Abstracts
A new inseminating fish species of the family Characidae, Bryconadenos tanaothoros, from tributaries of the upper rio Xingu and upper rio Tapajós basins, Mato Grosso, Brazil is described as the type species of a new genus. This new species and the genus are characterized by a glandular organ on the anterior region of the anal fin of sexually mature males, curved lower jaw teeth, and an inseminating reproductive mode. This new genus is hypothesized as most closely related to Attonitus, a genus with three inseminating species from Peru. Bryconadenos and Attonitus are suggested as related to certain inseminating, but undescribed characid species of uncertain relationships that are similar in certain respects to species of the glandulocaudine Planaltina and to the inseminating species of Knodus. These and a few other inseminating characids are included in a previous tentative characid subgroup designated as Clade A. No species among a relatively small sample of the many species of the Clade A genus Bryconamericus were found inseminating, except Bryconamericus pectinatus. However, newly collected specimens of B. pectinatus were found to have caudal-fin squamation like that of the species of Knodus and this species is here tentatively referred to Knodus. Our investigations indicate that at least several species of Knodus, including the type species, Knodus meridae, are not inseminating, but we found two inseminating apparently new characid species that currently would be referred to Knodus. These species lack the derived anal-fin rays present in the males of K. pectinatus. Other Clade A taxa known to be inseminating, such as two species of the large genus Creagrutus, three species of Monotocheirodon (two undescribed), and the species and genera of the characid subfamily Glandulocaudinae are briefly discussed regarding possible relationships to Attonitus and Bryconadenos. The anatomical aspects of the primary and secondary sexual characteristics of Bryconadenos and Attonitus are discussed in relation to certain other inseminating characids, such as the species of Brittanichthys and Hollandichthys, that are not currently hypothesized to belong to Clade A and presumably acquired insemination independently. It is concluded that much additional data regarding the reproductive modes as well as other anatomical/physiological systems of characids currently included in and excluded from Clade A are necessary before a reasonably supported phylogeny of Clade A characids and their possible outgroup relatives can be advanced. The anal-fin gland cells of sexually active male Bryconadenos specimens are histologically indistinguishable from club cells (also called alarm substance cells) found within the skin of cypriniforms, characiforms, catfishes, and other otophysan fishes. These cells occur at the skin's surface of the anal-fin gland in male Bryconadenos where they are organized into an organ. Many other adult male characids have club cells at the anal-fin's skin surface, often associated with anal-fin hooks, but were not found organized into an organ as in Bryconadenos. We hypothesize these cells to secrete a pheromone during courtship via holocrine secretion. Males of the genera Lophiobrycon , Glandulocauda, and Mimagoniates, tribe Glandulocaudini, were found to have club cells associated with their caudal-fin organ, but no specialized mucus cells were present as found in the caudal organ of males of the glandulocaudine Corynopoma riisei, tribe Stevardiini (= Corynopomini of past authors). In this species, males have hypertrophied mucus cells hypothesized to be modified for pheromone secretion. Evidence that the derived scales and fin rays of the caudal organ of males of the tribe Glandulocaudini are not homologous with that of other tribes of the Glandulocaudinae, as this subfamily was previously recognized, is discussed and it is concluded that the members of the tribe Glandulocaudini should be recognized as a separate subfamily, the Glandulocaudinae, with possible close relationships to some other Clade A inseminating characids that lack caudal-fin organs. The remaining tribes of the former Glandulocaudinae are here included in the subfamily name Stevardiinae. Many species of these two subfamilies and some of the other inseminating Clade A characids have modified sperm cells with an elongate "binding"cytoplasmic collar and mitochondria located along and beyond the nucleus. This may be an indication of a relationship at a unique level within Clade A characids. However, further research on the derived nature and homology of the sperm cells within inseminating Clade A characids and of the caudal organs of the tribes of the Stevardiinae must be undertaken in order to utilize sperm cell features as characters for studying phylogeny. Finally, the kinds of secretory cells and gross anatomical structures in the tail organs of the stevardiine tribes need detailed research in order to better present hypotheses of phylogeny for the tribes of the Stevardiinae as well as all inseminating Clade A characids.
Inseminating characid phylogeny; putative male pheromone cells; Attonitus; Bryconadenos; Knodus
Uma espécie com inseminação interna da família Characidae, Bryconadenos tanaothoros, de tributários das bacias do alto Tapajós e alto Xingu, Estado de Mato Grosso, Brasil é descrita como espécie nova e como espécie-tipo de um novo gênero. Esta nova espécie e o gênero são caracterizados pela posse de um órgão glandular na região anterior da nadadeira anal dos machos sexualmente maduros, dentes curvos na maxila inferior e um tipo de reprodução por inseminação. A hipótese mais viável indica que este novo gênero é mais intimamente relacionado a Attonitus, um gênero com três espécies inseminadoras do Peru. Sugere-se que Bryconadenos e Attonitus são relacionados a certas espécies inseminadoras mas não descritas de caracídeos de relações incertas, semelhantes em alguns aspectos a espécies do glandulocaudíneo Planaltina e as espécies inseminadoras de Knodus. Estas e alguns outros caracídeos inseminadores são incluídos em um subgrupo tentativamente reconhecido de caracídeos designado de Clado A. Nenhuma espécie de uma amostra relativamente pequena do numeroso grupo de espécies do gênero Bryconamericus revelou-se inseminadora, exceto Bryconamericus pectinatus. Entretanto, exemplares recentemente coletados desta espécie revelaram possuir um arranjo de escamas na nadadeira caudal semelhante áquele encontrado nas espécies de Knodus e por este motivo esta espécie foi tentativamente considerada como pertencendo ao gênero Knodus. Até agora, nossas investigações indicam que pelo menos algumas espécies de Knodus, inclusive a espécie-tipo, Knodus meridae, não são inseminadoras mas encontramos duas espécies inseminadoras de caracídeos aparentemente novas, que presentemente poderiam ser consideradas como pertencendo ao gênero Knodus. Esta espécies, entretanto, não possuem a condição derivada resultante da modificação dos raios da anal presente em K. pectinatus. Outros taxons do Clado A representados por espécies conhecidamente inseminadoras, como duas espécies do grande gênero Creagrutus, três espécies de Monotocheirodon (duas não descritas) e as espécies e gêneros da subfamília Glandulocaudinae são brevemente discutidos como possivelmente relacionados a Attonitus e Bryconadenos. Os aspectos anatômicos das características sexuais primárias e secundárias de Bryconadenos e Attonitus também são brevemente discutidos em relação a outros caracídeos inseminadores como as espécies de Brittanichthys e Hollandichthys que atualmente não pertencem, aparentemente, ao Clado A e presumivelmente tornaram-se inseminadoras independentemente. Conclui-se que muitos dados adicionais relativos aos modos reprodutivos dos caracídeos presentemente incluídos e alguns excluídos do Clado A são necessários antes que uma hipótese filogenética bem sustentada dos caracídeos do Clado A e seus parentes mais próximos possa ser construída. As células glandulares da nadadeira anal de exemplares machos sexualmente ativos de Bryconadenos são histologicamente indistinguíveis das células "club" (células de substância de alarme) encontradas dentro da pele de Cypriniformes, Characiformes, Siluriformes e outros peixes otofíseos. Estas células ocorrem na superfície da pele da glândula da nadadeira anal dos machos de Bryconadenos onde estão agrupadas em forma de órgão. Muitos outros machos adultos de caracídeos têm células "club" na superfície da pele da nadadeira anal, muitas vezes associadas a espinhos ou ganchos, mas nunca formando um órgão como em Bryconadenos. Lançamos a hipótese que estas células secretam um tipo de feromônio durante a corte nupcial, via secreção holócrina. Machos dos gêneros Lophiobrycon , Glandulocauda e Mimagoniates, tribo Glandulocaudini, têm células "club" associadas aos seus órgãos da nadadeira caudal, mas nenhum tipo de células mucosas especializadas foram encontradas como as que estão presentes no órgão caudal dos machos do glandulocaudineo Corynopoma riisei, tribo Stevardiini (= Corynopomini de autores prévios). Nesta espécie os machos possuem células mucosas especializadas, provavelmente modificadas para secreção de feromônio. A evidência de que as escamas e raios modificados das nadadeiras do órgão caudal dos machos da tribo Glandulocaudini não são homólogos àqueles de outras tribos de Glandulocaudinae, como esta subfamília foi previamente reconhecida, é discutida e conclui-se que os membros da tribo Glandulocaudini devem ser reconhecidos como uma subfamília separada, Glandulocaudinae, possivelmente intimamente relacionada com alguns outros caracídeos inseminadores do Clado A, que não possuem órgãos especializados na nadadeira caudal. As demais tribos da outrora subfamília Glandulocaudinae são aqui reunidas sob o nome de subfamília Stevardiinae. Muitas espécies destas duas subfamílias e alguns dos caracídeos inseminadores do Clado A têm células espermáticas modificadas com uma bainha citoplasmática alongada tipo aderida e mitocôndrias localizadas ao longo e além do núcleo. Isto pode significar uma indicação de parentesco em um nível único dentro dos caracídeos do Clado A. Entretanto, pesquisa adicional sobre a natureza derivada e homologia das células espermáticas nos caracídeos do Clado A e dos órgãos caudais das tribos de Stevardiinae precisa ser realizada para utilizar caraterísticas das células espermáticas como caracteres em estudos de filogenia. Finalmente, os tipos de células secretoras e estruturas anatômicas gerais nos órgãos caudais dos Stevardiíneos precisam ser pesquisados em detalhe para que seja possível apresentar hipóteses de relações filogenéticas das tribos de Stevardiinae e de todos os caracídeos inseminadores do clado A.
Putative relationships among inseminating and externally fertilizing characids, with a description of a new genus and species of Brazilian inseminating fish bearing an anal-fin gland in males (Characiformes: Characidae)
Stanley H. WeitzmanI; Naércio A. MenezesII; Hans-Georg EversIII; John R. BurnsIV
IDivision of Fishes, Department of Zoology, National Museum of Natural History, MRC 0159, PO Box 37012, Smithsonian Institution, Washington, D.C., 200013-7012, USA. e-mail: weitzman.stan@nmnh.si.edu
IIMuseu de Zoologia, Universidade de São Paulo, Caixa Postal 42494. 04218-970 São Paulo, SP, Brazil. e-mail: naercio@usp.br
IIIEdgar-Rob Strabe 21, D-20251 Hamburg, Germany. e-mail: hans-georg.evers@t-online.de
IVDepartment of Biological Sciences, George Washington University, Washington, D.C. 20052, USA. e-mail: jrburns@gwu.edu
ABSTRACT
A new inseminating fish species of the family Characidae, Bryconadenos tanaothoros, from tributaries of the upper rio Xingu and upper rio Tapajós basins, Mato Grosso, Brazil is described as the type species of a new genus. This new species and the genus are characterized by a glandular organ on the anterior region of the anal fin of sexually mature males, curved lower jaw teeth, and an inseminating reproductive mode. This new genus is hypothesized as most closely related to Attonitus, a genus with three inseminating species from Peru. Bryconadenos and Attonitus are suggested as related to certain inseminating, but undescribed characid species of uncertain relationships that are similar in certain respects to species of the glandulocaudine Planaltina and to the inseminating species of Knodus. These and a few other inseminating characids are included in a previous tentative characid subgroup designated as Clade A. No species among a relatively small sample of the many species of the Clade A genus Bryconamericus were found inseminating, except Bryconamericus pectinatus. However, newly collected specimens of B. pectinatus were found to have caudal-fin squamation like that of the species of Knodus and this species is here tentatively referred to Knodus. Our investigations indicate that at least several species of Knodus, including the type species, Knodus meridae, are not inseminating, but we found two inseminating apparently new characid species that currently would be referred to Knodus. These species lack the derived anal-fin rays present in the males of K. pectinatus. Other Clade A taxa known to be inseminating, such as two species of the large genus Creagrutus, three species of Monotocheirodon (two undescribed), and the species and genera of the characid subfamily Glandulocaudinae are briefly discussed regarding possible relationships to Attonitus and Bryconadenos. The anatomical aspects of the primary and secondary sexual characteristics of Bryconadenos and Attonitus are discussed in relation to certain other inseminating characids, such as the species of Brittanichthys and Hollandichthys, that are not currently hypothesized to belong to Clade A and presumably acquired insemination independently. It is concluded that much additional data regarding the reproductive modes as well as other anatomical/physiological systems of characids currently included in and excluded from Clade A are necessary before a reasonably supported phylogeny of Clade A characids and their possible outgroup relatives can be advanced.
The anal-fin gland cells of sexually active male Bryconadenos specimens are histologically indistinguishable from club cells (also called alarm substance cells) found within the skin of cypriniforms, characiforms, catfishes, and other otophysan fishes. These cells occur at the skin's surface of the anal-fin gland in male Bryconadenos where they are organized into an organ. Many other adult male characids have club cells at the anal-fin's skin surface, often associated with anal-fin hooks, but were not found organized into an organ as in Bryconadenos. We hypothesize these cells to secrete a pheromone during courtship via holocrine secretion.
Males of the genera Lophiobrycon , Glandulocauda, and Mimagoniates, tribe Glandulocaudini, were found to have club cells associated with their caudal-fin organ, but no specialized mucus cells were present as found in the caudal organ of males of the glandulocaudine Corynopoma riisei, tribe Stevardiini (= Corynopomini of past authors). In this species, males have hypertrophied mucus cells hypothesized to be modified for pheromone secretion. Evidence that the derived scales and fin rays of the caudal organ of males of the tribe Glandulocaudini are not homologous with that of other tribes of the Glandulocaudinae, as this subfamily was previously recognized, is discussed and it is concluded that the members of the tribe Glandulocaudini should be recognized as a separate subfamily, the Glandulocaudinae, with possible close relationships to some other Clade A inseminating characids that lack caudal-fin organs. The remaining tribes of the former Glandulocaudinae are here included in the subfamily name Stevardiinae. Many species of these two subfamilies and some of the other inseminating Clade A characids have modified sperm cells with an elongate "binding"cytoplasmic collar and mitochondria located along and beyond the nucleus. This may be an indication of a relationship at a unique level within Clade A characids. However, further research on the derived nature and homology of the sperm cells within inseminating Clade A characids and of the caudal organs of the tribes of the Stevardiinae must be undertaken in order to utilize sperm cell features as characters for studying phylogeny. Finally, the kinds of secretory cells and gross anatomical structures in the tail organs of the stevardiine tribes need detailed research in order to better present hypotheses of phylogeny for the tribes of the Stevardiinae as well as all inseminating Clade A characids.
Key words: Inseminating characid phylogeny, putative male pheromone cells, Attonitus , Bryconadenos , Knodus.
RESUMO
Uma espécie com inseminação interna da família Characidae, Bryconadenos tanaothoros, de tributários das bacias do alto Tapajós e alto Xingu, Estado de Mato Grosso, Brasil é descrita como espécie nova e como espécie-tipo de um novo gênero. Esta nova espécie e o gênero são caracterizados pela posse de um órgão glandular na região anterior da nadadeira anal dos machos sexualmente maduros, dentes curvos na maxila inferior e um tipo de reprodução por inseminação. A hipótese mais viável indica que este novo gênero é mais intimamente relacionado a Attonitus, um gênero com três espécies inseminadoras do Peru. Sugere-se que Bryconadenos e Attonitus são relacionados a certas espécies inseminadoras mas não descritas de caracídeos de relações incertas, semelhantes em alguns aspectos a espécies do glandulocaudíneo Planaltina e as espécies inseminadoras de Knodus. Estas e alguns outros caracídeos inseminadores são incluídos em um subgrupo tentativamente reconhecido de caracídeos designado de Clado A. Nenhuma espécie de uma amostra relativamente pequena do numeroso grupo de espécies do gênero Bryconamericus revelou-se inseminadora, exceto Bryconamericus pectinatus. Entretanto, exemplares recentemente coletados desta espécie revelaram possuir um arranjo de escamas na nadadeira caudal semelhante áquele encontrado nas espécies de Knodus e por este motivo esta espécie foi tentativamente considerada como pertencendo ao gênero Knodus. Até agora, nossas investigações indicam que pelo menos algumas espécies de Knodus, inclusive a espécie-tipo, Knodus meridae, não são inseminadoras mas encontramos duas espécies inseminadoras de caracídeos aparentemente novas, que presentemente poderiam ser consideradas como pertencendo ao gênero Knodus. Esta espécies, entretanto, não possuem a condição derivada resultante da modificação dos raios da anal presente em K. pectinatus. Outros taxons do Clado A representados por espécies conhecidamente inseminadoras, como duas espécies do grande gênero Creagrutus, três espécies de Monotocheirodon (duas não descritas) e as espécies e gêneros da subfamília Glandulocaudinae são brevemente discutidos como possivelmente relacionados a Attonitus e Bryconadenos. Os aspectos anatômicos das características sexuais primárias e secundárias de Bryconadenos e Attonitus também são brevemente discutidos em relação a outros caracídeos inseminadores como as espécies de Brittanichthys e Hollandichthys que atualmente não pertencem, aparentemente, ao Clado A e presumivelmente tornaram-se inseminadoras independentemente. Conclui-se que muitos dados adicionais relativos aos modos reprodutivos dos caracídeos presentemente incluídos e alguns excluídos do Clado A são necessários antes que uma hipótese filogenética bem sustentada dos caracídeos do Clado A e seus parentes mais próximos possa ser construída.
As células glandulares da nadadeira anal de exemplares machos sexualmente ativos de Bryconadenos são histologicamente indistinguíveis das células "club" (células de substância de alarme) encontradas dentro da pele de Cypriniformes, Characiformes, Siluriformes e outros peixes otofíseos. Estas células ocorrem na superfície da pele da glândula da nadadeira anal dos machos de Bryconadenos onde estão agrupadas em forma de órgão. Muitos outros machos adultos de caracídeos têm células "club" na superfície da pele da nadadeira anal, muitas vezes associadas a espinhos ou ganchos, mas nunca formando um órgão como em Bryconadenos. Lançamos a hipótese que estas células secretam um tipo de feromônio durante a corte nupcial, via secreção holócrina. Machos dos gêneros Lophiobrycon , Glandulocauda e Mimagoniates, tribo Glandulocaudini, têm células "club" associadas aos seus órgãos da nadadeira caudal, mas nenhum tipo de células mucosas especializadas foram encontradas como as que estão presentes no órgão caudal dos machos do glandulocaudineo Corynopoma riisei, tribo Stevardiini (= Corynopomini de autores prévios). Nesta espécie os machos possuem células mucosas especializadas, provavelmente modificadas para secreção de feromônio. A evidência de que as escamas e raios modificados das nadadeiras do órgão caudal dos machos da tribo Glandulocaudini não são homólogos àqueles de outras tribos de Glandulocaudinae, como esta subfamília foi previamente reconhecida, é discutida e conclui-se que os membros da tribo Glandulocaudini devem ser reconhecidos como uma subfamília separada, Glandulocaudinae, possivelmente intimamente relacionada com alguns outros caracídeos inseminadores do Clado A, que não possuem órgãos especializados na nadadeira caudal. As demais tribos da outrora subfamília Glandulocaudinae são aqui reunidas sob o nome de subfamília Stevardiinae. Muitas espécies destas duas subfamílias e alguns dos caracídeos inseminadores do Clado A têm células espermáticas modificadas com uma bainha citoplasmática alongada tipo aderida e mitocôndrias localizadas ao longo e além do núcleo. Isto pode significar uma indicação de parentesco em um nível único dentro dos caracídeos do Clado A. Entretanto, pesquisa adicional sobre a natureza derivada e homologia das células espermáticas nos caracídeos do Clado A e dos órgãos caudais das tribos de Stevardiinae precisa ser realizada para utilizar caraterísticas das células espermáticas como caracteres em estudos de filogenia. Finalmente, os tipos de células secretoras e estruturas anatômicas gerais nos órgãos caudais dos Stevardiíneos precisam ser pesquisados em detalhe para que seja possível apresentar hipóteses de relações filogenéticas das tribos de Stevardiinae e de todos os caracídeos inseminadores do clado A.
Introduction
Specimens belonging to the new characid genus and species described below, Bryconadenos tanaothoros, Figs. 1-4, were collected from various localities in the Serra do Roncador from the rio Suiá-Missu and its tributaries, the rio Suiazinho, all tributaries to the upper rio Xingu and from upper tributaries of the rio Tapajós, in Mato Grosso, Brazil. Lowe-McConnell (1991) was the first to study the ecology and present a tentative list of the species of the Serra do Roncador. Although some of her collecting localities were close to or the same as the type locality of B. tanaothoros, no species of characid that could be easily confused with our new fish was listed or illustrated by Lowe-McConnell. All her characiforms were identified by J. Géry and the only fish species in her Table that might be somewhat close in appearance to B. tanaothoros, was listed as "Knodus cf. breviceps." However, her specimens of this species were taken from the headwaters of the rio das Mortes a tributary of the rio Araguaia, not the drainages of the rio Xingu or the upper rio Tapajós where we report specimens of B. tanaothoros. We examined "syntypes" of Knodus breviceps Eigenmann, MCZ 20692 and other specimens from the rio Araguaia (see list of materials examined, Appendix II Appendix II ) and confirmed that this species does not have an anal-fin gland or other diagnostic features of B. tanaothoros.
The new species described herein first came to the attention of one of us, H.-G. E., in 1998 and 1999, as an aquarium fish export to Germany from the rio Suiá-Missu. These specimens were collected and exported in association with the Brazilian aquarium fish exporter, Marco Túlio Cortes de Lacerda, under a Brazilian aquarium fish export permit. Bryconadenos tanaothoros was previously collected and a report published by Werner (1992:121) with a photograph of live specimens that illustrated the anal-fin gland in the male. His specimens were from a water body near Canarana, between the upper portions of the rio Suiá-Missu and the rio Sete de Setembro, both tributaries of the upper rio Xingu. Werner presumed this fish to be a new genus and species, but did not describe it. Subsequent to the second appearance of this fish in the aquarium trade, many specimens were collected for scientific purposes from the same region by an expedition conducted by one of us, NAM.
Because H-G E observed that live specimens of this apparently new fish, when bred, appeared to have an inseminating reproductive mode, but the males lacked a caudal-fin organ as found in males of the inseminating characid subfamilies Glandulocaudinae and Stevardiinae and because the males of this new species had what appeared to be an anal-fin gland, he preserved a few specimens and submitted them for identification to SHW. When this species was first investigated, a routine identification that purposely ignored the presence of an anal-fin gland, keyed this fish to Knodus moenkhausii (Eigenmann & Kennedy) in both Eigenmann (1918:114) and Géry (1977:394). However, certain differences are noted below in addition to the absence of any previous record of an organized anal-fin gland, not only in Knodus Eigenmann, but also of any species of the Characidae.
We propose that B. tanaothoros is most closely related to the three species of Attonitus Vari & Ortega, all of which we found to be inseminating and with a concentration of club cells at the skin surface of the anterior region of the anal fin of sexually active males. However, Attonitus species do not have their club cells organized into a structured organ as found in Bryconadenos tanaothoros. In searching for other characids with an anal-fin organ or a concentration of club cells on the skin's surface of the anal fin of males in an attempt to evaluate the phylogenetic significance of such cells, we found that at least some non-glandulocaudine inseminating characids, for example some undescribed species that, at the present, would be ascribed to Knodus and the species of Monotocheirodon (two of them undescribed and all three inseminating) appear to belong to Clade A characids, a sub-group of the Characidae proposed by Malabarba & Weitzman (2003). Regarding Clade A, in their analysis of characiform relationships inferred from nuclear and mitochondrial gene sequences, Calcagnotto et al. (2005: fig 6) included six of the many Clade A genera recognized by Malabarba & Weitzman (2003). Of these six, Bryconamericus Eigenmann, Knodus , Creagrutus Günther, Hemibrycon Günther, Gephyrocharax Eigenmann, and Mimagoniates Regan, all were incorporated in a single clade in their studies, thus confirming at least in part the phylogenetic validity of Clade A. Further, information contained in the present study adds the new genus Bryconadenos to Clade A. Several of the Clade A genera included by Calcagnotto et al. (2005) are central to the discussion below regarding the relationships of Clade A genera and the origin of insemination in characid fishes.
Another recent study (Hubert et al., 2005), using mitochondrial ribosomal DNA data, although not the primary purpose of the work, produced a characiform cladogram expressing cladistic relationships. For the most part this study does not include data from characiform taxa pertinent to the relationship levels discussed here. Therefore this study will receive no further comment here except that one problem we see in their cladograms, figs 1 & 2, is that within the Characidae, especially regarding the use of the names Cheirodon and Tetragonopterus, both of which are the type genera of their respective subfamilies, these authors do not always place these genera within their respective subfamilies according the structure of their cladogram.
Additional preliminary research indicates that club cells, ordinarily found deep in the skin and designated as alarm substance cells, see Pfeiffer (1967 and 1977), are histologically indistinguishable from the surface club cells found in the anal fins of male Attonitus , Bryconadenos, or other characids. Further investigation found surface club cells in association with male anal-fin hooks in a wide variety of non-inseminating characids as well as many of the inseminating members of the Glandulocaudinae and Stevardiinae. However, we only found Bryconadenostanaothoros to have these cells organized into a structured gland.
We also found skin surface club cells associated with the caudal organ of the species of Lophiobrycon Castro et al. , Glandulocauda Eigenmann, and Mimagoniates, all the genera of the subfamily Glandulocaudinae. It is known that specialized mucus cells modified for pheromone secretion (Atkins & Fink, 1979) are associated with the differently organized and developmentally derived caudal organ of male Corynopoma riisei Gill, tribe Stevardiini (= Corynopomini of Weitzman & Menezes, 1998), and Weitzman & Fink (1985). Menezes and Weitzman (1990) discussed the developmental origin of the gross anatomy of the caudal organ of the Glandulocaudinae as this subfamily is interpreted here. We also discuss here the non-homology of the gross and to some degree the developmental anatomy of the caudal organ in species in the genera Lophiobrycon, Glandulocauda and Mimagoniates, subfamily Glandulocaudinae with some members of the subfamily Stevardiinae as interpreted here and noted that it is uniquely different than in other glandulo-caudines as the subfamily was understood at that time. This non-homology and the presence of differently derived secretory cells in the above three genera compared to at least some genera in the subfamily Stevardiinae suggest separate origin for the Glandulocaudinae and Stevardiinae. Therefore we tentatively recognize the former Glandulocaudinae of Weitzman & Menezes (1998), to include only the tribe Glandulocaudini. We tentatively consider the Stevardiinae to include all the other tribes of the former Glandulocaudinae. However, we suggest that further research regarding the distribution of skin surface club cells and specialized mucus cells as well as other secondary sexual features in male American characids especially those of the Stevardiinae and related outgroup characids will provide much needed and important data for phylogenetic studies of these fishes.
Methods and Materials
Count and measurement techniques follow Fink & Weitzman (1974: 1-2), Menezes & Weitzman (1990: 382-383), Weitzman et al. (1994:48), and Weitzman & Palmer (1997: 213-214). Counts of the holotype are given first followed in parentheses by the mean, (and median when the data are nonparametric), range and total number of specimens counted. Measurements in Table 1, other than standard length (SL), are expressed as a percentage of SL except for subunits of the head that are recorded as a percentage of head length. Total vertebral counts were taken from radiographs and alizarin cleared and stained specimens (C&S). These include the vertebrae of the Weberian apparatus as well as the complex caudal ossification, PU1 + U1 with the associated hypural bones and "half vertebrae" all counted as one element. Morphometric data for the holotype and paratypes are given in Table 1. Basic descriptive statistics and all graphs were prepared using SigmaPlot for Windows 3.0, 1995 and SigmaStat for Windows 2.0, 1995. The regression analyses follow the methods described by Weitzman & Palmer (1997:213-214) except that here the graphs are not presented in logarithmic format. All mature specimens of B. tanaothoros were identified to sex either by examination of their gonads or by examination of their anal-fin organs.
We utilized the osteological and other gross anatomical data reported by Vari & Ortega (2000) in their discussion of the relationships of Attonitus to other characids as well as additional data from specimens of Attonitus examined by us in our evaluation of the possible close relationship of Bryconadenos tanaothoros to Attonitus.
PAUP 3.1.1, Swofford (1993) was used only as an aid for discussion of the relationships of inseminating Clade A fishes because insufficient anatomical data, especially those related to sexual modes, were available from enough species and genera of not only the inseminating Clade A taxa, but also the apparently pertinent, but poorly known nominal genera Knodus and Bryconamericus and some undescribed species of Planaltina. We are unsure of what species of these three nominal genera are truly suitable for what we consider a meaningful hypothesis of phylogeny of Clade A inseminating taxa. Although we suggest that at least some species of Knodus and Bryconamericus may be outgroup taxa for an analysis of the phylogeny of the inseminating taxa such as the species in the glandulocaudine genera, as well as species of the genera Attonitus , Bryconadenos , Monotocheirodon Eigenmann, and possibly others discussed below such as the inseminating species of Knodus, non-inseminating species of Knodus and all species of Bryconamericus as currently recognized in the literature are not definable or necessarily monophyletic. They are in need of detailed reviews and phylogenetic studies before they can be considered wellorganized outgroup taxa suitable for the study of the phylogeny of their more derived putative relatives. The phylogenetic diagram presented in Fig. 11 is thus not an outcome of a PAUP 3.1.1 analysis, but is a simplified result of the discussion contained herin and avoids what we consider grossly conflicting PAUP 3.1.1 hypotheses that at many nodes suggest relationships that probably have no real phylogenetic significance.
In some cases when specimens of a given species in Appendix 2 Appendix II were present in quantity, entire mature male and female specimens were submitted for histological study of both primary and secondary sexual features. However, in most cases tissue samples for histology were taken only from particular organs. For example, in the case of the gonads, one entire gonad was removed from one side only, usually the right side. The lower limb of the first or anterior gill arch of the right side was submitted for histological study of gill glands. To obtain finray glandular tissue, the fin rays underlying that tissue were split sagittally so that the ray halves supporting the soft tissue of the right side of the fin were removed with their soft tissue and subjected to histological analysis. This allowed keeping the left side of the fin fully intact and attached to the fish.
For light microscopy (LM), tissues were fixed in 10% formalin for a minimum of one week and later transferred to 70% ethanol. Gill tissues were then decalcified overnight before proceeding. Fin tissues were treated in a similar manner or until decalcification was completed. Testes, ovaries, skin samples and some gill samples were dehydrated to 95% ethanol, infiltrated and embedded in glycol methacrylate, sectioned at 1-5 µm with a Sorvall Type JB-4 microtome, and stained with toluidine blue or periodic acid-Schiff reagent (PAS) and Harris hematoxylin (Quintero-Hunter et al., 1991). Some gill samples were dehydrated in an ethanol series, infiltrated and embedded in paraffin, sectioned at 7 µm, and stained with a modified Masson trichrome (Schreibman, 1964).
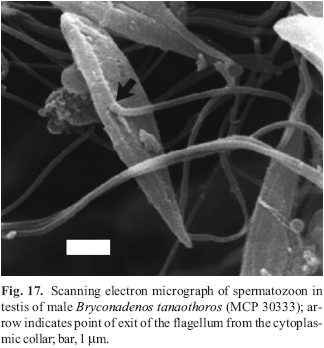
For scanning electron microscopy (SEM), the testis was fixed in Karnovsky's fixative and subsequently transferred to 70% ethanol. Pieces of testis were dried in a CPD 030 Bal-Tec critical point dryer. The dried specimen was then teased apart while being attached to a carbon tape, coated with gold in a SCD 005 Bal-Tec sputter-coater, and viewed with a JEOL-JSM 5800 scanning electron microscope. The voucher number of the specimen of B. tanaothoros used for SEM is MCP 30333. Due to specimen dissection before any measurements were made, a SL was not available.
For transmission electron microscopy (TEM), the testis samples were fixed in a modified Karnovsky's fixative (Ito & Karnovsky, 1968) and stored in this fixative under refrigeration until further processing was possible. Tissues were then rinsed in phosphate buffer and post-fixed in 1% osmium tetroxide in phosphate buffer for 30 min. They were then rinsed in phosphate buffer, dehydrated in an ethanol series, infiltrated and embedded in Araldite 502. Ultrathin sections were cut on a Sorvall MT5000 ultramicrotome, mounted on grids and stained with aqueous uranyl acetate and lead citrate. Sections were examined in a JEOL JEM 1200 electron microscope.
The following institutional abbreviations are used: ANSP, Academy of Natural Sciences, Philadelphia; CAS, California Academy of Sciences, San Francisco; IUM, Indiana University Museum; LIRP, Laboratório de Ictiologia de Ribeirão Preto, Faculdade de Filosofia, Ciências e Letras de Riberirão Preto; MCP, Museu de Ciências e Tecnologia, Pontifícia Universidade Católica do Rio Grande do Sul; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge; MUSM, Universidad Nacional Mayor de San Marcos, Lima; MZUSP, Museu de Zoologia da Universidade de São Paulo; MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro; USNM, National Museum of Natural History, Smithsonian Institution; and ZMB Museum für Naturkunde Berlin.
For comparative materal examined in addition to the specimens listed in the new species description, see Appendix 2 Appendix II .
Results
Family Characidae Agassiz, 1844
Bryconadenos, new genus
Type species.Bryconadenos tanaothoros, new species by monotypy and original designation.
The list of characters below is followed by a discussion comparing the characters listed with those of similar or the same structure in certain other characid genera.
Etymology. The first part of the name Bryconadenos is from the characid generic name Brycon that is from Greek bryko to eat greedily. The word brycon is an oftenused component of various generic names of small characids. The second part, adenos is from the Greek adenos meaning gland. Gender masculine.
Diagnosis. The following two characters are autapomorphic for Bryconadenos.
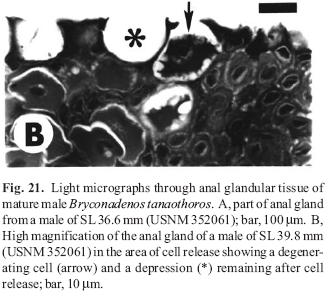
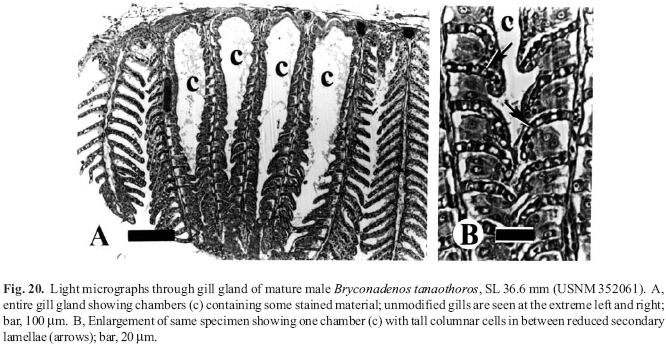
(1) Specimens of adult male, sexually active Bryconadenos have glandular club cells at the surface of the epidermis on the anterior part of the anal fin. These cells are organized into an organ whose cells apparently undergo holocrine secretion. Although certainly secretory, we suggest that this organ may be pheromonal in nature and associated with courtship activity. See Appendix 1Appendix 1 for histological details. Also, see the Discussion below for a review of the taxonomic distribution of the newly discovered club cells on the anal, pelvic, and caudal fins of male characids.
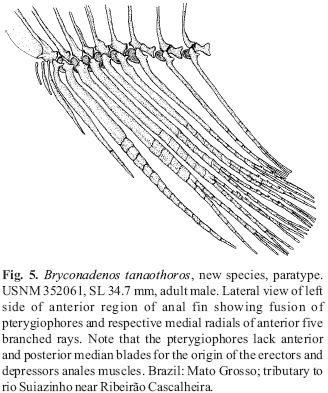
(2) There is a close articulation between the anterior and posterior facets of the basal portions of the anterior seven basal pterygiophores.
Distinguishing Characters. The remaining five characters, although useful for distinguishing Bryconadenos, are not unique to this genus.
(3) There is an increased development of the muscles involved in the movement of the anal-fin rays of males, in particular the erectors anales and the depressors anales in Bryconadenos compared to nearly all other species of the Characidae. This feature is also synapomorphy number 3 for the species of Attonitus, Vari & Ortega (2000: 120). Although this feature is present in Bryconadenos, it is not as welldeveloped as in Attonitus.
(4) Specimens of adult sexually active males of Bryconadenos lack pelvic-fin hooks.
(5) A band of dark chromatophores is located on the ventrolateral portion of the body wall above the anal-fin base, a pigment pattern more developed in males of Bryconadenos than in females. This was listed as synapomorphy # 6 for species of Attonitus by Vari & Ortega (2000: 120).
(6) Body wall somewhat convex proximate to anal-fin base. This was listed as synapomorphy # 1 for species of Attonitus, by Vari & Ortega (2000: 120).
(7) Lateral-line pores surrounded by a ring of dark chromatophores or at least associated with dark chromatophores. This character is a synapomorphy uniting the single species of Bryconadenos with the three species of Attonitus. However, it is somewhat better developed in Bryconadenos, being strongly present for the entire lateral-line length whereas in Attonitus it tends to be faded or weaker posteriorly. This feature was not proposed by Vari & Ortega (2000) as a synapomorphy for the species of Attonitus.
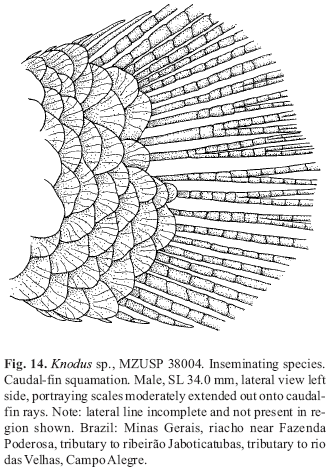
Discussion: Certain features that distinguish Bryconadenos from most other characid genera are in part shared with a few other characid genera, especially those in Clade A of Malabarba & Weitzman (2003). Sometimes these shared features differ in detail, opening questions about their homology and they often occur in different combinations among those genera. Possible phylogenetic relationships based on a cladistic analysis using these features are unfortunately clouded by inadequate sampling of characters of not only the Clade A genera, but many characid nominal genera. Certain large Clade A genera such as Bryconamericus and Knodus that are putatively plesiomorphic for the inseminating Clade A genera are little known phylogenetically, for example see remarks by Silva (2004:55 & 59), and are therefore difficult to use with confidence, except in a very simplistic fashion, as outgroup taxa for inseminating Clade A groups. Currently the Clade Agenera Knodus, with caudal fin squamation, and especially Bryconamericus, without caudal-fin squamation, lack the derived features that distinguish most other Clade A genera. At least a few species of Knodus are inseminating, indicating a possible relationships with at least some glandulocaudines, stevardiines, and other inseminating Clade A characids, but their primary sexual features, such as sperm cell structure, need further study to hypothesize a possible phylogeny of this genus and the relatively plesiomorphic genera of the Stevardiinae as those in the Diapomini.
The discussion below is arranged by character number as used for the list of Bryconadenos characters given above. Obviously this discussion is preliminary in nature, especially for those newly discovered features not previously known in characids.
(1) Only Bryconadenos appears to have club cells on the anterior part of the anal fin of sexually mature males organized into an organ, Figs. 1-2, 4 & 21 A & B, and that we suggest produces a pheromone used in courtship. This structure is absent in females, Fig. 3. A general survey of many species of stevardiines as well as Clade A and non-Clade A characids revealed that a wide variety of characids have club cells concentrated at the surface of the epidermis of parts of the anterior region of the anal fin of sexually mature males. These club cells are often associated with the bony hooks present on the anal-fin rays. Ordinarily in otophysans club cells are confined to the deeper skin layers of the epidermis in many parts of the body's surface and function as alarm substance pheromone cells. Hyphessobrycon diancistrus Weitzman is a good example of a characid with club cells at the surface of the epidermis associated with anal-fin hooks of adult males. The white tissue masses associated with the two remarkably large anal-fin hooks of males in this species were previously thought to be a collection of mucus cells, but they remained histologically uninvestigated. The presence and distribution of skin surface club cells in the anal-fin and some-times in the pelvic-fin, where they are often associated with fin-ray hooks in sexually mature males, remains to be investigated and recorded in detail in characid taxa. Menezes et al. (2003) recorded the presence of club cells at the surface of the pelvic-fin epidermis of Planaltina myersi Böhlke and especially P. glandipedis, both Clade A species currently placed in the Diapomini of the Stevardiinae. We recently discovered that the caudal-fin apparent pheromone cells of species of the glandulocaudine genera Lophiobrycon , Glandulocauda, and Mimagoniates, subfamily Glandulocaudinae, are actually club cells, not derived mucous cells as described for Corynopoma riisei, tribe Stevardiini, subfamily Stevardiinae, by Atkins & Fink (1979).
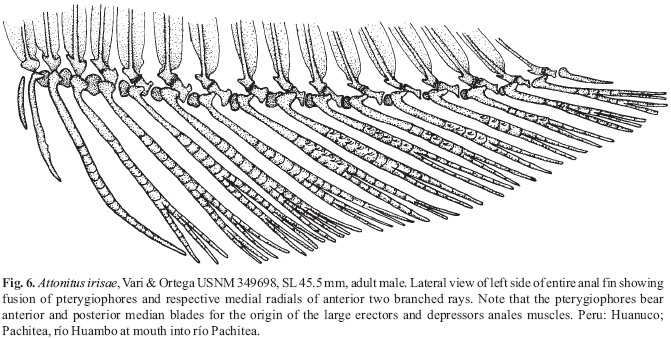
(2)Fig. 5 illustrates a close articulation between the basal anterior and posterior facets of the basal portions of the anterior seven basal anal-fin pterygiophores of an adult male Bryconadenos tanaothoros, a derived feature that includes more fin-ray bases in adult Bryconadenos males than in adult males of Attonitus, Fig. 6. Relatively long ligaments in most characids attach the posterior and anterior facets of these basal pterygiophores to each other. However, only relatively short ligaments attach the basal pterygiophores of the species of Attonitus. The first six anterior basal and the first six middle pterygiophores are fused to each other in Bryconadenos whereas in A. irisae at least, the first three anterior basal and the first three middle pterygiophores are solidly fused. Compare Figs. 5 & 6. The basal pterygiophores of Bryconadenos are not expanded in the sagittal plane as in the species of Attonitus.
(3)Compared to at least most other characids Bryconadenos has an increased development of the muscles involved in the movement of the anal-fin rays of males, in particular the erectors anales and the depressors anales. A similar but more developed modification is also present in the species of Attonitus as described by Vari & Ortega (2000). Bryconadenos as in the species of Attonitus has the soft connective tissues and ligaments associated with these muscles strongly developed compared to most characids (compare Figs. 5 & 6). Thus in these structures Bryconadenos apparently displays some of the features synapomorphic for the species of Attonitus, but with a more plesiomorphic condition in Bryconadenos.
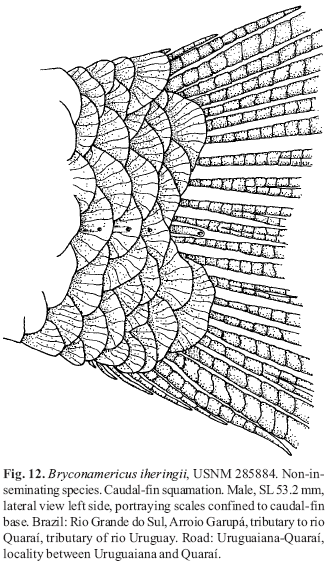
(4) Sexually active Bryconadenos males lack pelvic-fin hooks. There is much variation regarding the presence or absence of hooks on the pelvic fins of characid species, including the inseminating species of Clade A. Presumably the presence or absence of anal-fin hooks in males of these taxa is associated with variation in courtship activity. For example, all known species of Attonitus have numerous pelvic-fin hooks in adult sexually active males, but in the species of the nominal genus Bryconamericus and the apparently related species of the nominal genus Knodus, hooks are not consistently present among their respective species. However, because the phylogenetic relationships in these nominal genera essentially remain unstudied and uncertain and the possible presence versus absence of insemination in the species of these genera is nearly unknown, little can be interpreted from our incomplete survey of the species. The type species of Bryconamericus , Bryconamericus exodon Eigenmann (USNM 181813), so far as we could determine by histological examination, is a non-inseminating species, but does have pelvic-fin hooks in adult males as do adult males of Bryconamericus alpha Eigenmann (USNM 285343), and Bryconamericus iheringi (Boulenger) (USNM 285884). However, adult sexually active males of Bryconamericus deuterodontoides Eigenmann (USNM 349407) lack pelvic-fin hooks as do male specimens of Bryconamericus alfredae Eigenmann (ANSP 143357), and Bryconamericus stramineus Eigenmann (USNM 325698). The inseminating species of the non-Clade A Brittanichthys axelrodi Géry (USNM 198132, holotype) and presumably inseminating species Brittanichthys myersi Géry (USNM 198131, holotype) lack pelvic-fin hooks, but have highly derived autapomorphic caudal-fin structures bearing hooks that immediately distinguish this genus from any other characid genus (see also Malabarba & Weitzman, 1999: 425-426 and Géry 1965: fig. 6, and Burns & Weitzman, 2005). Note that histological examination of the caudal modifications in Brittanichthys axelrodi revealed no modified mucous cells or club cells. Most species of Creagrutus are known to have pelvic-fin hooks, including the two known to be inseminating, Creagrutus melasma Vari, Harold, & Taphorn (1994) and Creagrutus lepidus Vari et al. (1993), see Vari & Harold (2001). Sexually active adult males of Hollandichthys multifasciatus (Eigenmann & Norris) (USNM 297983 & USNM 320271) have pelvic-fin hooks and are inseminating, but do not belong to Clade A.
To further complicate phylogenetic implications of the features in the genera discussed here, inseminating and non-inseminating species occur in Knodus as it is currently defined. Adult males of K. meridae Eigenmann (USNM 121469), the type species of the genus which is apparently non-inseminating and has pelvic-fin hooks in sexually active adult males. Also, adult sexually active male K. septentrionalis Géry (USNM 361168) lack pelvic-fin hooks, but sexually active males of K. savenensis Géry (USNM 196088, holotype) and Knodus breviceps (IUM 17249), both apparently non-inseminating species, bear pelvic-fin hooks. One inseminating species currently assigned to Knodus (USNM 362386), has adult sexually active males with pelvic-fin hooks, while another inseminating species that may tentatively be considered a species of Knodus , K. pectinatus (Vari & Siebert) (USNM 303441, paratypes) is without pelvic-fin hooks in adult sexually active males. Finally adult sexually active males of at least two undescribed species of Monotocheirodon (MUSM 6756 & MUSM 11082) and sexually active males of M. pearsoni Eigenmann (CAS 59792, paratypes), all inseminating and perhaps related to inseminating Knodus species because of some sperm cell similarities that need further study, have derived pelvic-fin hooks that are unique to this genus so far as known. So far the presence or absence of club cells in association with the pelvic fins of these species remains unrecorded. An undescribed species referred to Monotocheirodon by Collette (1977: 238) and Zanata & Akama (2004:51) and described as having breeding tubercles is currently considered by us to belong to an undescribed species of Othonocheirodus. The specimens examined by Collette (1977) and referred to by Zanata & Akama (2004) are sexually mature adult males from ANSP 144106, Ecuador, Provence Zamora Chinchipe, backwaters of the río Zamora, tributary to the río Santiago, tributary to the río Marañon, 12 km northeast of the town of Zamora.
(5) Dark band of chromatophores along the ventral portion of body wall in the region of the anal-fin base. Vari & Ortega (2000:118) used this feature as a synapomorphy for the three species of Attonitus. We found this character in Bryconadenos, Figs. 3, 4, 7 & 8 of a male and female, to be similar to its occurrence in Attonitus. We tentatively agree with Vari & Ortega regarding the use of this feature as a possible synapomorphy for the obviously closely related species of Attonitus. However, we find this character problematic for use as a synapomorphy uniting Bryconadenos with Attonitus. A black pigment line along the anal-fin base of many characins is relatively common although often weakly developed. As in the species of Attonitus, this pigment feature is more weakly developed in females than males and it apparently is not equally developed among the species of Attonitus. This pattern of distribution of this pigment is widespread among characids and primarily occurs on the lateral surface of the articular bases of the anal-fin rays. Many non-Clade A characids such as some or a few species of Brycon , Astyanax , Moenkhausia , Hemigrammus , Paracheirodon, and Bryconops have this pigment pattern modestly developed. It seems likely that black pigment along the anal-fin base in the Characidae may have evolved independently or been lost independently many times. The intensity or full adult development of this pigment pattern varies much in preserved specimens of characids and this raises at least several categories of problems regarding its use in phylogenetic reconstruction. First, in specimens in collections intensity of dark pigment preservation initially depends on the method of fixation and the behavioral condition of the specimens when fixed in the field. Also dark pigment pattern intensity of specimens when caught can depend on local ecological factors such as clarity of the water and darkness or lightness of the substrate. Further, the possibility of fading during time spent in a collection and exposure to daylight or artificial light must be considered. These factors make it difficult to evaluate what may have been the natural intensity of a dark pigment in preserved fishes. However, in keeping literally hundreds of species of Neotropical characids alive for over sixty years, the senior author has noted much variation in the intensity of this and other dark color patterns in characids, depending on the species and genera as well as on the behavorial state of a particular species. Thus, although intensity of dark color patterns may certainly be a phylogenetically meaningful data source, they may be difficult to use. Nevertheless in some characids this pigment pattern has become considerably derived. For example, in the three miniature species of the cheirodontine genus Spintherobolus (Weitzman & Malabarba, 1999: figs. 15, 29-37) this pigment has become especially dark and presumably extended onto several of the posterior rays of the anal fin. This basal anal-fin pigment pattern is also dark and welldeveloped in some Clade A characids, but not so derived as in Spintherobolus. For example, see the stevardiine Ptychocharax rhyacophila Weitzman et al. (1994:40-47, figs. 1-2) where it is strongly developed. Also, the non-glandulocaudine Clade A characid Caiapobrycon tucurui Malabarba & Vari (2000:319, figs 2 and 3) has this pattern well-developed, but less so in the female. Because the Clade A cladogram of Malabarba & Weitzman (2003: 87, fig. 11) indicates that these two Clade A genera are not closely related to Attonitus or Bryconadenos and because a few dark chromatophores occur on the ray bases of many Clade A characids we believe it is best to use this character as a synapomorphy when it is distinctly derived as for example in the non-Clade A characid genus Spintherobolus.
(6) Convexity of body wall proximate to the anal-fin base. This feature was thoroughly discussed by Vari & Ortega (2000: 115-116) who found no outgroup characiforms that have a similar feature and that could be considered closely related to Attonitus. The only Clade A characid we found to have this characters is Bryconadenos which has this character much less developed than in the species of Attonitus, but may be indicative of a relationship.
(7) Lateral-line pores surrounded by a ring of dark chromatophores or at least associated with dark chromatophores. As noted above this character was not utilized by Vari & Ortega (2000), but we found no Clade A or other characids with this feature.
Note: The following three characters used as synapomorphies for the species of Attonitus by Vari & Ortega (2000: 120) are absent in the type species of Bryconadenos. These remain synapomorphies for the species of Attonitus. (1) Expansion of the anterior basal pterygiophores and realignment of the distal portion of the basal pterygiophores into a gentle arch in mature males. (2) Disparity in the relative size of the inner and outer premaxillary tooth rows, with a posterior curvature of the distal portions of the teeth on the inner tooth row and anterior curvature of the distal portions of the teeth in the outer tooth row. (3) An anteroventral curvature of the anterior portion of the dentary with a consequent anterodorsal orientation of the anterior dentary teeth and a distinct concavity of the ventral profile of the anterior portion of the dentary.
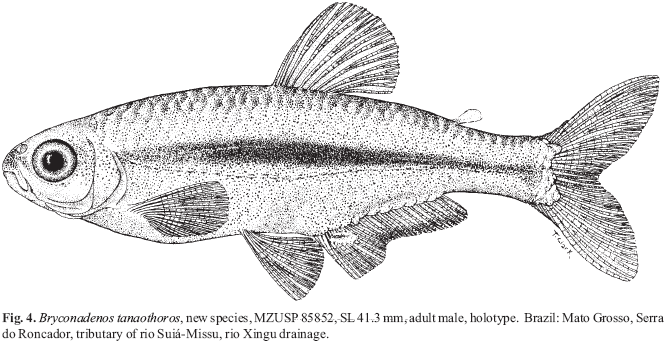
Bryconadenos tanaothoros, new species Figs. 1-4, Table 1
Holotype. MZUSP 85852, (41.3 mm SL), Brazil, Mato Grosso, Serra do Roncador, along shore line of rio Suiá-Missu, near Fazenda Terra do Sol, west of road BR-158 at 1250'90"S 5207'46"W; rio Suiá-Missu, flows into rio Suiá-Missu, a tributary of upper rio Xingu, by Paulo Valerio da Silva & Hans-Georg Evers, 23 June 1999.
Paratypes. The following lot collected with holotype: USNM 380150, 10, (11.3-38.9 mm SL); USNM 352061, 9, (20.8-36.3 m SL), Brazil, Mato Grosso, Serra do Roncador, two km from village of Ribeirão Cascalheira along road BR158, ribeirão Bonito and rio Suiá-Missu, all tributaries of rio Suiazinho, itself tributary of upper rio Xingu; GPS coordinates 12 57.24' S. 051 21.17' W; February 3, 1998 by Marco Tulio Cesar Lacerda, Paulo Valerio da Silva & Hans-Georg Evers. Note: The testis sample used for transmission electron microscopy (TEM) analysis described in Appendix 1 came from a male 50.0 mm SL previously used for breeding purposes by H.-G.E. The specimen was not retained, but was originally collected along with the specimens listed under USNM 352061. MZUSP 62102, 3, males, maturing adults, (26.9-28.5 mm SL) córrego Duas-Bocas, tributary of rio ribeirão Macuco, tributary of rio Teles-Pires, upper rio Tapajós basin, at 71.9 km north of the town of Sinop on the road BR-163, Mato Grosso, Brazil. Approximate coordinates 1117'S 5520'W; Cristiano L. R. Moreira & M. I. Landim. 21 Nov 1998. MCP 29467, 29 males, maturing-adults (32.3-43.7 mm SL),7 females maturing-adults (32.0-36.2 mm SL) rio Ferro on the road between Novo Mato Grosso and Nova Ubiratã, about 25 km SW of Novo Mato Grosso, upper rio Xingu basin, Mato Grosso, Brazil. GPS coordinates 133'32"S 552'12"W; January 30, 2002, R.E. Reis, L.R. Malabarba & E.H.L Pereira. MZUSP 79752, 4 immature males (32.0-33.0 mm SL) 2 immature females (28.0 and 32.5mm SL); LIRP 4087, 3 immature males (30.0-30.6 mm SL), 3 immature females (27.5-30.0 mm SL), confluence of rio Cervo and córrego do Gato on the bridge between Dona Rosa and Ribeirão Cascalheira, Município of Canarana, Mato Grosso, Brazil, upper rio Xingu basin. GPS coordinates 1309'13.6''S, 5155'18.7"W; January 21, 2002, L. Casatti, A. Melo, Hertz dos Santos & Fernando Gibran.
Diagnosis. As above for Bryconadenos.
Distinguishing characters. Some additional characters separating Bryconadenos tanaothoros from the species of Attonitus are as follows. The branched anal-fin ray count for B. tanaothoros is 18-21 with a mean of 19.6, while Vari & Ortega (2000:123) record a count of 11-14 with a mean of 12.75 for A. bounites, 14-17 with a mean of 15.22 for A. ephimeros, and a count of 14 to 17 with a mean of 15.33 for A. irisae, a clear difference. The pelvic fin-ray count for B. tanaothoros is i, 7 in all specimens of Bryconadenos tanaothoros while Vari & Ortega (2000:126) record a count of i, 5-6 with a mean of i, 5.9 for A. bounites and A.ephimeros. For A. irisae, they record i, 6 in all specimens, also a clear difference.
Description. Morphometric data of holotype and paratypes are presented in Table 1. Small tetragonopterine characid reaching at least 44.0 mm SL. Body laterally compressed; greatest depth at dorsal-fin origin. Dorsal profile of head anterior to nape slightly convex dorsal to nostril. Snout bluntly convex, tip about level with mid-point of orbit as determined by horizontal line congruent with SL. Lower jaw convex in profile and somewhat included below upper jaw. Ventral profile of head gently rounded, continuous with a gently convex belly that becomes more or less straight or concave in region of pelvic-fin origin. It then continues slightly convex or straight to anterior border of anus. Body profile along anal-fin base straight to slightly concave in females, somewhat concave in males dorsal to prominent anterior anal-fin lobe and then convex to posterior termination of anal fin. Ventral profile of caudal peduncle concave. Dorsal body profile between nape and dorsal-fin origin gently convex. Base of dorsal fin slightly concave and somewhat inclined posteroventrally. Body profile between termination of dorsal-fin base and origin of adipose fin slightly convex. At adipose-fin base this profile dips somewhat posteroventrally and then remains continuous with the concave dorsal profile of the caudal peduncle.
Dorsal-fin rays (ii, 8 in all specimens, n = 64); posterior ray not split to its base. Dorsal fin of about equal height in both sexes. Adipose fin present. Anal-fin rays iv, 21 (ivv, 18-21 branched rays, x = 19.6, median = 20, SD = 0.81, n = 64); posterior ray split to its base, counted as one ray. Anal fin with strongly developed anterior lobe with 4-5 unbranched rays and 56 branched rays in both sexes. Base of anterior lobe covered by anal-fin gland in sexually active males and with some glandular tissue present in females. See section on sexual dimorphism and Appendix 1Appendix 1 for histological description of anal-fin gland. Anal-fin of six sexually active males with bilateral bony hooks on branched rays 3-5, with about 4- 5 hooks on each side of each ray, but another specimen had 2 hooks on ray 2, 3 on ray 3, 5 on ray 4 and 3 on ray 5. Pectoral-fin rays i, 11 (i, 10-12 branched rays, x = 11.2, median = 12, SD= 0.50, n = 64). Tip of longest pectoral-fin ray falling short of almost reaching pelvic-fin origin, of about equal relative lengths in both sexes. Pectoral-fin rays without hooks. Pelvic-fin rays i, 7 (i, 7 in all 64 specimens examined). Sexually active males without pelvic-fin hooks. Pelvic-fin length of sexually mature specimens sexually dimorphic (see discussion below under sexual dimorphism). Principal caudal-fin ray count 10/9 in all specimens examined.
Scales cycloid with 0 to 5-6 radii along exposed posterior border. Lateral line complete, perforated scales 38 (range 3739, x = 38.1, median = 38, SD = 0.72, n = 64). Predorsal scales 11 (range 11-12, x = 11.5, median = 11, n = 64). Scale rows between dorsal-fin origin and anal-fin origin 10 (range 8-10, x = 8.8, median = 9, SD = 0.67, n = 64. Scale rows around caudal peduncle 14 in all specimens, n = 63.
Premaxillary teeth in two rows (see Fig. 9); outer row teeth 3 (range 1-4, x = 2.5, median = 3, SD = 0.73, n = 64). Outer row teeth more or less elongate, cylindrical and distally conical, with 2 small cusps on each side sometimes appearing only as small rounded eminence. Outer row teeth somewhat shorter than inner row teeth. Inner row teeth 4 in all specimens, compressed and flattened especially distally, somewhat concave on external surface; symphyseal tooth usually with 4, sometimes 5 cusps; following 3 teeth with 5 cusps graduated in size from smallest located anteriorly to usually with third cusp largest. Maxillary teeth 2 in all examined specimens, n = 64, compressed, flattened with 5 cusps, middle cusp being largest. Dentary with 4 large teeth followed more or less abruptly by 4 (range 4-5, x = 4.4, median = 4, SD = 0.49, n = 64) smaller teeth. Anterior 4 largest teeth with 5 cusps, middle cusp largest. These large teeth with thick circular bases, but distal half compressed with concave inner surface and convex outer surface. Anteriormost 2 of smaller teeth with 3 cusps, middle cusp largest. Subsequent small teeth with 2 or 3 cusps and posterior most tooth usually conical.
Vertebrae 38 (range 36-39, x = 37.3, median = 37, SD = 0.66, n = 20). Dorsal limb gill rakers 5 (range 5-7, x = 5.7, median = 6, SD = 0.58, n = 64); ventral limb gill rakers 11 (range 8-12, x = 10.6, median = 11, SD = 0.71, n = 64).
Branchiostegal rays 4 in one cleared and stained specimen, 3 rays originating on anterior and one on posterior ceratohyal. Anterior 2 rays each articulate in their own notch along ventral border of anterior ceratohyal, but part of the doanterior flat interior face of these rays articulates with the lateral face of the anterior ceratohyal. Third ray articulates with ventrolateral external surface of anterior ceratohyal and fourth branchiostegal ray articulates with the lateral surface of both anterior and posterior ceratohyal.
Color in alcohol. Description taken mostly from holotype, a fully adult male 41.3 mm SL, Fig. 4. Background body color pale to yellowishbrown, but dorsum of the body dark brown to black. Dark horizontal body stripe occurs mostly dorsal to or ventrally bordered by lateral line; broad stripe widest ventral to dorsal-fin origin and continues onto caudal-fin rays 9-12. Stripe darkest on rays 10-12 where dark pigment continues to distal tip of each ray. Obvious humeral mark or blotch not present, not distinguishable from anterior end of broad lateral stripe as it occurs just posterior to dorsal region of opercle. Pores of lateral line surrounded by obvious black slender circular line such that each pore appears as small dark circle. Relatively narrow line of dark chromatophores extending from near anus on body just dorsal to anal-fin base for about threefourths length of anal-fin base (see Figs. 7 & 8 of anal fin and adjoining body parts. Scattered dark chromatophores on the body sides ventral to lateral line (see Figs. 7 & 8). Abdominal and ventral regions of head mostly white.
Head medium brown in snout region dorsal to mouth. Lower jaw and area of head ventral to eye mostly without dark chromatophores. Some dark chromatophores extend in line ventrally along the upper approximate half of maxilla. Dorsal to maxilla, between it and eye occurs a line of dark chromatophores 3-4 chromatophores wide at its mid length and tapering at each end to about one chromatophore wide. Nostril without dark pigment, but area anterior and dorsal to area around nostril about the same color as dorsal area of the snout, medium brown. No dark pigment between nostril and eye. Area dorsal to eye pale yellow, but top of cranium black, especially areas dorsal to brain. Dorsal third of opercular area that appears black or dark in upper fish in Fig. 1 associated with gills and in some lights shows through mostly translucent opercle. Area of head and opercle posterior to approximately dorsal half of eye covered with dark scattered chromatophores; these for the most part contracted in holotype. Ventral part of head mostly white, except for some dark chromatophores in mid region of lower jaw just posterior to symphysis.
Anal fin appears mostly hyaline in our whole body photographs, but in drawing, Fig. 4, and close-up photographs, Figs. 7 & 8, of anal fin, some dark chromatophores on distal parts of fin can be seen, especially on anterior part of fin. Posteriorly on this fin dark chromatophores most dense on membranes between the fin rays. Remaining fins hyaline except for scattered dark chromatophores along fin rays and some on fin membranes, especially in dorsal, pelvic and caudal fins.
Color in life.Figs. 1-3. Dark pigment in life much like that in fixed specimens, except that broad lateral band multicolored as follows. Band same in both sexes except more intense in males. Band's ventral border outlined by lateral line with its series of pores, each circled by black as described above. Band consists of a brilliant reflective greenish gold, Fig.1, but may appear reflective blue in freshly preserved specimens in formalin. Body dorsal to band pale gray brown, but some-times with slight greenish cast. In freshly preserved specimens, back dorsal to color band is mostly clear because of contracted chromatophores. Borders of scales on back from nape posteriorly to dorsal-fin origin and from the posterior dorsal-fin insertion to adipose fin origin broadly bordered in black. Area ventral to body's broad band and posterior to abdomen colored like area dorsal to the body band, but may reflect a brilliant green as shown in the lower specimen in Fig.1. Dorsal region of opercle dark, but with some silvery reflective pigment. Just anterior to this region dorsal part of eye dark, but with some reflective red brown color. Remainder of eye globe silvery white, except for black pupil. Anterior to dark pigment of eye, snout region darkly pigmented. Ventral region of head and the abdomen silvery white. All fins appear essentially hyaline except for following. Distal thirds of first and second rays of dorsal fins are "soft" white in males only (Fig. 2), but this not always displayed (Fig. 1). First and second rays of pelvic fins white and entire longest unbranched ray and distal parts of first and second branched rays white in males only (Fig. 2). Areas of anterior lobe of anal fin covered by anal-fin gland white. Rays along basal one third of anal fin with some black pigment and black pigment on membranes occurs between distal one third of fin rays. This only shows well in male. Caudal fin much as described for specimens in alcohol except that males with dorsal most and especially ventral most principal rays with some white pigment. Adipose fin hyaline except for small amount of black pigment on anterior basal region and sometimes its leading border in males.
Sexual dimorphism.Bryconadenos tanaothoros is sexuallydimorphic in the comparative profile of the anterior anal-finlobe. See Fig. 1 for two males and Figs. 2 & 3 and 7 & 8 for comparison of males and females. The white color of the male anal-fin organ is nearly absent in females that always lack the organ, but have some club cells present. This difference in color can be compared by examining Figs. 2 & 3 and 7 & 8. Sexually active males have a gill gland whereas it is always absent in females. Adult males have longer pelvic fins than females. See Fig. 10 for a linear regression graph of male versus female pelvic-fin length.
Etymology. The name tanaothoros is derived from the Greek tanaos, meaning outstretched, and thoros, for seed of the male or semen. The words used together refer to the comparative elongate nature of the sperm cells of this species compared to those cells in the species of Attonitus. A noun in apposition.
Distribution. This species is known from the tributaries of the upper rio Xingu and upper rio Tapajós basins, Mato Grosso, Brazil.
Ecological notes. Live specimens of B. tanaothoros were collected from two localities in the Serra do Roncador, Mato Grosso. The type locality, where most of the aquarium and preserved specimens originated, at the date of collection, was a stream with fast flowing turbid water with a temperature of 27 C, a pH 5.0, and conductivity of 5µs/cm. The second locality, from an unnamed cortège located at 1334.70"S. 051 55.81"W, was a fast running clear water stream with a near surface temperature of 26.8 C, a pH of 5.0, an oxygen level of 9.8 mg/l, and a conductivity of 4µs/cm. The specimens of the various fish species, including B. tanaothoros, collected from the clear-water locality had more intense life colors, but in aquaria the life colors of specimens of B. tanaothoros from the two localities became of equal intensity, comparable to those of the specimens from the clear waters. Specimens of B. tanaothoros appeared to swim alone, never in schools and appeared to be uncommon, but not rare. The fishes were seined along the river's edge in relatively shallow water to 20 cm depth over a substrate consisting of sand, gravel, and submerged waterlogged wood. Individuals of B. tanaothoros appear to be rapid agile swimmers that when meeting conspecifics would chase each other for short distances. However, in 150 liter aquaria they do not seem to exhibt territoriality, but do appear occasionally somewhat aggressive toward one another. This species has an unusual swimming "style" somewhat like that of species of Creagrutus Günther in that they swim rapidly and tremble and quiver in the process. However, the species of Creagrutus that we have observed swim most often near the substrate, while B. tanaothoros swims in more open water above the substrate.
Discussion and Phylogeny. We discuss two topics: first, the relationships among the inseminating and some of the non-inseminating genera, tribes, and subfamilies of Clade A and second, the relationships of Bryconadenos to other inseminating Clade A genera. The first topic is essentially an overall discussion and evaluation of the problems still to be faced and kinds of data needed for a phylogenetically meaningful hypothesis of the relationships among inseminating and non-inseminating characids. This discussion was stimulated by the discovery that at least two kinds of skin secretory cells occur as male secondary sexual features in inseminating characids. The taxonomic distribution of these secretory cells suggested that the phylogeny of the glandulocaudine tribes of Weitzman & Menezes (1998) and their outgroups may be much more complex than previously assumed. Each of these kinds of cells serves as a distinctive feature when present on the caudal organ of some tribes, but not others of the former subfamily Glandulocaudinae. The distribution of these cell characteristics correlates with the taxonomic distribution of certain different gross anatomical specializations among the tribes of the former subfamily Glandulocaudinae. This discovery led to a reevaluation of the relationships among the tribes of the former Glandulocaudinae as well as the relationships of these tribes to other inseminating characids of Clade A. Further, it was felt that the problems facing a study of the relationships of Attonitus and Bryconadenos to other Clade A characids could be better understood once the outstanding problems of the phylogenetic relationships of the inseminating and non-inseminating Clade A characids had been reviewed and discussed with the new anatomical information acquired during the present study.
Relationships among inseminating and non-inseminating Clade Acharacids. We found Bryconadenos to be a member of the characid Clade A as proposed by Malabarba & Weitzman (2003: figs. 2 and 11) and to be related to Attonitus. Figure 11 illustrates the structure of Clade A as proposed here. Clade A consists of approximately 20 genera of characids plus those included in the subfamilies Glandulocaudinae and Stevardiinae. Nearly all Clade A characids have a dorsal fin count of ii, 8 and four teeth on the inner row of the premaxilla. While this is a relatively constant difference between Clade A characids and non-Clade A characids, there are derived exceptions within the tribe Glandulocaudini in which the dorsal-fin ray count is increased. For example in Mimagoniates rheocharis Menezes & Weitzman the count can reach as high as ii, 12. Note also that some miniature species in non-Clade A genera such as Hyphessobrycon Durbin may have Clade A characters independently derived via paedomorphosis. These apparent cases of convergence need investigation. As noted in the introduction above Calcagnotto et al. (2005) in part confirmed the existence of Clade A by finding that the 6 genera of Clade A that they investigated formed a phylogenetic entity in their extensive phylogenetic nuclear and mitochondrial gene sequence studies of characiforms.
All characid species having a supraorbital bone, for example those species in such genera as Brycon Müller & Troschel, Bryconops Kner, and Triportheus Cope are excluded from Clade A, but as indicated by Calcagnotto et al. (2005) these three genera are not particularly closely related characids according to their phylogenetic nuclear and mitochondrial gene sequence studies. Since it is currently assumed that the presence of a supraorbital bone is a relatively plesiomorphic characid character, their result is not surprising. Also, not all characids that lack a supraorbital bone belong to Clade A. Thus Clade A excludes a wide variety of so-called insertae sedis characid genera that lack a supraorbital bone as listed by Lima et al. in Reis et al. (2003). Also, the species of such characid subfamilies as the Aphyocharacinae, Characinae, Cheirodontinae, Iguanodectinae, Rhoadsiinae, Stethoprioninae, and the Tetragonopterinae (includes Tetragonopterus only as recognized by Reis in Reis et al., 2003) are excluded from Clade A. Note that Calcagnotto et al. (2005) found that the limited number of genera of the "old" Tetragonopterinae of Géry (1977) they included in their studies proved to be non-monophyletic. Currently, Clade A is a tentative phylogenetic hypothesis needing further investigation, but is helpful in exploring the possible phylogenetic relationships of Bryconadenos.
Species of both Bryconadenos and Attonitus share with the glandulocaudines and stevardiines certain histological and ultrastructural primary sexual features such as the structure of their sperm cells and insemination. Some of the reproductive features shared by Bryconadenos and Attonitus are also shared with inseminating species of the relatively plesiomorphic characid genus Knodus which appears in two places in our new Clade A diagram, Fig.11, one representing non-inseminating species and the other representing inseminating species. The inseminating species of Knodus are listed in Burns & Weitzman (2005: tabl. 1). Other inseminating non-glandulocaudine characids such as those of Brittanichthys Géry, Creagrutus (so far two species only), Monotocheirodon, and at least one species previously placed in Bryconamericus (B. pectinatus Vari & Siebert), but here referred to Knodus, also share certain histological and ultrastructural primary sexual features, such as the structure of the sperm cells, with glandulocaudines and most stevardiines (Burns & Weitzman, 2005). We found only two species of Creagrutus from Venezuela, C. lepidus, USNM 325045, and C. melasma, USNM 349411, are inseminating. However, some of the other 64 species of this genus so far examined for this feature show no evidence of insemination, for example Creagrutusfigueiredoi Vari & Harold (2001), USNM 292221; Creagrutus changae Vari & Harold (2001), (USNM 285276); Creagrutus affinis Steindachner (1880), USNM293247; Creagrutus britskii Vari & Harold, USNM 292226; and Creagrutus paralacus Harold & Vari (1994), (USNM 121505). Before comments can be made about the phylogenetic significance of insemination in Creagrutus, the distribution of insemination among its many species needs investigation as does the presence or absence of an elongate binding collar along the nucleus, mitochondria along the nucleus, a sperm storage area in the testis and the presence or absence of spermatozeugmata in the testis. Although Malabarba & Weitzman (2003) found Creagrutus to be a Clade A genus, its relationships to the inseminating genera of Clade A remain problematic and puzzling since both inseminating and non-inseminating species are known in this genus. Is insemination in this genus convergent with other inseminating Clade A genera? Perhaps detailed comparison of the ultrastructure of the sperm cells will shed light on this problem, but material for such a study is currently not available. The discoveries concerning possible and apparent convergence of insemination in several characid genera, including some apparently outside Clade A genera, suggest that detailed investigations of the histology and ultrastructure of sperm cells is necessary for a useful evaluation of the phylogenetic significance of insemination in characids. However, inseminating species in Clade A, especially the relatively plesiomorphic inseminating species of Knodus, may be suitable outgroup taxa for cladistic analyses relative to the combined Glandulocaudinae and Stevardiinae. Because this information was not known at the time of Weitzman & Menezes (1998), their within-group phylogeny and relationships of their Glandulocaudinae as well as other inseminating Clade A genera need reconsideration using species of the otherwise apparently plesiomorphic Clade A genera such as Hemibrycon , Knodus (non-inseminating as well as inseminating), and Bryconamericus as outgroup taxa in addition to species of the additional and the evidently more distantly related outgroup genus Astyanax.
There is an interesting progressive apomorphic condition in the glandulocaudine characids not found in the stevardiinae. No glandulocaudines have a hypertrophic extension of the body scales onto the rays of ventral caudal-fin lobe as is found in all the Stevardiinae and in the genus Knodus. Within the Stevardiinae, the various highly derived conditions of the lower caudalfin lobe, its squamation, and the lateral line has been described and illustrated by Weitzman & Fink (1985), Weitzman et al. (1994), Weitzman & Ortega (1995), Weitzman & Menezes (1998 & 2003). The caudal organs of the species in two of the glandulocaudine genera, Glandulocauda and Mimagoniates, have a hypertrophic extension of the upper lobe body scales onto the rays of dorsal caudal-fin lobe rather than the lower caudal-fin lobe as in the stevardiine tribes. Castro et al. (2003) found no extension of the squamation onto the upper and lower caudal-fin lobes in Lophiobrycon and we confirm their observations. This makes the upper lobe scale extension a synapomorphy for Glandulocauda and Mimagoniates, but not one for the Glandulocaudinae. Further, in Mimagoniates, the species have the upper caudal-fin lobe squamation involved with the caudal-fin rays that are modified into a glandular organ. Thus, the course of evolution of the gross anatomy the caudal organs in the Glandulocaudinae and Stevardiinae are very different. In addition, we now find that the caudal-gland cells of the caudal organ of the Glandulocaudinae consist of apparently specialized club cells, not the modified mucus cells reported for Corynopoma riisei and presumably present in other tribes of the Stevardiinae. See Weitzman et al. (1988: 384-413, figs 7-13, 16-17, and 23-24) for a discussion of the gross anatomy of the caudal-fin squamation and caudal-fin ray involvement in the caudal organ of the Glandulocaudinae other than Lophiobrycon. The two new species of Mimagoniates described by Menezes & Weitzman (1990) conform to this difference and have the specialized form of the glandulocaudin tail organ found in Mimagoniates.Weitzman & Menezes (1998: 183) further discussed the characters and distinctness of the Glandulocaudinae from the tribes that we here assign to the Stevardiinae, and finally the glandulocaudin Lophiobrycon weitzmani Castro et al. (2003) has a caudal organ similar to that of the two known species of Glandulocauda and is hypothesized plesiomorphic relative to that found in the species of Mimagoniates. Thus the caudal organs of the subfamilies Glandulocaudinae and Stevardiinae are not homologous regarding either their gross or their secretory cell anatomy. Therefore the Glandulocaudinae as previously recognized is a polyphyletic member of the Clade A characids and the tribe Glandulocaudini of that former Glandulocaudinae may be no closer related to the tribes of the Stevardiinae than to the relatively plesiomorphic members of such characid genera as the inseminating species of Knodus , Attonitus, and Bryconadenos. Thus we recommend the name Stevardiinae as the subfamily name for the former "glandulocaudine" tribes other than those in the former tribe Glandulocaudini which must now be considered as the subfamily Glandulocaudinae.
Calcagnotto et al. (2005) in a study of the relationships of characiforms using an analysis of nuclear and mitochondrial gene sequences used data from only two genera for the former Glandulocaudinae, Gephyrocharax (now in subfamily Stevardiinae) and Mimagoniates (now in subfamily Glandulocaudinae). These authors found these two taxa to be sister taxa compared to the other Clade A genera, Bryconamericus , Knodus , Creagrutus, and Hemibrycon that they also utilized in their analysis. The species (apparently one species in each case) of these genera they used were only identified to genus except Hemibrycon identified to H. cf. beni. Considering the problems regarding the relationships and identification among the species of especially Bryconamericus and Knodus as discussed here, the usefulness of the analysis of Calcagnotto et al. (2005) beyond con-firming in part the apparent phylogenetic significance of Clade A, would seem tenious at best. Nevertheless, a possible close relationship between the Stevardiinae and the Glandulocaudinae compared to other Clade A taxa needs a thorough study using as many as possible of the species of all the putatively involved genera.
The ontogeny of the modified caudal scales, in particular the pouch scale in the Stevardiinae, although apparently always developmentally derived from the scales at the base of the lower caudal-fin lobe, needs further comparative investigation regarding the homology of the caudal organ scales among the tribes of this subfamily. For example, the caudal scales of the Xenurobryconini are complex as discussed by Weitzman et al. (1994: 50-52) and by Weitzman & Ortega (1995: 139). Their developmental origin with the pouch scale originating as a derived scale of the horizontal scale row just ventral to the lateral-line scale row appears homologous with the probable origin of the derived pouch scale of the Stevardiini in which the pouch scale appears derived from the scale row just ventral to the lateral-line row in developing specimens of male Corynopoma riisei. Although we have examined this developmental pattern in many young to adult specimens of male Corynopoma riisei (see list of examined species below), this needs further documentation in the several species of Gephyrocharax and the two species of Pterobrycon. If this pouch scale developmental pattern is found consistent for the Stevardiini and Xenurobryconini, this would be a synapomorphy for these two tribes and perhaps others here tentatively assigned to the Stevardiinae if their pouch scales have a similar developmental origin. Interestingly, the pouch scale of the plesiomorphic xenurobryconin genus Argopleura incorporates what looks like a terminal lateral-line tube and thus its pouch scale looks superficially like a scale derived from the lateral-line scale series. See Weitzman & Fink (1985: 22-26, figs. 22 & 33). However, Weitzman et al. (1994: 50-52, figs. 5-7) provided developmental evidence that the pouch scale of another relatively plesiomorphic xenurobryconin genus, Ptychocharax, that also has a lateral-line tube incorporated in the pouch scale of the adult male, has the terminal lateral line tube secondarily incorporated into the developing pouch scale. Also, the developing pouch scale is derived from the scale row immediately ventral to the lateral line row. Finally, the pouch scale of another relatively plesiomorphic xenurobryconin genus Chrysobrycon appears derived from the scale row immediately below the lateral-line scale row. In this genus it does not incorporate a terminal lateral-line tube. See Weitzman & Menezes (1998: 187 & figs 11 & 12). Further research needs to be done regarding the development of the pouch scale in the species of Argopleura and those of the other genera of the Xenurobryconini. The development of the gross anatomy of the pouch scale of the Hysteronotini (Weitzman & Menezes, 1998: figs. 3-7) has not been approached in any detail, but the figures just cited indicate the possibility that the major pouch scale could be developmentally derived from the horizontal scale immediately below the lateral-line row. The developmental derivation of the pouch scales of the stevardiine tribes, the Landonini, Diapomini, and Phenacobryconini, have not been analyzed in any detail and need investigation.
Plesiomorphic caudal fin squamation for Clade A characids is here hypothesized to be like that in Bryconamericus iheringii. In this species, as in most characids, the lateral line is complete and the terminal posterior lateral-line scale is followed by a lateral line tube, Fig. 12. The three horizontal scale rows below the lateral-line scale series on ventral portion of the caudal peduncle appear to become complicated where they spread out and cover the base of the caudal peduncle and base of the lower lobe of the caudal fin. These three horizontal scale rows in Bryconamericus , Knodus, and young sexually immature male stevardiines appear to be increased in number at the point where the scales cover the base of the fin rays. This complexity makes determination of the horizontal scale rows from which the pouch scales and accessory pouch scales are derived during sexual maturation in males of the non-stevardiinin tribes of the Stevardiinae difficult to determine. One can only solve this problem by studying the sequence of development of the caudal squamation of males from non-sexually mature larvae to sexually mature adults. For example, see the description and discussion of the male complex caudal-fin squamation development and its anatomy at sexual maturity of Ptychocharax rhyacophila by Weitzman et al. (1994: 49-54). The caudal region of the non-inseminating Knodus meridae, Fig. 13, the type species of the genus, has a caudal-fin squamation not much different from that of Bryconamericus iheringii, except the scales are further extended onto the caudal fin and somewhat modified in shape. Variation of this pattern in K. meridae is found in other species of non-inseminating Knodus. The significance of caudal scale modification regarding cladistic relationships among the various species of Bryconamericus and Knodus remains unknown and we agree with Géry (1977) that recognition of these two genera on the basis of simple presence or absence of caudal-fin squamation, although it probably has a complex phylogenetic significance, currently remains a matter of identification and sorting convenience. Comparison of caudal-fin scale patterns among species of Bryconamericus and Knodus for phylogenetic purposes needs detailed ontogenetic study in these putative genera and in many other Clade A genera. For example, Figure 14 represents the caudal squamation of an undescribed inseminating species of Knodus. This species has an incomplete lateral line and no terminal lateral-line tube. Its caudal squamation is different from that illustrated for Knodus meridae, Fig. 13 that has a terminal lateral-line tube. We see no reason to "blindly" accept the simple extension of the caudal squamation onto the caudal-fin rays as an indication of phylogenetic relationships or taxonomic separation of two genera when this modification could possibly be convergent a number of times. However, obviously this does not mean that such a character should be excluded from a cladistic analysis wherein this and other characters may have phylogenetic significance at one to several different nodes. We suggest that there may be several as yet unexplored features in the caudal fins of males of Knodus , Bryconamericus and related Clade A characids. The genus Planaltina provides an example of the difficulty in detecting homologies without the aid of developmental studies of pouch scale components among putative members of the Stevardiinae. Menezes et al. (2003: figs. 19, 28, & 32) respectively illustrate the adult caudal-fin squamation of the three known species of Planaltina , P. myersi , P. glandipedis, and P. britskii. Examination of these illustrations does not provide the information needed to assign specific horizontal scale row derivation for the pouch scales of these fishes. Developmental information is needed to do this.
With the aid of developmental information of the kind discussed above, we suggest that the major single pouch scale of many of the Stevardiini and Xenurobryconini is derived from the scale row immediately ventral to the lateral-line row. Lacking the needed developmental information we cannot be certain of this for Planaltina and therefore are uncertain of its relationship with the stevardiines. Although the other genera of the Diapomini, Diapoma and Acrobrycon, appear to have pouch scales derived from the horizontal scale row below the lateral-line row, again, without developmental information we cannot evaluate this apparent adult pattern. We are convinced that gross developmental information of secondary sexual characters as well as information about the kinds of secretory cells among other things is essential to proposing testable hypotheses of phylogeny in Clade A fishes with derived caudal fins.
Thus the caudal secretory cells of all Clade A glandulocaudine and stevardiine tribes with some kind of caudal organ need histological and cell ultrastructure investigation to see if the caudal secretory cells of these tribes are truly homologous or instead evolved separately. For a list of the species and genera formerly placed in the Glandulocaudinae, see Weitzman (2003) and see Weitzman & Menezes (1998) for an earlier discussion of their phylogeny. See Malabarba (1998: 232) for a discussion of the inseminating Cheirodontinae and their relationships to other cheirodontines, Malabarba & Weitzman (1999: 424-426), and Malabarba & Weitzman (2003: 73-88) for discussions of characid inseminating and non-inseminating genera (Clade A characids) possibly related to the glandulocaudines and stevardiines.
Because the species of Attonitus lack the synapomorphies of the characid subfamily Cheirodontinae (Malabarba, 1998: 199) and the sperm cell synapomorphies of the inseminating fishes of the cheirodontine tribe Compsurini, as diagnosed by Malabarba, Weitzman & Burns in Malabarba (1998: 216-217), it appears that the "glandulocaudine" and related inseminating characids just discussed, including the the species of Attonitus and Bryconadenos, have no close relationships to the inseminating cheirodontine tribe Compsurini even though all these taxa are included in a clade lacking a suprorbital bone as expressed in the characid phylogenetic diagram of Malabarba & Weitzman (2003: Fig. 2). Thus insemination arose independently at least twice in characids lacking a supraorbital bone. This is further confirmed by the analysis of Calcagnotto et al. (2005). Most known inseminating characid fishes, members of what we here call the characid subfamily Stevardiinae have a caudal-fin secretory organ or at least a modification of the squamation of the base of the lower caudal-fin lobe of the males and rarely of the females. This correlates with our separation of Lophiobrycon , Glandulocauda and Mimagoniates into a separate subfamily, the Glandulocaudinae, with a possibly independent origin from a Clade A ancestor than that of the Stevardiinae. One other characid group, a single clade, the tribe Compsurini, within the characid subfamily Cheirodontinae, also has members with a caudal-fin secretory organ or at least a modification of the base of the caudal-fin in sexually mature males (Malabarba, Weitzman & Burns in Malabarba, 1998; Malabarba & Weitzman, 1999 & 2000, and Malabarba et al., 2004). Evidence so far accumulated indicates that insemination in the Compsurini has an independent origin from the taxa that we now consider the Glandulocaudinae and the Stevardiinae.
Certain features of the Stevardiinae and Glandulocaudinae such as insemination together with the presence of an elongate cytoplasmic collar binding the flagellum to the body of the elongated sperm cell (at least at some point during spermiogensis if not in fully developed sperm cells) and the presence of spermatozeugmata in some, may indicate a relationship with the inseminating Attonitus and Bryconadenos, both with no modified caudal-fin scales or with at least some scales extended onto the caudal fin. These two genera may also be related in some way to the inseminating species of Knodus and Planaltina, which apparently have no accumulation of secretory cells associated with scales and or fin rays in their tail fin. Currently we find that the only difference regarding the caudal-fin structures between the species of Planaltina and Knodus (both inseminating) is that the squamation on the tail of Planaltina forms a pouch while that of Knodus is adnate to the caudal-fin rays. Previous to Menezes et al. (2003) Knodus was not known to have inseminating species and at the present no study of relationships of the non-inseminating species of Knodus with the inseminating species of Knodus is available. That these fishes should all be included in the nominal genus Knodus is open to question. Currently we suggest that a detailed study of the non-inseminating and inseminating species of "Knodus," and their caudal-fin scales, and the possible presence of caudal-fin secretory cells, if any, may be crucial to the further study of the phylogenetic relationships of the Stevardiinae, the Glandulocaudinae, as well as the genus Planaltina. Other inseminating Clade A characids must also be included in such studies.
In conclusion and as emphasized above, large amounts of detailed information must be collected regarding the gross anatomy and its development of the male caudal-fin squamation, muscle, ligament, and fin ray modifications as well as other gross anatomical features such as the details of the primary and secondary sexual systems before the phylogenetic relationships of characids can be hypothesized with an great degree of confidence. Then such hypotheses should be compared with hypotheses of phylogeny based on molecular studies. It is our opinion that neither molecular nor anatomical data alone will provide information for satisfactory hypotheses of phylogeny. The basic problem with either may be the presence of convergence.
Bryconadenos relationships. Vari & Ortega (2000:115-122) extensively discussed the monophyly of Attonitus and its possible relationships with other characid genera. They provided a synapomorphy list for its species. Above we redefined Attonitus based on our discovery and description of Bryconadenos. Those characters unique to the three Peruvian species of Attonitus are briefly discussed, but the analysis of the relationships among them is discussed only in relation to the fact that A. irisae and A. ephimeros appear to have relatively plesiomorphic sperm nuclei compared to A. bounites. Although Vari & Ortega (2000) made extensive and detailed anatomical comparison of the species of Attonitus with species of characid genera that have somewhat similar gross anatomical characteristics, they concluded that none they examined are anatomically similar enough to be interpreted as sharing synapomorphies with the species of Attonitus.
The comparatively derived genus Attonitus was placed in the insertae sedis section of the Characidae by Lima et al. in Reis et al. (2003: 113). This insertae sedis collection of characid genera contains a wide variety of plesiomorphic and derived characids and was meant only to be a temporary "category" to include characid taxa that lack hypothesized relationships within the family.
All Attonitus species have sexually mature males with clubshaped anal-fin gland cells similar in location and structure with those of B. tanaothoros, but so far as we have examined their club cells are not organized into a glandular organ as in B. tanaothoros. The three species of Attonitus have curved lower jaw teeth, but the curvature of their teeth is entirely different from that of B. tanaothoros and the derived nature of the teeth of these two genera is here considered probably independently derived. Other characters, such as the dark pigment associated with the lateral-line pores in species of both genera, are here considered of phylogenetic significance and a synapomorphy. Gross anatomical, histological and ultrastructural (when possible) data were taken from the primary and secondary sexual anatomy of all species of all the inseminating genera mentioned above and the glandulocaudine and stevardiine species listed in Appendix 2 Appendix II . These data were added to the matrix used by Weitzman & Menezes (1998: 188) for an analysis of the phylogeny of the tribes and genera of the Glandulocaudinae, Stevardiinae and various Clade A inseminating characids. However, because of current lack of data from several presumably relevant taxa such as many species of Bryconamericus , Knodus , Hemibrycon, and some other Clade A genera, the resulting hypotheses are used here only for our discussion of the phylogenetic relationships of Bryconadenos with Attonitus, and, to a very limited extent, the relationships of these to other Clade Agenera.
Thus our new information, concerning the primary reproductive system and to some extent the putative pheromone organs of glandulocaudines and stevardiines and their apparent Clade A relatives, now make it possible to tentatively hypothesize phylogenetic relationships for the species of Attonitus to other characids. The discussion here of the primary and secondary sexual features of the species of Bryconadenos , Attonitus, some species of Bryconamericus and Knodus as well as some glandulocaudine and stevardiine genera and certain other inseminating characids now allows us to make some suggestions regarding future areas for phylogenetic investigation of those characid genera. We suggest that the new species, B. tanaothoros, is at least in some ways plesiomorphic relative to the species of the genus Attonitus although it has its own relatively derived features. We also suggest that Bryconadenos and Attonitus may be related to at least two inseminating species currently referred to the insertae sedis nominal genus Knodus (Burns & Weitzman, 2005). However, this statement at this time refers only to those species of this genus that have some, but not necessarily all, of the morphological synapomorphies of the primary sexual system used by Weitzman (2003) and Weitzman & Menezes (1998) to diagnose their Glandulocaudinae. A preliminary discussion of the primary and secondary sexual anatomy of the non-glandulocaudine and non-cheirodontine characid species so far known to be inseminating is included here. As noted above, we found all known species of Attonitus to be inseminating and suggest that a survey for the occurrence of insemination and its accompanying histological features of the gonads and fine structure characteristics of the sperm cells in the species of the nominal genera Knodus and perhaps Bryconamericus may lead to a reorganization of the phylogenetic relationships and generic assignments of at least some of the species currently placed in these two genera. The inseminating species of Knodus are here putatively considered phylogenetically related to the Glandulocaudinae and Stevardiinae, although an extensive survey of the primary and secondary sexual morphology and the reproductive modes of the species of Knodus and Bryconamericus must be made before the nature of their phylogenetic relationships to the other genera and tribes of Clade A characids can be hypothesized in any detail.
Although the species of Knodus frequently have been placed in Bryconamericus, for example Schultz (1944) and Román-Valencia (2000), they were retained in Knodus for "practical" reasons by some authors for example, see Géry (1977: 391) for a discussion. Also, they were retained separately by Lima et al. in Reis et al. (2003). The discovery that at least two species of Knodus are inseminating (Burns & Weitzman, 2005) as well as Knodus pectinatus would seem to raise new questions about the problem of generic placement and relationships of species currently assigned to Knodus and possibly Bryconamericus. We agree with the comments by Schultz (1944) and Roman-Valencia (2000) that the systematics of the characids that have been placed in Bryconamericus and Knodus are poorly known and that "intermediate" species exist with regard to the external anatomical characters separating these two nominal genera as proposed by Eigenmann (1917). See also Eigenmann & Myers (1929) for brief notes on these genera. We prefer at this time to recognize these nominal genera as separate because of the new information presented here regarding the complexities of the characters associated with the reproductive modes of some of these fishes included in Knodus at this time.
In this regard we note that contrary to Vari & Siebert (1990) Bryconamericus pectinatus of those authors has scales partly on the caudal-fin rays, similar to some of the species that sometimes have been placed in Knodus. In addition, Knodus pectinatus is inseminating. Therefore, although K. pectinatus is more derived regarding secondary sexual features as described by Vari & Siebert (1990) than the two undescribed but inseminating species of Knodus listed by Burns & Weitzman (2005), we here prefer to refer to B. pectinatus as Knodus pectinatus until the systematics of the species that have been assigned to Knodus and Bryconamericus can be studied in detail, especially regarding their reproductive modes. In this regard we have been unable to find any evidence of insemination in Knodus meridae, the type species of Knodus ac-cording to Eigenmann & Myers (1929). This has implications regarding the future usage of the generic name Knodus for the inseminating species once their phylogenetic relationships are better hypothesized.
The discovery and description of Bryconadenos tanaothoros with its prominent anal-fin gland in males led us to a renewed investigation of the integument associated secretory organs, presumably pheromonal in nature in glandulocaudine and stevardiine characids and their putative Clade A outgroup relatives such as some of the species currently included in the genera Bryconamericus and Knodus. This investigation remains in its infancy and we here report only an incomplete sampling of the data from some of the many species needing investigation in these nominal genera. The discussion below is divided into two parts. The first discusses the relationships of the new species to the previously known three species of Attonitus described by Vari & Ortega (2000). The second part discusses the phylogenetic implications of the primary and secondary sexual anatomy of certain characid species that display features that indicate apparent or possible relationships with the member taxa of the Glandulocaudinae and Stevardiinae.
The distribution of club cells on the surface of the anterior part of the anal fin of characid fishes apparently not belonging to Clade A characids should be noted here. For example, Hyphessobrycon diancistrus Weitzman (a miniature characid, but with ii, 9 dorsal-fin rays and with 3-4 teeth on the inner row of the premaxilla) has two large bony hooks on the anterior lobe of the anal fin and these are surrounded by a masses of white tissue. See Weitzman (1977: figs. 1 & 2). When this species was first described it was assumed that this white mass was mucus tissue. Now, reexamination shows that this tissue contains abundant club cells similar to those present in the anal fin of B. tanaothoros, although some scattered mucus cells are also present. However, investigation of the gonads of H. diancistrus found only aquasperm in the males and no evidence of sperm cells in the female's ovaries. This suggested that other, non-inseminating characid species may have concentrations of club cells at the skin surface associated with male's anal-fin hooks, something subsequently found to be true in several inseminating and non-inseminating characids.
Based on gross anatomical data, Bryconadenos tanaothoros is a sister species to the three previously described species of Attonitus , A. bounites , A. ephimeros, and A. irisae. As pointed out in the species description above B. tanaothoros lacks the derived jaws and teeth of the three species of Attonitus and differs from them in having at least one additional pelvic-fin ray (i, 7 versus i, 6 or occasionally 5). Bryconadenos tanaothoros has more branched anal-fin rays (18-21 versus a range of 14 17). Probably the pelvic fin-ray count of B. tanaothoros is more plesiomorphic than that of the three Peruvian species because the outgroup count for the Characidae as a whole is i, 7. However, to hypothesize the relative plesiomorphic versus derived nature of the anal fin-ray counts requires more outgroup information than is available to us at this time. We are in no position to evaluate the relative plesiomorphic versus derived condition of the anal fin ray counts of these two genera.
Vari & Ortega (2000: 118-120) extensively discussed evidence demonstrating that A. bounites is a sister species to A. ephimeros and A. irisae and that the later two species can be distinguished from the former by six synapomorphies. These six synapomorphies are absent in Bryconadenos as discussed above and this is consistent with considering A. ephimeros and A. irisae more derived than Attonitus bounties.
Vari & Ortega (2000) reported Attonitus bounites to be inseminating, but did not investigate the other two species in this respect. We here provide data that all three species are inseminating. In our investigation of insemination and the histology of the species of Attonitus (see Appendix 1Appendix 1), we found all species to be inseminating, but only A. bounites, to have elongate sperm cell nuclei. The sperm cells in B. tanaothoros are complex in having mitochondria (="midpiece") located alongside most of the nucleus and beyond as at least is found in species of most of the glandulocaudine and stevardiine tribes, Weitzman & Menezes (1998) and Burns et al. (1998). Unfortunately we do not have this kind of information for A. bounites and the apparent aquasperm cells of A. irisae and A. ephimeros available to us are so poorly fixed that it is difficult to be confident about their structure. The numerous anatomical features listed by Vari & Ortega (2000: 118-120) indicate that A. irisae is one of the two most derived species in Attonitus. However, a more complete analysis of this hypothesis awaits examination of better fixed specimens of all three species regarding sperm cell structure. The apparent absence in Attonitus and the inseminating species of Knodus of derived caudal-fin organs in males, such as are present in the species of the genera and tribes of the Stevardiinae and Glandulocaudinae might suggest that there is little evidence for a close phylogenetic relationship between Attonitus and Knodus on the one hand and the members of the Glandulocaudinae and Stevardiinae on the other hand. The presence of certain other anatomical features of the primary sexual system such as the highly derived structure of the sperm nuclei present in at least some of the species of Attonitus and Knodus and in the glandulocaudines and stevardiines suggests that the tribes of these two subfamilies may be a sister group of an inseminating clade that includes some species currently placed in Knodus , Attonitus, and Bryconadenos. We now assert that at least one of the tribes, the Glandulocaudini, is phylogenetically independent of the other tribes of the former Glandulocaudinae, Fig 11, and the remaining tribes, some perhaps only tentatively now in the Stevardiinae, may also have their sister group relationships with the inseminating species currently assigned to Knodus.
Using TEM preparations, examination of sperm cell ultrastructure of B. tanaothoros indicates a possible relationship with some of the more plesiomorphic species in the tribes of the Stevardiinae and Glandulocaudinae as well as the inseminating species of Knodus and/or inseminating Knodus related characids. Weitzman & Menezes (1998:180-188) placed the tribes of these two subfamilies in a single family in part based on the possession of insemination together with the presence of a cytoplasmic collar binding the anterior part of the flagellum to the body of the elongated sperm cell and the presence of spermatozeugmata in some. At that time, assuming all of the then glandulocaudine taxa had such sperm cell modifications, a strict consensus cladogram supported a hypothesis for the monophyly of the single subfamily Glandulocaudinae. However, we now find some of the characters of the "glandulocaudine" sperm cells more complexly distributed among Clade A taxa, even some without a caudal organ. For example two species of Attonitus , A. irisae and A. ephimeros, essentially have aquasperm while A. bounties has elongate sperm cells. Unfortunately TEM preparations were not available to investigate the comparative cell structure of the sperm cells of the species of this genus, but the single known species of the hypothesized close relative Bryconadenos has elongate sperm cells with mitochondria located along and beyond the nucleus as in at least many stevardiine in the tribes Hysteronotini, Stevardiini and Xenurobryconini. See also Pecio et al. (2005: 224 & fig. 4). The stevardiine tribe Phenacobryconini remains a problem because this genus has elongate sperm cells, but information about an elongate "binding" cytoplasmic collar or location of mitochondria are lacking and the females available for histological ovary inspection lacked sperm cells. The squamation of the apparently mature caudal organ (Weitzman & Fink, 1985: p. 20, fig. 30) suggests that the enlarged pouch scales could be derived from the horizontal scale row just ventral to the lateral-line scale row, but developmental information is lacking. Phenacobrycon could be a genus relatively closely related to the inseminating species of Knodus and at the same time related to the apparent stevardiine tribes Hysteronotini, Stevardiinae and Xenurobryconini, but confirming information is needed for such a hypothesis. Although several species of Creagrutus were found to be non-inseminating with sperm cells containing spherical nuclei (aquasperm), two are known to be inseminating and one of these, C. lepidus, has sperm cells with elongate nuclei and one, C. melasma, has ovoid nuclei. Again, no information is available regarding the ultrastructure of the sperm cells of these two species. Weitzman and Menezes (1998) hypothesized the tribes Landonini, Glandulocaudini and Diapomini each to be monophyletic and relatively plesiomorphic for their more inclusive Glandulocaudinae. However, the primary sexual features of the Landonini remain relatively unstudied and although the male of the single species has elongate sperm, its ultrastructure remains unknown and the available female ovaries contained no sperm and were apparently too immature to provide a useful sample regarding insemination. As noted above we now regard the inseminating species of the Glandulocaudini to be derived independently of the Stevardiinae. Concerning the possible monophyly of the Diapomini we are no longer convinced that this tribe is monophyletic. For example, the inseminating species of Planaltina (currently in the Diapomini of the Stevardiinae) have what are essentially aquasperm with slightly ovoid nuclei, while the species of the other two genera, Diapoma and Acrobrycon apparently have typical elongate sperm cells (Menezes, et al., 2003: 596).
Microscopic Analysis of Primary and Secondary Sex Characters of the Species of Attonitus and Bryconadenos.
Insemination. Histological analysis of mature ovaries confirmed the presence of spermatozoa in species of Attonitus and of Bryconadenos, Fig. 15, C & 16, A-C. No distinct sperm storage structures or regions were seen in the ovaries. Instead, spermatozoa could be found within folds of the ovarian cavity and within the remains of ovulated follicles. Similar findings have been reported for all inseminating characids analyzed to date (Burns et al., 1995, 1997, 2000).
Sperm and testis structure. Sperm nuclei, as determined from LM, were spherical to slightly ovoid in A. ephimeros and A. irisae, Fig. 16, A, B, and elongate in A. bounites Fig. 16, C, and B. tanaothoros (Fig. 15, C. Measurements of sperm nuclear length were made on LM sections under oil immersion using an ocular micrometer. In order to account for the possible ovoid shape of the sperm nuclei of A. ephimeros and A. irisae, only the greatest dimensions of these organelles were included in the calculations. The mean nuclear lengths (± one standard deviation) from 20 cells for each species were as follows: A. ephimeros, 1.81 ± 0.09 µm; A. irisae, 1.80 ± 0.08 µm; A. bounites, 2.85 ± 0.21 µm; B. tanaothoros, 6.67 ± 0.57 µm. The length and shape of the sperm nuclei of A. ephimeros and A. irisae are characteristic of the "aquasperm" of externally fertilizing characids. The only other inseminating characids analyzed to date that possess cells resembling aquasperm are species in the genus Planaltina (Burns et al., 1995; Menezes et al., 2003), Kolpotocheirodon theloura (Burns et al., 1997; Malabarba & Weitzman, 2000), and a species of Knodus (unpublished). All other inseminating characids have sperm nuclei that are elongate, as seen in A. bounites and B. tanaothoros. Elongate sperm nuclei and heads appear to be selectively advantageous for inseminating species (see Burns & Weitzman, 2005, for discussion).
Acknowledgments
The authors thank Tamara Clark for preparing Figures 4-6, 9, 11-14 with funds provided by the Herbert R. and Evelyn Axelrod Revolving Chair of the Division of Fishes, National Museum of Natural History, Smithsonian Institution. This source also provided funds for publication. Lisa Palmer prepared radiographic negatives and provided general curatorial assistance. Luiz Malabarba supplied valuable input regarding discussions of inseminating characid fishes. Marilyn Weitzman, Richard Vari, Eric Hilton, and Lisa Palmer provided valuable comments on the manuscript. Robert Javonillo provided histological expertise in preparing and examining several specimens for this research. Marco Tulio Caesar Lacerda and Paulo Valerio da Silva provided expert fieldwork and companionship for H-GE in collecting fishes of the Serra do Roncador. Cristiano L. R. Moreira supplied specimens of the new species from the upper rio Tapajós. We are gratefully indebted to all. This research was supported in part by the Neotropical Lowland Research Program of the Smithsonian Institution, and the project "Conhecimento, Conservação e Utilização Racional da Diversidade da Fauna de Peixes do Brasil" funded by the Financiadora de Estudos e Projetos (FINEP) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through Programa de Apoio a Núcleos de Excelência (PRONEX) No. 661058/1997-2, coordinated by NAM.
Literature Cited
Received May 2005
Accepted September 2005
Light microscopy was carried out on B. tanaothorus and the three species of Attonitus , A. irisae , A. ephimeros, and A. bounties. Electron microscopy was only carried out on B. tanaothoros due to the availability of fresh tissue for fixation. SEM showed that the sperm cell of B. tanaothorus is tapered at either end with the flagellum exiting the cell about midway along its length, Fig. 17. Fig. 18 presents transverse and longitudinal TEM sections through the spermatozoon of B. tanaothoros. Portions of the two centrioles are located within shallow nuclear fossae at the more flattened anterior part of the cell, Fig. 18, A. The nucleus, with its condensed chromatin, is also flattened in this region. Further posteriorly, both the cell and nucleus are more rounded in transverse section, Fig. 18, B & C. Nuclear elongation is mainly lateral and posterior to the centrioles. The flagellum is initially located within the cytoplasmic canal of an elongate cytoplasmic collar, Fig. 18, B & E, before exiting the collar about midway along the length of the cell as seen in the SEM, Fig. 17. Mitochondria are located along the length, Fig. 18, B, C, & F, of and slightly behind, Fig. 18, D, the nucleus, a character shared with all species of the subfamilies Glandulocaudinae and Stevardiinae whose sperm cells have been analyzed with TEM to date (Pecio & Rafiñski, 1994, 1999; Burns et al., 1998; Burns & Weitzman, 2005; and unpublished data). Accessory microtubules running longitudinally are also present at the periphery of the cell, Fig. 18, B, C, & D. Accessory microtubules, which have also been found in glandulocaudine species that produce distinct sperm packets (Pecio & Rafiñski, 1994, 1999; Burns et al., 1998; and unpublished data), may aid in the formation of these packets (Burns & Weitzman, 2005); B. tanaothoros also produces distinct sperm packets (spermatozeugmata).
Distinct, wide, aspermatogenic sperm storage areas were observed only in A. bounites and B. tanaothoros (Fig. 15, A & B), where the percent testis areas measured 15.4% and 17.6%, respectively (see Burns et al., 1995, for method). Similar sperm storage areas were observed in all species of the Glandulocaudinae and Stevardiinae analyzed histologically, where the percent testis areas occupied by these regions ranged from 17.6-92.6% (Burns et al., 1995). Comparable regions in externally fertilizing characids were narrower than the anterior spermatogenic portion of the testis, where the percent testis areas ranged from 4.3-12.2% (Burns et al., 1995). Thus, the sizes of the sperm storage areas in A. bounties and B. tanaothoros both of which have elongate sperm nuclei, tend to fall between those of externally fertilizing characids and the inseminating glandulocaudines and stevardiines. The cells lining the aspermatogenic storage areas in A. bounites were simple cuboidal, whereas in B. tanaothoros they varied from simple squamous to cuboidal.
Neither sperm packaging nor testicular secretions were observed in the testes of A. ephimeros , A. irisae or A. bounites. This was not the case with B. tanaothoros which produced very compact and organized sperm packets, Fig. 15, B, surrounded by an intensely PAS+ secretion, suggesting a carbohydrate component. Since the sperm packets are unencapsulated, they are classified as spermatozeugmata (Grier, 1981). Each spermatozeugma consists of multiple layers of parallel, overlapping spermatozoa. In longitudinal section the spermatozeugmata taper at either end (spindleshaped), whereas in transverse section they appear more rounded, Fig. 15, B. The intense PAS+ secretion seen in some specimens may aid in the formation and maintenance of the spermatozeugmata (Burns & Weitzman, 2005).
The precise location where spermatozeugmata are formed was difficult to determine in B. tanaothoros. In most parts of the anterior spermatogenic region the mature spermatozoa appeared to be already packaged into discrete packets upon release from the spermatocysts (spermiation). In other regions, however, unorganized masses of spermatozoa were observed within the sperm ducts of the spermatogenic region. The intense PAS+ secretion seen in the storage region (see Burns & Weitzman, 2005, for a color photomicrograph) was also often found within the sperm ducts of the spermatogenic region. Thus, it may be that loose spermatozoa or small clumps are released from the spermatocysts, but the PAS+ secretion may cause these to coalesce into the compact spermatozeugmata. Smaller, similarly shaped packets were also observed among the larger packets. The presence of both small and large sperm packets, their overall morphology, and the PAS+ secretions are all characters seen in species of the glandulocaudine genera, Glandulocauda and Mimagoniates (Pecio & Rafiñski, 1994, 1999: Burns et al., 1995).
An unusual structure found only in mature males of certain characid species is the gill gland (Burns & Weitzman, 1996). Gill glands develop from anterior gill filaments of the first gill arches and consist of chambers lined with an epithelium that varies from cuboidal to columnar (Burns & Weitzman, 1996; Bushmann et al., 2002). Gill glands were found in mature males of A. irisae , A. bounites and B. tanaothoros (A. ephimeros was not analyzed). The gill glands of the specimen of A. irisae appeared to be in the initial stages of formation, Fig. 19, C, because no hypertrophied cells were seen between the gill secondary lamellae, although a distal covering on three gill filaments formed two chambers. The gill glands of both A. bounties, Fig. 19, A & B, and B. tanaothoros, Fig. 20 were more highly developed. Each gill gland of A. bounites was formed from 14 gill filaments resulting in 13 chambers, Fig. 19, A, while that of B. tanaothoros comprised 5 filaments and 4 chambers, Fig. 20, A). Each gill gland chamber opened ventrally into the gill cavity. In both species the gill secondary lamellae within the gill gland chambers were greatly shortened, and tall columnar cells were present between each pair of secondary lamellae, Fig. 19, B & 20, B. Some secretory material was observed within the gill gland chambers as well. Although the function of gill glands has not yet been determined, their structure, location and secretory characteristics, as well as restriction to mature males, suggest a possible pheromonal function related to sexual behavior.
Two main types of glandular cells are found in the epidermis of ostariophysan fishes: mucous cells and club cells. Club cells, which are generally large cells with one or two nuclei located at or near the center of the cell, do not stain positive with the PAS technique (Pfeiffer, 1967, 1977). These club cells do not reach the skin surface, and they release their contents only if the skin is damaged (Pfeiffer, 1967). The substance released by these cells has been identified as hypoxanthine-3-N-oxide, which causes an alarm reaction in other fish (Pfeiffer et al., 1985; Brown et al., 2001). Therefore, these types of club cells have been called alarm substance cells ( Pfeiffer, 1967). A mass of tissue on the anal fin of B. tanaothoros is predominantly made up of essentially identical club cells that have generally one, centrally located nucleus and do not stain with PAS, Fig. 21, A. However, at specific regions on this tissue mass, the club cells do indeed reach the skin surface, and secretion is accomplished through degeneration of the entire cell, thus a type of holocrine secretion, Fig. 21, B. Although A. irisae and A. bounites do not have distinct glandular cell masses on their anal fins, histological analysis of the skin on their anal fins revealed abundant club cells that also showed characteristics of holocrine release. Thus, holocrine-releasing club cells on the anal fin may be characteristic of the species of Attonitus as well. Surface-releasing club cells have also been described in the catfish, Etropiella debauwi (Pfeiffer, 1967), and a holocrine release by surface club cells has been documented in the catfish, Rita rita (Mittal & Datta Munshi, 1970). These authors suggest that the function of these club cells may be other than the release of alarm substance. The holocrine release mechanism of the anal-fin club cells in B. tanaothoros and the three Attonitus species, coupled with the glandular mass being restricted to mature males in B. tanaothoros, points to a role of these cells in reproduction, perhaps via release of a pheromone.
Comparative specimens examined. Many of the specimens listed below, those in parentheses, were used to provide tissues samples (HE) for histological examination of gonads and in some cases other organs and tissues, for cell ultrastructure (CEUST), or for osteological comparison (C&S). In instances when over 25 specimens were available or when lots of the same species were available in quantity, whole specimens were submitted for histological examination of several tissues and organs such as the gill gland, the caudal organ, anal-fin tissue for the presence of secretory (probably pheromonal) cells. However, in most cases only tissue samples were taken from specimens (see under methods above). In the case of the species of Attonitus, lots examined for species comparisons were those given USNM numbers in Vari & Ortega (2000). One lot each of specimens of Planaltina britskii Menezes et al. and Planaltina myersi, both not recorded in Menezes et al. (2003), are recorded below. The species and generic identifications of the taxa list below, especially in those assigned to Bryconamericus and Knodus, are tentative pending future systematic reviews. Lima et al. (2004) have most recently outlined the problems associated with the taxonomy and systematics of the characid species variously assigned to especially Knodus and Bryconamericus. We agree with their evaluation of these genera and their currently included species. The species of these two genera are in desperate need of a taxonomic review and an investigation of their phylogenetic relationships. There may be several clades here, not just two as their current generic assignment suggests. Some new problems associated with insemination in some species currently assigned to one or the other of these two genera were mentioned by Menezes et al. (2003) as well as discussed here. The taxa listed in Bryconamericus and Knodus below were chosen for examination for a variety of reasons, but the major one was to get a preliminary sampling of the distribution of certain previously unexplored or only partly explored features found in the present investigation or in Menezes et al. (2003).
Attonitus bounties, paratypes, USNM 349701, 13 spms, 36.4-44.6 mm SL (2 mature males 41.9 and 44.6 mm SL, plus 1 mature female 42.4 mm SL, C&S) (1 mature male & 1 mature female, both 43.0 mm SL, HE); Peru, Puno, Provinca Carabaya, Zona Reservada Tambopata Candamo, río Candamo, 1324'S, 07001'W, 12 Aug. 1997 (type locality). Attonitus bounties, paratypes, USNM 349705, 1 spm, (mature female 41.2 mm SL, HE). Note: reported as a male in Vari & Ortega, (2000: 123); Peru, Cusco, Provinca Pucartambo, río Hospital, Pilcopata, 1304'S, 07110'W, 4 Jan. 1997. Attonitus bounites, paratypes, USNM 349715, 5 spms, 40.0-46.8 mm SL (mature male, 40.4 mm SL, HE), Peru, Puno, Provinca Sandia, Zona Reservada Tambopata Candamo, Cuenca Ebehuabaeji, río Explanada, 1324'40"S, 07000'19"W, 31 Jul, 1997. Attonitus bounites, USNM 363732, 2 immatures or young females 31.5-33.6 mm SL, no locality data. Attonitus ephimeros, paratypes, USNM 349696, 6 spms, 31.5-47.6 mm SL, (mature male, 47.6 mm SL, C&S), (1 female 31.5 mm SL & 1 male spm 45.7 mm SL, HE); Peru, Cusco, Provincia La Convencion, río Urubamba basin, río Picha, Puerto Huallana, Quebrada Mapichiriato, 1150'S, 07320'W, 15 Feb. 1997, (type locality). Attonitus ephimeros, paratypes, USNM 349708, 8 spms, 34.2-45.5 mm SL (1 immature 38.8 mm SL & 1 mature male 41.9 SL, C&S); Peru, Junin, Provincia Chanchamayo, La Merced, El Kimo río Chanchamayo, approximately 1103'S, 07519'W, 7 Dec. 1987. Attonitus irisae, paratypes, USNM 349698, 10 spms, 31.0-45.3 mm SL, (1 female 39.0 mm SL & 1 mature male 46.4 mm SL C&S); (1 mature male, 42.1 mm SL, & 1 mature female 38.2 mm SL, HE), Peru, Huanuco, Provincia Pachitea, mouth of río Huambo where it empties into río Pachitea, approximately 0939'S, 07456'W, 28 Jul, 1988 (type locality). Attonitus irisae, paratype, USNM 349704, 1 mature female, 36.3 mm SL HE), Peru, Ucayali, Provincia de Padre Adad, río Aguaytia, Boquerón Padre Abad, Velode Novia (approximately 0906'S, 07545'W); 13 Sept. 1994. Attonitus irisae, paratype, USNM 349697, 1 mature male, 38.7 mm SL, Peru, Ucayale, Provincia de Padre Adad, río Huacamayo, under bridge at km 155 on road from Tingo Maria to Pucallpa, 27 May 1983. Attonitus irisae, paratype, USNM 349710, 1 mature male, 36.7 mm SL, Peru, Ucayale, Provincia de Padre Adad, río Huacamayo, río Aguaytia basin, 13 Sept. 1994. Brittanichthys axelrodi, holotype, USNM 198132, male, 25.5 mm SL, Brazil, Amazonas, rio Itu, about 80 km upstream from Praia Bulufu, 26 Apr 1964. Brittanichthys axelrodi, USNM 221991, 62 spms, 16.0-24.8 mm SL, (14 spms, 16.0-24.8 mm SL C&S), (3 females, 20.9 & 23.9 mm SL & 2 mature males 22.3 & 12.9 mm SL, Note: In addition 4 whole specimens submitted for various histological and other tissue studies as follows: 2 females, 19.2 & 22.3 mm SL, 2 males 22.3 & 23.9 mmSL), Brazil, Amazonas, rio Negro, rio Urubaxi, 11 Feb. 1980. Brittanichthys axelrodi, USNM 326194, 2 spms, male 21.2 & female 23.0 mm SL, Brazil, Amazonas, rio Urubaxi, 11 Feb. 1980. Brittanichthys axelrodi, USNM 332486, 3 spms, 16.7-18.8 mm SL, Brazil, Amazonas, Lago da Rainha, rio Demini, 7 Feb. 1991. Brittanichthys axelrodi, USNM 374029, 8 spms, 18.0-19.8 mm SL, Colombia, Vichada, small lake near río Muzo, about 30 km west of San José de Ocune, río Vichada drainage, Jul 1974. Brittanichthys axelrodi, 2 live specimens, (male 22.3 mm SL and female 21.6 mm SL. No recorded locality, (euthanized and entire specimens preserved using CEUST fixative for HE & CEUST). Brittanichthys myersi Géry, holotype, USNM 198131, male, 31.5 mm SL, Brazil, Amazonas, south shore of rio Negro, ± 10 km west of Manaus, 7 Apr 1964. Bryconamericus alpha, USNM 285343, 20 spms, 35.9-48.2 mm SL (2 adult males, 42.9 & 43.3 mm SL & 1 adult female, 48.2 mm SL, HE), Venezuela, Barinas, rio Caparo, Mar 1980. Bryconamericus deuterodonoides Eigenmann, USNM 349383, 67 spms, 11.3-31.3 mm SL, (6 specimens, male & female, 19.7-31.3 mm SL, HE), Venezuela, Provincia Portuguesa, just upstream of Highway 5, 11km west by northwest of Guannare, 28 Feb 1998. Bryconamericus deuterodonoides, USNM 349407, 47 spms, 18.5-35.0 mm SL, Venezuela, Provincia Portuguesa, stream near town of Quebrada Seca, approximately 22 km north by northwest of Guarnare, 28 Feb. 1998. Bryconamericus exodon, USNM 232402, 7 spms, 31.5-44.0 mm SL, (1 mature male 34.4 mm SL & 1 mature female 41.9 mm SL, HE), Paraguay, Amambay, Parque Nacional Cerro Cora, rio Aquidaban, 13 Jan 1982. Bryconamericus iheringii, USNM 286884, 37 spms, 28.0-65.0 mm SL, (1 mature male 53.2 mm SL HE), Brazil, of Rio Grande do Sul, arroyo Garupa, tributary to rio Quarai and ultimately to rio Uruguay on road between Uruguayiana and Quari, R. 20 Dec 1986. Bryconamericus iheringii, USNM 337626, 21 spms, 28.0-65.0 mm SL, (2 mature males 65.0 mm SL and 44.4 mm SL, plus 1 mature female 39.1 mm SL, HE), Brazil, Rio Grande do Sul, Município de Lajeado, rio Forqueta at Marques de Sousa, approximately 2919'S, 05205'W, 7 Dec 1979. Bryconamericus pachacuti Eigenmann, MUSM 12016, 10 spms, 25.5-37.7 mm SL, (1 male 29.8 mm SL and 1 female 37.7 mm SL, HE), Peru, Cusco, Provincia Convencion, Quebrada Manitiari, río Parotori, tributary to río Urubamba, 20 May 1991. Bryconamericus sp. USNM 326937, 12 specimens 32.4-54.1 mm SL, (1adult male 38.5 mm SL and 1 adult female 53.2 mm SL, HE), Peru, Madre de Dios, Provincia Manu, Manu National Park, Pakitza, small stream named Panahua, 17 Feb. 1992. Cheirodon ortegai Vari & Géry (1980), MZUSP 25993, 3 spms (1 adult male 30.9 mm SL, 1 adult female 25.5 mm SL, HE), Peru, Ucayali, Provincia Coronel, Portillo, Lobococha, Masisea, 27 Feb 1976. Corynopoma riisei, USNM 338214, 375 spms, (young to adults, 11.8-36.9 mm SL), specimens in alcohol, but lightly stained with alizarin, second generation aquarium specimens from live specimens collected from Venezuela, Estado de Sucre, at Los Barrancas, approx, 1 ¼ km north of Tataracual, between Cumana and Cumanacoa, in río Manzanares, (approximately 1338'S, 04819'W) 06505˜ (approximately 1022'N 06505'W), 12 Mar 1977. Creagrutus affinis, USNM 293249, 121 spms, (1 adult male, 38.0 mm SL, & 1 adult female, 44.5 mm SL, HE), Panama, Provincia Darien, río Pucuro just above confluence with río Tuira, (approximately 0800'S, 07732'W), 16-18 Feb. Creagrutus britskii Vari & Harold, paratypes, USNM 292226, 13 spms, (1 adult male, 32.3 mm SL & 1 adult female 44.0 mm SL, HE), Brazil, Goiás, Minaçu, tributary of córrego Lageado, left bank tributary of rio Tocantins along road to Porto Rubião (approximately 1338'S, 04819'W), 16 Jan 1988. Creagrutus changae, paratypes, USNM 285276, 14 spms, (1 adult male, 53.0 mm SL & 1 adult female 58.0 mm SL, HE), Peru, Provincia Pachitea, río San Alejandro, tributary of río Sungarayacu, just above junction with río Sungarayacu (approximately 0923'S, 07511'W), 31 Jul 1975. Creagrutus lepidus, paratypes, USNM 325045, 10 spms, (1 mature male 41.75 mm SL & 1 mature female, 35.4 mm SL, HE), Venezuela, Provincia Yaracuy, Sierra de Aroa, río Aroa basin, Finca El Jaguar, quebrada El Charal, (approximately 1032'S, 06832'W), 24 Jul 1985. Creagrutus melasma, USNM 349411, 22 spms, (1 adult male, 26.0 mm SL, & 1 adult female, 26.5 mm SL, HE), Venezuela, Provincia Portuguesa, río Las Marias, at Quebrada Seca, approximately 45 minutes upstream by car from Highway 5, 22 km north west of Guanare, 28 Feb 1998. Creagrutus figueiredoi, paratypes, USNM 292221, 11 spms, (1 adult male, 46.5 mm SL & 1 adult female 52.5 mm SL, HE), Brazil, Goiás, Minaçu/Colinas do Sul, rio Tocantins, pools formed below dam of Usina Hidroelectic (UHE) de Serra da Mesa after closure of dam for filling, 28 Oct – 3 Nov 1996. Creagrutus paralacus, USNM 121505, 335 spms, (1 adult male, 49.0 mm SL, & 1 adult female, 62.5 mm SL, HE), Venezuela, Provincia Merida, Maracaibo basin, río Gonzales, tributary of río Chama at Gonzales, 29 Mar 1942. Knodus albolineatus Holly, NMW-83 365:1 "syntype," (68.2 ), Brazil, Amazonas, Piquirao. Listed as a synonym of Moenkhausia lepidura (Kner) by Benine in Lima et al. (2003). Knodus breviceps, paratypes, USNM 120274, 2, Brazil, Goyaz, exact locality not known, presumably rio Tocantins or rio Araguaia systems, (see Higuchi, 1996), 1867. Knodus breviceps, MCZ 20692, listed as "syntypes," 19, 51.2-66.6 mm SL, (mature male, 57.0 mm SL & mature female, 62.0 mm SL, HE), same locality as USNM 120274. Knodus breviceps, MCZ 92886, 11 spms, 28.6-37.5 mm SL, (male 31.6, female 32.2 mm SL HE), Brazil, Amazonas, Lago Jacaretinga, near Manaus, 25 Apr 1979. Knodus breviceps CAS 70411, 37 spms, 20.8-55.7 mm SL, Bolivia, La Paz, Espia at junction of río La Paz and río Miguilla to form río Bopi, 1-31 Jul 1921. Knodus breviceps CAS 70410 4 spms, 33.4-44.0 mm SL, Bolivia, Beni, mouth region of río Beni junction with río Madeira at Villa Bella, 5 Oct 1909. Knodus calliurus Ahl, ZMB 23684, holotype, (synonym of Moenkhausia lepidura according to Benine in Lima et al. 2003), (31.7 mm SL), Brazil, rio Capim, Oct 20, 1928. Knodus gamma Géry ZMH 1861, holotype, 48.5 mm SL, Ecuador, río Villano (Cururay), Selva Negra, 1 Jan 1963. Knodus gamma, ZMH 2211, paratypes, 2 spms, 44.8-47.6 mm SL, same locality as holotype. Knodus heteresthes Eigenmann, UF 103655, 10 spms, 33.9-38.9 mm SL, Brazil, Rondonia, rio Jaci-Paraná, approx. 40 river km upstream from town of Jaci-Paraná, rio Madeira system, 24 Jun 1994. Knodus heteresthes, UF 103694, 11 spms, 30.6-39.7 mm SL, Brazil, Rondonia, Corredeiras de Monte Cristo, near Ariquernes, rio Madeira system, 23 Jun 1994. Knodus meridae, holotype, BMNH 1911.5.29.148, (Note, label in bottle reads: 1908.5.29:148), 1, 43.5 mm SL, Venezuela, Merida, lago Maracaibo basin, presumably río Chama system, Merida, no collecting date. Knodus meridae, USNM 121473, 51, 2 adult males examined, 29.1-31.5 mm SL & 1 adult female, 35.7 mm SL, HE), Venezuela, Trujillo, lago Maracaibo basin, río San Juan, tributary of río Motatán, under bridge, south of Mene Grande, 17-20 Mar 1942. Knodus meridae, USNM 121469, 206, 15.2 – 43.1 mm SL, Venezuela, Trujillo, lago Maracaibo basin, río Motatán, 4 km above Motatán, 25 Mar 1942. Knodus moenkhausii (Eigenmann & Kennedy), AMNH 1463, "syntypes," 2, 26.1-27.7 mm SL, specimens dehydrated, Paraguay, Parana, De la Plata Basin, brook near arroyo Trementina, 1900-1901. Knodus moenkhausii, CAS 55104, 5, 21.8-26.5 mm SL, originally IU 10003, specimens severly dehydrated, same locality as AMNH 1463. Knodus moenkhausii, CAS 55103, originally IU 10002, paratype, 1, 33.5 mm SL, same locality as AMNH 1463. Knodus moenkhausii, FMNH 52601, paratypes, 2, 30.7-31.3 mm SL, specimens severly dehydrated, same locality as AMNH 1463. Knodus pectinatus, USNM 303441, paratypes, 5, 19.8-37.0 mm SL, (1 male, 34.8 mm SL, HE, 1 male 35.4 mm SL, C&S), Peru, Madre de Dios, Provincia Manú, Parque Nacional de Manú second large quebrada along trail 1, leading to east from Pakitza, tributary of río Manú, approximately 1150'S, 07121'W, 13 Sep 1988. Knodus pectinatus, USNM 303442, paratypes, 7 spms, 17.0-27.7 mm SL, (1 male 27.7 mm SL, C&S), Peru, Madre de Dios, Provincia Manú, second largest quebrada along trail 1 out of Pakitsa, río Manú drainage, 13 Sep 1988. Knodus pectinatus, MUSM 3708, 8, 19.5-40.2 mm SL, (1 male, 30.4 mm SL, & 1 female, 40.2 mm SL, HE), Peru, Madre de Dios, Provincia Pakitza, Parque Nacional de Manú, Picaflor stream, 26 Oct 1987. Knodus savannensis Géry USNM 196088, holotype, 30.4 mm SL, Brazil, savannas of northeastern Brazil between northeastern lower rio Tocantins and rio Capim, Sep 1959. Knodus septentrionalis ZMH 2261, holotype, 47.7 mm SL, Ecuador, Pastaza, río Capotazo, trib. of río Paztaza, "from Naundorff", 1963. Knodus septentrionalis USNM 361168, 13 spms, 24.8-47.8 mm SL, (1 male 34.0 & 1 female 35.3 mm SL, HE), Peru, Ucayali, Atayalya, Quebrada Campo Plata, 7 Apr 1998. Knodus septentrionalis USNM 330851, 48 spms, 21.5-37.5 mm SL, Peru, Loreto, Proviencia Arcadia, island in río Napo. 7 Nov 1993. Knodus septentrionalis USNM 328109, 10 spms (of 20 in bottle), 29.8-42.7 mm SL, Peru, Loreto, Proviencia Arcadia, río Napo, Maynas, 1 Nov 1993. Knodus sp. MZUSP 38004, 50 of 134, 22.8-36.6 mm SL, (1 adult male, 34.0 mm SL & 1 adult female, >35.5 mm SL, HE), Brazil, Minas Gerais, riacho near Fazenda Poderosa, tributary to ribeirão Jaboticatubas, tributary to rio das Velhas, Campo Alegre, Feb 1983. Knodus sp. 2, USNM 326277, 27 mm SL, (1 adult male, 39.0 mm SL, & 1 adult female 39.0 mm SL, HE), Peru, Madre de Dios, Parque Nacional Manu, Pakitza & vicinity, Oct 1987. Knodus sp. USNM 344440, 3, 29.6-35.6 mm SL, (1 adult male 32.2 mm SL, & 1 adult female 35.6 mm SL), Peru, Madre de Dios, Provencia Tambopata, Parque Nacional Manu, quebrada at 3 km off Tapir Trail, 28 Dec 1996. Knodus sp. MCZ 58466, 7 spms, 16.2-21.1 mm SL, (male ?, 21.1 mm SL, HE), Peru, Loreto, rio Ia, tributary to rio Ucayali, 12 Sep 1961. Knodus sp. USNM 361471, 61, 11.7-47.7 mm SL, Peru, Cusco, La Convencion, Echartea San Martin, Qda, Natsiringari, 1147'09''S, 07042'05''W, 31 Oct 1998. Knodus victoriae (Steindachner) CAS72053, 10 spms, 34.1-47.1 mm SL, Brazil, Goiaz, 10 spms, 34.1-47.2 mm SL, ribeirão Tacaurai, trib of rio das Almas, tributary of rio Maranhão, 25 Dec 1923. Lophiobrycon weitzmani. MZUSP 83353. paratypes, 30 spms, 10.9-25.3 mm SL. (2 spms, 1 adult male 25.3 mm SL & 1 female 19.7 mm SL partly dissected on right side for histology of primary and secondary sexual characters), from type locality, Bra-zil, Minas Gerais, rio Grande basin, Município de Delfinópolis, Estância Carmen Sílvia, córrego Bom Jesus, 20º12'10''S, 046º55'22''W, 20-21 Jul 1999. Monotocheirodon pearsoni "syntypes," CAS 59792, 7, 19.4-39.9 mm SL, (1 female, 32.1 mm SL, HE), Bolivia, La Paz, Espia where río La Paz and río Maquilla join to form río Bopi, tributary to río Beni, Jul 1921. Monotocheirodon sp., MUSM 6756, 75, mostly juveniles, but six larger specimens sent to USNM for examination, 23.1-39.0 mm SL, (3 adult males 35.3, 38.1, and 39.0 mm SL & 1 adult female 38.0 mm SL, HE & SEM), Peru, Puno, Sandia, Muspaypampa, río Inambari system, río Huasimayo, Santa Elena, 6 Jun 1994. Monotocheirodon sp., MUSM 11082, 9, 27.0-36.7 mm SL, (3 adult males, 27.0, 30.0, and 35.0 mm SL & 1 adult female, 36.7 mm SL, HE and CEUST fixative), Peru, Puno, Sandia, Zona Reservada Tambopata-Candamo, río Candamo, río Candamo, Ebebahuaeji stream (approximately 1324'90''S, 07000'96''W). Monotocheirodon sp., ANSP 143791, (1 adult male 33.9 mm SL, HE), Peru, Cuzco, río Hospital "mod-erate stream, 2 km west of Patria on north side of road, 12º53'S, 071º27'W, 12 Jul 1977. Monotocheirodon sp., ANSP 143792, 5, 30.7-40.0 mm SL, (1 adult female 38.5 mm SL, HE), Peru, Cuzco / Madre de Dios, mouth of río Carbon, below Atalaya on north side of road above and below ford, 12º53'S, 071º20'W, 18 Jul 1977. Paragoniates alburnus USNM 311357, 1, (mature male, 104.0 mm SL, HE), Ecuador, Napo, estero Culiyacu, left bank of río Tiputini, a branch of middle río Napo, no date, catalogued 10 Oct 1989. Planaltina britskii, USNM 362836, spms, 26.6-37.6 mm SL, (1 adult male, 33.2 mm SL & 1 adult female, 37.7 mm SL, HE), Brazil, Minas Gerais, córrego Julião, tributary to rio das Velhas, along road to Jaboticatubas, 1 Oct 1977. Planaltina myersi, UF 78149, 14 spms, 22.3-34.9 mm SL, Brazil, Distrito Federal, Ribeirão Santana, rio Parana drainage, 16º01'S, 047º49'W, 5 Agust 1988.
Appendix III
Menezes & Weitzman (2003: 562-563, figs. 1-3) published illustrations purported to be photographs of Planaltina myersi. Although all the data reported by Menezes and Weitzman (2003) were taken from specimens of Planaltina myersi, unfortunately the wrong photographs were accidentally substituted. These photographs and the ones of Planaltina myersi presented here as Fig. 22 were taken at the same time and soon after these fishes had been collected together in 1977. The illustrations published by Menezes & Weitzman (2003) are photographs of a species we currently assign to Knodus, but was recently described by Langeani et al. (2005) as Bryconamericus turiuba.
Fig. 22 - click to extend
In our discussions above we point out that the systematics of the nominal genera Bryconamericus and Knodus are much more complex than previously thought. We have 16 specimens, USNM 381498, 6 males and 10 females. Knodus turiuba appears to be a non-inseminating species based on histological examination of the gonads of one male (aquasperm present) and one female (no sperm found in the ovary). These specimens were sexually active when caught. We refer this species to Knodus based on the presence of scales on the caudal fin, but recognize that the systematics of Knodus and Bryconamericus are complex problems needing much clarification beyond the use of data taken from the usual morphometric, meristic, and gross anatomical features used by ichthyologists. See our discussion above regarding inseminating and non-inseminating species that we assigned to Knodus. See also Figs. 12 - 14 above for illustrations of the caudal-fin squamation of a species of Bryconamericus and one of a non-inseminating species of Knodus and one of a species found to be inseminating. Burns & Weitzman (2005: tabl. 1) also report and supply some data supporting insemination for Knodus sp., MZUSP 38004. Thus, considering the complications reported above in our discussions regarding the differences between and possible relationships of Bryconamericus and Knodus and the fact that some species of Knodus are inseminating, but others are, not including its type species, we prefer to currently recognize Knodus as distinct from Bryconamericus.
See Fig. 22 of the present publication for a photograph of Planaltina myersi, MNRJ 10634, a mature male, 32.5 and a female 36.8 mm SL,from Brazil: Distrito Federal, córrego Fumal, where crosses road between Brasília and Planaltina, near Planaltina, about 1520'S 4750'W, collected 11 Apr. 1982 by L.E. de Macedo Cordoso.
- Atkins, D. L. & W. L, Fink.1979. Morphology and histochemistry of the caudal gland of Corynopoma riisei Gill. Journal of Fish Biology, 14:465-469.
- Brown, G. E., J. C. Adrian, Jr. & M. L. Shih. 2001. Behavioral response of fathead minnows to hypoxanthine-3-N-oxide at varying concentrations. Journal of Fish Biology, 58:1465-1470.
- Burns J. R & S. H. Weitzman.1996. Novel gill-derived gland in the male swordtail characin, Corynopoma riisei (Teleostei: Characidae: Glandulocaudinae). Copeia, 1996: 627-633.
- Burns J. R & S. H. Weitzman. 2005. Insemination in ostariophysan fishes. Pp. 107-134. In: Grier, H. J. & M. C. Uribe (Eds.). Viviparous Fishes. New Life Publications, Homestead Florida.
- Burns, J. R., S. H. Weitzman, H. J. Greer, & N. A. Menezes. 1995. Internal fertilization, testis and sperm morphology in glandulocaudine fishes (Teleostei: Characidae: Glandulocaudinae). Journal of Morphology, 224: 224-145.
- Burns, J. R., S. H. Weitzman, K. R. Lange, & L. R. Malabarba. 1998. Sperm ultrastructure in characid fishes (Teleostei: Ostariophysi). Pp. 235-244. In: L. R. Malabarba, R. E. Reis, R. P. Vari, Z. M. Lucena & C. A. Lucena (Eds.). Phylogeny and Classification Neotropical Fishes. Porto Alegre, Edipucrs, 603 p.
- Burns, J. R., S. H. Weitzman & L. R. Malabarba. 1997. Insemination in eight species of cheirodontine fishes (Teleostei: Characidae: Cheirodontinae). Copeia, 1997: 4313-438.
- Burns, J. R., S. H. Weitzman, L. R. Malabarba, & A. Downing-Meisner. 2000. Sperm modifications in inseminating ostariophysan fishes, with new documentation of inseminating species. P. 255 in: Norberg, B., O. S. Kjesbu, G. L. Taranger, E. Andersson, & S. O. Stefansson (Eds.), Reproductive Physiology of Fish, Proceedings of the 6th International Symposium on the Reproductive Physiology of Fish, July 4-9, Institute of Marine Research and University of Bergen, Norway.
- Bushman, P. J., J. R. Burns & S. H. Weitzman. (2002). Gillderived glands in glandulocaudine fishes (Teleostei: Characidae: Glandulocaudinae). Journal of Morphology, 253: 187-185.
- Calcagnotto, D., S.A. Schaefer, & R. DeSalle. 2005. Relationships among characiforms fishes inferred from analysis of nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution. (Available online 5 Feb, 2005.)
- Castro, R. M. C., A. C. Ribeiro, R.C. Benine & A. L. A. Melo. 2003. Lophiobrycon weitzmani, a new genus and species of glandulocaudine fish (Characiformes: Characidae) from the rio Grande drainage, upper rio Paraná system, southeastern Brazil. Neotropical Ichthyology, 1(1): 11-19.
- Eigenmann, C. H. 1917. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43(1): 1-102.
- Eigenmann, C. H. 1918. The American Characidae, Memoirs of the Museum of Comparative Zoology, 43(2): 103-208.
- Eigenmann, C. H. & G.S. Myers.1929. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43(5): 429-515.
- Fink, W. L. & S. H. Weitzman. 1974. The so-called cheirodontin fishes of Central America with a description of two new species (Pisces: Characidae). Smithsonian Contributions to Zoology. 172: 1-46.
- Géry, J. 1965. A new genus from Brazil-Brittanichthys A new sexually-dimorphic characid genus with peculiar caudal ornament, from the Rio Negro, Brazil, with a discussion of certain cheirodontine genera and a description of two new species, B. axelrodi and B. myersi Tropical Fish Hobbyist, 13:13-23, 61-69.
- Géry, J. 1977. Characoids of the World. T.F. H. Publications, Neptune City, NJ, 672p.
- Grier, H. J. 1981. Cellular organization of the testis and spermatogenesis in fishes. American Zoologist, 21:345-357.
- Harold, A.S. & R.P. Vari. 1994. Systematics of the Trans-Andean species of Creagrutus (Ostariophysi: Characiformes: Characidae). Smithsonian Contributions to Zoology, 551:1-31.
- Hubert, N., C. Bonillo, & D. Paugy. 2005. Does elision account for molecular saturation: Case study based on mitochondrial ribosomal DNA among characiforms fishes (Teleostei: Ostariophysi). Molecular Phylogenetics and Evolution, 35(1): 300-308.
- Ito, S. & M. J. Karnovsky. 1968. Formaldehyde glutaraldeyhde fixatives containing trinitro compounds. Journal of Cell Biology, 36: 168.
- Lima, F. C. T, H. A. Britski & F.A. Machado. 2004. New Knodus (Ostariophysi: Characiformes: Characidae) from the upper Rio Paraguay Basin, Brazil. Copeia, 2004: 577-582.
- Lowe-McConnell, R. H. 1991. Natural history of fishes in Araguaia and Xingu Amazonian tributaries, Serra Roncador, Mato Grosso, Brazil. Ichthyolical Exploration of Freshwaters, 2(1): 63-82.
- Malabarba, L. R. 1998. Monophyly of the Cheirodontinae, characters and major clades (Ostariophysi: Characidae. Pp. 193-233. In: Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. Lucena, & C. A. Lucena (Eds.). Phylogeny and Classification Neotropical Fishes. Porto Alegre, Edipucrs, 603 p
- Malabarba, L. R. & S. H. Weitzman. 1999. A new genus and species of South American fish (Teleostei: Characidae: Cheirodontinae) with a derived caudal fin, including comments on inseminating cheirodontines. Proceedings of the Biological Society of Washington, vol. 112(2):411-432.
- Malabarba, L. R., & S. H. Weitzman. 2000. A new genus and species of inseminating fish (Teleostei: Cheirodontinae: Compsurini) from South America with uniquely derived caudal-fin dermal papillae. Proceedings of the Biological Society of Washington, 1139(1): 269-283.
- Malabarba, L. R. & R. P. Vari. 2000. Caiapobrycon tucurui, a new genus and species of characid from the rio Tocantins basin, Brazil. Ichthyolical Exploration of Freshwaters, 11(4):315-326.
- Malabarba, L. R., & S. H. Weitzman. 2003. Description of a new genus with six new species from Southern Brazil, Uruguay and Argentina, with a discussion of a putative characid clade (Teleostei: (Teleostei: Characiformes: Characidae). Comunicações do Museu de Ciências e Tecnologia, PUCRS, Série Zoologia, 16(1): 67-151.
- Malabarba, L. R., S. H. Weitzman, & T. Litz. 2004.Hyphessobrycon melanopleurus uruguayensis Messner, 1962, an available name and senior synonym of Cyanocharax macropinna Malabarba & Weitzman, 2003 (Ostariophysi: Characidae). Neotropical Ichthyology. 2(2): 99-102.
- Menezes, N. A. & S. H. Weitzman. 1990. Two new species of Mimagoniates (Teleostei: Characidae: Glandulocaudinae), their phylogeny and biogeography and a key to the glandulocaudin fishes of Brazil and Paraguay. Proceedings of the Biological Society of Washington, 103(2): 380-426.
- Menezes, N. A., S. H. Weitzman, & J. R. Burns. (2003). A systematic review of Planaltina (Teleostei: Characiformes: Characidae: Glandulocaudinae: Diapomini) with a description of two new species fromthe upper rio Paraná, Brazil. Proceedings of the Biological Society of Washington, 116(3):557-600.
- Mittal, A. K. & J. S. Datta Munshi. 1970. On the origin and cytomorphosis of 'club cells' in the skin of Rita rita (Ham.) (Bagridae, Pisces). Zeitschrift für Mikroscopie-Anatomie Forschung. 82:229-235.
- Pecio, A. & J. Rafiñski. 1994. Structure of the testes, spermatozoa and spermatozeugmata of Mimagoniates barberi Regan , 1907 (Teleostei: Characidae), an internally fertilizing oviparous fish. Acta Zoologica, Stockholm, 75: 179-185.
- Pecio, A., J. R. Burns and S. H. Weitzman. 2005. Sperm and spermatozeugma ultrastructure in the inseminating species Tyttocharax cochui , T. tambopatensis and Scopaeocharax rhinodus (Pisces: Teleostei: Characidae: Glandulocaudinae: Xenurobryconini). Journal of Morphology, 263:216-226.
- Pezzi de Silva, J. F. 2004. Two new species of Bryconamericus Eigenmann (Characiformes: Characidae) from southern Brazil. Neotropical Ichthyology, 2(2):55-60.
- Pfeiffer, W. 1967. Schreckreaktion und Schreckstoffzellen bei Ostariophysi und Gonorhynchiformes. Zeitschrift für vergleichende Physiolgie, 56:380-396.
- Pfeiffer, W. 1977. The distribution of fright reaction and alarm substance cells in fishes. Copeia 1977: 653-665.
- Pfeiffer, W., G. Riegelbauer, G. Meier & B. Scheibler. 1985. Effect of hypoxanthane-3(N)-oxide and hypoxanthine-1(N)-oxide on central nervous excitation of the black tetra Gymnocorymbus ternetzi (Characidae, Ostariophysi, Pisces) indicated by dorsal light response. Journal of Chemical Ecology. 11(4):507-523.
- Quintero-Hunter, I., Grier, H., & Muscato, M. 1991. Enhancement of histological detail using metinal yellow as counterstain in periodic acid Schiff's hematoxylin staining of glycol methcrylate tissue sections. Biotechnology and Histochemistry. 66:169-172.
- Reis, R. E., S. O. Kullander, & C. J. Ferraris (Eds.). 2003. Checklist of the Freshwater Fishes of South and Central America. Porto Alegre, Edipucrus, 729p.
- Román-Valencia. C. 2000. Tres nuevas especies de Bryconamericus (Ostariophysi: Characidae) de Colombia y diagnóstico del genero. Revista de Biologia Tropical, 482/3): 449-464.
- Schreibman M. P. 1964. Studies on the pituitary gland of Xipho-phorus maculatus (the platyfish). Zoologica 49:217-243.
- Schultz, L. P. 1944. The fishes of the family Characinidae from Venezuela, with descriptions of seventeen new forms. Proceedings of the United States national Museum, 95: 235-367.
- Steindachner, F. 1880. Zur Fisch-Fauna des Cauca und der Flüsse bei Guayquil. Denkschriften der Akademie der Wissenschaften, Wein, 42: 55-104, plates 1-9.
- Steindachner, F. 1915. Beiträge zur Kenntniss der Flussfische Südamerikas. V. Denkschriften der Akademie der Wissenschaften, Wein, 93: 15-106, plates 1-13.
- Swofford, D. 1993. PAUP: phylogenetic analysis using parsimony Version 3.1.1. Illinois Natural History Survey, Champaign.
- Vari, R .P. & A. Harold. 2001. Phylogenetic study of the Neotropical fish genera Creagrutus Günther and Piabina Reinhardt (Teleostei: Ostariophysi: Characiformes), with a Revision of the cis-Andean species. Smithsonian Contributions to Zoology, 613: i-v, 1-239.
- Vari, R. P. & J. Géry. 1980. Cheirodon ortegai, a new markedly sexually dimorphic Cheirodontinae (Pisces: Characoidea) from the Río Ucayali of Peru. Proceedings of the Biological Society of Washington., 93(1): 75-82.
- Vari, R. P. & H. Ortega. 2000. Attonitus a new genus of sexually dimorphic characiforms (Ostariophysi: Characidae) from western Amazonia; a phylogenetic definition and description of three new species. Ichthyological Exploration of Freshwaters, 11(2):113-140.
- Vari, R. P. & D. J. Siebert. 1990. A new, unusually sexually dimorphic species of Bryconamericus (Pisces: Ostariophysi: Characidae) from the Peruvian Amazon. Proceedings of the Biological Society of Washington, 103(3): 516524.
- Vari, R. P. A. S. Harold, C. A. Laso & A. Machado-Allison. 1993. Creagrutus lepidus, a new species from the Río Aroa system, Yaracuy State, Venezuela (Teleostei: Characiformes: Characidae). Ichthyological Exploration of Freshwaters, 4(4): 351-355.
- Vari, R. P., A. S. Harold, & D. C. Taphorn. 1994. Creagrutus melasma, a new species of characid fish (Teleostei: Characiformes) from upland streams of northern Venezuela. Proceedings of the Biological Society of Washington, 107(1): 90-96.
- Weitzman, S. H. 1977. A new species of characoid fish, Hyphes-sobrycon diancistrus, from the Río Vichada, Orinoco River drainage, Colombia, South America (Teleostei: Characidae). Proceedings of the Biological Society of Washington, 90(20): 348357.
- Weitzman, S. H. 2003. Subfamily Glandulocaudinae (characins, tetras). Pp. 222 230. In: Reis, R. E., S. O. Kullander, & C. J. Ferraris, Jr. Check List of the Freshwater Fishes of South and Central America. Porto Alegre, Edipucrs, 729 p.
- Weitzman, S. H. & S. V. Fink. 1985. Xenurobryconin phylogeny and putative pheromone pumps in glandulocaudine fishes (Teleostei: Characidae). Smithsonian Contributions to Zoology, 421: i-iii +1-121.
- Weitzman, S.H., N.A. Menezes, & M.J. Weitzman. 1988. Phylogenetic biogeography of the Glandulocaudini (Teleostei: Characiformes, Characidae) with comments on the distributions of other freshwater fishes in eastern and southeastern Brazil. pp. 379-427.In: Proceedings of a Workshop on Neotropical Distribution Patterns Held 12-16 January 1987. Academia Brasileira de Ciências, Rio de Janeiro.
- Weitzman, S. H., S. V. Fink, A. Machado-Allison & R. Royero L. 1994. A new genus and species of Glandulocaudinae (Teleostei: Characidae) from Southern Venezuela. Ichthyological Exploration of Freshwaters, 5(1): 45-64.
- Weitzman, S. H. & L. R. Malabarba. 1999. Systematics of Spintherobolus (Teleostei: Characidae: Cheirodontinae) from eastern Brazil. Ichthyological Exploration of Fresh-waters,10(1): 1-43.
- Weitzman, S. H. & N. A. Menezes. 1998. Relationships of the tribes and genera of the Glandulocaudinae (Ostariophysi: Characiformes: Characidae) with a description of a new genus, Chrysobrycon). Pp. 171-192. In: L. R. Malabarba, R. E. Reis, R. P. Vari, Z. M. Lucena & C. A. Lucena (eds.), Phylogeny and Classification Neotropical Fishes, Porto Alegre, Edipucrs, 603p.
- Weitzman, S. H. & H. Ortega. 1995. A new species of Tyttocharax (Teleostei: Characidae: Glandulocaudinae: Xenurobryconini) from the Río Madre de Dios basin of Peru. Ichthyological Exploration of Freshwaters, 6(2): 129-148.
- Weitzman, S. H. & L. Palmer. 1997. A new species of Hyphessobrycon (Teleostei: Characidae) from the Neblina region of Venezuela and Brazil, with comments on the putative 'rosy tetra clade. Ichthyological .
- Werner, U. 1992. Fishfangabenteuer Südamerika - Reisen in Sachen. Aquaristik. Landbuch Verlag, Hannover, p. 232.
- Zanata, A. M. & A. Akama. 2004. Myxiops aphos, new characid genus and species (Characiformes: Characidae) from the rio Lençóis, Bahia, Brazil. Neotropical Ichthyology, 2(2): 45-54.
Appendix 1
Appendix II
Publication Dates
-
Publication in this collection
12 Dec 2007 -
Date of issue
Sept 2005
History
-
Received
May 2005 -
Accepted
Sept 2005