ABSTRACT
Objective:
To characterize severe potential drug interactions in maternal intensive care, and to determine their frequency, risk factors and potential risk medications.
Methods:
An observational and longitudinal study conducted between December 2014 and December 2015 in a maternal intensive care unit. Clinical data were collected and severe potential drug interactions were identified on pregnant inpatients. The drug interactions were classified by type, prevalence and exposure rate. A multivariate logistic regression model was used to identify the severe potential drug interactions and the related drugs (p<0.05).
Results:
A total of 95.1% of patients were exposed to, at least, one potential drug interaction; in that, 91.7% 33.9% were related to, respectively, moderate and severe potential drug interactions. The patients were exposed, on average, on 69.2% of days they were in the intensive care unit. The main drugs involved in more severe drug interactions were magnesium sulfate, metoclopramide, propranolol and diazepam.
Conclusion:
The severe potential drug interactions were observed in almost all patients of the study, and, approximately one third of those interactions were related to greater severity and resulted in exposure during long hospital stay. The higher number of prescribed drugs and its previous use of medications at home increase the occurrence of severe potential drug interactions.
Keywords:
Drug interactions; Intensive care units; Maternal health; Drug therapy; Pregnancy
RESUMO
Objetivo:
Caracterizar as interações medicamentosas potenciais graves em terapia intensiva materna, e determinar sua frequência, os fatores e os medicamentos de risco associados à ocorrência dessas interações.
Métodos:
Estudo observacional e longitudinal executado entre dezembro de 2014 a dezembro de 2015, conduzido em uma unidade de terapia intensiva materna. Foram coletados dados clínicos e identificadas interações medicamentosas potenciais graves de gestantes admitidas. As interações medicamentosas foram caracterizadas quanto ao tipo, à prevalência e à taxa de exposição. Um modelo multivariado de regressão logística foi utilizado para identificação de fatores associados à ocorrência de interações medicamentosas potenciais graves e os medicamentos implicados (p<0,05).
Resultados:
Um total de 95,1% das pacientes foi exposto a, no mínimo, uma interação medicamentosa potencial, com 91,7% delas envolvidas com interações medicamentosas potenciais moderadas e 33,9% com as interações graves. As pacientes ficaram expostas, em média, em 69,2% dos dias que estiveram sob terapia intensiva. Os principais medicamentos implicados em interações medicamentosas de maior gravidade foram sulfato de magnésio, metoclopramida, propranolol e diazepam.
Conclusão:
As interações medicamentosas potenciais graves ocorreram na maioria das pacientes avaliadas. Aproximadamente um terço das interações foram graves e levaram à maior exposição por um longo período de internação. Maior número de fármacos prescritos e uso prévio domiciliar de medicamentos elevam a ocorrência de interações medicamentosas potenciais graves.
Descritores:
Interações de medicamentos; Unidade de terapia intensiva; Saúde materna; Tratamento farmacológico; Gravidez
INTRODUCTION
Care of critically ill patients in intensive care units (ICU) entails extensive use of medications and consequent polypharmacy. Prescription of several drugs increase occurrence of adverse events, especially drug interactions, leading to greater costs and longer hospital stay.(11. Uijtendaal EV, van Harssel LL, Hugenholtz GW, Kuck EM, Zwart-van Rijkom JE, Cremer OL, et al. Analysis of potential drug-drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34(3):213-9.–33. Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in cardiac and cardiothoracic intensive care units: an analysis of patients in an academic medical centre in the US. Drug Saf. 2010;33(10):879-88.) A potential drug-drug interaction (PDI) is defined as the concurrent administration of two potentially interactive drugs, with a prevalence of 46% to 80% among intensive care patients.(11. Uijtendaal EV, van Harssel LL, Hugenholtz GW, Kuck EM, Zwart-van Rijkom JE, Cremer OL, et al. Analysis of potential drug-drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34(3):213-9.,44. Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in the medical intensive care unit: an assessment of frequency, severity and the medications involved. Int J Pharm Pract. 2012;20(6):402-8.–66. Santibáñez C, Roque J, Morales G, Corrales R. [Characteristics of drug interactions in a pediatric intensive care unit]. Rev Chil Pediatr. 2014;85(5): 546-53. Spanish.) Because of clinical severity, critically ill patients have a lower capacity for drug elimination, increasing the risk of damage resulting from PDI.(33. Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in cardiac and cardiothoracic intensive care units: an analysis of patients in an academic medical centre in the US. Drug Saf. 2010;33(10):879-88.–55. Farzanegan B, Alehashem M, Bastani M, Baniasadi S. Potential drug-drug interactions in cardiothoracic intensive care unit of a pulmonary teaching hospital. J Clin Pharmacol. 2015;55(2):132-6.,77. Smithburger PL, Kane-Gill SL, Benedict NJ, Falcione BA, Seybert AL. Grading the severity of drug-drug interactions in the intensive care unit: a comparison between clinician assessment and proprietary database severity rankings. Ann Pharmacother. 2010;44(11):1718-24.)
The treatment of pregnant women at ICU is associated with emergency obstetric care.(88. Donati S, Senatore S, Ronconi A; Regional Maternal Mortality Working Group. Obstetric near-miss cases among women admitted to intensive care units in Italy. Acta Obstet Gynecol Scand. 2012;91(4):452-7.,99. Rios FG, Risso-Vázquez A, Alvarez J, Vinzio M, Falbo P, Rondinelli N, et al. Clinical characteristics and outcomes of obstetric patients admitted to the intensive care unit. Int J Gynaecol Obstet. 2012;119(2):136-40.) In developed countries, it is estimated that 2% or less of pregnant women are admitted to the ICU, while in developing countries this rate is approximately 10%.(99. Rios FG, Risso-Vázquez A, Alvarez J, Vinzio M, Falbo P, Rondinelli N, et al. Clinical characteristics and outcomes of obstetric patients admitted to the intensive care unit. Int J Gynaecol Obstet. 2012;119(2):136-40.) The main reasons for admission in maternal ICU are related to hemorrhage and hypertensive disorders of pregnancy.(88. Donati S, Senatore S, Ronconi A; Regional Maternal Mortality Working Group. Obstetric near-miss cases among women admitted to intensive care units in Italy. Acta Obstet Gynecol Scand. 2012;91(4):452-7.–1010. Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, Bateman BT. Epidemiology of obstetric-related ICU admissions in Maryland: 1999-2008. Crit Care Med. 2013;41(8):1844-52.) Other less common reasons include heart disease, genitourinary infection, obstetric complications, such as ectopic pregnancy and miscarriages, non-genitourinary infection, sepsis, cerebrovascular disease, and pulmonary embolism.(99. Rios FG, Risso-Vázquez A, Alvarez J, Vinzio M, Falbo P, Rondinelli N, et al. Clinical characteristics and outcomes of obstetric patients admitted to the intensive care unit. Int J Gynaecol Obstet. 2012;119(2):136-40.,1010. Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, Bateman BT. Epidemiology of obstetric-related ICU admissions in Maryland: 1999-2008. Crit Care Med. 2013;41(8):1844-52.)
The use of drugs in maternal ICU presents some peculiarities. Physiology changes during pregnancy, leading to modified actions of medications on the body.(1111. Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. Review.) Pregnant women present significant pharmacokinetic changes. The increase in adipose tissue increases the distribution volume and the half-life of lipophilic drugs. Other changes include increased activity of liver enzymes and glomerular filtration rate, with risk of fetal exposure to lipophilic drugs.(1111. Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. Review.–1313. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. Review.)
Most studies on PDI are limited to characterizing the frequency of medications and therapeutic classes involved, without highlighting which factors contribute to the occurrence of PDI and their possible consequences. However there is scarce literature on frequency of clinically significant PDI in pregnant women, in addition to characterization of these events in patients admitted to maternal ICU.
OBJECTIVE
To characterize severe potential drug-drug interactions in maternal intensive care units and determine the frequency, risks factors, and potential risk medications.
METHODS
An observational, descriptive, cross-sectional study conducted at the 6-bed maternal ICU of the Maternidade Escola Januário Cicco (MEJC), with an average of 53 admissions per month. All patients admitted to the ICU during the study period who stayed for more than 24 hours, and had more than two drugs prescribed were considered eligible. Patients previously included in the sample were excluded. A total of 348 patients were evaluated between December 2014 and December 2015.
This project was approved by the Research Ethics Committee of Hospital Universitário Onofre Lopes, protocol 496.656, CAAE: 21536713.0.0000.5292.
Clinical data related to previous pregnancies, comorbidity score as per the Mortality Probability Model (MMP) upon admission, clinical diagnosis according to the International Classification of Diseases and Related Health Problems - Tenth Edition (ICD-10), and prescribed drugs were collected. All patients were daily monitored for occurrence of drug interactions, assessed as to the prescribed items and time of exposure to the PDI throughout the hospitalization period.
The software Lexi-Interact was used to identify PDI in medical prescriptions. It consists of a database with restricted online access, with updated information on more than 8,000 unique drug terms. The database enables classifying the PDI as minor, moderate or severe, according to severity level. Severe PDI can be life-threatening and/or require intervention to reduce or prevent damage; moderate interactions imply exacerbation of the clinical condition, and possible changes in the prescription; and minor PDI imply a limited clinical effect with the possibility of worsening, but with no need for intervention.(1414. UptoDate. Drugs & Drug Interaction. Lexi-interact online [Internet]. Wolters Kluwer; 2018 [cited 2014 Dec 1]. Available from: https://www.uptodate.com/home/drugs-drug-interaction
https://www.uptodate.com/home/drugs-drug...
)
Statistical analysis was carried out using Stata release 11 (Stata Corporation, College Station, TX, USA). In the descriptive analysis, the characteristics of the patients were presented as mean and standard deviation, or relative or absolute frequencies, when appropriate. The rate of exposure to drug interactions was calculated by the ratio between the number of days exposed to the event and the total length of hospital stay. For each variable studied, the number of drug interactions per day of hospitalization was obtained by multiplying the number of events by the exposure rate. The identification of risk factors for severe drug interactions was conducted by univariate and multivariate analysis using logistic regression. Only the variables presenting a p value <0.10 were included in the multivariate model (p<0.05). To determine the prescription drugs at the highest risk of causing severe drug interactions we used regression adjusted by the risk factors identified and those already reported as being associated with drug interactions (number of medications, length of stay and age).
RESULTS
During the study, 348 patients were assessed (Table 1). The mean age was 26.4 years, with a mean of two pregnancies; 26.2% reported at least one miscarriage. In the sample, there was a predominance of cesarean section (57.8%), but roughly 37% were still pregnant. Gestational hypertension was characterized as the most frequent diagnosis (65.9%), followed by eclampsia (7.5%), and urinary infection (2.6%). Patients had a mean length of ICU stay of 3 days (standard deviation of 2.1 days).
Characteristics of the population (n=348) and profile of the potential drug interactions in relation to type and exposure rate of patients
Approximately 8±3.5 medications were administered during hospitalization. About 95.1% of patients were exposed to at least one PDI; in that, 52.9% of minor, and 91.7% of moderate severity (Table 2). About one-third of patients (33.9%) had a more severe PDI. Table 2 also emphasizes that during hospital stay patients were, on average, 69.2% of period exposed to at least one severe PDI.
Profile of the potential drug interactions in relation to type and exposure rate of patients
In the univariate logistic regression analysis, the occurrence of PDI was significantly associated with number of drugs administered, use of medications at home before hospitalization, age and diagnosis of urinary infection. These variables were included in the multivariate model (Table 3), in which a significant association was found between the occurrence of severe PDI and the number of drugs administered (odds ratio - OR: 1.106; 95% confidence interval - 95%CI: 1.073-1.139). There was also a significant association between the occurrence of severe PDI and the medications used at home (OR: 1.317; 95%CI: 1.022-1.696). The diagnosis of urinary tract infection was, however, less likely to be associated with occurrence of severe PDI (OR: 0.334; 95%CI: 0.136-0.854).
Univariate and multivariate logistic regression analysis of risk factors for occurrence of severe drug interactions
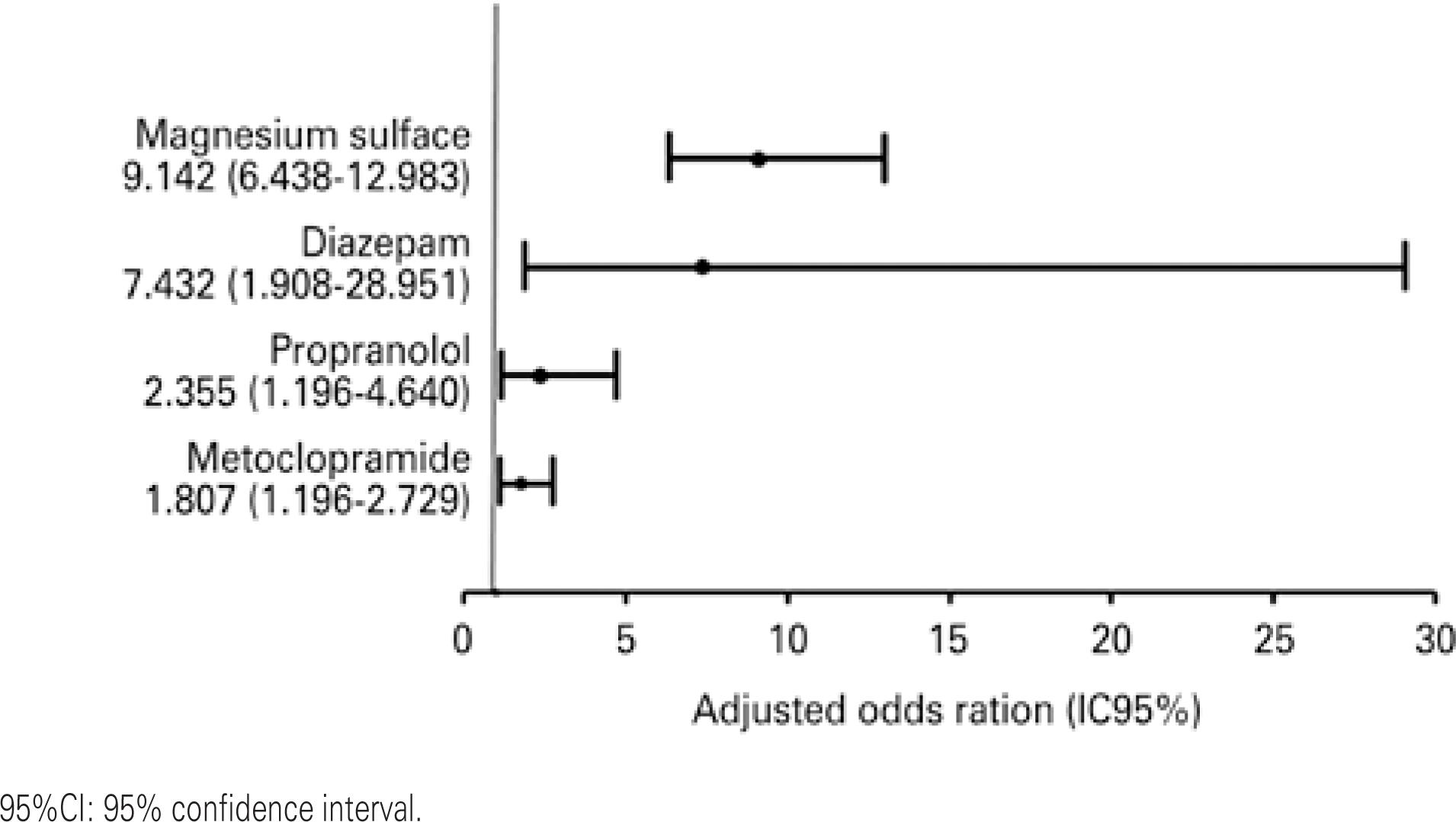
Among the drugs related to severe PDI, diazepam, magnesium sulfate, propranolol, and metoclopramide presented greater risk in pregnant women under intensive care (Figure 1), representing 66.5% of interactions. Magnesium sulfate accounted for 37.8% of severe PDI and presented the highest frequency, as depicted in table 4. Although it is a potentially dangerous drug, diazepam was only responsible for 7.1% of severe PDI detected, whereas propranolol and metoclopramide accounted for 4.9% and 16.6%, respectively.
Medications at risk of severe drug-drug interactions raves in pregnant women under intensive care (multiple regression model adjusted for age, number of prescribed medications, length of stay, use of medications before hospitalization, and diagnosis of urinary infection)
DISCUSSION
The occurrence of PDI in maternal intensive care is observed in almost all patients, and the exposure time covers 69.2% of hospitalization period. Compared to other intensive care modalities, the prevalence of PDI detected in pregnant women was markedly higher. For adult and cardiac ICU, e.g., the prevalence of patients exposed to at least one PDI ranges from 50 to 80%, and severe PDI occur in percentages lower than those found in this study.(33. Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in cardiac and cardiothoracic intensive care units: an analysis of patients in an academic medical centre in the US. Drug Saf. 2010;33(10):879-88.–55. Farzanegan B, Alehashem M, Bastani M, Baniasadi S. Potential drug-drug interactions in cardiothoracic intensive care unit of a pulmonary teaching hospital. J Clin Pharmacol. 2015;55(2):132-6.) A systematic review included nine studies and 3,150 intensive care patients evaluated, found that about 63% of patients were exposed to at least one PDI.(1515. Zheng WY, Richardson LC, Li L, Day RO, Westbrook JI, Baysari MT. Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):15-27.)
It is important to emphasize that prescription of drugs with potential drug interactions does not necessarily imply harm to patients. Nonetheless, the risk is higher when PDI are classified as severe.(55. Farzanegan B, Alehashem M, Bastani M, Baniasadi S. Potential drug-drug interactions in cardiothoracic intensive care unit of a pulmonary teaching hospital. J Clin Pharmacol. 2015;55(2):132-6.) In this study, about one-third of pregnant women had at least one severe PDI and were exposed for more than two-thirds of the hospitalization period. Obstetric patients present physiological changes that alter the pharmacokinetics and pharmacodynamics of numerous drugs, thus increasing the risk of harm associated with use of drugs, especially when there is interaction between them, which may affect the dyad mother-fetus.(1111. Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. Review.,1212. Nielsen OH, Maxwell C, Hendel J. IBD medications during pregnancy and lactation. Nat Rev Gastroenterol Hepatol. 2014;11(2):116-27. Review.,1616. Small MJ, James AH, Kershaw T, Thames B, Gunatilake R, Brown H. Near-miss maternal mortality: cardiac dysfunction as the principal cause of obstetric intensive care unit admissions. Obstet Gynecol. 2012;119(2 Pt 1):250-5.) Therefore, considering the prevalence and duration of exposure, severe PDI should be considered in the pharmacological approach of these patients.
Approximately 33.9% of patients presented severe PDI, and the prior use of medications before hospitalization and a high number of drugs prescribed during the hospitalization period were identified as risk factors for these events. Polypharmacy is a risk factor already described, and is related to the occurrence of drug interaction and other incidents associated with the use of medications.(1717. Cedraz KN, Santos Junior MC. Identificação e caracterização de interações medicamentosas em prescrições médicas da unidade de terapia intensiva de um hospital público da cidade de Feira de Santana, BA. Rev Soc Bras Clin Med. 2014;12(2):1-7.) The use of medications at home as a risk factor probably indicates greater clinical severity, leading to the use of multiple drugs during hospitalization. In contrast, urinary tract infection appeared as a reducing factor, which may be justified by the treatment approach applied in those cases in which a smaller number of drugs related to severe PDI are used.
Patients who were prescribed magnesium sulfate, diazepam, propranolol and metoclopramide were at increased risk for severe PDI. Magnesium sulfate is not commonly used in other intensive care modalities and is indicated for hypertensive disorders of pregnancy. The other drugs are more frequently related to more severe PDI in intensive care in general.(66. Santibáñez C, Roque J, Morales G, Corrales R. [Characteristics of drug interactions in a pediatric intensive care unit]. Rev Chil Pediatr. 2014;85(5): 546-53. Spanish.,1818. Ramos GV, Guaraldo L, Japiassú AM, Bozza FA. Comparison of two databases to detect potential drug-drug interactions between prescriptions of HIV/AIDS patients in critical care. J Clin Pharm Ther. 2015;40(1):63-7.)
Magnesium sulfate is the drug with the highest risk of causing severe PDI, especially when it is associated with nifedipine. The association can cause hypotension, hypocalcemia, neuromuscular blockade, myocardial depression, as well as pulmonary edema.(1919. Davis WB, Wells SR, Kuller JA, Thorp JM Jr. Analysis of the risks associated with calcium channel blockade: implications for the obstetrician-gynecologist. Obstet Gynecol Surv. 1997;52(3):198-201. Review.,2020. Xiao C, Gangal M, Abenhaim HA. Effect of magnesium sulfate and nifedipine on the risk of developing pulmonary edema in preterm births. J Perinat Med. 2014;42(5):585-9.) The mechanism of interaction involves the reduction of flow in the calcium channels in myocardial cells, in addition to blocking the adrenal release of norepinephrine, antagonizing the vasopressor effects.(2121. Neustein S, Dimich I, Shiang H, Bernstein H, Beilin Y. Cardiovascular consequences of the concomitant administration of nifedipine and magnesium sulfate in pigs. Int J Obstet Anesth. 1998;7(4):247-50.)
The association of propranolol and methyldopa is not recommended, since it may cause hypertensive reaction, tachycardia or arrhythmia due to peripheral blockade of beta-adrenergic receptors, warranting the effect of vasoconstriction by alpha adrenergic receptors.(2222. Nies AS, Shand DG. Hypertensive response to propranolol in a patient treated with methyldopa-a proposed mechansim. Clin Pharmacol Ther. 1973;14(5): 823-6.) Propranolol should also be avoided with cabergoline, due to block of normal sympathetic vasodilatation stimulated by beta-2 receptors, resulting in excessive vasoconstriction, and may cause hypertension or even peripheral ischemia.(2323. Venter CP, Joubert PH, Buys AC. Severe peripheral ischaemia during concomitant use of beta blockers and ergot alkaloids. Br Med J (Clin Res Ed). 1984;289(6440):288-9.,2424. Beeley L. Drug interactions and beta blockers. Br Med J (Clin Res Ed). 1984; 289(6455):1330-1.)
Severe PDI involving diazepam are associated with depression of neural activity and significant changes in drug metabolism. Diazepam and phenobarbital act to potentiate the gamma-aminobutyric acid (GABA) inhibitory effect, favoring the opening of the chlorine channels in the neural membrane.(2525. Sankar R. GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam. CNS Drugs. 2012;26(3):229-44. Review.–2727. Short TG, Galletly DC, Plummer JL. Hypnotic and anaesthetic action of thiopentone and midazolam alone and in combination. Br J Anaesth. 1991; 66(1):13-9.) Simultaneous use of these drugs may lead to potentiation of neuronal inhibition and, clinically, to central nervous system depression.(2727. Short TG, Galletly DC, Plummer JL. Hypnotic and anaesthetic action of thiopentone and midazolam alone and in combination. Br J Anaesth. 1991; 66(1):13-9.) Benzodiazepine, when associated with antipsychotic drugs, like levomepromazine, causes increased blocking of dopaminergic receptors, which may lead to extrapyramidal adverse events or other effects associated with these receptors.(2828. Meyer J. Drug-drug interactions with antipsychotics. CNS Spectr. 2007;12(12 Suppl 21):6-9. Review.)
Drug interactions between diazepam and phenytoin occur at the pharmacokinetic level, with hydantoin being metabolized by hepatic isoenzymes CYP2C9 and CYP2C19.(2929. Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, Klein TE, Callaghan JT; Clinical Pharmacogenetics Implementation Consortium. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther. 2014; 96(5):542-8.,3030. Bojanić Z, Bojanić N, Bojanić V, Lazović M. Drug interactions with diazepam. Acta Med Medianae. 2011;50(2):76-82.) Benzodiazepines are metabolized by a distinct isoform (CYP3A4), however, diazepam is specifically metabolized extensively by CYP2C19.(3131. Murphy A, Wilbur K. Phenytoin-diazepam interaction. Ann Pharmacother. 2003; 37(5):659-63.) Diazepam may, therefore, compete with the metabolizing enzyme and significantly increase phenytoin concentrations, inducing toxicity.(2929. Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, Klein TE, Callaghan JT; Clinical Pharmacogenetics Implementation Consortium. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther. 2014; 96(5):542-8.,3030. Bojanić Z, Bojanić N, Bojanić V, Lazović M. Drug interactions with diazepam. Acta Med Medianae. 2011;50(2):76-82.) Carbamazepine, may compromise the therapeutic action of diazepam through metabolic activity of the CYP3A4 isoenzymes.(3131. Murphy A, Wilbur K. Phenytoin-diazepam interaction. Ann Pharmacother. 2003; 37(5):659-63.–3434. Vajda FJ, Eadie MJ. The clinical pharmacology of traditional antiepileptic drugs. Epileptic Disord. 2014;16(4):395-408. Review.)
Metoclopramide also had a high frequency of more severe PDI, especially when used simultaneously with antipsychotics. The antiemetic action of metoclopramide is due to dopamine antagonism in D2 receptors.(3535. Mazhar F, Akram S, Haider N, Ahmed R. Overlapping of serotonin syndrome with neuroleptic malignant syndrome due to linezolid-fluoxetine and olanzapine-metoclopramide interactions: a case report of two serious adverse drug effects caused by medication reconciliation failure on hospital admission. Case Rep Med. 2016;2016:7128909.) The association with neuroleptics can cause intense dopaminergic blockade in the striatum and hypothalamus, favoring the occurrence of extrapyramidal effects and, in extreme cases, neuroleptic malignant syndrome.(3636. Wittmann O, Sadot E, Bisker-Kassif O, Scolnik D, Tavor O, Glatstein MM. Neuroleptic malignant syndrome associated with metoclopramide use in a boy. Am J Ther. 2016;23(5):e1246-9. Review.)
It is important to emphasize that this study characterizes PDI in pregnant women in intensive care, and data on this topic is scarcely found in the literature. Likewise other intensive care modalities, drug interactions are a major concern in medical practice, since they may have detrimental consequences for health care.(44. Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in the medical intensive care unit: an assessment of frequency, severity and the medications involved. Int J Pharm Pract. 2012;20(6):402-8.,66. Santibáñez C, Roque J, Morales G, Corrales R. [Characteristics of drug interactions in a pediatric intensive care unit]. Rev Chil Pediatr. 2014;85(5): 546-53. Spanish.,77. Smithburger PL, Kane-Gill SL, Benedict NJ, Falcione BA, Seybert AL. Grading the severity of drug-drug interactions in the intensive care unit: a comparison between clinician assessment and proprietary database severity rankings. Ann Pharmacother. 2010;44(11):1718-24.) The identification, description and monitoring of potential interactions are, therefore, important in order to minimize pharmacotherapy-related risks. Similar studies may contribute to establishing automated alert systems aimed to the multiprofessional team at ICU, enabling less exposure of patients to severe drug interactions. The findings also indicated that a clinical pharmacist should monitor the patients, adequately evaluating the occurrence of PDI in the prescription and making it possible to prevent damage.(11. Uijtendaal EV, van Harssel LL, Hugenholtz GW, Kuck EM, Zwart-van Rijkom JE, Cremer OL, et al. Analysis of potential drug-drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34(3):213-9.,3737. Riechelmann RP, Moreira F, Smaletz O, Saad ED. Potential for drug interactions in hospitalized cancer patients. Cancer Chemother Pharmacol. 2005;56(3):286-90.)
This paper presents some limitations. Data was collected in one single center, which may compromise generalizing the findings. Despite the prospective collection, some information may have been missed due to failures in the process of medical records. It is important to emphasize that the interactions described are potential, that is, they were classified from the medical prescription, and did not necessarily imply the occurrence of negative clinical outcomes. The detection of PDI, even those classified as severe, should be contextualized with the patient's clinical presentation, and the risk-benefit of the treatment should always be considered. Another aspect that may decrease the capacity of generalizing the results involves possible disagreement between the databases and compendia regarding the class and severity of drug interactions.(3838. Malone DC, Abarca J, Hansten PD, Grizzle AJ, Armstrong EP, Van Bergen RC, et al. Identification of serious drug-drug interactions: results of the partnership to prevent drug-drug interactions. J Am Pharm Assoc (2003). 2004;44(2):142-51.) However, the literature indicates that the software used in this study is solidly based on clinical evidence and is constantly updated.(3838. Malone DC, Abarca J, Hansten PD, Grizzle AJ, Armstrong EP, Van Bergen RC, et al. Identification of serious drug-drug interactions: results of the partnership to prevent drug-drug interactions. J Am Pharm Assoc (2003). 2004;44(2):142-51.,3939. Zheng WY, Richardson LC, Li L, Day RO, Westbrook JI, Baysari MT. Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):15-27.)
New investigations should be conducted in order to evaluate clinical outcomes related to drug interactions, especially correlating them with clinical parameters, length of stay, and mortality.
CONCLUSION
Almost all pregnant women in maternal intensive care are exposed to at least one potential drug-drug interaction. More than one-third of potential drug-drug interactions are severe and occur during most of the hospitalization period. The factors associated with severe potential drug-drug interactions are prior and use of medication at home, as well as a greater number of drugs prescribed during hospitalization, whereas the diagnosis of urinary tract infection is inversely related to the occurrence of potential drug-drug interaction. The drugs most implicated in potentially severe drug-drug interaction were magnesium sulfate, metoclopramide, propranolol and diazepam. It is important to emphasize that the identification, description, and monitoring of potential interactions are important to ensure safety of pregnant woman under intensive care.
REFERENCES
-
1Uijtendaal EV, van Harssel LL, Hugenholtz GW, Kuck EM, Zwart-van Rijkom JE, Cremer OL, et al. Analysis of potential drug-drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34(3):213-9.
-
2Ahn EK, Kam HJ, Park DK, Jung EY, Lee Y, Park RW. Differences among admitting departments in alerts and alert overrides for drug-drug interaction. Pharmacoepidemiol Drug Saf. 2014;23(4):390-7.
-
3Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in cardiac and cardiothoracic intensive care units: an analysis of patients in an academic medical centre in the US. Drug Saf. 2010;33(10):879-88.
-
4Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in the medical intensive care unit: an assessment of frequency, severity and the medications involved. Int J Pharm Pract. 2012;20(6):402-8.
-
5Farzanegan B, Alehashem M, Bastani M, Baniasadi S. Potential drug-drug interactions in cardiothoracic intensive care unit of a pulmonary teaching hospital. J Clin Pharmacol. 2015;55(2):132-6.
-
6Santibáñez C, Roque J, Morales G, Corrales R. [Characteristics of drug interactions in a pediatric intensive care unit]. Rev Chil Pediatr. 2014;85(5): 546-53. Spanish.
-
7Smithburger PL, Kane-Gill SL, Benedict NJ, Falcione BA, Seybert AL. Grading the severity of drug-drug interactions in the intensive care unit: a comparison between clinician assessment and proprietary database severity rankings. Ann Pharmacother. 2010;44(11):1718-24.
-
8Donati S, Senatore S, Ronconi A; Regional Maternal Mortality Working Group. Obstetric near-miss cases among women admitted to intensive care units in Italy. Acta Obstet Gynecol Scand. 2012;91(4):452-7.
-
9Rios FG, Risso-Vázquez A, Alvarez J, Vinzio M, Falbo P, Rondinelli N, et al. Clinical characteristics and outcomes of obstetric patients admitted to the intensive care unit. Int J Gynaecol Obstet. 2012;119(2):136-40.
-
10Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, Bateman BT. Epidemiology of obstetric-related ICU admissions in Maryland: 1999-2008. Crit Care Med. 2013;41(8):1844-52.
-
11Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. Review.
-
12Nielsen OH, Maxwell C, Hendel J. IBD medications during pregnancy and lactation. Nat Rev Gastroenterol Hepatol. 2014;11(2):116-27. Review.
-
13Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. Review.
-
14UptoDate. Drugs & Drug Interaction. Lexi-interact online [Internet]. Wolters Kluwer; 2018 [cited 2014 Dec 1]. Available from: https://www.uptodate.com/home/drugs-drug-interaction
» https://www.uptodate.com/home/drugs-drug-interaction -
15Zheng WY, Richardson LC, Li L, Day RO, Westbrook JI, Baysari MT. Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):15-27.
-
16Small MJ, James AH, Kershaw T, Thames B, Gunatilake R, Brown H. Near-miss maternal mortality: cardiac dysfunction as the principal cause of obstetric intensive care unit admissions. Obstet Gynecol. 2012;119(2 Pt 1):250-5.
-
17Cedraz KN, Santos Junior MC. Identificação e caracterização de interações medicamentosas em prescrições médicas da unidade de terapia intensiva de um hospital público da cidade de Feira de Santana, BA. Rev Soc Bras Clin Med. 2014;12(2):1-7.
-
18Ramos GV, Guaraldo L, Japiassú AM, Bozza FA. Comparison of two databases to detect potential drug-drug interactions between prescriptions of HIV/AIDS patients in critical care. J Clin Pharm Ther. 2015;40(1):63-7.
-
19Davis WB, Wells SR, Kuller JA, Thorp JM Jr. Analysis of the risks associated with calcium channel blockade: implications for the obstetrician-gynecologist. Obstet Gynecol Surv. 1997;52(3):198-201. Review.
-
20Xiao C, Gangal M, Abenhaim HA. Effect of magnesium sulfate and nifedipine on the risk of developing pulmonary edema in preterm births. J Perinat Med. 2014;42(5):585-9.
-
21Neustein S, Dimich I, Shiang H, Bernstein H, Beilin Y. Cardiovascular consequences of the concomitant administration of nifedipine and magnesium sulfate in pigs. Int J Obstet Anesth. 1998;7(4):247-50.
-
22Nies AS, Shand DG. Hypertensive response to propranolol in a patient treated with methyldopa-a proposed mechansim. Clin Pharmacol Ther. 1973;14(5): 823-6.
-
23Venter CP, Joubert PH, Buys AC. Severe peripheral ischaemia during concomitant use of beta blockers and ergot alkaloids. Br Med J (Clin Res Ed). 1984;289(6440):288-9.
-
24Beeley L. Drug interactions and beta blockers. Br Med J (Clin Res Ed). 1984; 289(6455):1330-1.
-
25Sankar R. GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam. CNS Drugs. 2012;26(3):229-44. Review.
-
26Löscher W, Rogawski MA. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia. 2012;53 Suppl 8:12-25. Review.
-
27Short TG, Galletly DC, Plummer JL. Hypnotic and anaesthetic action of thiopentone and midazolam alone and in combination. Br J Anaesth. 1991; 66(1):13-9.
-
28Meyer J. Drug-drug interactions with antipsychotics. CNS Spectr. 2007;12(12 Suppl 21):6-9. Review.
-
29Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, Klein TE, Callaghan JT; Clinical Pharmacogenetics Implementation Consortium. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther. 2014; 96(5):542-8.
-
30Bojanić Z, Bojanić N, Bojanić V, Lazović M. Drug interactions with diazepam. Acta Med Medianae. 2011;50(2):76-82.
-
31Murphy A, Wilbur K. Phenytoin-diazepam interaction. Ann Pharmacother. 2003; 37(5):659-63.
-
32Bachmann KA. Interações medicamentosas: um guia completo dos substratos, indutores e inibidores de enzimas do citocromo P450. 2a ed. São Paulo: Manole; 2006.
-
33Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43 Suppl 2:37-44. Review.
-
34Vajda FJ, Eadie MJ. The clinical pharmacology of traditional antiepileptic drugs. Epileptic Disord. 2014;16(4):395-408. Review.
-
35Mazhar F, Akram S, Haider N, Ahmed R. Overlapping of serotonin syndrome with neuroleptic malignant syndrome due to linezolid-fluoxetine and olanzapine-metoclopramide interactions: a case report of two serious adverse drug effects caused by medication reconciliation failure on hospital admission. Case Rep Med. 2016;2016:7128909.
-
36Wittmann O, Sadot E, Bisker-Kassif O, Scolnik D, Tavor O, Glatstein MM. Neuroleptic malignant syndrome associated with metoclopramide use in a boy. Am J Ther. 2016;23(5):e1246-9. Review.
-
37Riechelmann RP, Moreira F, Smaletz O, Saad ED. Potential for drug interactions in hospitalized cancer patients. Cancer Chemother Pharmacol. 2005;56(3):286-90.
-
38Malone DC, Abarca J, Hansten PD, Grizzle AJ, Armstrong EP, Van Bergen RC, et al. Identification of serious drug-drug interactions: results of the partnership to prevent drug-drug interactions. J Am Pharm Assoc (2003). 2004;44(2):142-51.
-
39Zheng WY, Richardson LC, Li L, Day RO, Westbrook JI, Baysari MT. Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):15-27.
Publication Dates
-
Publication in this collection
30 May 2019 -
Date of issue
2019
History
-
Received
11 Apr 2018 -
Accepted
12 Nov 2018