ifosfamide by bolus administration had some degree of OM.
The oral toxicity associated with conditioning regimens for HSCT is already well documented in the literature.(33. Kwon Y. Mecanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016;9:2007-16. Review.,2525. Blijlevens N, Schwenkglenks M, Bacon P, D'Addio A, Einsele H, Maertens J, Niederwieser D, Rabitsch W, Roosaar A, Ruutu T, Schouten H, Stone R, Vokurka S, Quinn B, McCann S; European Blood and Marrow Transplantation Mucositis Advisory Group. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy-European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26(9):1519-25.
26. Fadda G, Campus G, Lugliè P. Risk factors for oral mucositis in paediatric oncology patients receiving alkylant chemotherapy. BMC Oral Health. 2006;6:13.-2727. Castagna L, Magagnoli M, Balzarotti M, Sarina B, Siracusano L, Nozza A, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in refractory/relapsed Hodgkin's lymphoma: a monocenter prospective study. Am J Hematol. 2007;82(2):122-7.,3131. Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, et al. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002;30(12):893-8.,3939. Almeida VL, Leitão A, Reina LC, Montanari CA, Donnici CL, Lopes MT. [Cancer and cell cicle-specific and cell cicle nonspecific anticancer DNA-interactive agents: an introduction]. Quim Nova. 2005;28(1):118-29. Portuguese.) Despite the high incidence and the fact that oral damage caused by treatment regimens used in HSCT has prevention and treatment protocols guided by the MASCC/ISOO,(1212. Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S; Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453-61. Review. Erratum in: Cancer. 2015;121(8):1339.) there are still very few studies focusing on the effects of chemotherapeutics on the oral cavity. In this review, four articles(2525. Blijlevens N, Schwenkglenks M, Bacon P, D'Addio A, Einsele H, Maertens J, Niederwieser D, Rabitsch W, Roosaar A, Ruutu T, Schouten H, Stone R, Vokurka S, Quinn B, McCann S; European Blood and Marrow Transplantation Mucositis Advisory Group. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy-European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26(9):1519-25.
26. Fadda G, Campus G, Lugliè P. Risk factors for oral mucositis in paediatric oncology patients receiving alkylant chemotherapy. BMC Oral Health. 2006;6:13.-2727. Castagna L, Magagnoli M, Balzarotti M, Sarina B, Siracusano L, Nozza A, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in refractory/relapsed Hodgkin's lymphoma: a monocenter prospective study. Am J Hematol. 2007;82(2):122-7.,3131. Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, et al. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002;30(12):893-8.) addressing said protocols were selected. Blijlevens et al.,(2525. Blijlevens N, Schwenkglenks M, Bacon P, D'Addio A, Einsele H, Maertens J, Niederwieser D, Rabitsch W, Roosaar A, Ruutu T, Schouten H, Stone R, Vokurka S, Quinn B, McCann S; European Blood and Marrow Transplantation Mucositis Advisory Group. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy-European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26(9):1519-25.) and Castagna et al.,(2727. Castagna L, Magagnoli M, Balzarotti M, Sarina B, Siracusano L, Nozza A, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in refractory/relapsed Hodgkin's lymphoma: a monocenter prospective study. Am J Hematol. 2007;82(2):122-7.) compared the use of melphalan and the BEAM protocol as conditioning regimens for HSCT. Both found a high incidence of OM in association with both protocols, however it was slightly higher in patients submitted to conditioning with melphalan. The two papers found that, for both protocols, approximately 50% of patients developed severe OM. This demonstrates the marked oral toxicity resulting from HSCT conditioning. Kremens et al.,(3131. Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, et al. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002;30(12):893-8.) also demonstrated a high incidence of severe MO (50%) in patients on HSCT conditioning with melphalan for Wilms tumor, despite the small sample of only 20 subjects. Still on HSCT conditioning, Fadda et al.,(2626. Fadda G, Campus G, Lugliè P. Risk factors for oral mucositis in paediatric oncology patients receiving alkylant chemotherapy. BMC Oral Health. 2006;6:13.) looked at the effects of chemotherapeutics melphalan and busulfan and found that, of the patients receiving melphalan, 54.54% had some degree of OM, as described in previous studies. And as much as 81.82% of patients who received busulfan developed OM. Alkylating agents are known to form inter-filamentous bonds with DNA, i.e since DNA alkylation requires more complex repair mechanisms and may even inhibit DNA replication. This group of patients is often associated with the development of OM.(33. Kwon Y. Mecanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016;9:2007-16. Review.,3939. Almeida VL, Leitão A, Reina LC, Montanari CA, Donnici CL, Lopes MT. [Cancer and cell cicle-specific and cell cicle nonspecific anticancer DNA-interactive agents: an introduction]. Quim Nova. 2005;28(1):118-29. Portuguese.)
Few studies have demonstrated the relation between chemotherapy protocols used in hematological malignancies and OM severity. Abromowitch et al.,(2323. Abromowitch M, Sposto R, Perkins S, Zwick D, Siegel S, Finlay J, Cairo MS; Children's Oncology Group. Shortened intensified multiagent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: report from the Children's Oncology Group. Br J Haematol. 2008;143(2):261-7.) demonstrated toxicity resulting from each phase of leukemia treatment. No significant oral toxicities were reported, with no patient developing OM on induction, 6.1% presenting on consolidation, and less than 10% on maintenance phases. The incidence found by those authors is too low when compared with other studies. Bishop et al.,(3737. Bishop JF, Lowenthal RM, Joshua D, Mattews JP, Todd D, Cobcroft R, et al. Etoposide in acute nonlymphocytic leukemia. Australian Leukemia Study Group. Blood. 1990;75(1):27-32.) studied leukemia treatment with daunorubicin and etoposide (drugs used for induction and consolidation) and found that 26% of patients developed severe OM, i.e than that reported by Abromowitch et al.,(2323. Abromowitch M, Sposto R, Perkins S, Zwick D, Siegel S, Finlay J, Cairo MS; Children's Oncology Group. Shortened intensified multiagent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: report from the Children's Oncology Group. Br J Haematol. 2008;143(2):261-7.) Among studies addressing leukemia treatments, Lacayo et al.,(3232. Lacayo NJ, Lum BL, Becton DL, Weinstein H, Ravindranath Y, Chang MN, et al. Pharmacokinetic interactions of cyclosporine with etoposide and mitoxantrone in children with acute myeloid leukemia. Leukemia. 2002; 16(5):920-7.) assessed protocols with and without high-dose cytarabine for patients with acute myeloid leukemia. Patients who received high doses of cytarabine had significantly higher rates of severe OM. Wang et al.,(3535. Wang WS, Tzeng CH, Chiou TJ, Liu JH, Hsieh RK, Yen CC, et al. High-dose cytarabine and mitoxantrone as salvage therapy for refractory non-hodgkin's lymphoma. Jpn J Clin Oncol. 1997;27(3):154-7. Review.) also analyzed a chemotherapy protocol based on highdose cytarabine for treating refractory non-Hodgkin lymphoma. Although the number of patients enrolled was not very expressive (n=16), 37.5% of subjects had severe OM. This review also included another study by Shin et al.,(2424. Shin HJ, Chung JS, Lee JJ, Sohn SK, Choi YJ, Kim YK, et al. Treatment Outcomes with CHOP chemotherapy in adult patients with hemophagocytic lymphohistiocytosis. J Korean Med Sci. 2008;23(3):439-44.) with a small number of patients, assessing cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) protocol in 17 patients with hemophagocytic lymphohistiocytosis; of those, seven patients developed some degree of OM.
CONCLUSION
Although oral mucositis affects not only the patient's quality of life but also the course of antineoplastic treatment, few studies focused on the relation between the chemotherapy protocol and the incidence of the condition. In this review, we observed there are scarce studies assessing the relation between oral mucositis and the chemotherapy protocol used.
Through this integrative review, we further corroborate what has been frequently demonstrated in the literature: protocols for hematopoietic stem cell transplantation entail a high risk for development of oral mucositis. We also found that cytarabine, high-dose 5-FU, alkylating agents and platinum-based compounds are commonly associated with the development of oral mucositis. Therefore, when using the aforementioned protocols, attention must be paid to prevention and treatment of this condition. In addition, it is clear that further investigations are needed concerning the oral toxicity of different drugs, to allow for more effective prevention of this condition.
REFERENCES
-
1Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S. Oral mucositis and outcomes of autologous hematopoietic stem-cell transplantation following high-dose melphalan conditioning for multiple myeloma. J Support Oncol. 2007;5(5):231-5.
-
2Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stemcell transplantation. J Clin Oncol. 2001;19(8):2201-5.
-
3Kwon Y. Mecanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016;9:2007-16. Review.
-
4Suresh AV, Varma PP, Sinha S, Deepika S, Raman R, Srinivasan M, et al. Risk-scoring system for predicting mucositis in patients of head and neck cancer receiving concurrent chemoradiotherapy [rssm-hm]. J Cancer Res Ther. 2010;6(4):448-51.
-
5Pico JL, Avila-Garavito A, Naccache P. Mucositis: Its Occurrence, Consequences, and Treatment in Oncology Setting. Oncologist. 1998;3(6):446-51.
-
6Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol. 2007;5(9 Suppl 4):3-11. Review.
-
7Trucci VM, Veeck EB, Morosolli AR. Current strategies for the management of oral mucositis induced by radiotherapy or chemotherapy. Rev Odonto Cienc. 2009;24(3):309-14. Review.
-
8Sonis ST. Oral mucositis. Anticancer Drugs. 2011;22(7):607-12. Review.
-
9Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531-9.
-
10Bezinelli LM, de Paula Eduardo F, da Graça Lopes RM, Biazevic MG, de Paula Eduardo C, Correa L, et al. Cost-effectiveness of the introduction of specialized oral care with laser therapy in hematopoietic stem cell transplantation. Hematol Oncol. 2014;32(1):31-9.
-
11Eduardo FP, Bezinelli LM, Orsi MC, Rodrigues M, Ribeiro MS, Hamerschlak N, et al. The influence of dental care associated with laser therapy on oral mucositis during allogeneic hematopoietic cell transplant: retrospective study. einstein (São Paulo). 2011;9(2):201-6.
-
12Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S; Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453-61. Review. Erratum in: Cancer. 2015;121(8):1339.
-
13Chibaudel B, Lacave R, Lefevre M, Soussan P, Antoine M, Périé S, et al. Induction therapy with cetuximab plus docetaxel, cisplatin, and 5-fluorouracil (ETPF) in patients with resectable nonmetastatic stage III or IV squamous cell carcinoma of the oropharynx. A GERCOR phase II ECHO-07 study. Cancer Med. 2015;4(5):721-31.
-
14Bano N, Najam R, Qazi F, Mateen A. Gastrointestinal adverse effects in advanced colorectal carcinoma patients treated with different schedules of FOLFOX. Asian Pac J Cancer Prev. 2014;15(19):8089-93.
-
15Wang HM, Hsu CL, Hsieh CH, Fan KH, Lin CY, Chang JT. Concurrent chemoradiotherapy using cisplatin, tegafur, and leucovorin for advanced squamous cell carcinoma of the hypopharynx and oropharynx. Biomed J. 2014;37(3):133-40.
-
16Aapro M, Andre F, Blackwell K, Clavo E, Jahanzeb M, Papazisis K, et al. Adverse event management in patients with advanced cancer receiving oral everolimus: focus on breast cancer. Ann Oncol. 2014;25(4):763-73. Review.
-
17Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, et al. Long-term outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin J Cancer. 2013;32(10):525-32.
-
18Lin HX, Hua YJ, Chen QY, Luo DH, Sun R, Qiu F, et al. Randomized study of sinusoidal chronomodulated versus flat intermittent induction chemotherapy with cisplatin and 5-fluorouracil followed by traditional radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. 2013;32(9):502-11.
-
19Iwata H, Fujii H, Masuda N, Mukai H, Nishimura Y, Katsura K, et al. Efficacy, safety, pharmacokinetics and biomarker findings in patients with HER2-positive advanced or metastatic breast cancer treated with lapatinib in combination with capecitabine: results from 51 Japanese patients treated in a clinical study. Breast Cancer. 2015;22(2):192-200.
-
20Palappallil DS, Nair BL, Jayakumar KL, Puvathalil RT. Comparative study of the toxicity of 5-fluorouracil-adriamycin-cyclophosphamide versus adriamycincyclophosphamide followed by paclitaxel in carcinoma breast. Indian J Cancer. 2011;48(1):68-73.
-
21Baird R, Biondo A, Chhaya V, McLachlan J, Karpathakis A, Rahman S, et al. Toxicity associated with capecitabine plus oxaliplatin in colorectal cancer before and after an institutional policy of capecitabine dose reduction. Br J Cancer. 2011;104(1):43-50.
-
22Lee JO, Lee KW, Oh DY, Kim JH, Im SA, Kim TY, et al. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann Oncol. 2009;20(8):1402-7.
-
23Abromowitch M, Sposto R, Perkins S, Zwick D, Siegel S, Finlay J, Cairo MS; Children's Oncology Group. Shortened intensified multiagent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: report from the Children's Oncology Group. Br J Haematol. 2008;143(2):261-7.
-
24Shin HJ, Chung JS, Lee JJ, Sohn SK, Choi YJ, Kim YK, et al. Treatment Outcomes with CHOP chemotherapy in adult patients with hemophagocytic lymphohistiocytosis. J Korean Med Sci. 2008;23(3):439-44.
-
25Blijlevens N, Schwenkglenks M, Bacon P, D'Addio A, Einsele H, Maertens J, Niederwieser D, Rabitsch W, Roosaar A, Ruutu T, Schouten H, Stone R, Vokurka S, Quinn B, McCann S; European Blood and Marrow Transplantation Mucositis Advisory Group. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy-European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26(9):1519-25.
-
26Fadda G, Campus G, Lugliè P. Risk factors for oral mucositis in paediatric oncology patients receiving alkylant chemotherapy. BMC Oral Health. 2006;6:13.
-
27Castagna L, Magagnoli M, Balzarotti M, Sarina B, Siracusano L, Nozza A, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in refractory/relapsed Hodgkin's lymphoma: a monocenter prospective study. Am J Hematol. 2007;82(2):122-7.
-
28Oh DY, Kim TY, Kwon JH, Lee JJ, Joh Y, Kim DW, et al. Docetaxel + 5-fluorouracil + cisplatin 3-day combination chemotherapy as a first-line treatment in patients with unresectable gastric cancer. Jpn J Clin Oncol. 2005;35(7):380-5.
-
29Schmid P, Schippinger W, Nitsch T, Huebner G, Heilmann V, Schultze W, et al. Up-front tandem high-dose chemotherapy compared with standard chemotherapy with doxorubicin and paclitaxel in metastatic breast cancer: results of a randomized trial. J Clin Oncol. 2005;23(3):432-40.
-
30Saini A, Norman AR, Cunningham D, Chau I, Hill M, Tait D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer. 2003;88(12):1859-65.
-
31Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, et al. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002;30(12):893-8.
-
32Lacayo NJ, Lum BL, Becton DL, Weinstein H, Ravindranath Y, Chang MN, et al. Pharmacokinetic interactions of cyclosporine with etoposide and mitoxantrone in children with acute myeloid leukemia. Leukemia. 2002; 16(5):920-7.
-
33Leblond V, Lévy V, Maloisel F, Cazin B, Fermand JP, Harousseau JL, Remenieras L, Porcher R, Gardembas M, Marit G, Deconinck E, Desablens B, Guilhot F, Philippe G, Stamatoullas A, Guibon O; French Cooperative Group on Chronic Lymphocytic Leukemia and Macroglobulinemia. Multicenter, randomized comparative trial of fludarabine and the combination of cyclophosphamide-doxorubicin-prednisone in 92 patients with Waldenström macroglobulinemia in first relapse or with primary refractory disease. Blood. 2001;98(9):2640-4.
-
34Lissoni A, Gabriele A, Gorga G, Tumolo S, Landoni F, Mangioni C, et al. Cisplatin, epirubicin and paclitaxel-containing chemotherapy in uterine adenocarcinoma. Ann Oncol. 1997;8(10):969-72.
-
35Wang WS, Tzeng CH, Chiou TJ, Liu JH, Hsieh RK, Yen CC, et al. High-dose cytarabine and mitoxantrone as salvage therapy for refractory non-hodgkin's lymphoma. Jpn J Clin Oncol. 1997;27(3):154-7. Review.
-
36Anderson H, Hopwood P, Prendiville J, Radford JA, Thatcher N, Ashcroft L. A randomized study of bolous vs continuous pump infusion of ifosfamide and doxorubicin with oral etoposide for small cell lung cancer. Br J Cancer. 1993;67(6):1385-90.
-
37Bishop JF, Lowenthal RM, Joshua D, Mattews JP, Todd D, Cobcroft R, et al. Etoposide in acute nonlymphocytic leukemia. Australian Leukemia Study Group. Blood. 1990;75(1):27-32.
-
38Clavel M, Cognetti F, Dodion P, Wildiers J, Rosso R, Rossi A, et al. Combination chemotherapy with methotrexate, bleomycin, and vincristine with or without cisplatin in advanced squamous cell carcinoma of the head and neck. Cancer. 1987;60(6):1173-7.
-
39Almeida VL, Leitão A, Reina LC, Montanari CA, Donnici CL, Lopes MT. [Cancer and cell cicle-specific and cell cicle nonspecific anticancer DNA-interactive agents: an introduction]. Quim Nova. 2005;28(1):118-29. Portuguese.
Publication Dates
-
Publication in this collection
2018
History
-
Received
28 Jan 2017 -
Accepted
22 Aug 2017