Abstracts
Correct estimates of size at first maturity (L50) are useful for fish stock management and different methods have been proposed. In this study we propose the use of a modified logistic to estimate L50, including a variable asymptote (A). We also test the use of the Gonadossomatic Index (GSI) as a flag to establish the reproductive status of each female fish. The method is demonstrated using GSI data for four Neotropical fish species: Characiformes: Astyanax fasciatus (n = 473) and Oligosarcus robustus (n = 297); Siluriformes: Loricariichthys anus (n = 427) and Trachelyopterus lucenai (n = 195). The results were stable enough to propose this method be used for other fish species with different life histories and with a relatively unconstrained sampling programs. Nevertheless, a wide scale test program is desirable to identify any possible bias in this approach.
Fish; Reproduction; Patos lagoon; Logistic function
Estimativas precisas do tamanho de primeira maturação (L50) são úteis para a gestão de estoques naturais, existindo diversos métodos disponíveis na literatura. No presente estudo é proposto um método alternativo através de uma equação logística modificada, incluindo-se uma assíntota variável (A). Testou-se igualmente o uso do Índice Gonadossomático (GSI) como indicativo do status reprodutivo para fêmeas de peixes. O método é demonstrado com dados de quatro espécies de peixes Neotropicais: Characiformes: Astyanax fasciatus (n = 473) e Oligosarcus robustus (n = 297); Siluriformes: Loricariichthys anus (n = 427) e Trachelyopterus lucenai (n = 195). Os resultados obtidos foram suficientemente estáveis para propor-se o emprego do método para outras espécies de peixes com diferentes histórias de vida, mesmo que em programas de amostragem limitados. Sugere-se, entretanto, avaliação da metodologia em larga escala como forma de identificar-se possíveis desvios da abordagem proposta.
Estimating size at first maturity (L50) from Gonadossomatic Index (GSI) data
Nelson F. Fontoura; Aloísio S. Braun; Paulo Cesar C. Milani
Faculdade de Biociências, Pontifícia Universidade Católica do Rio Grande do Sul, Av. Ipiranga, 6681, P.O. Box 1429, 90619-900 Porto Alegre, RS, Brazil. nfontoura@pucrs.br
ABSTRACT
Correct estimates of size at first maturity (L50) are useful for fish stock management and different methods have been proposed. In this study we propose the use of a modified logistic to estimate L50, including a variable asymptote (A). We also test the use of the Gonadossomatic Index (GSI) as a flag to establish the reproductive status of each female fish. The method is demonstrated using GSI data for four Neotropical fish species: Characiformes: Astyanax fasciatus (n = 473) and Oligosarcus robustus (n = 297); Siluriformes: Loricariichthys anus (n = 427) and Trachelyopterus lucenai (n = 195). The results were stable enough to propose this method be used for other fish species with different life histories and with a relatively unconstrained sampling programs. Nevertheless, a wide scale test program is desirable to identify any possible bias in this approach.
Key words: Fish, Reproduction, Patos lagoon, Logistic function.
RESUMO
Estimativas precisas do tamanho de primeira maturação (L50) são úteis para a gestão de estoques naturais, existindo diversos métodos disponíveis na literatura. No presente estudo é proposto um método alternativo através de uma equação logística modificada, incluindo-se uma assíntota variável (A). Testou-se igualmente o uso do Índice Gonadossomático (GSI) como indicativo do status reprodutivo para fêmeas de peixes. O método é demonstrado com dados de quatro espécies de peixes Neotropicais: Characiformes: Astyanax fasciatus (n = 473) e Oligosarcus robustus (n = 297); Siluriformes: Loricariichthys anus (n = 427) e Trachelyopterus lucenai (n = 195). Os resultados obtidos foram suficientemente estáveis para propor-se o emprego do método para outras espécies de peixes com diferentes histórias de vida, mesmo que em programas de amostragem limitados. Sugere-se, entretanto, avaliação da metodologia em larga escala como forma de identificar-se possíveis desvios da abordagem proposta.
Introduction
Correct estimates of size at first maturity (L50 - length at which 50% of the fish are mature) are useful for fish stock management. Different methods have been proposed to estimate L50. Trippel & Harvey (1991) made a review on this subject, evaluating six different approaches. Nevertheless, with most methods, each individual fish should be identified as reproductive or non reproductive. This recognition is usually visual, and subjective descriptions of macroscopic aspects of ovaries and testes at different maturation stages have been published frequently (e. g. Gerritsen et al., 2003). A more unconstrained approach, such as the direct use of the Gonadosomatic Index (GSI), is restricted to the description of the seasonal cycle of reproduction.
Although different methods are available, most of the published papers apply some kind of logistic function to estimate L50 (e. g. Quaatey & Maravelias, 1999; Gonçalves & Erzini, 2000; Liu et al., 2001; Gerritsen et al., 2003; Dadebo et al., 2003; Lewis & Fontoura, 2005).
In this study the use of a modified logistic with a variable asymptote is proposed to estimate L50 from GSI data and different values of the Gonadosomatic Index (GSI) are tested as flag criteria to determine the reproductive status of female fish.
Material and Methods
To test the use of the Gonadossomatic Index (GSI) as a reproductive flag to estimate size at first maturation (L50), GSI data of four fish species were used: Characiformes: Astyanax fasciatus (n = 473) and Oligosarcus robustus (n = 297); Siluriformes: Loricariichthys anus (n = 427) and Trachelyopterus lucenai (n = 195). Animals were captured in lagoa do Casamento and lagoa dos Gateados; both connected marginal lagoons of the larger laguna dos Patos, Rio Grande do Sul State, Brazil. Samples were carried out from November 2002 to April 2004, totaling 17 monthly samples (samples were not taken in October 2003). Sampling was done using a beach seine net (20m long, 5mm mesh size) and gill nets (30m long, mesh size of 15, 20, 25, 30, 35, 40, 50, 60 and 70mm). The fish were weighed to the nearest 0.1g, total length was measured to the nearest mm, and gonads were weighed to the nearest 0.001 g.
The equation model to estimate size at first maturity is: P = A.(1+e(-r.(Lt-L50)))-1; where P is the proportion or ratio of reproductive females for each size class; A is the curve asymptote; r is a rate parameter related the speed of size change from non reproductive to reproductive status; Lt is the total length (cm) and L50 is the size at first maturity (cm). Parameter estimations were performed using the Non Linear Regression routine of SPSS 11.0 (Levenberg-Marquardt algorithm) from frequencies of reproductive to non reproductive females grouped in 1-2mm size classes. As a flag criteria of the reproductive status, individual GSI values were transformed into percentages of the maximum registered GSI for each species. As a simulation approach, flag values of 5%, 10%, 15%, 20%, 25%, and 30% of the maximum registered GSI were tested as criteria to identify engagement into reproduction.
Results
Logistic equations are used to estimate L50 because these simple mathematical functions can emulate a cumulative normal curve where L50 correspond to normal average. That is, L50 is the average size of an age-group when it starts to reproduce for the first time (age-group mean length in the middle of the reproductive period). Figure 1 shows an example of a growing age-group with temporal relative frequency of three different reproductive stages. For simplicity, each maturity stage has a standard deviation of one month and is one month apart. In this simulation L50 is 17.3cm, attained at age 10 months when most of the females are identified as reproductive (maturing + spawning + post-spawning). Nevertheless, it is interesting to note that even at the age 10, when most of the animals are engaged in reproductive activities, only 90% of the individuals could be identified as reproductive (Fig. 1). If computed all the animals captured during the three months of greatest reproductive activity, only 75% would be identifiable as reproductive in this simulation. Of course, this figure depends on how wide each reproductive stage is (standard deviation) and that frequencies do not necessarily follows a normal probability curve. In addition to the limitations of simple computer simulation, we should be aware that in some cases not all adult individuals can be identified as reproductive during the reproductive period. The central point is that regular logistic equations present asymptotic behavior which stabilizes at one (or 100%): from a certain size all individuals must be correctly identified as adults, and this can not be true (Trippel & Harvey, 1991).
Figure 2 presents three different logistic curves. The left curve shows a logistic curve with an asymptote at one and L50 of 17.3. The central curve represents real frequencies from the simulation of Fig. 1, considering animals captured in the three months of greatest reproductive activity (9 to 11). In this case, asymptote is at 0.75 (a maximum of 75% of animals could be identified as reproductive). The right curve results from trying to apply a regular logistic, with asymptote at one, to frequency data from the present simulation. Errors resulting from this approach could be significant, biasing L50 to greater values. Although this bias could be easily identified in Fig. 2, the error may be obscured by poor data series with large size classes.
Figure 3 presents GSI to length data for all females of Astyanax fasciatus, Oligosarcus robustus, Loricariichthys anus and Trachelyopterus lucenai captured throughout the sampling program. Gray boxes represent the range of available values of L50 for each species. For L. anus a single estimate of L50 is represented by a vertical line. Published values of L50 for these species can be seen at Table 1.
It is interesting to note the wide range of estimates of L50 for the same species. For A. fasciatus, different estimates of L50 could result from populations analyzed from distant basins, adapted to distinct environmental conditions, and probably presenting genetic differentiation (or even different species at the same designation). But it is not the case for O. robustus and T. lucenai with studies carried in the same basin and locations not far apart.
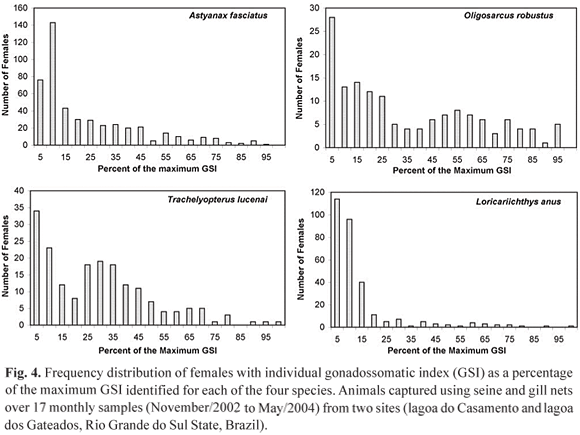
To test the use of GSI values to estimate L50, the starting point should be to categorize each animal as reproductive or non reproductive. The problem is where to establish the border line. The present approach is to consider as reproductive individuals with GSI values equal to or greater than a specific percentage of the maximum registered GSI for the species. Maximum registered values of GSI (percentage of the total body weight) for the analyzed species is as follow: A. fasciatus, 19.7%; O. robustus, 19.8%; L. anus, 11.8% and T. lucenai, 10.3%. Figure 4 presents the frequency distribution of individuals as percentages of the maximum GSI for each fish species. The intention was to identify a natural and clear threshold to distinguish between reproductive and non reproductive animals. Nevertheless, as can be seen in Fig. 4, there is no common pattern of frequency distribution and a natural threshold is unclear. The only observed pattern is that modal frequencies are always under 10% and in three of four cases, under 5%, which suggests that when not engaged in reproduction, ovaries present a GSI of less than 10% of the maximum possible GSI.
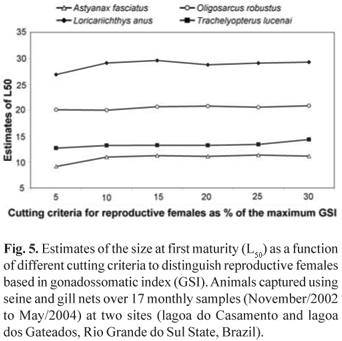
Results from testing different flag values of GSI to estimate L50 are presented in Table 2. It is interesting to observe that the selected cutting edge to classify animals as reproductive or non reproductive (5 - 30%) does not interfere in the estimated values of L50, as all resulting values are very similar, and most of them are equal, considering standard errors. As a general pattern, only the cutting edge of 5% apparently underestimates L50, as compared to greater cutting levels (Fig. 5). By increasing the cutting flag criteria to higher values, the smaller number of animals identified as reproductive is compensated by a lower asymptote value, maintaining the relative position of the logistic function to the X axis, and consequently does not interfering with the estimates of L50.
Discussion
The first point to be discussed is why change a well established methodology to estimate L50. In most Brazilian labs working with fish biology there are no technical personal to process samples. All the work is done by graduate and undergraduate students with varying levels of skill. Skills are developed at the same time that mistakes are being made and even well trained students can have doubts about the limits of a maturation stage. On the other hand, once the ovary is identified, measuring its weight is a very simple task, and is not prone to large methodological bias. The result is numerical, can be statistically inspected and is precise enough for most purposes.
While the present approach was being developed, the main concern was related to the cutting line or flag to establish an animal as reproductive or not. Two major problems were involved: (1) how big the GSI should be to consider the ovary as engaged in reproductive activities and (2) how sensitive the method is to this selected value. The first decision was that the flag GSI value should be species specific, and anchored as a fraction of the maximum registered GSI. A problem with this approach concerns the fact that the maximum GSI depends on the sample size, sampling site and sampling period. As the maximum GSI is not a stable parameter, how do the values obtained interfere with the estimates of L50? It was a surprise to identify that the estimated values of L50 were no different when using flag values from 5 to 30% of the maximum obtained GSI, indicating that precise information concerning maximum GSI is not critical to this methodological approach. Biased maximum GSI, by choosing different GSI flag values, will introduce variations in the estimates of the model asymptote (A), but the L50 will remain stable as demonstrated in Table 2.
Another problem concerns the "one" (or 100%) asymptote of the regular logistic function. Although this is a pattern usually found in biological data, especially in species with time-restricted reproduction, this is certainly not a rule. Comparing different methods to estimate L50, Trippel & Harvey (1991) identified four types of frequency distributions related to the different reproduction patterns. Distributions characterized by a failure to attain 100% maturity were classified as type IV by these authors. Figure 6 presents GSI data of adult females (larger then L50; 11cm) of A. fasciatus throughout the sample program. Note that except for June (and October with no sample), in all other months there are individuals with large GSI values. Even in months with more intense reproduction, as in September to February, there are also a lot of females with small GSI values and probably not easily identifiable as reproductive using visual inspection or numerical criteria. In this case, if regular logistic is applied, a biased L50 will be the result. Trippel & Harvey (1991) applied six different approaches to estimate L50 to this kind of data and according to these authors none of the methods yielded useful estimates.
In the past, the use of a regular logistic was also justified because this function has only two parameters, and could be easily linearized by log-transformation. Nowadays, with powerful statistical packages, there is no practical reason to continue to use a regular logistic. In this case, even classifying animals visually as reproductive and non reproductive based on ovary or testes morphological (even histological) aspects, the adjusted mathematical model should be a logistic with variable asymptote as a general rule, which in some cases will be estimated as one (or 100%).
Concluding, this findings support the idea that the proposed methodology to estimate L50, using GSI data and a logistic function with variable asymptote, is robust and stable enough to be applied to fish species with different life histories and with a more unconstrained sampling programs. Nevertheless, a wide scale test program would be desirable to identify any possible bias of this approach.
Acknowledgements
The authors would like to thank the students of the Laboratório de Ecologia Aquática da PUCRS, especially Camilla Marques, Camilla Barcelos and Tatiana Beras. Thanks also to FAPERGS (financial support), CAPES (fellowships to A. Braun) and CNPq (fellowships to P. Milani and N. Fontoura).
Literature Cited
Accepted March 2009
Published June 17, 2009
- Barbieri, G. & M. C. Barbieri. 1988. Curva de maturação, tamanho de primeira maturação gonadal e fecundidade de Astyanax bimaculatus e Astyanax fasciatus, da represa do Lobo, SP (Osteichthyes, Characidae). Revista Ceres, 35(197): 64-77.
- Becker, F. G. 2001. Observations on the reproduction, sex ratio and size composition of Trachelyopterus lucenai (Teleostei, Auchenipteridae) in lake Guaíba, RS, Brasil. Biociências, 9(2): 85-96.
- Bruschi Jr, W., A. C. Peret, J. R. Verani & C. B. Fialho. 1997. Reproducão de Loricariichthys anus (Valenciennes, 1840) da lagoa Emboaba, Osório, RS, Brasil. Revista Brasileira de Zoologia, 57: 677-685.
- Dadebo, E., G. Ahlgren & I. Ahlgren. 2003. Aspects of reproductive biology of Labeo horie Heckel (Pisces: Cyprinidae) in Lake Chamo, Ethiopia. African Journal of Ecology, 41: 31-38.
- Gerritsen, H. D., M. J. Armstrong, M. Allen, W. J. McCurdy & J. A. D. Peel. 2003. Variability in maturity and growth in a heavily exploited stock: whiting (Merlangius merlangus L.) in the Irish Sea. Journal of Sea Research, 49: 69-82.
- Gonçalves, J. M. S. & K. Erzini. 2000. The reproductive biology of the two-banded sea bream (Diplodus vulgaris) from the southwest coast of Portugal. Journal of Applied Ichthyology, 16: 110-116.
- Lewis D. S & N. F. Fontoura. 2005. Maturity and growth of Paralonchurus brasiliensis females in southern Brazil (Teleostei, Perciformes, Sciaenidae). Journal of Applied Ichthyology, 21: 94-100
- Liu, K. M., K. Y. Hung & C. T. Chen. 2001. Reproductive biology of the big eye Priacanthus macracanthus in the north-eastern waters off Taiwan. Fisheries Science, 67: 1008-1014.
- Nomura, H. 1975. Fecundidade, maturação sexual e índice gônado-somático de lambarís do gênero Astyanax Baird & Girard, 1854, relacionados com fatores ambientais. Revista Brasileira de Biologia, 35: 775-798.
- Nunes, D. M., M. Pellanda & S. M. Hartz. 2004. Dinâmica Reprodutiva de Oligosarcus jenynsii e Oligosarcus robustus na lagoa Fortaleza, Rio Grande do Sul, Brasil. Iheringia, Série Zoologia, 94(1): 5-11.
- Quaatey S. N. K. & C. D. Maravelias. 1999. Maturity and spawning pattern of Sardinella aurita in relation to water temperature and zooplankton abundance off Ghana, West Africa. Journal of Applied Ichthyology, 15: 63-69
- Trippel, E. A. & H. H. Harvey. 1991. Comparison of methods used to estimate age and length of fishes at sexual maturity using populations of white sucker (Catostomus commersoni). Canadian Journal of Fisheries and Aquatic Science, 48: 1446-1495.
Publication Dates
-
Publication in this collection
15 July 2009 -
Date of issue
June 2009
History
-
Accepted
17 June 2009 -
Received
Mar 2009