Abstracts
Cyphocharax gilbert infested by Riggia paranensis shows parasitic castration. The prevalence of parasitism in C. gilbert varied among different environments, being higher in the middle rio Itabapoana. Fish were collected monthly using two cast nets (thrown 30 times during the day) and gillnets kept in the river during 12 hour, from sunset to sunrise, between September 1997 and August 2000. Infestation pattern was investigated on 1358 specimens. Most of them were infested (57.9%), with one or two parasites; the majority (62.9%) was collected during the rainy season (spring-summer). The parasite did not show preference for sex or size of hosts. A total of 91.5% of the 511 examined parasites had a body size that represented 10.1% to 20% of host standard length. The reproductive condition of 311 specimens of R. paranensis was analyzed checking the presence of oocytes in the ovarian and eggs or embryos in the marsupium. Nearly 73% of them were at reproductive phase, and had a body size that represented 5.1% to 20% of host standard length. The size of the immature parasites varied from 0.1% to 5% of the host size. The results suggest that R. paranensis may adopt a fast growth rate strategy and increase the investment in reproduction when they occupy most of the host's pericardial space.
Body size; Prevalence of parasitism; Parasitic castration; Reproduction
Cyphocharax gilbert infestado por Riggia paranensis apresenta castração parasitária. A prevalência do parasito varia entre diferentes ambientes, sendo maior no trecho médio do rio Itabapoana. Os peixes foram coletados mensalmente neste rio usando duas tarrafas (lançadas por 30 vezes durante o dia) e redes de espera por 12 horas, do crepúsculo ao amanhecer, entre setembro de 1997 e agosto de 2000. O padrão de infestação foi investigado em 1358 peixes. Peixes infestados apresentaram um ou dois parasitos, representando 57,9% da população e a maioria deles (62,9%) foi coletada no período de maior precipitação (primavera-verão). O parasito não apresentou preferência por sexo ou tamanho do hospedeiro. No total, 91,5% de 511 parasitos examinados apresentaram um tamanho corpóreo que representou de 10,1% a 20% do comprimento padrão do hospedeiro. A condição reprodutiva em 311 espécimes R. paranensis foi analisada através da presença de oócitos no ovário e ovos ou embriões no marsúpio, sendo que cerca de 73% estavam em fase de reprodução, apresentando um tamanho corporal que variou entre 5,1% a 20% do comprimento padrão do hospedeiro. O tamanho dos parasitos imaturos variou de 0,1% a 5% do tamanho do hospedeiro. Os resultados sugerem que R. paranensis pode adotar uma estratégia de rápido crescimento e deve aumentar o investimento em reprodução quando ocupa grande parte do espaço disponível na cavidade pericardial do hospedeiro.
Infestation pattern and parasitic castration of the crustacean Riggia paranensis (Crustacea: Cymothoidea) on the fresh water fish Cyphocharax gilbert (Teleostei: Curimatidae)
Juliana de Souza AzevedoI; Leonardo Gomes da SilvaI; Carlos Roberto Silveira Fontenelle BizerriII; Marilvia Alencar Dansa-PetretskiI; Neuza Rejane Wille LimaIII
IUniversidade Estadual do Norte Fluminense, CBB, 28.015-620 Campos dos Goytacazes, RJ, Brazil.
IINP Consultoria Ambiental, Rua Carolina Alves, 55, 24.322-310 Niterói, RJ, Brazil.
IIIUniversidade Federal Fluminense, Instituto de Biologia, CP. 100.436, 24.001-970 Niterói, RJ, Brazil. e-mail: rejanewille@uol.com.br
ABSTRACT
Cyphocharax gilbert infested by Riggia paranensis shows parasitic castration. The prevalence of parasitism in C. gilbert varied among different environments, being higher in the middle rio Itabapoana. Fish were collected monthly using two cast nets (thrown 30 times during the day) and gillnets kept in the river during 12 hour, from sunset to sunrise, between September 1997 and August 2000. Infestation pattern was investigated on 1358 specimens. Most of them were infested (57.9%), with one or two parasites; the majority (62.9%) was collected during the rainy season (spring-summer). The parasite did not show preference for sex or size of hosts. A total of 91.5% of the 511 examined parasites had a body size that represented 10.1% to 20% of host standard length. The reproductive condition of 311 specimens of R. paranensis was analyzed checking the presence of oocytes in the ovarian and eggs or embryos in the marsupium. Nearly 73% of them were at reproductive phase, and had a body size that represented 5.1% to 20% of host standard length. The size of the immature parasites varied from 0.1% to 5% of the host size. The results suggest that R. paranensis may adopt a fast growth rate strategy and increase the investment in reproduction when they occupy most of the host's pericardial space.
Key words: Body size, Prevalence of parasitism, Parasitic castration, Reproduction.
RESUMO
Cyphocharax gilbert infestado por Riggia paranensis apresenta castração parasitária. A prevalência do parasito varia entre diferentes ambientes, sendo maior no trecho médio do rio Itabapoana. Os peixes foram coletados mensalmente neste rio usando duas tarrafas (lançadas por 30 vezes durante o dia) e redes de espera por 12 horas, do crepúsculo ao amanhecer, entre setembro de 1997 e agosto de 2000. O padrão de infestação foi investigado em 1358 peixes. Peixes infestados apresentaram um ou dois parasitos, representando 57,9% da população e a maioria deles (62,9%) foi coletada no período de maior precipitação (primavera-verão). O parasito não apresentou preferência por sexo ou tamanho do hospedeiro. No total, 91,5% de 511 parasitos examinados apresentaram um tamanho corpóreo que representou de 10,1% a 20% do comprimento padrão do hospedeiro. A condição reprodutiva em 311 espécimes R. paranensis foi analisada através da presença de oócitos no ovário e ovos ou embriões no marsúpio, sendo que cerca de 73% estavam em fase de reprodução, apresentando um tamanho corporal que variou entre 5,1% a 20% do comprimento padrão do hospedeiro. O tamanho dos parasitos imaturos variou de 0,1% a 5% do tamanho do hospedeiro. Os resultados sugerem que R. paranensis pode adotar uma estratégia de rápido crescimento e deve aumentar o investimento em reprodução quando ocupa grande parte do espaço disponível na cavidade pericardial do hospedeiro.
Introduction
The parasites might be selected to decrease or inhibit reproductive effort of host if they increase host survival and size, and thereby their own reproductive output (Keymer & Read, 1991; Hurd, 2001; Loot et al., 2002). Several species of the Cymothoidea are parasites and infest a variety of marine and fresh-water fish affecting the host physiology in different ways. For example, infested fish may have significantly lower erythrocyte counts, haematocrit and hemoglobin values, and significantly increased leukocyte counts, loss of weight and reduction of fecundity, increase in juveniles mortality, presence of serious lesions inside buccal cavity or tumor in the operculum, metaplasia on the area penetrated by the parasites, and exhibit parasitic castration (Huizinga, 1972; Romestand & Trilles, 1977; Thatcher, 1993; Adlard & Lester, 1994; Adlard & Lester, 1995; Marks et al., 1996; Thatcher, 2000; Azevedo et al., 2002; Horton & Okamura, 2003; Carvalho et al., 2004; Gomes da Silva et al., 2005; Rajkumar et al., 2005).
The specimens of Cyphocharax gilbert (Quoy & Gaimard, 1824) infested by the Cymothoidea Riggia paranensis Szidat, 1948 show parasitic castration, lacking gonadal development and two sex specific plasma proteins (Azevedo et al., 2002; Gomes da Silva et al., 2005). This parasite affected the body size of C. gilbert, enhanced host growth, mainly among the female hosts (Azevedo et al., 2002). However infested fish were similar to the no infested ones at the end of reproductive cycle in terms of size and number of melanomacrophages centers of spleen, an indicator of fish health (Meirelles, et al. 2003). Other similarity between infested females and post-spawning or initial gonadal development females was also observed for the plasmatic levels of the sexual hormone beta-estradiol and testosterone. These results all together suggest that the parasite seems to inhibit the initiation of host's reproduction (Meirelles et al., 2003).
Riggia paranensis is a hermaphrodite protandric species that is born as male and become female in the parasitic phase. Females obtain blood from the gills; males are smaller than females and live associated to them but are not blood feeders (Raibaut & Trilles, 1993; Bastos & Thatcher, 1997). Theykeep an opening in the host body to obtain oxygen and to deliver their larvae to the environment, being characterized as mesoparasites (Bergey et al., 2002). Larvae make one perforation close to the pectoral fin, become associated to the gills as females and grow inside a host-synthesized capsule at the pericardial area (Huizinga, 1972; Thatcher, 1991; Raibaut & Trilles, 1993).
The host, C. gilbert, inhabits the coastal drainages of eastern Brazil (Vari, 1992) and presents the highest fecundity among the members of the family Curimatidae, presenting multiple spawning and two reproductive periods that vary among ecosystems, but spring and summer seems to be the major period of reproduction (Azevedo et al., 1938; Menezes, 1994; Azevedo et al., 2002; Gomes da Silva et al., 2005). The prevalence of parasitism in C. gilbert varies among lagoons and rivers in the North Fluminense region, being higher in the middle rio Itabapoana (Azevedo et al., 2002).
This study describes the pattern of infestation in populations that inhabit the middle rio Itabapoana, in order to test if the parasite has any prevalence for sex or size of host, to verify if there is a relationship between host and parasites size, and to analyse parasite reproductive condition through the presence of oocytes in the ovary and eggs or embryos in the marsupium.
Material and Methods
Fish and parasites were collected monthly from September 1997 to August 2000 in the middle rio Itabapoana basin, Rio de Janeiro and Espírito Santo States, Brazil (21°15'S and 42°30'W). Six gillnets (three with 20 mm mesh, 25 m long and 1.5 m depth and three with 25 mm mesh, 25 m long and 1.5 m depth) were placed at river margins during 12 hours from crepuscule to the sunrise. Two cast nets (20 mm mesh and 2.5 m of diameter and 30 mm mesh and 4.0 m of diameter) were thrown 30 times during the day.
The standard body size of fish was measured as the length from the tip of snout to the fork of the caudal fin. The sex of fish was identified by ovaries and testis morphological characteristics (position, color and size of gonads) (Menezes, 1994; Azevedo et al., 2002; Gomes da Silva et al., 2005). The fish were analyzed registering the presence of parasites, number of parasites, and post-infested sign (cicatrization marks). Theywere classified into six categories, arranged in four groups: (1) without any parasitism signs (category a); (2) infested fish with one or two parasites (categories b or c); (3) fish with one cicatrization mark at one side of the body and with one parasite at the other side (category e), and (4) post-infested fish with one or two cicatrization marks (categories d or f) (Azevedo, 2002). The prevalence was calculated as the number of infested fish divided by the number of examined fish x 100%, following Bush et al. (1997).
The parasites were removed from their hosts to measure their sizes (length from the tip of head to the end of telson). They were preserved in ethanol solution (70%) for seven days and their oostegits were removed to observe the presence of oocytes inside the ovary and eggs or embryos inside the marsupium. Parasites without oocytes, eggs or embryos were classified as sexually immature (Azevedo, 2002).
The data were analyzed considering all the studied period as well as separately in two distinct periods of the studied years, the autumn and winter (from March to August) and the spring and summer (from September to February). Non-parametric analysis of variance (Kruskall-Wallis test) were performed to test preference of parasites (%) for host standard body lengths (Snedecor & Cochran, 1971). The sexual proportion between males and females (M:F) was calculated for both fish without parasites and infested or post-infested to verify if there was any prevalence of parasites (%) for sex of host. Spearman correlation test (rs) and the regression analysis (r2) were applied to verify if sizes of hosts and theirs parasites were related (Snedecor & Cochran, 1971).
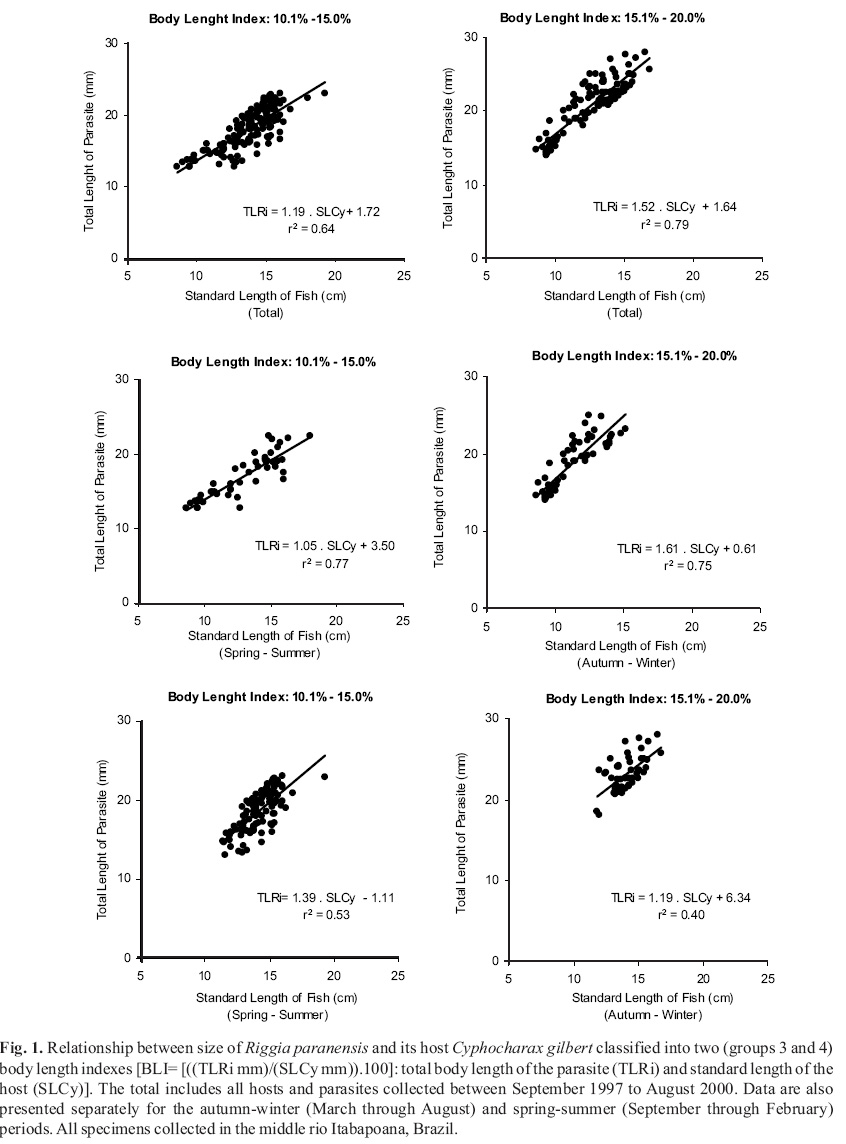
Azevedo (2002) verified that the standard body length of the hosts was only slightly related to the body length of R. paranensis due to the large variation of the body size of the parasites. Therefore the body length index was developed to circumvent this problem. The body length index (BLI) related the total length of R. paranensis (TLRi) and the standard length of C. gilbert (SLCy): BLI = [((TLRi mm)/(SLCy mm)). 100]. This index allowed the separation of the group of large hosts infested by small parasites from the group of small hosts invested by large parasites, allowing the application of correlation tests to verify the relationship between the sizes of host and parasite.
Four specimens (two males and two females) of C. gilbert infested with R. paranensis were deposited at Coleção Zoológica Didática da Universidade do Norte Fluminense (UENF/CY 1998/01-04). Additional vouchers were sent to Museu Nacional to Rio de Janeiro (MNRJ). Carcasses of the other specimens were frozen and have been used to measure heavy metals levels in the parasites and in the edible part and liver of fish.
Results
A total of 1358 fish were collected and 511 parasites were removed from hosts. Infested fish (n= 786) represented 57.9% of the total, and all of them did not present gonadal development. Most of the infested fish (63%) were collected during the spring and summer (Table 1). The frequencies of specimens with one parasite were very similar between the spring-summer and autumn-winter periods of the studied years. Fish with two parasites were relatively more frequent during the spring-summer period. Few fish were found with cicatrization only (3.1%). Small parasites were found growing inside a large host-synthesized capsule.
It was not observed a preference of parasites for host size (Table 1). The overall analysis of body length of infested fish was not significantly different from fish without parasites (H= 0.245; d.f.= 3; n= 1311; P>0.05). Similar results were observed when we compared separately the body length of fish collected during autumn and winter (H= 0.155; d.f.= 3; n= 483; P>0.05) or during spring-summer period (H= 0.203; d.f.= 3; n= 835; P>0.05). The body size of post-infested fish (with one or two cicatrization marks) did not vary significantly from fish without parasites signs (H= 0.103; d.f. = 2; n= 580; P>0.05) or from infested fish (with one or two parasites; H= 0.158; d.f. = 2; n= 784; P>0.05).
Non-preference of parasites for sex of host was also observed. The proportion between male and female (M:F) among fish without parasites (category a) was 49M:51F (n= 449). Among infested fish with only one parasite (category b) the sexual proportion was 48M: 52F (n= 132). Similar sexual proportions were observed for fish infested by two parasites (category c), fish with cicatrization (category d and f), and fish with one parasite and one cicatrization (category e).
Body length of fish and parasites varied from 80 mm to 220 mm and from 5 mm to 30 mm, respectively. The relationship between body sizes of the hosts and the parasites were analyzed for the total period of study (n= 511), for autumn and winter period (n= 120) or for the spring and summer period (n= 391).
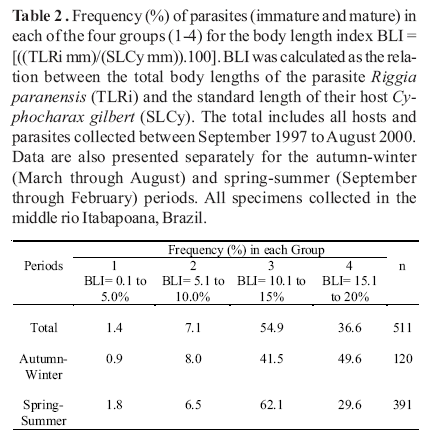
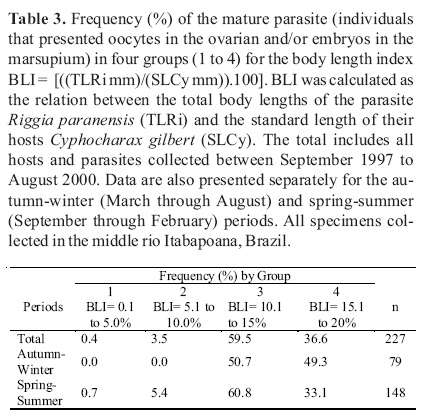
The body length index (BLI) applied revealed four natural groups of parasites. The first group involved parasites with body lengths ranging from 0.1% to 5.0% of its host body sizes. Parasites with body lengths ranging from 5.1% to 10.0% or from 10.1% to 15% or from 15.1% to 20% of the host body lengths represented the second, the third and the fourth groups, respectively. The results showed in Tables 2 and 3 and Fig. 1 followed these classifications.
The overall analysis revealed that most parasites (54.9%) belonged to the group 3 (Table 2). The partial analyses revealed that 62.1% of the parasites collected during the spring-summer period belonged to the group 3. A different pattern was observed for the autumn-winter period. During this period of the year, 49.6% of the parasites belonged to the group 4 and 41.5% belonged to the group 3. Few parasites belonged to the group 1 or 2 of the BLI. Only 1.4% and 7.1% of parasites belonged, respectively, to the group 1 and 2 for the overall analysis. The partial analyses revealed that 0.9% and 8% of parasites belonged, respectively, to the group 1 and 2 during autumn-winter period. During the spring-summer period it was observed that 1.8% and 6.5% parasites belonged to the group 1 and 2, respectively.
The reproductive condition of 311 specimens of R. paranensis was analyzed. A total of 227 specimens (73%) presented oocytes in the ovary and embryos inside the marsupium. The parasites that presented oocytes and embryos were more frequent during the spring-summer period (78%) than during the autumn-winter period (22%). Parasites at reproductive phases were less frequent during the autumn-winter period (57.5%) than during the spring-summer period (77.5%) (Table 3). The relationship between the body sizes of R. paranensis (TLRi) and C. gilbert (SLCv) was analyzed for mature and immature parasites separately. Most of the mature parasites belonged to the group 3 and 4 showing body sizes from 10.1 to 20% of their hosts (Table 3). The immature parasites (n= 84) did not present oocytes in the ovary and embryos inside the marsupium and presented body sizes that represented from 0.1% to 5% of host standard length.
Regression analysis between body sizes of host and their parasites were applied to verify if the coefficients were significant. Due to the limitation on sample size on groups 1 and 2 for the BLI, only the specimens belonging to the group 3 (BLI: 10.1% to 15%) and group 4 (BLI: 15.1% to 20%) were analyzed (Fig. 1). In the general analysis, the relationship between body size of parasites and their hosts were significant (P £ 0.05)for the specimens belonging to groups 3 (r2 = 0.64, n= 280, P £ 0.05)and 4 (r2 = 0.79, n= 187, P £ 0.05). Significant coefficients of regression (P £ 0.05)were also observed for the relationship between body size of parasites and it hosts (BLI group 3 and BLI group 4) in specimens collected during the autumn-winter and spring-summer periods (Fig. 1).
Discussion
The present study showed that in the middle rio Itabapoana around 60% specimens of C. gilbert were infested by one or two specimens of R. paranensis. Only 3.1% of fish presented one or two cicatrization marks, revealing an infrequent post-infested condition in the studied area. Small parasites growing inside a large host-synthesized capsule were found suggesting that fish can be infested again before the end of cicatrization. The prevalence of the parasite R. paranensis in the C. gilbert populations was lower (around 3.5) in other fluvial systems and lagoons in the northern of the Rio de Janeiro state (Azevedo et al., 2002).
The high prevalence of parasitism in the studied area might be a secondary impact of the introduction of exotic fish species from the rio Paraná into the basin of the rio Itabapoana (Bizerril & Lima, 2001; Azevedo et al., 2002). However environmental condition and spatial structure of the host population may also affect the prevalence of parasites (Grosholz & Ruiz, 1995; Carvalho et al., 2003). For example, the high rate of infestation of branchiurans in piranhas (Serrasalminae) seems to be a direct consequence of the proximity between individuals and of seasonal changes in water level and temperature (Carvalho et al., 2003).
Variations on prevalence of parasitism were observed in different periods of the year, increasing during rainy season (spring-summer) and decreasing during dry season (autumn-winter). Thus, the effect of water levels fluctuation in parasite prevalence observed by Carvalho et al. (2003) in the Pantanal wetland, may be not directly related to the variations on prevalence found in the studied area. The variations on prevalence verified in the middle rio Itabapoana might directly reflect the life cycle pattern of R. paranensis. The spring-summer was the major reproductive period of this parasite (Azevedo, 2002) and the larval infestation might be more effective during this period.
Seasonal variations on prevalence of parasitism might involve a preference by the sex, size or age of host(Radujkovic, 1991; Bragoni et al., 1983). The preference of parasite for male host or for fish at intermediate body size was presented by some species of Cymothoidea (Marks et al., 1996; Bello et al., 1997). However, R. paranensis did not present any preference for sex or size of the host.Non-preference for the host's sex was also observed in the parasitic isopods of Amblygaster sirm (Veerappan & Ramanathan, 1997). The non-preference for the host's sex associated to the consequences of parasitic castration seems to be a parasite adaptation rather than host survivorship success (Keymer & Read, 1991).
Positive correlation between body size of parasites and their hosts was observed in the present study. The same result was observed for the parasite Cymothoa exigua and it host Lutjanus peru (Álvares & Flores, 1997). Simultaneous growth of host and parasite was verified for other species of Cymothoidea and fish (Adlard & Lester, 1995), and suggested that once parasite is established in the body of a host there are no substitutions (Álvarez & Flores, 1997). The synchrony of parasite and host growth seems be a natural strategy that increases parasites reproductive output. The female isopod can also enhance host growth (Azevedo et al., 2002), increasing its own body size and reproductive output. Therefore the growth effects of parasites on their host might be a manipulation of the first one and an adaptive response of the second (Loot et al., 2002). Thus, the nutrients released from host as a result of parasitic castration will mainly depended on the amount of energy the hosts invest on reproduction (Keymer & Read, 1991).
Cyphocharax gilbert presents the highest fecundity among the members of the Curimatidae (Azevedo et al., 1938; Menezes, 1994; Azevedo, 2002). The infested individuals maintain the parasites reproductive output and the non-infested ones (around 40% of population) should keep the species continuities, suggesting coexistence and parasite-host coevolution (Toft & Aeschlimann, 1991). The reproductive patterns presented by the Cymothoidae parasites are also marked by a large fecundity (Raibaut & Trilles, 1993; Pouling, 1995). Riggia paranensis can present developed embryos in the marsupium and new oocytes in the ovary at the same time, especially during the rainy season. The reproductive mode showed by R. paranensis suggested thatit is a very prolific species (Azevedo, 2002).
The majority of the parasites showed a body size that represented 10% to 20% of the host standard length. Most of the parasites are mature when their body size hit up to 5% of host size. Immature parasites presented body sizes that represented from 0.1% to 5% of host standard length. The results of the presented study suggested that parasite might adopt a fast growth rate and should invest in reproductive efforts when they occupy most of the host's pericardial space, independent of sex or body size of host.
Acknowledgments
We acknowledge Antonio Pessanha and Luis Nascimento for technical support, and CNPq, FAPERJ, FENORTE, and IFS for the financial support.
Literature Cited
Received May 2006
Accepted August 2006
- Adlard, R. D. & R. J. G. Lester. 1994. Dynamics of the interaction between the parasitic isopod Anilocra pomacentri and the coral fish Chomis nitida Parasitology, 109(Part 3):311-324.
- Adlard, R. D. & R. J. G. Lester. 1995. The life-cycle and biology of Anilocra pomacentri (Isopoda, Cymothoidae), and ectoparasitic isopod of the coral-reef fish, Chromis nitida (Perciformes, Pomacentridae). Australian Journal of Zoology, 43(3):271-281.
- Álvares, F. & M. Flores. 1997. Cymothoa exígua (Isopoda: Cymothoidea) as parasite of Lutjamus peru (Pisces: Lutjamidae) Manzanillo, Colima, México. Revista de Biologia Tropical, 45(1B):391-394.
- Azevedo, P. de, M. V. Dias & B. B. Vieira. 1938. Biologia do sagüiru. Memórias do Instituto Oswaldo Cruz, 33(4):481-559.
- Azevedo, J. S. 2002. Dinâmica da interação parasito-hospedeiro Riggia paranensis (Crustacea, Cymothoidea) e Cyphocharax gilbert (Teleostei, Curimatidae) no rio Itabapoana, RJ/ES, e considerações sobre a biologia reprodutiva do parasito. Unpublished M.Sc. Dissertation, Universidade Estadual do Norte Fluminense, Campos dos Goytacazes, Rio de Janeiro. 111p.
- Azevedo, J. S., M. P. M. Thomé, L. Gomes da Silva, R. Novelli, M. Dansa-Petretski & N. R. W. Lima. 2002. Parasitismo de Riggia paranensis (Crustacea, Cymothoidea) em populações de Cyphocharax gilbert (Teleostei, Curimatidae) do norte do Estado do Rio de Janeiro. Boletim do Instituto de Pesca, 28(1):61-69.
- Bastos, P. B. & V. E. Thatcher. 1997. A redescription of Riggia paranensis Szidat, 1948 (Isopoda, Cymothoidae) based on thirty-two specimens from Curimatid fish of Rio de Janeiro, Brazil, with an Emendation of the genus. Memórias do Instituto Oswaldo Cruz, 92(6):755-760.
- Bello, G., A. Vaglio & G. Piscitelli. 1997. The reproductive cycle of Mothocya epimerica (Isopoda: Cymothoidae) a parasite of sand smelt Atherina boyeri (Osteichthyes: Atherinidae), in the Lesina Laggon, Italy. Journal of Natural History, 31(3):1055-1066.
- Bergey, L., J. S. Weis & P. Weis. 2002. Mercury uptake by the estuarine species Palaemonetes pugio and Fundulus heteroclitus compared with their parasites, Probopyrus pandalicola and Eustrongylides sp. Marine Pollution Bulletin, 44: 1046-1050.
- Bizerril, C. R. S. F. & N. R. W. Lima. 2001. Espécies de peixes introduzidas nos ecossistemas continentais do estado do Rio de Janeiro. Comunicações do Museu de Ciências e Tecnologia, PUCRS,14(1):43-59.
- Bragoni G., B. Romestand & J. P. Trilles. 1983. Cymothoadian parasitosis of the sea-dace (Dicentrarchus labrax Linnaeus, 1758) during breeding. II. Parasitic ecophysiology in the Diana pod. Annales de Parasitologie Humaine et Comparee, 58(6):593-609.
- Bush, A. O., K. D. Lafferty, J. M. Lozt & A. W. Shostak. 1997. Parasitology meets ecology on terms: Margolis et al., Revisited. Journal of Parasitology, 83(4):575-583.
- Carvalho, L. N., K. Del-Claro & R. M. Takemoto. 2003. Host-parasite interaction between branchiurans (Crustacea: Argulidae) and piranhas (Osteichthyes: Serrasalminae) in Pantanal wetland of Brazil. Environmental Biology of Fishes, 67(3): 289-296.
- Carvalho, L. N., R. Arruda & K. Del-Claro. 2004. Host-Parasite interactions between the piranha Pygocentrus nattereri (Characiformes, Characidae) and isopods and branchiurans (Crustacea) in the rio Araguaia basin, Brazil. Neotropical Ichthyology, 2(2): 93-98.
- Gomes da Silva, L., J. S. Azevedo, M. C. S. Lima Neto, N. R. W. Lima & M. Dansa-Petrestski. 2005. Effect of the parasitismon sex-specific plasmatic proteins of Cyphocharax gilbert (Teleostei). Parasitology,130(6):653-659.
- Grosholz, E. D. & G. M. Ruiz. 1995. Does spatial heterogeneity and genetic variation in population of the xanthid crab Rhitropanopeus harrisii (Gold) influence the prevalence of an introduced parasitic castrator? Journal of Experimental Marine Biology and Ecology, 187(1995):129-145.
- Horton, T. & B. Okamura 2003. Post-haemorrhagic anaemia in sea bass, Dicentrarchus labrax (L.), caused by blood feeding of Ceratothoa oestroides (Isopoda: Cymothoidae). Journal of Fish Diseases, 26(7):401-406.
- Huizinga, H. W. 1972. Pathobiology of Artistone trysibia Schioedte (Isopoda: Cyrimatidae) an endoparasitic isopoda of South American fresh water fishes. Journal of Wildlife Diseases, 8(7):225-232.
- Hurd, H. 2001. Host fecundity reduction: a strategy for damage limitation? Trends in Parasitology, 17(8): 363-368.
- Keymer, A. E. & A. Read. 1991. Behavioural ecology: the impact of parasitism. Pp. 37-61. In: C. A. Toft & L. Bolis (Eds.). Parasite-Host Associations. Coexistence or conflit? Oxford Science Publications, Oxford, 384p.
- Loot, G., L. R. Pouling, S. Lek & J-F. Guégan. 2002. The differential effects of Ligula intestinalis (L.) plerocercoids on host growth in three natural populations of roach, Rutilis rutilus (L.). Ecology of Freshwater Fish, 11(3): 168-177.
- Marks, R. E., F. Juares, J. A. Hare & D. O. Conover. 1996. Occurrence and the effect of the parasitic isopod, Lironeca ovalis (Isopoda: Cymothoidae), on young-of-the year bluefish, Pomatomus saltatrix (Pisces: Pomacentridae). Canadian Journal of Fish Aquatic Science, 53(9):2052-2057.
- Meirelles, M. E., J. S. Azevedo, R. M. Salgado, I. I. C. Silva, M. Dansa-Petretski, T. J. Sirotheau-Corrêa & N. R. W. Lima. 2003. Analysis of the melanomacrophage centres in the spleen of the fish Cyphocharax gilbert (Curimatidae, Teleost) infested by the parasite Riggia paranensis (Cymothoidea, Crustacea). Acta Microscopica, 12:417418, Supl. B and C.
- Menezes, M. S. 1994. Estrutura populacional e reprodução de Cyphocharax gilbert (Quoy & Gaimard, 1824) (Osteichthyes, Curimatidae) no trecho inferior do rio Paraíba do Sul (RJ, MG) e principais afluentes. Unpublished M.Sc. Dissertation, Universidade Federal do Paraná, Curitiba, Paraná. 111p.
- Poulin, R. 1995. Evolutionary influences on body-size in free-living and parasitic isopods. Biology ofJournal Linne Society, 54(3):231-244.
- Radujkovic, B. M. 1991. Parasitic crustaceans of Adriatic fishes. Wiad Parazytology, 37(2):149-150.
- Raibaut, A. & J. P. Trilles, 1993. The sexuality of parasitic crustaceans. Advances in Parasitology, 32: 367-444.
- Rajkumar M., K. P. K. Vasagam, P. Perumal & J. P. Trilles 2005. First record of Cymothoa indica (Crustacea, Isopoda, Cymothoidae) infecting the cultured catfish Mystus gulio in India. Diseases of Aquatic Organisms, 65 (3):269-272.
- Romestand, B. & J. P. Trilles. 1977. Influence of Cymothoidae (Crustacea, Isopoda, Flabellifera) on some hematological constants of host-fishes. Zeitschrift fur Parasitenkunde-Parasitology Research, 52 (1): 91-95.
- Snedecor, G. W. & W. G. Cochran. 1971. Statistical Methods. The Iowa State University Press., Iowa. 593p.
- Thatcher, V. E. 1991. Amazon fish parasites. Amazoniana,11(1): 263-572.
- Thatcher, V. E. 1993. Anphira branchialis gen. et sp. nov. (Crustacea, Isopoda, Cymothoidae) a gill cavity parasite of piranhas (Serrasalmus spp.) in the Brazilian Amazon. Acta Amazonica, 23(2-3): 297-307.
- Thatcher, V. E. 2000. The isopod parasites of South American Fishes. Pp. 193-226. In: G. Salgado-Maldonado, A. N. García Aldrete & V. M. Vidal-Martínez (Eds.). Metazoan parasites in the neotropics: a systematic and ecological perspective. Instituto de Biologia UNAM, México. 310p.
- Vari, R. P. 1992. Systematica of the netropical Characiform genus Cyphocharax Fowler(Pisces: Ostariophysi). Smithsonian Contributions to Zoology, (529):1-137.
- Veerappan, N. & M. Ramanathan. 1997. Report on some ectoparasitic isopods from the marine fish, Amblygaster sirm. Journal of Environmental Biology, 18(2):351-356.
Publication Dates
-
Publication in this collection
06 Aug 2007 -
Date of issue
Sept 2006
History
-
Accepted
Aug 2006 -
Received
May 2006