Abstracts
Spermiogenesis and sperm ultrastructure were analyzed in two species of characids with different modes of fertilization: externally fertilizing Hemigrammus erythrozonus and inseminating Corynopoma riisei. Spermiogenesis in H. erythrozonus is characterized by lateral development of the flagellum, nuclear rotation, formation of a shallow nuclear fossa, condensation of the chromatin by elimination of the electron-lucent area from the peripheral region of the nucleus, and renewal of the nuclear membrane. Multilammelated membrane and multivesicular bodies were also observed during elimination of the excess cytoplasm. The spermatozoon exhibits characters typical of "aquasperm," i.e. a spherical head containing a spherical nucleus with highly condensed chromatin, several small mitochondria located at the base of the nucleus within a cytoplasmic collar that extends into a long cytoplasmic sleeve surrounding the anterior part of the single flagellum, which is contained within a cytoplasmic canal. The flagellum lacks fins. The proximal and distal centrioles are nearly parallel to one another, with the anterior tips of both located within shallow nuclear fossae. Spermiogenesis in C. riisei is characterized by nuclear elongation alongside the forming flagellum, formation of an elongate cytoplasmic canal, displacement and elongation of the mitochondria, and uniform condensation of chromatin throughout the nucleus through enlargement of the diameter of the chromatin granules. The spermatozoon has an elongate nucleus with two elongate mitochondria localized to one side. Mitochondria are also located posterior to the nucleus forming a mitochondrial region. The single flagellum, which lacks fins, is lateral to the nucleus and initially contained within the greatly elongate cytoplasmic canal before exiting the canal at its posterior terminus. The spermatozoon of C. riisei exhibits several characters typical of "introsperm," such as an elongate nucleus and midpiece (mitochondrial region). The nuclear chromatin in the spermatozoon remains "flocculent" and is never as condensed as that seen in many characid sperm. Differences in spermiogenesis between externally fertilizing and inseminating characids are discussed.
Nuclear rotation; Aquasperm; Introsperm; Characids
Foram analisadas a espermiogênese e ultraestrutura dos espermatozóides de dois caracídeos com modos de fertilização distintos: fertilização externa em Hemigrammus erythrozonus e inseminação em Corynopoma riisei. A espermiogênese em H. erythrozonus é caracterizada pelo desenvolvimento lateral do flagelo, rotação nuclear, formação de uma fossa nuclear rasa, condensação de cromatina por eliminação da área elétron-lúcida na região periférica do núcleo e renovação da membrana nuclear. Membrana multilamelada e corpos multivesiculares foram observados durante a eliminação do excesso de citoplasma. O espermatozóide exibe os caracteres típicos do "aquaespermatozóide," com uma cabeça esférica que contem um núcleo esférico com cromatina muito condensada, várias mitocôndrias pequenas localizadas na base do núcleo e dentro de um colar citoplasmático, extedendo-se em uma bainha citoplasmática longa que rodeia a parte anterior do único flagelo, que está contido dentro de um canal citoplasmático. O flagelo carece de aletas. Os centríolos proximais e distais são quase paralelos, com as partes anteriores dos dois localizadas dentro de fossas nucleares pouco profundas. A espermiogênese em C. riisei é caracterizada pelo alongamento nuclear ao longo do flagelo, a formação de um canal citoplasmático longo, deslocamento e alongamento das mitocôndrias e uma condensação uniforme da cromatina por todo o núcleo por meio do aumento do diâmetro dos grânulos de cromatina. O espermatozóide tem um núcleo alongado com duas mitocôndrias alongadas dispostas em um lado. Algumas mitocôndrias localizam-se posteriormente ao núcleo formando uma região mitocondrial. O único flagelo, que carece de aletas, é lateral ao núcleo, posicionado anteriormente dentro de um canal citoplasmático muito alongado. O espermatozóide de C. riisei exibe vários caracteres típicos de "introespermatozóides" tais como um núcleo alongado e parte média alongada (região mitocondrial). A cromatina nuclear no espermatozóide permenece floculenta e nunca está tão condensada como nos espermatozóides de muitos outros caracídeos. Se discutem as diferenças na espermiogênese entre caracídeos com fertilização externa e os inseminadores.
Comparison of spermiogenesis in the externally fertilizing Hemigrammus erythrozonus and the inseminating Corynopoma riisei (Teleostei: Characiformes: Characidae)
Anna PecioI; John R. BurnsII; Stanley H. WeitzmanIII
IDepartment of Comparative Anatomy, Institute of Zoology, Jagiellonian University, Kraków, Poland. anna.pecio@uj.edu.pl
IIDepartment of Biological Sciences, George Washington University, Washington, DC 20052, USA. jrburns@gwu.edu
IIIDepartment of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-0109, USA. weitzmans@si.edu
ABSTRACT
Spermiogenesis and sperm ultrastructure were analyzed in two species of characids with different modes of fertilization: externally fertilizing Hemigrammus erythrozonus and inseminating Corynopoma riisei. Spermiogenesis in H. erythrozonus is characterized by lateral development of the flagellum, nuclear rotation, formation of a shallow nuclear fossa, condensation of the chromatin by elimination of the electron-lucent area from the peripheral region of the nucleus, and renewal of the nuclear membrane. Multilammelated membrane and multivesicular bodies were also observed during elimination of the excess cytoplasm. The spermatozoon exhibits characters typical of "aquasperm," i.e. a spherical head containing a spherical nucleus with highly condensed chromatin, several small mitochondria located at the base of the nucleus within a cytoplasmic collar that extends into a long cytoplasmic sleeve surrounding the anterior part of the single flagellum, which is contained within a cytoplasmic canal. The flagellum lacks fins. The proximal and distal centrioles are nearly parallel to one another, with the anterior tips of both located within shallow nuclear fossae. Spermiogenesis in C. riisei is characterized by nuclear elongation alongside the forming flagellum, formation of an elongate cytoplasmic canal, displacement and elongation of the mitochondria, and uniform condensation of chromatin throughout the nucleus through enlargement of the diameter of the chromatin granules. The spermatozoon has an elongate nucleus with two elongate mitochondria localized to one side. Mitochondria are also located posterior to the nucleus forming a mitochondrial region. The single flagellum, which lacks fins, is lateral to the nucleus and initially contained within the greatly elongate cytoplasmic canal before exiting the canal at its posterior terminus. The spermatozoon of C. riisei exhibits several characters typical of "introsperm," such as an elongate nucleus and midpiece (mitochondrial region). The nuclear chromatin in the spermatozoon remains "flocculent" and is never as condensed as that seen in many characid sperm. Differences in spermiogenesis between externally fertilizing and inseminating characids are discussed.
Key words: Nuclear rotation, Aquasperm, Introsperm, Characids.
RESUMO
Foram analisadas a espermiogênese e ultraestrutura dos espermatozóides de dois caracídeos com modos de fertilização distintos: fertilização externa em Hemigrammus erythrozonus e inseminação em Corynopoma riisei. A espermiogênese em H. erythrozonus é caracterizada pelo desenvolvimento lateral do flagelo, rotação nuclear, formação de uma fossa nuclear rasa, condensação de cromatina por eliminação da área elétron-lúcida na região periférica do núcleo e renovação da membrana nuclear. Membrana multilamelada e corpos multivesiculares foram observados durante a eliminação do excesso de citoplasma. O espermatozóide exibe os caracteres típicos do "aquaespermatozóide," com uma cabeça esférica que contem um núcleo esférico com cromatina muito condensada, várias mitocôndrias pequenas localizadas na base do núcleo e dentro de um colar citoplasmático, extedendo-se em uma bainha citoplasmática longa que rodeia a parte anterior do único flagelo, que está contido dentro de um canal citoplasmático. O flagelo carece de aletas. Os centríolos proximais e distais são quase paralelos, com as partes anteriores dos dois localizadas dentro de fossas nucleares pouco profundas. A espermiogênese em C. riisei é caracterizada pelo alongamento nuclear ao longo do flagelo, a formação de um canal citoplasmático longo, deslocamento e alongamento das mitocôndrias e uma condensação uniforme da cromatina por todo o núcleo por meio do aumento do diâmetro dos grânulos de cromatina. O espermatozóide tem um núcleo alongado com duas mitocôndrias alongadas dispostas em um lado. Algumas mitocôndrias localizam-se posteriormente ao núcleo formando uma região mitocondrial. O único flagelo, que carece de aletas, é lateral ao núcleo, posicionado anteriormente dentro de um canal citoplasmático muito alongado. O espermatozóide de C. riisei exibe vários caracteres típicos de "introespermatozóides" tais como um núcleo alongado e parte média alongada (região mitocondrial). A cromatina nuclear no espermatozóide permenece floculenta e nunca está tão condensada como nos espermatozóides de muitos outros caracídeos. Se discutem as diferenças na espermiogênese entre caracídeos com fertilização externa e os inseminadores.
Introduction
In fishes, the initial stages of spermatogenesis, which include the mitotic and meiotic divisions, exhibit limited variability among species. This contrasts sharply with the later stage of spermiogenesis when spermatids differentiate into the spermatozoa that are adapted to the particular fertilization mode of a given species. The greatest diversity of spermatozoa is observed within the Teleostei, the largest vertebrate group comprised of nearly 27,000 extant species (Nelson, 2006). Teleost reproductive modes vary from oviparous to viviparous, and from external to internal fertilization (Breder & Rosen, 1966). External fertilization is the dominant method of fertilization in nearly 97% of teleost species. In externally fertilizing species, spermatozoa are released into seawater or freshwater, which may occasionally be highly acidic, or into viscous secretions (e.g. in Blennidae) (Lahnsteiner et al., 1990). Fusion of egg and sperm (fertilization) occurs soon after release from the gonads once spermatozoa enter the micropyles of the eggs. Spermatozoa of externally fertilizing species, referred to as "aquasperm," generally possess spherical to ovoid heads containing spherical to ovoid nuclei, several small mitochondria located immediately posterior to the nucleus and within a cytoplasmic collar, and one or two flagella whose anterior portions are contained within a cytoplasmic canal of variable length (Jamieson, 1991).
Insemination, where sperm are introduced into the ovary, or internal fertilization, where sperm and egg actually fuse within the ovary, have been documented in about 3% of teleost species (Breder & Rosen, 1966; Koya et al., 2002; Burns & Weitzman, 2005). In males, adaptations for sperm transfer to the female have involved transformation of fins or other body regions into intromittent organs (Meisner, 2005; Burns & Weitzman, 2006), as well as modifications of both testis and sperm morphology (Burns et al., 1995, 2000). Spermatozoa of inseminating species, referred to as "introsperm," often become relatively complex with elongate nuclei and midpiece regions (Jamieson, 1991). Modifications of aquasperm into complex introsperm appear to have evolved independently in at least 24 teleostean families and even within congeneric species (e.g. Poeciliidae) (Reznick et al., 2002; Burns & Weitzman, 2005). Another modification related to the habits of insemination and internal fertilization is the production of sperm packets, both unencapsulated, referred to as spermatozeugmata, and encapsulated, called spermatophores (Grier & Parenti, 1994; Pecio et al., 2005). The anatomy and physiology of female reproductive systems have also been modified to permit the survival of introduced sperm for variable time periods, thus delaying fertilization and allowing for sperm competition among different males (Birkhead & Moller, 1997). All of these factors have probably influenced to some degree the evolution of modified sperm that are differentiated during the process of spermiogenesis.
In the family Characidae, insemination appears to have evolved independently at least three times: in members of the tribe Compsurini of the subfamily Cheirodontinae (Burns et al., 1997); in the subfamily Glandulocaudinae and in the subfamily Stevardiinae (Nelson, 1964; Burns et al., 1995; Weitzman et al., 2005). The species of the latter two subfamilies had previously all been included in the Glandulocaudinae, but a recent re-analysis of several morphological characters led to their being separated into the Glandulocaudinae (comprised of the genera Glandulocauda, Mimagoniates, and Lophiobrycon) and the Stevardiinae, containing all of the other genera previously included within the Glandulocaudinae (Weitzman et al., 2005).
Data on spermiogenesis in externally fertilizing species of the Characidae are very limited, having been described only for Brycon cephalus (Romagosa et al., 1999), Bryconops affinis (Andrade et al., 2001) and species in the genera Salminus and Brycon (Veríssimo-Silveira et al., 2006). However, some information on spermiogenesis is available for several other families belonging to the order Characiformes, including Erythrynidae (Quagio-Grassiotto et al., 2001), Curimatidae (Quagio-Grassiotto et al., 2003), Anostomidae (Pecio, 2003) and Alestidae (Shahin, 2006).
For inseminating characids, data on spermiogenesis is available only for the glandulocaudine Mimagoniates barberi (Pecio & Rafi ski, 1999). Descriptions of the ultrastructure of sperm, however, are available for the following characids: subfamily Stevardiinae, Diapoma speculiferum, Diapoma sp., Corynopoma riisei, and Pseudocorynopoma doriae (Burns & Weitzman, 2005; Burns et al., 1998) and Scopaeocharax rhinodus, Tyttocharax cochui and T. tambopatensis (Pecio et al., 2005); subfamily Glandulocaudinae, Mimagoniates barberi and M. microlepis (Pecio & Rafi
ski, 1999). Descriptions of the ultrastructure of sperm, however, are available for the following characids: subfamily Stevardiinae, Diapoma speculiferum, Diapoma sp., Corynopoma riisei, and Pseudocorynopoma doriae (Burns & Weitzman, 2005; Burns et al., 1998) and Scopaeocharax rhinodus, Tyttocharax cochui and T. tambopatensis (Pecio et al., 2005); subfamily Glandulocaudinae, Mimagoniates barberi and M. microlepis (Pecio & Rafi ski, 1994, 1999; Burns et al., 1998); subfamily Cheirodontinae, Macropsobrycon uruguayanae (Burns et al., 1998), and two species currently incertae sedis, Bryconadenos tanaothoros (Weitzman et al., 2005) and Brittanichthys axelrodi (Javonillo et al., 2007). Sperm morphology in these inseminating species can show marked variations, for example, in the orientation of the centrioles, degree of nuclear elongation, presence of accessory microtubules, or reduction of the cytoplasmic collar (Burns et al., 1998; Pecio et al., 2005).
ski, 1994, 1999; Burns et al., 1998); subfamily Cheirodontinae, Macropsobrycon uruguayanae (Burns et al., 1998), and two species currently incertae sedis, Bryconadenos tanaothoros (Weitzman et al., 2005) and Brittanichthys axelrodi (Javonillo et al., 2007). Sperm morphology in these inseminating species can show marked variations, for example, in the orientation of the centrioles, degree of nuclear elongation, presence of accessory microtubules, or reduction of the cytoplasmic collar (Burns et al., 1998; Pecio et al., 2005).
The main purpose of the present study is to provide information on spermiogenesis in the externally fertilizing Hemigrammus erythrozonus (incertae sedis) and the inseminating Corynopoma riisei (subfamily Stevardiinae). This analysis will demonstrate the differences between the differentiation of an aquasperm and that of an introsperm in two species of Characidae. The data presented for C. riisei is the first study on spermiogenesis in the subfamily Stevardiinae. This information will contribute to the growing database of ultrastructural characters that are being used in hypothesizing phylogenetic relationships among stevardiine and other characiform species (Weitzman & Menezes, 1998).
Materials and Methods
Four males of Hemigrammus erythrozonus, from a population originally in Guyana, were obtained from a local aquarist shop and kept in an aquarium (80x45x40cm) for several months on a natural photoperiod prior to this study. Fish were fed daily ad libitum with small crustaceans and Tubifex worms. Four mature males of SL 21-27 mm were killed by immersion in a 1% solution of tricaine methasulphonate (MS 222). The gonads were removed, cut into small fragments, and fixed in 3% glutaraldehyde in phosphate buffer (pH 7.4) overnight. The material was postfixed in 1% osmium tetroxide in the same buffer, dehydrated in alcohol and embedded in Epon 812. Ultra-thin sections were stained with uranyl acetate and lead citrate and were later examined under a JEOL JEM-100Sx transmission electron microscope. After dehydration, one small testis sample was dried in LADD CPD, then fractured and sputter-coated with gold and viewed in a JEOL JSM scanning electron microscope.
The specimen of Corynopoma riisei used for SEM was from the collection of the National Museum of Natural History, Smithsonian Institution (Washington, DC, USA), USNM 219619, SL 43 mm. This specimen had initially been fixed in 10% formalin and later transferred to 70% ethanol. A small sample of this testis was rinsed in tap water and postfixed in 1% osmium tetroxide in phosphate buffer for 2 hrs. After dehydration the testis was processed in the same manner as the sample of H. erythrozonus.
The testes of C. riisei used for TEM were from two aquarium specimens derived from stock originally collected by SHW in 1977 from the río Manzanares, between Cumaná and Cumanacoa, Sucre, Venezuela, SL 34.3 and 32.8 mm. After killing the specimens by immersion in a lethal dose of MS 222, the testes were removed and small pieces fixed in modified Karnovsky's fixative (Ito & Karnovsky, 1968), then rinsed in phosphate buffer and post-fixed in 1% osmium tetroxide in phosphate buffer for 30 min. Tissues were then rinsed in phosphate buffer, dehydrated in an ethanol series, infiltrated and embedded in Araldite 502. Ultra-thin sections were stained with uranyl acetate and lead citrate and examined under a JEOL JEM-100Sx transmission electron microscope.
Results
Spermiogenesis in Hemigrammus erythrozonus. The early spermatids of H. erythrozonus are spherical and possess nuclei with heterogeneous chromatin surrounded by a wide zone of cytoplasm (Fig. 1A). The centrioles are arranged nearly perpendicular one to another and located lateral to the spherical nucleus. The distal centriole (= basal body) extends to the flagellar axoneme, whose anterior portion is located within a cytoplasmic canal. During flagellar extension the mitochondria are located at the base of nucleus and possess a dark matrix (Fig. 1B). At this time, the orientation of the centrioles changes to a nearly parallel arrangement, while small fossae appear in the nucleus to accommodate the tips of both centrioles (Fig.1C). Before nuclear rotation chromatin is slightly mottled (Fig.1B) or finely granular (Fig. 1C). During the next stage of cell differentiation the nucleus rotates over the centriolar complex. This process is relatively synchronous given that within the same cyst, spermatids both before and after rotation can be seen (Fig.1D). After nuclear rotation, the head, midpiece and flagellum are arranged in a linear fashion (Fig.1E). The slight asymmetry of some nuclei relative to the long axis of the proximal centriole may be due to incomplete rotation. Most mitochondria are located at the side of the nucleus which does not rotate, forming an asymmetric midpiece.
During all of the above stages it was possible to observe the elimination of multivesicular bodies and lamellated membrane from the cytoplasm surrounding the nucleus (Fig.1D) and multilamellated membrane from the area of the cytoplasmic collar. In addition, nuclear chromatin became progressively more condensed in the spermatids within the cysts (Fig.1F), and this even continued after release of the cells into the lumen of the sperm ducts.
The nuclei of spermatozoa within the lumen of the sperm ducts possess highly condensed chromatin along with a more electron-lucent area at the periphery of the nucleus, which appears to be subsequently eliminated by the renewal of the nuclear membrane on the border of highly condensed chromatin (Fig. 2A). A similar electron-lucent area is also observed within the midpiece of spermatozoa. The nucleus of the spermatozoon of H. erythrozonus is roughly spherical and approximately 2 µm in diameter (Figs. 2B-C ). The chromatin is highly condensed and two shallow fossae contain the tips of each centriole. In frontally sectioned spermatozoa (Figs. 2B; Fig. 3A) the distal centriole (= basal body) is oriented at an angle of 146 degrees relative to the proximal centriole. Posterior to the nucleus, several mitochondria and vesicles are present in the cytoplasmic collar. The spherical to ovoid mitochondria, which have an electron-dense matrix, are distributed in irregular arrays (Fig. 2C) with their numbers decreasing more posteriorly (Figs. 2D-F). Posterior to the mitochondrial region on one side and just below the nucleus on the other, a thin cytoplasmic sleeve surrounds the anterior part of the flagellum (Figs. 2G-H; Fig. 3B). The axoneme of the flagellum forms the typical 9 + 2 pattern of microtubules, with no intratubular differentiation (Fig. 2I). The single flagellum lacks fins.
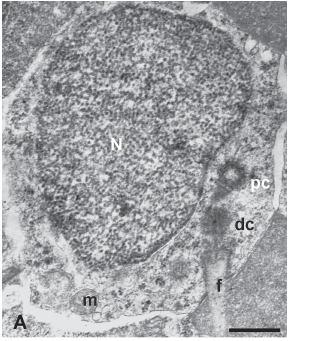
Spermiogenesis in Corynopoma riisei. As seen on a fractured surface with SEM, cysts containing spermatogonia, spermatocytes and spermatids at the beginning of spermiogenesis in C. riisei (Fig. 4A) are very similar to those observed in H. erythrozonus, with all cells completely filling the cysts and randomly distributed within them. The spermatids in more advanced stages of spermiogenesis, on the other hand, show significant elongation of their heads, which are oriented toward one pole of the cyst, with the flagella closely packed at the opposite pole (Fig. 4B). The arrangement of these cells resembles a "bouquet of flowers".
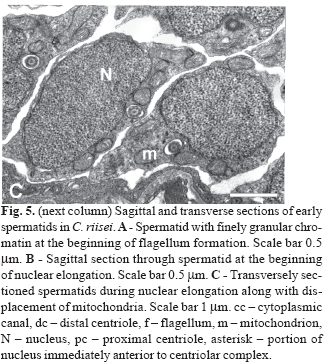
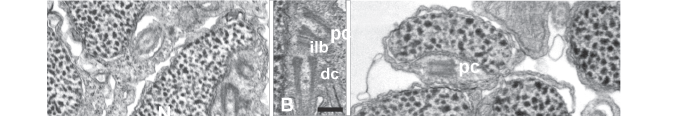
During the preliminary phase of flagellum formation, early spermatids are round with both centrioles in nearly perpendicular arrangement located tangential to the nucleus. The proximal centriole, part of which is located in a shallow fossa, has electron-dense material (intercentriolar lamellated body) on one side (Fig. 5A). At this point a slight degree of nuclear rotation may occur, as evidenced by the thin nuclear area directly anterior to the centriolar complex (Fig. 5B, asterisk). However, no further rotation is evident. Instead, the nucleus elongates lateral and posterior to the centriolar complex. During spermatid elongation the part of the nucleus located above the proximal centriole forms the anterior tip of the spermatid. The chromatin at this stage is finely granular (Fig. 5B). The spherical to ovoid mitochondria are located in the cytoplasm mainly on the flagellar side of the cell and below the nucleus (Fig. 5C).
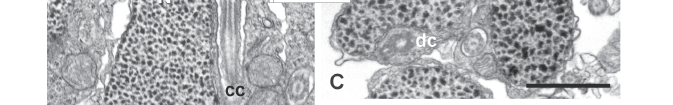
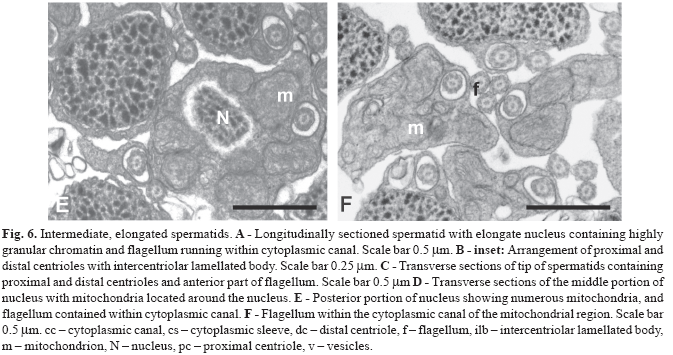
During the next stage the nucleus changes shape, becoming thinner and slightly flattened at the centriolar pole and thicker and more ovoid (in transverse section) posterior to the centrioles (Fig. 6A-B ). The number of mitochondria located alongside the anterior part of the nucleus is reduced to two, whereas in the posterior part of the nucleus multiple mitochondria are distributed around the nucleus (Fig. 6C-D). At this time the single flagellum also elongates. The anterior portion of the flagellum in located within an elongate cytoplasmic canal which runs the entire length of the spermatid (Fig. 6A, C-F). At later stages the nucleus of the spermatid elongates further with the chromatin condensing into larger "granules." The anterior part of the nucleus is flattened in the plane of the proximal centriole with a depression containing the distal centriole and proximal part of the flagellum (Fig. 7A). Mitochondria are located on the concave side of the nucleus and on either side of the flagellum (Fig. 7B). Posterior to the nucleus 4 -7 mitochondria are found, thus forming a mitochondrial region (Fig. 7C).
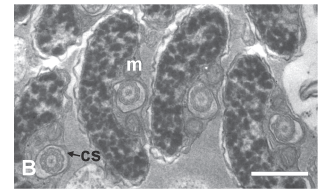
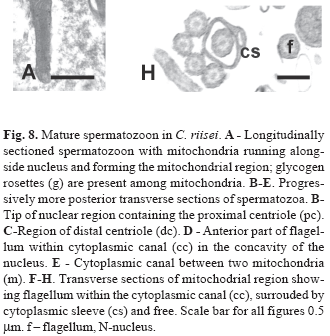
The spermatozoon of C. riisei is approximately 8.4 µm long and 1.5 µm wide (Fig. 8). All structures seen in the final stages of spermiogenesis are also present in the spermatozoon, where the incompletely condensed chromatin remains "flocculent". Along the nucleus elongate mitochondria are localized to one side, whereas posterior to the nucleus mitochondria are slightly larger and wider (Fig. 8A, E-F). All mitochondria possess multiple lamellar cristae, and glycogen rosettes can be seen among some of them (Fig. 8A). The shape of the nucleus changes from very flat at the anterior part to wider and more rounded toward the posterior part (Figs. 8B-E). The single flagellum is contained within the cytoplasmic canal along the entire nuclear and mitochondrial regions of the cell, extending beyond the mitochondrial region before exiting the end of the collar (Figs. 8D-H; Fig. 9). The flagellar axoneme consists of the typical 9 + 2 microtubule doublets, with no intratubular differentiation. The flagellum lacks fins.
Discussion
The introsperm seen in C. riisei probably evolved from an aquasperm similar to that seen in H. erythrozonus as an adaptation to the reproductive habit of insemination. The modifications to an aquasperm may have involved an inhibition of most of the process of nuclear rotation common to many aquasperm. Thus, instead of a symmetrical aquasperm where the flagellum is perpendicular to the nucleus as in H. erythrozonus, the new introsperm would have the flagellum running parallel to an elongate nucleus as in C. riisei. Most of the process of nuclear rotation may have been inhibited as well in all inseminating species analyzed thus far in the subfamily Glandulocaudinae (Mimagoniates barberi, M. microlepis) (Pecio & Rafi ski, 1994, 1999; Burns et al., 1998), subfamily Stevardiinae (Diapoma speculiferum, Diapoma sp., Pseudocorynopoma doriae, Scopaeocharax rhinodus, Tyttocharax tambopatensis and T. cochui ) (Burns et al., 1998; Pecio et al., 2005), and two species currently incertae sedis, Bryconadenos tanaothoros (Weitzman et al., 2005) and Brittanichthys axelrodi (Javonillo et al., 2007). In all of these species, spermatozoa have mitochondria located alongside and/or posterior to the elongate nuclei, centrioles located to one side near the anterior end of the nucleus, and a single flagellum running alongside the nucleus within a cytoplasmic canal of variable length (Pecio & Rafi
ski, 1994, 1999; Burns et al., 1998), subfamily Stevardiinae (Diapoma speculiferum, Diapoma sp., Pseudocorynopoma doriae, Scopaeocharax rhinodus, Tyttocharax tambopatensis and T. cochui ) (Burns et al., 1998; Pecio et al., 2005), and two species currently incertae sedis, Bryconadenos tanaothoros (Weitzman et al., 2005) and Brittanichthys axelrodi (Javonillo et al., 2007). In all of these species, spermatozoa have mitochondria located alongside and/or posterior to the elongate nuclei, centrioles located to one side near the anterior end of the nucleus, and a single flagellum running alongside the nucleus within a cytoplasmic canal of variable length (Pecio & Rafi ski, 1994, 1999; Burns et al., 1998; Pecio et al., 2005; Weitzman et al., 2005; Javonillo et al., 2007). The nuclear rotation observed in H. erythrozonus is typical of all externally fertilizing characiforms analyzed to date with the exception of Acestrorhynchus falcatus (Matos et al., 2000). The presence or absence of nuclear rotation has been described in the Teleostei by Mattei (1970), who classified two types of aquasperm. In Type I the nucleus rotates during spermiogenesis and as a result the flagellar axis is perpendicular to the base of the nucleus, whereas in type II there is no rotation and the flagellum remains parallel to the base of the nucleus. Mattei (1970) reported that spermiogenesis without nuclear rotation is more typical of aquasperm in more derived teleostean taxa, such as species in the order Perciformes. The only inseminating characid studied to date whose spermatozoon shows evidence of complete nuclear rotation is Macropsobrycon uruguayanae of the subfamily Cheirodontinae (Burns et al., 1998). The spermatozoon of C. riisei may actually represent a highly modified type I sperm that underwent only the beginning of nuclear rotation. The type II aquasperm described by Mattei (1970) has both centrioles located outside of any nuclear fossae. The location of at least part of the centrioles of C. riisei within nuclear fossae further supports its spermatozoon as being a modified type I sperm.
ski, 1994, 1999; Burns et al., 1998; Pecio et al., 2005; Weitzman et al., 2005; Javonillo et al., 2007). The nuclear rotation observed in H. erythrozonus is typical of all externally fertilizing characiforms analyzed to date with the exception of Acestrorhynchus falcatus (Matos et al., 2000). The presence or absence of nuclear rotation has been described in the Teleostei by Mattei (1970), who classified two types of aquasperm. In Type I the nucleus rotates during spermiogenesis and as a result the flagellar axis is perpendicular to the base of the nucleus, whereas in type II there is no rotation and the flagellum remains parallel to the base of the nucleus. Mattei (1970) reported that spermiogenesis without nuclear rotation is more typical of aquasperm in more derived teleostean taxa, such as species in the order Perciformes. The only inseminating characid studied to date whose spermatozoon shows evidence of complete nuclear rotation is Macropsobrycon uruguayanae of the subfamily Cheirodontinae (Burns et al., 1998). The spermatozoon of C. riisei may actually represent a highly modified type I sperm that underwent only the beginning of nuclear rotation. The type II aquasperm described by Mattei (1970) has both centrioles located outside of any nuclear fossae. The location of at least part of the centrioles of C. riisei within nuclear fossae further supports its spermatozoon as being a modified type I sperm.
During spermiogenesis in H. erythrozonus, the chromatin changes from having a finely granular or mottled appearance throughout the entire volume of the nucleus to being homogenously condensed and highly electron-dense in one area of the nucleus and more electron-lucent in a peripheral area, particularly in spermatozoa present in the lumen. In species of Curimatidae and in H. malabaricus (Erythrinidae) (Quagio-Grassiotto et al., 2001, 2003), all externally fertilizing, nuclear condensation is very similar to that observed in H. erythrozonus. However, the elimination of the electron-lucent area seen in H. erythrozonus during the final stage of condensation was only observed in Chilodus punctatus (Anostomidae) (Pecio, 2003). Nuclear condensation involving the gradual enlargement of chromatin granules described here for C. riisei is also typical for the other inseminating stevardiines, S. rhinodus and Tyttocharax spp. (personal observations), as well as the inseminating glandulocaudine, M. barberi (Pecio & Rafi ski, 1999; Pecio et al., 2005). The chromatin in the spermatozoon of most inseminating characids studied to date is highly condensed (Pecio & Rafi
ski, 1999; Pecio et al., 2005). The chromatin in the spermatozoon of most inseminating characids studied to date is highly condensed (Pecio & Rafi ski, 1994, 1999; Burns et al., 1998; Pecio et al., 2005). In C. riisei, however, the chromatin of the spermatozoon has a flocculent appearance more similar to that seen in another stevardiine, Pseudocorynopoma doriae (Burns et al., 1998; Burns & Weitzman, 2005). A similar type of chromatin condensation was also described in the externally fertilizing Salminus brasiliensis, Brycon microlepis and B. orbignyanus, primitive representatives of Characidae (Verissimo-Silveira, 2006). The variation in chromatin condensation seen among characids suggests the presence of different types of protamines associated with the DNA (Saperas et al., 1993).
ski, 1994, 1999; Burns et al., 1998; Pecio et al., 2005). In C. riisei, however, the chromatin of the spermatozoon has a flocculent appearance more similar to that seen in another stevardiine, Pseudocorynopoma doriae (Burns et al., 1998; Burns & Weitzman, 2005). A similar type of chromatin condensation was also described in the externally fertilizing Salminus brasiliensis, Brycon microlepis and B. orbignyanus, primitive representatives of Characidae (Verissimo-Silveira, 2006). The variation in chromatin condensation seen among characids suggests the presence of different types of protamines associated with the DNA (Saperas et al., 1993).
During spermiogenesis in the species of the present study, the nuclei assume very different shapes, resulting in sperm heads that are markedly different. The spherical shape of the nucleus in H. erythrozonus is a character typical not only of the aquasperm of all characiforms studied to date, but also of aquasperm of many other teleostean fishes (Jamieson, 1991). The slightly asymmetric shape of the nucleus, along with eccentrically located shallow nuclear fossae containing the tips of the nearly parallel centrioles, was also described in another characid species, Paracheirodon innesi (Jamieson, 1991). A nearly parallel arrangement of the centrioles was found in the inseminating genera, Scopaeocharax and Tyttocharax, whereas in other inseminating genera, such as Mimagoniates, Diapoma and Pseudocorynopoma, the centrioles are arranged perpendicular to one another as seen in C. riisei. In all of the above inseminating species, the centrioles are located near the anterior tip of the cell, lateral to the elongate nucleus (Burns et al., 1998; Pecio et al., 2005).
Elongate nuclei have been described in most inseminating characids studied to date (Pecio & Rafi ski, 1994, 1999; Burns et al., 1995, 1997, 1998, 2000; Azevedo et al., 2000; Burns & Weitzman, 2005, 2006; Weitzman et al., 2005; Javonillo et al., 2007), as well in many other internally fertilizing teleosts (Jamieson, 1991). However, inseminating species that produce spermatozoa more similar to aquasperm include Planaltina spp. (Burns et al., 1995; Menezes et al., 2003), Kolpotocheirodon theloura (Burns et al., 1997; Malabarba & Weitzman, 2000), Attonitus ephimeros and A. irisae (Weitzman et al., 2005), Monotocheirodon pearsoni (Burns & Weitzman, 2006) and Knodus sp. (Burns & Weitzman, 2005) among the Characidae and Ameca splendens, Aetonobius toweri, Characoden lateralis and Xenotoca eiseni among the Goodeidae (Jamieson, 1991).
ski, 1994, 1999; Burns et al., 1995, 1997, 1998, 2000; Azevedo et al., 2000; Burns & Weitzman, 2005, 2006; Weitzman et al., 2005; Javonillo et al., 2007), as well in many other internally fertilizing teleosts (Jamieson, 1991). However, inseminating species that produce spermatozoa more similar to aquasperm include Planaltina spp. (Burns et al., 1995; Menezes et al., 2003), Kolpotocheirodon theloura (Burns et al., 1997; Malabarba & Weitzman, 2000), Attonitus ephimeros and A. irisae (Weitzman et al., 2005), Monotocheirodon pearsoni (Burns & Weitzman, 2006) and Knodus sp. (Burns & Weitzman, 2005) among the Characidae and Ameca splendens, Aetonobius toweri, Characoden lateralis and Xenotoca eiseni among the Goodeidae (Jamieson, 1991).
Nuclear elongation during spermiogenesis to produce a final sperm cell that is long and thin has been described as an adaptation associated with insemination in many teleost families (Jamieson, 1989, 1991; Mattei 1991; Burns et al., 1998; Jamieson & Grier 1993; Burns & Weitzman; 2005). This streamlined shape may allow for more efficient movement of sperm cells through viscous fluids and narrow pathways in the ovary (Fawcett, 1970; Gardiner, 1978; Jamieson, 1991), as well as aid in the coalescence of spermatozoa into sperm packets. Formation of sperm clumps would result in greater densities of cells being transferred to the female, thus increasing the effectiveness of sperm transfer (Ginzburg, 1968). Histological sections of testes of C. riisei have shown sperm cells to be arranged into flowing patterns, although distinct spermatozeugmata could not be identified (Burns et al., 1995). However, Kutaygil (1959) made direct observations on living specimens and described the transfer of sperm "heaps" to the female. C. riisei may indeed produce sperm clumps that are embedded in a mucoid material, which can be seen in histological sections stained with the periodic acid/Schiff reagent (personal observations). Production of a type of spermatozeugma in this species is also supported by the distinct clumping of cells seen in Fig. 4. Inseminating characids known to produce distinct spermatozeugmata include the glandulocaudine genera Glandulocauda, Mimagoniates and Lophiobrycon (Pecio & Rafi ski, 1994, 1999, 2001; Burns et al., 1995; personal observation), the stevardiine genera Scopaeocharax, Tyttocharax and Xenurobrycon (Burns et al., 1995; Burns & Weitzman, 2005; Pecio et al., 2005) and two species currently incertae sedis, Bryconadenos tanaothoros (Weitzman et al., 2005) and Brittanichthys axelrodi (Javonillo et al., 2007).
ski, 1994, 1999, 2001; Burns et al., 1995; personal observation), the stevardiine genera Scopaeocharax, Tyttocharax and Xenurobrycon (Burns et al., 1995; Burns & Weitzman, 2005; Pecio et al., 2005) and two species currently incertae sedis, Bryconadenos tanaothoros (Weitzman et al., 2005) and Brittanichthys axelrodi (Javonillo et al., 2007).
Elongation of the nucleus in C. riisei proceeds without the aid of accessory microtubles. This contrasts with the situation in M. barberi where accessory microtubules, which are present around the nucleus during spermiogenesis when nuclear elongation occurs, persist in the mature spermatozoon as single row located at one side of the nucleus (Pecio & Rafi ski, 1999). Accessory microtubules are also present in mature spermatozoa of the stevardiine genera Scopaeocharax and Tyttocharax (Pecio et al., 2005), the cheirodontine Macropsobrycon uruguayanae (Burns & Weitzman, 2005) and the incertae sedis species Bryconadenos tanaothoros (Weitzman et al., 2005), although data on spermiogenesis are lacking. The appearance of accessory microtubules during spermiogenesis, coincident with nuclear elongation, was described in the viviparous poeciliids, Gambusia affinis, Poecilia latipinna and P. reticulata, where they appear during nuclear rotation (Billard, 1970; Grier, 1973, 1975). However, in the viviparous Anableps anableps of the Anablepidae, the sister group to Poeciliidae, during spermiogenesis no accessory microtubules are present when spermatid elongation occurs, in spite of the mature spermatozoon having a structure similar to that seen in poeciliids (personal observation).
ski, 1999). Accessory microtubules are also present in mature spermatozoa of the stevardiine genera Scopaeocharax and Tyttocharax (Pecio et al., 2005), the cheirodontine Macropsobrycon uruguayanae (Burns & Weitzman, 2005) and the incertae sedis species Bryconadenos tanaothoros (Weitzman et al., 2005), although data on spermiogenesis are lacking. The appearance of accessory microtubules during spermiogenesis, coincident with nuclear elongation, was described in the viviparous poeciliids, Gambusia affinis, Poecilia latipinna and P. reticulata, where they appear during nuclear rotation (Billard, 1970; Grier, 1973, 1975). However, in the viviparous Anableps anableps of the Anablepidae, the sister group to Poeciliidae, during spermiogenesis no accessory microtubules are present when spermatid elongation occurs, in spite of the mature spermatozoon having a structure similar to that seen in poeciliids (personal observation).
An elongate cytoplasmic collar, which appears during spermatid formation in C. riisei, persists in the spermatozoon, with the flagellum running the entire length of the head within the canal. An elongate collar is retained in the spermatozoon in the characid genera Diapoma and Pseudocorynpoma (Burns et al., 1998), and Brittanichthys (Javonillo et al., 2007), whereas in Mimagoniates (Pecio & Rafi ski, 1994, 1999; personal observations) and in S. rhinodus and T. tambopatensis (Pecio et al., 2005) most of the cytoplasmic collar degenerates during the final stage of spermiogenesis, and in the spermatozoon the collar is limited to the very anterior part of the cell.
ski, 1994, 1999; personal observations) and in S. rhinodus and T. tambopatensis (Pecio et al., 2005) most of the cytoplasmic collar degenerates during the final stage of spermiogenesis, and in the spermatozoon the collar is limited to the very anterior part of the cell.
Our results demonstrate marked differences in the process of spermiogenesis between an externally and an inseminating species of Characidae. Differences also exist for spermiogenesis among inseminating characid species, such as those belonging to the subfamilies Stevardiinae and Glandulocaudinae. One of the main goals of this study has been to uncover ultrastructural characters that may prove useful for future phylogenetic analyses of these fishes.
Acknowledgments
The authors thank Dr. D. Podkowa for advice in preparing the tables and Mrs. W. Jankowska for the sperm drawings. We thank also to the staff of the Department of Histology and Cytology (Institute of Zoology, Jagiellonian University) for providing electron microscope facilities. The study in SEM and TEM was supported by grant BW/IZ/64/2006.
Literature Cited
Submitted July 2007
Accepted November 2007
- Andrade, R. F., N. Bazzoli, E. Rizzo & Y. Sato. 2001. Continuous gametogenesis in the Neotropical freshwater teleost, Brycon affinis (Pisces: Characidae). Tissue & Cell, 33: 524-532.
- Azevedo, M. A., L. R. Malabarba & C. B. Fialho. 2000. Reproductive biology of the inseminating glandulocaudine Diapoma speculiferum Cope (Teleostei: Characidae). Copeia, 2000: 983-989.
- Billard, R. 1970. La spermatogenèse de Poecilia reticulata, IV La spermiogenèse. Etude ultrastructurale. Annales de Biologie Animale, 10: 493-510.
- Birkhead, T. R. & A. P. Moller. 1997. Sperm Competition and Sexual Selection. London. Academic Press.
- Breder, C. M. & D. E. Rosen. 1966. Modes of Reproduction in Fishes. T.F.H. Publications, Jersey City, NJ.
- Burns, J. R. & S. H. Weitzman. 2006. Intromittent organ in the genus Monotocheirodon (Characiformes: Characidae). Copeia, 2006: 529-534.
- Burns, J. R. & S.H. Weitzman, H. J. Grier & N. A. Menezes. 1995. Internal fertilization, testis and sperm morphology in glandulocaudine fishes (Teleostei: Characidae: Glandulocaudinae). Jounal of Morphology, 224: 131-145.
- Burns, J. R., S. H. Weitzman & L. R. Malabarba. 1997. Insemination in eight species of cheirodontine fishes (Teleostei: Characidae: Cheirodontinae). Copeia, 1997: 433-438.
- Burns, J. R., S.H. Weitzman, K. R. Lange & L. R. Malabarba. 1998. Sperm ultrastructure in characid fishes (Teleostei: Ostariophysi). Pp 235-244. In: Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.) Phylogeny and Classification of Neotropical Fishes. Porto Alegre, Brazil: Edipucrs, 603p.
- Burns, J. R., S. H. Weitzman, L. R. Malabarba & A. D. Meisner. 2000. Sperm modifications in inseminating ostariophysan fishes, with new documentation of inseminating species, p. 255. In: Proc. VI Intl. Symp. Reprod. Physiol. Fish. B. Norberg, O. S. Kjesbu, G. L. Taranger, E. Andersson and S. O. Stefansson (Eds.). Institute of Marine Research and University of Bergen, Bergen, Norway.
- Fawcett, D. W. 1970. A comparative view of sperm ultrastructure. Biology of Reproduction Supplement, 2: 90-127.
- Gardiner, D. M. 1978. Fine structure of the spermatozoon of the viviparous teleost, Cymatogaster aggregata Journal of Fish Biology, 13: 435-438.
- Ginzburg, A. S. 1968. Fertilization in Fishes and the Problem of Polyspermy. Academija Nauk SSSR, Moscow.
- Grier, H. J. 1973. Ultrastructure of the testis in the teleost Poecilia latipinna. Spermiogenesis with reference to the intercentriolar lamellated body. Journal of Ultrastructural Research, 45: 82-92.
- Grier, H. J. 1975. Spermiogenesis in the teleost Gambusia affinis with particular reference to the role played by microtubules. Cell and Tissue Research, 165: 89-102.
- Grier, H.J. & L. R. Parenti. 1994. Reproductive biology and systematics of phallostethid fishes as revealed by gonad structure. Environmental Biology of Fishes, 41: 287-299.
- Ito, S. & M. J. Karnovsky. 1968. Formaldehyde glutaraldehyde fixatives containing trinitro compounds. Journal of Cell Biology, 36:168.
- Jamieson, B. G. M. 1989. Complex spermatozoon of the live-bearing half-beak, Hemirhamphodon pogonognatus (Blecker): ultrastructural description (Euteleostei, Atherinomorpha, Beloniformes). Gamete Research, 24: 247-259.
- Jamieson, B. G. M. 1991. Fish Evolution and Systematics: Evidence from Spermatozoa. Cambridge Univ. Press, Cambridge.
- Jamieson, B. G. M. & H. J. Grier. 1993. Influences of phylogenetic position and fertilization biology on spermatozoal ultrastructure exemplified by excocoetoid and poeciliid fish. Hydrobiologia, 271: 11-25.
- Javonillo, R., J. R. Burns & S. H. Weitzman. 2007. Reproductive morphology of Brittanichthys axelrodi (Teleostei: Characidae), a miniature inseminating fish from South America. Journal of Morphology, 268: 23-32.
- Koya, Y., H. Munehara & K. Takano. 2002. Sperm storage and motility in the ovary of the marine sculpin Alcichthys alcicornis (Teleostei: Scorpaeniformes), with internal gametic association. Journal of Experimental Zoology, 292: 145-155.
- Kutaygil, N. 1959. Insemination, sexual differentiation and secondary sex characters in Stevardia albipinnis Gill. Hidriobiologie, Istanbul Universitat Fen Fakultesi Mecumuasi, ser. B, 24: 93-128.
- Lahnsteiner, F., U. Richtarski & R. A. Patzner. 1990. Function of the testicular glands in two blennid fishes, Salaria (=Blennius) pavo and Lipophrys (=Blennius) dalmatinus (Blennidae, Teleostei) as revealed by electron microscopy and enzyme biochemistry. Jounal of Fish Biology, 37: 85-97.
- Malabarba, L.R. & S. H.Weitzman. 2000. New genus and species of inseminating fish (Teleostei: Cheirodontinae: Compsurini) from South America with uniquely derived caudal fin dermal papillae. Proceedings of the Biological Society of Washington, 113: 269-283.
- Matos, E., P. Matos, L. Corral & C. Azevedo. 2000. Estrutura fina do espermatozóide de Acestrorhynchus falcatus Bloch (Teleostei, Characidae) da região norte do Brasil. Revista Brasileira de Zoologia, 17: 747-752.
- Mattei, X. 1970. Spermiogenèse comparée des poisons. Pp. 57-69. In: Comparative Spermatology (B. Baccetti ed.). Academic Press. New York.
- Mattei, X. 1991. Spermatozoon ultrastructure and its systematic implications in fishes. Canadian Journal of Zoology, 69:3038-3055.
- Meisner, A. D. 2005. Male modifications associated with insemination in teleosts. Pp. 165-190. In: Grier H.J. & M.C. Uribe (Eds). Viviparous Fishes. New Life Publications, Homestead Florida.
- Menezes, N.A., S. H. Weitzman & J. R. Burns. 2003. A systematic review of Planaltina (Teleostei: Characiformes: Characidae: Glandulocaudinae: Diapomini) with a description of two new species from the upper rio Paraná, Brazil. Proceedings of the Biological Society of Washington, 116: 557-600.
- Nelson, J. S. 2006. Fishes of the World. 4th Edit. John Wiley and Sons, New York.
- Nelson, K. 1964. Behavior and morphology in the glandulocaudine fishes (Ostariophysi, Characidae). University of Califoria Publications in Zoology, 75: 59-152.
- Pecio, A. 2003. Spermiogenesis and fine structure of the spermatozoon in a headstander, Chilodus punctatus (Teleostei, Characiformes, Anostomidae). Folia Biologica, 51: 55-62.
- Pecio, A. & J. Rafinski. 1994. Structure of the testis, spermatozoa and spermatozeugmata of Mimagoniates barberi Regan, 1907 (Teleostei: Characidae), an internally fertilizing, oviparous fish. Acta Zoologica, 75: 179-185.
- Pecio, A. & J. Rafinski. 1999. Spermiogenesis in Mimagoniates barberi (Teleostei: Ostariophysi: Characidae), an oviparous, internally fertilizing fish. Acta Zoologica, 80: 35-45.
- Pecio, A. & J. Rafinski. 2001. Spermatozeugmata formation in Mimagoniates barberi (Teleostei: Characidae). Journal of Morphology, 248: 270.
- Pecio, A., J. R. Burns & S. H. Weitzman. 2005. Sperm and spermatozeugma ultrastructure in the inseminating species Tyttocharax cochui, T. tambopatensis and Scopaeocharax rhinodus (Pisces:Teleostei: Characidae: Glandulocaudinae: Xenurobryconini). Journal of Morphology, 263: 216-226.
- Quagio-Grassiotto, I., J. N. C. Negrao, E. D. Carvelho & F. Forseti. 2001. Ultrastructure of spermatogenic cells and spermatozoa in Hoplias malabaricus (Teleostei, Characiformes, Erythrinidae). Journal of Fish Biology, 59: 1494-1502.
- Quagio-Grassiotto, I., M. C. Gameiro, T. Schneider, L. R. Malabarba & C. Oliveira. 2003. Spermiogenesis and spermatozoa ultrastructure in five species of the Curimatidae with some considerations on spermatozoal ultrastructure in Characiformes. Neotropical Ichthyology, 1: 35-45.
- Reznick, D.N., M. Mateos & M. S. Springer. 2002. Independent origins and rapid evolution of the placenta in the fish genus Poeciliopsis. Science, 298: 1018-1020.
- Romagosa, E., M. Y. Narahara, M. I. Borella, S. F. Parreira & N. Fenerich-Verani. 1999. Ultrastructure of the germ cells in the testis of matrinxã, Brycon cephalus (Teleostei, Characidae). Tissue & Cell, 31: 540-544.
- Saperas, N., E. Ribes, C. Buesa, F. Garcia-Hegart & M. Chiva. 1993. Differences in chromatin condensation during spermiogenesis in two species of fish with distinct protamines. Journal of Experimental Zoology, 265:185-194.
- Shahin, A. 2006. Spermatogenesis and spermatozoon ultrastructure in the Nile Pebblyfish Alestes dentex (Teleostei: Characiformes: Alestiidae) in Egypt. World Journal of Zoology, (1): 1-16.
- Veríssimo-Silveira, R., P. Gusmão-Pompiani, C. A. Vicentini & I. Quagio-Grassiotto. 2006. Spermiogenesis and spermatozoa ultrastructure in Salminus and Brycon, two primitive genera in Characidae (Teleostei: Ostariophysi: Characiformes). Acta Zoologica, 87:305-313.
- Weitzman, S.H. & N. A. Menezes. 1998. Relationships of the tribes and genera of the Glandulocaudinae (Ostariophysi: Characiformes: Characidae) with a description of a new genus Chrysobrycon Pp. 159-180. In: Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.) Phylogeny and Classification of Neotropical Fishes. Porto Alegre, Brazil: Edipucrs, 603p.
- Weitzman, S.H., N. A. Menezes, H. G. Evers & J. R. Burns. 2005. Putative relationships among inseminating and externally fertilizing characids, with a description of a new genus and species of Brazilian inseminating fish bearing an anal-fin gland in males (Characiformes: Characidae). Neotropical Ichthyology, 3: 323.
Publication Dates
-
Publication in this collection
15 Jan 2008 -
Date of issue
Dec 2007
History
-
Accepted
Nov 2007 -
Received
July 2007