Abstracts
The family Characidae is the most diverse among Neotropical fishes. Systematics of this family are mainly based on pre-cladistic papers, and only recently a phylogenetic hypothesis for Characidae was proposed by the author. That phylogeny was based on 360 morphological characters studied for 160 species, including representatives of families related to Characidae. This paper is based on that phylogenetic analysis, with the analyzed characters described herein and documented, accompanied by comparisons of their definition and coding in previous papers. Synapomorphies of each node of the proposed phylogeny are listed, comparisons with previous classifications provided, and autapomorphies of the analyzed species listed. Taxonomic implications of the proposed classification and the position of the incertae sedis genera within Characidae are discussed. A discussion of the phylogenetic information of the characters used in the classical systematics of the Characidae is provided.
Ostariophysi; Osteology; Morphology; Classification; Systematics
La familia Characidae es la más diversa entre los peces neotropicales. La sistemática de esta familia está basada principalmente en trabajos pre-cladísticos, y sólo recientemente una hipótesis filogenética para Characidae fue propuesta por el autor. Esa filogenia estaba basada en 360 caracteres estudiados en 160 especies, incluyendo representantes de familias relacionadas a Characidae. Este trabajo está basado en ese análisis filogenético, y los caracteres analizados son aquí descriptos y documentados, comparando su definición y codificación con trabajos previos. Las sinapomorfías de cada nodo de la filogenia propuesta son listadas, y se incluyen comparaciones con clasificaciones previas; también se listan las autapomorfías de las especies analizadas. Las implicancias taxonómicas de la clasificación propuesta y la posición de los géneros incertae sedis dentro de Characidae son discutidas. También se presenta una discusión de la información filogenética de los caracteres usados en la sistemática clásica de Characidae.
Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy
Juan Marcos Mirande
CONICET-Fundación Miguel Lillo, Miguel Lillo 251, 4000 San Miguel de Tucumán, Argentina. mcmirande@gmail.com
ABSTRACT
The family Characidae is the most diverse among Neotropical fishes. Systematics of this family are mainly based on pre-cladistic papers, and only recently a phylogenetic hypothesis for Characidae was proposed by the author. That phylogeny was based on 360 morphological characters studied for 160 species, including representatives of families related to Characidae. This paper is based on that phylogenetic analysis, with the analyzed characters described herein and documented, accompanied by comparisons of their definition and coding in previous papers. Synapomorphies of each node of the proposed phylogeny are listed, comparisons with previous classifications provided, and autapomorphies of the analyzed species listed. Taxonomic implications of the proposed classification and the position of the incertae sedis genera within Characidae are discussed. A discussion of the phylogenetic information of the characters used in the classical systematics of the Characidae is provided.
Key words: Ostariophysi, Osteology, Morphology, Classification, Systematics.
RESUMEN
La familia Characidae es la más diversa entre los peces neotropicales. La sistemática de esta familia está basada principalmente en trabajos pre-cladísticos, y sólo recientemente una hipótesis filogenética para Characidae fue propuesta por el autor. Esa filogenia estaba basada en 360 caracteres estudiados en 160 especies, incluyendo representantes de familias relacionadas a Characidae. Este trabajo está basado en ese análisis filogenético, y los caracteres analizados son aquí descriptos y documentados, comparando su definición y codificación con trabajos previos. Las sinapomorfías de cada nodo de la filogenia propuesta son listadas, y se incluyen comparaciones con clasificaciones previas; también se listan las autapomorfías de las especies analizadas. Las implicancias taxonómicas de la clasificación propuesta y la posición de los géneros incertae sedis dentro de Characidae son discutidas. También se presenta una discusión de la información filogenética de los caracteres usados en la sistemática clásica de Characidae.
Introduction
The order Characiformes includes more than 1,800 species, of which the family Characidae is the most diverse, with approximately 1,200 species (Reis et al., 2003); indeed, the Characidae is the fourth most diverse family of fishes, after the Cyprinidae, Cichlidae and Gobiidae (Eschmeyer & Fricke, 2009). Members of the Characidae occur from southern portions of the USA to northern Patagonia in Argentina, being especially diverse in the Amazon, Orinoco, and La Plata River basins.
According to the currently accepted phylogenetic hypotheses, based on both morphological and molecular data, the Cypriniformes constitute the sister group of (Characiformes (Siluriformes + Gymnotiformes)) (Fink & Fink, 1981, 1996; Dimmick & Larson, 1996). According to classifications prior to Mirande (2009), the Characiformes consisted of three African families (Citharinidae, Distichodontidae and Hepsetidae) (Géry, 1977, Calcagnotto et al., 2005), 14 Neotropical families (Acestrorhynchidae, Anostomidae, Characidae, Chilodontidae, Crenuchidae, Curimatidae, Ctenoluciidae, Cynodontidae, Erythrinidae, Gasteropelecidae, Hemiodontidae, Lebiasinidae, Parodontidae, Prochilodontidae, and Serrasalmidae) (Reis et al., 2003; Calcagnotto et al., 2005), and one trans-Atlantic family (Alestidae) (Zanata & Vari, 2005). The families Citharinidae and Distichodontidae constitute the suborder Citharinoidei, considered as the sister group of the Characoidei, which includes all the remaining Characiformes (Vari, 1979; Fink & Fink, 1981, 1996; Buckup, 1998; Calcagnotto et al., 2005). Among the Characoidei, the monophyly of a clade composed of the families Anostomidae, Chilodontidae, Curimatidae, and Prochilodontidae (Anostomoidea; Vari, 1983; Buckup, 1998), and a clade formed by the Neotropical families Ctenoluciidae, Erythrinidae and Lebiasinidae and the African family Hepsetidae (Erythrinoidea; Vari, 1995; Buckup, 1998) had been proposed. Relationships between these suprafamilial groups and the remaining Characiformes were unclear, and some hypotheses that conflicted in varying degrees were proposed (Uj, 1990; Ortí & Meyer, 1997; Buckup, 1998; Calcagnotto et al., 2005, Hubert et al., 2005).
Most families of the Characiformes have evidences of monophyly (Weitzman, 1954; Roberts, 1973, 1974; Vari, 1979, 1983, 1995; Buckup, 1998; Toledo-Piza, 2000; Zanata & Vari, 2005), whereas there are no consensus on the monophyly and composition of the Characidae. Most currently recognized subfamilial and generic groups in the Characidae are based on the pre-cladistic papers of Eigenmann (e. g. 1912, 1915, 1917, 1918, 1921, 1927) and Eigenmann & Myers (1929). Eigenmann (1917) was highly influential in terms of our present concepts of relationships within the Characidae. Eigenmann defined 17 characters with discrete alternative states, and used them in different combinations to diagnose the genera in the Characidae, considering the most frequent states as being primitive. The genus Astyanax Baird & Girard has the combination of the most frequent states of all these characters, and it was consequently considered by Eigenmann (1917) as primitive within the family. Eigenmann, however, recognized that the less frequent states could have independent origins in different species of the same genus, producing "polyphyletic" [sic] genera. Given the impossibility to classify the Characidae in a branching scheme, Eigenmann (1917) presented a radial pattern, identifying a "nucleus" of generalized morphology (represented by the genus Astyanax) and different lines of evolution diverging from it.
Eigenmann's classification was followed by Greenwood et al. (1966) and particularly by Géry (e. g. Géry, 1977). Géry also recognized the polyphyletic nature of this classification, and that this systematic scheme failed to reflect the phylogeny (Géry, 1972). In this pre-cladistic systematic classification, most genera of the Characidae, especially those with "generalized" morphology, were included in the subfamily Tetragonopterinae. The remaining genera were distributed across several subfamilies defined by the presence of somewhat arbitrarily chosen characters. Géry (1977), following the general classification of Eigenmann recognized the subfamilies (number of genera in parentheses) Agoniatinae (1), Rhaphiodontinae (2), Characinae (14), Bryconinae (6), Clupeacharacinae (1), Paragoniatinae (6), Aphyocharacinae (1), Glandulocaudinae (18), Stethaprioninae (3), Tetragonopterinae (49), Rhoadsiinae (2) and Cheirodontinae (13 genera sensu stricto and 36 sensu lato).
The first genus of the Characidae explicitly diagnosed by shared presumably apomorphic features was Bramocharax Gill (Rosen, 1972). Later, Vari (1977) presented evidence based on shared presumably derived features, supporting the monophyly of the subfamily Iguanodectinae. Weitzman & Fink (1983) explicitly explained the problems related with some generic characters used for the systematic schemes of Eigenmann and Géry, and the needing of a classification reflecting the phylogeny of the Characidae. A series of contributions proposing or corroborating the monophyly of some genera and subfamilies of the Characidae were published subsequently [Serrasalminae (Machado-Allison, 1983), Stethaprioninae (Reis, 1989), Glandulocaudinae (Weitzman & Fink, 1985; Weitzman & Menezes, 1998), Cheirodontinae (Malabarba, 1998a) and Paracheirodon Géry (Weitzman & Fink, 1983), Charax Scopoli (Lucena, 1987), Jupiaba Zanata (Zanata, 1997), Roestes Günther and Gilbertolus Eigenmann (Lucena & Menezes, 1998), Spintherobolus Eigenmann (Weitzman & Malabarba, 1999), Creagrutus Günther and Piabina Reinhardt (Vari & Harold, 1998, 2001), Deuterodon Eigenmann (Lucena & Lucena, 2002), Cyanocharax Malabarba & Weitzman (Malabarba & Weitzman, 2003), Attonitus Vari & Ortega (Vari & Ortega, 2000), and Bryconadenos Weitzman, Menezes, Evers & Burns (Weitzman et al., 2005)]. Most of these papers were focused on particular groups of the Characidae, without enough exploration of their relationships with the remaining Characidae. Malabarba (1998a) restricted the subfamily Cheirodontinae to a subset of the genera recognized in this subfamily by Géry (1977), leaving 33 genera as incertae sedis. Later, Reis (2003a) restricted the Tetragonopterinae to its type genus Tetragonopterus Cuvier, leaving many genera as incertae sedis within the Characidae. Lima et al. (2003) classified also several genera previously included in the subfamilies Bryconinae, Characinae, Cheirodontinae, and Paragoniatinae (Géry, 1977) as incertae sedis within the Characidae. The subfamilies and incertae sedis genera recognized in the last revision of the Characidae are as follows (number of genera in each group in parentheses): incertae sedis (88) (Lima et al., 2003), Agoniatinae (1) (Lima & Zanata, 2003), Clupeacharacinae (1) (Lima, 2003a), Iguanodectinae (2) (Moreira, 2003), Bryconinae (3) (Lima, 2003b), Serrasalminae (15) (Jégu, 2003), Aphyocharacinae (1) (Lima, 2003c), Characinae (12) (Lucena & Menezes, 2003), Stethaprioninae (4) (Reis, 2003a), Tetragonopterinae (1) (Reis, 2003b), Rhoadsiinae (3) (Cardoso, 2003a), Cheirodontinae (15) (Malabarba, 2003), and Glandulocaudinae (19) (Weitzman, 2003). The subfamily Rhaphiodontinae (sensu Géry, 1977) was included into the Cynodontidae (Lucena & Menezes, 1998; Toledo-Piza, 2003), and all the genera assigned to the Paragoniatinae by Géry (1977) were included in the incertae sedis-group (Lima et al., 2003). The Acestrorhynchidae was considered as a separate family (Lucena & Menezes, 1998, 2003).
Malabarba & Weitzman (2003) described Cyanocharax and proposed the monophyly of a group of genera (their clade A) including all members of Glandulocaudinae plus several incertae sedis genera. Later the glandulocaudin Lophiobrycon Castro, Ribeiro, Benine & Melo (Castro et al., 2003), and the incertae sedis genera Myxiops Zanata & Akama (Zanata & Akama, 2004), Nantis Mirande, Aguilera & Azpelicueta (Mirande et al., 2004, 2006a), Dectobrycon Zarske & Géry (Zarske & Géry, 2006), and Phallobrycon Menezes, Ferreira & Netto-Ferreira (Menezes et al., 2009) were described. The phylogeny of Calcagnotto et al. (2005) implicitly raised the Serrasalminae to the family level. Weitzman et al. (2005) described the incertae sedis genus Bryconadenos and restricted the Glandulocaudinae to Glandulocauda Eigenmann, Lophiobrycon and Mimagoniates Regan, shifting the remaining genera previously in the Glandulocaudinae to the subfamily Stevardiinae. Quevedo (2006) phylogenetically diagnosed the subfamily Paragoniatinae with a composition very similar to that proposed by Géry (1977) (the results of this and other recently completed theses are not discussed, pending their eventual publications).
There is no consensus about the phylogenetic relationships among subfamilies of the Characidae. Some phylogenetic analyses of different scope were performed, both from morphological and molecular data. The phylogenies of Uj (1990), Buckup (1991, 1998) and Lucena (1993) were based on morphological data. Uj (1990) did not perform a cladistic analysis; he just mapped character transformations on a "phylogenetic" tree obtained without specific criteria. This unpublished thesis, however, was an advance on compared morphological knowledge of the Characidae. The doctoral theses of Buckup (1991) and Lucena (1993) were the first cladistic analyses of the Characidae, with the main phylogenetic results of Buckup published later (Buckup, 1998). These analyses shared a high proportion of characters, but they had different objectives and, consequently, different taxon sampling. Most of the characters in those theses were analyzed by Mirande (2008, 2009) and discussed in the present paper. As the aim of Buckup (1991, 1998) was to obtain a hypothesis of relationships of the members of the Crenuchidae, he included only six genera of the Characidae. Given that the main objective of Lucena (1993) was to recover the phylogenetic relationships of the Characidae, the taxon sampling reflected the morphological diversity of the family, in a scope more similar to that of Mirande (2008, 2009). Most of the conclusions of Buckup (1991, 1998) and Lucena (1993) are included on the last classification of the Neotropical members of the Characiformes (Reis et al., 2003). Subsequently, several unpublished theses focused on phylogenies of specific groups within the Characidae (Moreira, 2002; Bertaco, 2003; Cardoso, 2003b; Serra, 2003; Benine, 2004; Bührnheim, 2006; Lima, 2006; Quevedo, 2006; Bertaco, 2008). Some of the characters used on these theses were also analyzed by Mirande (2008, 2009).
Molecular phylogenies of the Characiformes were proposed by Ortí & Meyer (1997), Hubert et al. (2005), and Calcagnotto et al. (2005). As in the cited morphological studies, the objectives of these analyses differed, and this affected the taxon and gene samplings and the methodologies used. Calcagnotto et al. (2005) published the most comprehensive molecular phylogeny of the Characiformes, including 27 taxa of the Characidae.
Mirande (2009) proposed the monophyly and a classification of the Characidae based on a phylogenetic analysis. Most incertae sedis genera were assigned to a subfamily or subfamilial-level clade, at least tentatively. The paper of Mirande (2009) was, however, mainly concerned with analytical issues, leaving most morphological descriptions and discussions, and comments on the taxonomic implications or the phylogeny for the present contribution. In the present study some characters were redefined or added from Mirande (2009) and the results herein obtained slightly differ to those of that paper.
Material and Methods
Osteological preparations
Osteological preparations were made following Taylor & van Dyke (1985) on one to five specimens of each species included in the analysis, according to their availability and observed intraspecific variability. Some characters involving musculature and soft tissues were observed with the aid of non-permanent Methylene Blue staining. A total of 23 species of 14 characiform families and one cypriniform form the outgroup, while 137 species of the Characidae form the ingroup of this study. Figures 1-124 illustrate most characters and character-states. Most figures are stacks of pictures at different focal depth, constructed with CombineZM software (Hadley, 2006), running under Linux through Wine software.
Taxon sampling
The taxonomic nomenclature of the Characoidea used in the present paper follows Mirande (2009), while that of remaining Characiformes follows Buckup (1998). Terminal taxa were included in the data matrix at species-level. The only exceptions are the root, and the superfamily Citharinoidei, which actually are compound taxa based primarily on Puntius tetrazona (Bleeker) and Distichodus maculatus Boulenger but allowing for documented variations within the Cyprinidae and Citharinoidei, respectively. Taxon sampling was done considering the inclusion of members of recognizedly monophyletic groups, representatives of the morphological variation within the family, members of the incertae sedis genera, species with special taxonomic interest (e. g. type species of the most diverse genera), and an outgroup including members of most families in the Characiformes. The taxon sampling focused in the inclusion of as many species as possible, with studies of intraspecific variations beyond the scope of this paper.
The analyses are rooted on the compound terminal taxon based on Puntius tetrazona (Cypriniformes, Cyprinidae). Cases in which the states observed in this species differed from those considered as plesiomorphic for Cypriniformes (Howes, 1978, 1979, 1980; Vari, 1979; Fink & Fink, 1981, 1996) were coded as polymorphic. Although there is enough consensus on the position of the characiform families Citharinidae and Distichodontidae (Citharinoidei) as the sister group of the remaining Characiformes (Characoidei) (Vari, 1979; Fink & Fink, 1981, 1996, Buckup, 1991, 1998; Calcagnotto et al., 2005), a root external to the Characiformes was used to test also such hypothesis.
A rather broad sampling of families related to the Characidae was carried out, to correctly optimize the characters and to test as rigorously as possible the monophyly of the Characidae. This test was improved analyzing some members of the families morphologically closer to Characidae, or included historically in this family, such as the Alestidae, Gasteropelecidae, and Serrasalmidae (Weitzman, 1954; Géry, 1977; Machado-Allison, 1983).
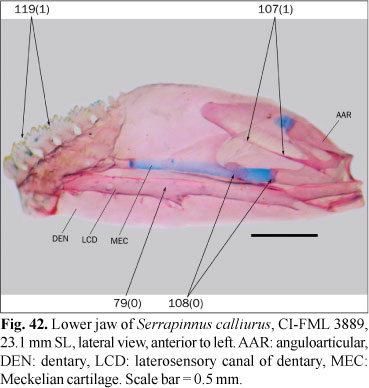
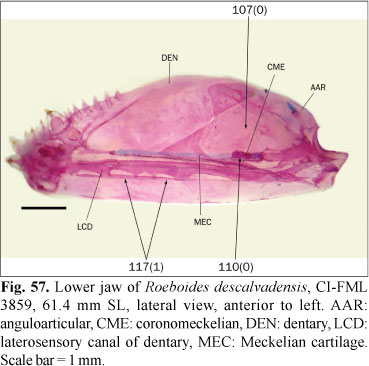
The ingroup is composed of members of all the subfamilies recognized in Reis et al. (2003) excepting the monotypic Clupeacharacinae; however, most of the effort was oriented towards sampling the incertae sedis genera which represented approximately two-thirds of the diversity of the Characidae, prior to Mirande (2009). The taxon termed "undescribed n. gen. and sp." by Mirande (2009) proved to be an undescribed species of Oligosarcus Günther. Thus, in this paper this species is named as Oligosarcus sp. leaving its description and discussion of relationships to be published elsewhere. The species named as Bryconamericus beta Eigenmann by Mirande (2009) is referred to as B. alpha Eigenmann in this paper, following the synonymy proposed by Román-Valencia (2003). The species named as Roeboides bonariensis (Steindachner) and R. paranensis by Mirande (2009) are referred to as R. microlepis (Reinhardt) and R. descalvadensis Fowler, following the synonymies proposed by Lucena (2003, 2007). Finally, the specimens referred to as Hemigrammus cf. rhodostomus Ahl by Mirande (2009) proved to be H. bleheri Géry & Mahnert. The list of examined material is shown in the Appendix 1 Appendix 1. List of examined material. Only C&S and alcohol specimens are listed. .
All species (with the exception of Brycon meeki Eigenmann & Hildebrand that was coded following Weitzman, 1962) were observed by the author. The coding of each species was made upon all its available information. If a particular state was observed in a species, but published data indicate the alternative condition in that species, such species were coded as polymorphic. Meristic characters (e. g. anal-fin rays counts) were coded according to the ranges cited in the literature.
Nomenclature and Abbreviations
Abbreviations mentioned on the list of examined material are as following: AI (Asociación Ictiológica, La Plata), ANSP (Academy of Natural Sciences of Philadelphia), CI-FML (Colección Ictiológica de la Fundación Miguel Lillo, Tucumán), LACMNH (Los Angeles County Museum of Natural History), MCNi (Colección ictiológica del Museo de Ciencias Naturales, Salta), MCP (Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre), MHNG (Muséum d'histoire naturelle, Genève), MNHN (Muséum national d'histoire naturelle, Paris), and MZUSP (Museu de Zoologia da Universidade de São Paulo).
Osteological nomenclature follows Weitzman (1962) with the modifications adopted by Zanata & Vari (2005), which are based principally on Nelson (1969), Patterson (1975), and Fink & Fink (1981, 1996). Abbreviations in character definitions are references to the following papers: EI (Eigenmann, 1917), FF (Fink & Fink, 1981, 1996), AM (Machado-Allison, 1983), UJ (Uj, 1990), VA (Vari, 1995), BU (Buckup, 1998), LU (Lucena, 1993), LC (Lucena, 1998), MA (Malabarba, 1998a), WM (Weitzman & Menezes, 1998), LM (Lucena & Menezes, 1998), CM (Malabarba, 1998b), TP (Toledo-Piza, 2000), VH (Vari & Harold, 2001), MO (Moreira, 2002), VB (Bertaco, 2003), CA (Cardoso, 2003b), SE (Serra, 2003), BE (Benine, 2004), ZV (Zanata & Vari, 2005), BÜ (Bührnheim, 2006), LI (Lima, 2006), QU (Quevedo, 2006), PZ (Toledo-Piza, 2007), MW(Menezes & Weitzman, 2009). The number following these abbreviations refers to the character number as used on the cited analysis; those cases in which the character states were modified from the cited paper are indicated with a "m", and those instances where the ordering of states were inverted are indicated with an "i". Some numbers of the list of characters of Malabarba (1998b) do not correspond with those of the data matrix; instead their correspondence was deduced from the information given in the text. In these cases the number in parentheses corresponds to the one deduced to have each particular character in the data matrix.
The principal objective of the proposed taxonomic nomenclature is to classify members of the Characidae in monophyletic units. The proposed nomenclature is as conservative as possible concerning to the creation of new names for taxonomic groupings, with all the names used in the recent literature which are compatible with the obtained phylogeny retained other than in cases when their preservation necessitates the creation of a number of new taxa. The new suprageneric names are rooted on the first described genus included within the clade. An evaluation of the monophyly and phylogeny of all genera is beyond the scope of this paper; therefore, new generic names are not proposed nor are species reassigned between genera.
Cladistic methodology
Additive characters were recoded as binaries and are represented by two or more character numbers; this improve greatly the efficiency of searches under self-weighting optimization (Goloboff, 1997) in terms of time and optimality. Binary coding of the additive characters has no effects on the results obtained under implied weighting and relatively small influence to the results under self-weighted optimization (see Mirande, 2009 for details). Conditions that resulted as intermediate between the defined states were coded as polymorphisms; although both situations are conceptually different, it was preferred over coding them as inapplicable or missing entries. Analyses were performed by parsimony, following the methods described by Hennig (1966) and developed by Farris (e. g. 1969, 1970, 1983) among others. Analyses under implied weighting (Goloboff, 1993) and self-weighting optimization (Goloboff, 1997) were performed with TNT software (Goloboff et al., 2003a, 2008). Details of this analysis were described elsewhere (Mirande, 2008, 2009), and they are not treated here. In this analysis the number of explored conditions were almost duplicated from Mirande (2009). In the present study, 21 values of k were used under each of the weighting schemes (vs. 11 in Mirande, 2009). Measures of stability and support are expressed in the discussion of each node. Stability measures consider all the range of explored parameters (see Mirande, 2009), while support measures were calculated for k = 13, under implied weighting. Those measures are, respectively, GC values as stability measures, relative frequencies, GC values as support measures (Goloboff et al., 2003b), and relative Bremer support (Bremer, 1994; Goloboff & Farris, 2001). Cases in which the support measures are (artificially) negative are indicated with a dash (-), whereas stability measures are indicated as negative.
Results and Discussion
Description of phylogenetic characters
Most analyzed characters are osteological (90%), while the remaining ones come from coloration, external features and reproductive biology. Of these, 135 were not described previously in the literature (published or not), and represent new definitions. Some characters about bony hooks on fins of adult males were redefined from Mirande (2009). The characters proposed by Menezes & Weitzman (2009) to be evidence for the monophyly of their Glandulocaudinae and Stevardiinae are herein analyzed together with the characters from Mirande (2009). Also, several missing entries in the analysis of Mirande (2009) were coded for this study. With that modifications, the data matrix herein analyzed has 365 characters and is provided as Appendix 2 Appendix 2 .
Neurocranium
Epiphyseal bar:
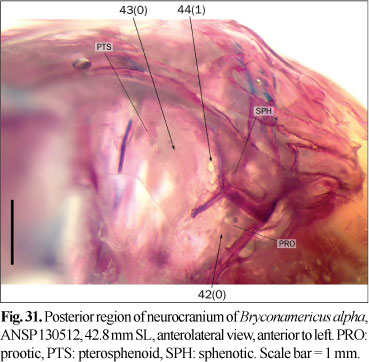
1. Posterior laminar expansion of epiphyseal bar: (0) absent; (1) present. (LU13i, LC4).
The frontals articulate each other via the epiphyseal bar, which transversely crosses the medial cranial fontanel. In most examined species, the epiphyseal bar is slender and approximately cylindrical in cross-section (state 0; Fig. 1), while a group of species has a laminar projection on the posterior margin of the epiphyseal bar, making it proportionally broader in dorsal view (state 1; Fig. 2). Lucena (1993, 1998) coded the presence of this expansion in several characins and Rhaphiodon vulpinus Agassiz. A small laminar expansion was also observed in examined specimens of Charax stenopterus (Cope) and Galeocharax humeralis (Valenciennes). Since Lucena (1998) noted the absence of such an expansion in these species, they are coded as polymorphic. Serrasalmus maculatus Kner has a broad epiphyseal bar that apparently lacks the laminar expansion. This condition is considered to be different from the states herein defined for this character, and this character is coded as inapplicable to S. maculatus. This character is also considered as inapplicable to the species in which the fontanel is completely covered by the frontals.
Basioccipital:
2. Ventral longitudinal lamellae of basioccipital: (0) falling short of posterior border of basioccipital; (1) reaching posterior border of cranium. (PZ24).
The prootic and basioccipital have two bilateral lamellae articulating with two longitudinal dorsal processes of the parasphenoid, forming the limits of the posterior myodome where part of the extrinsic musculature of the eye attaches, as described by Weitzman (1962: 24). In most examined members of the Characidae, these lamellae are restricted to the area of contact between the basioccipital and parasphenoid, and the surface of the basioccipital lacks any bilateral lamellae or ridges posterior to that region (state 0; Fig. 3). In most members of the outgroup and some of the Characidae these lamellae extend posteriorly to the parasphenoid in the region ventral to the lagenar capsules and reach the posterior margin of the cranium (state 1; Fig. 4).
Lagenar capsule:
3. Ventral projection of lagenar capsule: (0) not extending ventrally to horizontal through articulation between basioccipital and parasphenoid; (1) extending ventrally to articulation between basioccipital and parasphenoid. (UJ5m, ZV47m, PZ25m).
The extension of the lagenar capsules lateral to the cranial condyle is a synapomorphy of the Characiformes according to Fink & Fink (1981) and was observed in all the characiforms herein examined. The ventral extension of these capsules, in contrast, is variable among the examined species. In most species of the outgroup, the lagenar capsules do not extend ventrally to the articulation between the basioccipital and parasphenoid (state 0; Fig. 5), while in most species of the Characidae and some members of the outgroup these capsules are conspicuously extended, continuing ventrally beyond the area of articulation of those bones (state 1; Figs. 6 and 7).
4. Epioccipital bridge over posttemporal fossa: (0) absent; (1) present. (BÜ7i).
The posttemporal fossa in Characiformes is longitudinally crossed by the epioccipital bridge (state 1; Fig. 6) except in some miniature species of the Characidae and Crenuchidae (state 0; Weitzman & Fink, 1983: figs. 6, 8, 15, and 17). Although this character was considered to be related with miniaturization, its phylogenetic value has to be tested. The absence of an epioccipital bridge was herein observed only in Hasemania nana (Lütken) and Pyrrhulina australis Eigenmann & Kennedy. This character, however, is variable in the two examined specimens of the latter species, which is coded as polymorphic.
5. Form of epioccipital bridge: (0) cylindrical or vertically expanded in transverse section; (1) depressed in its middle region.
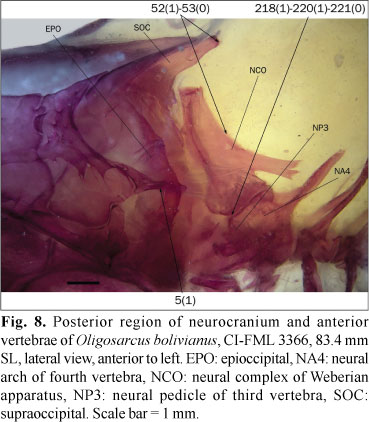
The epioccipital bridge over the posttemporal fossa is usually cylindrical or slightly expanded dorsally (state 0; Fig. 6). In the studied specimens of Bramocharax bransfordii Gill, Oligosarcus bolivianus (Fowler), O. cf. jenynsii (Günther), and O. sp., the middle region of this bridge is dorsoventrally depressed (state 1; Fig. 8).
6. Anterior articulation of epioccipital bridge: (0) with both parietal and pterotic; (1) only with parietal.
In most examined species the anterior region of the epioccipital bridge articulates with the parietal and pterotic (state 0; Fig. 6). In Grundulus cochae (Humboldt) and Paracheirodon axelrodi (Schultz), the epioccipital bridge is displaced dorsally and its anterior portion articulates only with the parietal (state 1; Weitzman & Fink, 1983: figs. 4, 5, and 7).
7. Posteriorly-oriented epioccipital spine: (0) present; (1) absent. (LU21m, LC3i).
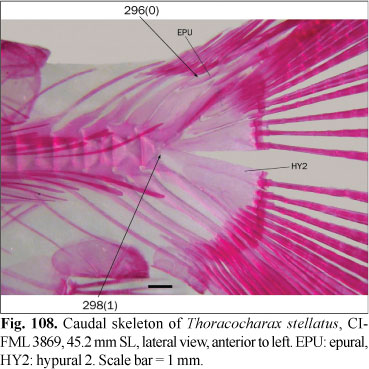
Most examined species lack projections on the posterior surface of the epioccipital (state 1; Fig. 5). A posterior projection of the epioccipital aligned with the epioccipital bridge that serves as a point of attachment of the epaxial musculature was observed in some species (state 0; Fig. 7). In Brycon orbignyanus (Valenciennes) and Salminus brasiliensis (Cuvier), the posterior tip of this process is rounded, differing from most species with state 0, in which it is pointed; these species are coded with state 0 regardless this difference. Weitzman (1962: fig. 3) illustrated a small lobe in Brycon meeki, similar to that herein observed in B. falcatus Müller & Troschel, B. pesu Müller & Troschel, Bryconexodon juruenae Géry, Cynopotamus argenteus (Valenciennes), Hemibrycon dariensis Meek & Hildebrand, Markiana nigripinnis (Perugia) and Moenkhausia xinguensis (Steindachner). These species are coded as polymorphic. Contrary to the observations of Lucena (1993), in the examined species of Aphyocharax Günther this process is absent, and they are coded as state 1. Puntius tetrazona, as in all other Cypriniformes, lacks a posttemporal fossa, and the form of the epioccipital differs slightly. This character was coded as inapplicable to the root of this analysis. In Carnegiella strigata (Günther) and Thoracocharax stellatus (Kner), the epineurals extend anteriorly to the cranium, reaching a position occupied by this spine when present. Indeed, the anteriormost epineurals are fused with the epioccipital. This character is also coded as inapplicable to these species.
8. Ventromedial opening of posttemporal fossa: (0) absent; (1) present. (UJ24, BU19, LU20, VH45m, TP23, BE17, ZV43m, LI35).
Most examined species have only two openings of the posttemporal fossa situated posterolateral in the cranium, with these separated by the epioccipital bridge (state 0). A third opening was described in the Citharinidae and Distichodontidae by Vari (1979). This opening was later referred as ventromedial opening of the posttemporal fossa by Buckup (1991, 1998) and Lucena (1993). This opening is situated posteriorly on the cranium, and is margined by the epioccipital and exoccipital or completely contained by the epioccipital (state 1; Zanata & Vari, 2005: fig. 10). The ventromedial opening of the posttemporal fossa was observed by Buckup (1998) and Lucena (1993) in members of the Alestidae, Crenuchidae, Curimatidae, Cynodontidae, Hemiodontidae, and Parodontidae. Vari & Harold (2001) and Zanata & Vari (2005) defined the different positions of this opening as two separate states, which is treated in the following character. Benine (2004) cited the presence of this opening in Moenkhausia barbouri Eigenmann, M. dichroura (Kner), and M. intermedia Eigenmann; however, this opening is absent in the examined species of Moenkhausia Eigenmann, and they are herein coded as state 0. According to Lucena (1993) this opening is present in Acestrorhynchus pantaneiro Menezes, although in the examined specimen it is absent, and the species is consequently coded as polymorphic.
9. Position of ventromedial opening of posttemporal fossa: (0) between epioccipital and exoccipital; (1) bordered entirely by epioccipital. (ZV43m, VH45m).
As previously mentioned, the ventrolateral opening of the posttemporal fossa is limited by the epioccipital and exoccipital in some species (state 0; Vari, 1979: fig. 15) while it is completely contained within the epioccipital in others (state 1; Roberts, 1974: figs. 5 and 59; Zanata & Vari, 2005: fig. 10). This opening is completely enclosed by the epioccipital in some species of Creagrutus (Vari & Harold, 2001) and most members of the Alestidae (except Chalceus Cuvier, among taxa examined here), Curimatidae, Hemiodontidae, and Parodontidae (Roberts, 1974; Zanata & Vari, 2005). A third opening partially margined by the exoccipital was cited for members of the Citharinidae, Crenuchidae, Cynodontidae, and Distichodontidae (Vari, 1979; Zanata & Vari, 2005). Species in which this opening is absent are coded as inapplicable to this character.
Sphenotic:
10. Length of sphenotic spine: (0) not extending ventrally to articulation between sphenotic and hyomandibula; (1) extending ventrally to articulation between sphenotic and hyomandibula. (VB28m, VB29m).
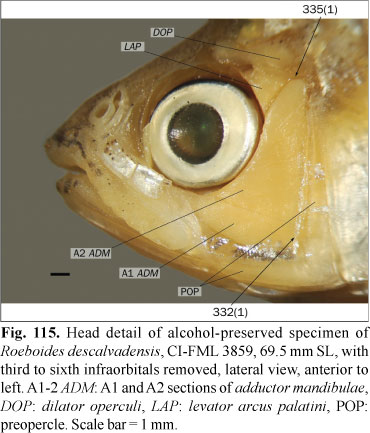
The sphenotic spine extends ventrally to the fossa for the dilator operculi, being bordered posteriorly by that muscle and partially by the levator arcus palatini. The ventral development of this spine is variable among the examined species. In some species it falls short of the ventral margin of main body of the sphenotic (state 0), whereas in other species this spine is longer, anteriorly bordering the levator arcus palatini and ventrally extending past the region of articulation of the sphenotic with the hyomandibula (state 1; Fig. 9). This character was only coded with states 0 or 1 in the species in which the sphenotic spine is clearly either not reaching or surpassing the articulation with the hyomandibula, respectively. In the examined specimens of Distichodus maculatus, Acestrorhynchus pantaneiro, Carlana eigenmanni (Meek), Mimagoniates rheocharis Menezes & Weitzman, Bryconamericus alpha, Cyanocharax alburnus (Hensel), Hemibrycon dariensis, Knodus breviceps Eigenmann, Odontostoechus lethostigmus Gomes, Aphyocharacidium bolivianum Géry, Aulixidens eugeniae Böhlke, Axelrodia lindeae Géry, Exodon paradoxus Müller & Troschel, Hemigrammus bleheri, Hollandichthys multifasciatus Eigenmann & Norris, Nematobrycon palmeri Eigenmann, Oligosarcus bolivianus, Parecbasis cyclolepis Eigenmann, Probolodus heterostomus Eigenmann, and Thayeria obliqua Eigenmann, the sphenotic spine is hardly reaching the articulation between this bone with the hyomandibula, and they coded as polymorphic. In Puntius tetrazona this spine reaches the ventral limit of the sphenotic, but it is variable in the Cypriniformes (Howes, 1978) and consequently the root of this analysis is also coded as polymorphic. In Astyanax lineatus (Perugia) this character is apparently variable during the growth. Examined juvenile specimens have state 1, while the adults have state 0, and this species is coded as polymorphic.
11. Position of sphenotic spine relative to hyomandibula: (0) rather aligned with anterior margin of hyomandibula; (1) displaced anteriorly relative to anterior margin of hyomandibula.
In most examined species, the sphenotic spine is aligned or slightly anterior to the anterior margin of the hyomandibula (state 0; Fig. 9), while in a group of species, such spine is anterior to the margin of the hyomandibula (state 1; Fig. 10). Bryconexodon juruenae and Hollandichthys multifasciatus have intermediate states that are coded as polymorphisms.
12. Position of sphenotic spine relative to the orbit: (0) bordering orbit posteriorly and aligned with anterior border of fourth and fifth infraorbitals; (1) distinctly posterior to orbital margin.
As stated in the previous character, the sphenotic spine is usually aligned with the anterior margin of the hyomandibula, thereby forming the posterior margin of the orbit (state 0). In a group of species the sphenotic spine is situated posterior to the anterior margin of the fourth and fifth infraorbitals and distant from the posterior margin of the orbit (state 1).
13. Temporal fossa: (0) well developed; (1) absent or much reduced. (VH41, LI27).
In most examined species the entire anterior margin of the pterotic articulates with the sphenotic, without an intervening space between these bones, or only a small pore (state 1; Figs. 6 and 11). The temporal fossa is an opening limited anteriorly by the sphenotic and posteriorly by the pterotic, and is present in some examined species (state 0; Weitzman, 1962: fig. 3). Vari & Harold (2001) reported the presence of this fossa in Piabina argentea Reinhardt and several species of Creagrutus not analyzed herein. Although Lima (2006) mentioned its absence in Brycon falcatus, among other species of the genus, this fossa is present in the examined specimen of this species, and is coded as polymorphic. In the examined specimens of Astyanax troya Azpelicueta, Casciotta & Almirón and Piabina argentea, this fossa has a size intermediate to the defined character states, and these species are coded as polymorphic for this character. In the examined specimens of Markiana nigripinnis the presence of this fossa is variable and this species is also coded as polymorphic.
Lateral ethmoid:
14. Form of anterior process of lateral ethmoid: (0) broad in ventral view, contacting proximal region of vomer in its entire length; (1) slender and separated from vomer. (CM10m).
In most examined species the lateral ethmoid has an anterior process oriented in the direction of the vomer. In many species of the outgroup and some examined members of the Characidae, this process is broad in ventral view and contacts the entire length of the parasphenoid and vomer in the region anterior to the main body of the lateral ethmoid (state 0). In most members of the ingroup this process is, in contrast, comparatively more slender and, as a consequence, leaves a broad space between the lateral ethmoid process and the lateral margin of the posterior portion of the vomer (state 1; Fig. 12). In Gymnocharacinus bergii Steindachner the process is much reduced, and this character is coded as inapplicable. In Aulixidens eugeniae and Engraulisoma taeniatum Castro this process is displaced medially, and it contacts the parasphenoid and vomer in its entire length. Although the origin of this condition seems to be different, resulting in a contact due to a different mechanism (medial displacement, rather than broadening of the process), these species are tentatively coded with state 0.
15. Lateral opening between ventral diverging lamellae of mesethmoid and anterior process of lateral ethmoid: (0) broad; (1) small, ovate and partially occluded by diverging lamellae of mesethmoid and anterior process of lateral ethmoid. (LU2).
The anterior process of the lateral ethmoid is situated approximately in the same plane as the corresponding diverging lamella of the mesethmoid (Weitzman, 1962) leaving, in most cases, a broad space between these structures, which is evident in lateral view (state 0; Fig. 13). In a few examined species, the diverging lamellae are much developed ventrally and both the vomer and the anterior process of the lateral ethmoid are expanded dorsally, with both articulating broadly with the mesethmoid. As a result the space delimited by these structures has an ovate shape and is much reduced compared with state 0 (state 1; Fig. 14). This character is coded as inapplicable in species lacking ventral lamellae of the mesethmoid.
16. Dorsal margin of lateral ethmoids: (0) aligned; (1) situated obliquely in dorsal view, converging in an anteriorly directed angle.
The dorsal margin of lateral ethmoids articulate with the frontals and, usually, with the ventral diverging lamellae of the mesethmoid. The medial region of the lateral ethmoids articulate with the roof of the mesethmoid through a cartilage. In most examined species the medial portion of the lateral ethmoids form a rather straight line from dorsal view, with its margin visible through the frontals and/or mesethmoid (state 0; Figs. 15 and 16). In a relatively small group of species the medial portions of the lateral ethmoids meet each other along an anterior angle (state 1; Figs. 17 and 18). Apparently such configuration of the lateral ethmoids allows an anterior displacement of the extrinsic musculature of the eye, which inserts in the anterior myodome. Engraulisoma taeniatum and Prodontocharax melanotus Pearson have intermediate situations that are coded as polymorphic for this character. In Inpaichthys kerri Géry & Junk and Mimagoniates rheocharis the medial portion of the lateral ethmoid is much reduced and this character is coded as inapplicable.
17. Articulation between medial region of lateral ethmoid and frontal or mesethmoid: (0) absent, lateral ethmoid articulated principally with ventral diverging lamellae of mesethmoid; (1) extensive articulation of entire lateral ethmoid dorsal margin.
The dorsal margin of the lateral ethmoid is synchondrally articulated with the ventral surface of the the lateral portion of the frontal, contacting also the orbital lamella of the frontal and the ventral diverging lamellae of the mesethmoid, when present. In most examined species the region of the lateral ethmoid situated just medial to the orbital lamella of the frontal does not articulates with the mesethmoid or the frontal (state 0; Fig. 19). In these species the variably broad space between the lateral ethmoid and the ventral surface of the frontal and mesethmoid is occupied by the ethmoid cartilage (Weitzman, 1962). Instead, in some species the entire dorsal margin of the lateral ethmoid articulates synchondrally with the frontal and/or mesethmoid, depending on the posterior extent of the mesethmoid under the frontal (state 1; Fig. 18). The species with state 1 have reduced ventral diverging lamellae of the mesethmoid; however, there are species in which these lamellae are much reduced, as some Cheirodontinae, with state 0 of this character.
Exoccipital:
18. Subtemporal fossa: (0) medially extended to middle exoccipital; (1) restricted to pterotic and prootic.
The subtemporal fossa is formed by an usually shallow depression of the pterotic and is limited posteromedially by the intercalar. In most examined species the intercalar is restricted to the region of articulation of the exoccipital and pterotic, and the subtemporal fossa is consequently excluded from the exoccipital (state 1; Fig. 3). In some species the intercalar is more medially situated and articulates principally with the exoccipital. In these cases the subtemporal fossa is partially formed by the exoccipital (state 0; Fig. 20). According to Miquelarena & Arámburu (1983) the intercalar articulates solely with the exoccipital in Gymnocharacinus bergii, corresponding with state 0; however, the position of this bone varies among the examined specimens, and this species is coded as polymorphic. In the examined specimens of Hasemania nana the intercalar is situated entirely on the pterotic; although this condition is not exactly that described in the state 1, this species is coded with such state given that the subtemporal fossa is also excluded from the exoccipital.
19. Ascending process on posterodorsal angle of exoccipital directed to neural complex of Weberian apparatus: (0) absent; (1) present.
The posterior margin of the exoccipital in almost all the examined species is smoothly rounded in the area situated just anterior to the neural complex of Weberian apparatus (state 0). The presence of a process on the exoccipital oriented to the neural complex (state 1; Uj, 1990: fig. 20d) was considered by Uj (1990) as a synapomorphy of his Aphyocharacidae (Aphyocharax + Prionobrama Fowler). This state was observed only in Aphyocharax anisitsi Eigenmann & Kennedy and this character is uninformative, although possibly a synapomorphy of some group within Aphyocharax.
Frontal:
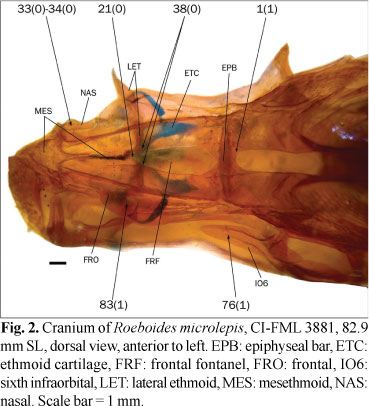
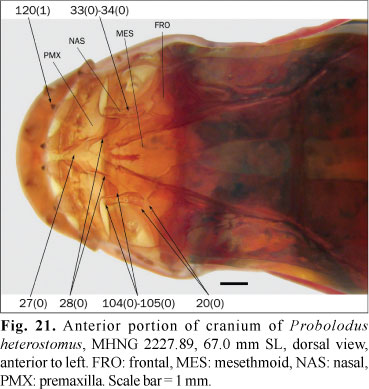
20. Anterior extension of frontal: (0) reaching posterior margin of nasal opening; (1) extending between nasals and reaching middle length of nasal opening.
The frontal reaches only to the posterior margin of the nasal opening in most examined species (state 0; Fig. 21). In the examined species of the Parodontidae and Leporinus striatus Kner, the frontals instead extend anteriorly between the nasals (state 1). In Puntius tetrazona the frontals are also anteriorly extended but this condition is variable within the Cypriniformes (Howes, 1978) and it is absent in basal Siluriformes, such as Diplomystes Bleeker. The root of this analysis is thus coded as polymorphic.
21. Contact between frontals anteriorly to frontal fontanel: (0) absent; (1) present. (VH37, MO35, SE2, BE9, ZV36m).
22. Frontal fontanel: (0) present; (1) totally occluded by frontals. (BU9, LU7, CM1(2), SE1i, ZV36m; PZ15).
In most examined species the frontals do not contact each other anterior to the epiphyseal bar and the anterior margin of the frontal fontanel is formed by the posterior margin of the mesethmoid (character 21, state 0; Fig. 2). In a number of species the frontals are rather in contact anterior to the frontal fontanel (character 21, state 1; character 22, state 0; Figs. 18 and 22). Both Puntius tetrazona and some morphologically generalized members of the Cypriniformes (Barilius Hamilton, Opsariichthys Bleeker, and Zacco Jordan & Evermann) completely lack a frontal fontanel (Howes, 1980: 155), while Diplomystes, which is considered a basal siluriform has a well developed fontanel (Arratia, 1987; Azpelicueta, 1994). The presence of a cranial fontanel is broadly distributed in Gymnotiformes except in the clade formed by Electrophorus Gill and Gymnotus Linnaeus, and it is optimized as present for the root of this order (Albert & Campos da Paz, 1998). Given the presence of a fully developed frontal fontanel in both the Gymnotiformes and Siluriformes, these characters are coded as polymorphic for the root of the present analysis, despite the absence of this fontanel in the Cypriniformes. The presence of a frontal fontanel is variable within the Characiformes. It is absent (character 22, state 1; Fig. 23) in the Erythrinidae, Gasteropelecidae, Hepsetidae, Lebiasinidae, and Parodontidae, and usually present in other families, such as the Alestidae and Characidae. This fontanel is absent in the examined specimen of Bryconaethiops macrops Boulenger, although it is present in this species according to Zanata & Vari (2005). These authors also described the ontogenetic occlusion of the frontal fontanel in Salminus brasiliensis and this character is coded as polymorphic for both species. The presence of contact between the frontals anterior to the frontal fontanel is variable or has some intermediate condition in examined specimens of Acestrocephalus sardina (Fowler), Astyanax mexicanus (De Filippi), Attonitus ephimeros Vari & Ortega, Bryconamericus alpha, Carlana eigenmanni, Deuterodon iguape Eigenmann, Distichodus maculatus, Hoplocharax goethei Géry, Odontostoechus lethostigmus, Phenagoniates macrolepis (Meek & Hildebrand), Pseudochalceus kyburzi Schultz and Rhoadsia altipinna Fowler, and it is coded as polymorphic in these species. According to Lima (2006) the frontals do not contact each other in Acestrorhynchus pantaneiro; however, in the examined specimen of this species these bones are in contact and this character is coded as polymorphic for this species. The frontal fontanel is limited anteriorly by the mesethmoid in the examined specimens of Engraulisoma taeniatum, Piabucus melanostomus Holmberg, and Piaractus mesopotamicus (Holmberg), contrary to the observations of Castro (1984), Moreira (2002), and Machado-Allison (1986), respectively; this character is coded as polymorphic also for these species.
23. Relative size of frontal and parietal fontanels: (0) length of frontal fontanel up to 2/3 length of parietal fontanel; (1) length of frontal fontanel 3/4 or more of length of parietal fontanel.
The cranial fontanel is divided by the epiphyseal bar to a frontal and a parietal fontanels. In most examined species, the frontal fontanel is conspicuously shorter than the parietal fontanel, reaching up 2/3 of the length of the latter opening (state 0). In other species the frontal fontanel is relatively longer, achieving 3/4 or more the length of the frontal fontanel (state 1). Intermediate or polymorphic conditions were observed in Metynnis maculatus (Kner), Aphyocharax nattereri (Steindachner), Acrobrycon tarijae Fowler, Bryconamericus exodon Eigenmann, B. rubropictus (Berg), B. thomasi Fowler, Hemigrammus unilineatus (Gill), Oligosarcus bolivianus, O. cf. jenynsii, Pristella maxillaris (Ulrey) and Thayeria boehlkei Weitzman, which are coded as polymorphisms. This character is considered as inapplicable to species in which the frontal fontanel is limited anteriorly by the frontals, because in these cases the shortening of the frontal fontanel is the consequence of a different arrangement than the one considered in the state 0.
24. Dilator fossa on lateral surface of frontal: (0) absent; (1) present. (BU13m, BU78m, LU11m).
In most examined species, the lateral margins of the frontal and the sphenotic spine form a depression where the anterior end of the dilator operculi muscle inserts, the dilator fossa (state 1; Fig. 24, Buckup, 1998: fig. 2). In some species the frontal projects laterally just dorsal to the dilator operculi, with that muscle inserting onto the ventral, rather than lateral surface of the neurocranium (state 0; Buckup, 1998: fig. 1). In Odontostilbe pequira (Steindachner) and the examined species of Aphyocharax and Moenkhausia, the dilator fossa is relatively small, but it is always present and these species are coded as state 1. This character is considered inapplicable to the species of Characidium Reinhardt, where the dilator operculi inserts posterior to the vertical through the orbit, in a situation not assignable to the states herein defined for this character. Buckup (1998) coded Characidium with a missing entry for this character for the same reason.
Mesethmoid:
25. Anterior end of mesethmoid: (0) trifurcate, with processes inserted into depressions on premaxillae; (1) not trifurcate, with a triangular anterior spine and articular processes reduced or absent. (UJ22m, UJ23, UJ38, BU1, LU1).
In some members of the outgroup the anterior portion of the mesethmoid has an anterior process of variable size and a pair of anterolateral processes inserting into small fossae on the premaxillae. These processes give to the anterior region of the mesethmoid a trifurcate form, or bifurcate in the cases where the medial process is reduced (state 0). In all members from the ingroup and some species from the outgroup, the mesethmoid has an anterior triangular process (the mesethmoid spine), and lateral wings that support the ascending processes of the premaxillae (state 1; Fig. 1). The situation in the Cypriniformes is not comparable due to the presence of a kinethmoid bone articulating with the premaxillae. However, the mesethmoid of the Siluriformes appears to correspond to state 0 and this state probably is ancestral for Characiformes. The root is herein coded as a missing entry pending further studies to resolve this issue. In the examined specimen of Hemiodus cf. thayeria (Böhlke), the mesethmoid has an anterior spine and reduced, but present, lateral wings. This condition is typically present in the Hemiodontidae with the exception of Argonectes Böhlke & Myers (Langeani, 1998) and that species is coded as state 1. In Phenacogaster tegatus (Eigenmann) and Roeboexodon geryi the mesethmoid spine has small lobes slightly projected to the medial margin of the premaxillary ascending process. These lobes are simultaneously present with the lateral wings of the mesethmoid, and they are herein considered to be non-homologous with the processes described in state 0.
26. Ventral projection of mesethmoid spine, forming a keel between premaxillae: (0) absent; (1) present. (VH35).
When present, the mesethmoid spine is rather pointed from lateral view and does not form a keel between the premaxillae (state 0). In Creagrutus spp., Piabina argentea, and Roeboexodon geryi, among the studied species, this spine has in addition a ventral laminar projection expanded between the premaxillae (state 1; Fig. 25). This state is diagnostic for a clade formed by Creagrutus and Piabina, according to Vari & Harold (2001). This character is considered as inapplicable for species in which the mesethmoid spine is completely absent.
27. Form of mesethmoid spine: (0) long, extending between premaxillae; (1) relatively short, with premaxillae articulating with each other anterior to mesethmoid. (BÜ1m).
The mesethmoid spine extends to varying degrees between the premaxillae. Usually, this spine is slender and long, almost completely separating the premaxillae, which consequently articulate with each other only at their anteroventral tips (state 0; Figs. 21 and 22). In a reduced number of species, the mesethmoid spine is much broader and shorter, approximating the form of an equilateral triangle from dorsal view and leaving a comparatively longer area of articulation between the premaxillae anteriorly (state 1; Fig. 26). This spine is relatively broad, but separates completely the premaxillae in Charax stenopterus, Heterocharax macrolepis Eigenmann, Hoplocharax goethei and Phenacogaster tegatus, which are coded as state 0.
28. Posterior portion of mesethmoid spine: (0) relatively slender; (1) as broad as lateral wings of mesethmoid.
In most examined species the posterior portion of the mesethmoid spine is rather broad but does not reach the tip of lateral wings (state 0; Figs. 21 and 22), which are visible as separate structures. Members of the Rhoadsiinae have the mesethmoid spine greatly expanded posteriorly, being approximately equal to the total width of the lateral wings of the mesethmoid. In this state, the lateral wings are not visible as discrete structures (state 1; Fig. 19). This character is coded as inapplicable for species in which the mesethmoid spine is absent.
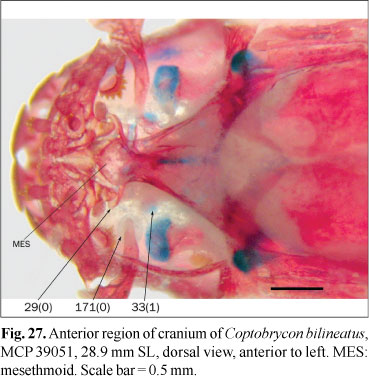
29. Lateral wings of mesethmoid: (0) present; (1) absent. (UJ21, BU2, LU3, BÜ6).
The lateral wings of mesethmoid ("lateral ethmoid wings" of Weitzman, 1962) are expansions that support the ascending process of the corresponding premaxilla. As mentioned above, in some members of the outgroup the mesethmoid is anteriorly trifurcate and has processes articulating with premaxillary fossae. In these cases, the lateral wings of mesethmoid are absent (state 1). Most species with a triangular anterior mesethmoid spine have lateral wings articulated with the premaxillary ascending processes (state 0; Fig. 27). An almost perfect correspondence exist between the absence of articular processes and the presence of lateral wings; however, the simultaneous absence of these structures in Hemiodus cf. thayeria, Leporinus striatus, and Pyrrhulina australis, among the examined species, justifies their inclusion as separate characters. This character is coded as inapplicable to the root of this analysis in light of the different configuration of the bones of the snout of the Cypriniformes and Siluriformes.
30. Ventral diverging lamellae of mesethmoid: (0) absent; (1) present. (LU0i, BU3i, TP8i, ZV22)
The ventral diverging lamellae of the mesethmoid were described by Weitzman (1962) for Brycon meeki. These paired lamellae are situated ventrally in the mesethmoid posterior to the lateral wings (state 1; Fig. 14) and are absent in most members of the outgroup (state 0). The condition observed in Puntius tetrazona is herein considered as non-comparable, and the root is coded as inapplicable for this character. In Aphyocharax spp., Paragoniates alburnus Steindachner, Phenagoniates macrolepis, Prionobrama paraguayensis (Eigenmann), and Xenagoniates bondi Myers, the nasal septum of the mesethmoid, which in other taxa is a single longitudinal medial lamella in the ventral surface of the mesethmoid, is, at least partially, formed by two parallel lamellae. In these taxa the ventral diverging lamellae of the mesethmoid, as observed in other species, are absent; however, it is probable that the nasal septum is formed partially by these lamellae. This character is coded as inapplicable for the mentioned species pending morphological studies to elucidate this situation. In the examined species of the Cheirodontinae these lamellae are reduced to small ridges of variable degree of development in different genera. They are coded as present in cheirodontin characids. In the examined specimens of Acestrorhynchus pantaneiro and Agoniates anchovia Eigenmann these lamellae are reduced in size but present and these species are coded as state 1. These lamellae are variably present among the examined specimens of Lonchogenys ilisha Myers and this character is coded as polymorphic.
31. Anterior convergence of ventral diverging lamellae with nasal septum of mesethmoid: (0) absent, or confluent near anterior end of nasal septum; (1) confluent at posterior end of nasal septum.
When present, the diverging lamellae of the mesethmoid usually extend anterior to the articulation with the vomer and are independent of the medial nasal septum of the mesethmoid (state 0; Fig. 1). In a group of species, principally composed of members of the clade A of Malabarba & Weitzman (2003), these lamellae converge near the posterior end of the nasal septum, with the olfactory capsules separated from each other by this composite septum (state 1; Fig. 28). In some examined species including most members of the Cheirodontinae, Hasemania nana, Hemigrammus erythrozonus Durbin, H. bleheri, Microschemobrycon casiquiare Böhlke, Paracheirodon axelrodi, Parecbasis cyclolepis, and Phenagoniates macrolepis, the ventral diverging lamellae converge with the nasal septum, but do so anteriorly rather than posteriorly, and the olfactory capsules are separated each other, at least partially, by the medial longitudinal nasal septum of the mesethmoid. This situation is coded as state 0. This character is inapplicable for species in which the ventral diverging lamellae of the mesethmoid are reduced or absent.
32. Nasal septum of mesethmoid: (0) single longitudinal lamella; (1) two parallel lamellae apparently formed, in part, by ventral diverging lamellae.
As previously mentioned, in most examined species the ventral diverging lamellae of the mesethmoid are independent each other, at least posteriorly, and the nasal septum is formed by a medial single lamella attached dorsally to the ventral surface of the mesethmoid (state 0; Figs. 1 and 28). In some species the nasal septum is formed by two closely-positioned and parallel lamellae that articulate posteriorly through cartilages with the medial region of lateral ethmoids. This condition can be observed dorsally through the somewhat transparent dorsal lamella of the mesethmoid (state 1; Fig. 18). As mentioned under character 30, in these species the ventral diverging lamellae of the mesethmoid are absent as separate structures, but probably partially form the composite nasal septum. Given that the identity of the ventral diverging lamellae as part of this nasal septum was not corroborated herein, this character is considered different from character 31. This character is coded as inapplicable to Aphyocharax nattereri in which the whole nasal septum is much reduced and Phenagoniates macrolepis in which the posterior portion of the lamellae forming the nasal septum diverge slightly at their posterior tips resulting in a not directly comparable condition. In Heterocharax macrolepis, Hoplocharax goethei, and Lonchogenys ilisha, the nasal septum resembles state 1, but the ventral diverging lamellae are present as separate structures. This character is coded as inapplicable to these species, pending future studies. The origin and homologies of the different structures forming the nasal septum and their relationships with the olfactory capsules remain to be studied in greater detail.
Nasal:
33. Nasal: (0) present; (1) absent. (ZV17).
The nasal bone is present in almost all the Characiformes as a tubular bone lateral to the mesethmoid (state 0; Figs. 2 and 21); its absence was cited among the examined phylogenies only in the alestid Lepidarchus adonis Roberts (Zanata & Vari, 2005) (state 1; Fig. 27). This bone is present in all the examined species except for Coptobrycon bilineatus (Ellis) and Hyphessobrycon elachys Weitzman. Absence of an ossified nasal is probably associated with miniaturization, although this bone is present in species of smaller adult sizes than Coptobrycon bilineatus.
34. Bony lamellae bordering sensory canal of nasal: (0) absent or more slender than tubular region; (1) wider at some point than tubular region. (VA17, LU31, MO48m, LI7, PZ10).
In most examined species the nasal is reduced to a tubular bone, lacking or with distinctly small associated lamellae (state 0; Figs. 2 and 21). Vari (1995) reported the presence of lamellae bordering the sensory canal of the nasal dorsally and ventrally (state 1) in members of the families Ctenoluciidae, Erythrinidae, Hepsetidae, and Lebiasinidae.
Orbitosphenoid:
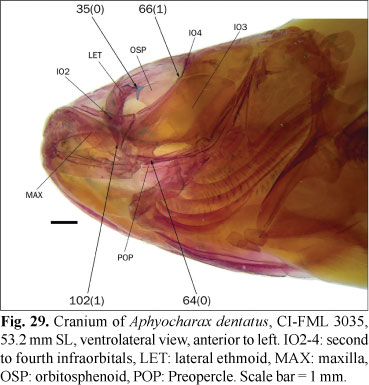
35. Synchondral articulation between lateral ethmoid and anterodorsal border of orbitosphenoid: (0) present; (1) absent, with orbitosphenoid distant from lateral ethmoid.
The anterodorsal tip of the orbitosphenoid (the orbitosphenoid wing, sensu Weitzman, 1962: 20) is usually distant from the lateral ethmoid, and the area between these bones is filled by the posterior projection of the ethmoid cartilage (sensu Weitzman, 1962: 20), which also limits anterolaterally the anterior myodome (state 1; Fig. 6). In some species, in contrast, the anterior margin of the orbitosphenoid is much closer to the lateral ethmoid and these bones articulate synchondrally (state 0; Fig. 29). This character is coded as inapplicable for Puntius tetrazona which, as is usual in the Cypriniformes, has an extensive contact between the orbitosphenoid and lateral ethmoid along the entire anterior margin of the orbitosphenoid. In Alestes cf. macrophthalmus Günther, Brycinus carolinae (Paugy & Levéquè), and Bryconaethiops macrops Boulenger, the orbitosphenoid articulates anteriorly with the lateral ethmoid, but contact in the region lateral of the olfactory nerve and ventral to the region considered in this character. These species are correspondingly coded as state 1. In the examined specimens of Bryconamericus cf. rubropictus this character is variable and is coded here as polymorphic.
36. Lateral bony coverage of olfactory nerve: (0) absent; (1) covered by posterior expansion of lateral ethmoid; (2) covered by an anterior tubular projection of orbitosphenoid; (3) covered laterally and ventrally by orbitosphenoid and lateral ethmoid, which do not form canal. (UJ13m, ZV29m).
The orbitosphenoid usually has, from anterior view, an anterior concavity partially containing the olfactory bulb of the brain, with the olfactory nerve directed anteriorly from the bulb to the olfactory capsule through a foramen in the lateral ethmoid. In most examined species, the olfactory bulb and nerve have no lateral bony coverage and they are visible through the orbit after the eye is removed (state 0; Fig. 13). In some species, the olfactory nerve is instead covered laterally in different modes. In the Parodontidae the lateral ethmoid has a posterior projection that laterally covers the olfactory nerve (state 1; Roberts, 1974: figs. 57, 61, and 63). In most species of the Alestidae, the olfactory nerves are covered by a tubular anterior projection of the orbitosphenoid that reaches the lateral ethmoid (state 2; Zanata & Vari, 2005: fig. 9). In Puntius tetrazona there is an extensive articulation between the orbitosphenoid and the lateral ethmoid that completely covers the olfactory bulb and tract. Distichodus Müller & Troschel and Xenocharax Günther, as is general in the Citharinoidei (Vari, 1979; Zanata & Vari, 2005), have a similar condition, but the lateral coverage of the anterior portion of the brain is not complete (state 3). The lateral coverage of the olfactory nerve by a tubular projection of the orbitosphenoid was considered as typical of the Alestidae by Géry (1977), and it was proposed as a synapomorphy for this family by Murray & Stewart (2002), and a synapomorphy for the African alestids by Zanata & Vari (2005).
37. Form of orbitosphenoid: (0) slender, relatively small and separate from parasphenoid; (1) massive, almost reaching parasphenoid ventrally. (UJ12, UJ36, LC1, TP18, BE25, BÜ9).
In most examined species of the Characidae the orbitosphenoid is slender, and its ventral margin is distant from the parasphenoid (state 0; Fig. 25), while in most members of the outgroup and some species of the Characidae, the orbitosphenoid is relatively massive and its ventral margin is close to the parasphenoid (state 1; Fig. 14). In the examined specimens of Acestrocephalus sardina, Chalceus macrolepidotus Cuvier, Hollandichthys multifasciatus, and Salminus brasiliensis the orbitosphenoid has an intermediate size and these species are coded as polymorphic.
38. Distance between posterodorsal margin of ethmoid cartilage and lateral ethmoids: (0) contacting, or almost contacting, lateral ethmoids; (1) distant from lateral ethmoids.
The dorsal region of the orbitosphenoid is margined anteriorly by a cartilage, the ethmoid cartilage of Weitzman (1962), which is arched in dorsal view and anteriorly limits the olfactory bulb of the brain and posteriorly the anterior myodome. This myodome contains part of the extrinsic musculature of the eye which attaches principally to the posterior wall of the lateral ethmoid. The position of the olfactory bulb relative to the lateral ethmoid and, consequently, the development and position of the anterior myodome varies among the examined species. The most obvious feature reflecting these differences is the position of the ethmoid cartilage, which is visible dorsally through the frontals. In most examined species the arch formed by this cartilage contacts, or almost contacts, the medial region of the lateral ethmoids (state 0; Fig. 2). In other species the ethmoid cartilage is distant from the lateral ethmoids and it is, instead, connected to the medial region of the lateral ethmoids through a longitudinal cartilage extending dorsally from the rhinosphenoid, when present (state 1; Fig. 15). In the Cypriniformes and Siluriformes the olfactory bulbs are broadly separated from the telencephalon and situated just posterior to the olfactory organ (Harder, 1975). Although this condition is not congruent with any of the states defined herein, it represents an opposite state to that described on state 1. Thus, the root is herein coded as state 0. State 1 was observed in a juvenile specimen of Salminus brasiliensis; however, Zanata & Vari (2005) described an anterior displacement of the olfactory bulb in Salminus Agassiz during the growth, and this character is coded as polymorphic for this species.
39. Opening between orbitosphenoid and pterosphenoid: (0) present, rounded or ovate, usually margined by frontal dorsally; (1) absent. (AM15m).
In most members of the outgroup the posterior margin of the orbitosphenoid is broadly articulated with the anterior margin of the pterosphenoid, leaving no gaps between these bones (state 1). Almost all the examined characids instead have incomplete articulations between these bones, resulting in an opening margined anteriorly by the orbitosphenoid, posteriorly by the pterosphenoid, and dorsally by the orbital lamella of the frontal (state 0; Figs. 6 and 30). Weitzman (1962) described a small foramen situated between the orbitosphenoid and pterosphenoid for Brycon meeki, which serves as passage for the trochlear nerve. The correspondence of the opening discussed herein and that described by Weitzman (1962) was not corroborated, and Brycon meeki is coded as a missing entry. A similar opening between the orbitosphenoid and pterosphenoid (i. e. not limited by the frontal), is present in Markiana nigripinnis and the examined species of Triportheus Cope. These species are coded with the state 0. The presence of this opening is variable in the examined specimens of Hoplocharax goethei and this species is coded as polymorphic.
Parasphenoid:
40. Anterior paired projections of parasphenoid: (0) absent; (1) present. (BE26).
The anterior region of the parasphenoid articulates with the posterior lamella of the vomer and the ventral margin of the lateral ethmoids. Usually, the lateral edges of the parasphenoid are entire and parallel in all their extent across the orbit, lacking any projections (state 0). The parasphenoid of some examined species instead has paired acute processes situated near its anterior end, oriented towards the posteroventral margin of each lateral ethmoid (state 1; Fig. 12). Benine (2004) defined three states for this character, considering an intermediate situation, in which such processes are present but reduced in size. Only the species in which these processes are present and well developed in all the examined specimens are herein coded as state 1, leaving as polymorphic those species in which these process are of variable occurrence or much reduced.
Parietal:
41. Parietal fontanel: (0) present in adults; (1) absent in adults. (BU15, LU12, ZV37, PZ16).
The parietal fontanel, when present, is limited laterally by the frontals and parietals, anteriorly by the epiphyseal bar, and posteriorly by the supraoccipital (state 0; Fig. 26). In some species, the contralateral frontals and parietals meet medially, occluding the parietal fontanel (state 1; Fig. 23). Among the members of the Characidae, Lucena (1998) mentioned the absence of this fontanel only in Brycon pesu, while Buckup (1998) coded it as present in B. guatemalensis Regan. According to Zanata & Vari (2005) this fontanel is present in specimens of B. pesu of 27.0 mm SL, and absent in specimens of 49.7 mm SL, indicating that at least in some species this character is variable during the growth. The parietal fontanel is present in specimens of 256 mm SL of B. meeki (Weitzman, 1962), and it is coded as present for this species. In the examined specimens of B. falcatus and B. orbignyanus this fontanel is present; as there is no published evidence indicating ontogenetic elimination of the parietal fontanel in these species, this character is also coded with state 0.
Prootic:
42. Trigemino-facialis foramen: (0) broad, largely limited by sphenotic dorsally; (1) narrow, as a cleft with sphenotic almost excluded from its margin.
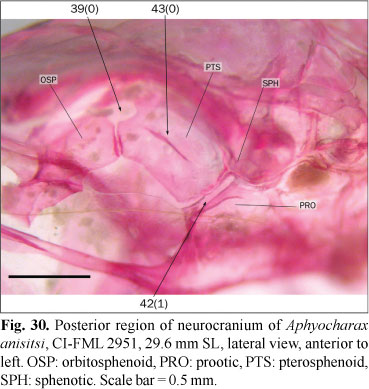
In most examined species the trigemino-facialis foramen is situated in an approximately triangular chamber on the anterior surface of the prootic, with the chamber limited dorsally by the sphenotic (state 0; Figs. 11 and 31). In the examined species of Aphyocharax the trigemino-facialis foramen is reduced to a cleft, narrow in anterolateral view and almost completely limited by the prootic and pterosphenoid (state 1; Fig. 30); in this state the dorsal margin of the trigemino-facialis foramen is much reduced and the sphenotic, which limits dorsally the foramen, is almost excluded from its margin.
Pterosphenoid:
43. Large foramen on pterosphenoid: (0) absent; (1) present, well developed.
In most examined species the lateral surface of the pterosphenoid is flat or has a shallow ridge ventrally limiting the supraorbital nerve, which is directed to the trigemino-facialis foramen (state 0; Figs. 11, 30, and 31). In the examined specimens of Aphyocharacidium bolivianum and Axelrodia lindeae there is a large foramen situated in the middle of the pterosphenoid (state 1; Fig. 32). The association of this foramen with blood vessels or nerves was not confirmed. The presence of this foramen is variable among the examined specimens of Microschemobrycon casiquiare and this species is coded as polymorphic.
44. Small foramen near posterior margin of pterosphenoid: (0) absent, or not pierced by nerves; (1) present, pierced by a branch of supraorbital nerve.
The supraorbital nerve runs through the orbit in the region of articulation between the frontal and orbitosphenoid/pterosphenoid, entering to the braincase through the trigemino-facialis foramen in most examined species (state 0). In some species, mostly of the Stevardiinae, a branch of this nerve enters to the braincase through a small foramen near the posteroventral margin of the pterosphenoid (state 1; Fig. 31). The examined alcohol-preserved specimens of Astyanax cf. eigenmanniorum (Cope), Cynopotamus argenteus, Hemibrycon surinamensis Géry, Hyphessobrycon bifasciatus Ellis, Moenkhausia sanctaefilomenae (Steindachner), Oligosarcus spp., and Roeboides microlepis have small pores in a similar position, but these pores are not pierced by nerves or blood vessels; therefore these species are coded with state 0.
Pterotic:
45. Dorsal process of pterotic where tendon from epaxial musculature attach: (0) absent; (1) present, projecting dorsally from tube for semicircular canal.
The examined species usually have a tendon from the epaxial musculature attached to the lateral margin of the pterotic tube for the horizontal semicircular canal, or to a process ventral to this tube (state 0). In a group of species, there is a small process directed dorsally from the tube for the semicircular canal, onto which this tendon attaches (state 1; Fig. 33). The presence of this process is variable among the examined specimens of Mimagoniates rheocharis, Nematobrycon palmeri, and Phenagoniates macrolepis which are coded as polymorphic.
46. Relative length of pterotic spine: (0) projected more posteriorly than attachment site of ligament from hyomandibula; (1) restricted to attachment region of hyomandibular ligament.
In all the examined members of the outgroup and some characids, the pterotic spine is projected posterior to the attachment site of a ligament from the hyomandibula (state 0; Figs. 5 and 7). In most examined members of the Characidae the pterotic spine is relatively reduced and its extension is limited to the posterior extension of the attachment site for the hyomandibular ligament (state 1).
Rhinosphenoid:
47. Rhinosphenoid: (0) absent; (1) present. (UJ35, BU7, LU8i, CM11(13), TP16i, VB15, SE15, BE20i, ZV31i, LI28, BÜ8, QU21i, PZ13).
The rhinosphenoid is a medial bone situated anterior to the orbitosphenoid, which is present in most characiforms (state 1; Figs. 6, 24, and 34) albeit is absent in many groups in the Characiformes and the remaining Ostariophysi (state 0; Fig. 14). Bührnheim (2006) mentioned the presence of rhinosphenoid, among others, in Charax stenopterus; this bone is, however, absent in the specimens of this species herein examined, and this species is coded as polymorphic. The presence of this bone is intraspecifically variable among the examined specimens of Astyanax correntinus (Holmberg), A. latens Mirande, Aguilera & Azpelicueta, Diapoma terofali (Géry), and Hyphessobrycon bifasciatus, in which this character is coded as polymorphic.
48. Dorsal expansion of rhinosphenoid: (0) absent; (1) present and forming a bony wall between olfactory nerves. (SE17, BE21).
The form of the rhinosphenoid is variable among the examined species. In some species this bone has a rectangular form and is situated completely ventral to the olfactory nerves (state 0; Figs. 6 and 34). In Characidium spp., Hemiodus cf. thayeria, and many species of the Characidae, the rhinosphenoid is expanded dorsally and so forming a bony wall between the olfactory nerves (state 1; Fig. 24). Benine (2004) coded, among others, Bario steindachneri (Eigenmann) and Tetragonopterus argenteus Cuvier as lacking a dorsally expanded rhinosphenoid, however a well developed process was observed in B. steindachneri and a relatively smaller process was found in T. argenteus and these two species are coded as polymorphic. A relatively reduced or intraspecifically variable dorsal projection of the rhinosphenoid was also observed in Aphyodite grammica Eigenmann, Hemigrammus unilineatus, Hyphessobrycon elachys, H. pulchripinnis Ahl, Microschemobrycon casiquiare, and Moenkhausia sanctaefilomenae which are coded as polymorphic. This character is coded as inapplicable to species lacking an ossified rhinosphenoid.
49. Posterior extension of rhinosphenoid cartilage: (0) projected only to middle horizontal length of orbitosphenoid, or less; (1) extended to vertical through region of articulation between orbitosphenoid and pterosphenoid.
The rhinosphenoid cartilage is situated between the rhinosphenoid, lateral ethmoid, and parasphenoid. In almost all of the examined species, this cartilage extends posteriorly along the dorsal margin of the parasphenoid to a point not surpassing the vertical through middle horizontal length of the orbitosphenoid (state 0; Fig. 35). In Inpaichthys kerri and Xenagoniates bondi, this cartilage extends more posteriorly and reaches the vertical through the region of articulation between the orbitosphenoid and pterosphenoid (state 1). The examined specimens of Hollandichthys multifasciatus have an intermediate situation that is coded as polymorphic.
50. Ventral border of rhinosphenoid: (0) distinctly separate from parasphenoid; (1) almost contacting parasphenoid.
In most examined species the rhinosphenoid is variably, but distinctly separated from the parasphenoid (state 0; Fig. 25). In a small group of species, the rhinosphenoid is situated near the parasphenoid, and its ventral margin is parallel to and almost contacts the dorsal margin of the parasphenoid (state 1). This character is coded as inapplicable in species without an ossified rhinosphenoid.
Supraoccipital:
51. Anterior margin of supraoccipital: (0) situated completely behind vertical through posterior orbital margin; (1) situated anterior to vertical through posterior orbital margin.
The anterior margin of the supraoccipital that forms the the posterior margin of the parietal fontanel, when that opening is present, is located posterior to the vertical through the posterior margin of the orbit in most examined species (state 0). In some species, in contrast, the anterior margin of the supraoccipital is situated anterior to the vertical through the posterior margin of the orbit (state 1; Fig. 24). The examined specimens of Hyphessobrycon socolofi Weitzman have an intermediate situation that is coded as polymorphic.
52. Length of supraoccipital spine: (0) extends dorsal of entire neural complex of Weberian apparatus; (1) extends dorsal of approximately one half extent of neural complex. (AM16m, CM8(9), MO34m, VB24m, SE18m, BE19m, QU25m).
53. Length of supraoccipital spine: (0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus; (1) extends only to anterior limit of neural complex. (LU23m, MO34m, VB24m, SE18m, BE19m, QU25m).
When it is present, the supraoccipital spine forms the posterior margin of the parietal fontanel and extends dorsal to the modified vertebrae of the Weberian apparatus to different degrees among the examined species. The length of the supraoccipital spine is herein compared to that of the neural complex of the Weberian apparatus. In some species the supraoccipital spine is greatly developed and extends dorsal to the entire neural complex (character 52, state 0; Fig. 24). In an intermediate state, it extends onto the middle of the length of the neural complex (character 52, state 1; character 53, state 0; Figs. 5 and 8), whereas in some species the supraoccipital spine covers only the anterior vertical portion of the neural complex (character 53, state 1; Fig. 35). Intermediate states of the character 52 were observed in Astyanax abramis (Jenyns), A. cf. abramis, A. correntinus, Brycon orbignyanus, Markiana nigripinnis, Piaractus mesopotamicus, Pseudochalceus kyburzi, and Rhoadsia altipinna, which are coded as polymorphic. The examined specimens of Hyphessobrycon socolofi and Roeboexodon geryi have intermediate states of the character 53, and this character is also coded as polymorphic in these species.
Vomer:
54. Dorsolateral processes of vomer: (0) absent; (1) present. (PZ7).
The vomer, in most species, has a medial longitudinal ridge in the dorsal surface aligned with a ridge on the ventral surface of the mesethmoid, together with there forming the nasal septum (state 0). In addition to this medial lamella, some species have paired dorsal lamellae on the vomer aligned with the ventral diverging lamellae of the mesethmoid (state 1; Toledo-Piza, 2007: fig. 5). These ridges were called dorsolateral processes of vomer by Toledo-Piza (2007).
Orbital region
Antorbital:
55. Antorbital: (0) present; (1) absent or fused with first infraorbital. (VA1, BU20, LU25, ZV1).
The antorbital is present as an independent ossification in almost all the Characiformes (state 0; Fig. 37), whereas it is presumably fused with the first infraorbital, forming the lachrymal, in the Cypriniformes and Siluriformes (Weitzman, 1962). The absence of antorbital (state 1) is not usual in Characiformes; it was mentioned for several genera (Lucena, 1993; Buckup, 1998) and proposed by Vari (1995) as a synapomorphy of a clade composed of the Ctenoluciidae and Erythrinidae. Hoplias cf. malabaricus (Bloch), Inpaichthys kerri, and the root of this analysis are coded with state 1, although based on its position and extension this bone appears to be fused in Hoplias cf. malabaricus and cyprinids and absent in I. kerri.
56. Position of antorbital relative to lateral ethmoid in lateral view: (0) antorbital entirely anterior to lateral ethmoid; (1) antorbital overlapping lateral ethmoid. (TP2).
In most examined species the antorbital is situated lateral to the olfactory capsules and bordering the nasal openings posteriorly but is positioned entirely anterior to the lateral ethmoid (state 0; Figs. 35 and 36). In some species the antorbital is relatively broader, extensively overlapping the lateral margin of the lateral ethmoid (state 1; Fig. 37). This broadening of the antorbital was mentioned by Castro (1984) as a possible synapomorphy of a proposed clade formed by Clupeacharax Pearson and Engraulisoma Castro.
Infraorbitals:
57. Relative position of anterior margin of antorbital and first infraorbital: (0) anterior margin of antorbital either aligned with or anterior to first infraorbital; (1) anterior margin of antorbital posterior to first infraorbital. (ZV6i).
The first infraorbital is located just ventral to the antorbital and extends to a varying degree along the longitudinal length of this bone. In most species the anterior margin of the first infraorbital reaches a point between the middle and anterior margin of the antorbital, but not projects anterior to this bone (state 0; Fig. 37). In several outgroups and Galeocharax humeralis the first infraorbital conspicuously projects anterior to the antorbital (state 1).
58. Bony lamellae bordering laterosensory canal of first infraorbital: (0) present; (1) absent. (ZV9m).
In most examined species the first infraorbital laterosensory canal is margined by a bony lamella both dorsally and ventrally, (state 0; Figs. 38 and 39). In some species the first infraorbital is reduced to the laterosensory canal, and these lamellae are lacking (state 1; Fig. 40). The lamellae of all the infraorbitals are much reduced in Gymnocharacinus bergii differing from other species lacking bony lamellae on the first infraorbital, in which the lamellae of the remaining infraorbitals are well developed. However, G. bergii is coded as state 1. The first infraorbital is absent in Carnegiella strigata and Coptobrycon bilineatus, and this character is coded as inapplicable to these species.
59. Extent of expansion of first infraorbital lateral to maxilla: (0) covering less than one half length of maxilla; (1) covering most of maxilla. (PZ30m).
The ventral margin of the first infraorbital forms a groove that receives the dorsal margin of the maxilla in most examined species. This grooves usually laterally covers as much as one half the length of the maxillary lamella when the mouth is closed (state 0). In Acestrorhynchus pantaneiro the first infraorbital entirely covers the maxilla in lateral view (state 1; e. g. Menezes, 1969: fig. 23), and the maxillary lamella is also significantly covered by the first infraorbital in Agoniates anchovia, Bryconexodon juruenae, Engraulisoma taeniatum, Exodon paradoxus, Heterocharax macrolepis, and Roeboexodon geryi, all of which are coded as state 1. This character is coded as inapplicable to Carnegiella strigata and Coptobrycon bilineatus, in which the infraorbitals are much reduced.
60. Lateral overlap of first infraorbital by anterior margin of second infraorbital: (0) absent; (1) present.
In most examined species the posterior margin of the first infraorbital and the anterior margin of the second infraorbital either are not contacting each other or the first infraorbital is overlapping laterally the second infraorbital (state 0; Figs. 40 and 41). The examined species of Bryconops Kner and Triportheus instead have the anterior margin of the second infraorbital extensively overlapping the posterior margin of the first infraorbital, especially in the region of the laterosensory canal, and covering it from lateral view (state 1; Fig. 39). This character is coded as inapplicable to Carnegiella strigata and Coptobrycon bilineatus in which the infraorbitals are reduced. This character differs with that described by Lucena & Menezes (1998: fig. 4) in which only the laterosensory canal of the second infraorbital overlaps laterally the lamella of the first one, whereas its lamella is situated clearly medial to that of the first infraorbital. In the state 1 of this character both the laterosensory canal and the lamella of the second infraorbital overlap laterally the lamella of the first infraorbital.
61. Overlap of maxilla by second infraorbital: (0) absent; (1) present. (LM5).
In most species of the ingroup the first infraorbital overlaps the maxilla to some degree, while the ventral margin of second infraorbital is situated dorsally and does not cover the maxilla laterally (state 0). In some examined species, both the first and second infraorbitals form part of a sheath that receives the dorsal margin of the maxilla when the mouth is closed (state 1; Zanata & Vari, 2005: fig. 3). In Apareiodon affinis (Steindachner), Characidium spp., Distichodus maculatus, Hemiodus cf. thayeria, Iguanodectes geisleri Géry, Leporinus striatus, Metynnis maculatus, Parodon nasus (Kner), Piabucus melanostomus, and Puntius tetrazona, the posterior tip of the maxilla falls short of the second infraorbital and this character is coded as inapplicable.
62. Articulation between second and third infraorbitals: (0) vertical; (1) anteroventrally angled; (2) posteroventrally angled.
The articulation between the second and third infraorbitals lacks interdigitations among the examined species. The main variation in this articulation is the relative angle of contact between these bones. In the most common situation the posterior margin of the second infraorbital and the anterior margin of the third infraorbital describe an approximately vertical line perpendicular to the horizontal arm of the preopercle (state 0; Fig. 41). In a relatively diverse group of species the second infraorbital has an approximately triangular shape, with an anteroventrally oblique posterior margin. In this state the third infraorbital partially borders the ventral edge of the second infraorbital (state 1; Fig. 40). The inverse situation is found in some species in which the posterior margin of the second infraorbital is posteroventrally oblique and the second infraorbital ventrally borders the anterior region of the third infraorbital (state 2; Fig. 39). The latter state is usually found in species with a long maxilla, although some species with a long maxilla have state 0 or even state 1, such as Paragoniates alburnus.
63. Anterior region of third infraorbital: (0) not much expanded relative to posterior region of second infraorbital; (1) abruptly expanded relative to posterior region of second infraorbital.
In most examined species the ventral margins of the second and third infraorbitals form a nearly continuous line, without conspicuous expansions of these bones (state 0; Fig. 41). In Hyphessobrycon eques (Boulenger), H. socolofi, and Pristella maxillaris, the anterior margin of the third infraorbital is much expanded ventrally relative to the posterior margin of the second infraorbital. Thus, the line formed by the ventral margins of these bones is not straight but rather has a sinusoidal shape in the region of articulation between these elements (state 1; Fig. 9).
64. Ventral extent of third infraorbital: (0) reaching horizontal arm of preopercle; (1) not reaching horizontal arm of preopercle, at least anteriorly. (EI5mi, AM23, UJ51, UJ53, BÜ19m).
The ventral extension of the third infraorbital was used in traditional characid systematics (Eigenmann, 1917) to discriminate some genera (e. g. Astyanax from Bryconamericus). In some species the third infraorbital is comparatively more developed, covering completely the cheek and reaching the horizontal arm of the preopercle (state 0; Fig. 29). In other species instead it is less developed and there is a "naked" area between the anterior region of this infraorbital and the preopercle (state 1; Fig. 33).
65. Posterior extent of third infraorbital: (0) covering angle of preopercle; (1) relatively reduced, angle of preopercle covered partially by fourth infraorbital. (MO44).
The posteroventral angle of the preopercle is bordered dorsally by the third infraorbital in most examined species (state 0; Weitzman, 1962: fig. 8, Zanata & Vari, 2005: fig. 3). In Hemiodus cf. thayeria, Micralestes stormsi, and Piabucus melanostomus, the third infraorbital is relatively less developed, articulating with the fourth infraorbital just dorsal to the angle of the preopercle (state 1; Roberts, 1974: fig. 6). Moreira (2002) mentioned the presence of state 1 as a synapomorphy for a clade including all the species of the Iguanodectinae except Iguanodectes geisleri and cited a parallel occurrence of this state in Micralestes acutidens (Peters). This character is coded as inapplicable in species with reduced infraorbital lamellae.
66. Fourth infraorbital: (0) present, well developed; (1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. (VA12, LU28m, LC7m, VB14, ZV12, QU16m, PZ34).
Most Characiformes have six infraorbitals (state 0; Figs. 40 and 41), with several reductions in different groups, being the fourth infraorbital most frequently the absent or reduced infraorbital (state 1; Fig. 29). Uj (1990) proposed the reduction or absence of such infraorbital as diagnostic for his Aphyocharacidae (including the genera Aphyocharax and Prionobrama), and Vari (1995) mentioned it as a synapomorphy of the Ctenoluciidae. The presence of the fourth infraorbital is variable among the examined specimens of Charax stenopterus, Mimagoniates rheocharis, Hyphessobrycon eques, and Nantis indefessus (Mirande, Aguilera & Azpelicueta), which are coded as polymorphic. The absence and the extreme reduction of the fourth infraorbital are variable within some species of Aphyocharax, and both conditions are very different to the presence of six well developed infraorbitals that is usually present in the Characidae. Therefore it is preferred to include both conditions in the same state, instead of coding this character for presence or absence of the fourth infraorbital. This character is coded as inapplicable to the species in which all the infraorbitals are reduced.
67. Form of fourth infraorbital: (0) approximately square or more developed longitudinally than dorsoventrally; (1) longer dorsoventrally than longitudinally. (SE25m, BE2, BÜ20).
The form of the fourth infraorbital is variable among the examined species. The fourth infraorbital in some species is more developed longitudinally than dorsoventrally or it is approximately square (state 0; Fig. 40), while in others it is more elongate dorsoventrally (state 1; Figs. 39 and 41). Contrary to the observations of Benine (2004), the examined specimens of Bario steindachneri and Bryconops melanurus (Bloch) have state 0 and these species are coded as polymorphic given possible intraspecific variability. The examined specimen of Knodus breviceps shows an intermediate state and this species is coded as polymorphic. The examined specimens of Moenkhausia sanctaefilomenae are variable in this character, and this species is also coded as polymorphic.
68. Posterior dorsoventral expansion of fourth infraorbital: (0) absent; (1) present. (PZ33).
In most examined species, the fourth infraorbital is approximately square or rectangular and bordered dorsally by the fifth infraorbital, whose posterior margin is situated just anterior to the exposed portion of the vertical arm of the preopercle (state 0). In Acestrocephalus sardina, Acestrorhynchus pantaneiro, Cynopotamus argenteus, Galeocharax humeralis, Oligosarcus spp., and Rhaphiodon vulpinus among the examined species, the posterior region of the fourth infraorbital is dorsoventrally expanded and the fifth infraorbital is displaced dorsally and relatively distant from the vertical arm of the preopercle (state 1; Figs. 10 and 34).
69. Lateral coverage of dilator fossa by sixth infraorbital: (0) almost complete, at least in its ventral border; (1) leaving a conspicuous naked area in anterior region of dilator fossa. (UJ49m, SE28m, PZ36m).
In most outgroups the sixth infraorbital laterally covers the fossa for the dilator operculi muscle (state 0). A group of species with well developed infraorbitals instead have the sixth infraorbital leaving a "naked" area in the anterior region of the dilator fossa (state 1). Uj (1990) mentioned the reduced size of the dermosphenotic (=sixth infraorbital) in a clade including his Aphyocharacidae (genera Aphyocharax and Prionobrama), Piabucidae (=Iguanodectinae), Paragoniatidae (=Paragoniatinae), and Tetragonopteridae (=Tetragonopterinae). This character is coded as inapplicable to the species in which the dilator fossa is absent or there is a reduction of the infraorbitals.
Supraorbital:
70. Supraorbital: (0) present; (1) absent. (UJ48, VA9, BU21, LU26, MO39, VB13, SE21, BE5, ZV2, QU15, PZ37).
The supraorbital, when present, is situated dorsal or anterodorsal to the orbit (state 0: Fig. 13; Zanata & Vari: figs. 1, 2, and 4). The absence of a supraorbital (state 1) was considered by Uj (1990) as a synapomorphy for the clade composed of his Aphyocharacidae (Aphyocharax and Prionobrama), Piabucidae (=Iguanodectinae), Paragoniatidae (=Paragoniatinae), and Tetragonopteridae (=Tetragonopterinae); however this character has an ambiguous optimization for that node according to the tree proposed by Uj. In addition, Vari (1995) mentioned the absence of the supraorbital as a parallelism for the Erythrinidae and Lebiasinidae. In the phylogenetic hypothesis of Buckup (1998), the absence of the supraorbital is a synapomorphy for a clade composed of Charax, Cynopotamus Valenciennes, Oligosarcus, Phenacogaster Eigenmann, and Tetragonopterus. According to Lucena (1993), the absence of this bone is a synapomorphy independently supporting a clade composed of Gnathocharax Fowler, Heterocharax Eigenmann, and Hoplocharax Géry, a clade including most members of the Characidae, and a node composed of the Erythrinidae and Lebiasinidae. Moreira (2002) mentioned the presence of the supraorbital in all known species of the Iguanodectinae, differing in that observation from Lucena (1993) and Malabarba & Weitzman (2003). Malabarba & Weitzman (2003) proposed a clade of characids lacking the supraorbital composed of the subfamilies Aphyocharacinae, Characinae, Cheirodontinae, Iguanodectinae, Rhoadsiinae, Stethaprioninae, Tetragonopterinae, some incertae sedis genera, and their clade A.
71. Contact between supraorbital and sixth infraorbital: (0) absent; (1) present. (AM19, UJ40, CM6(7)i, LU27i, MO40m, ZV5, LI4).
When present, the supraorbital is variably developed across the Characiformes. In some species it is longitudinally reduced and restricted to the anterior region of the dorsal margin of the orbit, not contacting the sixth infraorbital. In this state the orbit is margined dorsally by the frontal (state 0; Zanata & Vari, 2005: fig. 1A). In some species the posterior region of the supraorbital is relatively more developed, reaching to the anterior margin of the sixth infraorbital. In these cases the dorsal margin of the orbit is bordered by these two bones, which exclude the frontal from this margin (state 1; Fig. 13, Zanata & Vari, 2005: figs. 1B, 2, and 4). State 0 is coded only in the cases in which the supraorbital is present but not contacting the sixth infraorbital. This character is coded as inapplicable for species in which the supraorbital is absent.
Laterosensory system
Infraorbital canal:
72. Laterosensory canal in antorbital: (0) absent; (1) present. (UJ41, CM4(5), MO38, SE22m, BE1m).
In the examined species the infraorbitals usually bear laterosensory canal segments. Such canals are absent in the antorbital (state 0). The presence of a laterosensory canal in the antorbital (state 1) was proposed as a synapomorphy of Bryconops by Lucena (1993), and cited also for some species of Brycon Müller & Troschel and Iguanodectes Cope (Malabarba, 1998b; Moreira, 2002). This character is coded as inapplicable to the species lacking the antorbital.
73. Laterosensory canal of first infraorbital: (0) projects dorsally from main body of first infraorbital; (1) absent or does not projects dorsally. (LU30, CM2(3)i).
In most examined species the first infraorbital bears a laterosensory canal that extends dorsally to a point near the dorsal margin of the lamellar region of the bone (state 1). In some species this canal projects dorsal to the lamella of the first infraorbital (state 0; Fig. 13). This character is herein considered as inapplicable to Hoplias cf. malabaricus, in which the first infraorbital appears to be fused with the antorbital. Malabarba (1998b) coded this character with state 0, among others, in Brycon pesu and Salminus sp. In the examined specimens of B. pesu and S. brasiliensis this canal does not project dorsally. This character is coded as polymorphic in B. pesu, while S. brasiliensis is coded with state 0, given that the observations of Malabarba (1998b) are from an unidentified species of the genus, and the only available data about this character for S. brasiliensis is based on the observations made for the present paper.
74. Branching of laterosensory canals of fourth or fifth infraorbitals: (0) absent; (1) present. (BE3i, LI53m, PZ92).
The infraorbital laterosensory canal usually has a posterior branch oriented towards the dorsal end of the laterosensory canal in the preopercle or a pore in the vertical arm of the preopercle. In most examined species this canal is not ossified, but rather formed by soft tissue and it is situated superficially on the infraorbitals (state 0). In some species this canal is partially contained in the fourth or fifth infraorbitals and is evident as an ossified longitudinal branch of the laterosensory canal (state 1; Figs. 38, 39, and 41). This character is coded as polymorphic in Acestrorhynchus pantaneiro, Acestrocephalus sardina, Astyanax chico Casciotta & Almirón, Cyphocharax spilotus Vari, Deuterodon iguape, Hyphessobrycon pulchripinnis, Moenkhausia xinguensis, and Probolodus heterostomus, in which this branch is variably present. This character is coded as inapplicable for species in which the fourth and/or fifth infraorbitals are reduced or absent.
75. Direction of posterior branch of laterosensory canal of fourth or fifth infraorbital: (0) to a pore on preopercle near hyomandibular condyle; (1) to a pore conspicuously ventral to hyomandibular condyle.
In most examined species the posterior branch of the laterosensory canal of the fourth or fifth infraorbitals is directed towards the dorsal end of the preopercle near the region of articulation between the hyomandibula and opercle (state 0). In Chalceus macrolepidotus and Hemiodus cf. thayeria this branch is oriented to a point approximating half way down the vertical arm of the preopercle (state 1).
76. Laterosensory canal of sixth infraorbital: (0) not branched; (1) branched. (MO46m, ZV153, BÜ23, PZ93i).
In the generalized condition in the Characiformes described for Brycon meeki (Weitzman, 1962), the laterosensory canal of the sixth infraorbital is bifurcate, with one branch directed towards the neurocranium and other anteriorly (state 1; Figs. 2 and 38). Zanata & Vari (2005) mentioned that the state 1 is broadly distributed in Characiformes and cited the absence of this canal (state 0; Fig. 41) in several characids, Crenuchus spilurus Günther, Serrasalmus rhombeus (Linnaeus), and a group of alestids; as observed here this branch of the laterosensory canal is absent in most Characidae. The presence of this branch is variable among the examined specimens of Roeboexodon geryi, and this species is coded as polymorphic. This character is considered as inapplicable to Characidium rachovii Regan, Coptobrycon bilineatus, Grundulus cochae, Hoplocharax goethei, Hyphessobrycon elachys, H. luetkenii (Boulenger), Inpaichthys kerri, Pseudocorynopoma doriae Perugia, and Thoracocharax stellatus in which the sixth infraorbital is extremely reduced in size or absent.
77. Position of opening on neurocranium communicating with laterosensory canal of sixth infraorbital: (0) between frontal and pterotic; (1) in frontal. (VH38m, MO46m, ZV154m, PZ95).
In the condition illustrated by Weitzman (1962) and observed in most examined characiforms, the laterosensory canal of the sixth infraorbital communicates with an opening situated between the posterior margin of the frontal and the anterior margin of the pterotic (state 0; Fig. 34). In some species this opening is completely contained by the frontal and anterior to the articulation between that bone and the pterotic (state 1; Fig. 18). Zanata & Vari (2005) mentioned that state 1 is also present in Brycon pesu and some members of the family Alestidae. The analyzed specimens of Brycon pesu have the state 0, but this species is coded as polymorphic considering the observations of Zanata & Vari (2005). The examined specimens of Bryconamericus alpha, B. cf. iheringii (Boulenger), B. mennii Miquelarena, Protogino, Filiberto & López, B. cf. rubropictus, Nantis indefessus, and Piabina argentea are variable onto this character and are coded as polymorphic. In Hoplias cf. malabaricus the opening that receives the canal from the sixth infraorbital is situated completely in the pterotic, while in Characidium spp. this opening is situated between the frontal and sphenotic. This character is coded as inapplicable to both species.
78. Position of opening on neurocranium communicating with sixth infraorbital laterosensory canal: (0) lateral to or slightly anterior to sphenotic tube for vertical semicircular canal; (1) distinctly anterior to sphenotic tube for vertical semicircular canal.
The tube for the vertical semicircular canal is contained within the anterior region of the sphenotic and it is evident in diaphanized specimens both in lateral and dorsal views. In most examined species the laterosensory canal of the sixth infraorbital opens to the neurocranium in a position just lateral or slightly anterolateral to the sphenotic tube for the vertical semicircular canal (state 0; Fig. 32). In a small group of species this opening is instead displaced anterior to the sphenotic tube for a distance equal to the width of the tube (state 1; Fig. 18).
Dentary-preopercle canal:
79. Length of laterosensory canal of dentary: (0) piercing almost entire length of dentary; (1) reduced or absent.
In most examined species, there occurs a branch of the laterosensory canal running from the anterior region of the dentary to the posterior margin of the anguloarticular and exiting just dorsal to the region of articulation between the anguloarticular and the condyle of the quadrate (state 0; Fig. 42). In some species this canal is reduced, being either absent or restricted to the anterior half of the dentary (state 1). Although this character is probably related with miniaturization, some medium-sized species, as Bryconamericus rubropictus, have state 1.
80. Pores of laterosensory canal of lower jaw: (0) six or less; (1) seven or more.
Most examined species have three or four laterosensory canal pores piercing the dentary and one pore between the dentary and the anguloarticular (state 0; Fig. 43). In a small group of species there are six or more pores in the dentary plus one between the dentary and anguloarticular (state 1; Fig. 44). In Salminus brasiliensis there are many small tubules. As this condition is not directly comparable to any of the states described herein, this character is coded as inapplicable to this species. This character is also coded as inapplicable to species with laterosensory canals of the dentary reduced or absent.
81. Lateral surface of vertical canal of preopercle: (0) canal uncovered and situated posteriorly to musculature and infraorbitals; (1) covered by musculature and/or infraorbitals (ZV108i).
The vertical arm of the preopercle bears a canal of the laterosensory system. In most examined species this canal is situated superficially, posterior to the attachment site of the adductor mandibulae and the posterior margins of the third and fourth infraorbitals (state 0). Zanata & Vari (2005) described the anterior displacement of this canal, which is situated in a position close to the anterior margin of the preopercle (state 1; Roberts, 1974: fig. 6); in this state, the laterosensory canal of the vertical arm of preopercle is not visible laterally because it is covered by musculature and/or infraorbitals.
82. Dorsal end of laterosensory canal of preopercle and suprapreopercle: (0) not overlapping anterodorsal process of opercle; (1) overlapping anterodorsal process of opercle. (BU26, ZV107).
The laterosensory canal of the preopercle in most examined species is situated completely anterior to the anterodorsal margin of the opercle (state 0; Fig. 34), whereas in some species this canal bends laterally around the anterodorsal corner of the opercle which is often pointed in the form of an "opercular spine" as described by Vari (1979) (state 1; Fig. 45). This character is herein considered as inapplicable in the species in which this canal is reduced dorsally and does not reach the anterodorsal margin of the opercle. In the examined specimens of Aphyocharax anisitsi and Prodontocharax melanotus this canal slightly laterally overlaps the opercle. This condition is considered as intermediate between the states herein defined, and this character is coded as polymorphic for these species.
Frontoparietal canal:
83. Anterior region of laterosensory canal of frontal: (0) contained completely on frontal; (1) opens into a chamber limited dorsally by frontal and ventrally by lateral ethmoid.
The laterosensory canal of the frontal extends longitudinally in the region just medial to the orbit. In almost all the outgroups this canal reaches the anterior margin of the frontal and is anteriorly contiguous with the nasal canal (state 0; Fig. 23). In most members of the ingroup this canal opens anteriorly into a chamber, which is broad in dorsal and bordered dorsally by the frontal and posteroventrally by the lateral ethmoid (state 1; Fig. 2); this chamber is situated between the frontal and nasal canals. Intermediate conditions were observed in Micralestes stormsi and Engraulisoma taeniatum in which this character is coded as polymorphic.
84. Epiphyseal branch of supraorbital canal: (0) present; (1) absent.
In most examined species the epiphyseal branch of the supraorbital laterosensory canal extends medially dorsal to the epiphyseal bar and opens just lateral to the cranial fontanel (state 0; Figs. 1 and 46). In most members of the Stevardiinae and some other species this branch of the canal system is instead completely absent (state 1; Fig. 17).
85. Epiphyseal branch of corresponding supraorbital canals: (0) both aligned with epiphyseal bar; (1) oriented obliquely, opening posteriorly to epiphyseal bar.
The epiphyseal laterosensory canals are both aligned with the epiphyseal bar in most species included in this analysis (state 0; Fig. 1). Heterocharax macrolepis and Triportheus spp., in contrast, have these canals oriented posteromedially and opening in a position more posterior than the epiphyseal bar (state 1; Fig. 46). Intermediate states were observed in Galeocharax humeralis and Markiana nigripinnis, which are coded as polymorphic. This character is considered as inapplicable to the species in which the epiphyseal branch of the laterosensory canal is lacking.
86. Opening of epiphyseal laterosensory canals: (0) along margin of cranial fontanel; (1) canals continue dorsomedially in soft tissue, opening over or just lateral to the cranial fontanel.
The epiphyseal laterosensory canals in most examined species are included in the frontals and open dorsal to these bones or along the margin of the cranial fontanel (state 0). In a small group of species, these canals are continued dorsomedially by soft tissue and open over or just lateral to the cranial fontanel (state 1). An intermediate state was observed in Acestrocephalus sardina and Roeboides descalvadensis, in which the posterior projections of these canals are present but reduced in extension. This character is coded as polymorphic for these species.
87. Laterosensory canal on sphenotic: (0) absent; (1) present.
In most members of the Characiformes, the laterosensory canal of frontal continues posteriorly into the pterotic (state 0). As Buckup (1993) noted, in the Characidiinae this canal continues into the sphenotic, between the frontal and the pterotic segments (state 1).
Posttemporal canal:
88. Posterior branch of posttemporal laterosensory canal: (0) present; (1) absent. (PZ100).
A laterosensory canal passes through the extrascapular, posttemporal, and supracleithrum, posteriorly to the lateral line on the body. This canal has several branches in the extrascapular and usually is unbranched in the posttemporal and supracleithrum, although small tubules are usually present in these bones. Tubules of the supracleithrum are intraspecifically variable in form and number and are not considered in this paper. A small posterior branch of the posttemporal canal was observed as relatively intraspecifically stable in some species (state 0; Fig. 47). This branch is absent in most examined species (state 1; Fig. 48). The presence of such branch is variable among the examined specimens of Astyanax lineatus, Markiana nigripinnis, and Rhoadsia altipinna which are coded as polymorphic.
Lateral line:
89. Form of lateral line: (0) approximately straight; (1) curved ventrally in abdominal region. (EI12m).
In most examined outgroups the lateral line is straight along all of its length (state 0), whereas in the members of the ingroup it is variably curved ventrally in the abdominal region (state 1). In Puntius tetrazona the lateral line is straight, but this character is coded as polymorphic for the root of the analysis given that the lateral line is curved ventrally in the supposedly generalized Cypriniformes Opsariichthys and Zacco.
90. Degree of ventral curvature of lateral line: (0) straight or only slightly curved, with posterior portion aligned with middle caudal-fin rays; (1) distinctly curved and ventrally situated, with posterior lying within ventral half of caudal peduncle and aligned with lower lobe of caudal fin. (EI12m, BE89m, ZV164).
The lateral line is straight or slightly curved in most examined species, with the number of transverse scale series above the lateral line comparable to the count ventral of the lateral line. In these species the lateral line ends posteriorly between the middle caudal-fin rays (state 0). In a small group of examined species, the lateral line is distinctly curved ventrally anteriorly and runs ventrally on the lateral surface of the body flank. In these species there are only two or three scale series ventral to the lateral line and the lateral line runs along the ventral half of the caudal peduncle, terminating between the caudal-fin rays of the ventral lobe (state 1). In Thoracocharax stellatus the lateral line runs obliquely from its beginning to the anterior anal-fin rays. This condition is considered as non-homologous to the defined states, and this character is coded as inapplicable.
91. Lateral line: (0) complete; (1) interrupted. (EI2, VA72, MA60m, WM16, SE88, BE88, ZV162, BÜ153m, QU96).
The presence of a complete lateral line running from the cranium to the caudal peduncle (state 0) versus an interrupted lateral line (state 1) is one of the generic level characters used by Eigenmann (1917) and it is still used to discriminate various genera in the Characiformes. However, the reduction of various regions of the laterosensory system was mentioned for several groups, usually associated with miniaturization events (e. g. Weitzman & Fink, 1983; Buckup, 1993). Although this character is probably highly homoplastic, there are supraspecific taxa, as the genera Aphyocharax and Hollandichthys Eigenmann, with medium-sized species, that always have an interrupted lateral line. This is an evidence that, at least, in some level of analysis this character contains useful phylogenetic information. Variations of this character were observed in Psellogrammus kennedyi (Eigenmann), which is coded as polymorphic. This character is coded as inapplicable to Thoracocharax stellatus, in which the lateral line runs obliquely to the anterior anal-fin rays instead of running along the flank to the caudal peduncle.
92. Canal of lateral line on caudal-fin membrane: (0) absent; (1) present. (CM49m, ZV163i, LI58m, BÜ114m).
In most examined outgroups with a complete lateral line, the lateral line ends at the posteriormost perforated scale on the caudal peduncle (state 0). In many examined members of the Characidae with a complete lateral line an ossified canal continues the lateral line onto the membrane between the middle caudal-fin rays (state 1; Fig. 49). Although this canal is usually absent in species with an interrupted lateral line, in some of these species this canal is present. Therefore the presence or absence of this canal is coded independently from the extension of the lateral line. This character is coded as inapplicable to Thoracocharax stellatus, in which the lateral line is aligned towards the anal fin.
93. Length of caudal-fin canal of lateral line: (0) reaching only half of caudal-fin length; (1) almost reaching posterior margin of caudal fin. (LI58m, BÜ114m).
Upper jaw
Maxilla:
94. Anterior end of ascending process of maxilla: (0) with conspicuous notch; (1) pointed or rounded. (LU43, ZV67i).
In most examined species the tip of the ascending process of the maxilla is rounded or variably pointed (state 1; Fig. 50). Lucena (1993) described an anteriorly bifurcate ascending process of the maxilla (state 0: Zanata & Vari, 2005: figs. 17-18) in the African alestids he examined. Zanata & Vari (2005) mentioned that the anterior concavity of the ascending process serves as attachment site for the ligament that joints the maxilla with the mesethmoid. These authors mentioned the presence of such concavity in Alestes Müller & Troschel, Brycinus Valenciennes, Bryconaethiops Günther, Bryconalestes Hoedeman, and Triportheus albus. The anterior bifurcation of this process was herein observed in Micralestes stormsi, a species not included in the analysis of Zanata & Vari (2005). These authors coded as rounded the ascending process of four species of this genus. Within the Characidae, an anteriorly bifurcate ascending process of the maxilla was observed only in Aulixidens eugeniae. In the examined specimens of Triportheus this process is rounded.
95. Ventral margin of toothed region of maxilla: (0) approximately straight; (1) strongly concave.
The ventral margin of the toothed region of the maxilla is nearly straight in most examined species (state 0; Fig. 51), while the maxilla is strongly convex in this region in Phenagoniates macrolepis, and Xenagoniates bondi (state 1; Fig. 50). This character is considered as inapplicable for species with no or just one maxillary tooth.
96. Margins of toothed region of maxilla: (0) roughly parallel; (1) dorsally divergent.
In most examined species the margins of the maxilla run roughly parallel in the toothed region (state 0). Lucena & Lucena (2002) proposed the dorsal divergence of the margins of the maxillary lamellar portion as a synapomorphy of the genus Deuterodon (state 1; Fig. 52). Intermediate conditions were observed in Bryconamericus agna Azpelicueta & Almirón and Piabina argentea and are coded as polymorphic.
97. Expansion of lamellar portion of maxilla just posterior to toothed region: (0) absent or not pronounced; (1) very pronounced.
The ventral margin of the lamellar portion of maxilla, in most cases, describes a slightly sinusoidal line posteriorly to the toothed region, when maxillary teeth are present (state 0; Fig. 50). In a relatively small number of species, the lamellar portion of the maxilla is abruptly expanded posteriorly to the insertion of the teeth. In such cases, the ventral maxillary margin approximately follows the straight line anteriorly formed by the cusps of the maxillary teeth (state 1; Fig. 53). This character is coded as inapplicable in species lacking maxillary teeth. In Carlana eigenmanni and Rhoadsia altipinna this character is variable during the ontogeny; young specimens have state 1 and adults have state 0. These species are then coded as polymorphic.
98. Tubules for passage of blood vessels on lamellar portion of maxilla: (0) a single tubule, parallel to dorsal margin of maxilla; (1) tubule with anterior branch running parallel to anterior margin of maxilla and reaching one third of its length; (2) anastomosed tubules. (ZV75m).
In most examined species, the lamellar portion of the maxilla is pierced by one or a series of small tubules, which would serve as a passage for nerves and blood vessels (Menezes, 1976; Zanata & Vari, 2005). In many species, there is a single canal parallel to the dorsal margin of the maxilla (state 0; Figs. 52 and 53). In some species this canal instead is divided in two conspicuous branches with one of these tubules similar in form and position to that of state 0, while the other one is anteriorly-directed and runs parallel to the convex anterior margin of the maxilla in the edentulous region (state 1; Fig. 54). In these species the posterior branch is usually covered laterally by an expansion of the first infraorbital, while the anterior branch borders this bone when the mouth is closed. In other species these tubules are rather anastomosed (state 2; Fig. 55). Since state 1 refers to the arrangement of tubules in the toothless portion of the maxilla, this character is coded as inapplicable to the species with a completely toothed laminar region of the maxilla.
99. Posterior extent of maxilla: (0) not reaching second infraorbital; (1) reaching second infraorbital. (BU33, MA8m, VB5m, ZV72m, BÜ53m, QU4m).
In most examined members of the Characidae the maxilla reaches posterior to the second infraorbital (state 1; Figs. 33 and 56), while in some species the relatively shorter maxilla does not reach the second infraorbital (state 0; Zanata & Vari, 2005: fig. 2). The maxilla does not reach the second infraorbital in Puntius tetrazona, but Opsariichthys and basal Siluriformes have the state 1, and the root of this analysis is coded as polymorphic. This character is also coded as polymorphic for Distichodus maculatus; in this species the maxilla does not reach the second infraorbital, but this bone does reach the maxilla in Xenocharax spilurus Günther, which is considered a basal species of the Distichodontidae (Vari, 1979).
100. Length of maxilla relative to dentary: (0) maxilla reaching posterior end of Meckelian cartilage; (1) maxilla not reaching posterior end of Meckelian cartilage.
The length of the maxilla is usually correlated with the length of the dentary; however, variations in this correlation are considered in this character. In most examined species the lamellar portion of the maxilla extends to a point posterior to the posterior limit of the Meckelian cartilage (state 0; Fig. 24). In some species the lamellar portion of the maxilla is relatively reduced and does not reach the posterior end of the Meckelian cartilage when the mouth is closed (state 1; Figs. 35 and 56). This character is coded as polymorphic in Distichodus maculatus, which has state 1, whereas Xenocharax spilurus, a basal distichodontid, has state 0. This character is also coded as polymorphic in Carlana eigenmanni and Rhoadsia altipinna, which have state 1 when young and state 0 when adults.
101. Ontogenetic lengthening of maxilla: (0) absent; (1) present.
In most examined species growths of the maxilla during ontogeny is proportional with that of the remaining bones of the head, and its length is approximately constant in relation to the other bones (state 0). In members of the subfamily Rhoadsiinae, the proportional length of the maxilla increases during the ontogeny such that it becomes proportionally longer in larger individuals (state 1; Eigenmann, 1927: figs. 10, 13, and 14).
102. Dorsal projection of maxilla: (0) not overlaps second infraorbital; (1) overlaps second infraorbital.
The dorsal margin of the maxilla usually fits to a sheath formed only by the first infraorbital and, in some species, first and second infraorbitals when the mouth is closed (state 0). The inverse situation is present in the examined species of Aphyocharax, in which the maxilla laterally overlaps the second infraorbital when the mouth is closed (state 1; Fig. 29).
Premaxilla:
103. Interdigitations between premaxillae: (0) present; (1) absent. (LU37, ZV55i).
The mesethmoid spine usually separates almost completely the premaxillae. The premaxillae, in turn, are usually joined to each other anterior of the mesethmoid solely by connective tissue and lack bony interdigitations (state 1). Weitzman (1962) described the presence of bony interdigitations between the premaxillae of Brycon meeki (state 0; Weitzman, 1962: fig. 2). Vari (1979) later proposed the presence of bony interdigitations between premaxillae as a synapomorphy of the Citharinoidei.
104. Length of ascending process of premaxilla: (0) reaching at least one-third of length of nasal; (1) reaching just anterior end of nasal. (LU34mi).
The premaxillary ascending process usually reaches one third or more the length of the nasal, as described by Weitzman (1962) for Brycon meeki (state 0; Fig. 21). This condition is broadly distributed among the members of the Characidae. In most outgroups and a diverse group within the Characidae, the ascending process of the premaxilla is relatively shorter, reaching only the anterior end of the nasal (state 1; Figs. 18 and 26). Intermediate states were observed in Astyanax latens, Bryconamericus mennii, Diapoma terofali, Hasemania nana, and Pseudocorynopoma doriae, which are coded as polymorphic.
105. Alignment of ascending process of premaxilla: (0) aligned with medial margin of nasal; (1) medially shifted and separated from nasal.
The anterior process of the mesethmoid usually has a triangular shape with its posterior region as wide as the dorsal lamella of the mesethmoid, which is in turn situated posterior to the lateral wings. Since the anterior process medially borders the premaxilla and the dorsal lamella borders the nasal, the ascending process of premaxilla is usually aligned with the nasal, or contacts to it medially (state 0; Fig. 21). In Aphyocharax spp., Carnegiella strigata, Prionobrama paraguayensis, and Thoracocharax stellatus, the anterior process of the mesethmoid is much slender relative to the dorsal lamella of the mesethmoid. In these cases the nasal is consequently distinctly displaced laterally relative to the ascending process of the premaxilla (state 1; Fig. 18).
106. Form of posterolateral portion of premaxilla: (0) with notch; (1) with pedicle expanded laterally to maxilla. (LU36i, ZV52).
The posterolateral tip of the premaxillary alveolar arm in the members of the Characidae has a concave surface that receives the ventral margin of the ascending process of the maxilla (state 0; Zanata & Vari, 2005: fig. 14). Zanata & Vari (2005) described a projection of the posterior region of the premaxillary alveolar arm that borders the lateral surface of the proximal region of the maxilla (state 1; Zanata & Vari, 2005: fig. 13). These authors cited the presence of this pedicle-like process in most members of the Alestidae and in the serrasalmids Colossoma macropomum (Cuvier) and Piaractus mesopotamicus. In the examined specimen of Piaractus mesopotamicus the posterolateral end of the premaxilla has a concave surface where the maxilla articulates; the external lobe of this structure is relatively more developed but it does not laterally borders the maxilla and P. mesopotamicus is consequently coded as state 0 for this character. This character is not directly comparable in Puntius tetrazona and the root is coded as inapplicable.
Lower jaw
Anguloarticular:
107. Lateral ridge of anguloarticular: (0) absent; (1) present. (BÜ57m).
The anguloarticular has two anterior processes, a horizontal process and an oblique anterodorsal process. The horizontal process is situated along the longitudinal axis of the dentary and forms the posterior attachment site for the Meckelian cartilage; it is attached laterally to that cartilage and the coronomeckelian bone. The oblique process projects towards the posterodorsal margin of the dentary and usually is smooth or has longitudinal striae on its lateral surface (state 0; Fig. 57). In addition to these striae, some species have a bony ridge parallel to the anteroventral margin of the oblique process between its border and the ventral margin of the primordial ligament (state 1; Fig. 42).
108. Horizontal process of anguloarticular: (0) laterally covered by dentary only anteriorly; (1) broadly covered by dentary which reaches posterior border of Meckelian cartilage.
The anguloarticular processes articulate with the dentary such that the oblique dorsal process overlaps the dentary laterally and the horizontal process overlaps it medially. Both processes are visible laterally in most species with the oblique process completely visible, but the horizontal process only partially visible due to the overlap of the dentary which covers it laterally to different degrees. In most species the dentary laterally covers a relatively small portion of the horizontal process, which does not reach the vertical through posterior tip of the Meckelian cartilage (state 0; Figs. 42 and 58). In some species the dentary overlaps a longer portion of the horizontal process of the anguloarticular and reaches the posterior end of the Meckelian cartilage. In this state the Meckelian cartilage appears to be completely contained within the dentary from a lateral view, although it is clear in medial view that its posterior portion is in contact with the anguloarticular (state 1; Figs. 59 and 60).
109. Ventral margin of horizontal process of anguloarticular: (0) posteroventrally angled relative to laterosensory canal of dentary from medial view; (1) perpendicular to laterosensory canal of dentary from medial view.
The horizontal process of the anguloarticular articulates medially within the dentary in the Meckelian fossa, which in turns serves as the attachment area for the Aw section of the adductor mandibulae (Winterbottom, 1974; Howes, 1976). Fibers from the Aw section usually inserts posteriorly on a tendon from the A2 and A3 sections of the adductor mandibulae. The anterior portion of this tendon usually divides in two smaller tendons that attach to the retroarticular and the medial face of the dentary, respectively (Zanata & Vari, 2005). The tendon to the dentary inserts anteriorly or anteroventrally to the anteroventral margin of the anguloarticular at a point just ventral to the laterosensory canal of the dentary. In most examined species the anteroventral margin of the anguloarticular is angled with respect to the laterosensory canal of the dentary. In these cases the tendon from the adductor mandibulae attaches anteroventral to the anguloarticular (state 0; Fig. 58 and 60). In some species the anteroventral margin of the anguloarticular has a sinusoidal shape, bordering posteriorly the site of attachment of this tendon and crossing perpendicularly the laterosensory canal of the dentary (state 1; Figs. 59 and 61). This character is variable in Astyanax latens and A. cf. rutilus (Jenyns) which are coded as polymorphic.
Coronomeckelian:
110. Position of coronomeckelian: (0) situated mainly lateral to Meckelian cartilage; (1) situated mainly dorsal to Meckelian cartilage. (SE44).
The coronomeckelian serves as the attachment site of one of the anterior tendons of the adductor mandibulae muscle. The size and longitudinal position of the coronomeckelian are related with the degree of development of this tendon and the length of the lower jaw. The position of the coronomeckelian along the vertical is variable among the examined species. In some species the coronomeckelian bone is situated almost completely dorsal to the Meckelian cartilage (state 1; Fig. 61), while in others the bone is located mainly lateral to that cartilage (state 0; Figs. 57 and 58). Several intermediate cases were observed among the examined species which are coded as polymorphisms.
Dentary:
111. Interdigitations between dentaries: (0) absent; (1) present. (UJ18, BU35, LU46m, MA4, ZV82, LI10).
In the Cypriniformes, Citharinoidei, and some families of the Characoidei the articulation between the dentaries lacks interdigitations and these bones are joined together only by connective tissues (Vari, 1979) (state 0). This condition was considered as plesiomorphic for the Characiformes by Fink & Fink (1981). According to the phylogenetic hypothesis of Buckup (1998) the presence of bony interdigitations between dentaries (state 1; Figs. 60 and 61) is a synapomorphy for a clade composed of the Acestrorhynchidae, Alestidae, Characidae, Ctenoluciidae, Erythrinidae, Hepsetidae, and Lebiasinidae, with a reversal in Nannostomus Günther. The absence of interdigitations between dentaries was later proposed as a synapomorphy of a cheirodontin clade composed of Amazonspinther Bührnheim, Carvalho, Malabarba & Weitzman and Spintherobolus by Bührnheim et al. (2008).
112. Form of interdigitations between dentaries: (0) simple bony lamellae; (1) undulate lamellae.
The number of interdigitations between dentaries varies ontogenetically (Miquelarena, 1986), but some differences have been observed in the form of these lamellae. In most of the examined species the lamellae forming these interdigitations are simple, disposed horizontally, and parallel each other (state 0). Zanata & Vari (2005) described the undulation of margins of these lamellae (state 1; Zanata & Vari, 2005: fig. 22) for some African members of the family Alestidae. This character is coded as inapplicable in species lacking interdigitations between the dentaries.
113. Form and dentition of anterior region of dentary: (0) toothed and not depressed anteriorly; (1) edentulous and much depressed anteriorly.
The dentary is usually toothed and not depressed anteriorly in characiforms (state 0), while in the Parodontidae, the dentary is edentulous and much depressed anteriorly (state 1; e. g. Roberts, 1974: fig. 61). Some members of this family, however, have teeth along the lateral margins of the dentary. In Puntius tetrazona and the Cypriniformes in general, the dentary lacks teeth, but that bone is not anteriorly depressed as in the Parodontidae. The members of the Curimatidae lack dentary teeth when adults, but teeth are present in juveniles. Hemiodus cf. thayeria and almost all the genera of the Hemiodontidae lack dentary teeth, but the anterior margin of the dentary is not depressed as it is in the members of the Parodontidae (Roberts, 1974). All of these taxa were coded as state 0.
114. Medial anteroventral notch of dentary: (0) absent; (1) present. (BU37m, LU48m).
The presence of a notch along the anteroventral medial border of the dentary (state 1; Fig. 62) was observed in Iguanodectes geisleri, Phenagoniates macrolepis and Xenagoniates bondi, whereas in most examined species the ventral margin of the dentary is straight or slightly curved in this area (state 0). An anteroventral notch in the dentary was illustrated by Weitzman (1964) for Poecilobrycon harrisoni and this character was considered typical of the Pyrrhulinini (=Pyrrhulininae, Lebiasinidae) by him. However, as described by Weitzman (1964) this notch is continuous with the dentary foramen for the mental ramus of the mandibular branch of trigeminus nerve, while the notch herein considered is not related with the dentary foramen.
115. Medial process of dentary bordering Meckelian cartilage dorsally and medially: (0) absent; (1) present. (MO9).
According to Moreira (2002, 2003) the presence of a medial process in the dentary constitutes a synapomorphy for the Iguanodectinae (state 1; Fig. 62), with this process absent (state 0; Fig. 60) in the remaining species examined by him. This process was herein observed only in Iguanodectes geisleri and Piabucus melanostomus. In these species such a process medially borders a portion of the Meckelian cartilage and serves as an area of attachment for a tendon from the adductor mandibulae which inserts in the dentary, ventral to the Meckelian cartilage in other species.
116. Bony lamella covering dentary foramen laterally: (0) absent; (1) present.
The dentary foramen serves as a passage for nerves and blood vessels and is situated just dorsal and anterior to the anterior tip of the Meckelian cartilage. This foramen is usually evident in lateral view (state 0; Figs. 43 and 63). In Acestrocephalus sardina, Cynopotamus argenteus, and Galeocharax humeralis, among the examined species, this foramen is covered laterally by a bony lamella and is not evident from a lateral view (state 1; Fig. 44).
117. Longitudinal ridge covering laterosensory pores of dentary: (0) absent; (1) present.
The laterosensory canal of the dentary has a variable number of pores. These pores are evident in ventrolateral view in most examined species (state 0). In Charax stenopterus and the examined species of Roeboides Günther, there is a longitudinal bony ridge covering partially these pores which are thus visible only from a ventral view (state 1; Fig. 57).
Dentition
Generalities:
118. Morphology of premaxillary, maxillary, and dentary teeth: (0) all teeth conical, caniniform, or mamilliform; (1) some teeth multicuspidate or molariform. (FF44m, UJ2, BU72m, LU47m, LC23m, LU53m).
According to Uj (1990), the presence of multicuspidate teeth (state 1) is a synapomorphy of the Characiformes, although his conclusion was not based in a cladistic analysis but in the optimization of certain features on a tree arrived at without an explicit phylogenetic method. Lucena (1993) considered this variation under two different characters, involving the maxillary and dentary teeth respectively. According to the phylogeny proposed by Lucena (1993) the possession of multicuspidate teeth constitute independent synapomorphies for the Lebiasinidae and a clade including most of the Characidae, with a reversal to conical teeth in a clade composed of the genera Aphyocharax and Phenacogaster.
119. Premaxillary, maxillary, and dentary teeth: (0) not pedunculate, or pedunculate only in some of these bones; (1) pedunculate and uniformly shaped. (MA55m, MA56m, BÜ70m).
Multicuspidate teeth of most species, when present, have a broad base and somewhat variable form and size on the premaxilla, maxilla, and dentary (state 0). The presence of pedunculate teeth of similar form in the upper and lower jaws (state 1; Figs. 42 and 53) was mentioned as two independent synapomorphies for the Cheirodontinae by Malabarba (1998a). The presence of pedunculate teeth on the premaxilla, maxilla, and dentary was observed in this study in most of the examined members of the Cheirodontinae and in Gymnocharacinus bergii and Odontostoechus lethostigmus. Although the teeth of the latter two species are less compressed that those in members of the Cheirodontinae, they are coded with the state 1 based on overall form. The teeth in both jaws of Odontostilbe pequira are pedunculate, but having a slightly different morphology; this species, however, is also coded as state 1 given the small degree of that difference.
Premaxillary teeth:
120. Mamilliform teeth outside mouth: (0) absent; (1) present. (LU41mi).
The presence of teeth outside the mouth (state 1; Figs. 16 and 21) associated with lepidophagous habits has been cited for several genera in the Characiformes. Such dentition is absent in most examined species (state 0). The presence of three mamilliform teeth on the premaxilla oriented outside the oral cavity was proposed by Lucena (1993) as a synapomorphy of Roeboides. Exodon paradoxus and Roeboexodon geryi have only one or two mamilliform teeth in the premaxilla. Since the morphology of such teeth is very similar to those of Roeboides, these species are coded with the state 1 of this character. The teeth of Probolodus heterostomus have a similar overall shape to those present in Roeboides, but are tricuspidate. In Bryconexodon juruenae there is variation between unicuspidate and tricuspidate mamilliform teeth which are much similar to those of Exodon Müller & Troschel and Roeboexodon Géry and Probolodus Eigenmann, respectively. Given the extra-oral position of the teeth of Bryconexodon juruenae and Probolodus heterostomus and the rather similar shape of these teeth with those of the species of Roeboides both species are coded as state 1.
121. A pair of large conical teeth in premaxilla: (0) absent; (1) present. (LU40i).
In most examined characids there are one to three rows of teeth in the premaxilla; the teeth of each row are usually rather homogeneous in morphology (state 0). Lucena (1993) mentioned the presence of two conical teeth much larger than the remaining ones, situated near the tips of the premaxillary alveolar ramus (state 1; Fig. 51) in several characids. In the examined specimens of Charax stenopterus these teeth are slightly larger than the remaining ones, and this species is coded with state 1.
122. Number of rows of premaxillary teeth: (0) one; (1) two or three. (AM27m, LU38m, LU39m, BU32m, MA55m, CM37, MO3, VB75i, BE27, ZV57, LI11m, BÜ35m, QU80m).
123. Number of rows of premaxillary teeth: (0) one or two; (1) three. (EI4m, AM27m, LU38m, LU39m, BU32m, MA55m, CM37, MO3, VB75i, BE27, ZV57, LI11m, BÜ35m, QU80m).
The homology between the premaxillary rows of teeth among different species is not always easy to establish, especially when entire rows are lacking. The main criterion used herein to recognize, at least tentatively, teeth from different rows is developmental. The premaxillary teeth from the anterior two rows have intraosseous development (sensu Trapani, 2001), while those from the inner row have extraosseous development, growing in the soft connective tissue situated posterior to the alveolar ramus of the premaxilla. However, in some cases the discrimination between teeth from different rows could only be done following a topological criterion. Three states can be recognized among the examined species. In some species only one row of premaxillary teeth, with extraosseous development, is present (character 122, state 0). An anterior row of teeth, with intraosseous development, is present in most of the Characidae resulting in two rows of premaxillary teeth (character 122, state 1; character 123, state 0; Fig. 64). In a relatively small group of species there is an additional row of teeth, which also has intraosseous development but whose homology is difficult to establish and remains to be studied in detail. In the latter state there are three rows of premaxillary teeth (character 123, state 1; Zanata & Vari, 2005: fig. 15). As noted above, according to the hypothesis of Lucena (1993) the presence of two premaxillary rows of teeth is a synapomorphy for a clade corresponding to the Characidae, with parallel reversals to a single row in a clade composed of the genera Roestes, Lonchogenys Myers, Heterocharax, Gnathocharax, and Hoplocharax, and the clade composed of Hemigrammopetersius Pellegrin and Hydrocynus Cuvier. According to Lucena (1993) the possession of three rows of premaxillary teeth constitutes two independent synapomorphies for the clades composed of Creagrutus and Piabina and Brycon and Chalceus, respectively. In the hypothesis of Buckup (1998) the presence of two or three premaxillary rows of teeth is a synapomorphy for a group composed of the Alestidae, Characidae, Ctenoluciidae, Hepsetidae, Lebiasinidae, Erythrinidae, and Acestrorhynchidae, with a reversion in a subclade formed by the last five families. This is, however, just one of the possible most parsimonious optimizations for this character [the optimization produced by ACCTRAN (de Pinna, 1991)]. It is equally parsimonious to propose parallel acquisitions of two or more premaxillary rows of teeth in the Alestidae and Characidae without reversals. Malabarba (1998a) proposed the presence of only one functional row of smoothly aligned and similarly shaped premaxillary teeth as a synapomorphy of the subfamily Cheirodontinae. According to the phylogenetic hypothesis of Zanata & Vari (2005) the presence of two rows of premaxillary teeth is plesiomorphic for the Characiformes. The first of these characters is coded herein as inapplicable to Acestrorhynchus pantaneiro, Bryconexodon juruenae, Charax stenopterus, Cynopotamus argenteus, Exodon paradoxus, Galeocharax humeralis, Heterocharax macrolepis, Hoplias cf. malabaricus, Hoplocharax goethei, Lonchogenys ilisha, Oligosarcus cf. jenynsii, O. bolivianus, Rhaphiodon vulpinus, Roeboexodon geryi, Roeboides spp., and Serrasalmus maculatus. Although some of these species have a single premaxillary tooth row, these teeth are variable in size and/or morphology, probably corresponding to teeth from both the outer and the inner rows of other species. In Apareiodon affinis and Parodon nasus the premaxillary teeth originate from deep grooves separated by vertical lamellae; this condition is not comparable to that of the outer row of other species, and the character 122 is coded as inapplicable for these species. The single premaxillary teeth row of Leporinus striatus has an intraosseous development and it was thus considered as non-homologous with the outer row of other species. Quevedo (2006) coded Paragoniates alburnus as having just one premaxillary tooth row; however, in the specimen examined here, there are two teeth situated distinctly anterior of the remaining five teeth, as if they were in a separate anterior row. The first of these characters is coded as polymorphic for P. alburnus, considering possible intraspecific variations. A variable presence of one or two premaxillary tooth rows was mentioned in the description of Inpaichthys kerri (Géry & Junk, 1977). In the observed specimens all the premaxillary teeth have extraosseous development and would be homologous to the teeth from the inner row of other species. Inpaichthys kerri is consequently coded as state 0 of the character 122. The teeth of the genera Creagrutus and Piabina were described by Vari & Harold (2001) as composed of a triangular cluster of medial teeth (or triad), a primary row of teeth, and a single lateral tooth, which may be either present or absent. Although this characterization is useful to compare between species of these two genera, it is not easy to homologize the teeth of these genera with those of other characids based on these definitions. The primary row, as described by Vari & Harold (2001) is composed of teeth that have both intraosseous (those situated medially) and extraosseous development (those situated just lateral to the posterior teeth of the triad), which differs from the criteria used herein to recognize homologies between tooth rows. These definitions can be seen as homology statements which are useful at different levels of analysis; however, there is clearly still much uncertainty relative to the homologies between characid tooth rows. The analyzed species of Creagrutus are coded with the state 1 of the character 123, while Piabina argentea is coded as polymorphic for this character, given that its teeth could be interpreted as disposed in three rows or in two rows, with the anteriormost in a zigzag pattern. These characters are considered as inapplicable to species lacking premaxillary teeth.
Outer row of premaxillary teeth:
124. Alignment of teeth on anterior premaxillary row: (0) aligned; (1) not aligned, with one or two teeth situated anterior to remaining teeth.
In most species with two rows of premaxillary teeth, the anterior row is composed of teeth aligned in a shallow arch (state 0; Figs. 65). In Bryconamericus exodon and B. cf. exodon, there are one or two teeth relatively displaced anteriorly, giving to the outer row a zigzag-shape (state 1). The correspondence between these anteriorly displaced teeth with those of the outer row in species with three rows of premaxillary teeth is unclear; therefore, this character is considered inapplicable to species coded as having three premaxillary rows of teeth. The examined specimens of Bryconamericus scleroparius (Regan), Hemibrycon dariensis, H. surinamensis, and Knodus breviceps have intermediate conditions that are coded as polymorphisms. This character is coded as inapplicable to species lacking an outer row of premaxillary teeth with intraosseous development.
125. Cusps of teeth on outer premaxillary row: (0) one to three cusps; (1) five or more cusps. (BE28).
The premaxillary teeth of the outer tooth row are relatively slender and tricuspidate in most examined species (state 0; Fig. 55). In a relatively small group of species these teeth are instead expanded distally and have five or more cusps (state 1; Fig. 66). In the examined specimens of Astyanax cf. rutilus, Bryconamericus alpha, and Hemibrycon dariensis the teeth of the outer premaxillary row have five cusps, but the lateral ones are minute and the distal portion of teeth is not expanded. This condition is thus considered to be intermediate, and these species are coded as polymorphic. This character is coded as inapplicable to species with only one row of premaxillary teeth.
Inner row of premaxillary teeth:
126. Teeth on inner premaxillary row: (0) molariform; (1) with aligned in straight series or anteriorly concave pattern of cusps; (2) with anteriorly concave pattern plus anterior cusps. (ZV64m).
The cusps of teeth of the inner premaxillary row, when present, are either aligned in a straight line or form an anteriorly concave arch (state 1; Figs. 64, 65, and 67). In Alestes cf. macrophthalmus, Brycinus carolinae, and Bryconaethiops macrops, among the examined species, there is, in addition, a series of anterior cusps separated by a concave surface from the posterior cusps. The anterior series of cusps and the concave surface jointly give to these teeth a molariform aspect (state 2; Zanata & Vari, 2005: fig. 15). The teeth of the inner premaxillary row in Metynnis maculatus and Piaractus mesopotamicus have a molariform form, but their anterior margin is not formed by a series of cusps, but rather an even margin (state 0). This character is considered as inapplicable to species lacking a defined inner row of premaxillary teeth.
127. Alignment of cusps of medial teeth on inner premaxillary row: (0) forming anteriorly concave semicircle from ventral view; (1) forming shallow arch or aligned in straight series from ventral view. (ZV63i).
As mentioned above, the cusps of the inner premaxillary teeth row have an anteriorly concave arrangement from ventral view. In most examined species this concavity is relatively shallow, describing approximately one-fourth of a circle, or less, from ventral view (state 1; Figs. 64 and 67). Zanata & Vari (2005) mentioned that this concavity is much pronounced (state 0; Fig. 65) in some alestids and in the characid Triportheus albus. An intermediate state was herein observed in Bryconops affinis Günther, which is coded as polymorphic. This character is coded as inapplicable to species without a definite inner row of premaxillary teeth.
128. Form of teeth of inner premaxillary tooth row: (0) with cusps forming anteriorly concave arch; (1) with cusps aligned in straight series and without anterior concavity. (MO4).
As mentioned above the anterior surface of the teeth of the inner premaxillary row has an anteriorly oriented concavity in ventral view resulting from the arched position of the cusps on these teeth (state 0; Figs. 64 and 65). In a relatively small group of species these teeth are much compressed anteroposteriorly and, from a ventral view, their cusps follow an almost straight line (state 1; Fig. 67). This character and the preceding one represent a series of nested homologies and they can be seen as a single additive character with three states. This character is considered as inapplicable to those species lacking a defined inner row of premaxillary teeth. Both this character and the preceding one are coded as inapplicable to Agoniates anchovia, Aphyocharax spp., Aphyodite grammica, Axelrodia lindeae, Grundulus cochae, Inpaichthys kerri, Microschemobrycon casiquiare, Parecbasis cyclolepis, Prionobrama paraguayensis, Roeboides microlepis, and Salminus brasiliensis which have conical teeth or with minute lateral cusps.
129. Number of teeth in inner premaxillary row: (0) four or fewer; (1) five or more. (VB76, BE29i, ZV61i, BÜ41m).
130. Number of teeth in inner premaxillary row: (0) seven or fewer; (1) eight or more. (BÜ41m).
The presence of four teeth on the inner premaxillary row (character 129, state 0) was used in the generic key of Eigenmann (1917) to distinguish Argopleura Eigenmann, Bryconamericus, Ceratobranchia, Creagrutus, Hemibrycon, Knodus, Microgenys, Nematobrycon Eigenmann, and Piabina from the remaining characids, which usually have five teeth in that series (character 129, state 1; character 130, state 0; Fig. 64). Géry (1977) included most genera with four teeth on the inner premaxillary row in his tribe Tetragonopterini, within a subgroup therein called "genus Hemibrycon and allied genera" which included Boehlkea, Bryconacidnus, Bryconamericus, Carlastyanax Géry, Ceratobranchia, Coptobrycon Géry, Creagrudite Myers, Hemibrycon, Knodus, Microgenys, Nematobrycon, Piabarchus, Rhinobrycon, and Rhinopetitia. Although the phylogenetic importance of the possession of four premaxillary teeth on the inner row was not adequately tested, this character was later proposed by Malabarba & Weitzman (2003) to be a putative synapomorphy of their clade A. The presence of eight or more teeth on the inner premaxillary row (character 130, state 1; Fig. 68) was mentioned by Bührnheim (2006) for several small species of the Characidae. The first of these characters is coded as polymorphic in Bryconamericus cf. rubropictus, Cyanocharax alburnus, Diapoma terofali, and Hemibrycon surinamensis, and the second one in Aphyocharax dentatus Eigenmann & Kennedy, Inpaichthys kerri, and Pseudocorynopoma doriae.
131. Polymorphism of teeth on inner premaxillary row: (0) absent; (1) present, with two medial teeth somewhat larger and usually separated from remaining ones by a gap.
In most examined species the two medial teeth of the inner premaxillary tooth row are slightly larger than the remaining teeth, although the homology of these teeth in different species is unclear. These medial teeth are probably homologous to the posterior teeth of the triad described by Vari & Harold (2001) for Creagrutus and Piabina. Apparently this identification was followed by Zanata & Vari (2005: 34), who considered Brycon and Triportheus to have two teeth on the posteriormost premaxillary row of teeth, corresponding to the medial teeth of the inner row. Although the homology of the premaxillary teeth of different species remains to be studied, the teeth from the posteriormost premaxillary row of the examined species are usually concave anteriorly and have extraosseous development. In Brycon spp., Metynnis maculatus, Piaractus mesopotamicus, and Triportheus, among the examined species, these medial teeth are slightly larger and separated from the lateral ones by a gap (state 1). In other species the two medial teeth are not distinctly larger than the third tooth and the second and third tooth are separated each other in the same extent than the remaining pairs of teeth of the inner premaxillary tooth row (state 0).
132. Number of replacement tooth rows on premaxilla: (0) one; (1) two or more.
Most examined species have a single series of inner premaxillary row replacement teeth that demonstrate extraosseous development and which usually become functional simultaneously (state 0). Hemiodus cf. thayeria and the examined species of the Parodontidae have two or more rows of replacement teeth which apparently become functional sequentially (state 1; e. g. Roberts, 1974: fig. 7). Although the homology of particular teeth among Characiformes is uncertain, the simultaneous presence of two or more rows of replacement premaxillary teeth with, at least partially, extraosseous development is tentatively coded as present in the members of the Hemiodontidae and Parodontidae and as absent in the remaining species.
133. Fossa for inner row of replacement premaxillary teeth: (0) absent; (1) present.
The teeth of the inner premaxillary row in most examined species undergo a completely extraosseous development being formed in the soft connective tissue just posterior to the premaxilla (state 0). In a small group of species these teeth rather form within shallow cavities or fossae situated on the posterior surface of the premaxilla (state 1; Fig. 68).
Maxillary teeth:
134. Maxillary teeth: (0) absent; (1) present. (LU44m, MO6m, SE46m, BE34m, ZV78, LI12, BÜ54m).
The presence (state 1) or absence (state 0) of maxillary teeth was longly considered as significant in the classification of the Characidae. This character is coded as polymorphic in Hasemania nana, Hemigrammus bleheri, Moenkhausia dichroura, and Thayeria obliqua, in which there occurs either cited or herein observed variation in this character.
135. Number of maxillary teeth: (0) only one, or absent; (1) two or more. (LU45m, WM42m, CM38m, BE34m, VH7m, BÜ54m).
136. Number of maxillary teeth: (0) up to three; (1) four or more. (LU45m, WM42m, CM38m, BE34m, VH7m, BÜ54m).
The number of maxillary teeth has been considered in almost all published phylogenies of genera and subfamilies of the Characidae. The variation in number of maxillary teeth, however, is almost continuous among the examined species, and any definition of discrete states within this character would have some degree of subjectivity. The number of maxillary teeth is not distributed normally among the examined species, and many species have none (Fig. 54) to four teeth, while relatively few have more than ten teeth. In species with low number of maxillary teeth, this number is relatively more stable intraspecifically, and differences between one or two teeth could be phylogenetically informative, while species with high numbers of maxillary teeth also have relatively higher intraspecific variations in this feature, and the gaining or loss of one teeth has comparatively less correlation with phylogeny and species discrimination. The option of analyzing this character as a lineal continuous character, implemented in TNT (Goloboff et al., 2003, 2006), tends to overestimate the phylogenetic information of the transformations in groups with higher and variable number of maxillary teeth, in relation to groups with lower number of teeth (e. g. a transformation between one to three teeth will have lower weight than a transformation between 20 to 25 teeth). The coding of this character takes into account both personal observations and published information about the number of maxillary teeth in order to consider intraspecific variations, which in several species results in polymorphisms.
137. Extent of implantation of teeth along maxilla: (0) not reaching middle of maxillary lamella; (1) extending across almost entire maxillary lamella. (EI3, LU44m, MO6, VB79, SE46m, QU82).
The posterior extent of the area of implantation of the maxillary teeth is one of the "generic" level characters proposed by Eigenmann (1917). Eigenmann noted the presence of teeth along the entire anterior margin of the maxilla (state 1; Figs. 51 and 69) in Hollandichthys, Nematobrycon, Phenacogaster, and Pseudochalceus Kner. Lucena (1993) divided this character to three states, considering the absence of maxillary teeth, the presence of teeth only in the anterior half of the maxillary margin (state 0; Fig. 68), and the presence of teeth along the entire anterior margin of the maxilla. The examined specimens of Odontostoechus lethostigmus and Probolodus heterostomus have teeth only in the anterior half of the maxillary lamella, and these species are coded with state 0. In Carlana Strand and Rhoadsia Fowler, the maxilla is progressively longer and bears an increasingly number of teeth during ontogeny, with the dentition extending along the entire maxillary margin in adult specimens. Given that young specimens of Carlana eigenmanni and Rhoadsia altipinna have state 0 and adults exhibit state 1, these species are coded as polymorphic.
138. Number of cusps of anterior maxillary teeth: (0) conical, a single cusp; (1) three or more cusps. (LU43m, MO7m, VB81m, SE48m, BE35, BÜ55i, QU81m).
139. Number of cusps of anterior maxillary teeth: (0) up to three; (1) five or more cusps. (LU43m, MO7m, VB81m, SE48m, BE35, BÜ55i, QU81m).
Maxillary teeth usually have a similar morphology to that from the other jaw bones; however, some species with five or more cusps in the premaxillary and dentary teeth, have only conical (character 138, state 0; Fig. 69) or tricuspidate (character 138, state 1; character 139, state 0; Fig. 50) teeth on the maxilla. Although the number of cusps on the maxillary teeth is somewhat correlated with the number of cusps of other teeth, it is coded as a separate character as a function of the lack of correlation in some species. Five cusps were herein observed Bryconops melanurus instead of three, as reported by Benine (2004), and this species is coded as polymorphic. Benine (2004) mentioned the presence of five or more cusps in the maxillary teeth (character 139, state 1; Fig. 52), among others, in Bario steindachneri, Hemigrammus unilineatus, Hyphessobrycon eques, and Poptella paraguayensis (Eigenmann). This character is variable among the examined specimens of Bario steindachneri, Hyphessobrycon eques, and Poptella paraguayensis, which are coded as polymorphic for character 139. The examined specimen of Hemigrammus unilineatus has only three cusps on the maxillary teeth. This character is also coded as polymorphic for this species following the observations of Benine (2004). The maxillary teeth of Salminus brasiliensis are essentially conical but have lateral projections that appear to be rudimentary cusps. This condition is herein considered to be intermediate between the states defined for the character 138, and Salminus brasiliensis is coded as polymorphic for this character. Variation in the character 139 was observed in Astyanax chico, A. cf. eigenmanniorum, A. endy Mirande, Aguilera & Azpelicueta, A. lineatus, Bryconops affinis, Hyphessobrycon anisitsi (Eigenmann), H. eques, Prodontocharax melanotus, and Pseudocorynopoma doriae and this character is coded as polymorphic for these species.
140. Ontogenetic acquisition of conical teeth on maxilla: (0) absent; (1) present.
The proportional lengthening of the maxilla and the acquisition of conical maxillary teeth during the ontogeny (state 1; Fig. 70), were mentioned above as characteristic for members of the subfamily Rhoadsiinae by Fink & Weitzman (1974). Notwithstanding those observations, Fink & Weitzman classified the genus Carlana within the "so-called Cheirodontin fishes" due to the presence of only one row of premaxillary teeth. Carlana eigenmanni and Rhoadsia altipinna, the only analyzed members of Rhoadsiinae, are herein coded as state 1. This character is coded as unknown for Nematocharax venustus Weitzman, Menezes & Britski; although the maxillary dentition of adults of this species bear certain similarities with that of the Rhoadsiinae, an ontogenetic series of this species was unavailable for study and this character could not be analyzed.
Dentary teeth:
141. Orientation of anterior dentary teeth: (0) oriented dorsally or anterodorsally; (1) oriented anteriorly, almost parallel to main axis of dentary.
In most examined species the anterior teeth of dentary are situated perpendicular to or slightly oblique to the longitudinal axis of the bone, (state 0; Fig. 60). Some species instead have the anteriormost dentary teeth oriented anteriorly, in a plane almost parallel to the longitudinal axis of the dentary (state 1; Fig. 45).
142. Size and number of anterior dentary teeth: (0) four or five relatively broad teeth at front of dentary; (1) eight or more small and slender teeth at front of dentary.
Most examined species have four or five relatively broad teeth situated in the anterior region of the dentary (state 0; Fig. 59). In a relatively small group of species, the anterior dentary teeth are more slender, and eight or more teeth are situated at the anterior portion of the dentary (state 1). This character is coded as polymorphic in Grundulus cochae which has six or seven teeth in the anterior region of the dentary.
143. Inner row of dentary teeth: (0) present; (1) absent. (UJ50, BU36i, LU49i, CM41m, MO10, SE63m, LI13m).
The inner row of dentary teeth, when present, is situated just posterior to the replacement fossa for the anterior dentary teeth (Buckup, 1998) (state 0; Figs. 60 and 71). This inner row of dentary teeth is absent in most members of the Characidae (state 1). Distichodus and Triportheus were coded as state 1 by Buckup (1998) and Lucena (1993) respectively; however, an inner row of dentary teeth was observed in the examined species of both genera and they are herein coded as state 0. The members of the Crenuchidae analyzed by Buckup (1998) have two rows of teeth in the dentary; however the examined species of Characidium have only one row of dentary teeth and they are herein coded as state 1. The examined specimen of Hemiodus cf. thayeria lack dentary teeth. Juvenile specimens of this genus have teeth which are lost ontogenetically; although no juveniles of H. cf. thayeria were examined for this paper, the inner tooth row of dentary is absent in juveniles according to Langeani (1998), and this character is coded as state 1 for this species.
144. Symphyseal dentary teeth: (0) absent; (1) present. (UJ46, LU50i, CM41m, SE63m, ZV88, LI14).
In most species with two rows of dentary teeth, the posterior row is composed of numerous and minute conical teeth situated along the ridge posterior to the replacement fossa of the dentary (state 0; Fig. 71). In a group of species, the inner row of dentary teeth has a symphyseal tooth much larger than the remaining teeth, a broad diastema, and a posterior series of teeth similar to those present in state 0 (state 1; Fig. 60). This character is coded as inapplicable in species lacking the entire inner row of dentary teeth.
145. Articulation between dentary teeth: (0) absent; (1) present with associated processes and fossae. (AM6).
In most examined species, the dentary teeth are contiguously situated, without articulations among them (state 0). In some members of the Serrasalmidae, the posterior margin of each dentary tooth has a depression into which the anterior of the immediately posterior tooth inserts (Machado-Allison, 1983) (state 1; Fig. 63).
146. Position of anterior teeth of dentary: (0) along margin of dentary; (1) internally situated with dentary forming anterior ridge.
Dentary teeth are usually situated along the anterodorsal margin of the dentary (state 0). In Axelrodia lindeae the dentary teeth are displaced internally and their bases are bordered anteroventrally by a small continuous ridge of the dentary (state 1).
147. Separation between posterior dentary teeth: (0) less than width of these teeth; (1) more than width of these teeth.
The posterior teeth of the outer dentary row are usually conical and much reduced in size. In most species these teeth are closely situated each other, with only small spaces between them (state 0; Fig. 59). In the examined members of the Stethaprioninae (sensu Reis, 1989), and some other species, these teeth are comparatively more distant from each other, being separated by spaces broader than the width of each tooth (state 1; Fig. 43). This character is coded as polymorphic in Astyanax cf. eigenmanniorum1 and A. paris Azpelicueta, Almirón & Casciotta in which these spaces are of intermediate length.
148. Abrupt decrease in size of dentary teeth: (0) absent; (1) present. (EI8i, SE66m, SE67m, ZV85m, BÜ73m).
The outer row of dentary teeth is usually composed of four or five comparatively larger teeth with five or more cusps followed by a variable number of smaller teeth with fewer cusps. Eigenmann (1917) considered the size of the lateral dentary teeth as a generic character, distinguishing between abruptly smaller (state 1; Fig. 43) versus gradually smaller teeth (state 0; Fig. 62; Mirande et al., 2007: fig. 2). The presence of gradually decreasing dentary teeth was traditionally used to diagnose Deuterodon. This genus was later rediagnosed phylogenetically by Lucena & Lucena (2002) who did not utilize this character as a putative synapomorphy. Most of the herein examined species have a rather gradual decrease in the size of the dentary teeth, with a comparatively smaller group of species having four or five much larger anterior teeth and a posterior series of small, usually unicuspidate, teeth. According to the adopted definition of states, Charax stenopterus and Triportheus spp. are coded as state 0. This character is coded as polymorphic in Astyanax abramis, A. cf. asuncionensis Géry, A. lineatus, Brycon falcatus, B. pesu, Hemigrammus unilineatus, Jupiaba scologaster (Weitzman & Vari), and Pseudochalceus kyburzi, which have intermediate conditions.
Suspensorium
Quadrate:
149. Foramen on articular condyle of quadrate: (0) absent; (1) present. (LU59i).
The quadrate has an anterior condyle for articulation with the anguloarticular. The ventral surface of this condyle has, in medial view, a conspicuous vault that is not laterally visible in most species (state 0; Fig. 72). In some species this vault opens dorsally by way of a foramen situated on the dorsal surface of the condyle of the quadrate, with the foramen evident from a lateral view (state 1; Figs. 73 and 74). The root of this analysis is coded with state 0 because this foramen was not observed in Puntius tetrazona and is absent in Barilius and Opsariichthys according to Howes (1978: fig. 38). This character is variable among the examined specimens of Carlana eigenmanni, Hollandichthys multifasciatus, Odontostilbe paraguayensis Eigenmann & Kennedy, and Rhoadsia altipinna in which it is coded as polymorphic.
150. Form of quadrate: (0) with ventral portion longer than anterodorsal region; (1) with anterodorsal portion equal or longer than ventral region. (LU60m, LM26, MO28m, VB9, SE41m, BE45, ZV102m, QU8).
The quadrate in most examined species has rather well differentiated anterodorsal and ventral portions. The anterodorsal portion, which is situated anteriorly on the bone, contacts the ectopterygoid, mesopterygoid, and metapterygoid, while the ventral portion articulates with the symplectic and the horizontal arm of the preopercle. The quadrate borders the quadrate-metapterygoid fenestra anteriorly and ventrally. The ventral portion of the quadrate in most species is longer than the anterodorsal portion, and the fenestra is consequently longitudinally elongate (state 0; Fig. 72). In some species the anterodorsal portion is relatively more developed, equaling or surpassing the length of the ventral portion and the fenestra is rounded or dorsoventrally ovoid (state 1; Fig. 73).
151. Posterior extent of ventral process of quadrate: (0) reaching vertical through posterior margin of symplectic; (1) falling short of posterior margin of symplectic.
The ventral region of the quadrate articulates laterally with the horizontal arm of the preopercle via a variably elongate process, among the examined species. In many species this articulation reaches posteriorly to a vertical line through the posterior end of symplectic (state 0; Fig. 75), while in others it does not reach the posterior tip of the symplectic (state 1; Figs. 76 and 77). The degree of extension of the process may be related, at least in some groups, to the relative length of the horizontal arm of the preopercle or perhaps the size of the individuals. Such a correlation, if present, is not absolute because some relatively small species have this region much developed posteriorly, with the inverse also true. In the species with a dorsoventrally expanded metapterygoid-quadrate fenestra, the symplectic has an oblique orientation, and this character is not directly comparable. These cases are thus coded as inapplicable.
152. Longitudinal ridge in quadrate bordering adductor mandibulae muscle ventrally and, to some degree laterally: (0) absent; (1) present. (MO30).
The lateral surface of the quadrate medially borders to some degree the medial sections of the adductor mandibulae. In a group of taxa, the quadrate has a lateral bony longitudinal ridge that borders the adductor mandibulae both ventrally and ventrolaterally (state 1; Fig. 78). This ridge is, however, absent in most examined species (state 0). Moreira (2002) mentioned the presence of this bony ridge as a synapomorphy of the Iguanodectinae. A similar bony ridge was observed in Distichodus maculatus, but given its absence in Xenocharax spilurus, this character is coded as polymorphic for this terminal taxon.
153. Articulation between quadrate and anguloarticular: (0) anterior to or at vertical through lateral ethmoid; (1) posterior to lateral ethmoid. (LU62m, MO68, VB7m, SE42m, ZV93i, QU7m).
154. Articulation between quadrate and anguloarticular: (0) anterior to or at vertical through middle eye; (1) posterior to middle eye. (LU62m, VB7m, SE42m).
The posterior displacement of the articulation between the quadrate and the anguloarticular is related to the elongation of the lower jaw. The definition of states in this character follows Lucena (1993), who recognized three states. In most examined species of the Characidae this articulation is situated between the verticals through the lateral ethmoid and the middle of the eye (character 153, state 1; character 154, state 0), in some species it is located anterior to the vertical through the lateral ethmoid (character 153, state 0), whereas in other species it is posterior to the middle of the eye (character 154, state 1). The examined specimen of Distichodus maculatus has the state 0 in the character 153, but this compound outgroup taxon is coded as polymorphic for this character given that in Xenocharax the lower jaw is longer, corresponding to state 1. In Carlana eigenmanni and Rhoadsia altipinna, this articulation is situated posteriorly to the middle of the eye only in adult specimens, while in young individuals it is situated anterior to the middle of the eye; these species are coded as polymorphic for the character 154. Intermediate states between the states defined for the character 154 were observed in Brycon pesu and Nematocharax venustus, which are also coded as polymorphic.
155. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate: (0) absent; (1) present. (LU58mi, MO24, VB8m, SE34i).
A metapterygoid-quadrate fenestra is broadly distributed across the Characiformes. This fenestra is bordered dorsally and posteriorly by the metapterygoid and anteriorly and ventrally by the quadrate. The posteroventral margin of the fenestra is variably bordered by these bones or by a cartilage situated just dorsal to the symplectic. This portion of the quadrate has a process dorsally bordering the symplectic and posteriorly oriented towards the metapterygoid which also has a small anterior process directed anteriorly towards the quadrate in some species. In most examined species the processes from the quadrate and metapterygoid are separated by a cartilage, and consequently lack a common articular surface (state 0; Fig. 72); in other species, these processes are synchondrally articulated to each other (state 1; Fig. 73). The examined specimens of Brycon orbignyanus, Carlana eigenmanni, Nematocharax venustus, and Rhoadsia altipinna have intermediate states that are coded as polymorphisms.
Ectopterygoid:
156. Shape of ectopterygoid: (0) elongate; (1) triangular and much broadened anteriorly; (2) approximately square.
The ectopterygoid is usually elongate and situated just lateral and parallel to the mesopterygoid, in the area between the posterior margin of the palatine and the anterodorsal region of the quadrate (state 0). Among the examined species, this bone has a triangular shape in Aulixidens eugeniae, with its medial margin articulating with the mesopterygoid and the anterior margin with the palatine (state 1). In Engraulisoma taeniatum the ectopterygoid is relatively shorter and broader than in the state 0, having an approximately square shape (state 2). This character is not informative in the present analysis, other than involving autapomorphies of Aulixidens Böhlke and Engraulisoma.
157. Form of anterior portion of ectopterygoid: (0) broad and broadly articulating with palatine and connected to neurocranium by ligaments; (1) slender and articulating only to lateral margin of palatine, and lacking ligaments to neurocranium. (ZV97, ZV99).
In most examined members in the Characiformes, the ectopterygoid is broad anteriorly and broadly articulated with the palatine. In most species the ligaments joining the suspensorium to the neurocranium attach to the anterior portion of the ectopterygoid and to the palatine and are directed towards the vomer (state 0). Zanata & Vari (2005) analyzed the anterior reduction of the ectopterygoid and the presence or absence of ligaments to the neurocranium as separate characters; as these two features are correlated in the species examined, they are herein considered within the same character. It is important to note, however, that Zanata & Vari (2005) reported that the alestids Clupeocharax schoutedeni Pellegrin and Tricuspidalestes caeruleus (Matthes), which were not analyzed here, are exceptions to this correlation. Both the reduction of the anterior portion of the ectopterygoid and the absence of ligaments attached to this bone (state 1) were proposed by Zanata & Vari (2005) as synapomorphies for the family Alestidae.
158. Dorsal process of ectopterygoid oriented towards lateral ethmoid: (0) absent. (1) present.
The ventral margin of the lateral ethmoid articulates with the dorsal surfaces of the ectopterygoid and mesopterygoid through a cartilage. Most examined species lack dorsally oriented ectopterygoid processes towards the lateral ethmoid, with the exception of a rather shallow lateral bony ridge in some species (state 0). In Iguanodectes geisleri and Piabucus melanostomus there is instead a conspicuous dorsal expansion near the posterior end of this bony ridge with the expansion oriented in the direction of the lateral ethmoid (state 1; Fig. 78).
159. Ectopterygoid teeth row: (0) absent; (1) present. (VA53m, BU22mi, LU56m, MO16m, BE41, LI19, BÜ28, QU28, PZ50m).
In most examined species the ectopterygoid lacks teeth (state 0). Two patterns of teeth distribution were observed among species with ectopterygoid teeth. Some species have teeth forming a row along the lateral margin of the ectopterygoid, with these teeth rather similar in form to those of the jaws (state 1; e. g. Menezes, 1969: fig. 71). Some species have also a patch of minute teeth (see character 160). As both types of teeth are topologically and morphologically different from other and are simultaneously present in some species, they are coded as separate characters.
160. Patch of ectopterygoid teeth: (0) absent; (1) present. (LM25, TP30, PZ50m).
Ectopterygoid teeth are absent or restricted to a distinct row parallel to the lateral margin of the bone in most examined species (state 0). Some species have a patch of minute, conical, more medially positioned teeth relative to the ectopterygoid row of teeth, when the latter is present (state 1). This character is considered as independent from the preceding character because both types of teeth are simultaneously present in Hoplias cf. malabaricus.
161. Position of longitudinal cartilage dorsal to ectopterygoid: (0) bordered medially by mesopterygoid; (1) displaced laterally and separated from medial margin of mesopterygoid.
Most examined species have a cartilage dorsal to the ectopterygoid that medially borders the mesopterygoid (state 0). This cartilage is situated along all of the ectopterygoid, from its anterior articulation with the lateral ethmoid to a position near the anterodorsal region of the quadrate. In some species this cartilage is displaced laterally and does not border the medial margin of the mesopterygoid (state 1). Brycon pesu, Deuterodon langei Travassos, Jupiaba mucronata Eigenmann, J. scologaster, Triportheus nematurus (Kner), and T. pantanensis Malabarba have intermediate situations which are coded as polymorphisms.
162. Contact between ectopterygoid and anterodorsal region of quadrate: (0) present; (1) absent. (VH21, MO15, SE37, BE42m).
The ectopterygoid is situated just ventrolateral to the mesopterygoid, and usually articulates posteriorly with the anterodorsal region of the quadrate (state 0; Figs. 72 and 73). Vari & Harold (2001) mentioned the absence of contact between the ectopterygoid and quadrate in most species of Creagrutus (state 1; Fig. 45). Intraspecific variation was observed in Acrobrycon tarijae, Astyanax troya, Bryconamericus cf. iheringii, B. rubropictus, B. cf. rubropictus, B. thomasi, Cheirodon interruptus (Jenyns), Coptobrycon bilineatus, Deuterodon iguape, Jupiaba scologaster, Mimagoniates rheocharis, Nantis indefessus, N. cf. indefessus, Odontostilbe pequira, Phenacogaster tegatus, Stichonodon insignis (Steindachner), and Thayeria boehlkei, which are coded as polymorphisms.
Interopercle:
163. Anterior extension of interopercle: (0) extending anteriorly beyond terminus of horizontal arm of preopercle; (1) not extending anteriorly beyond terminus of horizontal arm of preopercle.
The interopercle in most examined species extends anteriorly beyond the anterior end of the preopercle and ventrally borders, at least partially, the region of articulation between the quadrate and anguloarticular (state 0; Fig. 75). In some species the interopercle is reduced anteriorly and does not reach the anterior margin of the horizontal arm of the preopercle (state 1; Fig. 74). In the examined specimens of Axelrodia lindeae and Carnegiella strigata the anterior margins of the interopercle and preopercle reach approximately the same vertical line, and this character is coded as polymorphic.
164. Abrupt posterior expansion of interopercle: (0) absent; (1) present.
In most examined species the interopercle gradually deepens to its posterior margin, which not overlaps the opercle in all its depth (state 0). In other species the posterior region of the interopercle is abruptly vertically expanded and its posterodorsal angle is conspicuously acute; the posterior margin of the interopercle, in these species, broadly overlaps the anteroventral margin of the opercle (state 1; Fig. 45). Intermediate situations were observed in Leporinus striatus and Odontostilbe pequira, which are coded with polymorphisms.
Mesopterygoid:
165. Mesopterygoid teeth: (0) absent; (1) present. (UJ68, VA54, BU23, LU55i, LC31, LM24, TP29, MO17, BÜ29m, PZ51).
The mesopterygoid lacks teeth in most species in the Characiformes (state 0). The presence of mesopterygoid teeth (state 1; Vari, 1995: fig. 8; Toledo-Piza, 2007: fig. 18) was proposed as a synapomorphy of a clade composed of Acestrorhynchus Eigenmann & Kennedy, Cynodon Cuvier, and Rhaphiodon Agassiz (Lucena, 1993), and was reported also in some other non-characid genera (Vari, 1995; Buckup, 1998). Lucena & Menezes (1998) also mentioned the presence of mesopterygoid teeth in these three genera, but under their phylogeny this character could be interpreted both as a synapomorphy of their Acestrorhynchidae plus Cynodontidae, with a reversion in the Roestinae, or as parallel autapomorphies of the Acestrorhynchidae and Cynodontidae. The examined specimen of Distichodus maculatus lacks mesopterygoid teeth; as these teeth were coded as present in this species by Buckup (1998), this character is coded as polymorphic.
Metapterygoid:
166. Anterodorsal lobe of metapterygoid oriented towards mesopterygoid: (0) absent or small and dorsally oriented; (1) present, conspicuous and anteriorly oriented. (CM9(11)).
In most examined species, the anterior portion of the metapterygoid has a variably developed lobe, oriented dorsally towards the mesopterygoid (state 0; Fig. 44). Malabarba (1998b) proposed the presence of a rounded anterior process as a synapomorphy of Triportheus (state 1; Fig. 76).
167. Shape of metapterygoid-quadrate fenestra: (0) rounded or ovate, anteriorly limited by anterodorsal region of quadrate; (1) anteriorly collapsed by convergence of metapterygoid and ventral region of quadrate. (MO28m).
When present, the metapterygoid-quadrate fenestra is anteriorly bordered by the anterodorsal portion of the quadrate, and has a rather ovate form (state 0; Fig. 44). In Iguanodectes geisleri and Piabucus melanostomus, among the examined species, the metapterygoid is expanded ventrally, articulating in some point of the ventral region of quadrate, limiting anteriorly such fenestra in instances when it is not completely occluded by the metapterygoid (state 1; Fig. 78).
168. Foramen in posterior region of metapterygoid: (0) absent; (1) present, encircled by metapterygoid or bordered partially by cartilage; (2) in form of incomplete arch, bordered posteriorly by hyomandibula (LU57m, VH23m, MO23m, SE35m, BE43m, LI22m, QU9m).
In most examined characids there is a foramen situated near the posterior margin of the metapterygoid which serves as passage for the ramus mandibularis trigeminus nerve. This foramen is usually situated entirely within the metapterygoid, although in some species, especially in young specimens, its posterior margin is formed by a cartilage (state 1; Figs. 44 and 73). In some species this foramen is not completely contained within the metapterygoid, and is posteriorly bordered by the hyomandibula (state 2; Figs. 72, 76, and 78), while in others it is completely absent (state 0). According to the phylogenetic hypothesis of Lucena (1993) the presence of this foramen is a synapomorphy of a clade including the Alestidae, Serrasalmidae, and Characidae (excepting Agoniates Müller & Troschel), with a reversion in a clade composed of Brycon, Bryconops, Triportheus, and the Alestidae and Serrasalmidae. Intermediate situations between the states 1 and 2 were observed in specimens of Heterocharax macrolepis and Hoplocharax goethei which are coded as polymorphisms between such states.
Opercle:
169. Posterior directed radial striae from articular region of opercle: (0) absent; (1) present.
A medial longitudinal bony ridge in the opercle serves the as site of attachment of the adductor operculi muscle, while the remaining medial surface of the opercle is smooth in most species (state 0). A series of striae situated in the medial surface of the opercle, oriented radially from the articular region of the opercle were observed in the Serrasalmidae and Prochilodus lineatus (Valenciennes) (state 1).
170. Length of medial bony ridge of opercle: (0) 60% or greater than opercular length; (1) less than 50% of opercular length.
As noted in the preceding character, the opercle has a medial bony ridge that receives fibers from the adductor operculi muscle. The length of this ridge is variable among the examined species. In most species it is less than one half the length of the opercle (state 1; Fig. 75), while in some species it achieves more than 60% of that length (state 0). Intermediate states were observed in specimens of Astyanax cf. abramis and A. asuncionensis, and these species are coded as polymorphic.
Palatine:
171. Ethmopalatine cartilage: (0) absent or reduced in size; (1) present and conspicuous.
The palatine articulates synchondrally with the vomer anteriorly and medially. Some species have a separate block of cartilage, the ethmopalatine cartilage of Fink & Fink (1981), just anterior to the palatine. When present, this receives the ascending process of the maxilla (state 1; Fig. 22). In most species this cartilage is absent or much reduced in size (state 0; Fig. 27).
172. Relative length of palatine: (0) approximately one-half length of ectopterygoid, or less; (1) distinctly longer than one-half length of ectopterygoid. (M020m).
The palatine is the most anterior bone of the suspensorium and partially forms the floor of the olfactory capsules. The size of the palatine is associated with the snout length, and the relative positions of the mouth and olfactory capsules. In some species the palatine is equal or shorter than half length of the ectopterygoid (state 0), whereas in other species the palatine is longer than half length of the ectopterygoid (state 1). Intermediate cases as coded as polymorphic.
173. Palatine foramen: (0) absent or reduced in size; (1) present and very conspicuous.
Most examined species have a palatine that is approximately rectangular in dorsal view and lacks a conspicuous foramen (state 0). A small group of species have a large foramen in the palatine, which is easily visible in both dorsal and ventral views (state 1; Fig. 79; Serra & Langeani, 2006: fig. 8). This foramen was described by Serra & Langeani (2006) for Bryconamericus exodon; however, in the examined specimens of B. exodon such foramen is variably present and this species is coded as polymorphic. Polymorphisms were also observed in Astyanax cf. abramis, A. asuncionensis, Bryconamericus cf. exodon, Diapoma terofali, and Mimagoniates rheocharis.
Preopercle:
174. Shape of posteroventral corner of preopercle: (0) acute; (1) rounded. (LU65).
The posteroventral corner of the preopercle is rounded in most examined species (state 1; Fig. 76). Lucena (1993) described an acute angle in the preopercle, present in Gnathocharax steindachneri Fowler, Heterocharax macrolepis, Hoplocharax goethei, Iguanodectes adujai Géry, Lonchogenys ilisha, and Roestes spp. (state 0).
Suprapreopercle:
175. Suprapreopercle: (0) fused to preopercle; (1) autogenous, separated from preopercle. (UJ20, BU27m, LU64i, BE49i).
The suprapreopercle is situated in contiguity or continuity with the laterosensory canal segment in the vertical arm of the preopercle and bears a canal of the laterosensory system. Usually this bone is absent or fused with the preopercle (state 0), whereas in some species the suprapreopercle is evident as an autogenous structure, completely separate from the preopercle (state 1; Fig. 34). In several species, although the suprapreopercle appears to be separate from the preopercle, it is situated very close to it. Since in these species is difficult to recognize the complete separation of the preopercle, state 1 is restricted only to cases in which the suprapreopercle is clearly separate, with an obvious gap with the preopercle. The suprapreopercle is present in Chanos Lacépède (Gonorynchiformes) and Diplomystes (Siluriformes) and was considered as primitive for Characiformes (Fink & Fink, 1981). The root of this analysis is thus coded as state 1. In the examined specimens of Charax stenopterus the suprapreopercle is not ossified and this species is coded with the state 0, although the presence of this bone is probably intragenerically variable according to the observations of Lucena (1993) for C. gibbosus. In the examined specimen of Galeocharax humeralis the laterosensory canal of the suprapreopercle is apparently autogenous but it is aligned with the preopercle. Given the coding of Lucena (1993) this character is coded herein as polymorphic. Intraspecific variation was observed in Astyanax troya, Creagrutus anary Fowler, Microschemobrycon casiquiare, and Oligosarcus sp. in which this character is similarly coded as polymorphic.
176. Bony lamellae bordering laterosensory canal of suprapreopercle: (0) absent; (1) present.
The suprapreopercle is usually limited to a tubular laterosensory canal without associated lamellae (state 0). Variably developed bony lamellae associated with the laterosensory canal of the suprapreopercle were observed in Markiana nigripinnis and Oligosarcus spp., (state 1; Fig. 34).
Branchial and hyoid arches
Anterior ceratohyal:
177. Anterior projection of anterior ceratohyal articulating laterally with hypohyals: (0) absent or much reduced; (1) present and achieving half length of hypohyals. (UJ69).
In most examined species, the proximal portion of the anterior ceratohyal articulates synchondrally with the hypohyals without bony contact between these bones (state 0). Uj (1990) mentioned the presence of a process laterally bordering the hypohyals in the African alestid Hydrocynus and the South American genera Acestrorhynchus, Agoniates, and Rhaphiodon (state 1). An intermediate state was observed in Heterocharax macrolepis among the examined species, which is coded as polymorphic.
178. Hyoid artery: (0) completely contained within anterior ceratohyal in passage from posterior ceratohyal to hypohyals; (1) emerging from anterior ceratohyal near its articulation with posterior ceratohyal. (BU25, BE50, ZV111).
A segment of the hyoid artery enters in the posterior ceratohyal and is oriented towards the dorsal hypohyal through the anterior ceratohyal. Castro (1984) and Buckup (1998) noted that in some taxa this artery is contained completely within the anterior ceratohyal as it passes to the dorsal hypohyal (state 0; Figs. 80 and 81), whereas in other species it exits by a pore near the posterodorsal margin of the anterior ceratohyal and then passes into an opening between the anterior ceratohyal and the dorsal hypohyal (state 1; Fig. 82). This state is present in most examined species of the Characidae, while state 0 is present in all members from the outgroup and some genera of the Characidae. A morphologically intermediate state was observed in Hoplocharax goethei and Piabucus melanostomus which are coded as polymorphic. In these species, the hyoid artery emerges at middle length of the anterior ceratohyal, but is contained in a deep open canal along the dorsal margin of the bone.
179. Ventral margin of anterior ceratohyal: (0) smooth and without notches; (1) with notches for articulation of branchiostegal rays. (LU68, BÜ79m).
The branchiostegal rays articulate with the ventral margin of the ceratohyals. Lucena (1993) mentioned the presence of notches along the margin of the anterior ceratohyal in most species of the Characidae (state 1; Figs. 81 and 82). These notches are absent (state 0; Fig. 80) in several characids and in most members of the outgroup. A series of small cartilages coincident in shape and position with the margins of the notches of other species were observed in the members of the Serrasalmidae. These cartilages are probably homologous with portions of the anterior ceratohyal forming these notches. Metynnis maculatus, Piaractus mesopotamicus, and Serrasalmus maculatus are thus coded as polymorphic for this character. Much reduced notches are present in the examined specimens of Hoplocharax goethei which is also coded as polymorphic.
180. Number of notches along ventral border of anterior ceratohyal: (0) zero to two; (1) three.
Species in the Characiformes usually have three branchiostegal rays articulating with the ventral margin of the anterior ceratohyal. In these species, the anterior two branchiostegal rays have a rather pedunculate anterior portion, which articulates with the notches of the ventral margin of the anterior ceratohyal. The third branchiostegal ray lacks a basal pedicle and articulates with the posteroventral surface of the anterior ceratohyal (state 0). In some members of the Cheirodontinae there is a third notch in the ventral margin of the anterior ceratohyal where the third branchiostegal ray articulates. This ray, however, lacks a developed anterior pedicle (state 1). The presence and development of this third notch is variable among the examined specimens of Aphyocharax dentatus and this species is coded as polymorphic.
181. Articulation between anterior and posterior ceratohyals: (0) synchondral, without bony interdigitations; (1) with bony interdigitations. (VA57, LI37).
In most examined species, the anterior ceratohyal articulates synchondrally with the posterior ceratohyal without any bony interdigitations between the bones (state 0; Figs. 80 and 82). Uj (1990) mentioned the presence of interdigitations between these bones (state 1; Fig. 83) as a synapomorphy of his Cynopotaminae (=Characinae, in part). Vari (1995) mentioned the presence of such interdigitations as a synapomorphy of a clade including the families Ctenoluciidae and Erythrinidae, and independently occurring in the lebiasinid genera Lebiasina Valenciennes and Piabucina Valenciennes.
Basibranchials:
182. First basibranchial: (0) absent or much reduced, not articulating anteriorly with basihyal; (1) well developed and articulating anteriorly with basihyal.
Members in the Characiformes have three or four ossified basibranchials situated medial to the hypobranchials. The anterior margin of the first basibranchial articulates with the posterior margin of the basihyal in almost all the examined taxa (state 1; Fig. 84). In some members of the outgroup, the first basibranchial is absent or much reduced, and it does not contact the posterior margin of the basihyal (state 0).
183. Contact between lamella on anterior portion of first basibranchial with lamella on posterior portion of second basibranchial: (0) absent; (1) present.
Basibranchials usually bear, anteriorly and/or posteriorly, thin bony lamellae which project dorsal to the cartilages situated between their main portions. These lamellae apparently develop as autogenous ossifications and usually fuses to the main portion of the basibranchials during ontogeny, as suggested by the presence of autogenous lamellae in some examined specimens. The bony lamellae are usually situated between the first and second and the second and third basibranchials. Some species have also a bony lamella situated dorsal to the fourth basibranchial, which is usually completely cartilaginous. The bony lamellae situated between the first and second basibranchials are usually absent or not contacting each other (state 0; Fig. 86), whereas in some species these lamellae form a bony bridge between the main portions of the first and second basibranchials (state 1; Fig. 85).
184. Bony lamellae between second and third basibranchials: (0) absent; (1) present. (PZ66m).
The presence of bony lamellae between the basibranchials is variable among the examined species and often independent in terms of occurrence between pairs of basibranchials; this character is therefore considered as not correlated with the preceding one. The bony lamellae between the second and third basibranchials are present in most examined species (state 1; Fig. 84). In some species these lamellae are completely absent, and the space between the main portion of the second and third basibranchials is filled only by cartilage (state 0; Fig. 86).
185. Bony lamella dorsal to fourth basibranchial: (0) present; (1) absent. (LU74, VH32, MO56, BE53i, LI43i, BÜ82i, PZ67i).
The fourth basibranchial is completely cartilaginous in most examined species. Lucena (1993) mentioned the presence of an ossified fourth basibranchial in many species of the Characidae; however, based on his coding, it is likely that Lucena was instead referring to the bony lamellae situated just dorsal to the fourth basibranchial (state 0; Figs. 84 and 87). In the phylogenetic hypothesis of Lucena (1993), the presence of this lamella is a synapomorphy of a clade including most characids, with a reversal in a clade composed of Creagrutus, Ctenobrycon Eigenmann, Iguanodectes, and Piabina. Contrary to the observations of Lucena (1993) and Benine (2004), this ossification is present in the examined specimens of Bryconamericus exodon, Deuterodon iguape, Hemigrammus unilineatus, and Poptella paraguayensis; these species are coded as polymorphic, in light of probable intraspecific variations. The presence of this bone is variable among the examined specimens of Astyanax cf. eigenmanniorum1 and Probolodus heterostomus and this character is also coded as polymorphic in these taxa. The bony lamella dorsal to the fourth basibranchial is absent (state 1; Fig. 88) in the examined specimens of Exodon paradoxus and Hyphessobrycon herbertaxelrodi Géry. Since Lucena (1993) and Benine (2004), respectively, noted the presence of this lamella in these species this character is coded as polymorphic for these taxa. In the examined specimen of Prionobrama paraguayensis the fourth basibranchial is ossified and bordered dorsally by the bony lamella, and this species is therefore coded as state 1.
186. Main portion of fourth basibranchial: (0) completely cartilaginous; (1) ossified.
As noted above, the fourth basibranchial is usually completely cartilaginous or has only a dorsal bony lamella (state 0; Figs. 87 and 88). In Phenagoniates macrolepis, Prionobrama paraguayensis, and Xenagoniates bondi among the examined species, the main portion of the fourth basibranchial is ossified (state 1). This character could not be examined in Paragoniates alburnus which is coded as a missing entry.
187. Teeth on lamella dorsal to fourth basibranchial: (0) absent; (1) present. (LM36, PZ68).
Lucena (1993) proposed the presence of teeth on the fourth basibranchial (state 1) as independent autapomorphies of Acestrorhynchus and Agoniates. Such teeth are usually absent (state 0) in the Characiformes. In the phylogenetic hypothesis of Lucena & Menezes (1998) the presence of teeth on the lamella dorsal to the fourth basibranchial is also optimized as a parallelism between Acestrorhynchus and Agoniates.
Basihyal:
188. Cartilages anterior to basihyal: (0) one or two blocks of cartilage, but anterior block much smaller; (1) two well developed blocks of cartilage. (VA56).
The anterior margin of the basihyal has a cartilaginous margin bordered anteriorly by an autogenous block of cartilage which supports the anterior portion of the primary tongue (state 0; Figs. 84 and 88). Some species have two independent autogenous blocks of cartilage anterior to the basihyal; these blocks are approximately of the same width and as wide as the basihyal (state 1; Fig. 87). In some species the anterior block of cartilage is much smaller and these cases were coded state 0. Both the homology between these cartilages and possible ontogenetic variations should be assessed in more focused studies. Intraspecific variation in this character were observed in Astyanax cf. eigenmanniorum2, A. endy, A. latens, A. troya, Cheirodon interruptus, Hollandichthys multifasciatus, Hyphessobrycon socolofi, and Psellogrammus kennedyi, which are coded as polymorphic.
189. Edentulous basihyal lamella: (0) absent; (1) present. (ZV109i, LI42).
Vari (1983: 24) described an "edentulous basihyal tooth plate" (state 1; Fig. 85; Vari, 1983: fig. 22) as a lamella situated just anterior and dorsal to the basihyal. This lamella is probably a serial homologous to the lamellae situated between the basibranchials. As this lamella, when present, lacks teeth in the Characiformes, is here referred as edentulous basihyal lamella. Such a lamella is absent in most examined species (state 0; Fig. 88). Zanata & Vari (2005) mentioned the presence of this lamella in Hepsetus odoe (Bloch), Hoplias microlepis, Triportheus albus, Xenocharax spilurus, and all of the alestids examined by them.
190. Anterior development of basihyal: (0) broadly extending beyond anterior margin of hypohyals; (1) slightly surpassing anterior margin of hypohyals.
The anterior margin of the basihyal usually extends distinctly anterior of the hypohyals, being the anteriormost element of the hyoid arch (state 0; Fig. 84). In the examined specimens of Carnegiella strigata, Thoracocharax stellatus, and Triportheus spp., the basihyal is relatively reduced, not extending anteriorly beyond the hypohyals, or at most slightly surpassing that elements (state 1; Fig. 85).
191. Form of anterior expansion of basihyal: (0) slender, with anterior margin less than two-thirds of its length; (1) expanded, with anterior margin with two-thirds or more of its length. (SE76m).
The anterior margin of the basihyal supports the anterior portion of the primary tongue. The basihyal expands laterally as it progresses anteriorly from its relatively slender articulation with the first basibranchial. In most species this expansion is limited, and the anterior margin of the basihyal have less than two-thirds of its length (state 0; Fig. 88). In some species the anterior region of the basihyal is much expanded laterally and the anterior margin have two-thirds or more of the length of this bone (state 1; Fig. 89). The basihyal in Bryconamericus cf. exodon, Cheirodon interruptus, Diapoma speculiferum, and Probolodus heterostomus has an intermediate state which is coded as polymorphic.
Gill rakers:
192. Rows of gill rakers on first ceratobranchial: (0) one; (1) two. (LU73i, LC34, SE83m, BÜ85, PZ61i).
193. Rows of gill rakers on second ceratobranchial: (0) one; (1) two. (LC35, SE83m, PZ62mi).
194. Rows of gill rakers on third and fourth ceratobranchials: (0) one; (1) two. (PZ63mi, PZ64i).
Most members of the outgroup have two rows of gill rakers on the first ceratobranchial (character 192, state 1), while most characids have only one row of gill rakers in this bone (character 192, state 0). The first row of gill rakers is situated along the leading margin of the ceratobranchial and the second row is located along the trailing margin. In almost all the examined species, there are two rows of gill rakers on the third and fourth ceratobranchials (character 194, state 1). The number of rows of gill rakers on the different ceratobranchials is not independent, according to the observations done for this paper. All species having two rows on the first ceratobranchial, have also two rows on the remaining ceratobranchials; correspondingly, all the species with two rows on the second ceratobranchial (character 193, state 1) have two rows on the third and fourth ceratobranchial, although not necessarily on the first ceratobranchial. In this way, this character can be seen as an ordered character with four states. The examined specimen of Cyanocharax alburnus has only one minute gill raker on the second row of the first ceratobranchial and this character is coded as polymorphic.
195. Number of gill rakers on first hypobranchial and ceratobranchial: (0) 16 or more; (1) 15 or fewer. (CM16(18)m, SE82m, BÜ90m).
196. Number of gill rakers on first hypobranchial and ceratobranchial: (0) 11 or more; (1) ten or fewer. (CM16(18)m, SE82m, BÜ90m).
The number of gill rakers is continuously variable among the examined taxa. Therefore, the limits of the states used in characters 195 and 196 are rather subjective and they were defined, in part, considering their congruence with the remaining characters under preliminary analyses. Malabarba (1998b) defined the states of this character with different ranges. According to her phylogeny, a high number of gill rakers (more than 20) (character 195, state 0) is a synapomorphy of Triportheus. Intraspecific variations in the character 196 was observed in Astyanax abramis, A. cf. abramis, and Prodontocharax melanotus, and in the character 195 in Astyanax troya, Coptobrycon bilineatus, Cynopotamus argenteus, Galeocharax humeralis, Grundulus cochae, Hasemania nana, Hemibrycon surinamensis, Hollandichthys multifasciatus, Hoplocharax goethei, Jupiaba mucronata, Micralestes stormsi, Mimagoniates rheocharis, Nematobrycon palmeri, Odontostoechus lethostigmus, Roeboides descalvadensis, and Thayeria boehlkei. These species are coded as polymorphic for the variable character.
197. Shape of first ceratobranchial gill rakers: (0) pointed and not anteroposteriorly compressed; (1) laminar and much compressed perpendicular to ceratobranchial; (2) short, broad and strongly denticulate. (UJ70m, LU71m, LM35m, TP38, PZ65m).
The gill rakers are morphologically rather homogeneous within the Characiformes, with a broad proximal region which articulates with the corresponding ceratobranchial and a progressively slender distal region (state 0; Fig. 83). Among the examined species of the Parodontidae the gill rakers are laminar, much reduced anteroposteriorly, and situated perpendicularly to the main axis of the ceratobranchial (state 1). Lucena (1993) mentioned the presence of short broad gill rakers bearing strong denticles in their dorsal surface (state 2; Toledo-Piza, 2000: fig. 12) in Acestrorhynchus pantaneiro, Cynodon gibbus (Agassiz), and Rhaphiodon vulpinus. This state is a synapomorphy of a clade composed on these genera in his phylogeny. According to Lucena & Menezes (1998), the optimization of this character is ambiguous, and this state could be a synapomorphy for their Acestrorhynchidae plus Cynodontidae, with a reversal in their Roestinae, or a parallelism between the Acestrorhynchidae and Cynodontidae. According to the phylogeny of Toledo-Piza (2007), the presence of the state 2 is a synapomorphy for a clade consisting of Acestrorhynchus, Cynodon, Hydrolycus Müller & Troschel, and Rhaphiodon.
198. Form of anterior gill rakers on first ceratobranchial: (0) not fused; (1) with fused bases forming plates extensively articulated with ceratobranchial.
The gill rakers are lanceolate and similar in form each other in most examined species (state 0). In Cynopotamus argenteus and Galeocharax humeralis the posterior gill rakers of the first ceratobranchial are similar to those of other examined species, while the anterior ones are progressively shorter and broader in these species. The gill rakers situated along the anterior one-third of the first ceratobranchial and on the first hypobranchial are fused to each other, thereby forming strongly denticulate plates (state 1; Fig. 86).
199. Lateral base of gill rakers on first ceratobranchial: (0) slender; (1) broad and laminar at least on anteriormost gill rakers.
The gill rakers are usually articulated with the first ceratobranchial by two slender anterolaterally and posteromedially oriented bases and aligned along an approximately transverse line relative to the main axis of the ceratobranchial (state 0; Fig. 83). In some species the anterolateral base of the gill rakers is expanded, forming a lamella extensively articulated with the first ceratobranchial (state 1; Fig. 81). This lamella is usually notched basally and appears to be composed of two parallel bony platelets. As mentioned under the preceding character, the anterior gill rakers of Cynopotamus argenteus and Galeocharax humeralis are expanded basally. This state, however, is not comparable to the states defined in this character, which is coded as inapplicable for those species. In Characidium spp., Carnegiella strigata, Puntius tetrazona, and Thoracocharax stellatus the anterolateral base of the gill rakers is reduced and this character is coded as inapplicable.
200. Form and degree of ossification of first ceratobranchial gill rakers: (0) laminar and not ossified distally; (1) rather thick and completely ossified distal region.
In most examined species, the gill rakers are slender and are not ossified distally (state 0; Mirande et al., 2007: fig. 3). In some species the gill rakers are relatively stronger and completely ossified (state 1; Fig. 83). This character has an intermediate state in Hollandichthys multifasciatus which is coded as polymorphic.
201. Denticles on gill rakers: (0) present; (1) absent. (CM17(19)m, SE80m, BÜ93m).
Denticles on the gill rakers (state 0; Mirande et al., 2007: fig. 3) are broadly distributed in the Characiformes, especially among the members of the Characidae. The presence and distribution of these denticles were considered by Bührnheim & Malabarba (2006) in the systematics of the genus Odontostilbe. The absence of these denticles was observed in most members of the outgroup and some characids (state 1).
202. Distribution of denticles on gill rakers: (0) restricted to margins, or absent; (1) along entire surface of gill rakers.
The denticles of the gill rakers, when present, are usually more densely distributed along the anterior and posterior margins of the rakers, where they are approximately aligned into a row along each margin (state 0). In some species these denticles are more densely distributed and situated also on the lateral surfaces of the gill rakers, (state 1), as mentioned by Mirande et al. (2007) for Astyanax chico, A. puka Mirande, Aguilera & Azpelicueta, and A. troya. This character, along with the preceding one can be considered as an additive character with three states; therefore the absence of gill rakers is coded in both characters. In Jupiaba mucronata and Moenkhausia xinguensis the denticles are largely restricted to margins of the gill-rakers, but some isolated denticles occur on their lateral surface and these species are coded as polymorphic.
203. Rows of gill rakers on first epibranchial: (0) one; (1) two. (LC38i).
In most examined species each of the four epibranchials has two rows of gill rakers (state 1). A single row of gill rakers on the first epibranchial (state 0) was observed in this study only in Acestrorhynchus pantaneiro, Agoniates anchovia, and Salminus brasiliensis.
Ceratobranchials:
204. Shape of dentigerous plate of fifth ceratobranchial: (0) rounded, with posterior notch; (1) elongated, without posterior notch. (VH33i, MO58i).
The fifth ceratobranchial bears a dentigerous plate in its posterior margin. This plate in most examined species is rather elongate, with obtuse angles with the main body of the fifth ceratobranchial both in their anterior and posterior regions (state 1; Vari & Harold, 2001: fig. 13A). In a small number of species the dentigerous plate has a rounded shape and its posterior region forms a straight or acute angle with the main body of the fifth ceratobranchial (state 0; Vari & Harold, 2001: fig. 13B). Uj (1990) mentioned the rounded shape of the dentigerous plate of the fifth ceratobranchial as a synapomorphy of his Piabucidae (= Iguanodectinae). Uj cited also this character state for the genus Creagrutus.
205. Teeth on fifth ceratobranchial: (0) present; (1) absent.
Most species in the Characiformes have teeth on the fifth ceratobranchial (state 0; Vari & Harold, 2001: fig. 13). The absence of such teeth (state 1) was proposed as a synapomorphy of the Curimatidae plus Prochilodontidae by Vari (1983) and it was observed in this study only in Cyphocharax spilotus and Prochilodus lineatus.
Pharyngobranchials:
206. Teeth on third pharyngobranchial: (0) present; (1) absent. (LC37, VH34, MO57, SE87m, BÜ84).
The third pharyngobranchial bears teeth in most members of the Characidae (state 0) and these teeth are absent in a relatively small group of examined species (state 1). Contrary to the reported by Moreira (2002), teeth on the third pharyngobranchial are present in the examined specimens of Brycon pesu and Pseudocorynopoma doriae, which are coded as polymorphic. Iguanodectes geisleri is also coded as polymorphic following the observations of Moreira (2002) for this species.
207. Teeth on fourth pharyngobranchial: (0) present; (1) absent. (BU41).
The fourth pharyngobranchial bears teeth in most species of the Characiformes (state 0). The absence of teeth in this bone (state 1) was proposed as a synapomorphy of the Anostomoidea (Anostomidae, Chilodontidae, Curimatidae, and Prochilodontidae) by Vari (1983). The ancestral condition for the Characiformes is unclear. The Cypriniformes lack dentigerous plates on the fourth and fifth pharyngobranchials (Fink & Fink, 1981, 1996) while Diplomystes, considered a basal siluriform, bears teeth on the third and fourth pharyngobranchials (Azpelicueta, 1994). This character is coded as missing for the root of this analysis.
208. Teeth on fifth pharyngobranchial: (0) present; (1) absent.
The fifth pharyngobranchial bears teeth in most taxa in the Characiformes (state 0). The absence or reduction of such teeth (state 1) is a synapomorphy of a clade composed of the Curimatidae and Prochilodontidae according to Vari (1983). For the same reasons as in character 207, the root is coded as missing.
209. Contact between fourth and fifth pharyngobranchial dentigerous plates: (0) absent; (1) present. (UJ81, BU42i).
In most characiforms the dentigerous plates of the fourth and fifth pharyngobranchials contact each other and form a continuous surface (state 0; Vari, 1983: fig. 13). The absence of contact between these pharyngobranchials (state 1; Vari, 1983: figs. 16-19) was considered by Vari (1983) as a synapomorphy of the Anostomoidea.
Interhyal:
210. Interhyal: (0) present; (1) absent.
The interhyal joins the suspensorium with the hyoid arch in almost all the examined species. This bone contacts dorsally with the symplectic and hyomandibula by means of cartilages; the interhyal contacts ventrally with the posterior ceratohyal (state 0). The interhyal is absent (state 1) in Aulixidens eugeniae and Engraulisoma taeniatum among the examined species.
211. Length of interhyal: (0) shorter than one-third of symplectic length; (1) equal to or longer than one-half of symplectic length.
The interhyal is a cylindrical bone much shorter than the symplectic in most examined species (state 0; Fig. 72). A configuration of the jaws and suspensorium including a shortening of the horizontal arm of the preopercle, the lengthening of the dentary, and a dorsal displacement of the posterior end of the symplectic was observed in some species. The dorsal displacement of the symplectic in this arrangement is correlated with an elongation of the interhyal to a relatively longer form (state 1). Although this character could be associated with predation, some apparently predatory genera such as Bramocharax and Oligosarcus have state 0.
Branchiostegal rays:
212. Number of branchiostegal rays: (0) three; (1) four or five. (VA61m, LU70, LM33m, TP33m, ZV112i, LI39).
213. Number of branchiostegal rays: (0) three or four; (1) five. (VA61m, LU70, LM33m, TP33m, ZV112i, LI39, PZ57).
Most characiforms have four branchiostegal rays (character 212, state 1; Character 213, state 0). Vari (1995) mentioned the presence of five branchiostegal rays (character 213, state 1) in Erythrinus Scopoli, Hoplerythrinus Gill and Hoplias and considered this state as a synapomorphy of the Erythrinidae. Lucena & Menezes (1998) considered the possession of five branchiostegal rays as a synapomorphy of the Cynodontinae. Variation between four and five branchiostegal rays was observed in Astyanax cf. asuncionensis, Cyanocharax alburnus, Knodus breviceps, and Nantis cf. indefessus with these species coded as polymorphic for character 213. The Cypriniformes has three branchiostegal rays, but both the Gymnotiformes and Siluriformes have more than five and the ancestral state for the Characiformes is unknown. Therefore, the root of this analysis is coded as missing.
214. Anterior portions of branchiostegal rays: (0) broad near their articulation with ceratohyals; (1) slender near their articulation with ceratohyals. (BE56).
In most examined species the branchiostegal rays are anteriorly expanded near their articulation with the ceratohyals (state 0; Fig. 80). In some characids, especially in members of the Characinae, the branchiostegal rays are slender along their entire length and lack the cited expansion (state 1; Fig. 82).
215. Attachment of first branchiostegal ray: (0) on proximal one-half length of anterior ceratohyal or anterior to that; (1) posterior to one-half length of anterior ceratohyal.
Brycon meeki has three branchiostegal rays attached to the ventral margin of the anterior ceratohyal (Weitzman, 1962). The first branchiostegal ray articulates to some point on the proximal one-half of the anterior ceratohyal, usually along its proximal one-third (state 0; Fig. 80). In the examined specimens of Triportheus spp. the attachment site of the branchiostegal rays is displaced posteriorly to the posterior half of the ceratohyal (state 1).
216. Distance between attachment site of first and second branchiostegal rays: (0) equal or shorter than distance between second and third rays; (1) longer than distance between second and third rays.
The first and second branchiostegal rays are usually closer to each other than are the second and third rays (state 0; Fig. 82). In some species, however, the first and second branchiostegal rays are relatively more distant from each other, being separated by a distance equal to or greater than the distance between the second and third rays (state 1; Fig. 83).
217. Number of branchiostegal rays attached to posterior ceratohyal: (0) one; (1) two. (VA60, LU69i, LM34, TP34, PZ58).
Most examined species share the possession of three branchiostegal rays articulated with the anterior ceratohyal, plus one attached to the posterior ceratohyal as described by Weitzman (1962) (state 0; Fig. 82). Vari (1995) mentioned the presence of two branchiostegal rays articulating with the posterior ceratohyal (state 1) in Ctenolucius spp, while Lucena (1993) also coded this state in Acestrorhynchus pantaneiro and Rhaphiodon vulpinus and considered the presence of two branchiostegal rays articulating with the posterior ceratohyal as a synapomorphy of a monophyletic clade composed of Acestrorhynchus, Cynodon, and Rhaphiodon, with a parallel occurrence in Ctenolucius. Lucena & Menezes (1998) proposed the inclusion of Gilbertolus and Roestes (subfamily Roestinae) in their family Cynodontidae, as sister group of Cynodon and Rhaphiodon. Since Gilbertolus and Roestes have state 0, the optimization of this character is ambiguous in the analysis of Lucena & Menezes (1998) for the clade containing the Acestrorhynchidae and Cynodontidae. This character could be optimized both as a synapomorphy of the Acestrorhynchidae and Cynodontidae, with a reversion in the Roestinae, or as parallelisms in the Acestrorhynchidae and Cynodontinae.
Vertebrae, ribs and Weberian apparatus
Weberian apparatus:
218. Form and articulation of neural pedicle of third vertebra: (0) pedicle well developed and articulating synchondrally with neural complex; (1) pedicle much smaller and without an articular surface with neural complex.
In most ingroup species the neural pedicle of the third vertebra is smaller relative to the condition described by Weitzman (1962) for Brycon meeki. In that species the posterior margin of the neural pedicle articulates with the neural arch of the fourth vertebra, and its dorsal margin articulates with the neural complex (state 0; Fig. 7; Weitzman, 1962: fig. 12). This situation was observed herein in most members of the outgroup and some representatives of the Characidae. In the remaining species the neural pedicle of the third vertebra is reduced to a furca extending dorsally to the neural complex but not articulated synchondrally with that bone. Consequently the ventral margin of the neural complex is articulated solely with the neural arch of the fourth vertebra (state 1; Fig. 8; Fink & Fink, 1981: fig. 16). In Puntius tetrazona and Opsariichthys (Fink & Fink, 1981), the neural arch of the third vertebra is well developed and broadly articulates with the anterior supraneurals. This situation is coded herein as state 0. In the gymnotiform Sternopygus Müller & Troschel, the situation is similar to that of Xenocharax and Brycon meeki (Fink & Fink, 1981; Weitzman, 1962), which constitutes additional evidence to consider state 0 as ancestral for the Characiformes. This character is coded as polymorphic in Galeocharax humeralis which demonstrates an intermediate state. Apparently, the neural arches of the third and fourth vertebrae of Engraulisoma taeniatum are fused each other, and this character is coded as inapplicable.
219. Development of transverse process of neural arch of third vertebra: (0) not reaching anterior margin of tripus; (1) well developed and extending beyond anterior margin of tripus. (VA71m, ZV114m).
The transverse process of third neural arch projects anteriorly or anterodorsally from the main body of the bone and receives the posterior tip of the intercalarium. Uj (1990) considered the great development of this process as diagnostic of his Agoniatidae (=Agoniatinae). Vari (1995) mentioned that this process is relatively reduced in the Ctenoluciidae and not reaching the posterior end of the intercalarium. In most species this process does not reach the anterior end of the tripus (state 0; Fig. 6) while in others it extends beyond the tripus anteriorly (state 1; Fig. 7). Intermediate states or intraspecific variability were observed in Bryconexodon juruenae, Diapoma speculiferum, Hoplocharax goethei, Lonchogenys ilisha, Moenkhausia xinguensis, and Odontostoechus lethostigmus which are coded as polymorphic.
220. Ascending process of neural pedicle of third vertebra: (0) absent; (1) present. (UJ9m, LU76m).
In most examined species, the neural pedicle of the third vertebra has a variably developed ascending process directed towards the neural complex (state 1; Fig. 8). In several outgroups this process is completely absent (state 0). A reduced dorsal expansion of the neural pedicle was observed in Characidium rachovii and Distichodus maculatus, and these species are coded as polymorphic.
221. Dorsal development of dorsal process of neural pedicle of third vertebra: (0) not broadly overlapping neural complex; (1) broadly overlapping neural complex. (UJ37m, BU45m, LU76m).
The dorsal process of the neural pedicle of the third vertebra, when present, is directed dorsally towards the neural complex. In most species this process falls short of the ventral margin of the neural complex, or only overlaps the margin slightly (state 0; Fig. 8), whereas in some species this process extensively overlaps the neural complex (state 1). Intermediate states were observed in Astyanax cf. rutilus, Carlana eigenmanni, Creagrutus anary, Hollandichthys multifasciatus, Hyphessobrycon luetkenii, Moenkhausia dichroura, and Oligosarcus sp. These species are coded as polymorphic. The cases in which this process is completely absent are coded as inapplicable.
222. Neural arch and vertebral centrum of fourth vertebra: (0) not fused and with autogenous fourth neural arch; (1) fused. (BU49).
Fink & Fink (1981) mentioned that the neural arch of the fourth vertebra is autogenous (state 0; Fink & Fink, 1981: figs. 14-15) in the Citharinidae and Distichodontidae and considered this state as plesiomorphic for the Characiformes, due to its presence in the Cypriniformes and Gymnotiformes. Among the examined species, the neural arch of the fourth vertebra is only autogenous in Puntius tetrazona and Distichodus maculatus, corroborating the observations of those authors. This neural arch is fused to the corresponding vertebral centrum in the remaining examined species (state 1; Weitzman, 1962: fig. 12).
Ribs:
223. Anteriorly directed spine at base of first rib: (0) absent; (1) present. (LU81m).
In most examined species, the base of the first rib posterior to the Weberian apparatus bears an expansion onto which a ligament directed towards the following rib and parapophysis attaches. This medially directed expansion is approximately triangular (state 0). In some species there is, additionally, a well-developed spine projected anteriorly from that expansion (state 1; Fig. 90). Zanata & Vari (2005) described a process in Acestrorhynchus microlepis (Jardine), Brycon spp., and Chalceus spp., which is medially directed and projects from the main body of the rib. That feature is non-homologous with the process described in the state 1 of this character.
224. Laminar bony ridge on dorsal margin of abdominal ribs: (0) absent; (1) present. (BE59).
The dorsal margin of the abdominal ribs lacks conspicuous projections in most examined species (state 0). Lucena (1993) mentioned the presence of dorsal bony ridges in the third to sixth abdominal ribs (state 1; Fig. 91) as parallel autapomorphies of Moenkhausia lepidura (Kner) and Parecbasis cyclolepis. Benine (2004) proposed the presence of bony ridges on abdominal ribs as a synapomorphy of a clade within Moenkhausia including M. lepidura.
225. Abdominal ribs on anterior caudal vertebrae: (0) absent; (1) present, associated to first and occasionally second caudal vertebrae.
In most species, the ribs are articulated to the abdominal and transitional vertebrae both of which lack haemal spines (state 0). In the examined species of the Serrasalmidae, the posterior ribs are associated with the first, or first and second, caudal vertebrae (state 1).
Vertebrae:
226. Relative number of precaudal vertebrae: (0) exceeding caudal vertebrae in two or more elements; (1) equal or less numerous than caudal vertebrae. (LU80m, MO87m).
The precaudal vertebrae include the abdominal and transitional vertebrae. In most examined species of the Characidae the caudal vertebrae are as numerous or less numerous than the precaudal vertebrae (state 1). In some members of the outgroup, in contrast, the precaudal vertebrae are more numerous than the caudal vertebrae (state 0).
227. Total number of vertebrae: (0) 40 or fewer; (1) 41 or more.
The number of vertebrae is almost continuously variable across the examined species. The limits of the states used herein are, therefore, rather subjective. Most examined species have a vertebral number comprised of between 35 and 38 vertebrae (state 0), while in some species a higher number was observed, exceeding 40 vertebrae (state 1). This character is coded as polymorphic for Distichodus maculatus, Galeocharax humeralis, and Hoplias cf. malabaricus which have ranges of vertebral counts overlapping the defined states. The examined specimen of Puntius tetrazona has 29 vertebrae, but the root of this analysis is coded as polymorphic because Opsariichthys, considered to be a generalized cypriniform by Fink & Fink (1981) has more than 40 vertebrae (Howes, 1978).
228. Total number of transitional vertebrae: (0) four or more; (1) three or fewer. (UJ47m, ZV121m).
Haemal processes are ventral projections of the vertebrae that form part of the haemal arch and the haemal spine of the caudal vertebrae. Transitional vertebrae have bilateral haemal processes but lack a haemal spine (Weitzman, 1962: fig. 14b). Most examined species have fewer than four transitional vertebrae, most often two (state 1), while some species have four or more (state 0). Variation between these states was observed in Acrobrycon tarijae and Iguanodectes geisleri, which are coded as polymorphic.
229. Transitional vertebrae with haemal canal: (0) present; (1) absent.
Additionally to the haemal processes, the posteriormost transitional vertebrae usually bear a haemal canal which is also present in the caudal vertebrae. This canal is formed by a transverse bony bridge between the contralateral haemal processes (state 0; Weitzman, 1962: figs. 14b and 14c). In some species this type of transitional vertebrae is absent, and the first vertebra that bears a haemal canal also has a haemal spine, being therefore the first caudal vertebra (state 1). This character is coded as polymorphic in Aphyocharacidium bolivianum, Astyanax latens, Bryconamericus cf. rubropictus, Carlana eigenmanni, Coptobrycon bilineatus, Hyphessobrycon pulchripinnis, and Psellogrammus kennedyi, which show variation between the defined states.
Pectoral girdle
Pectoral fins:
230. Margin of first pectoral ray in adult specimens: (0) not serrated; (1) conspicuously serrated. (VB41).
The external margin of the first pectoral and pelvic fins is formed by a cartilage situated between the hemiradii that constitute such ray. This margin is usually not serrated (state 0). Bertaco (2003) reported the presence of a serrated margin of this cartilage in Hollandichthys and Pseudochalceus as a synapomorphy for a clade composed of those genera (state 1). This condition was also observed in this study in Apareiodon affinis, Parodon nasus, and Characidium spp.
231. Base of second pectoral ray: (0) large and partially overlapping base of first pectoral ray from medial view; (1) similar in form and size to base of posterior rays. (ZV137i).
The base of each pectoral-fin ray has an expansion where several branches of the adductor radialis muscle attach (Winterbottom, 1974). In most examined species the base of the first pectoral-fin ray, usually the single unbranched ray, is much expanded in comparison to the bases of the posterior rays, all of which are of similar size (state 1). In a relatively small group of species, the base of the second pectoral-fin ray is much expanded and conspicuously laterally overlaps the base of the first fin ray (state 0; Fig. 92). Zanata & Vari (2005) mentioned that state 0 is broadly distributed in the Alestidae, being also present in several other characiforms. Zanata & Vari (2005) considered the state present in Hoplias microlepis as inapplicable, because the second pectoral-fin base is not conspicuously larger that those of the posterior rays, and it is oriented anteromedially rather than dorsally. A similar condition was observed in this study in Hoplias cf. malabaricus and Pyrrhulina australis in which this character is coded as inapplicable.
Cleithrum:
232. Anterior margin of cleithrum: (0) slightly sinuous; (1) with anterior pointed projection. (UJ64m, LU88, LC42m, PZ77m).
Lucena (1987) described a pointed projection of the anterior margin of the cleithrum lateral lamella (state 1; Fig. 93) of Charax. This projection extends laterally and posteriorly the surface where the sternohyoideus muscle attaches to the cleithrum. Uj (1990) mentioned the presence of such projection in his Cynopotamidae (genera Cynopotamus and Galeocharax Fowler) and Characidae (most of the Characinae). According to Lucena (1998), such a process is absent (state 0; Fig. 94) in, among others, Cynopotamus argenteus. The anterior process of the cleithrum was, however, observed in this species in this study and C. argenteus is coded as polymorphic for this character.
233. Form of posterior margin of cleithrum: (0) convex or slightly sinuous just dorsal to pectoral-fin insertion; (1) with notch just anterior to pectoral-fin insertion. (UJ64m, BU57, LU88, LC43, MO65, PZ78).
The lateral lamella of the cleithrum is situated just dorsal to the pectoral-fin insertion and usually has a slightly sinusoidal form that partially follows the anterior margin of this fin (state 0; Fig. 94). Lucena (1987) described the presence of a conspicuous notch broadly surrounding the anterior margin of the pectoral-fin insertion (state 1; Fig. 93) in Charax. Buckup (1998) coded this notch as present in Charax sp., Cynopotamus argenteus, and Phenacogaster microstictus Eigenmann, proposing it as a synapomorphy for a clade composed of these three species. A much reduced notch was observed in this study in Galeocharax humeralis and Hoplocharax goethei, which are coded as polymorphic.
234. Posterior margin of cleithrum: (0) without concavity ventral to first postcleithrum; (1) with concavity ventral to first postcleithrum. (BE68).
235. Posterior margin of cleithrum: (0) with concavity poorly pronounced or lacking; (1) with markedly concave margin, almost forming straight angle. (BE68).
In most examined species the posterior margin of cleithrum, is slightly concave in the region immediately ventral to the first postcleithrum. The position and degree of concavity of this region of the cleithrum is almost continuously variable among the examined species; however the cases in which the concavity is absent and those in which this concavity is much pronounced are clearly recognizable. The states herein defined follow in general those of Benine (2004), who differentiated a state in which the cleithrum lacks a concavity (character 234, state 0; Fig. 94), a state in which there is a slight concavity (character 234, state 1; character 235, state 0; Fig. 95) and a state in which this concavity is much marked, almost forming a straight angle (character 235, state 1; Fig. 96). The examined specimen of Hemigrammus unilineatus has the state 0 of the character 234, but this species is here coded as polymorphic given the observations of Benine (2004), who coded it with state 1.
236. Medial laminar expansion at dorsal tip of cleithrum: (0) absent; (1) present. (CM19(1?)).
Malabarba (1998b) proposed the presence of a medially oriented laminar blade-like projection at the dorsal tip of the cleithrum (state 1; Fig. 97) as a synapomorphy of Triportheus. This lamella is absent (state 0) in the remaining examined species.
237. Dorsal development of cleithrum: (0) much extended dorsally to mesocoracoid; (1) ending in a position just dorsal of tip of mesocoracoid. (LM20, CM18(10), PZ81).
The cleithrum, in most examined species, obviously projects dorsal of the tip of the mesocoracoid. The dorsal portion of the cleithrum in this condition is longer than one-half the length of the mesocoracoid (state 0). Malabarba (1998b) proposed a reduction of the dorsal extent of the cleithrum, which projects just slightly dorsal to mesocoracoid (state 1; Fig. 97) as a synapomorphy of Triportheus.
Coracoid:
238. Development of medial lamella of coracoid: (0) not expanded; (1) expanded as a keel. (CM21, MO63).
In most examined species, the medial lamellae of the coracoids do not articulate with each other but rather diverge posteriorly from a ventral view. Furthermore, in this condition the coracoids are not conspicuously expanded ventrally (state 0). In some species the coracoids lamellae are much expanded ventrally and medially contacting each other and forming a keel (state 1). Weitzman (1960) mentioned the presence of a pectoral keel in some phylogenetically distant groups as in the genera Ilisha Richardson, Odontognathus Lacépède, Opisthopterus Gill, and Raconda Gray (Clupeiformes: Clupeidae) and in Chela Hamilton (Cypriniformes: Cyprinidae). Within the Characiformes this author mentioned the presence of a pectoral keel in the genera Piabucus Oken, Pseudocorynopoma Perugia, Rhaphiodon, Triportheus, and the members of the Gasteropelecidae. A coracoid keel was also reported for Cynodon (Toledo-Piza, 2000) and Lignobrycon (Malabarba, 1998b). In Pseudocorynopoma doriae the coracoid lamellae are enlarged relative to other characids, but they do not articulate medially to form a keel and this species is coded as state 0.
239. Bony ridge of coracoid between base of mesocoracoid and ventral margin of interosseous space: (0) absent; (1) present.
The interosseous space (Starks, 1930) is a fenestra situated anterior to the region of articulation between the mesocoracoid and coracoid and is limited dorsally by the cleithrum and ventrally by the coracoid; this fenestra is present in most species in the Characiformes. In most examined species the lateral surface of the ventral lamella of the coracoid is relatively smooth and lacks obvious ridges in the region just anterior to its articulation with the mesocoracoid (state 0; Fig. 98). A bony ridge situated on the lateral surface of the coracoid between its articulation with the mesocoracoid and the interosseous space was observed in several species (state 1; Fig. 97). In Carnegiella strigata, Characidium spp., Hoplias cf. malabaricus, and Thoracocharax stellatus, the coracoid has a different set of modifications and this character is coded as inapplicable to these species. A broadening of the coracoid in a position similar to that of the ridge coded in this character was observed in Leporinus striatus. Given that the correspondence between these structures is doubtful, this character is also considered as inapplicable to L. striatus.
240. Anterior extension of coracoid ventral lamella: (0) reaching cleithrum; (1) not reaching cleithrum. (UJ34, BU55, LU84).
In most examined species the ventral lamella of the coracoid extends anteriorly to the cleithrum (state 0). According to Buckup (1998), the coracoid is reduced anteriorly in Crenuchus spilurus and Hoplias malabaricus and does not reach the cleithrum (state 1). Lucena (1993) mentioned that such a reduction in the anterior extension of the coracoid is present in Erythrinus erythrinus (Bloch), Hoplerythrinus unitaeniatus (Spix & Agassiz), and Hoplias malabaricus, among the taxa examined by him, and that it constitutes a synapomorphy of the Erythrinidae.
241. Ventral extension of coracoid lamella: (0) reaching ventral margin of cleithrum; (1) falling short of ventral margin of cleithrum.
The medial lamella of the coracoid projects ventrally to different degrees among the examined taxa. Usually its ventral margin reaches the body wall and it is visible through the skin and scales in some alcohol-preserved individuals. In such cases, the ventral margin of the coracoid reaches the ventral end of the cleithrum (state 0; Fig. 99). In Coptobrycon bilineatus, Grundulus cochae, Gymnocharacinus bergii, and Hoplias cf. malabaricus among the examined species, the medial lamella of the coracoid is much smaller ventrally and does not reach the ventral margin of the cleithrum (state 1; Fig. 100).
242. Anterior limit of interosseous space: (0) formed by dorsal margin of coracoid medial lamella and dorsal margin of cleithrum; (1) formed by dorsal margin of coracoid medial lamella and an oblique bony ridge located just ventral to dorsal margin of cleithrum.
In most examined species the interosseous space is limited anteriorly by the junction of the dorsal margin of the coracoid medial lamella and the dorsal margin of the cleithrum. The anterior end of the coracoid in such cases articulates through interdigitations with the medial margin of the cleithrum (state 0; Fig. 98). In some species the coracoid articulates anteriorly with an oblique bony ridge of the posterior surface of the anterior region of the cleithrum, and the interosseous space is limited anteriorly by these two elements. In these cases there are no interdigitations of the coracoid with the medial region of the cleithrum (state 1; Fig. 101). Although the interosseous space is absent in Characidium spp., the coracoid articulates anteriorly with a bony lamella in a mode comparable to that described in the state 1, and these species are coded as that state. The coracoid of Hoplias cf. malabaricus is much reduced and it does not articulate anteriorly with the cleithrum, and this character is coded as inapplicable for this species.
243. Coracoid foramen: (0) absent or reduced to small pore; (1) well developed. (LU85, CM20i, VB46i, PZ79m).
The coracoid foramen is a large rounded opening situated just anteroventral to the region of articulation of the mesocoracoid and coracoid (state 1; Fig. 99) as described for Brycon meeki by Weitzman (1962). This opening is absent or reduced to a small pore in most of the species examined here (state 0; Fig. 98).
Scapula:
244. Process of scapula forming anterior border of scapular foramen: (0) present; (1) absent. (LU87, VH48, ZV136).
The scapula borders posteriorly the scapular foramen in the Characiformes; this foramen in most examined species is also limited anteriorly by a ring-like projection of the anterolateral margin of the scapula (state 0; Fig. 97). In several characiforms such anterior process is reduced or absent and does not form the anterior margin of the scapular foramen (state 1). Intraspecific variability in this character was observed in this study in Aulixidens eugeniae, Bryconamericus agna, Bryconexodon juruenae, Diapoma terofali, Hyphessobrycon pulchripinnis, Nematobrycon palmeri, and Roeboides descalvadensis, which are coded as polymorphisms.
Mesocoracoid:
245. Articulation between ventral process of mesocoracoid and dorsal margin of scapula: (0) absent or small; (1) present and broad. (VH47).
In most species the mesocoracoid is columnar except in its dorsal region, which is variably expanded to articulate only with the cleithrum in most of the Characiformes (state 0; Fig. 98). As described by Vari & Harold (2001) in the species of Creagrutus this dorsal expansion of the mesocoracoid is projected ventrally to articulate synchondrally with the dorsal margin of the scapula (state 1; Fig. 102). Vari & Harold (2001) mentioned that such contact is also present in Triportheus, although they considered that condition as not homologous with that present in Creagrutus. A similar situation to that described for Creagrutus was observed here in Characidium spp., Gymnocharacinus bergii, and Hoplias cf. malabaricus. In Puntius tetrazona a projection of the middle region of the mesocoracoid to the scapula, ventral to its articulation with the cleithrum, was observed. Given that this state is different from those coded in this paper, the root of this analysis is coded as inapplicable. A cartilage of similar shape to the mesocoracoid process was observed in Pyrrhulina australis; however, since the correspondence of this cartilage to the ossified process is uncertain, this character is also coded as inapplicable for this species. In the lebiasinid Poecilobrycon (=Nannostomus) harrisoni (Eigenmann), illustrated by Weitzman (1964) this process is absent, at least as an ossification.
246. Ventral articulation of mesocoracoid: (0) anteriorly with coracoid and posteriorly with scapula; (1) only with coracoid.
The mesocoracoid in most examined species articulates ventrally with the coracoid and scapula (state 0; Fig. 98), whereas in the examined specimen of Prionobrama paraguayensis the mesocoracoid only articulates ventrally with the coracoid (state 1). This character constitutes an autapomorphy of this species in this analysis; however it is maintained in the data matrix considering its potential phylogenetic utility in future studies.
Postcleithra:
247. First postcleithrum: (0) present. (1) absent. (VH50, ZV132, QU43).
Three postcleithra are present in most members of the Characiformes (state 0). Among the examined species the first postcleithrum is absent (state 1) in Diapoma spp., Mimagoniates rheocharis, Pseudocorynopoma doriae, Puntius tetrazona, and Thoracocharax stellatus. In Puntius tetrazona, as is general in the Cypriniformes (Fink & Fink, 1981; Howes, 1978, 1979, 1980), there is a single postcleithrum, which corresponds in form and position to the second and third postcleithra which are apparently fused into one element. Fink & Fink (1981, 1996) proposed that the loss of the first postcleithrum occurred independently in the Gonorynchiformes, Cypriniformes, and Siluriformes. However, the most parsimonious optimizations of this character, according to the state distribution presented by those authors, is clearly ambiguous. It could be interpreted as a gain of the first postcleithrum in the (Characiformes (Siluriformes + Gymnotiformes)), with a secondary loss in the Siluriformes, or alternatively as a parallel acquisition of the first postcleithrum in the Characiformes and Gonorynchiformes. In both cases, the absence of the first postcleithrum is the most parsimonious optimization for the clade including the Cypriniformes and the remaining Otophysi. As mentioned, the ancestral state in the Characiformes is ambiguous, and this character is coded as polymorphic in the root of this analysis.
248. Second postcleithrum: (0) present; (1) absent. (VA65, BU58, LU89, LM30, CM25m, TP55, MO60, ZV133, QU45).
The presence (state 0) or absence (state 1) of the second postcleithrum was considered in several phylogenetic analyses concerning different groups of the Characiformes. Vari (1995) mentioned the absence of this postcleithrum in Boulengerella lateristriga (Boulenger), B. maculata (Valenciennes), Ctenolucius spp., and Hepsetus odoe. The absence of the second postcleithrum was also mentioned for the Gasteropelecidae (Weitzman, 1954), Engraulisoma taeniatum (Castro, 1984), Lignobrycon myersi (Miranda-Ribeiro) (Malabarba, 1998b), Gilbertolus atratoensis Schultz (Toledo-Piza, 2000), and the species of Rhabdalestes Hoedeman (Zanata & Vari, 2005). In Puntius tetrazona, as is general for the Cypriniformes (Fink & Fink, 1981, 1996), there is a single postcleithrum that would correspond to the fused second and third postcleithra based on its position and shape. The same condition is present in the Citharinidae and Distichodontidae (Vari, 1979). Given that the second postcleithrum is usually present in the remaining characiforms, the ancestral state of this character for the Characiformes is not clear and the root of this analysis is coded as polymorphic.
249. Third postcleithrum: (0) present; (1) absent. (VA64, BU59, LU90, LM31, CM25m, TP56, ZV134, QU46, PZ75).
The presence (state 0) or absence (state 1) of the third postcleithrum is usually associated to that of the second one. Vari (1995) mentioned the absence of the third postcleithrum in the genera Boulengerella, Ctenolucius, and Hepsetus Swainson. The absence of such postcleithrum was similarly cited for the members of the Gasteropelecidae (Weitzman, 1954), Engraulisoma taeniatum (Castro, 1984), Cynodon gibbus, Gnathocharax steindachneri, Hemigrammopetersius (=Rhabdalestes) rhodesiensis (Ricardo-Bertram), Paragoniates alburnus, Rhaphiodon vulpinus, Triportheus elongatus Günther [=T. auritus (Valenciennes)] (Lucena, 1993), the species of Chilodus Müller & Troschel (Vari et al., 1995), Pyrrhulina australis (Buckup, 1998), the species of Lignobrycon Eigenmann & Myers (Malabarba, 1998b), Gilbertolus atratoensis (Toledo-Piza, 2000), Clupeocharax schoutedeni, and Hemigrammopetersius barnardi (Herre) (Zanata & Vari, 2005).
250. Form of third postcleithrum: (0) slender, without associated lamella; (1) with a posterior lamella. (VB42, CA22m, SE70, BE69, ZV135i, QU47, PZ76).
The third postcleithrum is usually slender and rather sinuous as described by Weitzman (1962) for Brycon meeki. The degree of curvature of this bone is almost continuously variable among the examined species, and this variation is consequently not considered in this paper. In some species the third postcleithrum has an associated bony lamella dorsally (state 1; Fig. 93), while this lamella is absent in other species (state 0; Fig. 102). This character is coded as inapplicable to the species in which the third postcleithrum is absent or fused with the second postcleithrum. The presence of a lamella associated with the third postcleithrum is variable among the examined specimens of Grundulus cochae, and this species is coded as polymorphic.
251. Dorsal development of third postcleithrum: (0) projects dorsally to posterior region of scapula; (1) not projects dorsally to posterior region of scapula.
The third postcleithrum of most examined species reaches a position dorsal of the posterior projection of the scapula (state 0). In Agoniates anchovia, Aphyocharax spp., and Gymnocharacinus bergii this postcleithrum is smaller dorsally and reaches only the ventral margin of the posterior projection of the scapula (state 1). This character is coded as inapplicable in the species having the third postcleithrum absent or fused with the second. It is also coded as inapplicable to Prionobrama paraguayensis in which this postcleithrum is present only as a cartilaginous structure.
Posttemporal:
252. Position of ventral margin of posttemporal: (0) anterior to lateral margin of epioccipital; (1) lateral or posterior to lateral margin of epioccipital. (BE67m, BÜ94m).
253. Position of ventral end of posttemporal: (0) anterior or lateral to lateral margin of epioccipital; (1) posterior to lateral margin of epioccipital. (BE67m, BÜ94m).
The dorsal region of the posttemporal bone is situated laterally to the posttemporal fossa immediately anterior to the anterior margin of the epioccipital. From that location the posttemporal runs obliquely posteroventrally to its articulation with the cleithrum. The position of the ventral region of the posttemporal varies among the examined species. Benine (2004) mentioned that the posttemporal is usually situated just lateral to the epioccipital (character 252, state 1; character 253, state 0), while in some species it is situated anteriorly (character 252, state 0), or posteriorly (character 253, state 1). Benine (2004) however, did not refer to the position of some specific region of the posttemporal, and his observations are not directly comparable with those made here. Intermediate situations in the second of these characters were observed in Astyanax mexicanus, A. troya, Hyphessobrycon bifasciatus, H. eques, Nantis indefessus, N. cf. indefessus, and Piabucus melanostomus, which are coded as polymorphic.
Supracleithrum:
254. Ventral exit of laterosensory canal of supracleithrum: (0) covered by posterior lamella of supracleithrum and exiting medially; (1) ventral to lamella of supracleithrum and exiting on posterior margin of this bone. (SE71m).
The posterior margin of the supracleithrum overlaps to some degree the anterior scales of the body. This bone is pierced by a laterosensory canal that exits towards the first scale of the lateral line; however, with the region where the canal exits the supracleithrum variable. In most examined species the supracleithrum bears a lamellar posterior region dorsally margining, at least partially, the first scale of the lateral line. In these species the laterosensory canal exits near the posterior margin of the supracleithrum within a notch formed by the lamella and the main body of the supracleithrum (state 1; Fig. 48). In some species this notch is absent and the posterior lamella of the supracleithrum extensively laterally covers the first scale of the lateral line. In these species the laterosensory canal of the supracleithrum exits medial to the supracleithrum (state 0; Fig. 47). A different situation was observed in Pseudocorynopoma doriae, in which the laterosensory canal of the supracleithrum exits laterally to the bone. This character is coded as inapplicable for this species.
255. Fusion between posttemporal and supracleithrum: (0) absent; (1) present.
The posttemporal and supracleithrum are independent ossifications (state 0) in most examined species. Weitzman (1954) mentioned that in his Gasteropelecinae (=Gasteropelecidae) the posttemporal and supracleithrum are fused into a single ossification (state 1; Weitzman, 1954: fig. 7). This state was also mentioned by Castro (1984) for Engraulisoma taeniatum; this author considered this fusion as an autapomorphy of this species that is absent in Clupeacharax anchoveoides Pearson, the hypothesized sister taxon of E. taeniatum.
Pelvic girdle
Pelvic fin:
256. First pelvic-fin ray: (0) not branched; (1) branched. (VB55).
The first pelvic-fin ray is not branched in most examined species (state 0). Bertaco (2003) mentioned that this ray is branched in the species of Hollandichthys (state 1) and a synapomorphy of the genus. Quevedo (2006) reported the branching of this ray in Mimagoniates rheocharis, although the specimens of the species herein examined have state 0. This species is coded as polymorphic in light of possible intraspecific variability.
257. Relative length of first pelvic-fin ray of adult males: (0) not extending beyond margin of other rays; (1) extending beyond margin of other rays. (MA15, BÜ103).
The first, usually unbranched, pelvic-fin ray is slightly longer than the remaining rays in most examined species (state 0). Malabarba (1998a) mentioned that this ray extends distinctly beyond the remaining pelvic-fin rays in Odontostilbe fugitiva Cope, O. mitoptera (Fink & Weitzman), and O. pequira (state 1; Bührnheim & Malabarba, 2007: fig. 23). According to the phylogeny proposed by Malabarba (1998a), this state is a synapomorphy of Odontostilbe Cope.
258. Number of branched pelvic-fin rays: (0) six or less; (1) seven or more. (BU63m, LU95m, MA14i, MO68m, VB57m, BE70, ZV138m, QU59m).
259. Number of branched pelvic-fin rays: (0) seven or less; (1) eight or more. (BU63m, LU95m, MO68m, VB57m, ZV138m, QU59m).
Most examined species of the Characidae have seven branched pelvic-fin rays (character 258, state 1; character 259, state 0). The presence of only six branched pelvic-fin rays (character 258, state 0) was cited as diagnostic for Cyanocharax (Malabarba & Weitzman, 2003). Intraspecific variability between six and seven branched pelvic-fin rays was observed in Aphyocharax nattereri and Hemigrammus erythrozonus, which are coded as polymorphic for the character 258. The presence of eight or more branched pelvic-fin rays (character 259, state 1) was reported by some members of the Citharinidae, Crenuchidae, Distichodontidae, Hemiodontidae (Buckup, 1998), and Alestidae (Zanata & Vari, 2005). Variations between seven and eight branched pelvic-fin rays were observed in Astyanax paris, Hemigrammus bleheri, and Leporinus striatus, which are coded as polymorphic for the second of these characters.
Pelvic bone:
260. Pelvic bone: (0) not bifurcate anteriorly; (1) bifurcate with conspicuous notch. (UJ26, BU62i, MO69m, ZV139i).
In most examined species the pelvic bone has an overall cylindrical longitudinal axis and a variably developed medial lamella (state 0). Vari (1979) proposed the anterior bifurcation of the pelvic bone (state 1; Zanata & Vari, 2005: fig. 29) as a synapomorphy of the Citharinidae plus Distichodontidae. According to Fink & Fink (1981) these families form the sister group of the remaining characiforms and this shape of pelvic bone is a synapomorphy of the Otophysi and consequently plesiomorphic for the Characiformes. Buckup (1998) observed the anterior bifurcation of the pelvic bone in Crenuchus spilurus, Distichodus maculatus, and Xenocharax spilurus, coding it as absent in Citharinus gibbosus, among others, indicating some variability within the Citharinidae. Zanata & Vari (2005) mentioned a similar bifurcation in the examined species of Bryconaethiops.
261. Articulation between pelvic bones: (0) through ligaments; (1) with bony interdigitations between ischiatic processes.
In most examined species, the pelvic bones are loosely joined by ligaments (state 0). This situation is particularly pronounced in Acestrocephalus sardina, Cynopotamus argenteus, and Galeocharax humeralis, in which the pelvic bones are relatively broadly separated from each other; such variation, however, is not considered in this paper. In a relatively small group of species, the pelvic bones articulate each other by way of bony interdigitations of their ischiatic processes (state 1). The articulation of the pelvic bones was reported by Uj (1990) for Agoniates anchovia and by Winterbottom (1980) for the anostomid Pseudanos trimaculatus (Kner). Castro (1984) mentioned this condition also in Clupeacharax anchoveoides and Engraulisoma taeniatum; proposing it as a putative synapomorphy of a clade composed of these two taxa. Castro considered the state of these genera as different to that of Agoniates, in which such processes are articulated by way of a cartilage. The condition in Agoniates, Clupeacharax, and Engraulisoma are herein all coded as state 1.
262. Anterior extension of pelvic-bone along main axis: (0) not projecting anterior of lateral and medial lamellae; (1) projecting anterior of lateral and medial lamellae of pelvic bone. (CM26, ZV140, PZ86i).
The medial margin of the pelvic bone is formed by a cylindrical process, whose anterior tip terminates in a small cartilage in almost all the examined species. The anterior portion of the pelvic bone along its primary axis is situated near the anterior confluence of the lateral and medial pelvic bony lamellae. In these cases the primary axis of the pelvic bone does not extend anteriorly (state 0). Malabarba (1998b) considered the anterior projection of the primary axis of the pelvic bone beyond the lateral and medial lamellae (state 1; Malabarba, 1998b: fig. 12) as a synapomorphy for a clade composed of Lignobrycon and Triportheus. In Jupiaba spp., the anterior portion of the pelvic bone is modified as an acute point, and this character is coded as inapplicable. This character is also coded as inapplicable for the species in which the pelvic bone is anteriorly bifurcate.
263. Anterior tip of pelvic bone: (0) rounded and capped by a small cartilage; (1) pointed, lacking associated cartilage and frequently projecting outside body wall.
The anterior end of the pelvic-bone primary axis is rounded and capped by a small cartilage in most species in the Characiformes (state 0). In the species of Jupiaba this bone is very acute anteriorly and lacks this cartilage (Zanata, 1997) (state 1; Zanata, 1997: fig. 4A). In some species of this genus, the anterior tip of the pelvic bone pierces the body wall and projects outside as an externally visible spine. State 1 was observed only in the examined species of Jupiaba.
264. Dorsal longitudinal ridge on medial lamella of pelvic bone: (0) present; (1) absent.
The principal axis of the pelvic bone is bordered medially by a variably developed lamella. This lamella is usually uniformly thin (state 1; Fig. 103), whereas in some species the medial lamella has instead a dorsal longitudinal ridge that forms a secondary axis which diverges anteriorly from the primary axis (state 0; Fig. 104); this character is coded as inapplicable to species with an anteriorly bifurcate pelvic bone.
Epineurals, supraneurals, and dorsal fin skeleton
Dorsal fin:
265. Relative position of dorsal-fin anterior insertion: (0) anterior to or at vertical through pelvic-fin origin; (1) posterior to vertical through pelvic-fin origin. (CM29m).
In most examined characids, the dorsal fin is situated posterior to the vertical through the pelvic-fin origin (state 0). In some species, mostly in the outgroup, the dorsal-fin origin is situated at the vertical through the pelvic-fin insertion or slightly anterior of that line (state 1). This character varies among the examined specimens of Bryconops affinis and the A. eugeniae is coded as polymorphic. The cyprinids Opsariichthys spp., Puntius tetrazona, and Zacco spp. and, usually, the siluriforms have also the state 0. The root of this analysis was consequently coded as this state, although many species in the Cypriniformes have state 1 (Pflieger, 1997).
266. Dorsal-fin rays articulating with first dorsal pterygiophore: (0) two; (1) three or four. (LU98i, SE75, ZV124i).
The unbranched dorsal-fin rays articulate with the first proximal dorsal-fin pterygiophore with the number of rays articulating with that pterygiophore variable among the examined species. Lucena (1993) coded his functional outgroup and several species as having three or more rays articulating with this pterygiophore (state 1). Zanata & Vari (2005) mentioned the presence of only two rays associated with the first dorsal pterygiophore (state 0) in the members of the Alestidae and considered the possession of three rays as a common condition among the Neotropical characiforms. The presence of only two unbranched and eight branched dorsal-fin rays (ii+8) was considered by Malabarba & Weitzman (2003) as a diagnostic character of their clade A. This character is variable among the examined specimens of Aphyodite grammica, Astyanax paris, Brycon pesu, Carlana eigenmanni, Hollandichthys multifasciatus, Hyphessobrycon herbertaxelrodi, Inpaichthys kerri, Markiana nigripinnis, Moenkhausia cf. intermedia, Oligosarcus cf. jenynsii, Parecbasis cyclolepis, and Roeboides descalvadensis, which are coded as polymorphic.
267. Anteriorly oriented spine formed by first dorsal-fin ray: (0) absent; (1) present.
Among the species having three unbranched dorsal-fin rays, the first ray is usually similar to the posterior ones, but smaller (state 0). The Stethaprioninae was diagnosed by Géry (1964a) as having the first dorsal-fin ray oriented anteriorly to form a spine (state 1; Fig. 105); Reis (1989) considered this state to be a synapomorphy of this subfamily. Géry (1977: 367) illustrated an anterior projection of the first dorsal-fin ray of Prochilodus; however, as noted by this author, this projection have paired anterior processes, rather than a medial spine as in the Stethaprioninae of Reis (1989) and the situation in Prochilodus is considered non-homologous to that described in the state 1.
268. Anterior rays of dorsal fin of adult males: (0) not elongate; (1) elongate and reaching posteriorly to position close to adipose fin. (VB36, CA28).
The dorsal fin in most examined species is not sexually dimorphic, or when elongate it does not demonstrate the overall shape in males described for state 1. Cardoso (2003b) reported the posterior elongation of the first dorsal-fin rays (state 1) in adult males of Carlana eigenmanni, Nematocharax venustus, Parastremma pulchrum Dahl, Pseudochalceus spp., Pseudocorynopoma doriae, and Rhoadsia spp. In this condition, the last unbranched and first branched dorsal-fin rays reach posteriorly almost to the adipose fin. In Odontostilbe spp. only the last unbranched dorsal-fin ray is extended as a filament. This situation is herein considered as non-homologous to state 1 but rather treated in the following character.
269. Last unbranched dorsal-fin ray of adult males: (0) approximately as long as first branched ray; (1) distinctly longer than first branched ray and in the form of filament. (MA11, BÜ102).
In most examined species, the last unbranched and the first branched dorsal-fin rays are the longest rays with the dorsal-fin rays gradually decreasing in length posteriorly (state 0). Malabarba (1998a) noted that the posteriormost unbranched dorsal-fin ray is much longer than the remaining rays and extended as a filament (state 1; Bührnheim & Malabarba, 2007: fig. 23) in adult males of Odontostilbe fugitiva and Holoshesthes (=Odontostilbe) pequira . According to Malabarba (1998a), the elongation of this ray is a synapomorphy for the species of Odontostilbe.
270. Number of branched-rays on dorsal-fin: (0) eight or fewer; (1) nine or more. (AM2m, VB38m, BE64m, QU38m).
The number of branched dorsal-fin rays is rather uniform across the Characiformes with most examined species having nine rays (state 1). Malabarba & Weitzman (2003) partially diagnosed their clade A by the shared possession of only eight branched dorsal-fin rays (state 0) in almost all the members of that clade, with this state also present is Clupeacharax and Engraulisoma and variably in Paracheirodon, Piabucus, Serrabrycon Vari, and Tyttobrycon Géry. Malabarba & Weitzman (2003) mentioned the presence of nine branched dorsal-fin rays in an examined paratype of Aulixidens eugeniae; however the four examined specimens of this species have only eight rays and the species is coded here as polymorphic.
271. Relative length of anterior dorsal-fin rays: (0) not reaching tip of posterior rays when adpressed; (1) reaching tip of posterior rays when adpressed.
In Puntius tetrazona, as usual in the Cypriniformes, the length of the posterior dorsal-fin rays is similar to that of the anterior rays, consequently reaching more posteriorly in the adpressed fin (state 1). In most characid species, the anterior rays are proportionally longer and extend posteriorly to the tip of posterior rays, when the fin is adpressed (state 1).
272. Number of dorsal-fin rays on last pterygiophore: (0) one; (1) two, adnate. (BU64i, LU100i, MO78i, ZV126i).
Most examined characids have only one ray in the last dorsal-fin pterygiophore (state 0), while the last two dorsal-fin rays articulate with the last dorsal-fin pterygiophore (state 1) in several species of the outgroup. According to the phylogenetic hypothesis of Buckup (1998), the presence of only one ray articulating with the last pterygiophore is a synapomorphy of a clade composed of the Acestrorhynchidae, Characidae, Ctenoluciidae, Erythrinidae, Hepsetidae, and Lebiasinidae. Under the phylogeny of Lucena (1993), the presence of two rays on the last dorsal-fin pterygiophore is a synapomorphy of the Cynodontidae, that independently occurs in a clade composed of Alestes, Brycon, Chalceus, Rhabdalestes, Hydrocynus, and Serrasalmus Lacépède, with a reversal in Brycon.
Intermuscular bones:
273. Dorsal myorhabdoi: (0) absent; (1) present. (TP67).
The dorsal myorhabdoi are slender intermuscular bones situated dorsal to the epineurals (Chapman, 1944; Weitzman, 1954). These bones are present (state 1; Weitzman, 1954: fig. 2) in the Gasteropelecidae (Weitzman, 1954), the species of Citharinus Cuvier, and Rhaphiodon vulpinus (Toledo-Piza, 2000), but absent (state 0) in most members of the Characiformes.
274. Position of anteriormost epineurals: (0) lateral to fourth or fifth vertebrae; (1) reaching to cranium. (LM38, MO85m, ZV123m, PZ74).
In most examined species the anteriormost epineurals are situated posterior to the fourth or fifth vertebrae (state 0). In some taxa including species of Carnegiella Eigenmann, Thoracocharax Fowler (Weitzman, 1954), Clupeacharax (Castro, 1984), Acestrorhynchus, Agoniates, Chalceus, Cynodon, Gilbertolus, Lignobrycon, Rhaphiodon, Triportheus (Lucena & Menezes, 1998), Brycinus, Hydrocynus, and Micralestes Boulenger (Zanata & Vari, 2005), the anterior epineurals are situated along the posterior margin of the cranium, and in some of these species they originate in the posttemporal fossa (state 1; Fig. 5).
Dorsal pterygiophores:
275. Predorsal spine formed by first dorsal pterygiophore: (0) absent; (1) present. (AM21).
The presence of a predorsal spine formed by the first dorsal-fin pterygiophore was proposed by Machado-Allison (1983) as a synapomorphy of the Serrasalmidae (state 1). This spine is absent (state 0) in some Serrasalmidae and all non-serrasalmid Characiformes.
276. Number of dorsal pterygiophores: (0) nine; (1) 10 or more. (LU97m, VB38m, ZV125m).
277. Number of dorsal pterygiophores: (0) 10 or less; (1) 11 or more. (LU97m, VB38m, ZV125m).
278. Number of dorsal pterygiophores: (0) 11 or less; (1) 12 or more. (LU97m, VB38m, ZV125m).
Most examined species have 10 dorsal-fin pterygiophores and nine branched dorsal-fin rays (character 276, state 1). Lucena (1993) mentioned the presence of 11 or more dorsal pterygiophores (character 277, state 1) in Chalceus sp., Cynodon gibbus, Hoplias malabaricus, Rhaphiodon vulpinus, and Serrasalmus (=Pristobrycon) striolatus (Steindachner). Zanata & Vari (2005) mentioned the presence of 18 pterygiophores in Crenuchus Günther, 13 in Hoplias, 14 in Piaractus Eigenmann, 16 in Serrasalmus, and 18 in Xenocharax (character 278, state 1). Variations between 10 and 11 pterygiophores were herein observed in Hyphessobrycon herbertaxelrodi and Inpaichthys kerri, which are coded as polymorphic for character 277.
Supraneurals:
279. Supraneural anterior to neural spine of fourth vertebra: (0) absent or small; (1) present and vertically elongate. (BU47, LU96, MO74, SE98i, BE63i, ZV122, LI45, QU90, PZ76).
The supraneurals are situated between the neural spines anterior to the dorsal-fin pterygiophores. In most examined species the anteriormost supraneural is situated between the neural spines of the fourth and fifth vertebrae (state 1), whereas in some species there is a supraneural situated more anteriorly, anterior to the neural spine of the fourth vertebra (state 0; Fig. 7). According to Buckup (1998) the loss of this supraneural would be a synapomorphy of a clade composed of the genera Oligosarcus, Tetragonopterus, Phenacogaster, Charax, and Cynopotamus. Under the phylogenetic hypothesis of Lucena (1993) this supraneural was independently lost in four clades and a synapomorphy for a clade including most of the Characidae except for Agoniates, Brycon, Bryconops, Hemibrycon, Roeboexodon, and Triportheus. Zanata & Vari (2005) observed that in the Alestidae the absence of this supraneural is rather correlated with events of miniaturization. This supraneural is present in the examined specimen of Acestrorhynchus pantaneiro, contrary to the observations of Lucena (1993). This species is coded as polymorphic in light of possible intraspecific variability. In Apareiodon affinis, Parodon nasus, and Prochilodus lineatus, there is a large supraneural bounding anteriorly, dorsally, and posteriorly the neural spine of the fourth vertebra. Since the homology of this supraneural with the one treated here is uncertain, this character is coded as inapplicable for these species. In the examined specimens of Hasemania nana, there is an additional supraneural situated just posterior to the neural spine of the fourth vertebra. Given that the homology of this additional supraneural is unclear, this character is also coded as inapplicable for H. nana.
280. Number of supraneurals: (0) four or fewer; (1) five or more. (MO76m, SE95m, BE61m).
281. Number of supraneurals: (0) seven or fewer; (1) eight or more. (MO76m, SE95m, LI46, QU78m).
This character is related with the dorsal-fin position and the number of vertebrae; however, it is included as a separate feature given its potential phylogenetic informativeness at different levels. This character is coded considering the intraspecific variations mentioned in the original species descriptions. The examined specimen of Puntius tetrazona has only four supraneurals, but Barilius, a genus related with Opsariichthys has 13 (Howes, 1978), covering the entire range of variation considered for this character between these species. Therefore, the root of this analysis is coded as polymorphic for both these characters.
282. Bony lamellae associated with supraneurals: (0) absent or small; (1) wider than primary axis of supraneurals. (MO75m, SE96m, BE62, LI44).
The supraneurals usually have a more or less vertical, cylindrical, body, and variably developed anterior and posterior bony lamellae, which when present are wider dorsally. The degree of development of these lamellae is difficult to define as discrete states. The herein recognized states are lamellae that are absent or are narrower than the main cylindrical body of the supraneurals (state 0), and that in which the lamellae are wider than the body (state 1). This character is coded as polymorphic in the species in which these lamellae are variably present.
283. Position of last supraneural: (0) located two or fewer vertebrae in front of first dorsal pterygiophore; (1) located more than two vertebrae in front of first dorsal pterygiophore.
The posteriormost supraneural in most examined species is situated between the neural spines of the vertebrae immediately anterior to the first dorsal-fin pterygiophore (state 0). In a few species the posteriormost supraneural is distant three or more neural spines from the first dorsal pterygiophore (state 1).
Anal fin and pterygiophores
Anal fin:
284. Anal-fin position: (0) posterior or almost posterior to vertical through last dorsal-fin ray. (1) extended anteriorly ventral to dorsal fin. (EI16, MO77).
The anal-fin origin, in most examined species, is situated at or posterior to the vertical through the base of the posteriormost dorsal-fin ray (state 0), whereas in some species the anal fin extends anteriorly below the middle of the dorsal fin (state 1).
285. Number of unbranched anal-fin rays: (0) three or fewer; (1) four or more (ZV142m, LI51m).
Most examined species have four to six unbranched anal-fin rays (state 1), with a high intraspecific variation within this range. Zanata & Vari (2005) mentioned that most alestids have only three unbranched anal-fin rays (state 0), sharing this state with Crenuchus spilurus, Hepsetus odoe, and Hoplias microlepis, among the species examined by them, with most characids having four or five of such rays (state 1; Fig. 106). This character is herein coded as polymorphic in Aphyocharacidium bolivianum, in which a variation between three or four rays was observed.
286. Number of branched anal-fin rays: (0) 10 or less; (1) 11 or more. (UJ76m, BU65m, LU103m, SE92m, BE78m, LI52m).
287. Number of branched anal-fin rays: (0) 17 or less; (1) 18 or more. (LU103m, WM35i, MO80m, SE92m, BE78m, LI52m).
288. Number of branched anal-fin rays: (0) 24 or less; (1) 25 or more. (CM30m, MO80m, SE92m, BE78m).
289. Number of branched anal-fin rays: (0) 34 or less; (1) 35 or more. (BU66m, LU104m, CM30m, MO80m, SE92m, BE78m, QU61m).
The number of anal-fin rays is used in species-level systematic studies of many genera of the Characidae. However, this number is highly variable within some evidently monophyletic clades and its phylogenetic utility may be mostly restricted to the resolution of rather small clades. Notably, however, supposedly basal Cypriniformes and Siluriformes and most non-characid Characiformes have a relatively low number of branched anal-fin rays (character 286, state 0), while most species of the Characidae have usually more than 15 rays (character 286, state 1). Buckup (1998) proposed the presence of 19 or more total anal-fin rays as a synapomorphy of a clade including the Alestidae, Characidae, Acestrorhynchidae, Erythrinidae, Lebiasinidae, Hepsetidae, and Ctenoluciidae, with a reversal in a clade formed by the four latter families. According to the hypothesis of Lucena (1993) the presence of 14 or more anal-fin rays is a synapomorphy of a clade composed of the Acestrorhynchidae, Alestidae, Characidae, Cynodontidae, and Serrasalmidae. This character involves only the branched anal-fin rays, instead of total rays, as in Lucena (1993) and Buckup (1998). This coding system has advantages in terms of homology assessment, avoiding the coding of dissimilar arrangements as the same character state (e. g. ii+15 rays vs. v+12 rays). Coding of these four characters includes ranges of intraspecific variation taken from the original descriptions and systematic and faunistic revisions (Eigenmann, 1912, 1915, 1917, 1918, 1921, 1927; Eigenmann & Myers, 1929; Géry, 1977; Ringuelet et al., 1967; Reis, 1989; Vari & Harold, 2001; Zanata & Toledo-Piza, 2004). Most examined species have 18 to 35 branched anal-fin rays. The species having ranges of variation comprising more than one state were coded as polymorphic.
290. Form and length of anterior anal-fin rays: (0) similar to posterior rays; (1) longer and more compressed laterally than posterior rays. (BÜ109m, BÜ112m, BÜ113m).
In most examined species the anterior anal-fin rays are rather similar in form to the posterior rays (state 0). Bührnheim (2006) mentioned that the adult males of Axelrodia lindeae, the species of Heterocheirodon Malabarba, the species of Serrapinnus Malabarba, and two species of Spintherobolus have a series of modifications in the anterior anal-fin rays of males. These involve the presence of a lobe formed by rays bearing bony hooks, with the rays much compressed laterally and whose proximal portions are joined by strong ligaments (state 1; Fig. 106). These three characters are here considered a single feature, because they are functionally correlated, and given that the presence or absence of a lobe and the degree of development of the ligaments between the anal-fin rays are difficult to define as discrete states. In Acestrorhynchus pantaneiro the anterior anal-fin rays are much elongated relative to the posterior rays, forming a pronounced lobe. This state is not, however, exclusive to the males and it is not considered homologous with state 1 of this character. An intermediate condition between the defined states was observed in Aphyocharacidium bolivianum, which is coded as polymorphic.
291. Number of rays on last anal pterygiophore: (0) two; (1) one.
Most examined species have two rays articulating with the posteriormost anal-fin pterygiophore (state 0; Fig. 106). The examined specimens of Coptobrycon bilineatus and Hasemania nana instead have only one ray articulating with that pterygiophore (state 1).
Anal-fin pterygiophores:
292. Anterior notch on first anal pterygiophore: (0) absent; (1) present.
The cylindrical main body of the anteriormost anal pterygiophore, among the examined species, is limited anteriorly by a variably developed bony lamella. The anterior margin of this lamella lacks notches in most taxa (state 0). The examined members of the Parodontidae and Markiana nigripinnis instead have a marked notch (state 1) along the dorsal margin of the lamella.
293. Number of anal pterygiophores anterior to first haemal spine: (0) three or fewer; (1) four or more. (LU101, LC41).
In most species the dorsal portion of the first proximal anal-fin pterygiophore is situated between the anteriormost haemal spines (state 0). Lucena (1998) proposed that the anterior displacement of the anal fin relative to the haemal spines, with at least four anal pterygiophores anterior to the first haemal spine (state 1) is a synapomorphy of a clade composed of Charax and Roeboides. Lucena coded Cynopotamus spp. as state 0, but the specimen of C. argenteus herein examined has seven pterygiophores anterior to the first haemal spine, and is coded as state 1.
294. Proximal and medial radials of anal fins: (0) fused on anterior five pterygiophores; (1) fused in most pterygiophores; (2) medial radials absent or completely fused with proximal ones. (BE82m, ZV190m).
The anal pterygiophores are composed of three radials of which the proximal is the longest, extending dorsally between the haemal spines of caudal vertebrae. The medial and distal radials are much smaller and participate in the articulation of the pterygiophore with the corresponding anal-fin ray. In most characids the four or five anterior proximal anal pterygiophores are fused with the corresponding medial ones, while in the remaining pterygiophores the three radials are independent ossifications (state 0). In some species most pterygiophores, instead, have the proximal and medial pterygiophores fused and only a few posteriormost pterygiophores have three independent radials (state 1). In the examined specimens of Carnegiella strigata, Characidium spp., Hoplias cf. malabaricus, Metynnis maculatus, Piaractus mesopotamicus, Pyrrhulina australis, Serrasalmus maculatus, and Thoracocharax stellatus the medial radials are completely absent or fused with the proximal radials in all the pterygiophores (state 2). Zanata & Vari (2005) mentioned that in the Ctenoluciidae and Erythrinidae (as also herein observed in Characidium and Pyrrhulina) the form of the proximal radials suggests their fusion with the medial radials, while in the Serrasalmidae (as also herein observed in Carnegiella and Thoracocharax) the medial radials appear to be primarily absent. Both conditions are coded with the state 2, pending further studies.
295. Lateral lamellae on anterior anal pterygiophores: (0) absent; (1) present. (ZV144m).
In most examined species, the anal pterygiophores have two associated bony lamellae positioned anteriorly and posteriorly to their main body (state 0). Some species of the outgroup have, in addition, two lateral lamellae giving the pterygiophores a cross-shaped transverse section (state 1).
Caudal skeleton
Epurals:
296. Number of epurals: (0) one; (1) two or three. (BU68, LU106i, VH55, MO88m, BE85i, PZ90m).
297. Number of epurals: (0) one or two; (1) three. (BU69, LU107, CM33, MO88m, ZV148, PZ90m).
The number of epurals in the Characiformes varies from one to three, but most species have two epurals (character 296, state 1, character 297, state 0; Fig. 107). The presence of only one epural (character 296, state 0; Fig. 108) was mentioned for several species in the Characiformes (Lucena, 1993; Buckup, 1998; Moreira, 2002, Benine, 2004; Lima, 2006). Two epurals were observed herein in Acestrorhynchus pantaneiro, Gymnocorymbus ternetzi (Boulenger), Hemigrammus unilineatus, Hyphessobrycon eques, Parecbasis cyclolepis, and Piabina argentea, differing with observations of Lucena (1993), Moreira (2002), Benine (2004), and Lima (2006). These species are coded as polymorphic for character 296, in light of possible intraspecific variability. Variation between one and two epurals was observed in Pristella maxillaris which is coded as polymorphic for this character. The presence of three epurals (character 297, state 1; Fig. 109) was mentioned for several mostly non-characid Characiformes (Buckup, 1998; Moreira, 2002; Zanata & Vari, 2005; Lima, 2006).
Hypurals:
298. Fusion of hypural 2 to compound centrum: (0) absent; (1) present. (UJ28m).
The fusion of the hypural 2 with the compound centrum (state 1; Fig. 108) was proposed by Fink & Fink (1981, 1996) as a synapomorphy of the Otophysi. Zanata & Vari (2005: 122) mentioned that this fusion is present in most Characiformes, being secondarily absent (state 0; Zanata & Vari, 2005: fig. 30) in a few taxa which usually have the hypurals 1 and 2 fused to each other. According to Roberts (1969: figs. 56-58 and 60), the hypural 2 is not fused to the compound centrum in Ctenolucius, Hepsetus, Hoplias, and Hydrocynus. Roberts (1974: figs. 18, 38, and 39) illustrated such a condition also in the hemiodontids Argonectes, Bivibranchia Eigenmann, and Hemiodus Müller. Miquelarena (1982) mentioned that in Rhaphiodon the hypurals 2 and 3 are fused and articulated, but not fused, with the compound centrum. Zanata & Vari (2005), in the discussion on the phylogenetic position of †Mahengecharax carrolli Murray, indicated that the hypural 2 is fused with the compound centrum in the Alestidae with the exception of the miniature species Lepidarchus adonis. In this species both hypurals are fused each other but separated from the compound centrum; however they (Zanata & Vari, 2005: fig. 30) illustrated an autogenous hypural 2 in Brycinus macrolepidotus, and Murray (2004: fig. 13) illustrated six autogenous hypurals in Alestes stuhlmannii (Pfeffer). The absence of fusion between the hypural 2 and the compound centrum is usually correlated with the fusion of the hypurals 1 and 2 (Zanata & Vari, 2005); however, some exceptions were herein observed, and these characters are coded as separate in this paper. Intraspecific variability was observed in Heterocharax macrolepis, which is coded as polymorphic.
299. Fusion between hypurals 1 and 2: (0) absent; (1) present. (UJ28m, BU71, LU109).
As mentioned above, the fusion between the hypurals 1 and 2 (state 1) is often correlated with the lack of fusion between the hypural 2 and the compound centrum, but as this correlation is not perfect, these characters are coded as separate.
300. Posterior margin of hypural 3: (0) equal to or narrower than posterior margin of hypural 4; (1) deeper than posterior margin of hypural 4.
In the Characiformes and especially in the Characidae the posterior margin of the hypural 3 is usually deeper than that of the hypural 4 (state 1; Fig. 107), while in a group of species of the outgroup the posterior margin of the hypural 3 is equal or more slender than the hypural 4 (state 0; Fig. 109).
Procurrent rays:
301. Ventral procurrent caudal-fin rays of adult males: (0) slender; (1) laminar. (MA45m, BÜ127m, BÜ135).
In the generalized condition described by Weitzman (1962) for Brycon meeki, the lepidotrichia that form each procurrent ray are autogenous or are fused only at their ventral portion. In these cases the procurrent rays are similar to the principal caudal-fin rays but shorter or, if corresponding lepidotrichia are fused each other, they are as slender as the principal caudal-fin rays (state 0; Fig. 107). Malabarba (1998a) proposed the fusion and anteroventral expansion of the corresponding lepidotrichia of all the procurrent rays along their entire length, forming laminar structures (state 1; Fig. 110), as a synapomorphy of the tribe Cheirodontini.
302. Number of ventral procurrent caudal-fin rays: (0) 11 or fewer; (1) 12 or more. (MA42m, VB74m, BÜ124m).
The number of ventral procurrent rays is 11 or fewer in most examined species (state 0). Malabarba (1998a) considered the presence of more than 11 ventral procurrent rays (state 1) as a synapomorphy of the Cheirodontini (see previous character). Miquelarena (1982) mentioned a higher number of rays in Roeboides bonariensis (Steindachner) (=R. microlepis), Acestrorhynchus altus Menezes (=A. pantaneiro), Moenkhausia dichroura, and Tetragonopterus argenteus. Up to 11 ventral procurrent rays were observed in Roeboides microlepis (11 in 1 ex.), Acestrorhynchus pantaneiro (11 in 1 ex.), Moenkhausia dichroura (11 in 2 ex.), and Tetragonopterus argenteus (8-9 in 2 ex.) and these species are thus coded as polymorphic for this character, in light of possible intraspecific variability. Variation between the defined states was observed in Acestrocephalus sardina, Aphyocharacidium bolivianum, Axelrodia lindeae, Bryconamericus thomasi, and Gymnocharacinus bergii, which are coded as polymorphic.
303. Ventral procurrent caudal-fin rays of adult males: (0) not projecting through musculature and skin of peduncle; (1) projecting ventrally through peduncle musculature and skin. (MA47, BÜ129).
Most examined species have their anteriormost ventral procurrent rays contained completely within the skin and musculature of the caudal peduncle and not projecting ventrally as a keel (state 0). Malabarba (1998a) proposed as a synapomorphy of the Cheirodontini the ventral projection of such procurrent rays through the musculature and skin forming an externally visible keel (state 1; Fig. 110).
304. Caudal-fin bony stays: (0) absent; (1) present. (VA82, BU67, LU105, ZV150).
The presence of caudal stays (sensu Roberts, 1969) was mentioned several times in some studies treating the Ctenoluciidae (Vari, 1995) and Alestidae (Murray & Stewart, 2002; Zanata & Vari, 2005), in which they are particularly developed (state 1; Zanata & Vari, 2005: fig. 30). Bony stays are absent in the examined characids (state 0). Stays are longitudinal median bones situated at the base of the procurrent rays. whose homology with the procurrent rays is uncertain. Therefore, the presence or absence of stays is herein treated as different from the following character.
305. Anterior ventral procurrent caudal-fin rays: (0) paired, only distally fused; (1) fused in laminar medial bones.
Zanata & Vari (2005) mentioned that in the Characidae there are medial bones, similar to stays, but much anteriorly situated. These procurrent rays are situated in parallel; the posteriormost procurrent rays have separated lepidotrichia, which are progressively fused in the anterior ones. In this condition the anterior ventral procurrent rays are medial plates without remnants of separated lepidotrichia, articulated or situated between haemal spines of two or more vertebrae anterior to the compound centrum (state 1; Fig. 49). These plates have a different position than the caudal stays, which are longitudinal, perpendicular to the procurrent rays. In Puntius tetrazona and most representatives of the outgroup, the lepidotrichia of the anterior ventral procurrent rays are not fused to each other and do not reach the haemal spines (state 0).
Uroneurals:
306. Uroneurals: (0) absent or just one pair; (1) two pairs. (UJ54, BU70, LU108, MO89, ZV149, LI50).
The uroneurals are small paired bones aligned along the urostyle. Most examined taxa have only one pair of uroneurals (state 0), whereas the presence of two pairs of uroneurals (state 1; Weitzman, 1962: fig. 15) was observed in several mostly non-characid Characiformes. Among the examined species, the size of the second pair of uroneurals is variable when present, and in some cases they are much reduced. Nonetheless, all the species with a second pair of uroneurals present, regardless it size, are coded as state 1. In the examined specimens of Acestrocephalus sardina, Bryconamericus exodon, Carlana eigenmanni, Coptobrycon bilineatus, Moenkhausia cf. intermedia, M. sanctaefilomenae, Odontostoechus lethostigmus, and Roeboides descalvadensis, the second pair of uroneurals has a variable occurrence and these species are coded as polymorphic for this character. The complete lack of uroneurals was observed in Characidium rachovii, and this character is coded as inapplicable for that species.
Bony hooks
Distribution:
307. Bony hooks on fin rays: (0) absent; (1) present in adult males. (CM35m, CM36m, VB58, SE91i, LI60).
The presence of bony hooks on the fin rays (usually on the anal and pelvic fins) is a secondary sexual structure of males, that is broadly distributed in the Characidae and also present in the Gasteropelecidae and Serrasalmidae (Malabarba & Weitzman, 2003) (state 1; Figs. 103 and 104). Malabarba & Weitzman (2003) proposed the presence of bony hooks in adult males as a synapomorphy of a clade including the Gasteropelecidae, Serrasalmidae, and Characidae, excepting Agoniates, Clupeacharax, and Engraulisoma. Most species examined for this paper have bony hooks; however, the absence of bony hooks can not be assumed to be typical for a species based on the observation of a limited number of specimens of such species. Rather it should be coded only for species for which a good sample of individuals and/or undoubtedly adult males were examined. Serra (2003) coded Salminus as lacking bony hooks; although in the examined material no hooks were observed for this genus, S. brasiliensis is coded with the state 1 following Morais Filho & Schubart (1955). Weitzman & Malabarba (2003) mentioned the presence of bony hooks in Microschemobrycon Eigenmann citing Böhlke (1953a) and Géry (1973); however, Géry (1973) explicitly mentioned the absence of hooks in M. casiquiare and this character is coded as missing for this species. Additionally to personal observations, the absence of bony hooks is coded for Rhoadsia altipinna (Cardoso, 2002), Nematobrycon palmeri, Pseudochalceus kyburzi (Bertaco, 2003), Coptobrycon bilineatus, Hasemania nana (Serra, 2003), Paragoniates alburnus, Xenagoniates bondi (Quevedo, 2006) and their presence for Creagrutus anary, C. cf. taphorni, Piabina argentea (Vari & Harold, 2001), Roeboides microlepis (Lucena, 2003), Bryconexodon juruenae (Lima, 2006), Roeboides descalvadensis (Lucena, 2007), Hemibrycon dariensis and H. surinamensis (Bertaco, 2008) following literature.
308. Anal-fin bony hooks in adult males of species bearing hooks on fins: (0) absent; (1) present. (CM35m, CM36m).
Bony hooks, when present, are usually restricted to the anal and/or pelvic fins. A small group of species has bony hooks also on the other fins and this variation is considered below. Usually, there is an association between the presence of bony hooks in the pelvic and anal fins but this correlation is not perfect, and their presence in each of these fins is analyzed as separate characters. In most species whose mature males bear bony hooks, they are present in the anal fin (state 1), whereas in Characidium borellii and Nantis indefessus, among the examined species, these bony hooks are absent (state 0). This character is coded as inapplicable to species lacking bony hooks.
309. Pelvic-fin bony hooks in adult males of species bearing hooks on fins: (0) absent; (1) present. (CM35m, CM36m).
Pelvic-fin bony hooks are present (state 1) in most species having secondary sexual bony hooks. These hooks are absent, however, (state 0) in several species in which the anal-fin hooks are present. The absence of pelvic-fin bony hooks is somewhat related with the compressed body of some species, which is in turn usually associated with a small size of the pelvic fin.
310. Pectoral-fin bony hooks in adult males of species bearing hooks on fins: (0) absent; (1) present. (MA39m, MA41m, BÜ138m).
Bony hooks are usually absent in the pectoral (state 0), dorsal, and caudal fins. As the presence or absence of hooks on these fins is somewhat independent from each other, this character and the following two are coded as separate. Among the species bearing secondary sexual bony hooks, these structures are present in the pectoral fin in some species (state 1). All the examined species having pectoral-fin bony hooks have also hooks on the dorsal fin, excepting Phenacogaster tegatus.
311. Dorsal-fin bony hooks in adult males of species bearing hooks on fins: (0) absent; (1) present. (MA39m, MA41m, VB37, BÜ138m).
The presence of dorsal-fin bony hooks (state 1) is usually associated with the presence of hooks on the pectoral fin, as herein observed. However, the presence of bony hooks on the dorsal fin but their absence on the pectoral fin was herein observed in Bryconamericus thomasi and Hemigrammus erythrozonus. This character is coded as state 1 in Nematocharax venustus following Bertaco (2003).
312. Caudal-fin bony hooks in adult males of species bearing hooks on fins: (0) absent; (1) present.(MA39m, MA41m, BÜ138m).
The presence of caudal-fin bony hooks (state 1) is usually associated with the presence of these structures on the pectoral and dorsal fins. However, several species with bony hooks on those fins lack bony hooks on the caudal fin (state 0). The presence of caudal-fin bony hooks but their absence on pectoral and dorsal fins was observed herein only in Acrobrycon tarijae.
313. Bony hooks on base of pelvic-fin rays of adult males: (0) absent, or in small number compared to on segmented portion of rays; (1) as numerous as on segmented portion of rays.
Pelvic-fin bony hooks are usually more abundant on the branched region of the rays and are absent or much less concentrated in the basal portions of the rays (state 0). In some species the bony hooks are more densely concentrated along the basal portions of the pelvic-fin rays (state 1). Intermediate conditions were observed in Bryconamericus alpha, Hemigrammus erythrozonus, Knodus breviceps, Oligosarcus cf. jenynsii, and Prodontocharax melanotus, which are coded as polymorphic.
314. Bony hooks on last pelvic-fin ray of adult males: (0) absent or reduced in number; (1) as numerous as in other rays.
Bony hooks are usually absent or much less concentrated in the last pelvic-fin ray than on the remaining rays (state 0). In some species, bony hooks are also present and relatively abundant on the last pelvic-fin ray (state 1; Fig. 103). An intermediate situation was observed in Odontostoechus lethostigmus, which is coded as polymorphic.
315. Bony hooks on first pelvic-fin ray of adult males: (0) absent; (1) present.
The unbranched first pelvic-fin ray usually lacks bony hooks, even in species in which the hooks are abundant on other rays (state 0). The first pelvic-fin ray in a few species have, instead, relatively abundant bony hooks (state 1). This character is variable in Odontostoechus lethostigmus which is coded as polymorphic.
316. Position of anal-fin bony hooks of adult males: (0) paired and ordered laterally or posterolaterally; (1) medially positioned and oriented posteriorly; (2) asymmetrically disposed and irregularly arranged. (MA25m, BÜ145).
The anal-fin bony hooks are usually paired and oriented laterally or posterolaterally (state 0). Malabarba (1998a) proposed the presence of medial, posteriorly-oriented bony hooks, as a synapomorphy of a clade of the Cheirodontinae (state 1; Malabarba, 1998a: fig. 17). The presence of irregularly arranged and asymmetrically placed anal-fin bony hooks was proposed as a synapomorphy of the Stethaprioninae of Reis (1989) (state 2; Reis, 1989: fig. 13).
Scales
Morphology:
317. Scales: (0) cycloid; (1) ctenoid; (2) spinoid; (3) crenate. (EI10, LU119m).
Most characiforms have cycloid scales, without projections on their posterior field (state 0; Fig. 111). Vari (1979) reported the presence of ctenii along the posterior margin of the scales in the Distichodontidae and Citharidium Boulenger, and Lucena (1993) in Acestrocephalus sardina, Ctenobrycon hauxwellianus (Cope), Cynopotamus kincaidi (Schultz), and Galeocharax knerii (Steindachner). Following the classification of Roberts (1993), the scales of the Citharinidae and Distichodontidae are ctenoid (state 1), while those of Acestrocephalus, Cynopotamus and Galeocharax are spinoid (state 2; Fig. 112). The scales of Ctenobrycon (not analyzed here) and Psellogrammus are referred to as ctenoid in the literature; Roberts (1993) did not find ctenii in Ctenobrycon, differing with Lucena (1993), and did not examine specimens of Psellogrammus; thus, the type of squamation of that genera remained undiscussed by Lucena. The scales of the belly of Psellogrammus kennedyi have simple flattened serrations restricted to the margin of the scales similar to that of crenate scales in the classification of Roberts (1993) (state 3: e. g. Roberts, 1998: fig. 4G).
318. Anterior margin of scales: (0) uniformly curved or slightly undulated; (1) with conspicuous undulations. (LU121m).
The shape of the anterior margin of the scales is variable among the examined species. In many species this margin is almost straight, somewhat rounded, or slightly expanded along its central portion (state 0; Fig. 112). As the shape of the anterior margin of the scales is variable even in different regions of the same specimen, it is only considered here the case in which the undulations are much evident and regularly present on scales of different regions of the body (state 1; Fig. 111). Eigenmann (1917) mentioned the presence of crenate scales in Entomolepis (=Bario) steindachneri, while Géry (1977) indicated that the scales of B. steindachneri become undulated when specimens reach 55 mm SL. In the specimen examined for this paper (62 mm SL), the scales are only slightly undulated anteriorly; given this variation during the growth of this species, Bario steindachneri is coded as polymorphic for this character.
319. Circulii on posterior field of scales: (0) present; (1) absent. (BU80, LU118, ZV171m).
The circulii are concentric striae from the focus to the margins of the scales. In most characids the circulii are absent on the posterior field of the scales (state 1; Fig. 111), whereas most species of the outgroup and some characids have complete circulii reaching the posterior field of scales (state 0). The root of this analysis is coded as polymorphic because the circulii are absent in Puntius tetrazona but are present in other members of the Cypriniformes (Pflieger, 1997).
320. Radii on scales: (0) absent or reduced in number; (1) present and numerous on most scales.
The radii are radially disposed grooves on the surface of scales extending from the focus to the margins. In most species the radii are uniformly present in all regions of the body, at least in the posterior field of scales (state 1; Fig. 113). In some species such radii are either absent, much reduced in number, or variably present in different regions of body (state 0; Fig. 111).
321. Radii oriented towards anterior field of scales: (0) present; (1) only as longitudinal groove without defined margins; (2) absent. (ZV172m).
In most examined species the radii are only present on the posterior field of the scales (state 2), and do not converge towards the focus of the scales. Uj (1990) illustrated scales with radii oriented anteriorly (state 0) in some members of the Alestidae, Anostomidae, Ctenoluciidae, Erythrinidae, Hepsetidae, and Parodontidae. Zanata & Vari (2005) mentioned that in most alestids, along with the genera Hepsetus, Hoplias, and Triportheus, the radii are oriented from the focus in all directions, even to the anterior field. A much wider anteriorly-oriented groove without defined margins, (state 1, Fig. 113) was observed in Bario steindachneri and Moenkhausia sanctaefilomenae. This character is coded as inapplicable for species in which the radii are absent.
322. Radii of scales: (0) not converging at focus; (1) converging at focus.
Although the radii are oriented towards the focus, in most species they do not converge centrally, being independent each other (state 0). In some species the radii are in contact at the focus of the scales (state 1; Fig. 113). In Astyanax cf. abramis, A. asuncionensis, and A. cf. asuncionensis, this character is variable in different regions of the body of the same specimen, and this character is coded as polymorphic. This character is coded as inapplicable for species in which the radii are absent or reduced in number.
323. Semicircular grooves on posterior field of scales: (0) absent; (1) present.
As mentioned above, the posterior field of the scales usually has radii and/or circulii (state 0). Uj (1990) illustrated a few grooves similar to radii but semicircular in shape, situated on the posterior field on the scales of Stethaprion erythrops Cope (state 1). The presence of these grooves was corroborated in this paper. Although this character is autapomorphic for S. erythrops in this analysis, this character is included as potentially informative in the future.
Scale distribution:
324. Scales covering supraoccipital spine: (0) absent; (1) present and completely covering supraoccipital spine. (VA74, BU18, LU24, ZV168, LI61m, PZ102).
In most examined species the dorsal scales extend anteriorly to the posterior margin of the parietals and supraoccipital but do not cover the supraoccipital spine, which projects posteriorly between rows of scales (state 0). Vari (1995) mentioned that the anteriormost dorsal scales extend anteriorly dorsal to the supraoccipital spine and cover it completely from dorsal view (state 1) in the Ctenoluciidae, Erythrinidae, Hepsetidae, and Lebiasinidae; this state was considered by him as a synapomorphy for a clade composed of these four families. Vari (1995) mentioned a similar coverage of the supraoccipital spine in the Alestidae and Parodontidae, although he considered these cases as not comparable given the lack of a supraoccipital crest in the latter two families. This character is considered inapplicable to species lacking median predorsal scales.
325. Median predorsal scales: (0) covering entire predorsal region; (1) leaving naked area anterior to dorsal fin.(EI7, AM13, BE92).
The region between the posterior tip of the supraoccipital spine and the origin of the dorsal-fin origin is typically covered by scales (state 0) in most examined species. The absence of scales in the predorsal area (state 1) constitutes one of the "generic character" of Eigenmann (1917), and was used by that author to diagnose Gymnocorymbus Eigenmann. The absence of predorsal scales was also mentioned by Machado-Allison (1983) as a synapomorphy of the Serrasalminae (=Serrasalmidae). This author proposed a correlation between the absence of predorsal scales and the great development of the supraoccipital spine. The examined specimens of Gymnocharacinus bergii lack scales. Miquelarena & Arámburu (1983) described the squamation of this species, indicating that the scales are progressively reabsorbed in specimens of 42 mm SL or greater. According to those authors, the squamation is regular in smaller specimens, except along the middorsal line and the end of the caudal peduncle. Gymnocharacinus bergii is, thus, coded as state 1 following the literature.
326. Ventral serrae: (0) absent; (1) present. (AM1).
The presence of modified and aligned scales forming a ventral serrae (state 1) is diagnostic for the Serrasalminae (=Serrasalmidae) (Machado-Allison, 1983). These serrae are absent (state 0) in all remaining species in the Characiformes.
327. Scales covering anal-fin base: (0) one or two rows of scales covering anal-fin base; (1) several rows covering basal third of anal fin. (EI11, SE93m, BE93m).
Most examined species have one or two rows of scales partially covering the anal-fin base (state 0). In some species there are, instead, several rows of smaller scales covering more than one-third the length of the anal-fin rays (state 1). This character is coded as polymorphic for Poptella paraguayensis which has an intermediate state.
328. Scales covering caudal-fin lobes: (0) covering only their base; (1) covering one-third of their length. (EI1, CM34, SE89m, BE94).
The scales in most characids reach only the caudal-fin base (state 0), with the presence of scales over the caudal-fin lobes (state 1) used as a "generic character" by Eigenmann (1917). This character state is still used to diagnose several highly diverse genera in the Characidae. Cases in which the scales cover the medial caudal-fin rays, but not the lobes, as in the Serrasalmidae, Galeocharax humeralis, and Roeboides descalvadensis, are coded as state 0. Microschemobrycon casiquiare is coded as polymorphic given the observations of Géry (1977) about the intraspecific variability of this character. In the examined specimens of Bryconamericus alpha the scales cover part of the ventral caudal-fin lobe, but the dorsal lobe is completely naked. This character is coded as polymorphic for this species.
Muscles and ligaments
Cranial musculature:
329. Ventral division of tendon from adductor mandibulae inserted on dentary: (0) absent; (1) present.
The A2 and A3 sections of the adductor mandibulae form a strong tendon whose anterior region is usually divided in two smaller portions, one attached to the coronomeckelian bone and the second to the medial surface of the dentary just ventral to the Meckelian cartilage (state 1; Fig. 114). The ventral division of this tendon varies in degree of development and attachment site, which is considered below. In some species, such a division is completely absent, and the tendon from the adductor mandibulae attaches only in the coronomeckelian bone (state 0). This condition was observed in Puntius tetrazona and some representatives of the outgroup.
330. Longitudinal position of insertion of adductor mandibulae tendon on dentary: (0) on vertical through posterior half of Meckelian cartilage; (1) on vertical through middle or anterior half of Meckelian cartilage. (ZV92m).
The tendon from the adductor mandibulae usually attaches on the dentary on a vertical through some point of the posterior half of the Meckelian cartilage (state 0). In some species this tendon is comparatively stronger and inserts more anteriorly on the dentary (state 1). This anterior displacement of the attachment site is usually correlated with a shortening of the lower jaw, however this correlation is not perfect and these features have certain independence from each other.
331. Insertion of adductor mandibulae tendon on dentary: (0) ventral to Meckelian cartilage; (1) anterior to Meckelian cartilage; (2) on a medial process of the dentary. (ZV92m).
In most examined species the ventral division of the tendon from the adductor mandibulae inserts ventral to the Meckelian cartilage (state 0). In some species the insertion of this tendon is anteriorly displaced to the posterior wall of the dentary fossa for the replacement teeth (state 1; Fig. 114); this state was described by Zanata & Vari (2005) as synapomorphic for a clade including Alestes, Brycinus, and Bryconaethiops. The ventral division of the adductor mandibulae tendon in the Iguanodectinae is attached to an ascendant lobe of the inner surface of the dentary, immediately medial to Meckelian cartilage (Moreira, 2002) (state 2; Fig. 62).
332. Posterior attachment of A1 section of adductor mandibulae: (0) principally to vertical arm of preopercle; (1) restricted or almost restricted to horizontal arm of preopercle. (UJ65, LU111).
The A1 section is the most lateral division of the adductor mandibulae muscle. The posterior portion of this section broadly attaches to the horizontal and vertical arms of the preopercle (state 0). In several characids this section is relatively reduced and inserted almost exclusively on the horizontal arm of the preopercle (state 1; Fig. 115). Lucena (1993) coded Acestrorhynchus pantaneiro as state 0, while the state 1 was observed in the specimens herein examined, and this species is coded as polymorphic considering possible intraspecific variability. The root of this analysis is also coded as polymorphic due to variations in the attachment area within the Cypriniformes (Howes, 1978).
333. Attachment of medial tendon of A1 section of adductor mandibulae: (0) on quadrate near its articulation with preopercle; (1) on preopercle posterior to quadrate.
The ventral portion of the A1 section of the adductor mandibulae broadly attaches to the horizontal arm of the preopercle and the quadrate, with its dorsal surface bordered by the A2 section, which is situated more medially. The medial margin of the A1 section is limited dorsally by an oblique tendon which attaches anteriorly to other tendons from the A2 and A3 sections to form the large tendon described in the characters 329 to 331. This tendon usually inserts on the dorsal margin of the quadrate, near the articulation between that bone and the preopercle (state 0; Figs. 75 and 77). In some species this tendon, instead, attaches more posteriorly and dorsally on the preopercle posterior to the margin of the quadrate (state 1; Fig. 74). In several species the tendon in question could not be observed, and although apparently absent, this issue needs confirmation.
334. Anterior insertion of A1 section of adductor mandibulae: (0) on maxilla; (1) on coronoid process of dentary.
The A1 section of the adductor mandibulae attaches anteriorly to the maxilla (state 0) in the Cypriniformes and Citharinoidei (Howes, 1978; Vari, 1979). This condition was observed in Puntius tetrazona and Distichodus maculatus. In the remaining species this section rather attaches to the variably developed coronoid process of the lower jaw (state 1).
335. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi: (0) absent; (1) present. (LU113m, LU115m).
The dorsal margin of the adductor mandibulae is posterodorsally oblique and attaches to the dorsal region of the preopercle, while the dilator operculi is posteroventrally oriented from the neurocranium to the preopercle. In most species there is a triangular gap between these muscles immediately posterior to the orbit making the levator arcus palatini, which is medial to both muscles, visible from a lateral view. The ventral margin of the dilator operculi usually overlaps part of the adductor mandibulae dorsal margin reducing the laterally visible portion of the levator arcus palatini and covering the insertion of this muscle on the preopercle (state 1; Fig. 115). In some species, instead, the dilator operculi, does not cover the adductor mandibulae and the levator arcus palatini is visible to its insertion in the preopercle (state 0; Fig. 116). Lucena (1993) considered both the presence and degree of overlap of the dilator operculi over the adductor mandibulae as two separate characters. The degree of overlap of these muscles is apparently related to the degree of development of the musculature of jaws and its condition varies within some apparently monophyletic groups. In Aphyocharax dentatus, for example, these muscles broadly overlap, while in A. anisitsi, with a much weaker jaw musculature, they only slightly overlap. Thus, it is preferable herein to consider only the presence or absence of overlapping between the adductor mandibulae and the dilator operculi. Intraspecific variability in those features was observed in Aphyocharacidium bolivianum, Axelrodia lindeae, Hyphessobrycon luetkenii, Lonchogenys ilisha, and Piabina argentea which are coded as polymorphic for this character.
336. Anterior extension of adductor arcus palatini: (0) covering most of dorsal surface of mesopterygoid; (1) covering only half of dorsal surface of mesopterygoid. (LU114).
The adductor arcus palatini inserts anteriorly on the dorsal surface of the mesopterygoid. In most examined species, this muscle thinly covers only the posterior half of the mesopterygoid (state 1; Fig. 117). In some species both of the ingroup and the outgroup the adductor arcus palatini covers most of the dorsal surface of the mesopterygoid (state 0; Fig. 118).
337. Posterior region of levator arcus palatini: (0) limited laterally by adductor mandibulae and medially by adductor arcus palatini; (1) limited lateral and medially by A2 and A3 sections of adductor mandibulae. (LU110m, LU112m).
In most examined species the posterior portion of the levator arcus palatini is situated between the medial margin of the adductor mandibulae and the lateral surface of the adductor arcus palatini (state 0). In some species the A3 section of the adductor mandibulae (the most medial section) is instead conspicuously developed, and its dorsal margin extends between the levator arcus palatini and the adductor palatini. In these cases the levator arcus palatini is thus bordered medially and laterally by different sections of the adductor mandibulae (A2 and A3 sections) (state 1). Lucena (1993) treated the development of the A3 section of the adductor mandibulae and the relative position of the levator operculi as different characters. Both characters are herein combined as one because it was impossible to estimate the development of the A3 section independent of its relationship with the levator arcus palatini and the adductor arcus palatini muscles. A different situation was observed in Puntius tetrazona in which the A3 section of the adductor mandibulae is relatively more developed, although its dorsal margin is bordered laterally by fibers of the adductor arcus palatini; thus, the ventral margin of the levator operculi is margined laterally by both the adductor arcus palatini and the adductor mandibulae. The root of this analysis is thus coded as polymorphic for this character. Only a small portion of the levator arcus palatini is bordered medially by the A3 section of the adductor mandibulae in Aphyocharax dentatus. This is considered an intermediate condition and this species is coded as polymorphic.
338. Origin of dilator operculi: (0) anterior to vertical through posterior margin of eye; (1) completely posterior to vertical through posterior margin of eye.
In most examined species the dilator operculi inserts anteriorly in a fossa formed by the frontal and the sphenotic or on the ventral surface of the frontal. In both conditions it inserts just dorsal to the posterodorsal margin of the orbit (state 0). In Characidium spp., among the examined species, the anterior border of the dilator operculi is displaced to a point posterior to the vertical through the posterior margin of the orbit (state 1). In the Erythrinidae and plesiomorphically in the Lebiasinidae, the dilator operculi extends anteriorly through the sphenotic to the orbit (Vari, 1995). This condition is different to the states herein defined, and this character is coded as inapplicable to Hoplias cf. malabaricus and Pyrrhulina australis.
Postcranium:
339. Pseudotympanum limited by first pleural rib, lateralis superficialis, second pleural rib, obliquus inferioris and obliquus superioris: (0) absent; (1) present. (AM26m, MA1, ZV199m, BÜ163m).
In most examined species the musculature laterally covering the gas bladder is present, or if slightly reduced does not form a gap between the muscle layers (state 0). According to the phylogenetic hypothesis of Malabarba (1998a), the presence of a pseudotympanum limited anteriorly by the first rib, dorsally by the lateralis superficialis, posteriorly by the second rib, and ventrally by the obliquus inferioris and obliquus superioris (state 1; Malabarba, 1998a: fig. 3), constitutes a synapomorphy of the Cheirodontinae. This author mentioned that other characids have reductions in the musculature lateral to the anterior chamber of the gas bladder, but that the homology of such reductions with the pseudotympanum is doubtful. Malabarba (1998a) described different types of pseudotympani in Microschemobrycon sp., Rhoadsia altipinna, and Tetragonopterus chalceus Spix & Agassiz. This author mentioned the presence of a pseudotympanum similar to that of the Cheirodontinae also in Aphyocharacidium Géry, Charax, Leptagoniates Boulenger, Phenacogaster, Roeboides, and variably within the rosy tetra clade of Weitzman & Palmer (1997). Different types of such openings were herein observed in Galeocharax humeralis, Hyphessobrycon elachys, Metynnis maculatus, Paracheirodon axelrodi, Psellogrammus kennedyi, and Rhoadsia altipinna. This character is coded as inapplicable to these species.
340. Insertion of pterotic aponeurosis: (0) on pterotic spine or lateral surface of horizontal semicircular canal; (1) on a lobe situated dorsal to horizontal semicircular canal; (2) on pterotic or sphenotic, distinctly dorsal to horizontal semicircular canal
In most Characiformes the pterotic aponeurosis (sensu Weitzman & Fink, 1983), which attaches part of the epaxial musculature to the cranium, inserts on the pterotic spine or onto the lateral margin of the pterotic tube of the semicircular canal (state 0; Weitzman & Fink, 1983: figs. 9-11). In a small group of species this aponeurosis inserts immediately dorsal to the pterotic tube for the horizontal semicircular canal, on an ascending lobe situated in the angle formed by this canal and the pterotic laterosensory canal (state 1; Weitzman & Fink, 1983: figs. 12 and 14). As described by Weitzman & Fink (1983) for Paracheirodon, in some species the aponeurosis attaches to the pterotic or sphenotic approximately at the middle of the depth of the posttemporal fossa (state 2; Weitzman & Fink, 1983: figs. 6-8).
Coloration and miscellaneous characters
Coloration:
341. Humeral spot: (0) absent or vertically-elongate; (1) horizontally-ovate. (VH53m).
In most species the humeral spot is vertically elongate, with diffuse margins (state 0). Malabarba (1998a) proposed the total absence of a humeral spot as a synapomorphy of the Cheirodontinae; however, this state is not analyzed herein given that in some species the spot is extremely reduced, making the discrimination of that condition from the absence of spot difficult. Instead, in a group of species the humeral spot is ovate and horizontally elongated, with definite margins and surrounded anteriorly and posteriorly by somewhat clearer areas (state 1). The humeral spot of Astyanax correntinus is ovate, but much reduced in size and without definite margins, and this character is coded as polymorphic for the species. In Hemigrammus ulreyi (Boulenger) the humeral spot is extended posteriorly by a narrow lateral band. Since this condition differs from both states, this character is coded as inapplicable for this species.
342. Second humeral spot: (0) absent or diffuse; (1) present as a conspicuous vertical bar.
Most examined species have a variably developed anterior humeral spot and, occasionally, a very faint second one (state 0). In a small group of species both humeral spots are distinctly conspicuous vertical bars (state 1). This character is coded as polymorphic for Poptella paraguayensis, in which a second humeral spot is present but it is slightly more diffuse than the anterior bar.
343. Dark conspicuous spot on dorsal fin: (0) absent; (1) present. (MA65m, MA66m, MA67m, BE100, BÜ159).
In most species the dorsal fin is hyaline or have some yellowish to reddish coloration, lacking a distinct dark spot (state 0). Géry (1977) listed a group of Hyphessobrycon species [his H. callistus (Boulenger) group] as having a conspicuous dark spot in the dorsal fin (state 1); this group includes H. callistus (=H. eques) and H. pulchripinnis, among the species analyzed here. The constitution of this group of species is rather similar to that of the rosy tetra clade of Weitzman & Palmer (1997), which additionally includes H. socolofi and H. compressus, the type species of Hyphessobrycon, and probably also Hemigrammus unilineatus, the type species of Hemigrammus (Meek). This character is coded as polymorphic in Hemigrammus ulreyi in which this spot is usually present but fainter.
344. Horizontal line of chromatophores just dorsal to anal-fin base: (0) absent; (1) present.
In most species the region just dorsal to the anal-fin base has a rather uniform coloration (state 0). In a small group of species there is instead a well defined longitudinal line of chromatophores just dorsal to the anal-fin base (state 1). A similar line of chromatophores was mentioned by Malabarba (1998b) for Lignobrycon myersi.
345. Color of caudal-fin lobes: (0) symmetrically hyaline, yellowish, reddish, or violaceous; (1) ventral lobe orange or reddish and dorsal lobe hyaline; (2) ventral lobe dark brown or black and dorsal lobe hyaline; (3) both lobes dark brown or black. (BE103m).
In most examined species both lobes of the caudal fin have a similar coloration, being hyaline, yellowish, reddish, or violaceous (state 0). These colorations are usually variable intraspecifically, and are treated herein as the same state, although some discrimination between these situations could be done as a result of the study of specific clades. In most species of Aphyocharax the ventral lobe is orange to intense red and the dorsal lobe is hyaline (state 1). In Thayeria Eigenmann and Hemiodus, among the examined taxa, the ventral lobe is black and the dorsal lobe hyaline (state 2), whereas in a small group of species both lobes are black, usually with white tips (state 3).
346. Diffuse spots on flanks: (0) absent; (1) present, especially in young specimens.
Most characiforms have a rather uniform coloration except for the variable presence of humeral and peduncular spots and a lateral band (state 0). Usually the coloration of young specimens is similar to that of the mature adults. Machado-Allison (1983) proposed as a synapomorphy of the Serrasalminae (=Serrasalmidae) the presence of rounded blotches or diffuse spots on flanks (state 1). These blotches gradually disappear during the growth.
347. Little spot on each scale of flanks: (0) absent; (1) present.
In most species the distribution of melanophores is somewhat related with the size and distribution of the scales, being usually more concentrated along their margins and producing various reticulated patterns (state 0). Some species of Astyanax have highly concentrated chromatophores on the medial or distal surface of each scale, especially on those of the dorsolateral surface of the body, thereby producing a dotted appearance (state 1). This state was described and used in systematic studies by Eigenmann (1917), and subsequently used in the literature by several authors (e. g. Ringuelet et al., 1967; Géry, 1977) for the discrimination of a group of species including A. abramis and A. asuncionensis among those herein analyzed.
348. Dark spot covering entire depth of caudal peduncle: (0) absent; (1) present. (BE102m).
Many species have a more or less developed lateral band which is usually continuous with a spot on the caudal peduncle and extends to middle caudal-fin rays (state 0). A dorsally expanded dark caudal-peduncle spot (state 1) is present in Moenkhausia sanctaefilomenae among the examined species and, according to Benine (2004), in Bario steindachneri, Moenkhausia cotinho Eigenmann, M. oligolepis (Günther), and M. pyrophthalma Costa. This spot is barely visible in the examined specimens of B. steindachneri, and this species is coded as polymorphic.
Miscellaneous:
349. Ventral union of gill membranes: (0) joined anteriorly, but not covering the isthmus; (1) joined along length of isthmus but not attached to isthmus; (2) joined to each other and with isthmus. (MO91m).
The gill membranes, in most examined species, converge just anterior to the urohyal, leaving the entire isthmus visible from a ventral view (state 0). In some species the gill membranes are joined at the posterior end of the isthmus which is consequently covered ventrally by the membranes (state 1). In both cases, the gill membranes are free from the isthmus. In Puntius tetrazona and the Anostomoidea the gill membranes are instead joined each other, and are firmly attached to the isthmus (state 2).
350. Sclerotic bones: (0) single anteroventrally open bone; (1) two bones separated by cartilages.
The sclerotic bones are developed from a complete cartilaginous ring bordering the eye. In the condition typical for the Teleostei this cartilage forms two independent ossifications along the anterior and posterior margins of the eye that are separated by the remnants of the original cartilaginous ring (state 1; Fig. 119) (Franz-Odendaal & Hall, 2006). This condition was observed in most outgroup species and some members of the Characidae. In other characids these sclerotic bones apparently are fused dorsally, and only the anteroventral margin of the eye is limited by cartilage (state 0; Fig. 120). In Coptobrycon bilineatus there is a single bone completely encircling the eye, even anteroventrally. Given that this is considered to be an extreme case of ossification of the original cartilaginous ring, this species is coded with the state 0. The conservativeness or lack thereof of this character during the growth was not studied in detail, and this coding should be evaluated by more focused future studies. This character is variable among the examined specimens of Astyanax troya, Bryconamericus thomasi, Gymnocorymbus ternetzi, Nantis indefessus, Odontostilbe paraguayensis, and Serrapinnus calliurus, which are coded as polymorphic.
351. Nostrils: (0) rounded and divided only by skin fold; (1) nostrils distinctly separate.
In most characiforms the nostrils of each side of the snout are close each other, being only separated by a fold skin (state 0; Fig. 121). In Coptobrycon bilineatus and Grundulus cochae, among the ingroup species, there are instead two widely separated nostrils on each side of the snout (state 1; Fig. 122).
352. Gill-derived gland on males: (0) absent; (1) present.
Bushmann et al. (2002) described a gland formed by the anteriormost gill filaments (state 1) in adult males of some species of their Glandulocaudinae (Stevardiinae, in part). This gland was subsequently observed in Aphyocharacidium bolivianum, Aphyocharax anisitsi, Hemibrycon sp., Phenacogaster franciscoensis Eigenmann, and the Cheirodontinae (Bührnheim, 2006). As this gland is present only in males and is not easily visible in some species, the coding of this character is mostly based on literature. Personal observations of alcohol specimens were used only to code species in which this gland is undoubtedly present or absent in adult males. Although no seasonal variation in presence or absence of this gland was reported in the literature, this issue should be further evaluated.
353. Glandular tissue of granular appearance on caudal fin of mature males: (0) absent; (1) present (MW2).
According to Menezes & Weitzman (2009), the presence of glandular tissue in the caudal organ (state 1; e. g. Menezes & Weitzman, 2009: fig. 5) is unique to their Glandulocaudinae and Stevardiinae. They coded all remaining members of their clade A (=Stevardiinae, as treated here) as lacking the glandular tissue herein considered (state 0) even if they lack a caudal organ as such. The used definition of states is the same to that by Menezes & Weitzman (2009). A tissue similar to that described by Menezes & Weitzman (2009) was found in males of Piabucus melanostomus; although the glandular nature of this tissue was not confirmed, this species is also coded as state 1, pending further confirmation. This character is only coded in the species in which the presence of this glandular tissue was mentioned by Menezes & Weitzman (2009) or its presence or absence was observed in adult males. The remaining species are coded with missing entries. This character was not analyzed by Mirande (2009).
354. Hypertrophied ventral caudal-peduncle squamation: (0) absent; (1) present. (MW6)
The presence of a glandular fold on the ventral lobe of the caudal fin (state 1; Weitzman & Menezes, 1998: figs. 10-16) was considered as diagnostic for the Glandulocaudinae (Géry, 1977; Weitzman & Fink, 1985; Weitzman & Menezes, 1998). The monophyly of this subfamily was later questioned and the Stevardiinae was proposed to include most species of the former glandulocaudins (Weitzman et al., 2005). Weitzman & Menezes (2009) analyzed the glandular fold in two different characters, considering the origin of the scales involved in such fold. They considered the hypertrophy of the scales of the dorsal lobe of the caudal fin and that of the ventral lobe as different characters and proposed the first character to be diagnostic of their Glandulocaudinae and the second character to be synapomorphic for their Stevardiinae. The presence (state 1; e. g. Weitzman & Menezes, 1998: fig. 14) or absence of hypertrophy of scales of the caudal-fin ventral lobe is herein coded. This character was not included as such by Mirande (2009), who instead coded the presence or absence of a caudal fold of scales, without considering if the scales forming this fold were from the dorsal or ventral caudal-fin lobe.
355. Caudal gland cells consisting of modified mucous cells: (0) absent; (1) present. (MW5).
The presence of caudal gland cells formed by modified mucous cells (state 1) was proposed by Menezes & Weitzman (2009) as a synapomorphy of their Stevardiinae. No histological examination were made for this paper and the coding of this character is exclusively based on Menezes & Weitzman (2009). The presence or absence (state 0) of modified mucous cells was coded only at generic or suprageneric levels by Menezes & Weitzman (2009) and their presence or absence should be corroborated in each species. This character was not included by Mirande (2009).
356. Adipose fin: (0) present; (1) absent. (EI15, VA81, MA12, VB39, SE90m, QU41).
The presence of an adipose fin (state 0) was considered as a plesiomorphy of the Ostariophysi (Fink & Fink, 1981) that was independently lost in the Gonorynchiformes, Cypriniformes, and Gymnotiformes. However, according to the phylogenetic hypothesis of Fink & Fink (1981), the correct optimization of this character is ambiguous, and their interpretation of character-state evolution is equally parsimonious to the absence of the adipose fin in the ancestor of the Ostariophysi and its acquisition in the common ancestor of the Characiformes, Gymnotiformes, and Siluriformes. This character, thus, is coded as polymorphic to the root of this analysis. In most members of the Characiformes the adipose fin is present, while it is absent (state 1) in the Erythrinidae, Lebiasinidae, and some characids.
357. Papillae on tongue: (0) not aligned; (1) forming longitudinal rows anteriorly.
The epithelial papillae situated in the dorsal surface of the primary tongue are irregularly arranged in most examined species (state 0; Fig. 123). In some species these papillae are instead aligned, forming four to six conspicuous longitudinal rows on the anterior region of dorsal surface of the primary tongue (state 1; Fig. 124).
358. Insemination: (0) absent; (1) present. (MA70, WM2, BÜ168, QU92, MW1).
Although a detailed study of the reproductive biology of the species included in this analysis is beyond the scope of this paper, the available data about insemination is herein analyzed in a familial phylogenetic context. Although the list of characid inseminating species is surely far from complete, the published list of species in which insemination is known to be absent is even more incomplete. Thus, this and the following character, which is also related with reproductive biology, have a relatively high proportion of missing entries. Most characids have external fertilization (state 0), while the presence of insemination (state 1) was reported for several genera of the Cheirodontinae and Stevardiinae and the genus Hollandichthys (Bertaco, 2003; Burns et al., 1995, 1997, 1998; Burns & Weitzman, 2005; Castro et al., 2003; Menezes et al., 2003; Weitzman et al., 2005). This character is coded exclusively from literature.
359. Type of spermatozoa: (0) aquasperm; (1) introsperm. (MA71, WM47m, BÜ169, QU91).
The spermatozoa in externally fertilizing species are usually of the aquasperm type, with a rounded nucleus (state 0), while in the inseminating species they are usually of the introsperm type, with an elongated nucleus (state 1) (Burns & Weitzman, 2005). Although there is a correlation between the presence of insemination and introsperms, there are exceptions to such relationships (Burns & Weitzman, 2005). This character was coded only from literature, and many species are coded as missing entries.
360. Sperm storage area on testes: (0) absent or small; (1) present, as broad as spermatogenic area. (QU93).
The presence of a developed aspermatogenic region in the testicle, which serves as storage area (state 1) was proposed by Weitzman & Menezes (1998) as a synapomorphy of their Glandulocaudinae. A similar storage area was observed by Bertaco (2003) in the species of Hollandichthys. This storage area is absent or extremely reduced in most of the Characidae (state 0). This character was coded exclusively from literature.
361. Number of 2n chromosomes: (0) 36 to 40; (1) 46 or more.
362. Number of 2n chromosomes: (0) 48 or less; (1) 50 or more.
363. Number of 2n chromosomes: (0) 50 or less; (1) 52 or more.
364. Number of 2n chromosomes: (0) 52 or less; (1) 54 or more.
365. Number of 2n chromosomes: (0) 56 or less; (1) 58 or more.
Although several species analyzed herein have published information as to their chromosome number, this information was not previously included in phylogenetic analyses. The root of this analysis is coded as polymorphic for the characters 363, 364, and 365 because the number of chromosomes in the Cypriniformes usually ranges between 48 and 52. This character was coded from the literature (Arefjev, 1990a, b; Artoni & Bertollo, 2002; Bellafronte et al., 2005; Bertollo et al., 1986; Carvalho et al., 2002; Centofante et al., 2003; Cestari & Galetti, 1992; Falcão & Bertollo, 1985; Foresti et al., 1989; Galetti Jr. et al., 1981; ; Hinegardner & Rosen, 1972 ; Kirby et al., 1977; Paintner-Marques et al., 2002; Pauls & Bertollo, 1984; Portela et al., 1988; Porto et al., 1992; Silva & Maistro, 2006). Different numbers of chromosomes were reported for several species, which might indicate identification problems. These usually occurs in non-systematic papers; thus, several species are coded as polymorphic denoting some uncertainty as to identifications rather than polymorphisms per se.
Phylogenetic Results
The final hypothesis is the strict consensus between the most parsimonious trees obtained in a range of K-values under implied weighting (Goloboff, 1993). Details about the analysis itself, including the criteria by which this final hypothesis was constructed were already published (Mirande, 2009). The final hypothesis is presented in the Figs. 125-129.
Diagnosis of the obtained clades
The common synapomorphies of the individual trees are listed for each node of the final hypothesis. Those synapomorphic changes that are present only in some of the original trees are listed under "some trees". The node numbers correspond to those obtained from the TNT software and presented in the Figs. 125-129. GC values and relative frequencies as measures of stability, and GC values and relative Bremer support as measures of support are expressed between parentheses in each node (see Material and Methods, and Mirande, 2009).
OUTGROUP
Node 172: Characoidea (100 / 100 / 76 / 24)
Families Alestidae, Characidae, Gasteropelecidae, and Serrasalmidae.
The superfamily Characoidea was proposed by Buckup (1998) to include the Characidae (containing the Serrasalmidae) and Gasteropelecidae. That author did not include the Gasteropelecidae in his analysis, but classified that family with the Characidae based mainly on Géry (1977), who stated that the gasteropelecids have most characters in common with certain characids. The Characoidea is redefined in this paper to include, in addition, the former families Acestrorhynchidae and Cynodontidae (herein considered as subfamilies of the Characidae) and the Alestidae. In the publication by Buckup (1998) both the Acestrorhynchidae and Cynodontidae are included in the superfamily Cynodontoidea, while the Alestidae was classified in its own superfamily, the Alestoidea. Although no serrasalmids were included in the phylogeny of Buckup (1998), that author preferred to maintain this group as a subfamily of the Characidae in light of the lack of phylogenetic information refuting a close relationship of these families. In the molecular hypothesis of Calcagnotto et al. (2005) the Serrasalmidae, however, forms a clade separate from the Characidae. The hypothesis herein proposed also supports a familial level status for serrasalmids.
Synapomorphies:
1. Relative position of anterior margin of antorbital and first infraorbital (57): (1 > 0) anterior margin of antorbital either aligned with or anterior to first infraorbital. Reversed in node 184 and in Galeocharax humeralis.
2. Form of lateral line (89): (0 > 1) curved ventrally in abdominal region. Paralleled in Hemiodus cf. thayeria. Reversed in Rhaphiodon vulpinus.
3. Relative number of precaudal vertebrae (226): (0 > 1) equal or less numerous than caudal vertebrae. Reversed in Brycinus carolinae, Chalceus macrolepidotus, and Gymnocharacinus bergii.
4. Dorsal longitudinal ridge on medial lamella of pelvic bone (264): (0 > 1) absent. Reversed in the node 184.
5. Number of branched anal-fin rays (286): (0 > 1) 11 or more. Reversed in Chalceus macrolepidotus.
6. Number of branched anal-fin rays (287): (0 > 1) 18 or more. Reversed in the Alestidae, in nodes 280 and 290, and in Attonitus ephimeros and Prodontocharax melanotus. Some trees: Reversed in Hasemania nana. Paralleled in node 182.
7. Lateral lamellae on anterior anal pterygiophores (295): (1 > 0) absent. Reversed in the Alestidae.
Node 171: Gasteropelecidae (100 / 100 / 100 / 89)
Genera Carnegiella, Engraulisoma, Gasteropelecus Scopoli, and Thoracocharax; genus Clupeacharax?
Although the monophyly of the Gasteropelecidae, as traditionally defined, has long been obvious (Weitzman, 1954, 1960; Buckup, 1998), the relationships of this family with the remaining Characiformes had not been adequately tested. The great morphological divergence of the gasteropelecids probably complicated their inclusion in previous morphological phylogenies; however, no members of this family were included also in the molecular phylogeny of Calcagnotto et al. (2005). As herein proposed this family includes all genera traditionally considered in the Gasteropelecidae plus Engraulisoma (and tentatively also Clupeacharax). Castro (1984) proposed a close relationship between Engraulisoma taeniatum and Clupeacharax anchoveoides, the only member of the characid subfamily Clupeacharacinae (Géry, 1977; Lima, 2003a), based on seven putative synapomorphies: the possession of a foramen in the ventral surface of the pterotic, the short supraorbital, the fusion of the third and fourth infraorbitals (*), the antorbital with unique form and size within Characidae (*), the third branchiostegal ray articulated in the suture between the anterior and posterior ceratohyals, the absence of the second and third postcleithra (*), and the presence of interdigitations between the ischiatic processes of the pelvic bones. Synapomorphies marked with (*) are comparable to those defining the Gasteropelecidae in this analysis. The fusion of the posttemporal and supracleithrum, and the possession of only one epural, mentioned as autapomorphies of Engraulisoma taeniatum by Castro (1984), are shared with all the former Gasteropelecidae and with Thoracocharax stellatus, respectively. Similarly, the posteriorly situated dorsal fin is shared between Clupeacharax and the Gasteropelecidae sensu Weitzman (1954). Thus, although Clupeacharax anchoveoides was not included in this analysis, it can be tentatively included as incertae sedis within this clade, given the observations of Castro (1984). With conservativeness as a criterion, the genera Clupeacharax and Engraulisoma should be included in the Gasteropelecidae whether if the former genus is actually the sister group of Engraulisoma, as proposed by Castro (1984), or if Clupeacharax is basal to Engraulisoma and the remaining Gasteropelecidae. Under all possible scenarios Engraulisoma taeniatum and probably also Clupeacharax anchoveoides are removed from the Characidae and included in the Gasteropelecidae.
Synapomorphies:
1. Position of antorbital relative to lateral ethmoid in lateral view (56): (0 > 1) antorbital overlapping lateral ethmoid. Paralleled in node 302.
2. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae, in node 186, and in Aphyodite grammica, Hasemania nana, Hemigrammus erythrozonus, Hoplocharax goethei, Hyphessobrycon pulchripinnis, and Nematobrycon palmeri.
3. Laterosensory canal of first infraorbital (73): (1 > 0) projects dorsally from main body of first infraorbital. Paralleled in the Heterocharacinae.
4. Alignment of ascending process of premaxilla (105): (0 > 1) medially shifted and separated from nasal. Paralleled in node 193.
5. Position of coronomeckelian (110): (1 > 0) situated mainly lateral to Meckelian cartilage. Paralleled in nodes 176 and 206, and in Hoplias cf. malabaricus and Prochilodus lineatus.
6. Inner row of dentary teeth (143): (0 > 1) absent. Paralleled in nodes 166, 168, and 189, and in Rhaphiodon vulpinus and Serrasalmus maculatus.
7. Teeth on third pharyngobranchial (206): (0 > 1) absent. Paralleled in Piaractus mesopotamicus.
8. Second postcleithrum (248): (0 > 1) absent. Paralleled in node 302 and in Pseudocorynopoma doriae and Rhaphiodon vulpinus.
9. Third postcleithrum (249): (0 > 1) absent. Paralleled in node 302, and in Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
10. Fusion between posttemporal and supracleithrum (255): (0 > 1) present.
11. Anterior extension of pelvic-bone along main axis (262): (0 > 1) projecting anterior of lateral and medial lamellae of pelvic bone. Paralleled in node 302 and in Hoplias cf. malabaricus, Piabucus melanostomus, Rhaphiodon vulpinus, and Stethaprion erythrops.
12. Circulii on posterior field of scales (319): (0 > 1) absent. Paralleled in nodes 168 and 206, and in Agoniates anchovia. Some trees: Paralleled in node 302.
Autapomorphies of Engraulisoma taeniatum:
1. Extent of expansion of first infraorbital lateral to maxilla (59): (0 > 1) covering most of maxilla. Paralleled in node 277 and in Heterocharax macrolepis.
2. Shape of ectopterygoid (156): (0 > 2) approximately square.
3. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. Paralleled in nodes 184 and 242, and in Aphyocharax dentatus, Prionobrama paraguayensis, and Stichonodon insignis. Some trees: Paralleled in the Cheirodontinae and in Microschemobrycon casiquiare.
4. Interhyal (210): (0 > 1) absent. Paralleled in Aulixidens eugeniae.
5. Number of branchiostegal rays (212): (1 > 0) three. Paralleled in Apareiodon affinis, Leporinus striatus, and Pyrrhulina australis.
6. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. Paralleled in node 302, and in Agoniates anchovia, Cyanocharax alburnus, Deuterodon langei, Hemiodus cf. thayeria, Roeboexodon geryi, and Thayeria obliqua. Some trees: Paralleled in Microschemobrycon casiquiare and Parecbasis cyclolepis.
7. Transitional vertebrae with haemal canal (229): (0 > 1) absent. Paralleled in nodes 195 and 212, and in Aulixidens eugeniae, Metynnis maculatus, and Piabina argentea. Some trees: Paralleled in node 247 and in Bryconamericus alpha and Paracheirodon axelrodi.
8. Base of second pectoral ray (231): (0 > 1) similar in form and size to base of posterior rays. Paralleled in node 175. Some trees: Paralleled in node 302.
9. Bony ridge of coracoid between base of mesocoracoid and ventral margin of interosseous space (239): (1 > 0) absent. Paralleled in node 204.
10. Articulation between pelvic bones (261): (0 > 1) with bony interdigitations between ischiatic processes. Paralleled in node 302 and in Agoniates anchovia.
11. Relative length of anterior dorsal-fin rays (271): (0 > 1) reaching tip of posterior rays when adpressed. Paralleled in nodes 163 and 179 and in Pyrrhulina australis.
12. Position of last supraneural (283): (0 > 1) located more than two vertebrae in front of first dorsal pterygiophore. Paralleled in nodes 174 and 244 and in Gymnocharacinus bergii and Xenagoniates bondi.
13. Proximal and medial radials of anal fins (294): (2 > 0) fused on anterior five pterygiophores. Paralleled in node 179.
14. Posterior margin of hypural 3 (300): (1 > 0) equal to or narrower than posterior margin of hypural 4. Paralleled in node 181 and in the Alestidae.
15. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 184, 186, 209, 241, 261, and 270, and in Gymnocharacinus bergii.
Node 170: (100 / 100 / 100 / 65)
Genera Carnegiella, Gasteropelecus, and Thoracocharax.
This node includes the Gasteropelecidae as defined by Weitzman (1954), and recognized in subsequent papers (e. g. Géry, 1977). As Gasteropelecus was not analyzed, it is possible that some synapomorphies of this node actually correspond to a more restricted clade. Although the monophyly of a clade composed of Carnegiella, Gasteropelecus, and Thoracocharax was never tested in a phylogenetic context, it never was questioned, given the high resemblance of these genera and their morphological divergence from the remaining Characiformes (Weitzman, 1954; Weitzman & Palmer, 2003). The phylogenetic position of this clade, however, was unknown, because no previous higher-level phylogenies included species of the Gasteropelecidae. According to Géry (1977: 243), the members of this node "have most (characters) in common with the Characidae, chiefly with certain tetras"; however, this author maintained the Gasteropelecidae as a family, separated from the Characidae. Malabarba & Weitzman (2003) proposed a clade supported by the presence of bony hooks on the fin rays, which included the Characidae, Gasteropelecidae, and Serrasalmidae with the exception of the genera Agoniates, Clupeacharax, and Engraulisoma. According to their hypothesis both the Serrasalmidae and Gasteropelecidae should be included in the Characidae. However, such hypothesis was rather speculative, and not based on any published phylogenetic analysis.
Synapomorphies:
1. Ventral longitudinal lamellae of basioccipital (2): (1 > 0) falling short of posterior border of basioccipital. Paralleled in node 205 and in Serrasalmus maculatus.
2. Ventral projection of lagenar capsule (3): (0 > 1) extending ventrally to articulation between basioccipital and parasphenoid. Paralleled in the Serrasalmidae, in nodes 205, and 302, and in Cyphocharax spilotus and Micralestes stormsi.
3. Articulation between medial region of lateral ethmoid and frontal or mesethmoid (17): (0 > 1) extensive articulation of entire lateral ethmoid dorsal margin. Paralleled in node 193.
4. Form of orbitosphenoid (37): (1 > 0) slender, relatively small and separate from parasphenoid. Paralleled in the Characidae, in node 168, and in Hemiodus cf. thayeria.
5. Parietal fontanel (41): (0 > 1) absent in adults. Paralleled in nodes 162 and 181 and in Brycinus carolinae and Brycon pesu.
6. Supraorbital (70): (0 > 1) absent. Paralleled in nodes 185 and 205 and in Micralestes stormsi.
7. Lateral surface of vertical canal of preopercle (81): (1 > 0) canal uncovered and situated posteriorly to musculature and infraorbitals. Paralleled in nodes 175 and 204 and in Chalceus macrolepidotus and Metynnis maculatus.
8. Number of teeth in inner premaxillary row (130): (0 > 1) eight or more. Paralleled in the Aphyoditeinae and in Brycon orbignyanus, Grundulus cochae, Phenacogaster tegatus, Prionobrama paraguayensis, and Salminus brasiliensis.
9. Form of quadrate (150): (0 > 1) with anterodorsal portion equal or longer than ventral region. Paralleled in the Pseudochalceus clade, in nodes 176, 211, and 299, and in Exodon paradoxus and Hoplias cf. malabaricus.
10. Form of anterior portion of ectopterygoid (157): (0 > 1) slender and articulating only to lateral margin of palatine, and lacking ligaments to neurocranium. Paralleled in the Alestidae and in Agoniates anchovia and Attonitus ephimeros.
11. Bony lamella dorsal to fourth basibranchial (185): (1 > 0) present. Paralleled in nodes 168, 203, and 302 and in Phenacogaster tegatus.
12. Anterior development of basihyal (190): (1 > 0) broadly extending beyond anterior margin of hypohyals. Paralleled in node 302.
13. Rows of gill rakers on first ceratobranchial (192): (1 > 0) one. Paralleled in node 179.
14. Development of medial lamella of coracoid (238): (0 > 1) expanded as a keel. Paralleled in node 302 and in Paragoniates alburnus, Piabucus melanostomus, Pseudocorynopoma doriae, and Rhaphiodon vulpinus.
15. First postcleithrum (247): (0 > 1) absent. Paralleled in node 236.
16. Dorsal myorhabdoi (273): (0 > 1) present. Paralleled in Rhaphiodon vulpinus.
17. Position of anteriormost epineurals (274): (0 > 1) reaching to cranium. Paralleled in nodes 175 and 302 and in Distichodus maculatus and Piabucus melanostomus.
18. Number of supraneurals (281): (0 > 1) eight or more. Paralleled in node 207 and in Hemiodus cf. thayeria and Pyrrhulina australis.
19. Anal-fin position (284): (0 > 1) extended anteriorly ventral to dorsal fin. Paralleled in nodes 208, 212, and 236 and in Piabucus melanostomus.
Autapomorphies of Thoracocharax stellatus:
1. Opening between orbitosphenoid and pterosphenoid (39): (1 > 0) present, rounded or ovate, usually margined by frontal dorsally. Paralleled in nodes 205 and 302.
2. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Paralleled in nodes 168 and 180.
3. Number of maxillary teeth (135): (0 > 1) two or more.
4. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate (155): (0 > 1) present. Paralleled in nodes 176 and 299 and in Deuterodon langei, Heterocharax macrolepis, Pristella maxillaris, and Roeboides descalvadensis.
5. First basibranchial (182): (1 > 0) absent or much reduced, not articulating anteriorly with basihyal. Paralleled in node 163.
6. Number of branchiostegal rays (213): (0 > 1) five. Paralleled in Characidium borellii, Hoplias cf. malabaricus, Piaractus mesopotamicus, and Rhaphiodon vulpinus.
7. Number of dorsal pterygiophores (278): (0 > 1) 12 or more. Paralleled in the Serrasalmidae and in Characidium rachovii, Distichodus maculatus, and Hoplias cf. malabaricus.
8. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, and Stethaprion erythrops. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
9. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in nodes 210 and 221, and in Bario steindachneri, Markiana nigripinnis, Paragoniates alburnus, Rhaphiodon vulpinus, and Roeboides microlepis.
10. Ventral union of gill membranes (349): (0 > 1) joined along length of isthmus but not attached to isthmus. Paralleled in the Iguanodectinae and in node 162.
Autapomorphies of Carnegiella strigata:
1. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Aulixidens eugeniae, Carlana eigenmanni, Grundulus cochae, Odontostoechus lethostigmus, Paracheirodon axelrodi, Piabucus melanostomus, and Probolodus heterostomus.
2. Edentulous basihyal lamella (189): (0 > 1) present. Paralleled in the Alestidae and Heterocharacinae, in nodes 175 and 302, and in Bryconops affinis.
3. Adipose fin (356): (0 > 1) absent. Paralleled in the Gymnocharacinae, in node 181, and in Phenagoniates macrolepis.
Node 180: (100 / 100 / - / 12)
Families Alestidae, Characidae, and Serrasalmidae.
The monophyly of a clade equivalent to this grouping was not proposed in previous phylogenies. Uj (1990) proposed the monophyly of an African clade composed of the Alestidae, Citharinidae, and Distichodontidae, which was repeatedly contradicted in all subsequent phylogenies. The hypothesis of Buckup (1998) included the Erythrinoidea in a node similar to this one, making his results incompatible with those obtained in this study. In the hypothesis of Calcagnotto et al. (2005) the Alestidae are more related to the Crenuchidae and Erythrinoidea than to the Characidae. The families forming this node formed a trichotomy in the final hypothesis proposed by Mirande (2009), whereas the Serrasalmidae are herein proposed to be the sister group of the Alestidae and Characidae.
Synapomorphies:
1. Length of sphenotic spine (10): (0 > 1) extending ventrally to articulation between sphenotic and hyomandibula. Paralleled in Prochilodus lineatus. Reversed in the Characinae and in nodes 176 and 197.
2. Ventral diverging lamellae of mesethmoid (30): (0 > 1) present. Reversed in node 184.
3. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Paralleled in node 168 and in Thoracocharax stellatus. Reversed in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
4. Tubules for passage of blood vessels on lamellar portion of maxilla (98): (0 > 2) anastomosed tubules.
5. Horizontal process of anguloarticular (108): (0 > 1) broadly covered by dentary which reaches posterior border of Meckelian cartilage. Reversed in nodes 176 and 206.
6. Ascending process of neural pedicle of third vertebra (220): (0 > 1) present. Paralleled in Hemiodus cf. thayeria. Reversed in Rhaphiodon vulpinus.
7. Supraneural anterior to neural spine of fourth vertebra (279): (0 > 1) present and vertically elongate. Reversed in the Iguanodectinae, in node 204, and in Micralestes stormsi.
8. Uroneurals (306): (0 > 1) two pairs. Paralleled in node 163. Reversed in node 205.
Node 187: Serrasalmidae (100 / 100 / 100 / 59)
Genera Acnodon Eigenmann, Catoprion Müller & Troschel, Colossoma Eigenmann & Kennedy, †Megapiranha Cione, Dahdul, Lundberg & Machado-Allison, Metynnis Cope, Mylesinus Valenciennes, Myleus Müller & Troschel, Mylossoma Eigenmann & Kennedy, Ossubtus Jégu, Piaractus, Pristobrycon Eigenmann, Pygocentrus Müller & Troschel, Pygopristis Müller & Troschel, Serrasalmus, Tometes Valenciennes, and Utiaritichthys Miranda Ribeiro.
The monophyly of this clade was proposed by Machado-Allison (1983, 1986), supported by 27 synapomorphies, and subsequently corroborated by the molecular phylogenies by Ortí et al. (1996) and Calcagnotto et al. (2005). The taxonomic level of this group has been longly debated. Géry (1977) treated it as a family, while Machado-Allison (1982, 1983, 1985, 1986) and Jégu (2003) considered this clade as a subfamily of the Characidae. According to the phylogenetic hypothesis of Calcagnotto et al. (2005), this clade should be classified as a family, as herein proposed. As only a small sample of the serrasalmids are herein analyzed, some synapomorphies could correspond to more inclusive clades.
Synapomorphies:
1. Ventral projection of lagenar capsule (3): (0 > 1) extending ventrally to articulation between basioccipital and parasphenoid. Paralleled in nodes 170, 205, and 302 and in Cyphocharax spilotus and Micralestes stormsi.
2. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. Paralleled in nodes 182 and 205 and in Piabucus melanostomus and Prochilodus lineatus. Some trees: Paralleled in Brycon meeki and B. orbignyanus.
3. Teeth on inner premaxillary row (126): (1 > 0) molariform.
4. Polymorphism of teeth on inner premaxillary row (131): (0 > 1) present, with two medial teeth somewhat larger and usually separated from remaining ones by a gap. Paralleled in the Bryconinae.
5. Posterior directed radial striae from articular region of opercle (169): (0 > 1) present. Paralleled in Prochilodus lineatus.
6. Length of medial bony ridge of opercle (170): (1 > 0) 60% or greater than opercular length. Paralleled in node 210 and in Astyanax abramis, Creagrutus cf. taphorni, Hoplias cf. malabaricus, and Roeboides microlepis. Some trees: Paralleled in Acestrorhynchus pantaneiro and Salminus brasiliensis.
7. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in Attonitus ephimeros, Axelrodia lindeae, Hollandichthys multifasciatus, Hoplocharax goethei, Jupiaba scologaster, Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
8. Lateral base of gill rakers on first ceratobranchial (199): (0 > 1) broad and laminar at least on anteriormost gill rakers. Paralleled in in nodes 166 and 177 and in Hoplias cf. malabaricus. Reversed in Agoniates anchovia.
9. Abdominal ribs on anterior caudal vertebrae (225): (0 > 1) present, associated to first and occasionally second caudal vertebrae.
10. Number of dorsal pterygiophores (278): (0 > 1) 12 or more. Paralleled in Characidium rachovii, Distichodus maculatus, Hoplias cf. malabaricus, and Thoracocharax stellatus.
11. Bony lamellae associated with supraneurals (282): (0 > 1) wider than primary axis of supraneurals. Paralleled in Micralestes stormsi.
12. Fusion of hypural 2 to compound centrum (298): (1 > 0) absent. Paralleled in nodes 174 and 184 and in Distichodus maculatus, Hemiodus cf. thayeria, and Hoplias cf. malabaricus.
13.Radii on scales (320): (1 > 0) absent or reduced in number. Paralleled in the Iguanodectinae, in node 174, and in Cyphocharax spilotus, Distichodus maculatus, Markiana nigripinnis, and Phenagoniates macrolepis.
14. Median predorsal scales (325): (0 > 1) leaving naked area anterior to dorsal fin. Paralleled in node 284 and in Lonchogenys ilisha.
15. Ventral serrae (326): (0 > 1) present.
16. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in nodes 210 and 221 and in Bario steindachneri, Markiana nigripinnis, Paragoniates alburnus, Rhaphiodon vulpinus, Roeboides microlepis, and Thoracocharax stellatus.
17. Diffuse spots on flanks (346): (0 > 1) present, especially in young specimens.
18. Number of 2n chromosomes (363): (0 > 1) 52 or more. Paralleled in the Characinae, in node 196, and in Chalceus macrolepidotus, Hyphessobrycon herbertaxelrodi, Markiana nigripinnis, and Rhaphiodon vulpinus. Some trees: Paralleled in Hemigrammus unilineatus.
19. Number of 2n chromosomes (364): (0 > 1) 54 or more. Paralleled in Rhaphiodon vulpinus.
Autapomorphies of Piaractus mesopotamicus:
1. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 167, 177, 218, 260, and 276, and in Bryconamericus scleroparius and Chalceus macrolepidotus.
2. Canal of lateral line on caudal-fin membrane (92): (0 > 1) present. Paralleled in the Characidae.
3. Interdigitations between premaxillae (103): (1 > 0) present. Paralleled in the Bryconinae and in Distichodus maculatus.
4. Form of interdigitations between dentaries (112): (0 > 1) undulate lamellae. Paralleled in node 183.
5. Anterior extension of interopercle (163): (0 > 1) not extending anteriorly beyond terminus of horizontal arm of preopercle. Paralleled in the Heterocharacinae, in nodes 162, 174, and 212, and in Hoplias cf. malabaricus.
6. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax latens, A. cf. rutilus, A. pelegrini Eigenmann, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Parecbasis cyclolepis, and Stichonodon insignis.
7. Teeth on third pharyngobranchial (206): (0 > 1) absent. Paralleled in the Gasteropelecidae.
8. Number of branchiostegal rays (213): (0 > 1) five. Paralleled in Characidium borellii, Hoplias cf. malabaricus, Rhaphiodon vulpinus, and Thoracocharax stellatus.
Node 186: (100 / 100 / 90 / 54)
Genera Acnodon?, Catoprion?, Colossoma?, †Megapiranha?, Metynnis, Mylesinus?, Myleus?, Mylossoma?, Ossubtus?, Pristobrycon?, Pygocentrus?, Pygopristis?, Serrasalmus, Tometes?, and Utiaritichthys?
The internal relationships of the Serrasalmidae are unresolved, and several hypotheses has been proposed regarding this issue. Machado-Allison (1983) proposed the monophyly of a clade including the genera Catoprion, Metynnis, Pygocentrus, Pygopristis, Pristobrycon, and Serrasalmus. In the hypothesis of Calcagnotto et al. (2005), Piaractus is the sister group of the remaining analyzed members of the family, which include species of Metynnis and Serrasalmus. Both hypotheses are compatible with the one herein obtained, although the synapomorphies of this node should be further evaluated, because it is likely that some of those features are diagnostic of more or less inclusive clades. The fossil genus †Megapiranha was proposed to be the sister group of Pygopristis, Pristobrycon, Pygocentrus, and Serrasalmus (Cione et al., 2009), and is tentatively included at this node.
Synapomorphies:
1. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae and Gasteropelecidae, and in Aphyodite grammica, Hasemania nana, Hemigrammus erythrozonus, Hoplocharax goethei, Hyphessobrycon pulchripinnis, and Nematobrycon palmeri.
2. Articulation between dentary teeth (145): (0 > 1) present with associated processes and fossae.
3. Predorsal spine formed by first dorsal pterygiophore (275): (0 > 1) present.
4. Number of branched anal-fin rays (288): (0 > 1) 25 or more. Paralleled in the Characidae.
5. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 184, 209, 241, 261, and 270, and in Engraulisoma taeniatum and Gymnocharacinus bergii.
6. Insertion of adductor mandibulae tendon on dentary (331): (0 > 1) anterior to Meckelian cartilage. Paralleled in nodes 183 and 253 and in Xenagoniates bondi.
7. Number of 2n chromosomes (365): (0 > 1) 58 or more.
Autapomorphies of Serrasalmus maculatus:
1. Ventral longitudinal lamellae of basioccipital (2): (1 > 0) falling short of posterior border of basioccipital. Paralleled in nodes 170 and 205.
2. Position of sphenotic spine relative to hyomandibula (11): (0 > 1) displaced anteriorly relative to anterior margin of hyomandibula. Paralleled in nodes 162 and 211 and in Acestrorhynchus pantaneiro, Piabina argentea, and Salminus brasiliensis.
3. Dorsal process of pterotic where tendon from epaxial musculature attach (45): (0 > 1) present, projecting dorsally from tube for semicircular canal. Paralleled in the Heterocharacinae, in node 193, and in Rhoadsia altipinna.
4. Posterior branch of posttemporal laterosensory canal (88): (0 > 1) absent. Paralleled in Characidium rachovii.
5. Inner row of dentary teeth (143): (0 > 1) absent. Paralleled in the Gasteropelecidae, in nodes 166, 168, and 189, and in Rhaphiodon vulpinus.
6. Ectopterygoid teeth row (159): (0 > 1) present. Paralleled in nodes 168 and 300 and in Acestrorhynchus pantaneiro, Distichodus maculatus, Hoplias cf. malabaricus, and Xenagoniates bondi.
7. Fusion between hypurals 1 and 2 (299): (0 > 1) present. Paralleled in Distichodus maculatus and Hemiodus cf. thayeria.
Autapomorphies of Metynnis maculatus:
1. Contact between frontals anteriorly to frontal fontanel (21): (1 > 0) absent. Paralleled in node 167. Some trees: Paralleled in nodes 175 and 206 and in Brycon meeki.
2. Anterior margin of supraoccipital (51): (0 > 1) situated anterior to vertical through posterior orbital margin. Paralleled in node 220 and in Cynopotamus argenteus.
3. Lateral surface of vertical canal of preopercle (81): (1 > 0) canal uncovered and situated posteriorly to musculature and infraorbitals. Paralleled in nodes 170, 175, and 204 and in Chalceus macrolepidotus.
4. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
5. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. Paralleled in nodes 244 and 299 and in Hasemania nana, Hyphessobrycon bifasciatus, Odontostilbe microcephala Eigenmann, and Roeboides descalvadensis. Some trees: Paralleled in node 265.
6. Transitional vertebrae with haemal canal (229): (0 > 1) absent. Paralleled in nodes 195 and 212, and in Aulixidens eugeniae, Engraulisoma taeniatum, and Piabina argentea. Some trees: Paralleled in node 247 and in Bryconamericus alpha and Paracheirodon axelrodi.
7. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (0 > 1) three or four. Paralleled in nodes 203 and 276 and in Salminus brasiliensis. Some trees: Paralleled in Brycon orbignyanus.
8. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
Node 179: (-16 / 90 / - / 21)
Families Alestidae and Characidae.
The family Alestidae was longly considered as related with the Characidae or even included in it (e. g. Greenwood et al., 1966; Weitzman & Malabarba, 1998). In the hypothesis of Buckup (1998), the Alestidae is the sister group of a clade composed of the Acestrorhynchidae, Characidae, and Erythrinoidea. In the phylogenetic hypothesis of Lucena (1993), the Alestidae is included in the Characidae, being related with Brycon and Serrasalmus (the only serrasalmid included in his analysis). Murray & Stewart (2002) proposed both the monophyly and the familial level of the Alestidae. In the phylogeny of Calcagnotto et al. (2005) the African Alestidae are monophyletic and included in a node also containing the Erythrinoidea and Crenuchidae. Zanata & Vari (2005) published a comprehensive phylogeny of the Alestidae including several members of other characiform families in their analysis. The sister-group relationships of the Alestidae, however, were not hypothesized by Zanata & Vari (2005). In the present analysis the Alestidae are proposed to be the sister group of the Characidae. However, this hypothesis has low stability across the different analyses made in this study given that under most conditions, but not in the globally more stable hypotheses, the alestids are instead the sister group of the serrasalmids.
Synapomorphies:
1. Form of anterior process of lateral ethmoid (14): (0 > 1) slender and separated from vomer. Paralleled in Distichodus maculatus and Hemiodus cf. thayeria. Reversed in Aulixidens eugeniae, Rhaphiodon vulpinus, and Salminus brasiliensis.
2. Distance between cartilage anterior to orbitosphenoid and lateral ethmoids (38): (1 > 0) contacting, or almost contacting, lateral ethmoids. Paralleled in node 168 and in Hemiodus cf. thayeria and Hoplias cf. malabaricus. Reversed in node 204.
3. Rows of gill rakers on first ceratobranchial (192): (1 > 0) one. Paralleled in node 170. Reversed in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Reversed in node 249 and in Attonitus ephimeros.
4. Coracoid foramen (243): (0 > 1) well developed. Reversed in node 205 and in Salminus brasiliensis. Some trees: Reversed in node 302 and in Brycon orbignyanus.
5. Relative length of anterior dorsal-fin rays (271): (0 > 1) reaching tip of posterior rays when adpressed. Paralleled in node 163 and in Engraulisoma taeniatum and Pyrrhulina australis. Reversed in Mimagoniates rheocharis.
6. Proximal and medial radials of anal fins (294): (2 > 0) fused on anterior five pterygiophores. Paralleled in Engraulisoma taeniatum. Transformed to state 1 in nodes 184, 208, 218, and 221 and in Psellogrammus kennedyi and Pseudocorynopoma doriae. Some trees: Transformed to state 1 in node 295.
Node 185: Alestidae (100 / 100 / 46 / 19)
Genera Alestes, Alestopetersius Hoedeman, Arnoldichthys Myers, Bathyaethiops Fowler, Brachypetersius Hoedeman, Brycinus, Bryconaethiops, Bryconalestes, Chalceus, Clupeocharax Pellegrin, Duboisialestes Poll, Hemigrammopetersius, Hydrocynus, Ladigesia Géry, Lepidarchus Roberts, Micralestes, Nannopetersius Hoedeman, Petersius Hilgendorf, Phenacogrammus Eigenmann, Rhabdalestes, Tricuspidalestes Poll, and Virilia Roberts.
The monophyly of the Alestidae as composed of the "African characids" of Greenwood et al. (1966) was not greatly discussed in the literature. The phylogenetic analysis of the Alestidae by Zanata & Vari (2005) represented a great improvement in the knowledge of the morphology and phylogeny of the family. These authors defined the monophyly of the family and proposed the Neotropical genus Chalceus as the sister group of the remaining alestids. Thus, at present, Alestidae is the unique trans-Atlantic family within the Characiformes. The results obtained herein concerning this group are congruent with the hypothesis of Zanata & Vari (2005).
Synapomorphies:
1. Overlap of maxilla by second infraorbital (61): (1 > 0) absent. Paralleled in node 205. Some trees: Paralleled in node 302.
2. Dorsal end of laterosensory canal of preopercle and suprapreopercle (82): (0 > 1) overlapping anterodorsal process of opercle. Paralleled in node 230 and in Bario steindachneri, Hyphessobrycon eques, Parecbasis cyclolepis, Pristella maxillaris, and Stichonodon insignis.
3. Degree of ventral curvature of lateral line (90): (0 > 1) distinctly curved and ventrally situated, with posterior lying within ventral half of caudal peduncle and aligned with lower lobe of caudal fin. Paralleled in the Bryconops clade. Reversed in Bryconaethiops macrops.
4. Form of anterior portion of ectopterygoid (157): (0 > 1) slender and articulating only to lateral margin of palatine, and lacking ligaments to neurocranium. Paralleled in node 170 and in Agoniates anchovia and Attonitus ephimeros.
5. Edentulous basihyal lamella (189): (0 > 1) present. Paralleled in the Heterocharacinae, in nodes 175 and 302, and in Bryconops affinis and Carnegiella strigata.
6. Number of branched anal-fin rays (287): (1 > 0) 17 or less. Reversal of synapomorphy 6 of the Characoidea. Paralleled in nodes 280 and 290, and in Attonitus ephimeros and Prodontocharax melanotus. Some trees: Reversed in Hasemania nana. Paralleled in node 182.
7. Lateral lamellae on anterior anal pterygiophores (295): (0 > 1) present. Reversal of synapomorphy 7 of the Characoidea.
8. Number of epurals (297): (0 > 1) three. Paralleled in node 167 and in Brycon meeki.
9. Posterior margin of hypural 3 (300): (1 > 0) equal to or narrower than posterior margin of hypural 4. Paralleled in Engraulisoma taeniatum and in node 181.
Autapomorphies of Chalceus macrolepidotus:
1. Form of mesethmoid spine (27): (0 > 1) relatively short, with premaxillae articulating with each other anterior to mesethmoid. Paralleled in nodes 225 and 234 and in Paracheirodon axelrodi.
2. Laterosensory canal in antorbital (72): (0 > 1) present. Paralleled in the Bryconops clade and in Brycon falcatus and Iguanodectes geisleri.
3. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 167, 177, 218, 260, and 276, and in Bryconamericus scleroparius and Piaractus mesopotamicus.
4. Direction of posterior branch of laterosensory canal of fourth or fifth infraorbital (75): (0 > 1) to a pore conspicuously ventral to hyomandibular condyle. Paralleled in Hemiodus cf. thayeria.
5. Lateral surface of vertical canal of preopercle (81): (1 > 0) canal uncovered and situated posteriorly to musculature and infraorbitals. Paralleled in nodes 170, 175, and 204 and in Metynnis maculatus.
6. Number of rows of premaxillary teeth (123): (0 > 1) three. Paralleled in the Bryconinae and in Bryconaethiops macrops.
7. Number of maxillary teeth (136): (0 > 1) four or more. Paralleled in nodes 177, 181, and 205 and in Hemiodus cf. thayeria.
8. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Paralleled in nodes 177 and 205 and in Hoplias cf. malabaricus.
9. Contact between lamella on anterior portion of first basibranchial with lamella on posterior portion of second basibranchial (183): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 168, 177, and 216, and in Distichodus maculatus, Hemiodus cf. thayeria, Hoplias cf. malabaricus, and Piabina argentea.
10. Relative number of precaudal vertebrae (226): (1 > 0) exceeding caudal vertebrae in two or more elements. Reversal of synapomorphy 3 of Characoidea. Paralleled in Brycinus carolinae and Gymnocharacinus bergii.
11. Number of branched anal-fin rays (286): (1 > 0) 10 or less. Reversal of synapomorphy 5 of the Characoidea.
12. Number of 2n chromosomes (363): (0 > 1) 52 or more. Paralleled in the Characinae and Serrasalmidae, in node 196, and in Hyphessobrycon herbertaxelrodi, Markiana nigripinnis, and Rhaphiodon vulpinus. Some trees: Paralleled in Hemigrammus unilineatus.
Node 184: (100 / 100 / 99 / 26)
Genera Alestes, Alestopetersius, Arnoldichthys, Bathyaethiops, Brachypetersius, Brycinus, Bryconaethiops, Bryconalestes, Clupeocharax, Duboisialestes, Hemigrammopetersius, Hydrocynus, Ladigesia, Lepidarchus, Micralestes, Nannopetersius, Petersius, Phenacogrammus, Rhabdalestes, Tricuspidalestes, and Virilia.
This clade corresponds to the family Alestidae as recognized before the paper of Zanata & Vari (2005), including only the African members of the clade. As a relatively small number of alestids are included in this analysis, the proposed synapomorphies could actually be diagnostic for more or less restricted nodes, as is the situation with the Gasteropelecidae and Serrasalmidae.
Synapomorphies:
1. Ventromedial opening of posttemporal fossa (8): (0 > 1) present. Paralleled in the Heterocharacinae and in node 175.
2. Ventral diverging lamellae of mesethmoid (30): (1 > 0) absent. Reversal of synapomorphy 2 of node 180.
3. Relative position of anterior margin of antorbital and first infraorbital (57): (0 > 1) anterior margin of antorbital posterior to first infraorbital. Reversal of synapomorphy 1 of the Characoidea. Paralleled in Galeocharax humeralis.
4. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
5. Anterior end of ascending process of maxilla (94): (1 > 0) with conspicuous notch. Paralleled in Aulixidens eugeniae.
6. Form of posterolateral portion of premaxilla (106): (0 > 1) with pedicle expanded laterally to maxilla.
7. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. Paralleled in node 242, and in Aphyocharax dentatus, Engraulisoma taeniatum, Prionobrama paraguayensis, and Stichonodon insignis. Some trees: Paralleled in the Cheirodontinae and in Microschemobrycon casiquiare.
8. Dorsal longitudinal ridge on medial lamella of pelvic bone (264): (1 > 0) present. Reversal of synapomorphy 4 of the Characoidea.
9. Number of dorsal pterygiophores (276): (1 > 0) nine. Paralleled in the Stevardiinae and in Hoplocharax goethei and Piabucus melanostomus.
10. Proximal and medial radials of anal fins (294): (0 > 1) fused in most pterygiophores. Paralleled in nodes 208, 218, and 221 and in Psellogrammus kennedyi and Pseudocorynopoma doriae. Some trees: Paralleled in node 295.
11. Fusion of hypural 2 to compound centrum (298): (1 > 0) absent. Paralleled in the Serrasalmidae, in node 174, and in Distichodus maculatus, Hemiodus cf. thayeria, and Hoplias cf. malabaricus.
12. Caudal-fin bony stays (304): (0 > 1) present.
13. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 186, 209, 241, 261, and 270, and in Engraulisoma taeniatum and Gymnocharacinus bergii.
Autapomorphies of Micralestes stormsi:
1. Ventral projection of lagenar capsule (3): (0 > 1) extending ventrally to articulation between basioccipital and parasphenoid. Paralleled in the Serrasalmidae, in nodes 170, 205, and 302, and in Cyphocharax spilotus.
2. Posterior extent of third infraorbital (65): (0 > 1) relatively reduced, angle of preopercle covered partially by fourth infraorbital. Paralleled in Hemiodus cf. thayeria, Piabucus melanostomus, and Pyrrhulina australis.
3. Supraorbital (70): (0 > 1) absent. Paralleled in nodes 170, 185, and 205.
4. Laterosensory canal of sixth infraorbital (76): (1 > 0) not branched. Paralleled in the Iguanodectinae, in node 203, and in Charax stenopterus, Cyphocharax stellatus, and Phenacogaster tegatus.
5. Position of opening on neurocranium communicating with laterosensory canal of sixth infraorbital (77): (0 > 1) in frontal. Paralleled in nodes 193 and 249 and in Attonitus ephimeros.
6. Ventral margin of horizontal process of anguloarticular (109): (0 > 1) perpendicular to laterosensory canal of dentary from medial view. Paralleled in node 199 and in Bario steindachneri, and Bramocharax bransfordii.
7. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. Paralleled in nodes 265 and 294 and in Brycon orbignyanus, Bryconops melanurus, Gymnocharacinus bergii, and Nematocharax venustus. Some trees: Paralleled in Bryconamericus agna.
8. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
9. Ventral margin of anterior ceratohyal (179): (0 > 1) with notches for articulation of branchiostegal rays. Paralleled in nodes 167 and 189 and in Brycon orbignyanus and Salminus brasiliensis.
10. Total number of transitional vertebrae (228): (0 > 1) three or fewer. Paralleled in node 205 and in Acestrorhynchus pantaneiro, Characidium rachovii, Cyphocharax spilotus, and Triportheus pantanensis.
11. Posterior margin of cleithrum (234): (0 > 1) with concavity ventral to first postcleithrum. Paralleled in node 189.
12. Form of third postcleithrum (250): (1 > 0) slender, without associated lamella.
13. Supraneural anterior to neural spine of fourth vertebra (279): (1 > 0) absent or small. Reversal of synapomorphy 7 of node 180. Paralleled in the Iguanodectinae and in node 204.
14. Bony lamellae associated with supraneurals (282): (0 > 1) wider than primary axis of supraneurals. Paralleled in the Serrasalmidae.
Node 183: (100 / 100 / 100 / 100)
Genera Alestes, Brycinus, and Bryconaethiops.
The monophyly of a clade including the species of Alestes, Brycinus, and Bryconaethiops was proposed by Zanata & Vari (2005) as supported by four synapomorphies.
Synapomorphies:
1. Lateral bony coverage of olfactory nerve (36): (0 > 2) covered by an anterior tubular projection of orbitosphenoid.
2. Form of interdigitations between dentaries (112): (0 > 1) undulate lamellae. Paralleled in Piaractus mesopotamicus.
3. Teeth on inner premaxillary row (126): (1 > 2) with anteriorly concave pattern plus anterior cusps.
4. Alignment of cusps of medial teeth on inner premaxillary row (127): (1 > 0) forming anteriorly concave semicircle from ventral view. Paralleled in node 262 and in Moenkhausia dichroura. Some trees: Paralleled in node 302.
5. Articulation between quadrate and anguloarticular (153): (1 > 0) anterior to or at vertical through lateral ethmoid. Paralleled in the Iguanodectinae and in node 166.
6. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in node 177 and in Astyanax latens, A. cf. rutilus, A. pelegrini, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Parecbasis cyclolepis, Piaractus mesopotamicus, and Stichonodon insignis.
7. Insertion of adductor mandibulae tendon on dentary (331): (0 > 1) anterior to Meckelian cartilage. Paralleled in nodes 186 and 253 and in Xenagoniates bondi.
Autapomorphies of Brycinus carolinae:
1. Frontal fontanel (22): (0 > 1) totally occluded by frontals. Paralleled in Brycon pesu.
2. Parietal fontanel (41): (0 > 1) absent in adults. Paralleled in nodes 162, 170, and 181 and in Brycon pesu.
3. Relative number of precaudal vertebrae (226): (1 > 0) exceeding caudal vertebrae in two or more elements. Reversal of synapomorphy 3 of Characoidea. Paralleled in Chalceus macrolepidotus and Gymnocharacinus bergii.
Node 182: (100 / 100 / 90 / 100)
Genera Alestes and Bryconaethiops.
The monophyly of a clade including the species of Alestes and Bryconaethiops was previously proposed by Zanata & Vari (2005) and supported by two characters not analyzed here: the presence of well-developed indentations between the fifth and sixth infraorbitals in the margin of the eye, and a well-developed eyelid covering part of the eye. This node was also obtained in the present analysis based on two different characters.
Synapomorphies:
1. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. Paralleled in the Serrasalmidae, in node 205, and in Piabucus melanostomus and Prochilodus lineatus. Some trees: Paralleled in Brycon meeki and B. orbignyanus.
2. Number of branched anal-fin rays (287): (0 > 1) 18 or more. Reversal of synapomorphy 6 of the Alestidae.
Autapomorphies of Bryconaethiops macrops:
1. Degree of ventral curvature of lateral line (90): (1 > 0) straight or only slightly curved, with posterior portion aligned with middle caudal-fin rays. Reversal of synapomorphy 3 of the Alestidae.
2. Number of rows of premaxillary teeth (123): (0 > 1) three. Paralleled in the Bryconinae and in Chalceus macrolepidotus.
3. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
4. Pelvic bone (260): (0 > 1) bifurcate with conspicuous notch.
5. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in nodes 211, 223, and 262 and in Bramocharax bransfordii, Hyphessobrycon bifasciatus, and Nematocharax venustus. Some trees: Paralleled in the Aphyoditeinae.
No autapomorphies found for Alestes cf. macrophthalmus.
INGROUP
Node 178: Characidae (100 / 100 / 44 / 17)
Subfamilies Acestrorhynchinae, Agoniatinae, Aphyocharacinae, Aphyoditeinae, Bryconinae, Characinae, Cheirodontinae, Cynodontinae, Gymnocharacinae, Heterocharacinae, Iguanodectinae, Rhoadsiinae, Salmininae, Stevardiinae, and Tetragonopterinae; Astyanax clade, Astyanax paris clade, Bramocharax clade, Bryconamericus scleroparius clade, Bryconops clade, Hyphessobrycon anisitsi clade, and Pseudochalceus clade.
The composition and diagnosis of the Characidae are among the most problematic issues in the phylogeny of the Characiformes, and no explicit diagnosis based on synapomorphic features was proposed under previous phylogenetic studies of the family (Uj, 1990; Lucena, 1993; Buckup, 1998). This node is not completely congruent with some clade from previous phylogenies, but it is the one that requires the fewest nomenclatural changes relative to previous definitions of the family (e. g. Géry, 1977). The present definition of the Characidae is largely compatible with the hypothesis of Uj (1990) and Buckup (1998), but the node H of the hypothesis of Uj (1990) also includes the Serrasalmidae, while the node 14 of Buckup (1998) excludes Acestrorhynchus from the Characidae. This hypothesis is relatively less congruent with that of Lucena (1993), in which the node most similar to the Characidae of this study excludes Acestrorhynchus, Cynodon, and Rhaphiodon, and includes the Alestidae and Serrasalmidae. Results of molecular analyses are also rather different from the the results of the present hypothesis. The node of Ortí & Meyer (1997) most similar to the Characidae of this paper excludes the Cynodontinae and includes the Alestidae and Ctenoluciidae, while the node referred to as "Neotropical characids" by Calcagnotto et al. (2005) excludes the Cynodontinae but includes the genus Chalceus, in the Alestidae of this study. It is notable that both molecular phylogenies agree in the exclusion of the Cynodontinae from the Characidae, and in the non-monophyly of Alestidae, with Chalceus separated from the African alestids. Both the monophyly of a clade composed of the Acestrorhynchinae and Cynodontinae, and the sister-group relationship between Chalceus and the African Alestidae are supported by morphological phylogenies (Lucena & Menezes, 1998; Zanata & Vari, 2005), and are corroborated herein. In the present analysis the monophyly of the Characidae is supported by one additional unambiguous character (ch. 92) relative to the analysis of Mirande (2009).
Synapomorphies:
1. Form of orbitosphenoid (37): (1 > 0) slender, relatively small and separate from parasphenoid. Paralleled in nodes 168 and 170 and in Hemiodus cf. thayeria. Reversed in node 193 and in Markiana nigripinnis, Rhaphiodon vulpinus, and Roeboides microlepis.
2. Rhinosphenoid (47): (0 > 1) present. Paralleled in nodes 168 and 170 and in Hemiodus cf. thayeria. Reversed in nodes 207, 260, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
3. Canal of lateral line on caudal-fin membrane (92): (0 > 1) present. Paralleled in Piaractus mesopotamicus. Reversed in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
4. Total number of vertebrae (227): (0 > 1) 41 or more. Paralleled in Apareiodon affinis, Hemiodus cf. thayeria, and Prochilodus lineatus. Reversed in nodes 205 and 302 and in Brycon pesu.
5. Number of branched anal-fin rays (288): (0 > 1) 25 or more. Paralleled in node 186. Reversed in nodes 200, 277, and 300 and in Acestrorhynchus pantaneiro and Iguanodectes geisleri.
6. Anterior ventral procurrent caudal-fin rays (305): (0 > 1) fused in laminar medial bones.
7.Radii of scales (322): (1 > 0) not converging at focus. Paralleled in node 168 and in Hemiodus cf. thayeria. Reversed in node 273 and in Stichonodon insignis and Tetragonopterus argenteus. Some trees: Reversed in node 302 and in Microschemobrycon casiquiare.
8. Attachment of medial tendon of A1 section of adductor mandibulae (333): (1 > 0) on quadrate near its articulation with preopercle. Reversed in node 211.
Some trees:
9.Radii oriented towards anterior field of scales (321): (0 > 2) absent. Reversed in node 302.
Node 177: (100 / 100 / 7 / 10)
Subfamilies Acestrorhynchinae, Agoniatinae, Bryconinae, Cynodontinae, and Salmininae.
The monophyly of a group equivalent with this clade was not proposed in previous phylogenies. The Acestrorhynchinae and Cynodontinae were recently considered as families, separate from the Characidae (as the Acestrorhynchidae and Cynodontidae; e. g. Buckup, 1998; Menezes, 2003; Toledo-Piza, 2003). In the analysis by Buckup (1998), Acestrorhynchus is the sister group of the Erythrinoidea, and both the Acestrorhynchidae and Cynodontidae were included in the superfamily Cynodontoidea. In the present paper both clades are included as subfamilies of the Characidae. According to the hypothesis of Uj (1990), his Agoniatidae (=Agoniatinae) and Bryconidae (=Bryconinae and Salmininae) are sequentially arranged at the base of a clade corresponding to the Characidae of this study. In the analysis of Buckup (1998), Brycon is the sister group of the remaining members attributable to the Characidae; that author did not analyze the position of Agoniates and Salminus. In the analysis of Lucena (1993) the Acestrorhynchinae and Cynodontinae form the sister group of a clade including most genera of the Characidae. Agoniates is the sister group of the remaining members of this clade, while Brycon and Triportheus are situated more distally. This node includes most of the characids having a supraorbital bone, and only Bryconops, some of the Heterocharacinae, and the Iguanodectinae have an ossified supraorbital among the members of the Characidae not included in this clade.
Synapomorphies:
1. Posteriorly-oriented epioccipital spine (7): (1 > 0) present. Paralleled in nodes 162, 177, and 218. Reversed in node 174.
2. Contact between supraorbital and sixth infraorbital (71): (0 > 1) present. Paralleled in node 162 and in Prochilodus lineatus. Reversed in Agoniates anchovia.
3. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 167, 218, 260, and 276, and in Bryconamericus scleroparius, Chalceus macrolepidotus, and Piaractus mesopotamicus. Reversed in node 302.
4. Length of caudal-fin canal of lateral line (93): (0 > 1) almost reaching posterior margin of caudal fin. Paralleled in node 216 and in Astyanax pelegrini and Tetragonopterus argenteus.
5. Number of maxillary teeth (136): (0 > 1) four or more. Paralleled in nodes 181 and 205 and in Chalceus macrolepidotus and Hemiodus cf. thayeria. Reversed in node 302.
6. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Paralleled in node 205 and in Chalceus macrolepidotus and Hoplias cf. malabaricus. Reversed in node 302.
7. Contact between lamella on anterior portion of first basibranchial with lamella on posterior portion of second basibranchial (183): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 168 and 216, and in Chalceus macrolepidotus, Distichodus maculatus, Hemiodus cf. thayeria, Hoplias cf. malabaricus, and Piabina argentea. Reversed in Agoniates anchovia.
8. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in node 183 and in Astyanax latens, A. cf. rutilus, A. pelegrini, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Parecbasis cyclolepis, Piaractus mesopotamicus, and Stichonodon insignis. Reversed in Brycon pesu.
9. Lateral base of gill rakers on first ceratobranchial (199): (0 > 1) broad and laminar at least on anteriormost gill rakers. Paralleled in the Serrasalmidae, in node 166, and in Hoplias cf. malabaricus. Reversed in Agoniates anchovia.
10. Position of ventral end of posttemporal (253): (0 > 1) posterior to lateral margin of epioccipital. Paralleled in nodes 211 and 228 and in Bryconamericus scleroparius, Hoplias cf. malabaricus, Markiana nigripinnis, Probolodus heterostomus, and Prochilodus lineatus. Reversed in Brycon pesu.
Node 176: (100 / 100 / 52 / 23)
Subfamilies Acestrorhynchinae, Agoniatinae, Cynodontidae, and Salmininae.
The monophyly of this assemblage was not previously proposed. The relationships of Salminus were superficially treated by Roberts (1974), who considered this genus to be a primitive and relatively unspecialized characid. Roberts highlighted what he thought were close morphological resemblance between Salminus and Brycon, although he suggested that such resemblance could be produced by the persistence in both genera of plesiomorphic features. This supposition is congruent with the results obtained in this paper.
Synapomorphies:
1. Length of sphenotic spine (10): (1 > 0) not extending ventrally to articulation between sphenotic and hyomandibula. Reversal of synapomorphy 1 of node 180. Paralleled in the Characinae and in node 197.
2. Articulation between second and third infraorbitals (62): (0 > 2) posteroventrally angled. Paralleled in the Characinae, in node 300, and in Bryconops melanurus and Hollandichthys multifasciatus.
3. Horizontal process of anguloarticular (108): (1 > 0) laterally covered by dentary only anteriorly. Reversal of synapomorphy 5 of node 180. Paralleled in node 206.
4. Position of coronomeckelian (110): (1 > 0) situated mainly lateral to Meckelian cartilage. Paralleled in the Gasteropelecidae, in node 206, and in Hoplias cf. malabaricus and Prochilodus lineatus. Reversed in Rhaphiodon vulpinus.
5. Form of quadrate (150): (0 > 1) with anterodorsal portion equal or longer than ventral region. Paralleled in the Pseudochalceus clade, in nodes 170, 211, and 299, and in Exodon paradoxus and Hoplias cf. malabaricus.
6. Articulation between quadrate and anguloarticular (154): (0 > 1) posterior to middle eye. Paralleled in Hoplias cf. malabaricus.
7. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate (155): (0 > 1) present. Paralleled in node 299 and in Deuterodon langei, Heterocharax macrolepis, Pristella maxillaris, Roeboides descalvadensis, and Thoracocharax stellatus.
8. Form and degree of ossification of first ceratobranchial gill rakers (200): (0 > 1) rather thick and completely ossified distal region. Paralleled in nodes 212 and 299 and in Hoplias cf. malabaricus, Prionobrama paraguayensis, and Pristella maxillaris.
9. Rows of gill rakers on first epibranchial (203): (1 > 0) one. Reversed in Rhaphiodon vulpinus.
Salmininae:
Genus Salminus
The Salmininae was proposed by Eigenmann (1917) as a line diverging from the Cheirodontinae. Roberts (1969) alternatively considered Salminus to be a basal characid. Géry (1977) included this genus in the tribe Salminini, as part of the subfamily Bryconinae. The genus Salminus was classified as incertae sedis within the Characidae by Lima et al. (2003), and it is herein proposed to be removed from that group and classified in its own subfamily.
Autapomorphies of Salminus brasiliensis:
1. Position of sphenotic spine relative to hyomandibula (11): (0 > 1) displaced anteriorly relative to anterior margin of hyomandibula. Paralleled in nodes 162 and 211 and in Acestrorhynchus pantaneiro, Piabina argentea, and Serrasalmus maculatus.
2. Temporal fossa (13): (1 > 0) well developed. Paralleled in node 300 and in Brycon meeki and Bryconexodon juruenae.
3. Form of anterior process of lateral ethmoid (14): (1 > 0) broad in ventral view, contacting proximal region of vomer in its entire length. Reversal of synapomorphy 1 of node 179. Paralleled in Aulixidens eugeniae and Rhaphiodon vulpinus.
4. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Hollandichthys multifasciatus, and Pseudocorynopoma doriae.
5. Number of teeth in inner premaxillary row (130): (0 > 1) eight or more. Paralleled in the Aphyoditeinae, in node 170, and in Brycon orbignyanus, Grundulus cochae, Phenacogaster tegatus, and Prionobrama paraguayensis.
6. Foramen in posterior region of metapterygoid (168): (2 > 1) present, encircled by metapterygoid or bordered partially by cartilage. Paralleled in node 205.
7. Ventral margin of anterior ceratohyal (179): (0 > 1) with notches for articulation of branchiostegal rays. Paralleled in nodes 167 and 189 and in Brycon orbignyanus and Micralestes stormsi.
8. Articulation between anterior and posterior ceratohyals (181): (0 > 1) with bony interdigitations. Paralleled in node 210 and in Hoplias cf. malabaricus and Rhaphiodon vulpinus.
9. Coracoid foramen (243): (1 > 0) absent or reduced to small pore. Reversal of synapomorphy 4 of node 179. Paralleled in node 205. Some trees: Paralleled in node 302 and in Brycon orbignyanus.
10. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (0 > 1) three or four. Paralleled in nodes 203 and 276 and in Metynnis maculatus. Some trees: Paralleled in Brycon orbignyanus.
11. Number of ventral procurrent caudal-fin rays (302): (0 > 1) 12 or more. Paralleled in the Bryconops clade and in nodes 229 and 252.
12. Anterior extension of adductor arcus palatini (336): (1 > 0) covering most of dorsal surface of mesopterygoid. Paralleled in node 166 and in Creagrutus anary and Markiana nigripinnis. Some trees: Paralleled in Brycon orbignyanus.
13. Sclerotic bones (350): (1 > 0) single anteroventrally open bone.
Some trees:
14. Length of medial bony ridge of opercle (170): (1 > 0) 60% or greater than opercular length. (k11-14). Paralleled in the Serrasalmidae, in node 210, and in Astyanax abramis, Creagrutus cf. taphorni, Hoplias cf. malabaricus, and Roeboides microlepis. Some trees: Paralleled in Acestrorhynchus pantaneiro.
Node 175: (100 / 100 / 62 / 60)
Subfamilies Acestrorhynchinae, Agoniatinae, and Cynodontinae.
Several hypotheses about the relationships of Agoniates have been proposed, all of which agree in the relatively basal position of this genus within the Characidae (Géry, 1963, 1972; Castro, 1984; Uj, 1990; Lucena, 1993). Uj (1990) proposed the Agoniatidae (=Agoniatinae) to be the sister group of the remaining members of his clade H (the most compatible of his groupings with the Characidae as herein recognized). Uj partially based his hypotheses in the proposal by Géry (1963). In the hypothesis of Lucena (1993), Agoniates is the sister group of a clade which includes most of the Characidae, with the exceptions of the Acestrorhynchinae and Cynodontinae. A close relationship between the monogeneric Agoniatinae and the Acestrorhynchinae and Cynodontinae was not previously proposed.
Synapomorphies:
1. Ventromedial opening of posttemporal fossa (8): (0 > 1) present. Paralleled in the Heterocharacinae and in node 184.
2. Lateral surface of vertical canal of preopercle (81): (1 > 0) canal uncovered and situated posteriorly to musculature and infraorbitals. Paralleled in nodes 170 and 204 and in Chalceus macrolepidotus and Metynnis maculatus.
3. Anterior projection of anterior ceratohyal articulating laterally with hypohyals (177): (0 > 1) present and achieving half length of hypohyals.
4. Edentulous basihyal lamella (189): (0 > 1) present. Paralleled in the Alestidae and Heterocharacinae, in node 302, and in Bryconops affinis and Carnegiella strigata.
5. Base of second pectoral ray (231): (0 > 1) similar in form and size to base of posterior rays. Paralleled in Engraulisoma taeniatum. Some trees: Paralleled in node 302.
6. Position of anteriormost epineurals (274): (0 > 1) reaching to cranium. Paralleled in nodes 170 and 302 and in Distichodus maculatus and Piabucus melanostomus.
Some trees:
7. Contact between frontals anteriorly to frontal fontanel (21): (1 > 0) absent. (k11-14). Paralleled in node 167 and in Metynnis maculatus. Some trees: Paralleled in node 206 and in Brycon meeki.
Agoniatinae:
Genus Agoniates
The relationships of the monogeneric subfamily Agoniatinae were analyzed by Géry (1963, 1972), who considered the presence in this genus of some presumably "primitive" and "adaptive" characters. Géry proposed that Agoniates was related with Clupeacharax. Castro (1984) proposed a sister-group relationship of Clupeacharax and Engraulisoma, contrary to the relationships suggested by Géry (1963) for Agoniates. Zanata (2000) later proposed a close relationship of Agoniates with Lignobrycon, and Triportheus, but Lima & Zanata (2003) maintained the Agoniatinae as valid and monogeneric. Lima et al. (2003) classified Lignobrycon and Triportheus as incertae sedis within Characidae. The results of the present study do not support a close relationship between Agoniates and Triportheus. Perhaps the inclusion of Lignobrycon in a broader phylogenetic analysis could resolve these discrepancies.
Autapomorphies of Agoniates anchovia:
1. Position of ventromedial opening of posttemporal fossa (9): (0 > 1) bordered entirely by epioccipital. Paralleled in node 163.
2. Dorsal expansion of rhinosphenoid (48): (1 > 0) absent. Paralleled in nodes 201, 212, and 300.
3. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
4. Contact between supraorbital and sixth infraorbital (71): (1 > 0) absent. Reversal of synapomorphy 2 of node 177.
5. Opening of epiphyseal laterosensory canals (86): (0 > 1) canals continue dorsomedially in soft tissue, opening over or just lateral to the cranial fontanel.
6. Form of anterior portion of ectopterygoid (157): (0 > 1) slender and articulating only to lateral margin of palatine, and lacking ligaments to neurocranium. Paralleled in the Alestidae, in node 170, and in Attonitus ephimeros.
7. Contact between lamella on anterior portion of first basibranchial with lamella on posterior portion of second basibranchial (183): (1 > 0) absent. Reversal of synapomorphy 7 of node 177.
8. Lateral base of gill rakers on first ceratobranchial (199): (1 > 0) slender. Reversal of synapomorphy 9 of node 177.
9. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. Paralleled in node 302, and in Cyanocharax alburnus, Deuterodon langei, Engraulisoma taeniatum, Hemiodus cf. thayeria, Roeboexodon geryi, and Thayeria obliqua. Some trees: Paralleled in Microschemobrycon casiquiare and Parecbasis cyclolepis.
10. Anteriorly directed spine at base of first rib (223): (0 > 1) present. Paralleled in the Heterocharacinae. Some trees: Paralleled in node 302.
11. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162, 247, and 253 and in Attonitus ephimeros, Characidium borellii, Iguanodectes geisleri, Moenkhausia cf. intermedia, Prionobrama paraguayensis, and Xenagoniates bondi.
12. Dorsal development of third postcleithrum (251): (0 > 1) not projects dorsally to posterior region of scapula. Paralleled in node 192 and in Gymnocharacinus bergii.
13. Articulation between pelvic bones (261): (0 > 1) with bony interdigitations between ischiatic processes. Paralleled in node 302 and in Engraulisoma taeniatum.
14. Number of epurals (296): (1 > 0) one. Paralleled in Prionobrama paraguayensis.
15.Circulii on posterior field of scales (319): (0 > 1) absent. Paralleled in the Gasteropelecidae and in nodes 168 and 206. Some trees: Paralleled in node 302.
16. Posterior attachment of A1 section of adductor mandibulae (332): (0 > 1) restricted or almost restricted to horizontal arm of preopercle. Paralleled in the Iguanodectinae, in node 211, and in Aphyodite grammica and Pyrrhulina australis.
Node 174: (100 / 100 / 99 / 73)
Subfamilies Acestrorhynchinae and Cynodontinae.
The monophyly of a clade composed of the Acestrorhynchinae and Cynodontinae was proposed in several morphological phylogenies (Uj, 1990; Lucena, 1993; Lucena & Menezes, 1998); however, this clade was not resolved as a monophyletic unit in the molecular analyses of Ortí & Meyer (1990) and Calcagnotto et al. (2005). This analysis corroborates a sister-group relationship between these two subfamilies, which is supported by numerous synapomorphies.
Synapomorphies:
1. Posteriorly-oriented epioccipital spine (7): (0 > 1) absent. Reversal of synapomorphy 1 of node 177.
2. Dilator fossa on lateral surface of frontal (24): (1 > 0) absent. Paralleled in node 181.
3. Bony lamellae bordering sensory canal of nasal (34): (0 > 1) wider at some point than tubular region. Paralleled in node 181 and in Galeocharax humeralis, Leporinus striatus, and Roeboexodon geryi.
4. Ventral border of rhinosphenoid (50): (0 > 1) almost contacting parasphenoid. Paralleled in node 193.
5. Posterior dorsoventral expansion of fourth infraorbital (68): (0 > 1) present. Paralleled in nodes 210 and 299.
6. Morphology of premaxillary, maxillary, and dentary teeth (118): (1 > 0) all teeth conical, caniniform or mamilliform. Paralleled in the Heterocharacinae, in nodes 181 and 211, and in Axelrodia lindeae, Grundulus cochae, and Exodon paradoxus.
7. A pair of large conical teeth in premaxilla (121): (0 > 1) present. Paralleled in node 299.
8. Anterior extension of interopercle (163): (0 > 1) not extending anteriorly beyond terminus of horizontal arm of preopercle. Paralleled in the Heterocharacinae, in nodes 162 and 212, and in Hoplias cf. malabaricus and Piaractus mesopotamicus.
9. Mesopterygoid teeth (165): (0 > 1) present.
10. Shape of first ceratobranchial gill rakers (197): (0 > 2) short, broad and strongly denticulated.
11. Length of interhyal (211): (0 > 1) equal to or longer than one-half of symplectic length. Paralleled in node 211 and in Hoplias cf. malabaricus and Pseudochalceus kyburzi.
12. Number of branchiostegal rays attached to posterior ceratohyal (217): (0 > 1) two.
13. Position of last supraneural (283): (0 > 1) located more than two vertebrae in front of first dorsal pterygiophore. Paralleled in node 244 and in Engraulisoma taeniatum, Gymnocharacinus bergii, and Xenagoniates bondi.
14. Fusion of hypural 2 to compound centrum (298): (1 > 0) absent. Paralleled in the Serrasalmidae, in node 184, and in Distichodus maculatus, Hemiodus cf. thayeria, and Hoplias cf. malabaricus.
15.Radii on scales (320): (1 > 0) absent or reduced in number. Paralleled in the Iguanodectinae and Serrasalminae and in Cyphocharax spilotus, Distichodus maculatus, Markiana nigripinnis, and Phenagoniates macrolepis.
Cynodontinae:
Genera Cynodon, Gilbertolus?, Hydrolycus, Rhaphiodon, and Roestes?
Hydrolycus and Rhaphiodon (including Cynodon) were included in the characid subfamily Rhaphiodontinae by Géry (1977). Lucena & Menezes (1998) proposed this clade as the sister group of the Acestrorhynchidae, and the familial name Cynodontidae (already used by Greenwood et al., 1966) was given to this clade plus the genera Gilbertolus and Roestes (not analyzed here). The phylogeny of Acestrorhynchus undertaken by Toledo-Piza (2007) included the genera of the Cynodontinae as part of the outgroup. In her paper Gilbertolus and Roestes are not closely related with Cynodon, Hydrolycus, and Rhaphiodon, but rather related with other members of the Characidae. Therefore, the position of Gilbertolus and Roestes is currently uncertain. Toledo-Piza (2007), however, argued that not all the known evidence relating these five genera was used in her analysis. Thus, these genera are herein tentatively maintained within the Cynodontinae. The monophyly of the Cynodontinae (as Cynodontidae) was corroborated by Lucena & Menezes (1998) and Toledo-Piza (2000).
Autapomorphies of Rhaphiodon vulpinus:
1. Posterior laminar expansion of epiphyseal bar (1): (0 > 1) present. Paralleled in Piabucus melanostomus.
2. Form of anterior process of lateral ethmoid (14): (1 > 0) broad in ventral view, contacting proximal region of vomer in its entire length. Reversal of synapomorphy 1 of node 179. Paralleled in Aulixidens eugeniae and Salminus brasiliensis.
3. Synchondral articulation between lateral ethmoid and anterodorsal border of orbitosphenoid (35): (1 > 0) present. Paralleled in the Aphyocharacinae and in Leporinus striatus, Mimagoniates rheocharis, Pristella maxillaris, and Rhaphiodon vulpinus.
4. Form of orbitosphenoid (37): (0 > 1) massive, almost reaching parasphenoid ventrally. Reversal of synapomorphy 1 of the Characidae. Paralleled in node 193 and in Markiana nigripinnis and Roeboides microlepis.
5. Pores of laterosensory canal of lower jaw (80): (0 > 1) seven or more. Paralleled in nodes 211 and 299.
6. Form of lateral line (89): (1 > 0) approximately straight. Reversal of synapomorphy 2 of the Characoidea.
7. Position of coronomeckelian (110): (0 > 1) situated mainly dorsal to Meckelian cartilage. Reversal of synapomorphy 4 of node 176.
8. Inner row of dentary teeth (143): (0 > 1) absent. Paralleled in the Gasteropelecidae, in nodes 166, 168, and 189, and in Serrasalmus maculatus.
9. Patch of ectopterygoid teeth (160): (0 > 1) present. Paralleled in Hoplias cf. malabaricus.
10. Suprapreopercle (175): (0 > 1) autogenous, separated from preopercle. Paralleled in nodes 210 and 302 and in Markiana nigripinnis and Roeboides microlepis.
11. Articulation between anterior and posterior ceratohyals (181): (0 > 1) with bony interdigitations. Paralleled in node 210 and in Hoplias cf. malabaricus and Salminus brasiliensis.
12. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Attonitus ephimeros, Axelrodia lindeae, Hollandichthys multifasciatus, Hoplocharax goethei, Jupiaba scologaster, Piabucus melanostomus, Pyrrhulina australis, and Xenagoniates bondi.
13. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, and Prodontocharax melanotus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
14. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 210, 225, 276, and 297 and in Brycon orbignyanus, Hoplocharax goethei, and Hyphessobrycon elachys.
15. Rows of gill rakers on first epibranchial (203): (0 > 1) two. Reversal of synapomorphy 9 of node 176.
16. Number of branchiostegal rays (213): (0 > 1) five. Paralleled in Characidium borellii, Hoplias cf. malabaricus, Piaractus mesopotamicus, and Thoracocharax stellatus.
17. Anterior portions of branchiostegal rays (214): (0 > 1) slender near their articulation with ceratohyals. Paralleled in nodes 212 and 256.
18. Ascending process of neural pedicle of third vertebra (220): (1 > 0) absent. Reversal of synapomorphy 6 of node 180.
19. Dorsal development of cleithrum (237): (0 > 1) ending in a position just dorsal of tip of mesocoracoid. Paralleled in node 302.
20. Development of medial lamella of coracoid (238): (0 > 1) expanded as a keel. Paralleled in nodes 170 and 302 and in Paragoniates alburnus, Piabucus melanostomus, and Pseudocorynopoma doriae.
21. Second postcleithrum (248): (0 > 1) absent. Paralleled in the Gasteropelecidae, in node 302, and in Pseudocorynopoma doriae.
22. Third postcleithrum (249): (0 > 1) absent. Paralleled in the Gasteropelecidae, in node 302, and in Piabucus melanostomus, Pyrrhulina australis, and Xenagoniates bondi.
23. Ventral exit of laterosensory canal of supracleithrum (254): (0 > 1) ventral to lamella of supracleithrum and exiting on posterior margin of this bone. Paralleled in node 205.
24. Anterior extension of pelvic-bone along main axis (262): (0 > 1) projecting anterior of lateral and medial lamellae of pelvic bone. Paralleled in the Gasteropelecidae, in node 302, and in Hoplias cf. malabaricus, Piabucus melanostomus, and Stethaprion erythrops.
25. Number of dorsal-fin rays on last pterygiophore (272): (0 > 1) two, adnate.
26. Dorsal myorhabdoi (273): (0 > 1) present. Paralleled in node 170.
27. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
28. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in nodes 210 and 221, and in Bario steindachneri, Markiana nigripinnis, Paragoniates alburnus, Roeboides microlepis, and Thoracocharax stellatus.
29. Posterior region of levator arcus palatini (337): (0 > 1) limited lateral and medially by A2 and A3 sections of adductor mandibulae. Paralleled in Piabina argentea.
30. Number of 2n chromosomes (363): (0 > 1) 52 or more. Paralleled in the Characinae and Serrasalmidae, in node 196, and in Chalceus macrolepidotus, Hyphessobrycon herbertaxelrodi, and Markiana nigripinnis. Some trees: Paralleled in Hemigrammus unilineatus.
31. Number of 2n chromosomes (364): (0 > 1) 54 or more. Paralleled in the Serrasalmidae.
Acestrorhynchinae:
Genus Acestrorhynchus
The subfamily Acestrorhynchinae was originally proposed to include Acestrorhynchus and Oligosarcus by Menezes (1969). Géry (1977) classified these two genera plus Bramocharax in the tribe Acestrorhynchini, as part of the subfamily Characinae. That author even stated that the Acestrorhynchini was "one of the best-known characid groups" (Géry, 1977: 323). This hypothesis of a close relationship was challenged by Menezes & Géry (1983) and refuted by Buckup (1998). The latter author found that Oligosarcus and Acestrorhynchus are only distantly related. In his phylogeny Oligosarcus is deeply nested in the Characidae, while Acestrorhynchus was not included in the Characidae, but rather in its own family, the Acestrorhynchidae. The Acestrorhynchidae was also considered as valid by Lucena & Menezes (1998) and Menezes (2003), among others. The monophyly of Acestrorhynchus was tested by Toledo-Piza (2007), who obtained a sister-group relationship between this genus and the Cynodontidae as previously proposed by Lucena & Menezes (1998). This clade containing Acestrorhynchus and Rhaphiodon is nested in a group composed of the Agoniatinae and Salmininae, which is the sister group of the Bryconinae. All of these were traditionally included in the Characidae. Consequently, both the Acestrorhynchinae and Cynodontinae are herein proposed to be subfamilies of the Characidae.
Autapomorphies of Acestrorhynchus pantaneiro:
1. Position of sphenotic spine relative to hyomandibula (11): (0 > 1) displaced anteriorly relative to anterior margin of hyomandibula. Paralleled in nodes 162 and 211 and in Piabina argentea, Salminus brasiliensis, and Serrasalmus maculatus.
2. Position of sphenotic spine relative to the orbit (12): (0 > 1) distinctly posterior to orbital margin. Paralleled in nodes 193 and 299 and in Attonitus ephimeros, Cynopotamus argenteus, and Gymnocharacinus bergii.
3. Anterior region of laterosensory canal of frontal (83): (0 > 1) opens into a chamber limited. dorsally by frontal and ventrally by lateral ethmoid.
4. Ectopterygoid teeth row (159): (0 > 1) present. Paralleled in nodes 168 and 300 and in Distichodus maculatus, Hoplias cf. malabaricus, Serrasalmus maculatus, and Xenagoniates bondi.
5. Rows of gill rakers on third and fourth ceratobranchials (194): (1 > 0) one.
6. Total number of transitional vertebrae (228): (0 > 1) three or fewer. Paralleled in node 205 and in Characidium rachovii, Cyphocharax spilotus, Micralestes stormsi, and Triportheus pantanensis.
7. Number of branched anal-fin rays (288): (1 > 0) 24 or less. Reversal of synapomorphy 5 of the Characidae. Paralleled in nodes 200, 277, and 300 and in Iguanodectes geisleri.
8. Humeral spot (341): (0 > 1) horizontally-ovate. Paralleled in node 259 and in Brycon orbignyanus, Jupiaba mucronata, and Roeboides microlepis.
Some trees:
9. Length of medial bony ridge of opercle (170): (1 > 0) 60% or greater than opercular length. (k11-14). Paralleled in the Serrasalmidae, in node 210, and in Astyanax abramis, Creagrutus cf. taphorni, Hoplias cf. malabaricus, and Roeboides microlepis. Some trees: Paralleled in Salminus brasiliensis.
Node 190: Bryconinae (86 / 97 / 1 / 10)
Genera Brycon, Chilobrycon Géry & de Rham, Henochilus Garman, Lignobrycon, and Triportheus.
The subfamily Bryconinae was considered by Géry (1977) to be composed of the tribes Bryconini, Salminini, and Triportheini. Although a close relationship between Brycon and Triportheus has been suggested several times in the literature (e. g. Géry, 1977), only Malabarba (1998b) obtained evidence supporting this hypothesis. In her phylogeny, Lignobrycon is the sister group of Triportheus, supported by eight synapomorphies. Lima (2003b), mainly based in the analysis of Zanata (2000) restricted the Bryconinae to Brycon, Chilobrycon, and Henochilus, leaving Lignobrycon, Salminus, and Triportheus as incertae sedis within the Characidae (Lima et al., 2003). This subfamily is herein redefined to include the previously incertae sedis genera Lignobrycon and Triportheus. The included species of Brycon form a polytomy in this node, and the monophyly of the genus is not supported in this analysis. However, only a small sample of this genus was analyzed, and a study focused on the question of the monophyly of Brycon lies far beyond the scope of this paper.
Synapomorphies:
1. Interdigitations between premaxillae (103): (1 > 0) present. Paralleled in Distichodus maculatus and Piaractus mesopotamicus.
2. Number of rows of premaxillary teeth (123): (0 > 1) three. Paralleled in Bryconaethiops macrops and Chalceus macrolepidotus.
3. Polymorphism of teeth on inner premaxillary row (131): (0 > 1) present, with two medial teeth somewhat larger and usually separated from remaining ones by a gap. Paralleled in the Serrasalmidae.
Autapomorphies of Brycon pesu:
1. Frontal fontanel (22): (0 > 1) totally occluded by frontals. Paralleled in Brycinus carolinae.
2. Parietal fontanel (41): (0 > 1) absent in adults. Paralleled in nodes 162, 170, and 181 and in Brycinus carolinae.
3. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
4. Number of gill rakers on first hypobranchial and ceratobranchial (195): (0 > 1) 15 or fewer. Reversal of synapomorphy 8 of node 177.
5. Total number of vertebrae (227): (1 > 0) 40 or fewer. Reversal of synapmorphy 4 of the Characidae. Paralleled in nodes 205 and 302.
6. Position of ventral end of posttemporal (253): (1 > 0) anterior or lateral to lateral margin of epioccipital. Reversal of synapomorphy 10 of node 177.
7. Scales covering supraoccipital spine (324): (0 > 1) present and completely covering supraoccipital spine. Paralleled in Prochilodus lineatus.
Autapomorphies of Brycon orbignyanus:
1. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Bryconamericus scleroparius, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
2. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. Paralleled in nodes 265 and 294 and in Bryconops melanurus, Gymnocharacinus bergii, Micralestes stormsi, and Nematocharax venustus. Some trees: Paralleled in Bryconamericus agna.
3. Number of teeth in inner premaxillary row (130): (0 > 1) eight or more. Paralleled in the Aphyoditeinae, in node 170, and in Grundulus cochae, Phenacogaster tegatus, Prionobrama paraguayensis, and Salminus brasiliensis.
4. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273, 283, and 294, and in Bramocharax bransfordii, Gymnocharacinus bergii, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
5. Ventral margin of anterior ceratohyal (179): (0 > 1) with notches for articulation of branchiostegal rays. Paralleled in nodes 167 and 189 and in Micralestes stormsi and Salminus brasiliensis.
6. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
7. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 210, 225, 276, and 297 and in Hoplocharax goethei, Hyphessobrycon elachys, and Rhaphiodon vulpinus.
8. Humeral spot (341): (0 > 1) horizontally-ovate. Paralleled in node 259 and in Acestrorhynchus pantaneiro, Jupiaba mucronata, and Roeboides microlepis.
Some trees:
9. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. (k11-14). Paralleled in the Serrasalmidae, in nodes 182 and 205, and in Piabucus melanostomus and Prochilodus lineatus. Some trees: Paralleled in Brycon meeki.
10. Coracoid foramen (243): (1 > 0) absent or reduced to small pore. (k11-14). Reversal of synapomorphy 4 of node 179. Paralleled in node 205 and in Salminus brasiliensis. Some trees: Paralleled in node 302.
11. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (0 > 1) three or four. (k9-10). Paralleled in nodes 203 and 276 and in Metynnis maculatus and Salminus brasiliensis.
12. Anterior extension of adductor arcus palatini (336): (1 > 0) covering most of dorsal surface of mesopterygoid. (k9-10). Paralleled in node 166 and in Creagrutus anary, Markiana nigripinnis, and Salminus brasiliensis.
Autapomorphies of Brycon meeki:
1. Temporal fossa (13): (1 > 0) well developed. Paralleled in node 300 and in Bryconexodon juruenae and Salminus brasiliensis.
2. Number of epurals (297): (0 > 1) three. Paralleled in the Alestidae and in node 167.
Some trees:
3. Contact between frontals anteriorly to frontal fontanel (21): (1 > 0) absent. (k11-14). Paralleled in node 167 and in Metynnis maculatus. Some trees: Paralleled in nodes 175 and 206.
4. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. (k11-14). Paralleled in the Serrasalmidae, in nodes 182 and 205, and in Piabucus melanostomus and Prochilodus lineatus. Some trees: Paralleled in Brycon orbignyanus.
Autapomorphy of Brycon falcatus:
1. Laterosensory canal in antorbital (72): (0 > 1) present. Paralleled in the Bryconops clade and in Chalceus macrolepidotus and Iguanodectes geisleri.
Node 302: (100 / 100 / 100 / 47)
Genera Lignobrycon and Triportheus.
The monophyly of Triportheus was generally accepted even before the cladistic analysis of Malabarba (1998b), who identified four synapomorphies for Triportheus and proposed Lignobrycon as the sister group of the genus. Some of the synapomorphies found here for the two analyzed species of Triportheus could be applicable to more or less restricted clades within this genus, or to the node composed of Lignobrycon plus Triportheus.
Synapomorphies:
1. Ventral projection of lagenar capsule (3): (0 > 1) extending ventrally to articulation between basioccipital and parasphenoid. Paralleled in the Serrasalmidae, in nodes 170 and 205, and in Cyphocharax spilotus and Micralestes stormsi.
2. Opening between orbitosphenoid and pterosphenoid (39): (1 > 0) present, rounded or ovate, usually margined by frontal dorsally. Paralleled in node 205 and in Thoracocharax stellatus.
3. Position of antorbital relative to lateral ethmoid in lateral view (56): (0 > 1) antorbital overlapping lateral ethmoid. Paralleled in the Gasteropelecidae.
4. Lateral overlap of first infraorbital by anterior margin of second infraorbital (60): (0 > 1) present. Paralleled in the Bryconops clade.
5. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, and 225, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
6. Form of fourth infraorbital (67): (0 > 1) longer dorsoventrally than longitudinally. Paralleled in node 189.
7. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (1 > 0) absent. Reversal of synapomorphy 3 of node 177.
8. Epiphyseal branch of corresponding supraorbital canals (85): (0 > 1) oriented obliquely, opening posteriorly to epiphyseal bar. Paralleled in Heterocharax macrolepis.
9. Number of maxillary teeth (136): (1 > 0) up to three. Reversal of synapomorphy 5 of node 177.
10. Extent of implantation of teeth along maxilla (137): (1 > 0) not reaching middle of maxillary lamella. Reversal of synapomorphy 6 of node 177.
11. Anterodorsal lobe of metapterygoid oriented towards mesopterygoid (166): (0 > 1) present, conspicuous and anteriorly oriented.
12. Relative length of palatine (172): (0 > 1) distinctly longer than one-half length of ectopterygoid. Paralleled in nodes 197 and 261 and in Hyphessobrycon pulchripinnis and Paracheirodon axelrodi.
13. Suprapreopercle (175): (0 > 1) autogenous, separated from preopercle. Paralleled in node 210 and in Markiana nigripinnis, Roeboides microlepis, and Rhaphiodon vulpinus.
14. Bony lamella dorsal to fourth basibranchial (185): (1 > 0) present. Paralleled in nodes 168, 170, and 203 and in Phenacogaster tegatus.
15. Edentulous basihyal lamella (189): (0 > 1) present. Paralleled in the Alestidae and Heterocharacinae, in node 175, and in Bryconops affinis and Carnegiella strigata.
16. Anterior development of basihyal (190): (1 > 0) broadly extending beyond anterior margin of hypohyals. Paralleled in node 170.
17. Distribution of denticles on gill rakers (202): (1 > 0) restricted to margins, or absent.
18. Attachment of first branchiostegal ray (215): (0 > 1) posterior to one-half length of anterior ceratohyal.
19. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. Paralleled in Agoniates anchovia, Cyanocharax alburnus, Deuterodon langei, Engraulisoma taeniatum, Hemiodus cf. thayeria, Roeboexodon geryi, and Thayeria obliqua. Some trees: Paralleled in Microschemobrycon casiquiare and Parecbasis cyclolepis.
20. Total number of vertebrae (227): (1 > 0) 40 or fewer. Reversal of synapmorphy 4 of the Characidae. Paralleled in node 302 and in Brycon pesu.
21. Medial laminar expansion at dorsal tip of cleithrum (236): (0 > 1) present.
22. Dorsal development of cleithrum (237): (0 > 1) ending in a position just dorsal of tip of mesocoracoid. Paralleled in Rhaphiodon vulpinus.
23. Development of medial lamella of coracoid (238): (0 > 1) expanded as a keel. Paralleled in node 170 and in Paragoniates alburnus, Piabucus melanostomus, Pseudocorynopoma doriae, and Rhaphiodon vulpinus.
24. Second postcleithrum (248): (0 > 1) absent. Paralleled in the Gasteropelecidae and in Pseudocorynopoma doriae and Rhaphiodon vulpinus.
25. Third postcleithrum (249): (0 > 1) absent. Paralleled in the Gasteropelecidae and in Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
26. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, and 280, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
27. Articulation between pelvic bones (261): (0 > 1) with bony interdigitations between ischiatic processes. Paralleled in Agoniates anchovia and Engraulisoma taeniatum.
28. Anterior extension of pelvic-bone along main axis (262): (0 > 1) projecting anterior of lateral and medial lamellae of pelvic bone. Paralleled in the Gasteropelecidae and in Hoplias cf. malabaricus, Piabucus melanostomus, Rhaphiodon vulpinus, and Stethaprion erythrops.
29. Position of anteriormost epineurals (274): (0 > 1) reaching to cranium. Paralleled in nodes 170 and 175 and in Distichodus maculatus and Piabucus melanostomus.
Some trees:
30. Overlap of maxilla by second infraorbital (61): (1 > 0) absent. (k9-10). Paralleled in the Alestidae and in node 205.
31. Alignment of cusps of medial teeth on inner premaxillary row (127): (1 > 0) forming anteriorly concave semicircle from ventral view. (k9-10). Paralleled in nodes 183 and 262 and in Moenkhausia dichroura.
32. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. (k 9-10). Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae.
33. Anteriorly directed spine at base of first rib (223): (0 > 1) present. (k9-10). Paralleled in the Heterocharacinae and in Agoniates anchovia.
34. Base of second pectoral ray (231): (0 > 1) similar in form and size to base of posterior rays. (k9-10). Paralleled in node 175 and in Engraulisoma taeniatum.
35. Coracoid foramen (243): (1 > 0) absent or reduced to small pore. (k11-14). Reversal of synapomorphy 4 of node 179. Paralleled in node 205 and in Salminus brasiliensis. Some trees: Paralleled in Brycon orbignyanus.
36.Circulii on posterior field of scales (319): (0 > 1) absent. (k9-10). Paralleled in the Gasteropelecidae, in nodes 168 and 206, and in Agoniates anchovia.
37.Radii oriented towards anterior field of scales (321): (2 > 0) present. (k9-10). Reversal of synapomorphy 9 of the Characidae.
38.Radii of scales (322): (0 > 1) converging at focus. (k9-10). Reversal of synapomorphy 7 of the Characidae. Paralleled in node 273 and in Stichonodon insignis and Tetragonopterus argenteus. Some trees: Paralleled in Microschemobrycon casiquiare.
Autapomorphy of Triportheus pantanensis:
1. Total number of transitional vertebrae (228): (0 > 1) three or fewer. Paralleled in node 205 and in Acestrorhynchus pantaneiro, Characidium rachovii, Cyphocharax spilotus, and Micralestes stormsi.
No autapomorphies found for Triportheus nematurus.
Node 189: (100 / 100 / 61 / 20)
Subfamilies Aphyocharacinae, Aphyoditeinae, Characinae, Cheirodontinae, Gymnocharacinae, Heterocharacinae, Iguanodectinae, Rhoadsiinae, Stethaprioninae, Stevardiinae, and Tetragonopterinae; Astyanax clade, Astyanax paris clade, Bramocharax clade, Bryconamericus scleroparius clade, Bryconops clade, Hyphessobrycon anisitsi clade, and Pseudochalceus clade.
This node is congruent with clade 15 of Buckup (1998), which included the genera Bryconops, Charax, Cynopotamus, Oligosarcus, Phenacogaster, and Tetragonopterus. According to that author, this clade is supported by two synapomorphies: the frontal expanded lateral to the supraorbital laterosensory canal and the absence of an inner dentary row of teeth. The former of these synapomorphies is related with the presence and size of the supraorbital bone, and it is coded differently in this study. The second synapomorphy is corroborated here.
Synapomorphies:
1. Form of fourth infraorbital (67): (0 > 1) longer dorsoventrally than longitudinally. Paralleled in node 302. Reversed in nodes 200, 210, 228, 277, and 282.
2. Inner row of dentary teeth (143): (0 > 1) absent. Paralleled in the Gasteropelecidae, in nodes 166 and 168, and in Rhaphiodon vulpinus and Serrasalmus maculatus. Reversed in the Heterocharacinae, in node 276, and in Aphyocharacidium bolivianum.
3. Ventral margin of anterior ceratohyal (179): (0 > 1) with notches for articulation of branchiostegal rays. Paralleled in node 167 and in Brycon orbignyanus, Micralestes stormsi, and Salminus brasiliensis. Reversed in node 211 and in Stichonodon insignis.
4. Form and articulation of neural pedicle of third vertebra (218): (0 > 1) pedicle much smaller and without an articular surface with neural complex.
5. Dorsal development of dorsal process of neural pedicle of third vertebra (221): (1 > 0) not broadly overlapping neural complex. Reversed in node 212.
6. Posterior margin of cleithrum (234): (0 > 1) with concavity ventral to first postcleithrum. Paralleled in Micralestes stormsi. Reversed in node 204.
Node 188: Iguanodectinae (100 / 100 / 100 / 57)
Genera Iguanodectes and Piabucus.
The monophyly of the Iguanodectinae was proposed by Vari (1977), based in three characters of the gas-bladder; several new synapomorphies are added in this paper, although some of them could correspond to inner nodes within this subfamily. The monophyly of the Iguanodectinae was corroborated by Moreira (2002), who also studied the composition and internal relationships of this clade.
Synapomorphies:
1. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
2. Lateral coverage of dilator fossa by sixth infraorbital (69): (0 > 1) leaving a conspicuous naked area in anterior region of dilator fossa. Paralleled in the Iguanodetinae, in node 197, and in Charax stenopterus, Hoplocharax goethei, Phenacogaster tegatus, and Psellogrammus kennedyi.
3. Laterosensory canal of sixth infraorbital (76): (1 > 0) not branched. Paralleled in node 203 and in Charax stenopterus, Cyphocharax stellatus, Micralestes stormsi, and Phenacogaster tegatus.
4. Posterior extent of maxilla (99): (1 > 0) not reaching second infraorbital.
5. Medial process of dentary bordering Meckelian cartilage dorsally and medially (115): (0 > 1) present.
6. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
7. Longitudinal ridge in quadrate bordering adductor mandibulae muscle ventrally and, to some degree, laterally (152): (0 > 1) present. Paralleled in nodes 209 and 252.
8. Articulation between quadrate and anguloarticular (153): (1 > 0) anterior to or at vertical through lateral ethmoid. Paralleled in nodes 166 and 183.
9. Dorsal process of ectopterygoid oriented towards lateral ethmoid (158): (0 > 1) present.
10. Shape of metapterygoid-quadrate fenestra (167): (0 > 1) anteriorly collapsed by convergence of metapterygoid and ventral region of quadrate.
11. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in node 280 and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
12. Shape of dentigerous plate of fifth ceratobranchial (204): (1 > 0) rounded, with posterior notch. Paralleled in node 252 and in Axelrodia lindeae.
13. Supraneural anterior to neural spine of fourth vertebra (279): (1 > 0) absent or small. Reversal of synapomorphy 7 of node 180. Paralleled in node 204 and in Micralestes stormsi.
14. Number of anal pterygiophores anterior to first haemal spine (293): (0 > 1) four or more. Paralleled in in node 214 and in Cynopotamus argenteus and Gymnocorymbus ternetzi.
15.Radii on scales (320): (1 > 0) absent or reduced in number. Paralleled in the Serrasalminae, in node 174, and in Cyphocharax spilotus, Distichodus maculatus, Markiana nigripinnis, and Phenagoniates macrolepis.
16. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in nodes 184, 186, 209, 241, 261, and 270 and in Engraulisoma taeniatum and Gymnocharacinus bergii.
17. Insertion of adductor mandibulae tendon on dentary (331): (0 > 2) on a medial process of the dentary.
18. Posterior attachment of section A1 from adductor mandibulae (332): (0 > 1) restricted or almost restricted to horizontal arm of preopercle. Paralleled in node 211 and in Agoniates anchovia, Aphyodite grammica, and Pyrrhulina australis.
19. Ventral union of gill membranes (349): (0 > 1) joined along length of isthmus but not attached to isthmus. Paralleled in node 162 and in Thoracocharax stellatus.
Autapomorphies of Piabucus melanostomus:
1. Posterior laminar expansion of epiphyseal bar (1): (0 > 1) present. Paralleled in Rhaphiodon vulpinus.
2. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. Paralleled in the Serrasalmidae, in nodes 182 and 205, and in Prochilodus lineatus. Some trees: Paralleled in Brycon meeki and B. orbignyanus.
3. Posterior extent of third infraorbital (65): (0 > 1) relatively reduced, angle of preopercle covered partially by fourth infraorbital. Paralleled in Hemiodus cf. thayeria, Micralestes stormsi, and Pyrrhulina australis.
4. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Aulixidens eugeniae, Carlana eigenmanni, Carnegiella strigata, Grundulus cochae, Odontostoechus lethostigmus, Paracheirodon axelrodi, and Probolodus heterostomus.
5. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Attonitus ephimeros, Axelrodia lindeae, Hollandichthys multifasciatus, Hoplocharax goethei, Jupiaba scologaster, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
6. Form of posterior margin of cleithrum (233): (0 > 1) with notch just anterior to pectoral-fin insertion.
7. Development of medial lamella of coracoid (238): (0 > 1) expanded as a keel. Paralleled in nodes 170 and 302 and in Paragoniates alburnus, Pseudocorynopoma doriae, and Rhaphiodon vulpinus.
8. Third postcleithrum (249): (0 > 1) absent. Paralleled in the Gasteropelecidae, in node 302, and in Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
9. Anterior extension of pelvic-bone along main axis (262): (0 > 1) projecting anterior of lateral and medial lamellae of pelvic bone. Paralleled in the Gasteropelecidae, in node 302, and in Hoplias cf. malabaricus, Rhaphiodon vulpinus, and Stethaprion erythrops.
10. Number of branched-rays on dorsal-fin (270): (1 > 0) eight or fewer. Paralleled in the Stevardiinae and in Coptobrycon bilineatus, and Hoplocharax goethei.
11. Position of anteriormost epineurals (274): (0 > 1) reaching to cranium. Paralleled in nodes 170, 175, and 302 and in Distichodus maculatus.
12. Number of dorsal pterygiophores (276): (1 > 0) nine. Paralleled in the Stevardiinae, in node 184, and in Hoplocharax goethei.
13. Anal-fin position (284): (0 > 1) extended anteriorly ventral to dorsal fin. Paralleled in nodes 170, 208, 212, and 236.
14. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Pseudocorynopoma doriae, Rhaphiodon vulpinus , Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
15. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi (335): (1 > 0) absent. Paralleled in Creagrutus anary, Inpaichthys kerri, Prionobrama paraguayensis, Pristella maxillaris, Prodontocharax melanotus, and Pyrrhulina australis. Some trees: Paralleled in Hyphessobrycon elachys.
Autapomorphies of Iguanodectes geisleri:
1. Relative length of pterotic spine (46): (0 > 1) restricted to attachment region of hyomandibular ligament. Paralleled in node 205.
2. Laterosensory canal in antorbital (72): (0 > 1) present. Paralleled in the Bryconops clade and in Brycon falcatus and Chalceus macrolepidotus.
3. Medial anteroventral notch of dentary (114): (0 > 1) present. Paralleled in node 209.
4. Maxillary teeth (134): (1 > 0) absent. Paralleled in Aulixidens eugeniae, Coptobrycon bilineatus, Parecbasis cyclolepis, and Stichonodon insignis. Some trees: Paralleled in Hyphessobrycon elachys and Psellogrammus kennedyi.
5. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in the Astyanax clade, in nodes 284 and 290, and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, and Hyphessobrycon bifasciatus. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
6. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. Paralleled in the Characinae and in Bryconops melanurus, Hyphessobrycon pulchripinnis, and Moenkhausia sanctaefilomenae. Some trees: Paralleled in node 196 and in Hemigrammus erythrozonus and Hyphessobrycon herbertaxelrodi.
7. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162, 247, and 253 and in Agoniates anchovia, Attonitus ephimeros, Characidium borellii, Moenkhausia cf. intermedia, Prionobrama paraguayensis, and Xenagoniates bondi.
8. Number of unbranched anal-fin rays (285): (1 > 0) three or fewer. Paralleled in node 252 and in Paracheirodon axelrodi.
9. Number of branched anal-fin rays (288): (1 > 0) 24 or less. Reversal of synapomorphy 5 of the Characidae. Paralleled in nodes 200, 277, and 300 and in Acestrorhynchus pantaneiro.
Node 206: (-2 / 97 / - / 26)
Subfamilies Aphyocharacinae, Aphyoditeinae, Characinae, Cheirodontinae, Gymnocharacinae, Heterocharacinae, Rhoadsiinae, Stethaprioninae, Stevardiinae, and Tetragonopterinae; Astyanax clade, Astyanax paris clade, Bramocharax clade, Bryconamericus scleroparius clade, Bryconops clade, Hyphessobrycon anisitsi clade, and Pseudochalceus clade.
Mirande (2009) obtained a trichotomy composed of the Iguanodectinae, the Bryconops clade, and a large clade containing the Heterocharacinae and the characids lacking a supraorbital. This trichotomy is resolved in this study, being the Iguanodectinae the sister group of the remaining two clades, which form in the present study a monophyletic group herein described. Although this node was obtained in the globally more stable hypothesis, in most analyses under self-weighted optimization (see Mirande, 2009) the Iguanodectinae is the sister group of the Bryconops clade, a resolution which is incongruent with this node.
Synapomorphies:
1. Horizontal process of anguloarticular (108): (1 > 0) laterally covered by dentary only anteriorly. Reversal of synapomorphy 5 of node 180. Paralleled in node 176. Reversed in nodes 246, 253, and 261 and in Xenagoniates bondi.
2. Position of coronomeckelian (110): (1 > 0) situated mainly lateral to Meckelian cartilage. Paralleled in the Gasteropelecidae, in node 176, and in Hoplias cf. malabaricus and Prochilodus lineatus. Reversed in nodes 200, 282, and 290 and in Hemigrammus erythrozonus.
3.Circulii on posterior field of scales (319): (0 > 1) absent. Paralleled in the Gasteropelecidae, in node 168, and in Agoniates anchovia. Some trees: Paralleled in node 302. Reversed in node 261 and in Exodon paradoxus, Phenagoniates macrolepis, and Roeboides microlepis.
Some trees:
4. Contact between frontals anteriorly to frontal fontanel (21): (1 > 0) absent. (k11-14). Paralleled in node 167 and in Metynnis maculatus. Some trees: Paralleled in node 175 and in Brycon meeki. Reversed in Bario steindachneri, Exodon paradoxus, Galeocharax humeralis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Reversed in Knodus breviceps.
Node 278: Bryconops clade (100 / 100 / 100 / 30)
Genus Bryconops.
Monophyly of Bryconops had not previously been tested. Three subgenera were proposed within this genus, Brycochandus Eigenmann, Bryconops, and Creatochanes Günther (Géry, 1977). The two species analyzed here correspond to the subgenus Creatochanes according to that classification, and the diagnosis provided for this clade may be different if species of the other subgenera were included. In most analyses under self-weighted optimization this clade is the sister group of the Iguanodectinae, and Bryconops, even if monophyletic, will not justify a subfamilial-level name. Thus, both the monophyly and the proposed relationships of Bryconops should be further corroborated.
Synapomorphies:
1. Lateral overlap of first infraorbital by anterior margin of second infraorbital (60): (0 > 1) present. Paralleled in node 302.
2. Laterosensory canal in antorbital (72): (0 > 1) present. Paralleled in Brycon falcatus, Chalceus macrolepidotus, and Iguanodectes geisleri.
3. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in nodes 167, 177, 218, 260, and 276 and in Bryconamericus scleroparius, Chalceus macrolepidotus, and Piaractus mesopotamicus.
4. Degree of ventral curvature of lateral line (90): (0 > 1) distinctly curved and ventrally situated, with posterior lying within ventral half of caudal peduncle and aligned with lower lobe of caudal fin. Paralleled in the Alestidae.
5. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
6. Abrupt decrease in size of dentary teeth (148): (0 > 1) present. Paralleled in nodes 222, 255, and 299, and in Astyanax latens and A. paris.
7. Contact between lamella on anterior portion of first basibranchial with lamella on posterior portion of second basibranchial (183): (0 > 1) present. Paralleled in nodes 168, 177, and 216 and in Chalceus macrolepidotus, Distichodus maculatus, Hemiodus cf. thayeria, Hoplias cf. malabaricus, and Piabina argentea.
8. Number of ventral procurrent caudal-fin rays (302): (0 > 1) 12 or more. Paralleled in nodes 229 and 252 and in Salminus brasiliensis.
9. Number of 2n chromosomes (362): (1 > 0) 48 or less. Paralleled in node 181.
Autapomorphies of Bryconops melanurus:
1. Articulation between second and third infraorbitals (62): (0 > 2) posteroventrally angled. Paralleled in the Characinae, in nodes 176 and 300, and in Hollandichthys multifasciatus.
2. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. Paralleled in nodes 265 and 294 and in Brycon orbignyanus, Gymnocharacinus bergii, Micralestes stormsi, and Nematocharax venustus. Some trees: Paralleled in Bryconamericus agna.
3. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. Paralleled in the Characinae and in Hyphessobrycon pulchripinnis, Iguanodectes geisleri, and Moenkhausia sanctaefilomenae. Some trees: Paralleled in node 196 and in Hemigrammus erythrozonus and Hyphessobrycon herbertaxelrodi.
Autapomorphy of Bryconops affinis:
1. Edentulous basihyal lamella (189): (0 > 1) present. Paralleled in the Alestidae and Heterocharacinae, in nodes 175 and 302, and in Carnegiella strigata.
Node 205: (100 / 100 / 92 / 26)
Subfamilies Aphyocharacinae, Aphyoditeinae, Characinae, Cheirodontinae, Gymnocharacinae, Heterocharacinae, Rhoadsiinae, Stethaprioninae, Stevardiinae, and Tetragonopterinae; Astyanax clade, Astyanax paris clade, Bramocharax clade, Bryconamericus scleroparius clade, Hyphessobrycon anisitsi clade, and Pseudochalceus clade.
The monophyly of this clade had not been proposed in previous phylogenies. A comparable clade obtained by Lucena (1993) included, in addition the, families Alestidae and Serrasalmidae, the subfamily Bryconinae, and the genera Bryconops and Roestes.
Synapomorphies:
1. Ventral longitudinal lamellae of basioccipital (2): (1 > 0) falling short of posterior border of basioccipital. Paralleled in node 170 and in Serrasalmus maculatus.
2. Ventral projection of lagenar capsule (3): (0 > 1) extending ventrally to articulation between basioccipital and parasphenoid. Paralleled in the Serrasalmidae, in nodes 170 and 302, and in Cyphocharax spilotus and Micralestes stormsi. Reversed in node 210 and in Rhoadsia altipinna.
3. Opening between orbitosphenoid and pterosphenoid (39): (1 > 0) present, rounded or ovate, usually margined by frontal dorsally. Paralleled in node 302 and in Thoracocharax stellatus.
4. Relative length of pterotic spine (46): (0 > 1) restricted to attachment region of hyomandibular ligament. Paralleled in Iguanodectes geisleri. Reversed in Pseudocorynopoma doriae.
5. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. Paralleled in the Serrasalmidae, in node 182, and in Piabucus melanostomus and Prochilodus lineatus. Some trees: Paralleled in Brycon meeki and B. orbignyanus. Reversed in nodes 272 and 287 and in Bramocharax bransfordii, Deuterodon iguape, and Lonchogenys ilisha.
6. Overlap of maxilla by second infraorbital (61): (1 > 0) absent. Paralleled in the Alestidae. Some trees: Paralleled in node 302. Reversed in Hollandichthys multifasciatus and Mimagoniates rheocharis.
7. Supraorbital (70): (0 > 1) absent. Paralleled in nodes 170 and 185 and in Micralestes stormsi. Reversed in Lonchogenys ilisha.
8. Number of maxillary teeth (136): (0 > 1) four or more. Paralleled in nodes 177 and 181 and in Chalceus macrolepidotus and Hemiodus cf. thayeria. Reversed in nodes 232 and 246 and in Aulixidens eugeniae.
9. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Paralleled in node 177 and in Chalceus macrolepidotus and Hoplias cf. malabaricus. Reversed in node 202.
10. Foramen in posterior region of metapterygoid (168): (2 > 1) present, encircled by metapterygoid or bordered partially by cartilage. Paralleled in Salminus brasiliensis. Reversed in Bryconamericus scleroparius and Pseudocorynopoma doriae.
11. Total number of vertebrae (227): (1 > 0) 40 or fewer. Reversal of synapomorphy 4 of the Characidae. Paralleled in node 302 and in Brycon pesu. Reversed in node 209.
12. Total number of transitional vertebrae (228): (0 > 1) three or fewer. Paralleled in Acestrorhynchus pantaneiro, Characidium rachovii, Cyphocharax spilotus, Micralestes stormsi, and Triportheus pantanensis.
13. Coracoid foramen (243): (1 > 0) absent or reduced to small pore. Reversal of synapomorphy 4 of node 179. Paralleled in Salminus brasiliensis. Some trees: Paralleled in node 302 and in Brycon orbignyanus. Reversed in Heterocharax macrolepis.
14. Ventral exit of laterosensory canal of supracleithrum (254): (0 > 1) ventral to lamella of supracleithrum and exiting on posterior margin of this bone. Paralleled in Rhaphiodon vulpinus. Reversed in node 193 and in Markiana nigripinnis.
15. Uroneurals (306): (1 > 0) absent or just one pair. Reversal of synapomorphy 8 of node 180. Reversed in the Tetragonopterinae, in nodes 276 and 300, and in Bryconamericus scleroparius, Galeocharax humeralis, and Markiana nigripinnis.
Node 217: Heterocharacinae (100 / 100 / 99 / 35)
Genera Gnathocharax?, Heterocharax, Hoplocharax, and Lonchogenys.
The monophyly of a clade composed of Gnathocharax (not analyzed here), Heterocharax, Hoplocharax, and Lonchogenys was proposed by Lucena (1998). According to his hypothesis, this clade also included the subfamily Characinae. The tentative inclusion of Gnathocharax in this subfamily follows Lucena (1998). Lucena & Menezes (2003) followed this classification and included these genera in the Characinae. The relationships of the miniature genus Priocharax Weitzman & Vari (not analyzed here) are uncertain. In the phylogeny of Lucena (1998) Priocharax is the sister group of the remaining members of the Characinae (including the members of this clade). Lucena & Menezes (2003) also included Priocharax in the Characinae, and that classification is followed herein as far as the placement of this genus pending focused studies of this question. Toledo-Piza (2007) obtained a sister-group relationship between Gnathocharax and the cynodontin Roestes separate from the remaining taxa of the Cynodontinae. She stated, however, that not all the information relating Roestes with the remaining Cynodontinae was included in her analysis. Until the relationships of Gnathocharax be further studied it is tentatively included in this clade.
Synapomorphies:
1. Ventromedial opening of posttemporal fossa (8): (0 > 1) present. Paralleled in nodes 175 and 184.
2. Dorsal process of pterotic where tendon from epaxial musculature attach (45): (0 > 1) present, projecting dorsally from tube for semicircular canal. Paralleled in node 193 and in Rhoadsia altipinna and Serrasalmus maculatus.
3. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
4. Laterosensory canal of first infraorbital (73): (1 > 0) projects dorsally from main body of first infraorbital. Paralleled in the Gasteropelecidae.
5. Morphology of premaxillary, maxillary, and dentary teeth (118): (1 > 0) all teeth conical, caniniform or mamilliform. Paralleled in nodes 174, 181, and 211 and in Axelrodia lindeae, Grundulus cochae, and Exodon paradoxus.
6. Inner row of dentary teeth (143): (1 > 0) present. Reversal of synapomorphy 2 of node 189. Paralleled in node 276 and in Aphyocharacidium bolivianum.
7. Anterior extension of interopercle (163): (0 > 1) not extending anteriorly beyond terminus of horizontal arm of preopercle. Paralleled in nodes 162, 174, and 212 and in Hoplias cf. malabaricus and Piaractus mesopotamicus.
8. Shape of posteroventral corner of preopercle (174): (1 > 0) acute.
9. Edentulous basihyal lamella (189): (0 > 1) present. Paralleled in the Alestidae, in nodes 175 and 302, and in Bryconops affinis and Carnegiella strigata.
10. Anteriorly directed spine at base of first rib (223): (0 > 1) present. Paralleled in Agoniates anchovia. Some trees: Paralleled in node 302.
11. Horizontal line of chromatophores just dorsal to anal-fin base (344): (0 > 1) present. Paralleled in Coptobrycon bilineatus.
Autapomorphies of Hoplocharax goethei:
1. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae and Gasteropelecidae, in node 186, and in Aphyodite grammica, Hasemania nana, Hemigrammus erythrozonus, Hyphessobrycon pulchripinnis, and Nematobrycon palmeri.
2. Lateral coverage of dilator fossa by sixth infraorbital (69): (0 > 1) leaving a conspicuous naked area in anterior region of dilator fossa. Paralleled in the Iguanodetinae, in node 197, and in Charax stenopterus, Phenacogaster tegatus, and Psellogrammus kennedyi.
3. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 229, 279, 288, and 294 and in Characidium rachovii, Hyphessobrycon anisitsi, Moenkhausia sanctaefilomenae, Phenacogaster tegatus, and Pyrrhulina australis.
4. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Attonitus ephimeros, Axelrodia lindeae, Hollandichthys multifasciatus, Jupiaba scologaster, Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
5. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
6. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 210, 225, 276, and 297 and in Brycon orbignyanus, Hyphessobrycon elachys, and Rhaphiodon vulpinus.
7. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
8. Number of branched-rays on dorsal-fin (270): (1 > 0) eight or fewer. Paralleled in the Stevardiinae and in Coptobrycon bilineatus, and Piabucus melanostomus.
9. Number of dorsal pterygiophores (276): (1 > 0) nine. Paralleled in the Stevardiinae, in node 184, and in Piabucus melanostomus.
10. Ventral procurrent caudal-fin rays of adult males (303): (0 > 1) projecting ventrally through peduncle musculature and skin. Paralleled in node 229 and in Axelrodia lindeae.
Node 216: (72 / 95 / 12 / 29)
Genera Heterocharax and Lonchogenys.
A sister-group relationship of the monotypic genera Heterocharax and Lonchogenys were previously proposed by Lucena (1998) and is corroborated here.
Synapomorphies:
1. Length of caudal-fin canal of lateral line (93): (0 > 1) almost reaching posterior margin of caudal fin. Paralleled in node 177 and in Astyanax pelegrini and Tetragonopterus argenteus.
2. Contact between lamella on anterior portion of first basibranchial with lamella on posterior portion of second basibranchial (183): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 168 and 177, and in Chalceus macrolepidotus, Distichodus maculatus, Hemiodus cf. thayeria, Hoplias cf. malabaricus, and Piabina argentea.
Autapomorphies of Lonchogenys ilisha:
1. Length of supraoccipital spine (53): (0 > 1) extends only to anterior limit of neural complex. Reversal of synapomorphy 5 of node 205. Paralleled in nodes 272 and 287 and in Bramocharax bransfordii and Deuterodon iguape.
2. Supraorbital (70): (1 > 0) present. Reversal of synapomorphy 7 of node 205.
3. Foramen on articular condyle of quadrate (149): (0 > 1) present. Paralleled in nodes 168, 211, and 231 and in Grundulus cochae, Hasemania nana, Hyphessobrycon eques.
4. Median predorsal scales (325): (0 > 1) leaving naked area anterior to dorsal fin. Paralleled in the Serrasalmidae and in node 284.
Autapomorphies of Heterocharax macrolepis:
1. Extent of expansion of first infraorbital lateral to maxilla (59): (0 > 1) covering most of maxilla. Paralleled in node 277 and in Engraulisoma taeniatum.
2. Epiphyseal branch of corresponding supraorbital canals (85): (0 > 1) oriented obliquely, opening posteriorly to epiphyseal bar. Paralleled in node 302.
3. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate (155): (0 > 1) present. Paralleled in nodes 176 and 299 and in Deuterodon langei, Pristella maxillaris, Roeboides descalvadensis, and Thoracocharax stellatus.
4. Coracoid foramen (243): (0 > 1) well developed. Reversal of synapomorphy 13 of node 205.
Node 204: (100 / 100 / 59 / 7)
Subfamilies Aphyocharacinae, Aphyoditeinae, Characinae, Cheirodontinae, Gymnocharacinae, Rhoadsiinae, Stethaprioninae, Stevardiinae, and Tetragonopterinae; Astyanax clade, Astyanax paris clade, Bramocharax clade, Bryconamericus scleroparius clade, Hyphessobrycon anisitsi clade, and Pseudochalceus clade.
This clade includes most characids without a supraorbital. A monophyletic group containing the characids lacking a supraorbital was proposed by Malabarba & Weitzman (2003: fig. 2). Most species of this clade are morphologically highly conservative, and some of the important factors leading to variation between species involve diet or miniaturization. These usually have poor correlations with phylogeny and relationships between the members of this clade are among the less resolved within the Characiformes. The molecular phylogeny of Calcagnotto et al. (2005) shows a polytomy in the clade containing this group of characids, suggesting that molecular data are not necessarily better than morphology in assessing the internal relationships within this clade.
Synapomorphies:
1. Distance between cartilage anterior to orbitosphenoid and lateral ethmoids (38): (0 > 1) distant from lateral ethmoids. Reversal of synapomorphy 2 of node 179. Reversed in node 223 and in Bryconexodon juruenae and Galeocharax humeralis.
2. Lateral surface of vertical canal of preopercle (81): (1 > 0) canal uncovered and situated posteriorly to musculature and infraorbitals. Paralleled in nodes 170 and 175 and in Chalceus macrolepidotus and Metynnis maculatus. Reversed in Markiana nigripinnis.
3. Hyoid artery (178): (0 > 1) emerging from anterior ceratohyal near its articulation with posterior ceratohyal.
4. Posterior margin of cleithrum (234): (1 > 0) without concavity ventral to first postcleithrum. Reversal of synapomorphy 6 of node 189. Reversed in nodes 196 and 289 and in Probolodus heterostomus and Roeboexodon geryi.
5. Bony ridge of coracoid between base of mesocoracoid and ventral margin of interosseous space (239): (1 > 0) absent. Paralleled in Engraulisoma taeniatum.
6. Form of third postcleithrum (250): (0 > 1) with a posterior lamella. Reversed in node 242 and in Gymnocharacinus bergii, Pseudochalceus kyburzi, and Rhoadsia altipinna.
7. Supraneural anterior to neural spine of fourth vertebra (279): (1 > 0) absent or small. Reversal of synapomorphy 7 of node 180. Paralleled in the Iguanodectinae and in Micralestes stormsi.
Node 213: Characinae (67 / 95 / 7 / 4)
Genera Acanthocharax Eigenmann, Acestrocephalus Eigenmann, Bryconexodon Géry, Charax, Cynopotamus, Exodon, Galeocharax, Phenacogaster, Priocharax?, Roeboexodon, and Roeboides.
Exodon and Roeboexodon were included in the subfamily Characinae by Géry (1977), but later considered as incertae sedis by Lima et al. (2003), along with Bryconexodon and many other genera. Exodon is the sister group of Roeboides in the analysis by Calcagnotto et al. (2005), whereas it is related to Oligosarcus according to Lucena (1993). Lucena & Menezes (2003) included Gnathocharax, Heterocharax, Hoplocharax, and Lonchogenys in the Characinae but these are classified here, at least preliminarily in the case of Gnathocharax, in the subfamily Heterocharacinae. According to Lucena (1998) the miniature genus Priocharax is the sister group of a clade including Gnathocharax, Heterocharax, Hoplocharax, Lonchogenys, and the remaining characins. This position, basal to Heterocharacinae plus Characinae, would exclude Priocharax from the latter subfamily. Priocharax, however, was not analyzed and it is provisionally maintained in the Characinae pending specific studies.
Synapomorphies:
1. Length of sphenotic spine (10): (1 > 0) not extending ventrally to articulation between sphenotic and hyomandibula. Reversal of synapomorphy 1 of node 180. Paralleled in nodes 176 and 197. Reversed in node 218.
2. Articulation between second and third infraorbitals (62): (0 > 2) posteroventrally angled. Paralleled in nodes 176 and 300 and in Bryconops melanurus and Hollandichthys multifasciatus.
3. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. Paralleled in Bryconops melanurus, Hyphessobrycon pulchripinnis, Iguanodectes geisleri, and Moenkhausia sanctaefilomenae. Some trees: Paralleled in node 196 and in Hemigrammus erythrozonus and Hyphessobrycon herbertaxelrodi.
4. Number of 2n chromosomes (363): (0 > 1) 52 or more. Paralleled in the Serrasalmidae, in node 196, and in Chalceus macrolepidotus, Hyphessobrycon herbertaxelrodi, Markiana nigripinnis, and Rhaphiodon vulpinus. Some trees: Paralleled in Hemigrammus unilineatus.
Node 277: (100 / 100 / 55 / 16)
Genera Bryconexodon, Exodon, and Roeboexodon.
The genera Bryconexodon, Exodon, and Roeboexodon share with Catoprion, Probolodus, Roeboides, and Serrabrycon the presence of teeth oriented outside the mouth, which are presumably associated with lepidophagous habits (Lucena, 1998). Catoprion is a member of the Serrasalmidae (Jégu, 2003), while Probolodus is herein included in the Tetragonopterinae. Although Roeboides is a member of the Characinae, the possession of teeth outside the mouth is a parallelism between the present clade and that genus, something already proposed by Lucena (1998). The relationships of Serrabrycon magoi Vari are unknown, although Vari (1986) mentioned the possibility of a close relationship between that genus and Bryconexodon, Exodon, Probolodus, Roeboexodon, and/or Roeboides. According to his description, Serrabrycon magoi shares with these genera the possession of teeth outside the mouth, which are associated with a lepidophagous habit. Serrabrycon, however, is considered here as incertae sedis given the homoplastic nature of this character within the order. The three genera included in this clade are rather dissimilar each other; however this node is well supported.
Synapomorphies:
1. Extent of expansion of first infraorbital lateral to maxilla (59): (0 > 1) covering most of maxilla. Paralleled in Engraulisoma taeniatum and Heterocharax macrolepis.
2. Form of fourth infraorbital (67): (1 > 0) approximately square or more developed longitudinally than dorsoventrally. Reversal of synapomorphy 1 of node 189. Paralleled in 200, 210, 228, and 282.
3. Mamilliform teeth outside mouth (120): (0 > 1) present. Paralleled in node 218 and in Probolodus heterostomus.
4. Number of branched anal-fin rays (288): (1 > 0) 24 or less. Reversal of synapomorphy 5 of the Characidae. Paralleled in nodes 200 and 300 and in Acestrorhynchus pantaneiro and Iguanodectes geisleri.
Autapomorphies of Roeboexodon geryi:
1. Lateral opening between ventral diverging lamellae of mesethmoid and anterior process of lateral ethmoid (15): (0 > 1) small, ovate and partially occluded by diverging lamellae of mesethmoid and anterior process of lateral ethmoid. Paralleled in Markiana nigripinnis and Roeboides descalvadensis.
2. Ventral projection of mesethmoid spine, forming a keel between premaxillae (26): (0 > 1) present. Paralleled in node 253.
3. Bony lamellae bordering sensory canal of nasal (34): (0 > 1) wider at some point than tubular region. Paralleled in nodes 174 and 181 and in Galeocharax humeralis and Leporinus striatus.
4. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, and Stichonodon insignis. Some trees: Reversed in node 292.
5. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. Paralleled in node 302, and in Agoniates anchovia, Cyanocharax alburnus, Deuterodon langei, Engraulisoma taeniatum, Hemiodus cf. thayeria, and Thayeria obliqua. Some trees: Paralleled in Microschemobrycon casiquiare and Parecbasis cyclolepis.
6. Posterior margin of cleithrum (234): (0 > 1) with concavity ventral to first postcleithrum. Reversal of synapomorphy 4 of node 204. Paralleled in nodes 196 and 289 and in Probolodus heterostomus.
Node 276: (67 / 95 / 57 / 16)
Genera Bryconexodon and Exodon.
The monophyly of a clade composed of Bryconexodon and Exodon was not previously proposed. In the analysis by Mirande (2009) these two genera formed a trichotomy along with Roeboexodon.
Synapomorphies:
1. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 167, 177, 218, and 260, and in Bryconamericus scleroparius, Chalceus macrolepidotus, and Piaractus mesopotamicus.
2. Inner row of dentary teeth (143): (1 > 0) present. Reversal of synapomorphy 2 of node 189. Paralleled in the Heterocharacinae and in Aphyocharacidium bolivianum.
3. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 210, 225, and 297 and in Brycon orbignyanus, Hoplocharax goethei, Hyphessobrycon elachys, and Rhaphiodon vulpinus.
4. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (0 > 1) three or four. Paralleled in node 203 and in Metynnis maculatus and Salminus brasiliensis. Some trees: Paralleled in Brycon orbignyanus.
5. Uroneurals (306): (0 > 1) two pairs. Reversal of synapomorphy 15 of node 205. Paralleled in the Tetragonopterinae, in node 300, and in Bryconamericus scleroparius, Galeocharax humeralis, and Markiana nigripinnis.
Autapomorphies of Exodon paradoxus:
1. Contact between frontals anteriorly to frontal fontanel (21): (0 > 1) present. Reversal of synapomorphy 4 of node 206. Paralleled in Bario steindachneri, Galeocharax humeralis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in Knodus breviceps.
2. Morphology of premaxillary, maxillary, and dentary teeth (118): (1 > 0) all teeth conical, caniniform or mamilliform. Paralleled in the Heterocharacinae, in nodes 174, 181, and 211, and in Axelrodia lindeae and Grundulus cochae.
3. Form of quadrate (150): (0 > 1) with anterodorsal portion equal or longer than ventral region. Paralleled in the Pseudochalceus clade, in nodes 170, 176, 211, and 299, and in Hoplias cf. malabaricus.
4. Position of longitudinal cartilage dorsal to ectopterygoid (161): (0 > 1) displaced laterally and separated from medial margin of mesopterygoid. Paralleled in nodes 211 and 300.
5. Relative position of dorsal-fin anterior insertion (265): (1 > 0) anterior to or at vertical through pelvic-fin origin. Paralleled in node 282 and in Creagrutus anary, Moenkhausia xinguensis, and Parecbasis cyclolepis.
6. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
7.Circulii on posterior field of scales (319): (1 > 0) present. Reversal of synapomorphy 3 of node 206. Paralleled in node 261 and in Phenagoniates macrolepis and Roeboides microlepis.
Autapomorphies of Bryconexodon juruenae:
1. Temporal fossa (13): (1 > 0) well developed. Paralleled in node 300 and in Brycon meeki and Salminus brasiliensis.
2. Distance between cartilage anterior to orbitosphenoid and lateral ethmoids (38): (1 > 0) contacting, or almost contacting, lateral ethmoids. Reversal of synapomorphy 1 of node 204. Paralleled in node 223 and in Galeocharax humeralis.
3. Anterior paired projections of parasphenoid (40): (0 > 1) present. Paralleled in Bryconexodon juruenae.
4. Dorsolateral processes of vomer (54): (0 > 1) present. Paralleled in nodes 215 and 218 and in Markiana nigripinnis.
5. Articulation between second and third infraorbitals (62): (2 > 1) anteroventrally angled.
6. Articulation between quadrate and anguloarticular (154): (1 > 0) anterior to or at vertical through middle eye. Paralleled in node 202 and Phenacogaster tegatus.
Node 212: (100 / 100 / 57 / 16)
Genera Acanthocharax, Acestrocephalus, Charax, Cynopotamus, Galeocharax, Phenacogaster, Priocharax?, and Roeboides.
This node corresponds to the Characinae of Lucena & Menezes (2003), with the exclusion of the Heterocharacinae. As previously mentioned, the inclusion of Priocharax in this clade is tentative.
Synapomorphies:
1. Dorsal expansion of rhinosphenoid (48): (1 > 0) absent. Paralleled in nodes 201 and 300 and in Agoniates anchovia.
2. Anterior extension of interopercle (163): (0 > 1) not extending anteriorly beyond terminus of horizontal arm of preopercle. Paralleled in the Heterocharacinae, in nodes 162 and 174, and in Hoplias cf. malabaricus and Piaractus mesopotamicus.
3. Form and degree of ossification of first ceratobranchial gill rakers (200): (0 > 1) rather thick and completely ossified distal region. Paralleled in nodes 176 and 299 and in Hoplias cf. malabaricus, Prionobrama paraguayensis, and Pristella maxillaris.
4. Anterior portions of branchiostegal rays (214): (0 > 1) slender near their articulation with ceratohyals. Paralleled in node 256 and in Rhaphiodon vulpinus.
5. Dorsal development of dorsal process of neural pedicle of third vertebra (221): (0 > 1) broadly overlapping neural complex. Reversal of synapomorphy 5 of node 189.
6. Transitional vertebrae with haemal canal (229): (0 > 1) absent. Paralleled in node 195 and in Aulixidens eugeniae, Engraulisoma taeniatum, Metynnis maculatus, and Piabina argentea. Some trees: Paralleled in node 247 and in Bryconamericus alpha and Paracheirodon axelrodi.
7. Anal-fin position (284): (0 > 1) extended anteriorly ventral to dorsal fin. Paralleled in nodes 170, 208, and 236 and in Piabucus melanostomus.
8. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in node 207 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
Autapomorphies of Phenacogaster tegatus:
1. Lateral coverage of dilator fossa by sixth infraorbital (69): (0 > 1) leaving a conspicuous naked area in anterior region of dilator fossa. Paralleled in the Iguanodectinae, in node 197, and in Charax stenopterus, Hoplocharax goethei, and Psellogrammus kennedyi.
2. Laterosensory canal of sixth infraorbital (76): (1 > 0) not branched. Paralleled in the Iguanodectinae, in node 203, and in Charax stenopterus, Cyphocharax stellatus, and Micralestes stormsi.
3. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 229, 279, 288, and 294 and in Characidium rachovii, Hoplocharax goethei, Hyphessobrycon anisitsi, Moenkhausia sanctaefilomenae, and Pyrrhulina australis.
4. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, and Inpaichthys kerri.
5. Length of ascending process of premaxilla (104): (0 > 1) reaching just anterior end of nasal. Paralleled in nodes 225 and 294 and in Charax stenopterus, and Stichonodon insignis. Some trees: Paralleled in Bryconamericus agna.
6. Number of teeth in inner premaxillary row (130): (0 > 1) eight or more. Paralleled in the Aphyoditeinae, in node 170, and in Brycon orbignyanus, Grundulus cochae, Prionobrama paraguayensis, and Salminus brasiliensis.
7. Articulation between quadrate and anguloarticular (154): (1 > 0) anterior to or at vertical through middle eye. Paralleled in node 202 and Bryconexodon juruenae.
8. Ethmopalatine cartilage (171): (0 > 1) present and conspicuous. Paralleled in Charax stenopterus and Tetragonopterus argenteus.
9. Bony lamella dorsal to fourth basibranchial (185): (1 > 0) present. Paralleled in nodes 168, 170, 203, and 302.
10. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent. Paralleled in Creagrutus anary, Hyphessobrycon eques, H. luetkenii, Pseudocorynopoma doriae, and Stethaprion erythrops. Some trees: Paralleled in Markiana nigripinnis and Psellogrammus kennedyi.
11. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus cf. iheringii, B. rubropictus, Hyphessobrycon luetkenii, and H. socolofi.
12. Gill-derived gland on males (352): (0 > 1) present. Paralleled in node 196.
Node 211: (100 / 100 / 98 / 59)
Genera Acanthocharax?, Acestrocephalus, Charax, Cynopotamus, Galeocharax, and Roeboides.
The monophyly of a clade composed of Acanthocharax (not analyzed in this study), Acestrocephalus, Charax, Cynopotamus, Galeocharax, and Roeboides, as the sister group of Phenacogaster, was proposed by Lucena (1998); the tentative inclusion of Acanthocharax in this clade follows that paper.
Synapomorphies:
1. Position of sphenotic spine relative to hyomandibula (11): (0 > 1) displaced anteriorly relative to anterior margin of hyomandibula. Paralleled in node 162 and in Acestrorhynchus pantaneiro, Piabina argentea, Salminus brasiliensis, and Serrasalmus maculatus.
2. Subtemporal fossa (18): (1 > 0) medially extended to middle exoccipital. Paralleled in Hyphessobrycon luetkenii.
3. Pores of laterosensory canal of lower jaw (80): (0 > 1) seven or more. Paralleled in node 299 and in Rhaphiodon vulpinus.
4. Morphology of premaxillary, maxillary, and dentary teeth (118): (1 > 0) all teeth conical, caniniform or mamilliform. Paralleled in the Heterocharacinae, in nodes 174 and 181, and in Axelrodia lindeae, Grundulus cochae, and Exodon paradoxus.
5. Foramen on articular condyle of quadrate (149): (0 > 1) present. Paralleled in nodes 168 and 231 and in Grundulus cochae, Hasemania nana, Hyphessobrycon eques, and Lonchogenys ilisha.
6. Form of quadrate (150): (0 > 1) with anterodorsal portion equal or longer than ventral region. Paralleled in the Pseudochalceus clade, in nodes 170, 176, and 299, and in Exodon paradoxus and Hoplias cf. malabaricus.
7. Position of longitudinal cartilage dorsal to ectopterygoid (161): (0 > 1) displaced laterally and separated from medial margin of mesopterygoid. Paralleled in node 300 and in Exodon paradoxus.
8. Ventral margin of anterior ceratohyal (179): (1 > 0) smooth and without notches. Reversal of synapomorphy 3 of node 189. Paralleled in Stichonodon insignis.
9. Length of interhyal (211): (0 > 1) equal to or longer than one-half of symplectic length. Paralleled in node 174 and in Hoplias cf. malabaricus and Pseudochalceus kyburzi.
10. Distance between attachment site of first and second branchiostegal rays (216): (0 > 1) longer than distance between second and third rays.
11. Anterior margin of cleithrum (232): (0 > 1) with anterior pointed projection.
12. Position of ventral end of posttemporal (253): (0 > 1) posterior to lateral margin of epioccipital. Paralleled in node 228 and in Bryconamericus scleroparius, Hoplias cf. malabaricus, Markiana nigripinnis, Probolodus heterostomus, and Prochilodus lineatus.
13. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in nodes 223 and 262 and in Bramocharax bransfordii, Bryconaethiops macrops, Hyphessobrycon bifasciatus, and Nematocharax venustus. Some trees: Paralleled in the Aphyoditeinae.
14. Posterior attachment of A1 section of adductor mandibulae (332): (0 > 1) restricted or almost restricted to horizontal arm of preopercle. Paralleled in the Iguanodectinae and in Agoniates anchovia, Aphyodite grammica, and Pyrrhulina australis.
15. Attachment of medial tendon of A1 section of adductor mandibulae (333): (0 > 1) on preopercle posterior to quadrate. Reversal of synapomorphy 8 of the Characidae.
Node 214: (100 / 100 / 54 / 49)
Genera Acanthocharax?, Charax, and Roeboides.
A close relationship between Acanthocharax (not analyzed here), Charax, and Roeboides was proposed by Lucena (1998). Acanthocharax is tentatively included in this clade following his hypothesis. The analyzed species of Charax and Roeboides form a monophyletic clade, partially corroborating the hypothesis of Lucena (1998). Charax stenopterus is the only species of this genus herein analyzed. This species was included in a different genus (Asiphonichthys Cope) by Géry (1977) due to having only one row of premaxillary teeth and an incomplete lateral line. Perhaps the inclusion of a species of Charax with more generalized morphology could affect the list of synapomorphies of this and/or the following node.
Synapomorphies:
1. Length of supraoccipital spine (52): (1 > 0) extends dorsal of entire neural complex of Weberian apparatus. Paralleled in node 221 and in Psellogrammus kennedyi.
2. Longitudinal ridge covering laterosensory pores of dentary (117): (0 > 1) present.
3. Number of anal pterygiophores anterior to first haemal spine (293): (0 > 1) four or more. Paralleled in the Iguanodectinae and in Cynopotamus argenteus and Gymnocorymbus ternetzi.
Autapomorphies of Charax stenopterus:
1. Lateral coverage of dilator fossa by sixth infraorbital (69): (0 > 1) leaving a conspicuous naked area in anterior region of dilator fossa. Paralleled in the Iguanodectinae, in node 197, and in Hoplocharax goethei, Phenacogaster tegatus, and Psellogrammus kennedyi.
2. Laterosensory canal of sixth infraorbital (76): (1 > 0) not branched. Paralleled in the Iguanodectinae, in node 203, and in Cyphocharax stellatus, Micralestes stormsi, and Phenacogaster tegatus.
3. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
4. Length of ascending process of premaxilla (104): (0 > 1) reaching just anterior end of nasal. Paralleled in nodes 225 and 294 and in Phenacogaster tegatus and Stichonodon insignis. Some trees: Paralleled in Bryconamericus agna.
5. Size and number of anterior dentary teeth (142): (0 > 1) eight or more small and slender teeth at front of dentary. Paralleled in the Aphyoditeinae and in Pyrrhulina australis.
6. Ethmopalatine cartilage (171): (0 > 1) present and conspicuous. Paralleled in Phenacogaster tegatus and Tetragonopterus argenteus.
Node 218: (100 / 100 / 84 / 82)
Genus Roeboides.
The monophyly of Roeboides was tested in a cladistic context by Lucena (1998). Members of Roeboides share modifications of the dentition associated with its lepidophagous habit, a feeding mode also mentioned for Bryconexodon, Exodon, and Roeboexodon, among the Characinae. The hypothesis of Lucena (1998) that this kind of dentition and the lepidophagous habit were originated in parallel in these genera and in Roeboides is corroborated in this study.
Synapomorphies:
1. Posteriorly-oriented epioccipital spine (7): (1 > 0) present. Paralleled in nodes 162 and 177.
2. Length of sphenotic spine (10): (0 > 1) extending ventrally to articulation between sphenotic and hyomandibula. Reversal of synapomorphy 1 of the Characinae.
3. Dorsolateral processes of vomer (54): (0 > 1) present. Paralleled in node 215 and in Bryconexodon juruenae and Markiana nigripinnis.
4. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 167, 177, 260, and 276, and in Bryconamericus scleroparius, Chalceus macrolepidotus, and Piaractus mesopotamicus.
5. Mamilliform teeth outside mouth (120): (0 > 1) present. Paralleled in node 277 and in Probolodus heterostomus.
6. Proximal and medial radials of anal fins (294): (0 > 1) fused in most pterygiophores. Paralleled in nodes 184, 208, and 221 and in Psellogrammus kennedyi and Pseudocorynopoma doriae. Some trees: Paralleled in node 295.
Autapomorphies of Roeboides descalvadensis:
1. Lateral opening between ventral diverging lamellae of mesethmoid and anterior process of lateral ethmoid (15): (0 > 1) small, ovate and partially occluded by diverging lamellae of mesethmoid and anterior process of lateral ethmoid. Paralleled in Markiana nigripinnis and Roeboexodon geryi.
2. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate (155): (1 > 0) absent. Paralleled in nodes 176 and 299 and in Deuterodon langei, Heterocharax macrolepis, Pristella maxillaris, and Thoracocharax stellatus.
3. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. Paralleled in nodes 244 and 299 and in Hasemania nana, Hyphessobrycon bifasciatus, Metynnis maculatus, and Odontostilbe microcephala. Some trees: Paralleled in node 265.
Autapomorphies of Roeboides microlepis:
1. Form of orbitosphenoid (37): (0 > 1) massive, almost reaching parasphenoid ventrally. Reversal of synapomorphy 1 of the Characidae. Paralleled in node 193 and in Markiana nigripinnis and Rhaphiodon vulpinus.
2. Length of medial bony ridge of opercle (170): (1 > 0) 60% or greater than opercular length. Paralleled in the Serrasalmidae, in node 210, and in Astyanax abramis, Creagrutus cf. taphorni, and Hoplias cf. malabaricus. Some trees: Paralleled in Acestrorhynchus pantaneiro and Salminus brasiliensis.
3. Suprapreopercle (175): (0 > 1) autogenous, separated from preopercle. Paralleled in nodes 210 and 302 and in Markiana nigripinnis and Rhaphiodon vulpinus.
4.Circulii on posterior field of scales (319): (1 > 0) present. Reversal of synapomorphy 3 of node 206. Paralleled in node 261 and in Exodon paradoxus and Phenagoniates macrolepis.
5. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in nodes 210 and 221, and in Bario steindachneri, Markiana nigripinnis, Paragoniates alburnus, Rhaphiodon vulpinus, and Thoracocharax stellatus.
6. Humeral spot (341): (0 > 1) horizontally-ovate. Paralleled in node 259 and in Acestrorhynchus pantaneiro, Brycon orbignyanus, and Jupiaba mucronata.
Node 210: (100 / 100 / 85 / 52)
Genera Acestrocephalus, Cynopotamus, and Galeocharax.
Members of this node were included in the genus Cynopotamus by Géry (1977), who considered three subgenera: Acestrocephalus (including Galeocharax), Hybocharax Géry & Vu-Tân-Tuê, and Cynopotamus. This author mentioned that the members of this clade (his Cynopotamus) "look exactly like a big Charax with rough scales" (Géry, 1977: 306), undoubtedly referring to the spinoid scales which characterizes the members of this group. The monophyly of a clade composed of Acestrocephalus, Cynopotamus, and Galeocharax was proposed by Lucena (1998).
Synapomorphies:
1. Ventral projection of lagenar capsule (3): (1 > 0) not extending ventrally to horizontal through articulation between basioccipital and parasphenoid. Reversal of synapomorphy 2 of node 205. Paralleled in Rhoadsia altipinna.
2. Form of fourth infraorbital (67): (1 > 0) approximately square or more developed longitudinally than dorsoventrally. Reversal of synapomorphy 1 of node 189. Paralleled in 200, 228, 277, and 282.
3. Posterior dorsoventral expansion of fourth infraorbital (68): (0 > 1) present. Paralleled in nodes 174 and 299.
4. Bony lamella covering dentary foramen laterally (116): (0 > 1) present.
5. Length of medial bony ridge of opercle (170): (1 > 0) 60% or greater than opercular length. Paralleled in the Serrasalmidae and in Astyanax abramis, Creagrutus cf. taphorni, Hoplias cf. malabaricus, and Roeboides microlepis. Some trees: Paralleled in Acestrorhynchus pantaneiro and Salminus brasiliensis.
6. Suprapreopercle (175): (0 > 1) autogenous, separated from preopercle. Paralleled in nodes 210 and 302 and in Markiana nigripinnis, Roeboides microlepis, and Rhaphiodon vulpinus.
7. Articulation between anterior and posterior ceratohyals (181): (0 > 1) with bony interdigitations. Paralleled in Hoplias cf. malabaricus, Rhaphiodon vulpinus, and Salminus brasiliensis.
8. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 225, 276, and 297 and in Brycon orbignyanus, Hoplocharax goethei, Hyphessobrycon elachys, and Rhaphiodon vulpinus.
9. Scales (317): (0 > 2) spinoid.
10. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in node 221, and in Bario steindachneri, Markiana nigripinnis, Paragoniates alburnus, Rhaphiodon vulpinus, Roeboides microlepis, and Thoracocharax stellatus.
11. Sclerotic bones (350): (0 > 1) two bones separated by cartilages. Paralleled in nodes 208, 221, 250, and 259.
Autapomorphy of Acestrocephalus sardina:
1. Bony lamellae associated with supraneurals (282): (1 > 0) absent or small. Paralleled in nodes 197, 272, and 299 and in Nematocharax venustus.
Node 215: (100 / 100 / 61 / 77)
Genera Cynopotamus and Galeocharax.
Géry (1977) considered Galeocharax to be a synonym of Acestrocephalus (both treated by him as subgenera of Cynopotamus), implicitly suggesting a closer relationship between these two genera than with Cynopotamus. This same relationship was found by Lucena (1998), who obtained a clade formed by Acestrocephalus and Galeocharax. According to the present hypothesis, however, Galeocharax is the sister group of Cynopotamus.
Synapomorphies:
1. Dorsolateral processes of vomer (54): (0 > 1) present. Paralleled in node 218 and in Bryconexodon juruenae and Markiana nigripinnis.
2. Form of anterior gill rakers on first ceratobranchial (198): (0 > 1) with fused bases forming plates extensively articulated with ceratobranchial.
Autapomorphies of Galeocharax humeralis:
1. Contact between frontals anteriorly to frontal fontanel (21): (0 > 1) present. Reversal of synapomorphy 4 of node 206. Paralleled in Bario steindachneri, Exodon paradoxus, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in Knodus breviceps.
2. Bony lamellae bordering sensory canal of nasal (34): (0 > 1) wider at some point than tubular region. Paralleled in nodes 174 and 181 and in Leporinus striatus and Roeboexodon geryi.
3. Distance between cartilage anterior to orbitosphenoid and lateral ethmoids (38): (1 > 0) contacting, or almost contacting, lateral ethmoids. Reversal of synapomorphy 1 of node 204. Paralleled in node 223 and in Bryconexodon juruenae.
4. Relative position of anterior margin of antorbital and first infraorbital (57): (0 > 1) anterior margin of antorbital posterior to first infraorbital. Reversal of synapomorphy 1 of Characoidea. Paralleled in node 184.
5. Uroneurals (306): (0 > 1) two pairs. Reversal of synapomorphy 15 of node 205. Paralleled in the Tetragonopterinae, in nodes 276 and 300, and in Bryconamericus scleroparius and Markiana nigripinnis.
Autapomorphies of Cynopotamus argenteus:
1. Position of sphenotic spine relative to the orbit (12): (0 > 1) distinctly posterior to orbital margin. Paralleled in nodes 193 and 299 and in Acestrorhynchus pantaneiro, Attonitus ephimeros, and Gymnocharacinus bergii.
2. Anterior margin of supraoccipital (51): (0 > 1) situated anterior to vertical through posterior orbital margin. Paralleled in node 220 and in Metynnis maculatus.
3. Bony lamellae between second and third basibranchials (184): (0 > 1) present.
4. Number of anal pterygiophores anterior to first haemal spine (293): (0 > 1) four or more. Paralleled in the Iguanodectinae, in node 214, and in Gymnocorymbus ternetzi.
Node 203: (25 / 89 / 5 / 11)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, Rhoadsiinae, Stethaprioninae, Stevardiinae, and Tetragonopterinae; Astyanax clade, Astyanax paris clade, Bramocharax clade, Bryconamericus scleroparius clade, Hyphessobrycon anisitsi clade, and Pseudochalceus clade.
This node is incongruent with the results by Mirande (2009) and was not previously proposed. In that analysis the Jupiaba clade was the sister group of a clade including the remaining characids lacking a supraorbital. In the present study the subfamily Characinae is the sister group of that clade of characids, whereas the Jupiaba clade of Mirande (2009) is included in the Tetragonopterinae.
Synapomorphies:
1. Relative size of frontal and parietal fontanels (23): (1 > 0) length of frontal fontanel up to 2/3 length of parietal fontanel. Reversed in node 264.
2. Laterosensory canal of sixth infraorbital (76): (1 > 0) not branched. Paralleled in the Iguanodectinae and in Charax stenopterus, Cyphocharax stellatus, Micralestes stormsi, and Phenacogaster tegatus. Reversed in Markiana nigripinnis, Odontostilbe microcephala, Oligosarcus cf. jenynsii, and Tetragonopterus argenteus.
3. Bony lamella dorsal to fourth basibranchial (185): (1 > 0) present. Paralleled in nodes 168, 170, and 302 and in Phenacogaster tegatus. Reversed in node 296 and in Axelrodia lindeae, Gymnocharacinus bergii, Hollandichthys multifasciatus, Mimagoniates rheocharis, Nematocharax venustus, Paracheirodon axelrodi, and Prodontocharax melanotus.
4. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (0 > 1) three or four. Paralleled in node 276 and in Metynnis maculatus and Salminus brasiliensis. Some trees: Paralleled in Brycon orbignyanus. Reversed in Bario steindachneri and Thayeria obliqua. Some trees: Reversed in Hyphessobrycon elachys and Paracheirodon axelrodi.
Node 228: (13 / 57 / - / 4)
Subfamily Rhoadsiinae; Bramocharax clade and Pseudochalceus clade.
This node is incongruent with the final hypothesis by Mirande (2009), in which the Rhoadsiinae was related with the Characinae, rather than with the Bramocharax and Pseudochalceus clades. However, this node was obtained in the most stable hypothesis under self-weighted optimization by Mirande (2009: fig. 4).
Synapomorphies:
1. Form of fourth infraorbital (67): (1 > 0) approximately square or more developed longitudinally than dorsoventrally. Reversal of synapomorphy 1 of node 189. Paralleled in 200, 210, 277, and 282.
2. Position of ventral end of posttemporal (253): (0 > 1) posterior to lateral margin of epioccipital. Paralleled in node 211 and in Bryconamericus scleroparius, Hoplias cf. malabaricus, Markiana nigripinnis, Probolodus heterostomus, and Prochilodus lineatus. Reversed in Nematocharax venustus.
Node 275: Bramocharax clade (86 / 98 / 42 / 48)
Genera Bramocharax and Oligosarcus.
The genera Bramocharax and Oligosarcus were related with Hollandichthys and Pseudochalceus in the hypothesis proposed by Mirande (2009), and the latter two genera were included in the Bramocharax clade. Hollandichthys and Pseudochalceus are instead related with the Rhoadsiinae in this study. Rosen (1972) considered Bramocharax to be monophyletic and derived from some species of Astyanax. However, a recent molecular analysis proposed that this genus is not monophyletic and that each species of Bramocharax is related with different populations of Astyanax from Central America (Ornelas-García et al., 2008). The hypothesis of non-monophyly of Bramocharax is surprising giving the morphological resemblance between its species; however, the high congruence of that hypothesis with the geographical distribution of the clades proposed by Ornelas García et al. (2008) and the morphological plasticity of some characids make this hypothesis plausible. These authors proposed parallel evolution of "Bramocharax ecomorphs" from different species or populations of Astyanax and suggested that "the morphotype of Bramocharax represents a recurrent trophic adaptation" (Ornelas-García et al., 2008). Only one species of Bramocharax was analyzed in this study, and this analysis is insufficient to adequately test the hypothesis of the non-monophyly of Bramocharax proposed by those authors. The close relationship between Bramocharax and Astyanax is not, however, supported by the present hypothesis, which instead relates Bramocharax with Oligosarcus. The inclusion of some Mesoamerican species of Astyanax with relatively high number of maxillary teeth, such as A. nasutus Meek and some morphologically conservative species of Bramocharax, such as B. bailey Rosen, would be useful to test the monophyly and position of this clade.
Synapomorphy:
1. Form of epioccipital bridge (5): (0 > 1) depressed in its middle region.
Autapomorphies of Bramocharax bransfordii:
1. Length of supraoccipital spine (53): (0 > 1) extends only to anterior limit of neural complex. Reversal of synapomorphy 5 of node 205. Paralleled in nodes 272 and 287 and in Deuterodon iguape and Lonchogenys ilisha.
2. Ventral margin of horizontal process of anguloarticular (109): (0 > 1) perpendicular to laterosensory canal of dentary from medial view. Paralleled in node 199 and in Bario steindachneri and Micralestes stormsi.
3. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273, 283, and 294, and in Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
4. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in nodes 211, 223, and 262, and in Bryconaethiops macrops, Hyphessobrycon bifasciatus, and Nematocharax venustus. Some trees: Paralleled in the Aphyoditeinae.
Node 300: (100 / 100 / 79 / 39)
Genus Oligosarcus.
The genus Oligosarcus was traditionally considered to be related with Acestrorhynchus (e. g. Menezes, 1969). Géry (1977), classified both genera in the tribe Acestrorhynchini of the subfamily Characinae. The phylogeny of Buckup (1998), however, did not yield a close relationship between these two genera, and Acestrorhynchus was excluded from the Characidae. The monophyly of Oligosarcus was not tested in the literature, although the species of this genus share some unique or unusual characters within Characidae, mostly involving their dentition (Menezes, 1969). The morphologically most generalized described species of this genus, Oligosarcus pintoi Campos is not herein analyzed, and probably some of the listed synapomorphies would apply to a subclade within Oligosarcus. According to the present analysis, the undescribed new species included in this clade (treated as an undescribed new genus and species by Mirande, 2009) should be described as a species of Oligosarcus.
Synapomorphies:
1. Temporal fossa (13): (1 > 0) well developed. Paralleled in Brycon meeki, Bryconexodon juruenae, and Salminus brasiliensis.
2. Dorsal expansion of rhinosphenoid (48): (1 > 0) absent. Paralleled in nodes 201 and 212 and in Agoniates anchovia.
3. Articulation between second and third infraorbitals (62): (0 > 2) posteroventrally angled. Paralleled in the Characinae, in node 176, and in Bryconops melanurus and Hollandichthys multifasciatus.
4. Ectopterygoid teeth row (159): (0 > 1) present. Paralleled in node 168 and in Acestrorhynchus pantaneiro, Distichodus maculatus, Hoplias cf. malabaricus, Serrasalmus maculatus, and Xenagoniates bondi.
5. Position of longitudinal cartilage dorsal to ectopterygoid (161): (0 > 1) displaced laterally and separated from medial margin of mesopterygoid. Paralleled in node 211 and in Exodon paradoxus.
6. Bony lamellae bordering laterosensory canal of suprapreopercle (176): (0 > 1) present. Paralleled in Markiana nigripinnis.
7. Number of branched anal-fin rays (288): (1 > 0) 24 or less. Reversal of synapomorphy 5 of the Characidae. Paralleled in nodes 200 and 277 and in Acestrorhynchus pantaneiro and Iguanodectes geisleri.
8. Uroneurals (306): (0 > 1) two pairs. Reversal of synapomorphy 15 of node 205. Paralleled in the Tetragonopterinae, in node 276, and in Bryconamericus scleroparius, Galeocharax humeralis, and Markiana nigripinnis.
Oligosarcus sp.:
1. Posterior branch of posttemporal laterosensory canal (88): (1 > 0) present.
Node 299: (100 / 100 / 98 / 53)
Oligosarcus bolivianus, O. jenynsii, other Oligosarcus?
The species of Oligosarcus are rather morphologically homogeneous, and the monophyly of this genus as currently composed was accepted even without a phylogeny supporting it. The synapomorphies of this node are perhaps applicable to a more inclusive clade composed of most species of the genus.
Synapomorphies:
1. Position of sphenotic spine relative to the orbit (12): (0 > 1) distinctly posterior to orbital margin. Paralleled in node 193 and in Acestrorhynchus pantaneiro, Attonitus ephimeros, Cynopotamus argenteus, and Gymnocharacinus bergii.
2. Posterior dorsoventral expansion of fourth infraorbital (68): (0 > 1) present. Paralleled in nodes 174 and 210.
3. Pores of laterosensory canal of lower jaw (80): (0 > 1) seven or more. Paralleled in node 211 and in Rhaphiodon vulpinus.
4. A pair of large conical teeth in premaxilla (121): (0 > 1) present. Paralleled in node 174.
5. Abrupt decrease in size of dentary teeth (148): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 222 and 255, and in Astyanax latens and A. paris.
6. Form of quadrate (150): (0 > 1) with anterodorsal portion equal or longer than ventral region. Paralleled in the Pseudochalceus clade, in nodes 170, 176, and 211, and in Exodon paradoxus and Hoplias cf. malabaricus.
7. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate (155): (0 > 1) present. Paralleled in node 176 and in Deuterodon langei, Heterocharax macrolepis, Pristella maxillaris, Roeboides descalvadensis, and Thoracocharax stellatus.
8. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. Paralleled in node 244 and in Hasemania nana, Hyphessobrycon bifasciatus, Metynnis maculatus, Odontostilbe microcephala, and Roeboides descalvadensis. Some trees: Paralleled in node 265.
9. Form and degree of ossification of first ceratobranchial gill rakers (200): (0 > 1) rather thick and completely ossified distal region. Paralleled in nodes 176 and 212 and in Hoplias cf. malabaricus, Prionobrama paraguayensis, and Pristella maxillaris.
10. Bony lamellae associated with supraneurals (282): (1 > 0) absent or small. Paralleled in nodes 197 and 272 and in Acestrocephalus sardina and Nematocharax venustus.
11. Bony hooks on last pelvic-fin ray of adult males (314): (0 > 1) as numerous as in other rays. Paralleled in nodes 232, 240, and 258 and in Axelrodia lindeae.
Autapomorphy of Oligosarcus cf. jenynsii:
1. Laterosensory canal of sixth infraorbital (76): (0 > 1) branched. Reversal of synapomorphy 2 of node 203. Paralleled in Markiana nigripinnis, Odontostilbe microcephala, and Tetragonopterus argenteus.
No autapomorphies found for Oligosarcus bolivianus.
Node 227: (32 / 84 / - / 3)
Subfamily Rhoadsiinae; Pseudochalceus clade.
Hollandichthys and Pseudochalceus was related to Bramocharax and Oligosarcus in the hypothesis by Mirande (2009) and included in his Bramocharax clade. In the present study the former genera are rather related with the Rhoadsiinae and included in the Pseudochalceus clade. It is herein preferred not to include Hollandichthys and Pseudochalceus in the Rhoadsiinae given the low support of this node. However this clade is rather stable among the analyses performed for this study and its monophyly should be further evaluated.
Synapomorphies:
1. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 229, 279, 288, and 294 and in Characidium rachovii, Hoplocharax goethei, Hyphessobrycon anisitsi, Moenkhausia sanctaefilomenae, Phenacogaster tegatus, and Pyrrhulina australis.
2. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
Node 293: Pseudochalceus clade (69 / 81 / 50 / 14)
Genera Hollandichthys and Pseudochalceus.
Hollandichthys was considered to be a synonym of Pseudochalceus by Géry (1977), implying a close relationship between these two genera. The sister-group relationship between Hollandichthys and Pseudochalceus was subsequently proposed by Bertaco (2003).
Synapomorphies:
1. Form of quadrate (150): (0 > 1) with anterodorsal portion equal or longer than ventral region. Paralleled in nodes 170, 176, 211, and 299 and in Exodon paradoxus and Hoplias cf. malabaricus.
2. Margin of first pectoral ray in adult specimens (230): (0 > 1) conspicuously serrated. Paralleled in nodes 162 and 168.
Autapomorphies of Pseudochalceus kyburzi:
1. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
2. Denticles on gill rakers (201): (0 > 1) absent. Paralleled in the Gymnocharacinae, in nodes 245 and 253, and in Axelrodia lindeae. Some trees: Paralleled in Hyphessobrycon elachys and H. herbertaxelrodi.
3. Length of interhyal (211): (0 > 1) equal to or longer than one-half of symplectic length. Paralleled in nodes 174 and 211 and in Hoplias cf. malabaricus.
4. Form of third postcleithrum (250): (1 > 0) slender, without associated lamella. Reversal of synapomorphy 6 of node 204. Paralleled in node 242 and in Gymnocharacinus bergii and Rhoadsia altipinna.
5. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Autapomorphies of Hollandichthys multifasciatus:
1. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Pseudocorynopoma doriae, and Salminus brasiliensis.
2. Overlap of maxilla by second infraorbital (61): (0 > 1) present. Reversal of synapomorphy 6 of node 205. Paralleled in Mimagoniates rheocharis.
3. Articulation between second and third infraorbitals (62): (0 > 2) posteroventrally angled. Paralleled in the Characinae, in nodes 176 and 300, and in Bryconops melanurus.
4. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Attonitus ephimeros, Axelrodia lindeae, Hoplocharax goethei, Jupiaba scologaster, Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
5. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in node 296 and in Axelrodia lindeae, Gymnocharacinus bergii, Mimagoniates rheocharis, Nematocharax venustus, Paracheirodon axelrodi, and Prodontocharax melanotus.
6. First pelvic-fin ray (256): (0 > 1) branched.
7. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
8. Insemination (358): (0 > 1) present. Paralleled in node 239.
9. Type of spermatozoa (359): (0 > 1) introsperm. Paralleled in node 237.
10. Sperm storage area on testes (360): (0 > 1) present, as broad as spermatogenic area.
Node 226: Rhoadsiinae (44 / 91 / 7 / 5)
Genera Carlana, Nematocharax Weitzman, Menezes & Britski, Parastremma Eigenmann, and Rhoadsia.
The close relationship between Carlana, Parastremma (not analyzed), and Rhoadsia, which form the subfamily Rhoadsiinae was proposed and previously discussed in the literature (e. g. Géry, 1977). Although this subfamily was not phylogenetically diagnosed, it was recognized as monophyletic in the last revision of the family (Cardoso, 2003b). The close relationship of the monotypic Nematocharax to the Rhoadsiinae had been previously proposed by Mirande (2009) and corroborated in this study. According to the present results, the nomenclatural alternatives are to define a new subfamily for Nematocharax, or include this genus in the Rhoadsiinae. The latter of these alternatives is preferred herein as more conservative than is the creation of new names.
Synapomorphies:
1. Form of teeth of inner premaxillary tooth row (128): (0 > 1) with cusps aligned in straight series and without anterior concavity. Paralleled in nodes 195, 245, and 280 and in Hemigrammus bleheri and Odontostoechus lethostigmus.
2. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in nodes 273, 283, and 294 and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
Autapomorphies of Nematocharax venustus:
1. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. Paralleled in nodes 265 and 294 and in Brycon orbignyanus, Bryconops melanurus, Gymnocharacinus bergii, and Micralestes stormsi. Some trees: Paralleled in Bryconamericus agna.
2. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
3. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in node 296 and in Axelrodia lindeae, Gymnocharacinus bergii, Hollandichthys multifasciatus, Mimagoniates rheocharis, Paracheirodon axelrodi, and Prodontocharax melanotus.
4. Position of ventral end of posttemporal (253): (1 > 0) anterior or lateral to lateral margin of epioccipital. Reversal of synapomorphy 2 of node 228.
5. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in nodes 211, 223, and 262, and in Bramocharax bransfordii, Bryconaethiops macrops, and Hyphessobrycon bifasciatus. Some trees: Paralleled in the Aphyoditeinae.
6. Bony lamellae associated with supraneurals (282): (1 > 0) absent or small. Paralleled in nodes 197, 272, and 299 and in Acestrocephalus sardina.
7. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, and Probolodus heterostomus.
8. Scales covering caudal-fin lobes (328): (0 > 1) covering one-third of their length. Paralleled in node 222 and in Aulixidens eugeniae, Distichodus maculatus, and Markiana nigripinnis. Some trees: Paralleled in Knodus breviceps.
Node 225: (100 / 100 / 100 / 76)
Genera Carlana, Parastremma, and Rhoadsia.
The composition of this node corresponds to the subfamily Rhoadsiinae according to Cardoso (2003b) and previous authors. The inclusion of Parastremma in this clade was not tested, but the genus is listed in this clade following Cardoso (2003b). It is probable, however, that some of the reported synapomorphies of this node actually apply to a more inclusive clade.
Synapomorphies:
1. Form of mesethmoid spine (27): (0 > 1) relatively short, with premaxillae articulating with each other anterior to mesethmoid. Paralleled in node 234 and in Chalceus macrolepidotus and Paracheirodon axelrodi.
2. Posterior portion of mesethmoid spine (28): (0 > 1) as broad as lateral wings of mesethmoid.
3. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
4. Ontogenetic lengthening of maxilla (101): (0 > 1) present.
5. Length of ascending process of premaxilla (104): (0 > 1) reaching just anterior end of nasal. Paralleled in node 294 and in Charax stenopterus, Phenacogaster tegatus, and Stichonodon insignis. Some trees: Paralleled in Bryconamericus agna.
6. Orientation of anterior dentary teeth (141): (0 > 1) oriented anteriorly, almost parallel to main axis of dentary. Paralleled in Prodontocharax melanotus.
7. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 210, 276, and 297 and in Brycon orbignyanus, Hoplocharax goethei, Hyphessobrycon elachys, and Rhaphiodon vulpinus.
Autapomorphies of Rhoadsia altipinna:
1. Ventral projection of lagenar capsule (3): (1 > 0) not extending ventrally to horizontal through articulation between basioccipital and parasphenoid. Reversal of synapomorphy 2 of node 205. Paralleled in node 210.
2. Dorsal process of pterotic where tendon from epaxial musculature attach (45): (0 > 1) present, projecting dorsally from tube for semicircular canal. Paralleled in the Heterocharacinae, in node 193, and in Serrasalmus maculatus.
3. Margins of toothed region of maxilla (96): (0 > 1) dorsally divergent. Paralleled in nodes 162, 209, 254, and 282 and in Prodontocharax melanotus.
4. Form of third postcleithrum (250): (1 > 0) slender, without associated lamella. Reversal of synapomorphy 6 of node 204. Paralleled in node 242 and in Gymnocharacinus bergii and Pseudochalceus kyburzi.
5. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, and Pseudochalceus kyburzi. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Autapomorphies of Carlana eigenmanni:
1. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Aulixidens eugeniae, Carnegiella strigata, Grundulus cochae, Odontostoechus lethostigmus, Paracheirodon axelrodi, Piabucus melanostomus, and Probolodus heterostomus.
2. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
Node 202: (6 / 82 / - / 3)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, Stevardiinae, and Tetragonopterinae; Astyanax clade, Astyanax paris clade, Bryconamericus scleroparius clade, and Hyphessobrycon anisitsi clade.
This node is incongruent with the hypothesis by Mirande (2009). As with node 202 such incongruence is due to the relative positions of Jupiaba and the Characinae.
Synapomorphies:
1. Extent of implantation of teeth along maxilla (137): (1 > 0) not reaching middle of maxillary lamella. Reversal of synapomorphy 9 of node 205. Reversed in node 208 and in Grundulus cochae, Hemibrycon surinamensis, Nematobrycon palmeri, and Prodontocharax melanotus.
2. Articulation between quadrate and anguloarticular (154): (1 > 0) anterior to or at vertical through middle eye. Paralleled in Bryconexodon juruenae and Phenacogaster tegatus.
Node 224: Tetragonopterinae (-9 / 69 / - / 4)
Genera Bario Myers, Brachychalcinus Boulenger, Deuterodon, Gymnocorymbus, Hasemania Ellis, Hemigrammus Gill, Hyphessobrycon Durbin, Jupiaba, Moenkhausia, Myxiops?, Paracheirodon, Orthospinus Reis, Petitella Géry & Boutière?, Poptella Eigenmann, Pristella Eigenmann, Probolodus, Stethaprion Cope, Stichonodon Eigenmann, Tetragonopterus, and Thayeria.
The subfamily Tetragonopterinae was the most diverse among the Characidae under the classical systematics of the family (e. g. Géry, 1977). Reis (2003b) later restricted this subfamily to the genus Tetragonopterus due to the lack of presumably apomorphic features shared by its members. In that classification, most tetragonopterines were classified as incertae sedis within Characidae (Lima et al., 2003). Both in the phylogeny by Mirande (2009) as in the present study Tetragonopterus argenteus is the sister group of the Stethaprioninae. In the phylogeny of Mirande (2009) the Tetragonopterinae and Stethaprioninae form a monophyletic unit which is the sister group of a large clade of characids. In that hypothesis both subfamilies were valid and Hemigrammus, along with several other genera, formed the Hemigrammus clade. In the phylogenetic hypothesis herein obtained both the Stethaprioninae and Tetragonopterinae are nested in a large clade composed additionally by the members of the Hemigrammus clade of Mirande (2009). This monophyletic assemblage is named Tetragonopterinae, which has precedence over Stethaprioninae. This clade includes also the type-species of the highly diverse genera Hemigrammus and Moenkhausia, and presumably also Hyphessobrycon. Weitzman & Palmer (1997) and Weitzman & Malabarba (1998) discussed the possible polyphyly of Hyphessobrycon, and the paraphyly of Hemigrammus. This analysis corroborates their findings that a phylogenetic classification of Hemigrammus and Hyphessobrycon would result in generic reassignments of many species currently in those genera. Probably the same statement is applicable to Moenkhausia. Petitella (not analyzed herein) shares with Hemigrammus bleheri a presumably apomorphic coloration, with an intensely red head and the presence of three conspicuous black bars in the caudal fin. Indeed, Petitella georgiae Géry & Boutière is mainly distinguished from Hemigrammus bleheri as having only one row of premaxillary teeth (vs. two rows). Thus, Petitella is tentatively included in this node. Myxiops, as discussed below, shares several presumably apomorphic features with Deuterodon and it is also tentatively included in this clade. Most internal clades of the Characidae are poorly supported, and this node is not an exception. The monophyly and composition of the Tetragonopterinae as herein defined, thus, should be further tested.
Synapomorphy:
1. Uroneurals (306): (0 > 1) two pairs. Reversal of synapomorphy 15 of node 205. Paralleled in nodes 276 and 300 and in Bryconamericus scleroparius, Galeocharax humeralis, and Markiana nigripinnis. Reversed in node 288 and in Gymnocorymbus ternetzi and Jupiaba scologaster.
Autapomorphies of Probolodus heterostomus:
1. Mamilliform teeth outside mouth (120): (0 > 1) present. Paralleled in nodes 218 and 277.
2. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Aulixidens eugeniae, Carlana eigenmanni, Carnegiella strigata, Grundulus cochae, Odontostoechus lethostigmus, Paracheirodon axelrodi, and Piabucus melanostomus.
3. Number of teeth in inner premaxillary row (129): (1 > 0) four or fewer. Paralleled in node 198 and in Markiana nigripinnis.
4. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
5. Posterior margin of cleithrum (234): (0 > 1) with concavity ventral to first postcleithrum. Reversal of synapomorphy 4 of node 204. Paralleled in nodes 196 and 289 and in Roeboexodon geryi.
6. Position of ventral end of posttemporal (253): (0 > 1) posterior to lateral margin of epioccipital. Paralleled in nodes 211 and 228 and in Bryconamericus scleroparius, Hoplias cf. malabaricus, Markiana nigripinnis, and Prochilodus lineatus.
7. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, and Nematocharax venustus.
8. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in node 268 and in Acrobrycon tarijae, Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Hyphessobrycon luetkenii, and H. socolofi.
Node 223: (9 / 70 / - / 1)
Genera Bario, Brachychalcinus, Deuterodon, Gymnocorymbus, Hasemania, Hemigrammus, Hyphessobrycon, Jupiaba, Moenkhausia, Myxiops?, Paracheirodon, Orthospinus, Petitella, Poptella, Pristella, Stethaprion, Stichonodon, Tetragonopterus, and Thayeria.
Synapomorphies:
1. Distance between cartilage anterior to orbitosphenoid and lateral ethmoids (38): (1 > 0) contacting, or almost contacting, lateral ethmoids. Reversal of synapomorphy 1 of node 204. Paralleled in Bryconexodon juruenae and Galeocharax humeralis. Reversed in node 286 and in Moenkhausia sanctaefilomenae.
2. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in nodes 211 and 262, and in Bramocharax bransfordii, Bryconaethiops macrops, Hyphessobrycon bifasciatus, and Nematocharax venustus. Some trees: Paralleled in the Aphyoditeinae. Reversed in Hemigrammus unilineatus. Some trees: Reversed in Hasemania nana.
Node 283: (25 / 53 / 17 / 1)
Genera Deuterodon, Jupiaba, and Myxiops?.
The monophyly of a clade including the genera Deuterodon and Jupiaba had not been previously proposed. However, Jupiaba acanthogaster (Eigenmann), Jupiaba minor (Travassos), and Jupiaba pinnata (Eigenmann), were originally described as members of Deuterodon, suggesting some resemblance between these two genera. The sister-group relationship of Deuterodon and Jupiaba was herein obtained without analyzing those three species, whose study may be useful to test both the monophyly of this node and that of Deuterodon and Jupiaba. Myxiops shares several presumably apomorphic features with Deuterodon and is tentatively included in this node.
Synapomorphies:
1. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273 and 294, and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
2. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Node 296: (100 / 100 / 69 / 71)
Genus Jupiaba.
The monophyly of Jupiaba was proposed by Zanata (1997) and supported by unique modifications of the pelvic bone. In the final hypothesis of Mirande (2009) the species of Jupiaba were the sister group of the remaining distal characids lacking a supraorbital bone, and was included in the Jupiaba clade. In the present hypothesis Jupiaba is nested within the Tetragonopterinae as the sister group of Deuterodon.
Synapomorphies:
1. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in Axelrodia lindeae, Gymnocharacinus bergii, Hollandichthys multifasciatus, Mimagoniates rheocharis, Nematocharax venustus, Paracheirodon axelrodi, and Prodontocharax melanotus.
2. Anterior tip of pelvic bone (263): (0 > 1) pointed, lacking associated cartilage and frequently projecting outside body wall.
Autapomorphies of Jupiaba scologaster:
1. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Attonitus ephimeros, Axelrodia lindeae, Hollandichthys multifasciatus, Hoplocharax goethei, Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
2. Uroneurals (306): (1 > 0) absent or just one pair. Reversal of synapomorphy 1 of the Tetragonopterinae. Paralleled in node 288 and in Gymnocorymbus ternetzi.
Autapomorphy of Jupiaba mucronata:
1. Humeral spot (341): (0 > 1) horizontally-ovate. Paralleled in node 259 and in Acestrorhynchus pantaneiro, Brycon orbignyanus, and Roeboides microlepis.
Node 282: (100 / 100 / 89 / 69)
Genera Deuterodon and Myxiops?
The monophyly of Deuterodon was proposed by Lucena & Lucena (2002), who based their hypothesis in some unique features of the maxilla shared by the members of this genus. Prior to that paper some species of Deuterodon were included in Astyanax (Eigenmann, 1917; Géry, 1977). An evaluation on the monophyly of Deuterodon is beyond the scope of this paper, and only two species of this genus are herein analyzed. The genus Myxiops (not analyzed here) shares all the synapomorphies of the genus Deuterodon as proposed by Lucena & Lucena (2002). Myxiops was principally diagnosed by Zanata & Akama (2004) based on autapomorphies and by the possession of only one row of premaxillary teeth (vs. two in Deuterodon), a moderately homoplastic character within Characidae. The placement of Myxiops should be tested in the future, but the information from its description permits its inclusion, at least tentatively, in this clade.
Synapomorphies:
1. Form of fourth infraorbital (67): (1 > 0) approximately square or more developed longitudinally than dorsoventrally. Reversal of synapomorphy 1 of node 189. Paralleled in 200, 210, 228, and 277.
2. Margins of toothed region of maxilla (96): (0 > 1) dorsally divergent. Paralleled in nodes 162, 209, and 254 and in Prodontocharax melanotus and Rhoadsia altipinna.
3. Position of coronomeckelian (110): (0 > 1) situated mainly dorsal to Meckelian cartilage. Reversal of synapomorphy 2 of node 206. Paralleled in nodes 200 and 290 and in Hemigrammus erythrozonus.
4. Relative position of dorsal-fin anterior insertion (265): (1 > 0) anterior to or at vertical through pelvic-fin origin. Paralleled in Creagrutus anary, Exodon paradoxus, Moenkhausia xinguensis, and Parecbasis cyclolepis.
Autapomorphies of Deuterodon langei:
1. Expansion of lamellar portion of maxilla just posterior to toothed region (97): (0 > 1) very pronounced. Paralleled in node 232. Some trees: Paralleled in Paracheirodon axelrodi.
2. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate (155): (0 > 1) present. Paralleled in nodes 176 and 299 and in Heterocharax macrolepis, Pristella maxillaris, Roeboides descalvadensis, and Thoracocharax stellatus.
3. Distribution of denticles on gill rakers (202): (0 > 1) along entire surface of gill rakers. Paralleled in node 269 and in Astyanax correntinus, Hoplias cf. malabaricus, Hyphessobrycon eques, and Pristella maxillaris.
4. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. Paralleled in node 302, and in Agoniates anchovia, Cyanocharax alburnus, Engraulisoma taeniatum, Hemiodus cf. thayeria, Roeboexodon geryi, and Thayeria obliqua. Some trees: Paralleled in Microschemobrycon casiquiare and Parecbasis cyclolepis.
5. Process of scapula forming anterior border of scapular foramen (244): (0 > 1) absent. Paralleled in nodes 193 and 252 and in Aphyodite grammica, Hoplias cf. malabaricus, Hyphessobrycon herbertaxelrodi, Leporinus striatus, Odontostilbe paraguayensis, and Thayeria obliqua.
Autapomorphy of Deuterodon iguape:
1. Length of supraoccipital spine (53): (0 > 1) extends only to anterior limit of neural complex. Reversal of synapomorphy 5 of node 205. Paralleled in nodes 272 and 287 and in Bramocharax bransfordii and Lonchogenys ilisha.
Node 222: (-4 / 68 / - / 1)
Genera Bario, Brachychalcinus, Gymnocorymbus, Hasemania, Hemigrammus, Hyphessobrycon, Moenkhausia, Paracheirodon, Orthospinus, Petitella, Poptella, Pristella, Stethaprion, Stichonodon, Tetragonopterus, and Thayeria.
Synapomorphies:
1. Abrupt decrease in size of dentary teeth (148): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 255 and 299, and in Astyanax latens and A. paris. Reversed in Stichonodon insignis.
2. Scales covering caudal-fin lobes (328): (0 > 1) covering one-third of their length. Paralleled in Aulixidens eugeniae, Distichodus maculatus, Markiana nigripinnis, and Nematocharax venustus. Some trees: Paralleled in Knodus breviceps. Some trees: Reversed in node 285.
Node 221: (95 / 100 / 55 / 17)
Genera Brachychalcinus, Gymnocorymbus, Orthospinus, Poptella, Stethaprion, Stichonodon, and Tetragonopterus.
The close relationship between the genera assigned in the literature to Stethaprioninae (Brachychalcinus, Orthospinus, Poptella, and Stethaprion) and the genera Gymnocorymbus, Stichonodon, and Tetragonopterus was suggested by Reis (1989), who examined the three latter genera as part of his outgroup in a phylogenetic diagnosis of the Stethaprioninae. According to Géry (1977: 451) Gymnocorymbus "represent the adaptation of some deep Moenkhausia toward a disciform body, accompanied by a naked predorsal line". That hypothesis about the relationships of Gymnocorymbus was only partially tested here, given that only four, non-deep-bodied, species of Moenkhausia were included in the analysis. Géry (1977) also noted the resemblance of Stichonodon and Tetragonopterus with Gymnocorymbus and Poptella, and some deep-bodied Moenkhausia, respectively, and considered them to be closely related. This node was composed of the subfamilies Tetragonopterinae and Stethaprioninae by Mirande (2009) whereas it is included in the Tetragonopterinae according to the final hypothesis herein proposed.
Synapomorphies:
1. Length of supraoccipital spine (52): (1 > 0) extends dorsal of entire neural complex of Weberian apparatus. Paralleled in node 214 and in Psellogrammus kennedyi.
2. Separation between posterior dentary teeth (147): (0 > 1) more than width of these teeth. Paralleled in Astyanax cf. rutilus, Aulixidens eugeniae, and Pristella maxillaris.
3. Proximal and medial radials of anal fins (294): (0 > 1) fused in most pterygiophores. Paralleled in nodes 184, 208, and 218 and in Psellogrammus kennedyi and Pseudocorynopoma doriae. Some trees: Paralleled in node 295.
4. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in node 210, and in Bario steindachneri, Markiana nigripinnis, Paragoniates alburnus, Rhaphiodon vulpinus, Roeboides microlepis, and Thoracocharax stellatus.
5. Sclerotic bones (350): (0 > 1) two bones separated by cartilages. Paralleled in nodes 208, 210, 250, and 259.
Autapomorphies of Tetragonopterus argenteus:
1. Laterosensory canal of sixth infraorbital (76): (0 > 1) branched. Reversal of synapomorphy 2 of node 203. Paralleled in Markiana nigripinnis, Odontostilbe microcephala, Oligosarcus cf. jenynsii.
2. Length of caudal-fin canal of lateral line (93): (0 > 1) almost reaching posterior margin of caudal fin. Paralleled in nodes 177 and 216 and in Astyanax pelegrini.
3. Tubules for passage of blood vessels on lamellar portion of maxilla (98): (0 > 1) tubule with anterior branch running parallel to anterior margin of maxilla and reaching one third of its length. Paralleled in node 201.
4. Ethmopalatine cartilage (171): (0 > 1) present and conspicuous. Paralleled in Charax stenopterus and Phenacogaster tegatus.
5. Base of second pectoral ray (231): (1 > 0) large and partially overlapping base of first pectoral ray from medial view. Paralleled in nodes 164 and 301 and in Moenkhausia dichroura and Prionobrama paraguayensis.
6. Anterior margin of scales (318): (0 > 1) with conspicuous undulations. Paralleled in Markiana nigripinnis.
7.Radii of scales (322): (0 > 1) converging at focus. Reversal of synapomorphy 7 of the Characidae. Paralleled in node 273 and in Stichonodon insignis. Some trees: Paralleled in node 302 and in Microschemobrycon casiquiare.
8. Second humeral spot (342): (0 > 1) present as a conspicuous vertical bar. Paralleled in Gymnocorymbus ternetzi and Hyphessobrycon bifasciatus.
Node 220: (11 / 91 / 17 / 3)
Genera Brachychalcinus, Gymnocorymbus, some Moenkhausia?, Orthospinus, Poptella, Stethaprion, and Stichonodon.
The monophyly of the Stethaprioninae, including Brachychalcinus, Orthospinus, Poptella, and Stethaprion, was proposed by Reis (1989). This author recognized the significant resemblance of these genera to other deep-bodied characids, although he raised the possibility that this feature arose independently in different lineages of the Characidae. The Stethaprioninae, as redefined by Mirande (2009) was composed of the genera listed by Reis (1989) plus Gymnocorymbus (both species of Gymnocorymbus share the presumably apomorphic naked predorsal line and this genus is assumed here to be monophyletic) and the monotypic genus Stichonodon. The inclusion of Stichonodon in the Stethaprioninae was originally proposed by Eigenmann (1907), who later classified this genus in its own subfamily Stichonodontinae (e. g. Eigenmann, 1910, as Stichanodontinae). As mentioned above this node is included in the Tetragonopterinae for temporal precedence over both Stethaprioninae and Stichonodontinae. The Stethaprioninae was hypothesized to be related with some deep-bodied Moenkhausia (Géry, 1977). Both that issue and the evaluation of the monophyly of Moenkhausia lie beyond the scope of this paper, but it is probable that some species of Moenkhausia should be included in this subfamily (although Moenkhausia xinguensis, the type species of the genus, is included in a clade discussed below).
Synapomorphies:
1. Anterior margin of supraoccipital (51): (0 > 1) situated anterior to vertical through posterior orbital margin. Paralleled in Cynopotamus argenteus and Metynnis maculatus.
2. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 236, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Node 284: (11 / 77 / 2 / 3)
Genera Gymnocorymbus and Stichonodon; some Moenkhausia?
As noted above, the marked resemblance between Gymnocorymbus and Stichonodon was highlighted by Géry (1977) and Reis (1989), but the monophyly of a clade comprising these two genera was not previously proposed. The monophyly of Gymnocorymbus was not tested, although both species of this genus share the absence of scales along the predorsal line, an unusual feature within the Characidae, and this genus is provisionally treated as monophyletic. Some species of Moenkhausia, however, share a naked predorsal line (e. g. M. dorsinuda Zarske & Géry; not included in this study), and could be related to or included in this clade.
Synapomorphies:
1. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in the Astyanax clade, in node 290, and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
2. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 289 and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
3. Median predorsal scales (325): (0 > 1) leaving naked area anterior to dorsal fin. Paralleled in the Serrasalmidae and in Lonchogenys ilisha.
Autapomorphies of Stichonodon insignis:
1. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, and Roeboexodon geryi. Some trees: Reversed in node 292.
2. Dorsal end of laterosensory canal of preopercle and suprapreopercle (82): (0 > 1) overlapping anterodorsal process of opercle. Paralleled in the Alestidae, in node 230, and in Bario steindachneri, Hyphessobrycon eques, Parecbasis cyclolepis, and Pristella maxillaris.
3. Length of ascending process of premaxilla (104): (0 > 1) reaching just anterior end of nasal. Paralleled in nodes 225 and 294 and in Charax stenopterus and Phenacogaster tegatus. Some trees: Paralleled in Bryconamericus agna.
4. Fossa for inner row of replacement premaxillary teeth (133): (0 > 1) present.
5. Maxillary teeth (134): (1 > 0) absent. Paralleled in Aulixidens eugeniae, Coptobrycon bilineatus, Iguanodectes geisleri, and Parecbasis cyclolepis. Some trees: Paralleled in Hyphessobrycon elachys and Psellogrammus kennedyi.
6. Abrupt decrease in size of dentary teeth (148): (1 > 0) absent. Reversal of synapomorphy 1 of node 222.
7. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. Paralleled in nodes 184 and 242, and in Aphyocharax dentatus, Engraulisoma taeniatum, and Prionobrama paraguayensis. Some trees: Paralleled in the Cheirodontinae and in Microschemobrycon casiquiare.
8. Ventral margin of anterior ceratohyal (179): (1 > 0) smooth and without notches. Reversal of synapomorphy 3 of node 189. Paralleled in node 211.
9. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax latens, A. cf. rutilus, A. pelegrini, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Parecbasis cyclolepis, and Piaractus mesopotamicus.
10. Laminar bony ridge on dorsal margin of abdominal ribs (224): (0 > 1) present. Paralleled in node 297 and in Parecbasis cyclolepis.
11. Number of dorsal pterygiophores (277): (0 > 1) 11 or more. Paralleled in Stethaprion erythrops.
12.Radii of scales (322): (0 > 1) converging at focus. Reversal of synapomorphy 7 of the Characidae. Paralleled in node 273 and in Tetragonopterus argenteus. Some trees: Paralleled in node 302 and in Microschemobrycon casiquiare.
Autapomorphies of Gymnocorymbus ternetzi:
1. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in nodes 207 and 212 and in Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
2. Number of anal pterygiophores anterior to first haemal spine (293): (0 > 1) four or more. Paralleled in the Iguanodectinae, in node 214, and in Cynopotamus argenteus.
3. Uroneurals (306): (1 > 0) absent or just one pair. Reversal of synapomorphy 1 of the Tetragonopterinae. Paralleled in node 288 and in Jupiaba scologaster.
4. Second humeral spot (342): (0 > 1) present as a conspicuous vertical bar. Paralleled in Hyphessobrycon bifasciatus and Tetragonopterus argenteus.
Node 219: (100 / 100 / 61 / 49)
Genera Brachychalcinus, Orthospinus, Poptella, and Stethaprion.
The monophyly of this clade (as the Stethaprioninae) was proposed by Reis (1989), supported by the presence of a predorsal spine, and the possession of randomly distributed anal-fin bony hooks in mature males. The composition of this clade follows Reis (1989).
Synapomorphy:
1. Anteriorly oriented spine formed by first dorsal-fin ray (267): (0 > 1) present.
Autapomorphies of Stethaprion erythrops:
1. Lateral coverage of dilator fossa by sixth infraorbital (69): (1 > 0) almost complete, at least in its ventral border. Paralleled in Hyphessobrycon pulchripinnis.
2. Anterior extension of pelvic-bone along main axis (262): (0 > 1) projecting anterior of lateral and medial lamellae of pelvic bone. Paralleled in the Gasteropelecidae, in node 302, and in Hoplias cf. malabaricus, Piabucus melanostomus, and Rhaphiodon vulpinus.
3. Number of dorsal pterygiophores (277): (0 > 1) 11 or more. Paralleled in Stichonodon insignis.
4. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
5. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent. Paralleled in Creagrutus anary, Hyphessobrycon eques, H. luetkenii, Phenacogaster tegatus, and Pseudocorynopoma doriae. Some trees: Paralleled in Markiana nigripinnis and Psellogrammus kennedyi.
6. Semicircular grooves on posterior field of scales (323): (0 > 1) present.
No autapomorphies found for Poptella paraguayensis.
Node 274: (-4 / 71 / - / 1)
Genera Bario, Hasemania, Hemigrammus, Hyphessobrycon, Moenkhausia, Paracheirodon, Petitella?, Pristella, and Thayeria.
Synapomorphy:
1. Bony hooks on first pelvic-fin ray of adult males (315): (0 > 1) present. Paralleled in Aphyocharacidium bolivianum, Aphyocharax anisitsi, Aulixidens eugeniae, and Nantis indefessus. Some trees: Reversed in node 291.
Node 273: (11 / 83 / 19 / 3)
Genera Bario and Moenkhausia.
This clade includes the analyzed species of Bario and Moenkhausia. Although a relationship between these genera was previously proposed (e. g. Géry, 1977), no published phylogenetic hypothesis supported that proposal. The genus Moenkhausia has not been demonstrated to be monophyletic; indeed, some of its species were related with other genera (e. g. M. georgiae Géry with Tetragonopterus; Géry, 1977). This genus probably is not monophyletic even with the inclusion of Bario steindachneri, as suggest the results herein obtained. The monophyly and phylogeny of Moenkhausia were studied in a still unpublished thesis (Benine, 2004), the results of which when published will surely help in resolve the taxonomy of this diverse genus.
Synapomorphies:
1. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 283 and 294, and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
2.Radii of scales (322): (0 > 1) converging at focus. Reversal of synapomorphy 7 of the Characidae. Paralleled in Stichonodon insignis and Tetragonopterus argenteus. Some trees: Paralleled in node 302 and in Microschemobrycon casiquiare.
Autapomorphy of Moenkhausia xinguensis:
1. Relative position of dorsal-fin anterior insertion (265): (1 > 0) anterior to or at vertical through pelvic-fin origin. Paralleled in node 282 and in Creagrutus anary, Exodon paradoxus, and Parecbasis cyclolepis.
Node 272: (100 / 100 / 30 / 12)
Bario steindachneri, Moenkhausia sanctaefilomenae, other Moenkhausia?
Synapomorphies:
1. Length of supraoccipital spine (53): (0 > 1) extends only to anterior limit of neural complex. Reversal of synapomorphy 5 of node 205. Paralleled in node 287 and in Bramocharax bransfordii, Deuterodon iguape, and Lonchogenys ilisha.
2. Bony lamellae associated with supraneurals (282): (1 > 0) absent or small. Paralleled in nodes 197 and 299 and in Acestrocephalus sardina and Nematocharax venustus.
3.Radii oriented towards anterior field of scales (321): (2 > 1) only as longitudinal groove without defined margins.
Autapomorphies of Moenkhausia sanctaefilomenae:
1. Distance between cartilage anterior to orbitosphenoid and lateral ethmoids (38): (0 > 1) distant from lateral ethmoids. Reversal of synapomorphy 1 of node 223. Paralleled in node 286.
2. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
3. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 229, 279, 288, and 294 and in Characidium rachovii, Hoplocharax goethei, Hyphessobrycon anisitsi, Phenacogaster tegatus, and Pyrrhulina australis.
4. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
5. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. Paralleled in the Characinae and in Bryconops melanurus, Hyphessobrycon pulchripinnis, and Iguanodectes geisleri. Some trees: Paralleled in node 196 and in Hemigrammus erythrozonus and Hyphessobrycon herbertaxelrodi.
Autapomorphies of Bario steindachneri:
1. Contact between frontals anteriorly to frontal fontanel (21): (0 > 1) present. Reversal of synapomorphy 4 of node 206. Paralleled in Exodon paradoxus, Galeocharax humeralis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in Knodus breviceps.
2. Dorsal end of laterosensory canal of preopercle and suprapreopercle (82): (0 > 1) overlapping anterodorsal process of opercle. Paralleled in the Alestidae, in node 230, and in Hyphessobrycon eques, Parecbasis cyclolepis, Pristella maxillaris, and Stichonodon insignis.
3. Ventral margin of horizontal process of anguloarticular (109): (0 > 1) perpendicular to laterosensory canal of dentary from medial view. Paralleled in node 199 and in Bramocharax bransfordii and Micralestes stormsi.
4. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (1 > 0) two. Reversal of synapomorphy 4 of node 203. Paralleled in Thayeria obliqua. Some trees: Paralleled in Hyphessobrycon elachys and Paracheirodon axelrodi.
5. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bryconamericus cf. iheringii, B. rubropictus, Hyphessobrycon luetkenii, H. socolofi, and Phenacogaster tegatus.
6. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax cf. asuncionensis, A. lineatus, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, Nematocharax venustus, and Probolodus heterostomus.
7. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in node 268 and in Acrobrycon tarijae, Astyanax cf. asuncionensis, A. lineatus, Hyphessobrycon luetkenii, H. socolofi, and Probolodus heterostomus.
8. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in nodes 210 and 221, and in Markiana nigripinnis, Paragoniates alburnus, Rhaphiodon vulpinus, Roeboides microlepis, and Thoracocharax stellatus.
Node 289: (-16 / 61 / - / 1)
Genera Hasemania?, Hemigrammus, Hyphessobrycon, Paracheirodon, Petitella?, Pristella, and Thayeria; some Moenkhausia.
The monophyly of a group of species congruent with the taxa in this node was not previously proposed. In the hypothesis by Mirande (2009) the clade composed of Moenkhausia dichroura and M. cf. intermedia are the sister group of the clade composed of Bario steindachneri, Moenkhausia sanctaefilomenae and M. xinguensis, the type species of the genus. In that hypothesis the analyzed species of Moenkhausia formed a monophyletic unit except for the inclusion of Bario steindachneri. In the present hypothesis, Moenkhausia is composed of two lineages not forming a monophyletic group. As the type species of the genus is M. xinguensis, related to Bario and M. sanctaefilomenae, the species of Moenkhausia included in this node should be transferred to a new genus, or to Hemigrammus, as discussed below.
Synapomorphies:
1. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284 and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
2. Posterior margin of cleithrum (234): (0 > 1) with concavity ventral to first postcleithrum. Reversal of synapomorphy 4 of node 204. Paralleled in node 196 and in Probolodus heterostomus and Roeboexodon geryi. Some trees: Reversed in node 295.
Node 297: (100 / 100 / 82 / 40)
Moenkhausia bonita Benine, Castro & Sabino?, M. dichroura, M. gracilima Eigenmann?, M. lepidura?, M. intermedia, and other Moenkhausia?
The species included in this clade were considered within the Moenkhausia lepidura group by Géry (1977), characterized by the possession of a low body. An assessment of the monophyly of this group of species, however, is beyond the scope of this paper. Géry (1977) mentioned that Moenkhausia dichroura, M. gracilima, M. intermedia, and M. lepidura, among the species of his lepidura group share the dark coloration of the caudal-fin dorsal lobe. Moenkhausia bonita shares with M. dichroura and M. intermedia the dark coloration of both caudal-fin lobes, and it is tentatively included in this clade.
Synapomorphies:
1. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 210, 225, and 276 and in Brycon orbignyanus, Hoplocharax goethei, Hyphessobrycon elachys, and Rhaphiodon vulpinus.
2. Laminar bony ridge on dorsal margin of abdominal ribs (224): (0 > 1) present. Paralleled in Parecbasis cyclolepis and Stichonodon insignis.
3. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
4. Color of caudal-fin lobes (345): (0 > 3) both lobes dark brown or black. Paralleled in Bryconamericus exodon.
Autapomorphies of Moenkhausia cf. intermedia:
1. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162, 247, and 253 and in Agoniates anchovia, Attonitus ephimeros, Characidium borellii, Iguanodectes geisleri, Prionobrama paraguayensis, and Xenagoniates bondi.
2. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. Paralleled in nodes 162, 285, and 301 and in Aulixidens eugeniae, Creagrutus anary, Diapoma speculiferum, and Pyrrhulina australis. Some trees: Paralleled in node 247 and in Knodus breviceps.
Autapomorphies of Moenkhausia dichroura:
1. Alignment of cusps of medial teeth on inner premaxillary row (127): (1 > 0) forming anteriorly concave semicircle from ventral view. Paralleled in nodes 183 and 262. Some trees: Paralleled in node 302.
2. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax latens, A. cf. rutilus, A. pelegrini, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Parecbasis cyclolepis, Piaractus mesopotamicus, and Stichonodon insignis.
3. Base of second pectoral ray (231): (1 > 0) large and partially overlapping base of first pectoral ray from medial view. Paralleled in nodes 164 and 301 and in Prionobrama paraguayensis and Tetragonopterus argenteus.
Node 288: (-2 / 55 / - / 1)
Genera Hasemania?, Hemigrammus, Hyphessobrycon, Paracheirodon, Petitella?, Pristella, and Thayeria.
Following the nomenclatural preference against the creation of new names, all of the genera included in this node should be synonymized to Hemigrammus, which has temporal precedence over the remaining genera, excepting perhaps Petitella which was not analyzed in this paper and whose relationships are uncertain. An alternative solution would imply the creation of at least two new generic names, for Moenkhausia dichroura and M. intermedia (and species related to them) and Hemigrammus ulreyi. The present phylogenetic hypothesis would imply many generic reassignments, especially in the species of the highly diverse genus Hyphessobrycon. If this hypothesis is further corroborated, Hemigrammus would become the most diverse genus of the Characidae, with approximately 200 species. The synonymies and generic reassignments implied for this contribution should be further corroborated by more concerned papers, analyzing a higher number of species of this diverse clade.
Synapomorphies:
1. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 229, 279, and 294 and in Characidium rachovii, Hoplocharax goethei, Hyphessobrycon anisitsi, Moenkhausia sanctaefilomenae, Phenacogaster tegatus, and Pyrrhulina australis.
2. Uroneurals (306): (1 > 0) absent or just one pair. Reversal of synapomorphy 1 of the Tetragonopterinae. Paralleled in Gymnocorymbus ternetzi and Jupiaba scologaster.
No autapomorphies found for Hemigrammus ulreyi.
Node 287: (-4 / 54 / - / 1)
Genera Hasemania?, Hemigrammus (excepting, at least, H. ulreyi), Hyphessobrycon, Paracheirodon, Petitella?, Pristella, and Thayeria.
This clade is composed of a group of species putatively included in the rosy tetra clade of Weitzman & Palmer (1997), or which are related to it, according to those authors.
Synapomorphies:
1. Length of supraoccipital spine (53): (0 > 1) extends only to anterior limit of neural complex. Reversal of synapomorphy 5 of node 205. Paralleled in node 272 and in Bramocharax bransfordii, Deuterodon iguape, and Lonchogenys ilisha.
2. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 294 and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
Node 290: (44 / 89 / 22 / 4)
Genera Petitella? and Thayeria; Hemigrammus bleheri, H. rhodostomus?, other Hemigrammus?
The relationship between Hemigrammus bleheri and Thayeria was not proposed prior to Mirande (2009). As noted above, the species of Thayeria should be transferred to Hemigrammus under the present hypothesis of relationships. Petitella georgiae and Hemigrammus rhodostomus share with H. bleheri a presumably derived coloration; an intense red head and the caudal fin transversed by black and white markings. These species are, therefore, tentatively included in this clade. The monotypic genus Petitella was not analyzed here; however, if it were included in this node it should also be transferred to Hemigrammus.
Synapomorphies:
1. Position of coronomeckelian (110): (0 > 1) situated mainly dorsal to Meckelian cartilage. Reversal of synapomorphy 2 of node 206. Paralleled in nodes 200 and 282 and in Hemigrammus erythrozonus.
2. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in the Astyanax clade, in node 284, and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
3. Number of branched anal-fin rays (287): (1 > 0) 17 or less. Reversal of synapomorphy 6 of the Characoidea. Paralleled in the Alestidae, in node 280, and in Attonitus ephimeros and Prodontocharax melanotus. Some trees: Paralleled in Hasemania nana.
Autapomorphy of Hemigrammus bleheri:
1. Form of teeth of inner premaxillary tooth row (128): (0 > 1) with cusps aligned in straight series and without anterior concavity. Paralleled in the Rhoadsiinae, in nodes 195, 245, and 280, and in Odontostoechus lethostigmus.
Node 301: (100 / 100 / 92 / 43)
Genus Thayeria.
As previously exposed, according to the hypothesis of relationships proposed herein, Thayeria should be transferred to Hemigrammus.
Synapomorphies:
1. Base of second pectoral ray (231): (1 > 0) large and partially overlapping base of first pectoral ray from medial view. Paralleled in node 164 and in Moenkhausia dichroura, Prionobrama paraguayensis, and Tetragonopterus argenteus.
2. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. Paralleled in nodes 162 and 285 and in Aulixidens eugeniae, Creagrutus anary, Diapoma speculiferum, Moenkhausia cf. intermedia, and Pyrrhulina australis. Some trees: Paralleled in node 247 and in Knodus breviceps.
3. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, and 297, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
4. Color of caudal-fin lobes (345): (0 > 2) ventral lobe dark brown or black and dorsal lobe hyaline. Paralleled in Hemiodus cf. thayeria.
Autapomorphies of Thayeria obliqua:
1. Form of anterior expansion of basihyal (191): (0 > 1) expanded, with anterior margin with two-thirds or more of its length. Paralleled in Aulixidens eugeniae, Cyphocharax spilotus, and Prodontocharax melanotus.
2. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. Paralleled in node 302, and in Agoniates anchovia, Cyanocharax alburnus, Deuterodon langei, Engraulisoma taeniatum, Hemiodus cf. thayeria, and Roeboexodon geryi. Some trees: Paralleled in Microschemobrycon casiquiare and Parecbasis cyclolepis.
3. Process of scapula forming anterior border of scapular foramen (244): (0 > 1) absent. Paralleled in nodes 193 and 252 and in Aphyodite grammica, Deuterodon langei, Hoplias cf. malabaricus, Hyphessobrycon herbertaxelrodi, Leporinus striatus, and Odontostilbe paraguayensis.
4. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (1 > 0) two. Reversal of synapomorphy 4 of node 203. Paralleled in Bario steindachneri. Some trees: Paralleled in Hyphessobrycon elachys and Paracheirodon axelrodi.
Autapomorphy of Thayeria boehlkei:
1. Length of laterosensory canal of dentary (79): (0 > 1) reduced or absent. Paralleled in node 279 and in Aphyocharax nattereri, Bryconamericus rubropictus, Hyphessobrycon elachys, H. luetkenii, and Nantis cf. indefessus.
Node 286: (-4 / 45 / - / 1)
Genera Hasemania?, Hemigrammus (excepting, at least, H. bleheri and H. ulreyi), Hyphessobrycon, Paracheirodon, Petitella?, and Pristella.
Synapomorphy:
1. Distance between cartilage anterior to orbitosphenoid and lateral ethmoids (38): (0 > 1) distant from lateral ethmoids. Reversal of synapomorphy 1 of node 223. Paralleled in Moenkhausia sanctaefilomenae.
Node 292: (48 / 76 / 27 / 8)
Genus Pristella; Hemigrammus unilineatus, Hyphessobrycon amandae Géry & Uj?, H. axelrodi (Travassos)?, H. bentosi Durbin?, H. copelandi Durbin?, H. compressus?, H. ecuadoriensis Eigenmann & Henn?, H. eques, H. erythrostigma (Fowler)?, H. georgettae Géry?, H. haraldschultzi Travassos?, H. hasemani Fowler?, H. loweae Costa & Géry?, H. melasemion Fowler?, H. micropterus (Eigenmann)?, H. milleri Durbin?, H. minor Durbin?, H. panamensis Durbin?, H. pulchripinnis, H. pyrrhonotus Burgess?, H. rosaceus Durbin?, H. roseus (Géry)?, H. simulatus (Géry)?, H. socolofi, H. sweglesi (Géry)?, H. takasei Géry?, H. uruguayensis (Fowler)?, H. werneri Géry & Uj? Moenkhausia hemigrammoides Géry?, and M. pittieri Eigenmann?
The rosy tetra clade of Weitzman & Palmer (1997) is putatively composed of Hyphessobrycon axelrodi, H. bentosi, H. copelandi, H. eques, H. compressus (the type-species of Hyphessobrycon), H. ecuadoriensis, H. erythrostigma, H. georgettae, H. haraldschultzi, H. loweae, H. melasemion, H. micropterus, H. milleri, H. minor, H. panamensis, H. pyrrhonotus, H. rosaceus, H. roseus, H. simulatus, H. socolofi, H. sweglesi, H. takasei, H. uruguayensis, and H. werneri. These authors, also listed Hemigrammus unilineatus, Hyphessobrycon amandae, H. hasemani, H. pulchripinnis, Moenkhausia hemigrammoides, M. pittieri, and Pristella maxillaris as probable relatives to the rosy tetra clade. Most species of this node are included in this list of species; although the rosy tetra clade was based mainly on certain shared coloration patterns, and no phylogenetic analysis supports its monophyly, the species composing it are listed with question marks.
Synapomorphies:
1. Dark conspicuous spot on dorsal fin (343): (0 > 1) present.
Some trees:
2. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. (k13-14). Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Reversed in Hemigrammus unilineatus.
Autapomorphies of Pristella maxillaris:
1. Synchondral articulation between lateral ethmoid and anterodorsal border of orbitosphenoid (35): (1 > 0) present. Paralleled in the Aphyocharacinae and in Leporinus striatus, Mimagoniates rheocharis, and Rhaphiodon vulpinus.
2. Anterior paired projections of parasphenoid (40): (1 > 0) absent. Some trees: Paralleled in Hasemania nana.
3. Anterior region of third infraorbital (63): (0 > 1) abruptly expanded relative to posterior region of second infraorbital. Paralleled in node 295.
4. Dorsal end of laterosensory canal of preopercle and suprapreopercle (82): (0 > 1) overlapping anterodorsal process of opercle. Paralleled in the Alestidae, in node 230, and in Bario steindachneri, Hyphessobrycon eques, Parecbasis cyclolepis, and Stichonodon insignis.
5. Number of cusps of anterior maxillary teeth (138): (1 > 0) conical, a single cusp. Some trees: Paralleled in Aphyodite grammica and Axelrodia lindeae.
6. Separation between posterior dentary teeth (147): (0 > 1) more than width of these teeth. Paralleled in node 221 and in Astyanax cf. rutilus and Aulixidens eugeniae.
7. Articulation between ventral margin of metapterygoid and posterodorsal margin of quadrate (155): (0 > 1) present. Paralleled in nodes 176 and 299 and in Deuterodon langei, Heterocharax macrolepis, Roeboides descalvadensis, and Thoracocharax stellatus.
8. Form and degree of ossification of first ceratobranchial gill rakers (200): (0 > 1) rather thick and completely ossified distal region. Paralleled in nodes 176, 212, and 299 and in Hoplias cf. malabaricus and Prionobrama paraguayensis.
9. Distribution of denticles on gill rakers (202): (0 > 1) along entire surface of gill rakers. Paralleled in node 269 and in Astyanax correntinus, Deuterodon langei, Hoplias cf. malabaricus, and Hyphessobrycon eques.
10. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi (335): (1 > 0) absent. Paralleled in Creagrutus anary, Inpaichthys kerri, Piabucus melanostomus, Prionobrama paraguayensis, Prodontocharax melanotus, and Pyrrhulina australis. Some trees: Paralleled in Hyphessobrycon elachys.
Node 291: (34 / 90 / 0 / 2)
Hemigrammus unilineatus, Hyphessobrycon eques, H. pulchripinnis, H. socolofi, other Hyphessobrycon?, some Moenkhausia?
Among the analyzed species, in this node is Hemigrammus unilineatus (the type species of Hemigrammus), Hyphessobrycon eques, H. pulchripinnis, and H. socolofi. The former species and H. pulchripinnis were considered as related to the rosy tetra clade, while H. eques and H. socolofi were included in that clade by Weitzman & Palmer (1997). One of the species also included in that clade is Hyphessobrycon compressus (the type species of Hyphessobrycon; not analyzed herein). As noted by Weitzman & Palmer (1997) Hemigrammus has temporal precedence over Hyphessobrycon, and the latter genus maybe would have to be synonymized with Hemigrammus.
Synapomorphies:
Some trees:
1. Number of branched anal-fin rays (288): (0 > 1) 25 or more. (k9-12).
2. Bony hooks on first pelvic-fin ray of adult males (315): (1 > 0) absent. (k9-12). Reversal of synapomorphy 1 of node 274.
3. Insertion of pterotic aponeurosis (340): (0 > 1) on a lobe situated dorsal to horizontal semicircular canal. (k13-14). Paralleled in Hyphessobrycon bifasciatus.
Autapomorphies of Hyphessobrycon pulchripinnis:
1. Contact between frontals anteriorly to frontal fontanel (21): (0 > 1) present. Reversal of synapomorphy 4 of node 206. Paralleled in Bario steindachneri, Exodon paradoxus, Galeocharax humeralis, and Odontostoechus lethostigmus. Some trees: Paralleled in Knodus breviceps.
2. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae and Gasteropelecidae, in node 186, and in Aphyodite grammica, Hasemania nana, Hemigrammus erythrozonus, Hoplocharax goethei, and Nematobrycon palmeri.
3. Lateral coverage of dilator fossa by sixth infraorbital (69): (1 > 0) almost complete, at least in its ventral border. Paralleled in Stethaprion erythrops.
4. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273, 283, and 294, and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
5. Relative length of palatine (172): (0 > 1) distinctly longer than one-half length of ectopterygoid. Paralleled in nodes 197, 261, and 302 and in Paracheirodon axelrodi.
6. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. Paralleled in the Characinae and in Bryconops melanurus, Iguanodectes geisleri, and Moenkhausia sanctaefilomenae. Some trees: Paralleled in node 196 and in Hemigrammus erythrozonus and Hyphessobrycon herbertaxelrodi.
Autapomorphies of Hemigrammus unilineatus:
1. Number of supraneurals (280): (0 > 1) five or more. Reversal of synapomorphy 2 of node 223. Some trees: Paralleled in Hasemania nana.
Some trees:
2. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. (k13-14). Reversal of synapomorphy 2 of node 292.
3. Length of ascending process of premaxilla (104): (1 > 0) reaching at least one-third of length of nasal. (k13-14).
4. Number of 2n chromosomes (363): (0 > 1) 52 or more. (k9-12). Paralleled in the Characinae and Serrasalmidae, in node 196, and in Chalceus macrolepidotus, Hyphessobrycon herbertaxelrodi, Markiana nigripinnis, and Rhaphiodon vulpinus.
Node 295: (100 / 100 / 73 / 18)
Hyphessobrycon eques, H. socolofi, other Hyphessobrycon?, some Moenkhausia?
Synapomorphies:
1. Anterior region of third infraorbital (63): (0 > 1) abruptly expanded relative to posterior region of second infraorbital. Paralleled in Pristella maxillaris.
Some trees:
2. Posterior margin of cleithrum (234): (1 > 0) without concavity ventral to first postcleithrum. (k9-12). Reversal of synapomorphy 2 of node 289.
3. Proximal and medial radials of anal fins (294): (0 > 1) fused in most pterygiophores. (k9-12). Paralleled in nodes 184, 208, 218, and 221 and in Psellogrammus kennedyi and Pseudocorynopoma doriae.
4. Position of anal-fin bony hooks of adult males (316): (0 > 2) asymmetrically disposed and irregularly arranged. (k13-14). Paralleled in Hyphessobrycon luetkenii.
5. Pseudotympanum limited by first pleural rib, lateralis superficialis, second pleural rib, obliquus inferioris, and obliquus superioris (339): (0 > 1) present. (k9-12). Paralleled in node 234 and in Characidium rachovii.
Autapomorphies of Hyphessobrycon socolofi:
1. Relative size of frontal and parietal fontanels (23): (1 > 0) length of frontal fontanel up to 2/3 length of parietal fontanel. Some trees: Paralleled in Hyphessobrycon elachys.
2. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax latens, A. cf. rutilus, A. pelegrini, Hoplias cf. malabaricus, Moenkhausia dichroura, Parecbasis cyclolepis, Piaractus mesopotamicus, and Stichonodon insignis.
3. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus cf. iheringii, B. rubropictus, Hyphessobrycon luetkenii, and Phenacogaster tegatus.
4. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, Nematocharax venustus, and Probolodus heterostomus.
5. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in node 268 and in Acrobrycon tarijae, Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Hyphessobrycon luetkenii, and Probolodus heterostomus.
Autapomorphies of Hyphessobrycon eques:
1. Dorsal end of laterosensory canal of preopercle and suprapreopercle (82): (0 > 1) overlapping anterodorsal process of opercle. Paralleled in the Alestidae, in node 230, and in Bario steindachneri, Parecbasis cyclolepis, Pristella maxillaris, and Stichonodon insignis.
2. Foramen on articular condyle of quadrate (149): (0 > 1) present. Paralleled in nodes 168, 211, and 231 and in Grundulus cochae, Hasemania nana, and Lonchogenys ilisha.
3. Distribution of denticles on gill rakers (202): (0 > 1) along entire surface of gill rakers. Paralleled in node 269 and in Astyanax correntinus, Deuterodon langei, Hoplias cf. malabaricus, and Pristella maxillaris.
4. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent. Paralleled in Creagrutus anary, Hyphessobrycon luetkenii, Phenacogaster tegatus, Pseudocorynopoma doriae, and Stethaprion erythrops. Some trees: Paralleled in Markiana nigripinnis and Psellogrammus kennedyi.
Node 285: (-2 / 53 / - / 1)
Genus Paracheirodon; Hasemania nana, other Hasemania?, Hemigrammus erythrozonus, other Hemigrammus?, Hyphessobrycon elachys, H. herbertaxelrodi, other Hyphessobrycon?
Synapomorphies:
1. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. Paralleled in nodes 162 and 301 and in Aulixidens eugeniae, Creagrutus anary, Diapoma speculiferum, Moenkhausia cf. intermedia, and Pyrrhulina australis. Some trees: Paralleled in node 247 and in Knodus breviceps.
Some trees:
2. Scales covering caudal-fin lobes (328): (1 > 0) covering only their base. (k9-12). Reversal of synapomorphy 2 of node 222.
Autapomorphies of Paracheirodon axelrodi:
1. Form of mesethmoid spine (27): (0 > 1) relatively short, with premaxillae articulating with each other anterior to mesethmoid. Paralleled in nodes 225 and 234 and in Chalceus macrolepidotus.
2. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Aulixidens eugeniae, Carlana eigenmanni, Carnegiella strigata, Grundulus cochae, Odontostoechus lethostigmus, Piabucus melanostomus, and Probolodus heterostomus.
3. Relative length of palatine (172): (0 > 1) distinctly longer than one-half length of ectopterygoid. Paralleled in nodes 197, 261, and 302 and in Hyphessobrycon pulchripinnis.
4. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in node 296 and in Axelrodia lindeae, Gymnocharacinus bergii, Hollandichthys multifasciatus, Mimagoniates rheocharis, Nematocharax venustus, and Prodontocharax melanotus.
5. Number of unbranched anal-fin rays (285): (1 > 0) three or fewer. Paralleled in node 252 and in Iguanodectes geisleri.
Some trees:
6. Anterior articulation of epioccipital bridge (6): (0 > 1) only with parietal. (k12-14). Paralleled in Grundulus cochae.
7. Expansion of lamellar portion of maxilla just posterior to toothed region (97): (0 > 1) very pronounced. (k12-14). Paralleled in node 232 and in Deuterodon langei.
8. Number of maxillary teeth (135): (1 > 0) only one, or absent. (k12-14). Paralleled in the Astyanax clade, in nodes 284 and 290, and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana and Parecbasis cyclolepis.
9. Transitional vertebrae with haemal canal (229): (1 > 0) present. (k9-11). Paralleled in nodes 195 and 212, and in Aulixidens eugeniae, Engraulisoma taeniatum, Metynnis maculatus, and Piabina argentea. Some trees: Paralleled in node 247 and in Bryconamericus alpha.
10. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (1 > 0) two. (k12-14). Reversal of synapomorphy 4 of node 203. Paralleled in Bario steindachneri and Thayeria obliqua. Some trees: Paralleled in Hyphessobrycon elachys.
11. Insertion of pterotic aponeurosis (340): (0 > 2) on pterotic or sphenotic, distinctly dorsal to horizontal semicircular canal. (k12-14). Some trees: Paralleled in Hyphessobrycon elachys.
Autapomorphies of Hyphessobrycon herbertaxelrodi:
1. Number of maxillary teeth (136): (0 > 1) four or more.
2. Abrupt decrease in size of dentary teeth (148): (0 > 1) present.
3. Ethmopalatine cartilage (171): (1 > 0) absent or reduced in size.
4. Process of scapula forming anterior border of scapular foramen (244): (0 > 1) absent. Paralleled in nodes 193 and 252 and in Aphyodite grammica, Deuterodon langei, Hoplias cf. malabaricus, Leporinus striatus, Odontostilbe paraguayensis, and Thayeria obliqua.
5. Number of 2n chromosomes (363): (0 > 1) 52 or more. Paralleled in the Characinae and Serrasalmidae, in node 196, and in Chalceus macrolepidotus, Hyphessobrycon herbertaxelrodi, Markiana nigripinnis, and Rhaphiodon vulpinus. Some trees: Paralleled in Hemigrammus unilineatus.
Some trees:
6. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. (k12-14). Paralleled in the Characinae and in Bryconops melanurus, Hyphessobrycon pulchripinnis, Iguanodectes geisleri, and Moenkhausia sanctaefilomenae. Some trees: Paralleled in node 196 and in Hemigrammus erythrozonus.
7. Denticles on gill rakers (201): (0 > 1) absent. (k9-11). Paralleled in the Gymnocharacinae, in nodes 245 and 253, and in Axelrodia lindeae and Pseudochalceus kyburzi. Some trees: Paralleled in Hyphessobrycon elachys.
Autapomorphies of Hyphessobrycon elachys:
1. Nasal (33): (0 > 1) absent. Paralleled in Coptobrycon bilineatus.
2. Length of laterosensory canal of dentary (79): (0 > 1) reduced or absent. Paralleled in node 279 and in Aphyocharax nattereri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, Nantis cf. indefessus, and Thayeria boehlkei.
3. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
4. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two. Paralleled in nodes 210, 225, 276, and 297 and in Brycon orbignyanus, Hoplocharax goethei, and Rhaphiodon vulpinus.
5. Anterior rays of dorsal fin of adult males (268): (0 > 1) elongate and reaching posteriorly to position close to adipose fin. Paralleled in Pseudocorynopoma doriae.
Some trees:
6. Relative size of frontal and parietal fontanels (23): (1 > 0) length of frontal fontanel up to 2/3 length of parietal fontanel. (k9-11). Paralleled in Hyphessobrycon socolofi.
7. Maxillary teeth (134): (1 > 0) absent. (k9-11). Paralleled in Aulixidens eugeniae, Coptobrycon bilineatus, Iguanodectes geisleri, Parecbasis cyclolepis, and Stichonodon insignis. Some trees: Paralleled in Psellogrammus kennedyi.
8. Denticles on gill rakers (201): (0 > 1) absent. (k9-11). Paralleled in the Gymnocharacinae, in nodes 245 and 253, and in Axelrodia lindeae and Pseudochalceus kyburzi. Some trees: Paralleled in Hyphessobrycon herbertaxelrodi.
9. Number of branched pelvic-fin rays (258): (1 > 0) six or less. (k9-11). Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana.
10. Dorsal-fin rays articulating with first dorsal pterygiophore (266): (1 > 0) two. (k12-14). Reversal of synapomorphy 4 of node 203. Paralleled in Bario steindachneri and Thayeria obliqua. Some trees: Paralleled in Paracheirodon axelrodi.
11. Bony hooks on fin rays (307): (1 > 0) absent. (k9-11). Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana.
12. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi (335): (1 > 0) absent. (k12-14). Paralleled in Creagrutus anary, Inpaichthys kerri, Piabucus melanostomus, Prionobrama paraguayensis, Pristella maxillaris, Prodontocharax melanotus, and Pyrrhulina australis.
13. Insertion of pterotic aponeurosis (340): (0 > 2) on pterotic or sphenotic, distinctly dorsal to horizontal semicircular canal. (k12-14). Some trees: Paralleled in Paracheirodon axelrodi.
Autapomorphies of Hemigrammus erythrozonus:
1. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae and Gasteropelecidae, in node 186, and in Aphyodite grammica, Hasemania nana, Hoplocharax goethei, Hyphessobrycon pulchripinnis, and Nematobrycon palmeri.
2. Position of coronomeckelian (110): (0 > 1) situated mainly dorsal to Meckelian cartilage. Reversal of synapomorphy 2 of node 206. Paralleled in nodes 200, 282, and 290.
Some trees:
3. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. (k12-14). Paralleled in the Characinae and in Bryconops melanurus, Hyphessobrycon pulchripinnis, Iguanodectes geisleri, and Moenkhausia sanctaefilomenae. Some trees: Paralleled in node 196 and in Hyphessobrycon herbertaxelrodi.
Autapomorphies of Hasemania nana:
1. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
2. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae and Gasteropelecidae, in node 186, and in Aphyodite grammica, Hemigrammus erythrozonus, Hoplocharax goethei, Hyphessobrycon pulchripinnis, and Nematobrycon palmeri.
3. Foramen on articular condyle of quadrate (149): (0 > 1) present. Paralleled in nodes 168, 211, and 231 and in Grundulus cochae, Hyphessobrycon eques, and Lonchogenys ilisha.
4. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. Paralleled in nodes 244 and 299 and in Hyphessobrycon bifasciatus, Metynnis maculatus, Odontostilbe microcephala, and Roeboides descalvadensis. Some trees: Paralleled in node 265.
5. Posterior margin of cleithrum (234): (1 > 0) without concavity ventral to first postcleithrum.
6. Number of rays on last anal pterygiophore (291): (0 > 1) one. Paralleled in Coptobrycon bilineatus.
Some trees:
7. Anterior paired projections of parasphenoid (40): (1 > 0) absent. (k9-11). Paralleled in Pristella maxillaris.
8. Number of maxillary teeth (135): (1 > 0) only one, or absent. (k9-11). Paralleled in the Astyanax clade, in nodes 284 and 290, and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Paracheirodon axelrodi and Parecbasis cyclolepis.
9. Number of branched pelvic-fin rays (258): (1 > 0) six or less. (k9-11). Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hyphessobrycon elachys.
10. Number of supraneurals (280): (0 > 1) five or more. (k9-11). Reversal of synapomorphy 2 of node 223. Paralleled in Hemigrammus unilineatus.
11. Number of branched anal-fin rays (287): (1 > 0) 17 or less. (k9-11). Reversal of synapomorphy 6 of the Characoidea. Paralleled in the Alestidae, in nodes 280 and 290, and in Attonitus ephimeros and Prodontocharax melanotus.
12. Bony hooks on fin rays (307): (1 > 0) absent. (k9-11). Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hyphessobrycon elachys.
Node 201: (-9 / 82 / - / 1)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, and Stevardiinae; Astyanax clade, Astyanax paris clade, Bryconamericus scleroparius clade, and Hyphessobrycon anisitsi clade.
This node is incongruent with the hypothesis of Mirande (2009). In that hypothesis the Astyanax clade included the Astyanax paris, Bryconamericus scleroparius, and Hyphessobrycon anisitsi clades, as proposed herein. Also the relative position of the Bramocharax clade of Mirande (2009) is different than in the present hypothesis.
Synapomorphies:
1. Dorsal expansion of rhinosphenoid (48): (1 > 0) absent. Paralleled in nodes 212 and 300 and in Agoniates anchovia.
2. Tubules for passage of blood vessels on lamellar portion of maxilla (98): (0 > 1) tubule with anterior branch running parallel to anterior margin of maxilla and reaching one third of its length. Paralleled in Tetragonopterus argenteus. Reversed in node 197. Transformed to state 2 in Markiana nigripinnis.
Node 271: Hyphessobrycon luetkenii clade (46 / 81 / - / 2)
Astyanax latens, other Astyanax?, Hyphessobrycon bifasciatus, H. luetkenii, other Hyphessobrycon?
The species in this clade were included in the Astyanax clade in the hypothesis of Mirande (2009). According to the present hypothesis a new genus should be assigned to these species, given that the type species of Astyanax (A. mexicanus) is included in the Astyanax clade, and the type species of Hyphessobrycon (H. compressus), although not herein analyzed, is presumably included in the Tetragonopterinae along with the members of the rosy tetra clade of Weitzman & Palmer (1997). This node is rather stable across the analyses done for this study, however, its position is variable, lying within the Astyanax clade in some of them. Thus, both the monophyly and position of this clade should be further tested.
Synapomorphy:
1. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
Autapomorphies of Astyanax latens:
1. Abrupt decrease in size of dentary teeth (148): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 222, 255, and 299, and in Astyanax paris.
2. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax cf. rutilus, A. pelegrini, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Parecbasis cyclolepis, Piaractus mesopotamicus, and Stichonodon insignis.
Node 294: (76 / 91 / 27 / 11)
Hyphessobrycon bifasciatus, H. luetkenii, some Astyanax?
Synapomorphies:
1. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 229, 279, and 288 and in Characidium rachovii, Hoplocharax goethei, Hyphessobrycon anisitsi, Moenkhausia sanctaefilomenae, Phenacogaster tegatus, and Pyrrhulina australis.
2. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
3. Length of ascending process of premaxilla (104): (0 > 1) reaching just anterior end of nasal. Paralleled in node 225 and in Charax stenopterus, Phenacogaster tegatus, and Stichonodon insignis. Some trees: Paralleled in Bryconamericus agna.
4. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. Paralleled in node 265 and in Brycon orbignyanus, Bryconops melanurus, Gymnocharacinus bergii, Micralestes stormsi, and Nematocharax venustus. Some trees: Paralleled in Bryconamericus agna.
5. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273 and 283, and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
Autapomorphies of Hyphessobrycon luetkenii:
1. Subtemporal fossa (18): (1 > 0) medially extended to middle exoccipital. Paralleled in node 211.
2. Length of laterosensory canal of dentary (79): (0 > 1) reduced or absent. Paralleled in node 279 and in Aphyocharax nattereri, Bryconamericus rubropictus, Hyphessobrycon elachys, Nantis cf. indefessus, and Thayeria boehlkei.
3. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, and Hoplocharax goethei. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
4. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent. Paralleled in Creagrutus anary, Hyphessobrycon eques, Phenacogaster tegatus, Pseudocorynopoma doriae, and Stethaprion erythrops. Some trees: Paralleled in Markiana nigripinnis and Psellogrammus kennedyi.
5. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus cf. iheringii, B. rubropictus, Hyphessobrycon socolofi, and Phenacogaster tegatus.
6. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon socolofi, Nematocharax venustus, and Probolodus heterostomus.
7. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in node 268 and in Acrobrycon tarijae, Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Hyphessobrycon socolofi, and Probolodus heterostomus.
8. Position of anal-fin bony hooks of adult males (316): (0 > 2) asymmetrically disposed and irregularly arranged. Some trees: Paralleled in node 295.
Autapomorphies of Hyphessobrycon bifasciatus:
1. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in the Astyanax clade, in nodes 284 and 290, and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
2. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. Paralleled in nodes 244 and 299 and in Hasemania nana, Metynnis maculatus, Odontostilbe microcephala, and Roeboides descalvadensis. Some trees: Paralleled in node 265.
3. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in nodes 211, 223, and 262, and in Bramocharax bransfordii, Bryconaethiops macrops, and Nematocharax venustus. Some trees: Paralleled in the Aphyoditeinae.
4. Insertion of pterotic aponeurosis (340): (0 > 1) on a lobe situated dorsal to horizontal semicircular canal. Some trees: Paralleled in node 291.
5. Second humeral spot (342): (0 > 1) present as a conspicuous vertical bar. Paralleled in Gymnocorymbus ternetzi and Tetragonopterus argenteus
Node 200: (-23 / 80 / - / 1)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, and Stevardiinae; Astyanax clade, Astyanax paris clade, and Bryconamericus scleroparius clade.
In the hypothesis of Mirande (2009) the Astyanax clade included the Astyanax paris and the Bryconamericus scleroparius clades, differing with the results herein obtained. As with most of the internal nodes of the distal characids, this node is rather unstable across the analyses done for this study.
Synapomorphies:
1. Form of fourth infraorbital (67): (1 > 0) approximately square or more developed longitudinally than dorsoventrally. Reversal of synapomorphy 1 of node 189. Paralleled in 210, 228, 277, and 282. Reversed in node 266. Some trees: Reversed in nodes 233 and 256.
2. Position of coronomeckelian (110): (0 > 1) situated mainly dorsal to Meckelian cartilage. Reversal of synapomorphy 2 of node 206. Paralleled in nodes 282 and 290 and in Hemigrammus erythrozonus. Reversed in node 195 and in Astyanax cf. eigenmanniorum1.
3. Number of branched anal-fin rays (288): (1 > 0) 24 or less. Reversal of synapomorphy 5 of the Characidae. Paralleled in nodes 277 and 300 and in Acestrorhynchus pantaneiro and Iguanodectes geisleri. Reversed in nodes 208, 255, and 263 and in Bryconamericus scleroparius and Nematobrycon palmeri.
Astyanax paris clade
Astyanax paris, other Astyanax?
Astyanax paris is rather dissimilar to other species of Astyanax in the lacking of secondary sexual bony hooks on the fins of adult males and the possession of several maxillary teeth. However, these features are not exclusive of this species within Astyanax, and the position of this species in a different node than the type species of the genus should be further tested. According to this results, A. paris should be transferred to a new genus.
Autapomorphies of Astyanax paris:
1. Abrupt decrease in size of dentary teeth (148): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 222, 255, and 299, and in Astyanax latens.
2. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Node 199: (-23 / 80 / - / 1)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, and Stevardiinae; Astyanax clade, and Bryconamericus scleroparius clade.
This node differs with the node 200 of Mirande (2009) by the exclusion of the herein defined Hyphessobrycon luetkenii and Astyanax paris clades and the Bramocharax and Pseudochalceus clades. This node is supported by a single synapomorphy of a moderately homoplastic character and is rather unstable across the analyses made for this paper, as most internal nodes of the distal characids.
Synapomorphy:
1. Ventral margin of horizontal process of anguloarticular (109): (0 > 1) perpendicular to laterosensory canal of dentary from medial view. Paralleled in Bario steindachneri, Bramocharax bransfordii, and Micralestes stormsi. Reversed in nodes 195, 280, and 298 and in Attonitus ephimeros.
Node 267: Astyanax clade (-9 / 95 / - / 3)
Genera Astyanax, Ctenobrycon?, Markiana Eigenmann, and Psellogrammus; Hyphessobrycon anisitsi and other Hyphessobrycon?
This node would require a subfamilial category according to the hypothesis herein proposed. However, given the relatively low taxon sampling and some variations obtained under different searches, an informal provisional name is given to this clade, pending specific contributions. According to this analysis, this clade is composed of the genera Astyanax, Markiana, and Psellogrammus, plus some species currently classified in the genus Hyphessobrycon. The internal relationships of this clade, however indicate that all species of this node should be transferred to Astyanax. According to the classical systematics of the family (Eigenmann, 1917), Hyphessobrycon differs from Astyanax only by having an interrupted lateral line. Both Hyphessobrycon anisitsi as H. bifasciatus and H. luetkenii, treated above, are not related with the rosy tetra clade nor even included in the Tetragonopterinae in the present hypothesis. Some species of Hyphessobrycon not included in this paper, such as H. auca Almirón, Casciotta, Bechara & Ruiz-Díaz and H. boulengeri (Eigenmann) share certain overall resemblances with species of this clade and the Hyphessobrycon luetkenii clade, but not sufficient to include them in some clade, even putatively. According to its traditional definition, Ctenobrycon is only distinguished from Psellogrammus by the presence of a complete lateral line and both genera share the presence of ctenoid scales (Eigenmann, 1917). Lima et al. (2003) listed Tetragonopterus correntinus Holmberg and Astyanax pelegrini as species inquirendae within Ctenobrycon. These species lack ctenoid scales, and were included in Astyanax by Mirande et al. (2006b). Both A. correntinus and A. pelegrini are included in this clade, while Ctenobrycon is listed with a question mark as a probable member of the clade.
Synapomorphy:
1. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in nodes 284 and 290 and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
Autapomorphy of Astyanax mexicanus:
1. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
Node 266: (-9 / 93 / - / 3)
Genera Astyanax (excepting, at least, A. latens, A. mexicanus, and A. paris), Ctenobrycon?, Markiana, and Psellogrammus; Hyphessobrycon anisitsi, other Hyphessobrycon?
Synapomorphy:
1. Form of fourth infraorbital (67): (0 > 1) longer dorsoventrally than longitudinally. Reversal of synapomorphy 1 node 200. Some trees: Paralleled in nodes 233 and 256. Reversed in node 260 and in Astyanax troya.
Autapomorphies of Hyphessobrycon anisitsi:
1. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 229, 279, 288, and 294 and in Characidium rachovii, Hoplocharax goethei, Moenkhausia sanctaefilomenae, Phenacogaster tegatus, and Pyrrhulina australis.
2. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Inpaichthys kerri, and Phenacogaster tegatus.
Node 265: (-9 / 91 / - / 3)
Genera Astyanax (excepting, at least, A. latens, A. mexicanus, and A. paris), Ctenobrycon?, Markiana, and Psellogrammus; some Hyphessobrycon?
Synapomorphies:
1. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. Paralleled in node 294 and in Brycon orbignyanus, Bryconops melanurus, Gymnocharacinus bergii, Micralestes stormsi, and Nematocharax venustus. Some trees: Paralleled in Bryconamericus agna. Reversed in node 261.
Some trees:
2. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. (some trees under k9-14). Paralleled in nodes 244 and 299 and in Hasemania nana, Hyphessobrycon bifasciatus, Metynnis maculatus, Odontostilbe microcephala, and Roeboides descalvadensis.
No autapomorphies found for Astyanax cf. eigenmanniorum2.
Node 270: (76 / 93 / 28 / 23)
Astyanax chico, A. endy, A. puka, A. troya, other Astyanax?, some Hyphessobrycon?
Synapomorphy:
1. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 184, 186, 209, 241, and 261, and in Engraulisoma taeniatum and Gymnocharacinus bergii.
No autapomorphies found for Astyanax endy.
Node 269: (76 / 96 / 26 / 100)
Astyanax chico, A. puka, A. troya, other Astyanax?, some Hyphessobrycon?
Synapomorphy:
1. Distribution of denticles on gill rakers (202): (0 > 1) along entire surface of gill rakers. Paralleled in Astyanax correntinus, Deuterodon langei, Hoplias cf. malabaricus, Hyphessobrycon eques, and Pristella maxillaris.
No autapomorphies found for Astyanax puka.
Node 268: (97 / 99 / 83 / 19)
Astyanax chico, A. troya, other Astyanax?, some Hyphessobrycon?
The presence of bony hooks on all fins was cited in the literature for some species of Astyanax, including A. chico, A. elachylepis Bertaco & Lucinda, A. hermosus Miquelarena, Protogino & López, A. ojiara Azpelicueta & García, A. pynandi Casciotta, Almirón, Bechara, Roux & Ruiz-Díaz, A. stenohalinus Messner, and A. troya (Azpelicueta & García, 2000; Azpelicueta et al., 2002; Bertaco & Lucinda 2005; Casciotta et al., 2003; Casciotta & Almirón, 2004; Miquelarena et al., 2005). The presence of hooks on all the fins excepting the pectorals was cited for A. tumbayaensis Miquelarena & Menni by Miquelarena & Menni (2005). The presence of bony hooks on all the fins excepting the caudal was similarly mentioned for Hyphessobrycon hamatus Bertaco & Malabarba (Bertaco & Malabarba, 2005). The development or even the presence or absence of bony hooks on the fins could be variable across the year during different reproductive stages. Thus, the characters supporting this clade as a monophyletic unit must be further evaluated across samples collected throughout the year.
Synapomorphies:
1. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus cf. iheringii, B. rubropictus, Hyphessobrycon luetkenii, H. socolofi, and Phenacogaster tegatus.
2. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in node 248 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, Nematocharax venustus, and Probolodus heterostomus.
3. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in Acrobrycon tarijae, Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Hyphessobrycon luetkenii, H. socolofi, and Probolodus heterostomus.
Autapomorphy of Astyanax troya:
1. Form of fourth infraorbital (67): (1 > 0) approximately square or more developed longitudinally than dorsoventrally. Reversal of synapomorphy 1 of node 266. Paralleled in node 260.
No autapomorphies found for Astyanax chico.
Node 264: (-6 / 87 / - / 3)
Genera Ctenobrycon?, Markiana, and Psellogrammus; Astyanax cf. abramis, A. asuncionensis, A. correntinus, A. cf. eigenmanniorum, A. lineatus, A. pelegrini, A. cf. rutilus, other Astyanax?, some Hyphessobrycon?
Synapomorphy:
1. Relative size of frontal and parietal fontanels (23): (0 > 1) length of frontal fontanel 3/4 or more of length of parietal fontanel. Reversal of synapomorphy 1 of node 203. Reversed in node 261.
Autapomorphy of Astyanax cf. eigenmanniorum1:
1. Position of coronomeckelian (110): (1 > 0) situated mainly lateral to Meckelian cartilage. Reversal of synapomorphy 2. Paralleled in node 195.
Node 263: (-6 / 90 / - / 3)
Genera Ctenobrycon?, Markiana, and Psellogrammus; Astyanax cf. abramis, A. asuncionensis, A. correntinus, A. lineatus, A. pelegrini, A. cf. rutilus, other Astyanax?, some Hyphessobrycon?
Synapomorphy:
1. Number of branched anal-fin rays (288): (0 > 1) 25 or more. Reversal of synapomorphy 3 of node 200. Paralleled in nodes 208 and 255 and in Bryconamericus scleroparius and Nematobrycon palmeri.
Autapomorphies of Astyanax cf. rutilus:
1. Separation between posterior dentary teeth (147): (0 > 1) more than width of these teeth. Paralleled in node 221 and in Aulixidens eugeniae and Pristella maxillaris.
2. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
3. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax latens, A. pelegrini, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Parecbasis cyclolepis, Piaractus mesopotamicus, and Stichonodon insignis.
Node 262: (-2 / 94 / 21 / 4)
Genera Ctenobrycon?, Markiana, and Psellogrammus; Astyanax cf. abramis, A. asuncionensis, A. correntinus, A. lineatus, A. pelegrini, other Astyanax?
Eigenmann (1921) defined three subgenera in Astyanax, based on differences in squamation. The subgenus Poecilurichthys Gill included those species with irregularly arranged predorsal scales. Astyanax abramis, A. asuncionensis (as A. bimaculatus paraguayensis Eigenmann), A. correntinus, and A. pelegrini are among the species listed for this subgenus by Eigenmann (1921). All these species are contained in this node, along with Astyanax lineatus (included in the subgenus Astyanax by Eigenmann, 1921), Markiana nigripinnis, and Psellogrammus kennedyi. The subgenus Poecilurichthys could be redefined as containing the species of this node; however, the topology of the Astyanax clade does not justify such subgeneric division.
Synapomorphies:
1. Alignment of cusps of medial teeth on inner premaxillary row (127): (1 > 0) forming anteriorly concave semicircle from ventral view. Paralleled in node 183 and in Moenkhausia dichroura. Some trees: Paralleled in node 302. Reversed in Markiana nigripinnis.
2. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in nodes 211 and 223, and in Bramocharax bransfordii, Bryconaethiops macrops, Hyphessobrycon bifasciatus, and Nematocharax venustus. Some trees: Paralleled in the Aphyoditeinae. Reversed in Markiana nigripinnis.
3. Papillae on tongue (357): (0 > 1) forming longitudinal rows anteriorly.
Autapomorphy of Astyanax correntinus:
1. Distribution of denticles on gill rakers (202): (0 > 1) along entire surface of gill rakers. Paralleled in node 269 and in Deuterodon langei, Hoplias cf. malabaricus, Hyphessobrycon eques, and Pristella maxillaris.
Node 261: (-11 / 93 / 6 / 4)
Genera Ctenobrycon?, Markiana, and Psellogrammus; Astyanax cf. abramis, A. asuncionensis, A. lineatus, A. pelegrini, other Astyanax?
Synapomorphies:
1. Relative size of frontal and parietal fontanels (23): (1 > 0) length of frontal fontanel up to 2/3 length of parietal fontanel. Reversal of synapomorphy 1 of node 264.
2. Horizontal process of anguloarticular (108): (0 > 1) broadly covered by dentary which reaches posterior border of Meckelian cartilage. Reversal of synapomorphy 1 of node 206. Paralleled in nodes 246 and 253 and in Xenagoniates bondi.
3. Cusps of teeth on outer premaxillary row (125): (1 > 0) one to three cusps. Reversal of synapomorphy 1 of node 265.
4. Relative length of palatine (172): (0 > 1) distinctly longer than one-half length of ectopterygoid. Paralleled in nodes 197 and 302 and in Hyphessobrycon pulchripinnis and Paracheirodon axelrodi. Reversed in node 259.
5.Circulii on posterior field of scales (319): (1 > 0) present. Reversal of synapomorphy 3 of node 206. Paralleled in Exodon paradoxus, Phenagoniates macrolepis, and Roeboides microlepis. Reversed in Markiana nigripinnis.
6. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 184, 186, 209, 241, and 270, and in Engraulisoma taeniatum and Gymnocharacinus bergii.
Some trees:
7. Number of branched anal-fin rays (289): (0 > 1) 35 or more. (some trees under k9-14). Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in Markiana nigripinnis.
Autapomorphies of Psellogrammus kennedyi:
1. Length of supraoccipital spine (52): (1 > 0) extends dorsal of entire neural complex of Weberian apparatus. Paralleled in nodes 214 and 221.
2. Lateral coverage of dilator fossa by sixth infraorbital (69): (0 > 1) leaving a conspicuous naked area in anterior region of dilator fossa. Paralleled in the Iguanodetinae, in node 197, and in Charax stenopterus, Hoplocharax goethei, and Phenacogaster tegatus.
3. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
4. Proximal and medial radials of anal fins (294): (0 > 1) fused in most pterygiophores. Paralleled in nodes 184, 208, 218, and 221 and in Pseudocorynopoma doriae. Some trees: Paralleled in node 295.
5. Scales (317): (0 > 3) crenate.
Some trees:
6. Maxillary teeth (134): (1 > 0) absent. (some trees under k9-14). Paralleled in Aulixidens eugeniae, Coptobrycon bilineatus, Iguanodectes geisleri, Parecbasis cyclolepis, and Stichonodon insignis. Some trees: Paralleled in Hyphessobrycon elachys.
7. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent. (some trees under k9-14). Paralleled in Creagrutus anary, Hyphessobrycon eques, H. luetkenii, Phenacogaster tegatus, Pseudocorynopoma doriae, and Stethaprion erythrops. Some trees: Paralleled in Markiana nigripinnis.
Autapomorphies of Astyanax pelegrini:
1. Length of caudal-fin canal of lateral line (93): (0 > 1) almost reaching posterior margin of caudal fin. Paralleled in nodes 177 and 216 and in Tetragonopterus argenteus.
2. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax latens, A. cf. rutilus, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Parecbasis cyclolepis, Piaractus mesopotamicus, and Stichonodon insignis.
Node 260: (-37 / 87 / - / 7)
Genus Markiana; Astyanax cf. abramis, A. asuncionensis, A. lineatus, other Astyanax?
Although a close relationship between Bryconamericus scleroparius and Markiana nigripinnis is not supported in the present hypothesis, these species, despite having much different overall morphologies, share the absence of an ossified rhinosphenoid, an overlap of the horizontal arm of the preopercle by the third infraorbital, the possession of only four teeth on the inner premaxillary row, and the presence of two uroneurals. These characters determine that these two species constitute a monophyletic clade in the analyses performed under self-weighted optimization both in Mirande (2009: fig. 4) and in several analyses performed for the present study. The variable relative positions of B. scleroparius and Markiana contributes to the low stability of this node and most of the remaining nodes of the Astyanax clade.
Synapomorphies:
1. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
2. Form of fourth infraorbital (67): (1 > 0) approximately square or more developed longitudinally than dorsoventrally. Reversal of synapomorphy 1 of node 266. Paralleled in Astyanax troya. Some trees: Reversed in A. asuncionensis and A. cf. asuncionensis.
3. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 167, 177, 218, and 276, and in Bryconamericus scleroparius, Chalceus macrolepidotus, and Piaractus mesopotamicus.
Autapomorphies of Markiana nigripinnis:
1. Lateral opening between ventral diverging lamellae of mesethmoid and anterior process of lateral ethmoid (15): (0 > 1) small, ovate and partially occluded by diverging lamellae of mesethmoid and anterior process of lateral ethmoid. Paralleled in Roeboexodon geryi and Roeboides descalvadensis.
2. Form of orbitosphenoid (37): (0 > 1) massive, almost reaching parasphenoid ventrally. Reversal of synapomorphy 1 of the Characidae. Paralleled in node 193 and in Rhaphiodon vulpinus and Roeboides microlepis.
3. Dorsolateral processes of vomer (54): (0 > 1) present. Paralleled in nodes 215 and 218 and in Bryconexodon juruenae.
4. Articulation between second and third infraorbitals (62): (0 > 1) anteroventrally angled. Paralleled in node 196 and in Pyrrhulina australis.
5. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 198, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in node 292.
6. Laterosensory canal of sixth infraorbital (76): (0 > 1) branched. Reversal of synapomorphy 2 of node 203. Paralleled in Odontostilbe microcephala, Oligosarcus cf. jenynsii, and Tetragonopterus argenteus.
7. Lateral surface of vertical canal of preopercle (81): (0 > 1) covered by musculature and/or infraorbitals. Reversal of synapomorphy 2 of node 204.
8. Tubules for passage of blood vessels on lamellar portion of maxilla (98): (1 > 2) anastomosed tubules.
9. Alignment of cusps of medial teeth on inner premaxillary row (127): (0 > 1) forming shallow arch or aligned in straight series from ventral view. Reversal of synapomorphy 1 of node 262.
10. Number of teeth in inner premaxillary row (129): (1 > 0) four or fewer. Paralleled in node 198 and in Probolodus heterostomus.
11. Suprapreopercle (175): (0 > 1) autogenous, separated from preopercle. Paralleled in nodes 210 and 302 and in Roeboides microlepis and Rhaphiodon vulpinus.
12. Bony lamellae bordering laterosensory canal of suprapreopercle (176): (0 > 1) present. Paralleled in node 300.
13. Position of ventral end of posttemporal (253): (0 > 1) posterior to lateral margin of epioccipital. Paralleled in nodes 211 and 228 and in Bryconamericus scleroparius, Hoplias cf. malabaricus, Probolodus heterostomus, and Prochilodus lineatus.
14. Ventral exit of laterosensory canal of supracleithrum (254): (1 > 0) covered by posterior lamella of supracleithrum and exiting medially. Reversal of synapomorphy 14 of node 205. Paralleled in node 193.
15. Number of supraneurals (280): (0 > 1) five or more. Reversal of synapomorphy 2 of node 262.
16. Anterior notch on first anal pterygiophore (292): (0 > 1) present. Paralleled in node 162.
17. Uroneurals (306): (0 > 1) two pairs. Reversal of synapomorphy 15 of node 205. Paralleled in the Tetragonopterinae, in nodes 276 and 300, and in Bryconamericus scleroparius and Galeocharax humeralis.
18. Anterior margin of scales (318): (0 > 1) with conspicuous undulations. Paralleled in Tetragonopterus argenteus.
19.Circulii on posterior field of scales (319): (0 > 1) absent. Reversal of synapomorphy 5 of node 261.
20.Radii on scales (320): (1 > 0) absent or reduced in number. Paralleled in the Iguanodectinae and Serrasalminae, in node 174, and in Cyphocharax spilotus, Distichodus maculatus, and Phenagoniates macrolepis.
21. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in nodes 210 and 221, and in Bario steindachneri, Paragoniates alburnus, Rhaphiodon vulpinus, Roeboides microlepis, and Thoracocharax stellatus.
22. Scales covering caudal-fin lobes (328): (0 > 1) covering one-third of their length. Paralleled in node 222 and in Aulixidens eugeniae, Distichodus maculatus, and Nematocharax venustus. Some trees: Paralleled in Knodus breviceps.
23. Anterior extension of adductor arcus palatini (336): (1 > 0) covering most of dorsal surface of mesopterygoid. Paralleled in node 166 and in Creagrutus anary and Salminus brasiliensis. Some trees: Paralleled in Brycon orbignyanus.
24. Number of 2n chromosomes (363): (0 > 1) 52 or more. Paralleled in the Characinae and Serrasalmidae, in node 196, and in Chalceus macrolepidotus, Hyphessobrycon herbertaxelrodi, and Rhaphiodon vulpinus. Some trees: Paralleled in Hemigrammus unilineatus.
Some trees:
25. Number of branched anal-fin rays (289): (0 > 1) 35 or more. (some trees under k9-14). Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261.
26. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent.(some trees under k9-14). Paralleled in Creagrutus anary, Hyphessobrycon eques, H. luetkenii, Phenacogaster tegatus, Pseudocorynopoma doriae, and Stethaprion erythrops. Some trees: Paralleled in Psellogrammus kennedyi.
Autapomorphies of Astyanax lineatus:
1. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, Bario steindachneri, Bryconamericus cf. iheringii, B. rubropictus, Hyphessobrycon luetkenii, H. socolofi, and Phenacogaster tegatus.
2. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax cf. asuncionensis, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, Nematocharax venustus, and Probolodus heterostomus.
3. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in node 268 and in Acrobrycon tarijae, Astyanax cf. asuncionensis, Bario steindachneri, Hyphessobrycon luetkenii, H. socolofi, and Probolodus heterostomus.
Some trees:
4. Maxillary teeth (134): (0 > 1) present. (some trees under k 9-14).
Node 259: (100 / 100 / 44 / 32)
Astyanax abramis, A. argyrimarginatus Garutti?, A. asuncionensis, A. bimaculatus (Linnaeus)?, A. goyacensis Eigenmann?, A. lacustris (Lütken)?, A. maculisquamis Garutti & Britski?, A. orthodus Eigenmann?, A. paraguayensis (Fowler)?, A. saltor Travassos?, A. superbus Myers?, A. validus Géry, Planquette & Le Bail?, other Astyanax?
This clade corresponds to the Astyanax bimaculatus group of Garutti (1999), which is characterized by a rounded or ovate humeral spot and a peduncular spot that projects posteriorly over the median caudal-fin rays. The monophyly of this group was not tested, although the form of such a humeral spot is infrequent in the Characidae. Thus, the species included in this group by Garutti (1999) are listed with question marks pending specific studies.
Synapomorphies:
1. Relative length of palatine (172): (1 > 0) approximately one-half length of ectopterygoid, or less. Reversal of synapomorphy 4 of node 261.
2. Humeral spot (341): (0 > 1) horizontally-ovate. Paralleled in Acestrorhynchus pantaneiro, Brycon orbignyanus, Jupiaba mucronata, and Roeboides microlepis. Reversal of synapomorphy 4 of node 261.
3. Little spot on each scale of flanks (347): (0 > 1) present.
Some trees:
4. Sclerotic bones (350): (0 > 1) two bones separated by cartilages. (some trees under k 9-14). Paralleled in nodes 208, 210, 221, and 250.
Autapomorphy of Astyanax asuncionensis:
Some trees:
1. Form of fourth infraorbital (67): (0 > 1) longer dorsoventrally than longitudinally. Reversal of synapomorphy 2 of node 260. (some trees under k9-14). Paralleled in Astyanax cf. asuncionensis.
No autapomorphies found for Astyanax cf. abramis.
Node 258: (-11 / 72 / - / 7)
Astyanax abramis, A. cf. asuncionensis, other Astyanax?
Synapomorphy:
1. Bony hooks on last pelvic-fin ray of adult males (314): (0 > 1) as numerous as in other rays. Paralleled in nodes 232, 240, and 299 and in Axelrodia lindeae.
Autapomorphies of Astyanax cf. asuncionensis:
1. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax lineatus, Bario steindachneri, Bryconamericus cf. iheringii, B. rubropictus, Hyphessobrycon luetkenii, H. socolofi, and Phenacogaster tegatus.
2. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, Nematocharax venustus, and Probolodus heterostomus.
3. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in node 268 and in Acrobrycon tarijae, Astyanax lineatus, Bario steindachneri, Hyphessobrycon luetkenii, H. socolofi, and Probolodus heterostomus.
Some trees:
4. Form of fourth infraorbital (67): (0 > 1) longer dorsoventrally than longitudinally. (some trees under k9-14). Reversal of synapomorphy 2 of node 260. Paralleled in Astyanax asuncionensis.
5. Sclerotic bones (350): (1 > 0) single anteroventrally open bone. (some trees under k9-14).
Autapomorphy of Astyanax abramis:
1. Length of medial bony ridge of opercle (170): (1 > 0) 60% or greater than opercular length. Paralleled in the Serrasalmidae, in node 210, and in Creagrutus cf. taphorni, Hoplias cf. malabaricus, and Roeboides microlepis. Some trees: Paralleled in Acestrorhynchus pantaneiro and Salminus brasiliensis.
Node 198: (-27 / 94 / - / 1)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, and Stevardiinae; Bryconamericus scleroparius clade.
This node differs to the hypothesis of Mirande (2009) only in the position of Bryconamericus scleroparius, which was included in the Astyanax clade in that paper. The sister-group relationship between B. scleroparius and Markiana nigripinnis under several of the analyses performed in this study reduces the stability of this node.
Synapomorphies:
1. Dorsal margin of lateral ethmoids (16): (0 > 1) situated obliquely in dorsal view, converging in an anteriorly directed angle. Paralleled in node 162. Reversed in the Aphyoditeinae and in Nematobrycon palmeri.
2. Ventral extent of third infraorbital (64): (1 > 0) reaching horizontal arm of preopercle. Reversal of synapomorphy 3 of node 180. Paralleled in the Heterocharacinae and Iguanodectinae, in nodes 184, 225, and 302, and in Agoniates anchovia, Brycon pesu, Hasemania nana, Markiana nigripinnis, Moenkhausia sanctaefilomenae, Pseudochalceus kyburzi, Roeboexodon geryi, and Stichonodon insignis. Some trees: Reversed in nodes 280 and 298 and in Aphyodite grammica, Axelrodia lindeae, Bryconamericus rubropictus, and Creagrutus cf. taphorni.
3. Number of teeth in inner premaxillary row (129): (1 > 0) four or fewer. Paralleled in node Markiana nigripinnis and Probolodus heterostomus. Reversed in nodes 195 and 235 and in Grundulus cochae.
Bryconamericus scleroparius clade
Bryconamericus brevirostris (Günther)?, B. emperador (Eigenmann & Ogle)?, B. guaytarae Eigenmann & Henn?, B. loisae (Géry)?, B. multiradiatus Dahl?, B. peruanus (Müller & Troschel)?, B. scleroparius, B. simus (Boulenger)?, B. terrabensis Meek?, B. zeteki Hildebrand?.
Bryconamericus scleroparius was included in the Astyanax clade in the hypothesis of Mirande (2009). In this study instead this species is the sister group of a large clade of characids composed of the subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, and Stevardiinae. Bryconamericus scleroparius shares with B. brevirostris, B. emperador (with eight or nine branched dorsal-fin rays), B. peruanus, B. scopiferus guaitarae (=B. guaytarae), and B. simus (Eigenmann, 1917) the presence of nine branched dorsal-fin rays and a relatively high number of anal-fin rays. Géry (1977) included these species plus B. caucanus Eigenmann, B. loisae, B. multiradiatus, B. phoenicopterus (Cope), B. terrabensis, and B. zeteki in a Bryconamericus peruanus group, defined by the relatively high number of anal-fin rays, but not necessarily having nine branched dorsal-fin rays. Among these species, Bryconamericus caucanus and B. phoenicopterus have only eight branched dorsal-fin rays (as in true Bryconamericus), according to Eigenmann (1917), and these two species are not putatively considered in this clade. Román-Valencia (2000) mentioned that Bryconamericus dahli has between 8 and 10 branched dorsal-fin rays. Such intraspecific variation is rare among characids, but this species is also tentatively included in this clade. As Bryconamericus exodon, the type species of the genus, is included in the Stevardiinae, B. scleroparius and close relatives should be transferred to another genus. Eretmobrycon bayano Fink, transferred to Bryconamericus by Román-Valencia (2000) has also 9 branched dorsal-fin rays, at least in some individuals (Román-Valencia, 2002). Bryconamericus bayano is phylogenetically related to Bryconamericus emperador and B. scleroparius according to Román-Valencia & Vanegas-Ríos (2009). Although that hypothesis was based only in a molecular Maximum Likelihood approach and they did not propose synapomorphies relating these species, it constitute the single reference about the relationships of B. bayano. According to the results obtained herein and considering the hypothesis of Román-Valencia & Vanegas-Ríos (2009), it is possible that Eretmobrycon should have to be resurrected to include not only B. bayano but also B. scleroparius and close relatives.
Autapomorphies of Bryconamericus scleroparius:
1. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
2. Branching of laterosensory canals of fourth or fifth infraorbitals (74): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 167, 177, 218, 260, and 276, and in Chalceus macrolepidotus and Piaractus mesopotamicus.
3. Foramen in posterior region of metapterygoid (168): (1 > 2) in form of incomplete arch, bordered posteriorly by hyomandibula. Reversal of synapomorphy 10 of node 205. Paralleled in Pseudocorynopoma doriae.
4. Position of ventral end of posttemporal (253): (0 > 1) posterior to lateral margin of epioccipital. Paralleled in nodes 211 and 228 and in Hoplias cf. malabaricus, Markiana nigripinnis, Probolodus heterostomus, and Prochilodus lineatus.
5. Number of branched anal-fin rays (288): (0 > 1) 25 or more. Reversal of synapomorphy 3 of node 200. Paralleled in nodes 208, 255, and 263 and in Nematobrycon palmeri.
6. Uroneurals (306): (0 > 1) two pairs. Reversal of synapomorphy 15 of node 205. Paralleled in the Tetragonopterinae, in nodes 276 and 300, and in Galeocharax humeralis and Markiana nigripinnis.
Node 197: (16 / 95 / - / 3)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, Gymnocharacinae, and Stevardiinae.
Synapomorphies:
1. Length of sphenotic spine (10): (1 > 0) not extending ventrally to articulation between sphenotic and hyomandibula. Reversal of synapomorphy 1 of node 180. Paralleled in the Characinae and in node 176. Reversed in Creagrutus anary and Inpaichthys kerri. Some trees: Reversed in Aphyodite grammica.
2. Lateral coverage of dilator fossa by sixth infraorbital (69): (0 > 1) leaving a conspicuous naked area in anterior region of dilator fossa. Paralleled in the Iguanodetinae and in Charax stenopterus, Hoplocharax goethei, Phenacogaster tegatus, and Psellogrammus kennedyi. Reversed in node 232 and in Parecbasis cyclolepis.
3. Tubules for passage of blood vessels on lamellar portion of maxilla (98): (1 > 0) a single tubule, parallel to dorsal margin of maxilla. Reversal of synapomorphy 2 of node 201.
4. Relative length of palatine (172): (0 > 1) distinctly longer than one-half length of ectopterygoid. Paralleled in nodes 261 and 302 and in Hyphessobrycon pulchripinnis and Paracheirodon axelrodi. Reversed in node 255 and in Acrobrycon tarijae, Aphyocharax nattereri, Gymnocharacinus bergii, Parecbasis cyclolepis, and Piabina argentea.
5. Bony lamellae associated with supraneurals (282): (1 > 0) absent or small. Paralleled in nodes 272 and 299 and in Acestrocephalus sardina and Nematocharax venustus. Reversed in Parecbasis cyclolepis and Prodontocharax melanotus.
Node 281: Gymnocharacinae (37 / 91 / 9 / 4)
Genera Coptobrycon, Grundulus Valenciennes, Gymnocharacinus Steindachner, and Nematobrycon.
The subfamily Gymnocharacinae was proposed by Eigenmann (1910) as containing only Gymnocharacinus bergii. This group was maintained by Géry (1977) as the tribe Gymnocharacini; that author (Géry, 1977: 535) mentioned that this species could be related with some "generalized Hemibrycon-like tetragonopterine". Miquelarena & Arámburu (1983) listed 22 characters distinguishing Gymnocharacinus bergii from the remaining Tetragonopterinae of Géry (1977). According to the present hypothesis some of those characters are synapomorphies of this clade. Miquelarena & Arámburu (1983) justified the validity of the monotypic subfamily Gymnocharacinae based on the mentioned list of characters. However, the number of synapomorphies itself is not an appropriate basis to assign a group a particular nomenclatural category. Such decision requires an underlying phylogenetic hypothesis. In the present analysis this clade also includes Coptobrycon bilineatus, Grundulus cochae, and Nematobrycon palmeri. A close relationship between these four species was not previously proposed. Mirande (2009) proposed the Gymnocharacinae to be composed of Coptobrycon, Grundulus, and Gymnocharacinus, leaving Nematobrycon in the monotypic Nematobrycon clade. The monophyly of this clade was obtained in the analyses under self-weighted optimization by Mirande (2009) and in the final hypothesis herein proposed. Although this node is relatively less stable than that of the Gymnocharacinae of Mirande (2009), it is herein preferred to redefine this subfamily to include also Nematobrycon. Among the genera included in this node, Coptobrycon and Gymnocharacinus are monotypic, while both Grundulus and Nematobrycon are composed of few species that greatly resemble each other and which are much different from the remaining Characidae. Although the monophyly of Grundulus and Nematobrycon were not proposed in terms of shared synapomorphies, these genera are treated as monophyletic in this paper. The disjunct geographic distribution of the genera included in this clade is noteworthy. The species of Grundulus and Nematobrycon inhabit northwestern South America, Coptobrycon bilineatus lives in the upper basin of the río Paraná, in eastern Brazil, and Gymnocharacinus bergii is the most austral member of the family Characidae, inhabiting the Argentine province of rio Negro in the northeastern Patagonia. Given the present state of knowledge it is premature to advance conclusions about the biogeography of this subfamily.
Synapomorphies:
1. Denticles on gill rakers (201): (0 > 1) absent. Paralleled in nodes 245 and 253 and in Axelrodia lindeae and Pseudochalceus kyburzi. Some trees: Paralleled in Hyphessobrycon elachys and H. herbertaxelrodi.
2. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in nodes 207, 283, 297, and 301 and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
3. Adipose fin (356): (0 > 1) absent. Paralleled in node 181 and in Carnegiella strigata and Phenagoniates macrolepis.
Autapomorphies of Nematobrycon palmeri:
1. Dorsal margin of lateral ethmoids (16): (1 > 0) aligned. Reversal of synapomorphy 1 of node 198. Paralleled in the Aphyoditeinae.
2. Anterior paired projections of parasphenoid (40): (0 > 1) present. Paralleled in Bryconexodon juruenae.
3. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae and Gasteropelecidae, in node 186, and in Aphyodite grammica, Hasemania nana, Hemigrammus erythrozonus, Hoplocharax goethei, and Hyphessobrycon pulchripinnis.
4. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Reversal of synapomorphy 1 of node 202. Paralleled in node 208 and in Grundulus cochae, Hemibrycon surinamensis, and Prodontocharax melanotus.
5. Number of branched anal-fin rays (288): (0 > 1) 25 or more. Reversal of synapomorphy 3 of node 200. Paralleled in nodes 208, 255, and 263 and in Bryconamericus scleroparius.
Node 280: (100 / 100 / 83 / 33)
Genera Coptobrycon, Grundulus, and Gymnocharacinus.
The monophyly of these genera was proposed by Mirande (2009) as the resurrected and redefined subfamily Gymnocharacinae. Although the relationship of Nematobrycon with these three genera is somewhat unstable across the analyses herein performed, the node composed of Coptobrycon, Grundulus, and Gymnocharacinus is supported by several synapomorphies and is stable across all the explored analytical conditions.
Synapomorphies:
1. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
2. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Reversal of synapomorphy 2 of node 198. Paralleled in node 298 and in Aphyodite grammica, Axelrodia lindeae, Bryconamericus rubropictus, and Creagrutus cf. tahorni.
3. Ventral margin of horizontal process of anguloarticular (109): (1 > 0) posteroventrally angled relative to laterosensory canal of dentary from medial view. Reversal of synapomorphy 1 of node 199. Paralleled in nodes 195 and 298 and in Attonitus ephimeros.
4. Form of teeth of inner premaxillary tooth row (128): (0 > 1) with cusps aligned in straight series and without anterior concavity. Paralleled in the Rhoadsiinae, in nodes 195 and 245, and in Hemigrammus bleheri and Odontostoechus lethostigmus.
5. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
6. Ventral extension of coracoid lamella (241): (0 > 1) falling short of ventral margin of cleithrum. Paralleled in Hoplias cf. malabaricus.
7. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
8. Number of branched anal-fin rays (287): (1 > 0) 17 or less. Reversal of synapomorphy 6 of the Characoidea. Paralleled in the Alestidae, in node 290, and in Attonitus ephimeros and Prodontocharax melanotus. Some trees: Paralleled in Hasemania nana.
Autapomorphies of Gymnocharacinus bergii:
1. Position of sphenotic spine relative to the orbit (12): (0 > 1) distinctly posterior to orbital margin. Paralleled in nodes 193 and 299 and in Acestrorhynchus pantaneiro, Attonitus ephimeros, and Cynopotamus argenteus.
2. Bony lamellae bordering laterosensory canal of first infraorbital (58): (0 > 1) absent. Paralleled in node 208.
3. Premaxillary, maxillary, and dentary teeth (119): (0 > 1) pedunculate and uniformly shaped. Paralleled in node 232 and in Odontostoechus lethostigmus.
4. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. Paralleled in nodes 265 and 294 and in Brycon orbignyanus, Bryconops melanurus, Micralestes stormsi, and Nematocharax venustus. Some trees: Paralleled in Bryconamericus agna.
5. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273, 283, and 294, and in Bramocharax bransfordii, Brycon orbignyanus, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
6. Relative length of palatine (172): (1 > 0) approximately one-half length of ectopterygoid, or less. Reversal of synapomorphy 4 of node 197. Paralleled in node 255 and in Acrobrycon tarijae, Aphyocharax nattereri, Parecbasis cyclolepis, and Piabina argentea.
7. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in node 296 and in Axelrodia lindeae, Hollandichthys multifasciatus, Mimagoniates rheocharis, Nematocharax venustus, Paracheirodon axelrodi, and Prodontocharax melanotus.
8. Relative number of precaudal vertebrae (226): (1 > 0) exceeding caudal vertebrae in two or more elements. Reversal of synapomorphy 3 of Characoidea. Paralleled in Brycinus carolinae and Chalceus macrolepidotus.
9. Articulation between ventral process of mesocoracoid and dorsal margin of scapula (245): (0 > 1) present and broad. Paralleled in node 252.
10. Form of third postcleithrum (250): (1 > 0) slender, without associated lamella. Reversal of synapomorphy 6 of node 204. Paralleled in node 242 and in Pseudochalceus kyburzi and Rhoadsia altipinna.
11. Dorsal development of third postcleithrum (251): (0 > 1) not projects dorsally to posterior region of scapula. Paralleled in node 192 and in Agoniates anchovia.
12. Position of last supraneural (283): (0 > 1) located more than two vertebrae in front of first dorsal pterygiophore. Paralleled in nodes 174 and 244 and in Engraulisoma taeniatum and Xenagoniates bondi.
13. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 184, 186, 209, 241, 261, and 270, and in Engraulisoma taeniatum and Gymnocharacinus bergii.
Node 279: (76 / 96 / 17 / 5)
Genera Coptobrycon and Grundulus.
Synapomorphies:
1. Length of laterosensory canal of dentary (79): (0 > 1) reduced or absent. Paralleled in Aphyocharax nattereri, Bryconamericus rubropictus, Hyphessobrycon elachys, H. luetkenii, Nantis cf. indefessus, and Thayeria boehlkei.
2. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 229, 288, and 294 and in Characidium rachovii, Hoplocharax goethei, Hyphessobrycon anisitsi, Moenkhausia sanctaefilomenae, Phenacogaster tegatus, and Pyrrhulina australis.
3. Nostrils (351): (0 > 1) nostrils distinctly separate. Paralleled in node 168 and in Leporinus striatus.
Autapomorphies of Grundulus cochae:
1. Anterior articulation of epioccipital bridge (6): (0 > 1) only with parietal. Some trees: Paralleled in Paracheirodon axelrodi.
2. Morphology of premaxillary, maxillary, and dentary teeth (118): (1 > 0) all teeth conical, caniniform or mamilliform. Paralleled in the Heterocharacinae, in nodes 174, 181, and 211, and in Axelrodia lindeae and Exodon paradoxus.
3. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Aulixidens eugeniae, Carlana eigenmanni, Carnegiella strigata, Odontostoechus lethostigmus, Paracheirodon axelrodi, Piabucus melanostomus, and Probolodus heterostomus.
4. Number of teeth in inner premaxillary row (129): (0 > 1) five or more. Reversal of synapomorphy 3 of node 198. Paralleled in nodes 195 and 235.
5. Number of teeth in inner premaxillary row (130): (0 > 1) eight or more. Paralleled in the Aphyoditeinae, in node 170, and in Brycon orbignyanus, Prionobrama paraguayensis, and Salminus brasiliensis.
6. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Reversal of synapomorphy 1 of node 202. Paralleled in node 208 and in Hemibrycon surinamensis, Nematobrycon palmeri, and Prodontocharax melanotus.
7. Foramen on articular condyle of quadrate (149): (0 > 1) present. Paralleled in nodes 168, 211, and 231 and in Hasemania nana, Hyphessobrycon eques, and Lonchogenys ilisha.
Autapomorphies of Coptobrycon bilineatus:
1. Anterior convergence of ventral diverging lamellae with nasal septum of mesethmoid (31): (1 > 0) absent, or confluent near anterior end of nasal septum. Paralleled in Aulixidens eugeniae and Knodus breviceps.
2. Nasal (33): (0 > 1) absent. Paralleled in Hyphessobrycon elachys.
3. Maxillary teeth (134): (1 > 0) absent. Paralleled in Aulixidens eugeniae, Iguanodectes geisleri, Parecbasis cyclolepis, and Stichonodon insignis. Some trees: Paralleled in Hyphessobrycon elachys and Psellogrammus kennedyi.
4. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in the Astyanax clade, in nodes 284 and 290, and in Aulixidens eugeniae, Cheirodon interruptus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
5. Number of branched-rays on dorsal-fin (270): (1 > 0) eight or fewer. Paralleled in the Stevardiinae and in Hoplocharax goethei, and Piabucus melanostomus.
6. Number of rays on last anal pterygiophore (291): (0 > 1) one. Paralleled in Hasemania nana.
7. Horizontal line of chromatophores just dorsal to anal-fin base (344): (0 > 1) present. Paralleled in the Heterocharacinae.
Node 196: (44 / 97 / - / 4)
Subfamilies Aphyocharacinae, Aphyoditeinae, Cheirodontinae, and Stevardiinae.
The monophyly of this node was originally proposed by Mirande (2009).
Synapomorphies:
1. Articulation between second and third infraorbitals (62): (0 > 1) anteroventrally angled. Paralleled in Markiana nigripinnis and Pyrrhulina australis. Reversed in Acrobrycon tarijae.
2. Posterior margin of cleithrum (234): (0 > 1) with concavity ventral to first postcleithrum. Reversal of synapomorphy 4 of node 204. Paralleled in node 289 and in Probolodus heterostomus and Roeboexodon geryi. Reversed in node 298 and in Cheirodon interruptus and Inpaichthys kerri.
3. Gill-derived gland on males (352): (0 > 1) present. Paralleled in Phenacogaster tegatus. Reversed in nodes 236 and 251 and in Aphyocharax nattereri.
4. Number of 2n chromosomes (363): (0 > 1) 52 or more. Paralleled in the Characinae and Serrasalmidae and in Chalceus macrolepidotus, Hyphessobrycon herbertaxelrodi, Markiana nigripinnis, and Rhaphiodon vulpinus. Some trees: Paralleled in Hemigrammus unilineatus.
Some trees:
5. Number of gill rakers on first hypobranchial and ceratobranchial (196): (0 > 1) ten or fewer. (k13-14). Paralleled in the Characinae and in Bryconops melanurus, Hyphessobrycon pulchripinnis, Iguanodectes geisleri, and Moenkhausia sanctaefilomenae. Some trees: Paralleled in Hemigrammus erythrozonus and Hyphessobrycon herbertaxelrodi. Reversed in node 237 and in Bryconamericus cf. exodon and B. cf. iheringii. Some trees: Reversed in the Cheirodontinae and in Cyanocharax alburnus.
Node 195: (95 / 99 / 26 / 13)
Subfamilies Aphyocharacinae, Aphyoditeinae, and Cheirodontinae.
The monophyly of this node was also proposed by Mirande (2009). Most genera contained in this clade were included in the Cheirodontinae by Eigenmann (1915), along with several other genera. That classification was partially followed by Géry (1977), which included the genera herein included in the Aphyoditeinae in his Cheirodontinae sensu lato.
Synapomorphies:
1. Ventral margin of horizontal process of anguloarticular (109): (1 > 0) posteroventrally angled relative to laterosensory canal of dentary from medial view. Reversal of synapomorphy 1 of node 199. Paralleled in nodes 280 and 298 and in Attonitus ephimeros. Reversed in Xenagoniates bondi.
2. Position of coronomeckelian (110): (1 > 0) situated mainly lateral to Meckelian cartilage. Reversal of synapomorphy 2. Paralleled in Astyanax eigenmanniorum1.
3. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in Aulixidens eugeniae, Carlana eigenmanni, Carnegiella strigata, Grundulus cochae, Odontostoechus lethostigmus, Paracheirodon axelrodi, Piabucus melanostomus, and Probolodus heterostomus.
4. Form of teeth of inner premaxillary tooth row (128): (0 > 1) with cusps aligned in straight series and without anterior concavity. Paralleled in the Rhoadsiinae, in nodes 245 and 280, and in Hemigrammus bleheri and Odontostoechus lethostigmus.
5. Number of teeth in inner premaxillary row (129): (0 > 1) five or more. Reversal of synapomorphy 3 of node 198. Paralleled in node 235 and in Grundulus cochae.
6. Transitional vertebrae with haemal canal (229): (0 > 1) absent. Paralleled in node 212 and in Aulixidens eugeniae, Engraulisoma taeniatum, Metynnis maculatus, and Piabina argentea. Some trees: Paralleled in node 247 and in Bryconamericus alpha and Paracheirodon axelrodi. Reversed in Parecbasis cyclolepis and Serrapinnus calliurus.
Node 194: Aphyocharacinae (100 / 100 / 64 / 66)
Genera Aphyocharax, Inpaichthys Géry & Junk, Leptagoniates?, Paragoniates Steindachner, Phenagoniates Eigenmann & Wilson, Prionobrama, Rachoviscus Myers?, and Xenagoniates Myers.
As classically treated, the subfamily Aphyocharacinae included only Aphyocharax (Eigenmann, 1909). Géry (1977), however, mentioned the pronounced resemblance between Aphyocharax and the members of his Paragoniatinae (genera Leptagoniates, Paragoniates, Phenagoniates, Prionobrama, Rachoviscus, and Xenagoniates), especially with Prionobrama and Rachoviscus. A similar group of genera was obtained as monophyletic by Quevedo (2006). Leptagoniates was not analyzed in this paper, but it shares an overall resemblance with Xenagoniates and, in the case of its type-species, L. steindachneri Boulenger, a very long anal-fin, with more than 60 rays. This unusual character state could be an evidence of close relationship between Leptagoniates and the Aphyocharacinae. The relationships of Rachoviscus are also unclear. Géry (1977) mentioned that R. crassiceps Myers has non-aligned premaxillary teeth, which seems to form two independent rows in some specimens. This condition is comparable to that of Inpaichthys kerri among the species herein analyzed. Rachoviscus additionally shares with Inpaichthys certain resemblances in overall form and coloration. Both Leptagoniates and Rachoviscus are provisionally included in this node based on the long anal-fin and the position of the premaxillary teeth which probably relate these genera with Xenagoniates and Inpaichthys, respectively. Thus, this node includes the subfamilies Aphyocharacinae and Paragoniatinae of Géry (1977), plus Inpaichthys kerri as sister group of these former subfamilies. The nomenclatural options derivable from these results are to conserve as valid both the Aphyocharacinae and Paragoniatinae, add a new monotypic subfamily for Inpaichthys, or join together all members of this clade in the same subfamily. The latter option is preferred so as to be conservative in the creation of new categories, and avoid the potential need to create other subfamilies for species eventually resolved at the base of this clade. Among the available names, Aphyocharacinae Eigenmann, 1909 has temporal precedence over Paragoniatinae Géry, 1972.
Synapomorphies:
1. Synchondral articulation between lateral ethmoid and anterodorsal border of orbitosphenoid (35): (1 > 0) present. Paralleled in Leporinus striatus, Mimagoniates rheocharis, Pristella maxillaris, and Rhaphiodon vulpinus.
2. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Gasteropelecidae, in node 186, and in Aphyodite grammica, Hasemania nana, Hemigrammus erythrozonus, Hoplocharax goethei, Hyphessobrycon pulchripinnis, and Nematobrycon palmeri. Reversed in node 207.
3. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in nodes 220, 236, 280, and 302 and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys. Reversed in Aphyocharax dentatus and Paragoniates alburnus.
Autapomorphies of Inpaichthys kerri:
1. Length of sphenotic spine (10): (0 > 1) extending ventrally to articulation between sphenotic and hyomandibula. Reversal of synapomorphy 1 of node 197. Paralleled in Creagrutus anary. Some trees: Paralleled in Aphyodite grammica.
2. Posterior extension of rhinosphenoid cartilage (49): (0 > 1) extended to vertical through region of articulation between orbitosphenoid and pterosphenoid.
3. Antorbital (55): (0 > 1) absent or fused with first infraorbital. Paralleled in Hoplias cf. malabaricus.
4. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, and Phenacogaster tegatus.
5. Abrupt posterior expansion of interopercle (164): (0 > 1) present. Paralleled in the Cheirodontinae and in Apareiodon affinis, and Pyrrhulina australis.
6. Posterior margin of cleithrum (234): (1 > 0) without concavity ventral to first postcleithrum. Reversal of synapomorphy 2 of node 196. Paralleled in node 298 and in Cheirodon interruptus.
7. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
8. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi (335): (1 > 0) absent. Paralleled in Creagrutus anary, Piabucus melanostomus, Prionobrama paraguayensis, Pristella maxillaris, Prodontocharax melanotus, and Pyrrhulina australis. Some trees: Paralleled in Hyphessobrycon elachys.
Node 193: (100 / 100 / 98 / 74)
Genera Aphyocharax, Leptagoniates?, Paragoniates, Phenagoniates, Prionobrama, Rachoviscus?, and Xenagoniates.
Géry (1977) proposed a close relationship between his Aphyocharacinae and Paragoniatinae, although he did not propose shared synapomorphies common to these two groups. The high support and stability of this clade corroborate his proposal.
Synapomorphies:
1. Position of sphenotic spine relative to the orbit (12): (0 > 1) distinctly posterior to orbital margin. Paralleled in node 299 and in Acestrorhynchus pantaneiro, Attonitus ephimeros, Cynopotamus argenteus, and Gymnocharacinus bergii. Reversed in Paragoniates alburnus.
2. Articulation between medial region of lateral ethmoid and frontal or mesethmoid (17): (0 > 1) extensive articulation of entire lateral ethmoid dorsal margin. Paralleled in node 170.
3. Form of orbitosphenoid (37): (0 > 1) massive, almost reaching parasphenoid ventrally. Reversal of synapomorphy 1 of the Characidae. Paralleled in Markiana nigripinnis, Rhaphiodon vulpinus, and Roeboides microlepis.
4. Dorsal process of pterotic where tendon from epaxial musculature attach (45): (0 > 1) present, projecting dorsally from tube for semicircular canal. Paralleled in the Heterocharacinae and in Rhoadsia altipinna and Serrasalmus maculatus.
5. Ventral border of rhinosphenoid (50): (0 > 1) almost contacting parasphenoid. Paralleled in node 174.
6. Position of opening on neurocranium communicating with laterosensory canal of sixth infraorbital (77): (0 > 1) in frontal. Paralleled in node 249 and in Attonitus ephimeros and Micralestes stormsi. Reversed in Phenagoniates macrolepis.
7. Position of opening on neurocranium communicating with sixth infraorbital laterosensory canal (78): (0 > 1) distinctly anterior to sphenotic tube for vertical semicircular canal.
8. Alignment of ascending process of premaxilla (105): (0 > 1) medially shifted and separated from nasal. Paralleled in the Gasteropelecidae.
9. Process of scapula forming anterior border of scapular foramen (244): (0 > 1) absent. Paralleled in node 252 and in Aphyodite grammica, Deuterodon langei, Hoplias cf. malabaricus, Hyphessobrycon herbertaxelrodi, Leporinus striatus, Odontostilbe paraguayensis, and Thayeria obliqua. Reversed in Xenagoniates bondi.
10. Ventral exit of laterosensory canal of supracleithrum (254): (1 > 0) covered by posterior lamella of supracleithrum and exiting medially. Reversal of synapomorphy 14 of node 205. Paralleled in Markiana nigripinnis.
Node 192: (100 / 100 / 92 / 86)
Genus Aphyocharax.
The monophyly of Aphyocharax was not adequately tested, although the species of this genus share some modifications in the infraorbitals which suggest its monophyly (Lima, 2003c). This is partially corroborated in this paper, although a complete phylogeny of the genus is still necessary.
Synapomorphies:
1. Trigemino-facialis foramen (42): (0 > 1) narrow, as a cleft with sphenotic almost excluded from its margin.
2. Dorsal projection of maxilla (102): (0 > 1) overlaps second infraorbital.
3. Dorsal development of third postcleithrum (251): (0 > 1) not projects dorsally to posterior region of scapula. Paralleled in Agoniates anchovia and Gymnocharacinus bergii.
Autapomorphies of Aphyocharax nattereri:
1. Contact between frontals anteriorly to frontal fontanel (21): (1 > 0) absent. Paralleled in Aphyodite grammica and Axelrodia lindeae.
2. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, 280, and 298 and in Attonitus ephimeros, Bryconamericus scleroparius, Brycon orbignyanus, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
3. Length of laterosensory canal of dentary (79): (0 > 1) reduced or absent. Paralleled in node 279 and in Bryconamericus rubropictus, Hyphessobrycon elachys, H. luetkenii, Nantis cf. indefessus, and Thayeria boehlkei.
4. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
5. Relative length of palatine (172): (1 > 0) approximately one-half length of ectopterygoid, or less. Reversal of synapomorphy 4 of node 197. Paralleled in node 255 and in Acrobrycon tarijae, Gymnocharacinus bergii, Parecbasis cyclolepis, and Piabina argentea.
6. Gill-derived gland on males (352): (1 > 0) absent. Reversal of synapomorphy 6 of node 196. Paralleled in nodes 236 and 251.
Node 191: (100 / 100 / 68 / 35)
Aphyocharax anisitsi, A. dentatus, other Aphyocharax?
The sister-group relationship between Aphyocharax anisitsi and A. dentatus, as obtained herein, should be tested in a phylogenetic analysis of this genus. Both species have a reddish ventral caudal-fin lobe, a character-state shared with other species of Aphyocharax, which could correspond to a more inclusive node.
Synapomorphies:
1. Lateral ridge of anguloarticular (107): (0 > 1) present. Paralleled in the Cheirodontinae and in Aphyocharacidium bolivianum and Parecbasis cyclolepis.
2. Color of caudal-fin lobes (345): (0 > 1) ventral lobe orange or reddish, and dorsal, lobe hyaline.
Autapomorphies of Aphyocharax dentatus:
1. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. Paralleled in nodes 184 and 242, and in Engraulisoma taeniatum, Prionobrama paraguayensis, and Stichonodon insignis. Some trees: Paralleled in the Cheirodontinae and in Microschemobrycon casiquiare.
2. Number of branched pelvic-fin rays (258): (0 > 1) seven or more. Reversal of synapomorphy 3 of the Aphyocharacinae. Paralleled in Paragoniates alburnus.
Autapomorphies of Aphyocharax anisitsi:
1. Ascending process on posterodorsal angle of exoccipital directed to neural complex of Weberian apparatus (19): (0 > 1) present.
2. Bony hooks on base of pelvic-fin rays of adult males (313): (0 > 1) as numerous as on segmented portion of rays. Paralleled in node 229.
3. Bony hooks on first pelvic-fin ray of adult males (315): (0 > 1) present. Paralleled in node 274 and in Aphyocharacidium bolivianum, Aulixidens eugeniae, and Nantis indefessus.
Node 208: (100 / 100 / 94 / 65)
Genera Leptagoniates?, Paragoniates, Phenagoniates, Prionobrama, and Xenagoniates.
This clade includes the Paragoniatinae of Géry (1972, 1977) as a highly supported and stable group, which, as already mentioned, is herein included in the Aphyocharacinae. Leptagoniates is tentatively included in this clade, as explained at node 193.
Synapomorphies:
1. Bony lamellae bordering laterosensory canal of first infraorbital (58): (0 > 1) absent. Paralleled in Gymnocharacinus bergii.
2. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Reversal of synapomorphy 1 of node 202. Paralleled in Grundulus cochae, Hemibrycon surinamensis, Nematobrycon palmeri, and Prodontocharax melanotus.
3. Main portion of fourth basibranchial (186): (0 > 1) ossified.
4. Anal-fin position (284): (0 > 1) extended anteriorly ventral to dorsal fin. Paralleled in nodes 170, 212, and 236 and in Piabucus melanostomus.
5. Number of branched anal-fin rays (288): (0 > 1) 25 or more. Reversal of synapomorphy 3 of node 200. Paralleled in nodes 255 and 263 and in Bryconamericus scleroparius and Nematobrycon palmeri.
6. Proximal and medial radials of anal fins (294): (0 > 1) fused in most pterygiophores. Paralleled in nodes 184, 218, and 221 and in Psellogrammus kennedyi and Pseudocorynopoma doriae. Some trees: Paralleled in node 295.
7. Sclerotic bones (350): (0 > 1) two bones separated by cartilages. Paralleled in nodes 210, 221, 250, and 259.
Autapomorphies of Prionobrama paraguayensis:
1. Number of teeth in inner premaxillary row (130): (0 > 1) eight or more. Paralleled in the Aphyoditeinae, in node 170, and in Brycon orbignyanus, Grundulus cochae, Phenacogaster tegatus, and Salminus brasiliensis.
2. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. Paralleled in nodes 184 and 242, and in Aphyocharax dentatus, Engraulisoma taeniatum, and Stichonodon insignis. Some trees: Paralleled in the Cheirodontinae and in Microschemobrycon casiquiare.
3. Form and degree of ossification of first ceratobranchial gill rakers (200): (0 > 1) rather thick and completely ossified distal region. Paralleled in nodes 176, 212, and 299 and in Hoplias cf. malabaricus and Pristella maxillaris.
4. Base of second pectoral ray (231): (1 > 0) large and partially overlapping base of first pectoral ray from medial view. Paralleled in nodes 164 and 301 and in Moenkhausia dichroura and Tetragonopterus argenteus.
5. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162, 247, and 253 and in Agoniates anchovia, Attonitus ephimeros, Characidium borellii, Iguanodectes geisleri, Moenkhausia cf. intermedia, and Xenagoniates bondi.
6. Ventral articulation of mesocoracoid (246): (0 > 1) only with coracoid.
7. Number of epurals (296): (1 > 0) one. Paralleled in Agoniates anchovia.
8. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi (335): (1 > 0) absent. Paralleled in Creagrutus anary, Inpaichthys kerri, Piabucus melanostomus, Pristella maxillaris, Prodontocharax melanotus, and Pyrrhulina australis. Some trees: Paralleled in Hyphessobrycon elachys.
Node 207: (39 / 92 / 64 / 48)
Genera Leptagoniates?, Paragoniates, Phenagoniates, and Xenagoniates.
Although no synapomorphies for this group of genera were proposed, Géry (1977) included them in his Paragoniatinae. That author considered Prionobrama to be a basal member of this group, and highlighted its resemblance to Aphyocharax. Uj (1990) included Prionobrama in his Aphyocharacidae (=Aphyocharacinae) and not in his Paragoniatidae (=Paragoniatinae) together with the remaining genera of the Paragoniatinae of Géry (1977). However, Uj did not provided explicit reasons supporting that hypothesis. In the present study Prionobrama is the sister group of this clade.
Synapomorphies:
1. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 260, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
2. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. Paralleled in Odontostilbe paraguayensis and Parecbasis cyclolepis.
3. Fourth infraorbital (66): (1 > 0) present, well developed. Reversal of synapomorphy 2 of the Aphyocharacinae.
4. Number of supraneurals (281): (0 > 1) eight or more. Paralleled in node 170 and in Hemiodus cf. thayeria and Pyrrhulina australis.
5. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in node 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Pseudocorynopoma doriae, Rhaphiodon vulpinus, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
6. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 283, 297, and 301, and in Astyanax paris, Bryconamericus mennii, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Autapomorphies of Paragoniates alburnus:
1. Position of sphenotic spine relative to the orbit (12): (1 > 0) bordering orbit posteriorly and aligned with anterior border of fourth and fifth infraorbitals. Reversal of synapomorphy 1 of node 193.
2. Development of medial lamella of coracoid (238): (0 > 1) expanded as a keel. Paralleled in nodes 170 and 302 and in Piabucus melanostomus, Pseudocorynopoma doriae, and Rhaphiodon vulpinus.
3. Number of branched pelvic-fin rays (258): (0 > 1) seven or more. Reversal of synapomorphy 3 of the Aphyocharacinae. Paralleled in Aphyocharax dentatus.
4. Scales covering anal-fin base (327): (0 > 1) several rows covering basal third of anal fin. Paralleled in the Serrasalmidae, in nodes 210 and 221, and in Bario steindachneri, Markiana nigripinnis, Rhaphiodon vulpinus, Roeboides microlepis, and Thoracocharax stellatus.
Node 209: (100 / 100 / 100 / 100)
Genera Leptagoniates?, Phenagoniates, and Xenagoniates.
The close relationship between Phenagoniates and Xenagoniates was previously proposed by Quevedo (2006). Probably Leptagoniates is also related with this clade, and some of these synapomorphies actually correspond to a more inclusive clade.
Synapomorphies:
1. Ventral margin of toothed region of maxilla (95): (0 > 1) strongly concave.
2. Margins of toothed region of maxilla (96): (0 > 1) dorsally divergent. Paralleled in nodes 162, 254, and 282 and in Prodontocharax melanotus and Rhoadsia altipinna.
3. Medial anteroventral notch of dentary (114): (0 > 1) present. Paralleled in Iguanodectes geisleri.
4. Longitudinal ridge in quadrate bordering adductor mandibulae muscle ventrally and, to some degree, laterally (152): (0 > 1) present. Paralleled in the Iguanodectinae and in node 252.
5. Total number of vertebrae (227): (0 > 1) 41 or more. Reversal of synapomorphy 11 of node 205.
6. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 184, 186, 241, 261, and 270, and in Engraulisoma taeniatum and Gymnocharacinus bergii.
Autapomorphies of Xenagoniates bondi:
1. Lateral line (91): (1 > 0) complete.
2. Horizontal process of anguloarticular (108): (0 > 1) broadly covered by dentary which reaches posterior border of Meckelian cartilage. Reversal of synapomorphy 1 of node 206. Paralleled in nodes 246, 253, and 261.
3. Ventral margin of horizontal process of anguloarticular (109): (0 > 1) perpendicular to laterosensory canal of dentary from medial view. Reversal of synapomorphy 1 of Xenagoniates bondi.
4. Ectopterygoid teeth row (159): (0 > 1) present. Paralleled in nodes 168 and 300 and in Acestrorhynchus pantaneiro, Distichodus maculatus, Hoplias cf. malabaricus, and Serrasalmus maculatus.
5. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Attonitus ephimeros, Axelrodia lindeae, Hollandichthys multifasciatus, Hoplocharax goethei, Jupiaba scologaster, Piabucus melanostomus, Pyrrhulina australis, and Rhaphiodon vulpinus.
6. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162, 247, and 253 and in Agoniates anchovia, Attonitus ephimeros, Characidium borellii, Iguanodectes geisleri, Moenkhausia cf. intermedia, and Prionobrama paraguayensis.
7. Process of scapula forming anterior border of scapular foramen (244): (1 > 0) present. Reversal of synapomorphy 9 of node 193.
8. Third postcleithrum (249): (0 > 1) absent. Paralleled in the Gasteropelecidae, in node 302, and in Piabucus melanostomus, Pyrrhulina australis, and Rhaphiodon vulpinus.
9. Position of last supraneural (283): (0 > 1) located more than two vertebrae in front of first dorsal pterygiophore. Paralleled in nodes 174 and 244 and in Engraulisoma taeniatum and Gymnocharacinus bergii.
10. Insertion of adductor mandibulae tendon on dentary (331): (0 > 1) anterior to Meckelian cartilage. Paralleled in nodes 183, 186, and 253.
Autapomorphies of Phenagoniates macrolepis:
1. Position of opening on neurocranium communicating with laterosensory canal of sixth infraorbital (77): (1 > 0) between frontal and pterotic. Reversal of synapomorphy 6 of node 193.
2.Circulii on posterior field of scales (319): (1 > 0) present. Reversal of synapomorphy 3 of node 206. Paralleled in node 261 and in Exodon paradoxus and Roeboides microlepis.
3.Radii on scales (320): (1 > 0) absent or reduced in number. Paralleled in the Iguanodectinae and Serrasalminae, in node 174, and in Cyphocharax spilotus, Distichodus maculatus, and Markiana nigripinnis.
4. Adipose fin (356): (0 > 1) absent. Paralleled in the Gymnocharacinae, in node 181, and in Carnegiella strigata.
Node 234: (100 / 100 / 27 / 21)
Subfamilies Aphyoditeinae and Cheirodontinae.
Géry (1965a) proposed the tribe Aphyoditeini, as part of the Cheirodontidi (=Cheirodontinae), in which he later (Géry, 1973) included a group of small species with only one row of conical or tricuspid premaxillary teeth. This group was composed of Aphyocharacidium, Aphyodite Eigenmann, Axelrodia Géry, Brittanichthys Géry, Macropsobrycon Eigenmann, Microschemobrycon, Thrissobrycon Böhlke, and Tyttobrycon. Géry (1977) included in his "Cheirodontinae and allied genera" a group of "Cheirodontinae sensu stricto" and several groups of genera supposedly related with Cheirodontinae; among them, Géry (1977) included an Aphyodite group or Aphyoditeina, composed of the same genera listed by Géry (1973) in addition to Atopomesus Myers, Leptobrycon Eigenmann, Oligobrycon Eigenmann, Oxybrycon Géry, Paracheirodon, Parecbasis Eigenmann, and Prodontocharax Eigenmann & Pearson. The genera Macropsobrycon and Prodontocharax were included in Cheirodontinae by Malabarba (1998a), which considered the remaining genera of the Aphyoditeina as not included in the Cheirodontinae, becoming incertae sedis within the Characidae. That classification was retained by Lima et al. (2003). A close relationship between the Aphyoditeinae and Cheirodontinae was proposed several times in the literature, but never in a phylogenetic context. The subfamily Aphyoditeinae was not phylogenetically diagnosed. Although a more detailed study is necessary, it is resurrected as the sister group of the Cheirodontinae, containing, at least, the genera Aphyocharacidium, Aphyodite, Axelrodia, Microschemobrycon, and Parecbasis, included in this analysis.
Synapomorphies:
1. Form of mesethmoid spine (27): (0 > 1) relatively short, with premaxillae articulating with each other anterior to mesethmoid. Paralleled in node 225 and in Chalceus macrolepidotus and Paracheirodon axelrodi.
2. Length of maxilla relative to dentary (100): (0 > 1) maxilla not reaching posterior end of Meckelian cartilage. Paralleled in node 245. Reversed in Odontostilbe pequira.
3. Pseudotympanum limited by first pleural rib, lateralis superficialis, second pleural rib, obliquus inferioris, and obliquus superioris (339): (0 > 1) present. Paralleled in Characidium rachovii. Some trees: Paralleled in node 295.
Node 257: Aphyoditeinae (100 / 100 / 60 / 19)
Genera Aphyocharacidium, Aphyodite, Axelrodia, Leptobrycon?, Microschemobrycon, Oxybrycon?, Parecbasis, and Tyttobrycon?
As mentioned above, this is the first published phylogenetic diagnosis of the Aphyoditeinae. According to Géry (1977) both Atopomesus pachyodus Myers and Oligobrycon microstomus Eigenmann have strong teeth, which are not compressed anteroposteriorly and the former genus has seven premaxillary teeth, while the latter has only four. Both genera are considered here as incertae sedis within the Characidae. The genus Leptobrycon has numerous premaxillary teeth (14) (Géry, 1977), and it is included tentatively in this clade pending further studies. According to Géry (1977), Oxybrycon has two rows of dentary teeth, as does the aphyoditein Aphyocharacidium. This character state is unique to these species among the characids without a supraorbital bone, being interpreted herein as a potential synapomorphy of a clade containing Aphyocharacidium and Oxybrycon. Leptobrycon and Oxybrycon have a long maxilla that reaches the posterior end of Meckelian cartilage and differ in that from the species included in the Aphyoditeinae or Cheirodontinae. This character state, however, could be a synapomorphy of these two genera. Given the available information, Leptobrycon and Oxybrycon are tentatively included in the Aphyoditeinae. The species of Tyttobrycon look like the Aphyoditeinae, with a very short maxilla not reaching the posterior end of the Meckelian cartilage (with the exception of T. xeruini Géry) (Géry, 1973), and they have eight or nine premaxillary teeth (excepting T. dorsimaculatus Géry, with six or seven). This genus is also included in the Aphyoditeinae at least provisionally. All the genera of Aphyoditeinae lack phylogenetic diagnoses and their monophyly was not tested, but they are herein treated provisionally as monophyletic.
Synapomorphies:
1. Dorsal margin of lateral ethmoids (16): (1 > 0) aligned. Reversal of synapomorphy 1 of node 198. Paralleled in Nematobrycon palmeri.
2. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
3. Number of teeth in inner premaxillary row (130): (0 > 1) eight or more. Paralleled in node 170, and in Brycon orbignyanus, Grundulus cochae, Phenacogaster tegatus, Prionobrama paraguayensis, and Salminus brasiliensis.
4. Size and number of anterior dentary teeth (142): (0 > 1) eight or more small and slender teeth at front of dentary. Paralleled in Charax stenopterus and Pyrrhulina australis.
Some trees:
5. Number of supraneurals (280): (1 > 0) four or fewer. Paralleled in in nodes 211, 223, and 262 and in Bramocharax bransfordii, Bryconaethiops macrops, Hyphessobrycon bifasciatus, and Nematocharax venustus. Some trees: Reversed in Aphyodite grammica.
Autapomorphies of Parecbasis cyclolepis:
1. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. Paralleled in node 207 and in Odontostilbe paraguayensis.
2. Lateral coverage of dilator fossa by sixth infraorbital (69): (1 > 0) almost complete, at least in its ventral border. Reversal of synapomorphy 2 of node 197. Paralleled in node 232.
3. Dorsal end of laterosensory canal of preopercle and suprapreopercle (82): (0 > 1) overlapping anterodorsal process of opercle. Paralleled in the Alestidae, in node 230, and in Bario steindachneri, Hyphessobrycon eques, Pristella maxillaris, and Stichonodon insignis.
4. Lateral ridge of anguloarticular (107): (0 > 1) present. Paralleled in the Cheirodontinae, in node 191, and in Aphyocharacidium bolivianum.
5. Maxillary teeth (134): (1 > 0) absent. Paralleled in Aulixidens eugeniae, Coptobrycon bilineatus, Iguanodectes geisleri, and Stichonodon insignis. Some trees: Paralleled in Hyphessobrycon elachys and Psellogrammus kennedyi.
6. Relative length of palatine (172): (1 > 0) approximately one-half length of ectopterygoid, or less. Reversal of synapomorphy 4 of node 197. Paralleled in node 255 and in Acrobrycon tarijae, Aphyocharax nattereri, Gymnocharacinus bergii, and Piabina argentea.
7. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
8. Number of gill rakers on first hypobranchial and ceratobranchial (195): (1 > 0) 16 or more. Paralleled in nodes 177 and 183 and in Astyanax latens, A. cf. rutilus, A. pelegrini, Hoplias cf. malabaricus, Hyphessobrycon socolofi, Moenkhausia dichroura, Piaractus mesopotamicus, and Stichonodon insignis.
9. Laminar bony ridge on dorsal margin of abdominal ribs (224): (0 > 1) present. Paralleled in node 297 and in Stichonodon insignis.
10. Transitional vertebrae with haemal canal (229): (1 > 0) present. Reversal of synapomorphy 6 of node 195. Paralleled in Serrapinnus calliurus.
11. Relative position of dorsal-fin anterior insertion (265): (1 > 0) anterior to or at vertical through pelvic-fin origin. Paralleled in node 282 and in Creagrutus anary, Exodon paradoxus, and Moenkhausia xinguensis.
12. Bony lamellae associated with supraneurals (282): (0 > 1) wider than primary axis of supraneurals. Reversal of synapomorphy 5 of node 197. Paralleled in Prodontocharax melanotus.
Some trees:
13. Number of maxillary teeth (135): (1 > 0) only one, or absent. (k9-12). Paralleled in the Astyanax clade, in nodes 284 and 290, and in Aulixidens eugeniae, Cheirodon interruptus, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana and Paracheirodon axelrodi.
14. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. (k13-14). Paralleled in node 302, and in Agoniates anchovia, Cyanocharax alburnus, Deuterodon langei, Engraulisoma taeniatum, Hemiodus cf. thayeria, Roeboexodon geryi, and Thayeria obliqua. Some trees: Paralleled in Microschemobrycon casiquiare.
Autapomorphies of Microschemobrycon casiquiare:
1. Epiphyseal branch of supraorbital canal (84): (0 > 1) absent. Paralleled in the Stevardiinae.
Some trees:
2. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. (k9-12). Paralleled in nodes 184 and 242, and in Aphyocharax dentatus, Engraulisoma taeniatum, Prionobrama paraguayensis, and Stichonodon insignis. Some trees: Paralleled in the Cheirodontinae.
3. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. (k 13-14). Paralleled in node 302, and in Agoniates anchovia, Cyanocharax alburnus, Deuterodon langei, Engraulisoma taeniatum, Hemiodus cf. thayeria, Roeboexodon geryi, and Thayeria obliqua. Some trees: Paralleled in Parecbasis cyclolepis.
4.Radii of scales (322): (0 > 1) converging at focus. (k9-12). Reversal of synapomorphy 7 of the Characidae. Paralleled in node 273 and in Stichonodon insignis and Tetragonopterus argenteus. Some trees: Paralleled in node 302.
Autapomorphies of Aphyodite grammica:
1. Contact between frontals anteriorly to frontal fontanel (21): (1 > 0) absent. Paralleled in Aphyocharax nattereri and Axelrodia lindeae.
2. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Reversal of synapomorphy 2 of node 198. Paralleled in nodes 280 and 298 and in Axelrodia lindeae, Bryconamericus rubropictus, and Creagrutus cf. tahorni.
3. Fourth infraorbital (66): (0 > 1) absent or much reduced and bordered posteriorly by third and fifth infraorbitals. Paralleled in the Aphyocharacinae and Gasteropelecidae, in node 186, and in Hasemania nana, Hemigrammus erythrozonus, Hoplocharax goethei, Hyphessobrycon pulchripinnis, and Nematobrycon palmeri.
4. Process of scapula forming anterior border of scapular foramen (244): (0 > 1) absent. Paralleled in nodes 193 and 252 and in Deuterodon langei, Hoplias cf. malabaricus, Hyphessobrycon herbertaxelrodi, Leporinus striatus, Odontostilbe paraguayensis, and Thayeria obliqua.
5. Posterior attachment of A1 section of adductor mandibulae (332): (0 > 1) restricted or almost restricted to horizontal arm of preopercle. Paralleled in the Iguanodectinae, in node 211, and in Agoniates anchovia and Pyrrhulina australis.
Some trees:
6. Length of sphenotic spine (10): (0 > 1) extending ventrally to articulation between sphenotic and hyomandibula. Reversal of synapomorphy 1 of node 197. Paralleled in Creagrutus anary and Inpaichthys kerri.
7. Number of cusps of anterior maxillary teeth (138): (1 > 0) conical, a single cusp. Paralleled in Pristella maxillaris. Some trees: Paralleled in Axelrodia lindeae.
8. Number of supraneurals (280): (0 > 1) five or more. Reversal of synapomorphy 5 of the Aphyoditeinae.
Node 256: (100 / 100 / 60 / 21)
Genera Aphyocharacidium, Axelrodia, Leptobrycon?, Oxybrycon? and Tyttobrycon?
Although the monophyly and relationships of the Aphyoditeinae are well supported in the present analysis, the internal relationships of this subfamily are poorly resolved. The only obtained sister-group relationship within this subfamily is that between Aphyocharacidium bolivianum and Axelrodia lindeae.
Synapomorphies:
1. Anterior portions of branchiostegal rays (214): (0 > 1) slender near their articulation with ceratohyals. Paralleled in node 212 and in Rhaphiodon vulpinus.
Some trees:
2. Large foramen on pterosphenoid (43): (0 > 1) present, well developed. (k9-12).
3. Form of fourth infraorbital (67): (0 > 1) longer dorsoventrally than longitudinally. (k13-14). Reversal of synapomorphy 1 node 200. Paralleled in node 266. Some trees: Paralleled in node 233.
Autapomorphies of Axelrodia lindeae:
1. Contact between frontals anteriorly to frontal fontanel (21): (1 > 0) absent. Paralleled in Aphyocharax nattereri and Aphyodite grammica.
2. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Reversal of synapomorphy 2 of node 198. Paralleled in nodes 280 and 298 and in Aphyodite grammica, Bryconamericus rubropictus, and Creagrutus cf. tahorni.
3. Morphology of premaxillary, maxillary, and dentary teeth (118): (1 > 0) all teeth conical, caniniform or mamilliform. Paralleled in the Heterocharacinae, in nodes 174, 181, and 211, and in Grundulus cochae and Exodon paradoxus.
4. Position of anterior teeth of dentary (146): (0 > 1) internally situated with dentary forming anterior ridge.
5. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Attonitus ephimeros, Hollandichthys multifasciatus, Hoplocharax goethei, Jupiaba scologaster, Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
6. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in node 296 and in Gymnocharacinus bergii, Hollandichthys multifasciatus, Mimagoniates rheocharis, Nematocharax venustus, Paracheirodon axelrodi, and Prodontocharax melanotus.
7. Denticles on gill rakers (201): (0 > 1) absent. Paralleled in the Gymnocharacinae, in nodes 245 and 253, and in Pseudochalceus kyburzi. Some trees: Paralleled in Hyphessobrycon elachys and H. herbertaxelrodi.
8. Shape of dentigerous plate of fifth ceratobranchial (204): (1 > 0) rounded, with posterior notch. Paralleled in the Iguanodectinae and in node 252.
9. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
10. Ventral procurrent caudal-fin rays of adult males (301): (0 > 1) laminar. Paralleled in node 229.
11. Ventral procurrent caudal-fin rays of adult males (303): (0 > 1) projecting ventrally through peduncle musculature and skin. Paralleled in node 229 and in Hoplocharax goethei.
12. Bony hooks on last pelvic-fin ray of adult males (314): (0 > 1) as numerous as in other rays. Paralleled in nodes 232, 240, 258, and 299.
Some trees:
13. Number of cusps of anterior maxillary teeth (138): (1 > 0) conical, a single cusp. (k9-12). Paralleled in Pristella maxillaris. Some trees: Paralleled in Aphyodite grammica.
14. Rows of gill rakers on second ceratobranchial (193): (1 > 0) one. (k13-14). Paralleled in nodes 238 and 253 and in Bryconamericus exodon.
Autapomorphies of Aphyocharacidium bolivianum:
1. Lateral ridge of anguloarticular (107): (0 > 1) present. Paralleled in the Cheirodontinae, in node 191, and in Parecbasis cyclolepis.
2. Inner row of dentary teeth (143): (1 > 0) present. Reversal of synapomorphy 2 of node 189. Paralleled in the Heterocharacinae and in node 276.
3. Bony hooks on first pelvic-fin ray of adult males (315): (0 > 1) present. Paralleled in node 274 and in Aphyocharax anisitsi, Aulixidens eugeniae, and Nantis indefessus.
Some trees:
4. Anterior convergence of ventral diverging lamellae with nasal septum of mesethmoid (31): (0 > 1) confluent at posterior end of nasal septum. (k13-14).
Node 233: Cheirodontinae (100 / 100 / 75 / 41)
Genera Acinocheirodon Malabarba & Weitzman, Amazonspinther, Aphyocheirodon Eigenmann, Cheirodon, Cheirodontops Schultz, Compsura Eigenmann, Heterocheirodon, Kolpotocheirodon Malabarba & Weitzman, Macropsobrycon, †Megacheirodon, Nanocheirodon, Odontostilbe, Prodontocharax, Pseudocheirodon Meek & Hildebrand, Saccoderma Schultz, Serrapinnus, and Spintherobolus.
The Cheirodontinae is maybe the best studied subfamily of the Characidae. Its monophyly was cladistically proposed by Malabarba (1998a), supported by the presence of a pseudotympanum between the anterior two ribs, the absence of a humeral spot, the presence of distally expanded teeth with narrow base, and the presence of only one row of premaxillary teeth, which are aligned each other and have similar shapes. The monophyly of this subfamily was corroborated in the unpublished thesis of Bührnheim (2006). Thus, testing the monophyly of this clade is not a primary objective of this paper, and a relatively small sample of the species in this subfamily was included. The composition of this subfamily follows Malabarba (1998a), Bührnheim (2006), and Bührnheim et al. (2008).
Synapomorphies:
1. Lateral ridge of anguloarticular (107): (0 > 1) present. Paralleled in in node 191 and in Aphyocharacidium bolivianum and Parecbasis cyclolepis.
2. Abrupt posterior expansion of interopercle (164): (0 > 1) present. Paralleled in Apareiodon affinis, Inpaichthys kerri, and Pyrrhulina australis.
3. Relative length of first pelvic-fin ray of adult males (257): (0 > 1) extending beyond margin of other rays. Reversed in node 229.
Some trees:
4. Form of fourth infraorbital (67): (0 > 1) longer dorsoventrally than longitudinally. (k13-14). Reversal of synapomorphy 1 node 200. Paralleled in node 266. Some trees: Paralleled in node 256.
5. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. (some trees under k9-14). Paralleled in nodes 184 and 242, and in Aphyocharax dentatus, Engraulisoma taeniatum, Prionobrama paraguayensis, and Stichonodon insignis. Some trees: Paralleled in Microschemobrycon casiquiare.
6. Number of gill rakers on first hypobranchial and ceratobranchial (196): (1 > 0) 11 or more. (k13-14). Reversal of synapomorphy 5 of node 196. Paralleled in node 237 and in Bryconamericus cf. exodon and B. cf. iheringii. Some trees: Paralleled in Cyanocharax alburnus.
Autapomorphies of Prodontocharax melanotus:
1. Margins of toothed region of maxilla (96): (0 > 1) dorsally divergent. Paralleled in nodes 162, 209, 254, and 282 and in Rhoadsia altipinna.
2. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Reversal of synapomorphy 1 of node 202. Paralleled in node 208 and in Grundulus cochae, Hemibrycon surinamensis, and Nematobrycon palmeri.
3. Orientation of anterior dentary teeth (141): (0 > 1) oriented anteriorly, almost parallel to main axis of dentary. Paralleled in node 225.
4. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in node 296 and in Axelrodia lindeae, Gymnocharacinus bergii, Hollandichthys multifasciatus, Mimagoniates rheocharis, Nematocharax venustus, and Paracheirodon axelrodi.
5. Form of anterior expansion of basihyal (191): (0 > 1) expanded, with anterior margin with two-thirds or more of its length. Paralleled in Aulixidens eugeniae, Cyphocharax spilotus, and Thayeria obliqua.
6. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
7. Bony lamellae associated with supraneurals (282): (0 > 1) wider than primary axis of supraneurals. Reversal of synapomorphy 5 of node 197. Paralleled in Parecbasis cyclolepis.
8. Number of branched anal-fin rays (287): (1 > 0) 17 or less. Reversal of synapomorphy 6 of the Characoidea. Paralleled in the Alestidae, in nodes 280 and 290, and in Attonitus ephimeros. Some trees: Paralleled in Hasemania nana.
9. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi (335): (1 > 0) absent. Paralleled in Creagrutus anary, Inpaichthys kerri, Piabucus melanostomus, Prionobrama paraguayensis, Pristella maxillaris, and Pyrrhulina australis. Some trees: Paralleled in Hyphessobrycon elachys.
Node 232: (100 / 100 / 61 / 18)
Genera Acinocheirodon?, Amazonspinther?, Aphyocheirodon?, Cheirodon, Cheirodontops?, Compsura?, Heterocheirodon?, Kolpotocheirodon?, Macropsobrycon?, †Megacheirodon?, Nanocheirodon?, Odontostilbe, Pseudocheirodon?, Saccoderma?, Serrapinnus, and Spintherobolus?
Prodontocharax is the sister group of the remaining Cheirodontinae according to the present analysis, which is in agreement with the hypothesis of Malabarba (1998a). However, it is probable that some of the synapomorphies found for this node actually correspond to a more inclusive clade.
Synapomorphies:
1. Lateral coverage of dilator fossa by sixth infraorbital (69): (1 > 0) almost complete, at least in its ventral border. Reversal of synapomorphy 2 of node 197. Paralleled in Parecbasis cyclolepis. Reversed in Cheirodon interruptus.
2. Expansion of lamellar portion of maxilla just posterior to toothed region (97): (0 > 1) very pronounced. Paralleled in Deuterodon langei. Some trees: Paralleled in Paracheirodon axelrodi.
3. Premaxillary, maxillary, and dentary teeth (119): (0 > 1) pedunculate and uniformly shaped. Paralleled in Gymnocharacinus bergii and Odontostoechus lethostigmus.
4. Number of maxillary teeth (136): (1 > 0) up to three. Reversal of synapomorphy 8 of node 205. Paralleled in node 246 and in Aulixidens eugeniae.
5. Last unbranched dorsal-fin ray of adult males (269): (0 > 1) distinctly longer than first branched ray and in the form of filament. Reversed in node 229.
6. Bony hooks on last pelvic-fin ray of adult males (314): (0 > 1) as numerous as in other rays. Paralleled in nodes 240, 258, and 299 and in Axelrodia lindeae.
Autapomorphy of Odontostilbe pequira:
1. Length of maxilla relative to dentary (100): (1 > 0) maxilla reaching posterior end of Meckelian cartilage. Reversal of synapomorphy 2 of node 234.
Node 231: (90 / 99 / 7 / 32)
Genera Acinocheirodon?, Amazonspinther?, Aphyocheirodon?, Cheirodon, Cheirodontops?, Compsura?, Heterocheirodon?, Kolpotocheirodon?, Macropsobrycon?, †Megacheirodon?, Nanocheirodon?, Pseudocheirodon?, Saccoderma?, Serrapinnus, and Spintherobolus?; Odontostilbe microcephala, O. paraguayensis, and other Odontostilbe?
Synapomorphies:
1. Foramen on articular condyle of quadrate (149): (0 > 1) present. Paralleled in nodes 168 and 211 and in Grundulus cochae, Hasemania nana, Hyphessobrycon eques, and Lonchogenys ilisha.
2. Number of notches along ventral border of anterior ceratohyal (180): (0 > 1) three.
Autapomorphies of Odontostilbe microcephala:
1. Laterosensory canal of sixth infraorbital (76): (0 > 1) branched. Reversal of synapomorphy 2 of node 203. Paralleled in Markiana nigripinnis, Oligosarcus cf. jenynsii, and Tetragonopterus argenteus .
2. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. Paralleled in nodes 244 and 299 and in Hasemania nana, Hyphessobrycon bifasciatus, Metynnis maculatus, and Roeboides descalvadensis. Some trees: Paralleled in node 265.
3. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
Node 230: (90 / 98 / - / 100)
Genera Acinocheirodon?, Amazonspinther?, Aphyocheirodon?, Cheirodon, Cheirodontops?, Compsura?, Heterocheirodon?, Kolpotocheirodon?, Macropsobrycon?, †Megacheirodon?, Nanocheirodon?, Pseudocheirodon?, Saccoderma?, Serrapinnus, and Spintherobolus?; Odontostilbe paraguayensis and other Odontostilbe?
Synapomorphy:
1. Dorsal end of laterosensory canal of preopercle and suprapreopercle (82): (0 > 1) overlapping anterodorsal process of opercle. Paralleled in the Alestidae and in Bario steindachneri, Hyphessobrycon eques, Parecbasis cyclolepis, Pristella maxillaris, and Stichonodon insignis.
Autapomorphies of Odontostilbe paraguayensis:
1. Length of supraoccipital spine (53): (1 > 0) extends posteriorly to, at least, middle length of neural complex of Weberian apparatus. Paralleled in node 207 and in Parecbasis cyclolepis.
2. Process of scapula forming anterior border of scapular foramen (244): (0 > 1) absent. Paralleled in nodes 193 and 252 and in Aphyodite grammica, Deuterodon langei, Hoplias cf. malabaricus, Hyphessobrycon herbertaxelrodi, Leporinus striatus, and Thayeria obliqua.
Node 229: (100 / 100 / 93 / 29)
Genera Acinocheirodon?, Amazonspinther, Cheirodon, Compsura?, Heterocheirodon, Kolpotocheirodon?, Macropsobrycon?, †Megacheirodon, Nanocheirodon, Saccoderma?, Serrapinnus, and Spintherobolus.
The genera Cheirodon and Serrapinnus were included in the tribe Cheirodontini by Malabarba (1998a), along with Heterocheirodon, †Megacheirodon, Nanocheirodon, and Spintherobolus. The genera Acinocheirodon and Kolpotocheirodon were described posteriorly to that paper (Malabarba & Weitzman, 1999, 2000) and these two genera were included in the tribe Compsurini. As the relationships between the Compsurini and the Cheirodontini are unresolved (Malabarba, 1998a) the species of the Compsurini are included with question marks. The genus Amazonspinther is the sister group of Spintherobolus, forming a clade supported by 15 synapomorphies (Bührnheim et al., 2008); indeed it was originally treated as a species of this genus (Bührnheim, 2006). Both Amazonspinther and Spintherobolus are included in Cheirodontini according to Bührnheim et al. (2008), and they are listed in this clade.
Synapomorphies:
1. Lateral line (91): (0 > 1) interrupted. Paralleled in nodes 227, 279, 288, and 294 and in Characidium rachovii, Hoplocharax goethei, Hyphessobrycon anisitsi, Moenkhausia sanctaefilomenae, Phenacogaster tegatus, and Pyrrhulina australis.
2. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
3. Relative length of first pelvic-fin ray of adult males (257): (1 > 0) not extending beyond margin of other rays. Reversal of synapomorphy 3 of the Cheirodontinae.
4. Last unbranched dorsal-fin ray of adult males (269): (1 > 0) approximately as long as first branched ray. Reversal of synapomorphy 5 of node 232.
5. Ventral procurrent caudal-fin rays of adult males (301): (0 > 1) laminar. Paralleled in Axelrodia lindeae.
6. Number of ventral procurrent caudal-fin rays (302): (0 > 1) 12 or more. Paralleled in the Bryconops clade, in node 252, and in Salminus brasiliensis.
7. Ventral procurrent caudal-fin rays of adult males (303): (0 > 1) projecting ventrally through peduncle musculature and skin. Paralleled in Axelrodia lindeae and Hoplocharax goethei.
8. Bony hooks on base of pelvic-fin rays of adult males (313): (0 > 1) as numerous as on segmented portion of rays. Paralleled in Aphyocharax anisitsi.
Autapomorphies of Serrapinnus calliurus:
1. Transitional vertebrae with haemal canal (229): (1 > 0) present. Reversal of synapomorphy 6 of node 195. Paralleled in Parecbasis cyclolepis.
2. Form and length of anterior anal-fin rays (290): (0 > 1) longer and more compressed laterally than posterior rays.
3. Position of anal-fin bony hooks of adult males (316): (0 > 1) medially positioned and oriented posteriorly.
Autapomorphies of Cheirodon interruptus:
1. Lateral coverage of dilator fossa by sixth infraorbital (69): (0 > 1) leaving a conspicuous naked area in anterior region of dilator fossa. Reversal of synapomorphy 1 of the Cheirodontinae.
2. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in the Astyanax clade, in nodes 284 and 290, and in Aulixidens eugeniae, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
3. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249 and in Attonitus ephimeros.
4. Posterior margin of cleithrum (234): (1 > 0) without concavity ventral to first postcleithrum. Reversal of synapomorphy 2 of node 196. Paralleled in node 298 and in Inpaichthys kerri.
5. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Axelrodia lindeae, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Node 243: Stevardiinae (74 / 97 / 43 / 9)
Genera Acrobrycon Eigenmann & Pearson, Argopleura, Attonitus, Aulixidens, Boehlkea?, Bryconacidnus?, Bryconadenos?, Bryconamericus, Caiapobrycon?, Ceratobranchia ?, Chrysobrycon Weitzman & Menezes, Corynopoma Gill, Creagrutus, Cyanocharax, Diapoma, Gephyrocharax Eigenmann, Glandulocauda, Hemibrycon, Hypobrycon?, Hysteronotus Eigenmann, Iotabrycon Roberts, Knodus, Landonia Eigenmann & Henn, Lophiobrycon, Microgenys?, Mimagoniates, Monotocheirodon?, Nantis, Odontostoechus, Othonocheirodus?, Phallobrycon?, Phenacobrycon Eigenmann, Piabarchus?, Piabina, Planaltina Böhlke, Pseudocorynopoma, Pterobrycon Eigenmann, Ptychocharax Weitzman, Fink, Machado-Allison & Royero, Rhinobrycon?, Rhinopetitia?, Scopaeocharax Weitzman & Fink, Tyttocharax Fowler, and Xenurobrycon Myers & Miranda Ribeiro.
Géry (1977) included in his tribe Tetragonopterini a group named Hemibrycon and allied genera, based on the presence of only four teeth in the inner premaxillary row. Géry mentioned that this character is usually associated with a great development of the third supraorbital, which reaches the horizontal arm of the preopercle. This group was composed of Boehlkea, Bryconacidnus, Bryconamericus, Carlastyanax, Ceratobranchia, Coptobrycon, Creagrudite, Hemibrycon, Knodus, Microgenys, Nematobrycon, Piabarchus, Rhinobrycon, and Rhinopetitia. All these genera were classified as incertae sedis within the Characidae by Lima et al. (2003). Malabarba & Weitzman (2003) observed that most species of these genera also share the possession of only eight branched dorsal-fin rays. Those authors proposed a putatively monophyletic clade (their clade A) which comprised the members of the Glandulocaudinae of Weitzman (2003) and the incertae sedis genera Attonitus, Boehlkea, Bryconacidnus, Bryconamericus, Caiapobrycon, Ceratobranchia, Creagrutus, Cyanocharax, Hemibrycon, Hypobrycon, Knodus, Microgenys, Monotocheirodon, Odontostoechus, Othonocheirodus, Piabarchus, Piabina, Rhinobrycon, and Rhinopetitia. Weitzman et al. (2005) later described Bryconadenos within this clade, and split the glandulocaudin characids into the subfamilies Glandulocaudinae and Stevardiinae. Ruiz-C. & Román-Valencia (2006) subsequently synonymized Carlastyanax with Astyanax. This clade appears as monophyletic in the molecular phylogeny of Calcagnotto et al. (2005), as composed of the genera Bryconamericus, Creagrutus, Gephyrocharax, Hemibrycon, Knodus, and Mimagoniates. In the present hypothesis this node includes Aulixidens and Nantis, along with the analyzed genera of the clade A of Malabarba & Weitzman (2003). The genus Nantis was described after Malabarba & Weitzman (2003) and in its description some similarities with members of the clade A were mentioned. These include the sharing of only eight branched dorsal-fin rays, but Nantis was considered as incertae sedis because of the possession of five teeth in the inner premaxillary row, instead of four (Mirande et al., 2004; 2006a). Aulixidens eugeniae was examined by Malabarba & Weitzman (2003), who observed nine branched dorsal-fin rays in this species, differing from the eight rays herein observed. Aulixidens eugeniae is included in this clade even after coding that character as polymorphic for the species. Most genera included in this subfamily lack phylogenetic diagnoses; however all the genera are herein treated as monophyletic, considering the position of their type-species. For example, according to the present analysis, Bryconamericus is not monophyletic, and at least one of its species (B. scleroparius) is excluded from the Stevardiinae; however, as B. exodon, its type-species, is included in the Stevardiinae, this genus is considered as part of it. The genera included in the clade A by Malabarba & Weitzman (2003) but not analyzed here are listed with question marks. All members of the former subfamilies Glandulocaudinae and Stevardiinae are included in this clade because these clades were phylogenetically treated (as the Glandulocaudinae) by Weitzman & Menezes (1998). The name Stevardiinae Eigenmann, 1909 is used for this clade due to priority over Glandulocaudinae Eigenmann, 1914 and Hemibryconini Géry, 1966. Diapomini Eigenmann, 1909, although proposed in the same year than Stevardiinae, was a subclade of Stevardiinae prior to Mirande (2009); thus, the latter name is preferred for this clade. In this manner, the subfamily Stevardiinae is herein redefined to include all the genera of the clade A of Malabarba & Weitzman (2003) plus Aulixidens and Nantis.
Synapomorphies:
1. Epiphyseal branch of supraorbital canal (84): (0 > 1) absent. Paralleled in Microschemobrycon casiquiare. Reversed in node 253.
2. Number of branched-rays on dorsal-fin (270): (1 > 0) eight or fewer. Paralleled in Coptobrycon bilineatus, Hoplocharax goethei, and Piabucus melanostomus. Reversed in node 235.
3. Number of dorsal pterygiophores (276): (1 > 0) nine. Paralleled in node 184 and in Hoplocharax goethei and Piabucus melanostomus. Reversed in Mimagoniates rheocharis.
Node 255: (100 / 100 / 90 / 22)
Genera Boehlkea? and Hemibrycon.
The monophyly of Hemibrycon was treated in the unpublished doctoral thesis of Bertaco (2008) and this issue lies beyond the scope of this paper. The two species of this genus form a monophyletic group that is the sister group of the remaining members of the Stevardiinae. According to their traditional definitions, Boehlkea (not analyzed here) is differentiated from Hemibrycon only in having the caudal fin covered by scales. Given that this character is rather homoplastic in this phylogeny, Boehlkea is potentially related or even included in Hemibrycon, and tentatively listed in this clade.
Synapomorphies:
1. Abrupt decrease in size of dentary teeth (148): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 222 and 299, and in Astyanax latens and A. paris.
2. Relative length of palatine (172): (1 > 0) approximately one-half length of ectopterygoid, or less. Reversal of synapomorphy 4 of node 197. Paralleled in Acrobrycon tarijae, Aphyocharax nattereri, Gymnocharacinus bergii, Parecbasis cyclolepis, and Piabina argentea.
3. Palatine foramen (173): (0 > 1) present and very conspicuous. Paralleled in Acrobrycon tarijae.
4. Number of branched anal-fin rays (288): (0 > 1) 25 or more. Reversal of synapomorphy 3 of node 200. Paralleled in nodes 208 and 263 and in Bryconamericus scleroparius and Nematobrycon palmeri.
Autapomorphy of Hemibrycon surinamensis:
1. Extent of implantation of teeth along maxilla (137): (0 > 1) extending across almost entire maxillary lamella. Reversal of synapomorphy 1 of node 202. Paralleled in node 208 and in Grundulus cochae, Nematobrycon palmeri, and Prodontocharax melanotus.
Autapomorphy of Hemibrycon dariensis:
1. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273, 283, and 294, and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Some trees: Paralleled in node 246.
Node 242: (39 / 96 / 26 / 9)
Genera Acrobrycon, Argopleura, Attonitus, Aulixidens, Boehlkea?, Bryconacidnus?, Bryconadenos?, Bryconamericus, Caiapobrycon?, Ceratobranchia?, Chrysobrycon, Corynopoma, Creagrutus, Cyanocharax, Diapoma, Gephyrocharax, Glandulocauda, Hypobrycon?, Hysteronotus, Iotabrycon, Knodus, Landonia, Lophiobrycon, Microgenys?, Mimagoniates, Monotocheirodon?, Nantis, Odontostoechus, Othonocheirodus?, Phallobrycon?, Phenacobrycon, Piabarchus?, Piabina, Planaltina, Pseudocorynopoma, Pterobrycon, Ptychocharax, Rhinobrycon?, Rhinopetitia?, Scopaeocharax, Tyttocharax, and Xenurobrycon.
The situation of Hemibrycon as the sister group of the remaining Stevardiinae was not mentioned explicitly, but a basal position of this genus, based on its generalized morphology was proposed in the literature (e. g. Géry, 1977). The Stevardiinae, as recognized herein, is not monophyletic in the analysis of Lucena (1993), with Hemibrycon being in a relatively basal position within the Characidae, as the sister group of a clade which includes most members of the family. In the analysis of Calcagnotto et al. (2005) Hemibrycon is the sister group of Creagrutus in a terminal node within the Stevardiinae, as recognized here. This node includes all the Stevardiinae excepting Hemibrycon, and maybe Boehlkea, which is, however, listed with a question mark.
Synapomorphies:
1. Small foramen near posterior margin of pterosphenoid (44): (0 > 1) present, pierced by a branch of supraorbital nerve. Reversed in Mimagoniates rheocharis.
2. Contact between ectopterygoid and anterodorsal region of quadrate (162): (0 > 1) absent. Paralleled in node 184 and in Aphyocharax dentatus, Engraulisoma taeniatum, Prionobrama paraguayensis, and Stichonodon insignis. Some trees: Paralleled in the Cheirodontinae and in Microschemobrycon casiquiare.
3. Form of third postcleithrum (250): (1 > 0) slender, without associated lamella. Reversal of synapomorphy 6 of node 204. Paralleled in Gymnocharacinus bergii, Pseudochalceus kyburzi, and Rhoadsia altipinna.
Autapomorphies of Cyanocharax alburnus:
1. Development of transverse process of neural arch of third vertebra (219): (0 > 1) well developed and extending beyond anterior margin of tripus. Paralleled in node 302, and in Agoniates anchovia, Deuterodon langei, Engraulisoma taeniatum, Hemiodus cf. thayeria, Roeboexodon geryi, and Thayeria obliqua. Some trees: Paralleled in Microschemobrycon casiquiare and Parecbasis cyclolepis.
2. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 236, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Some trees:
3. Number of gill rakers on first hypobranchial and ceratobranchial (196): (1 > 0) 11 or more. (k13-14). Reversal of synapomorphy 5 of node 196. Paralleled in node 237 and in Bryconamericus cf. exodon and B. cf. iheringii. Some trees: Paralleled in the Cheirodontinae.
Node 241: (6 / 93 / 5 / 22)
Genera Acrobrycon, Argopleura, Attonitus, Aulixidens, Boehlkea?, Bryconacidnus?, Bryconadenos?, Bryconamericus, Caiapobrycon?, Ceratobranchia?, Chrysobrycon, Corynopoma, Creagrutus, Diapoma, Gephyrocharax, Glandulocauda, Hypobrycon?, Hysteronotus, Iotabrycon, Knodus, Landonia, Lophiobrycon, Microgenys?, Mimagoniates, Monotocheirodon?, Nantis, Odontostoechus, Othonocheirodus?, Phallobrycon?, Phenacobrycon, Piabarchus?, Piabina, Planaltina, Pseudocorynopoma, Pterobrycon, Ptychocharax, Rhinobrycon?, Rhinopetitia?, Scopaeocharax, Tyttocharax, and Xenurobrycon.
Synapomorphy:
1. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (0 > 1) on vertical through middle or anterior half of Meckelian cartilage. Paralleled in the Iguanodectinae, in nodes 184, 186, 209, 261, and 270, and in Engraulisoma taeniatum and Gymnocharacinus bergii. Reversed in nodes 237 and 298 and in Attonitus ephimeros.
Node 246: (-11 / 73 / - / 100)
Bryconamericus agna, B. alpha, B. exodon, other Bryconamericus?, Knodus breviceps, other Knodus?; Phallobrycon?
The monophyly of Knodus has been repeatedly challenged in the literature (e. g. Géry, 1977), and this genus was even proposed to be synonymized with Bryconamericus (Román-Valencia, 2000). A study of the monophyly and relationships of Knodus is beyond the scope of this paper. This clade includes Bryconamericus exodon, the type species of this genus, along with B. agna, B. alpha, and Knodus breviceps, the only species of that genus herein analyzed. This clade has a basal polytomy, having as a possible solution, the sister-group relationship between Knodus and the true Bryconamericus. However, both the resolution of this clade and the position of Knodus meridae Eigenmann, the type species of the genus need to be resolved to arrive at conclusions about the validity and relationships of Knodus. Although the relationships of Phallobrycon are unknown, Menezes et al. (2009) tentatively hypothesized the close relationship of this genus with the inseminating species of Knodus, recognizing however that such a hypothesis is an oversimplification of a very complex problem. The inclusion of Phallobrycon in this node is tentative, because it would depend both on the corroboration of the hypothesis proposed by Menezes et al. (2009) and the position of the inseminating species of Knodus, which were not analyzed in this paper.
Synapomorphies:
1. Horizontal process of anguloarticular (108): (0 > 1) broadly covered by dentary which reaches posterior border of Meckelian cartilage. Reversal of synapomorphy 1 of node 206. Paralleled in nodes 253 and 261 and in Xenagoniates bondi.
2. Number of maxillary teeth (136): (1 > 0) up to three. Reversal of synapomorphy 8 of node 205. Paralleled in node 232 and in Aulixidens eugeniae.
Some trees:
3. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. (k9-11). Paralleled in the Rhoadsiinae, in nodes 273, 283, and 294, and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus. Reversed in node 247.
Autapomorphies of Knodus breviceps:
1. Anterior convergence of ventral diverging lamellae with nasal septum of mesethmoid (31): (1 > 0) absent, or confluent near anterior end of nasal septum. Paralleled in Aulixidens eugeniae and Coptobrycon bilineatus.
Some trees:
2. Contact between frontals anteriorly to frontal fontanel (21): (0 > 1) present. (k9-11). Reversal of synapomorphy 4 of node 206. Paralleled in Bario steindachneri, Exodon paradoxus, Galeocharax humeralis, Hyphessobrycon pulchripinnis, and Odontostoechus lethostigmus.
3. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. (k12-14). Paralleled in nodes 162, 285, and 301 and in Aulixidens eugeniae, Creagrutus anary, Diapoma speculiferum, Moenkhausia cf. intermedia, and Pyrrhulina australis. Some trees: Paralleled in node 247.
4. Scales covering caudal-fin lobes (328): (0 > 1) covering one-third of their length. (k9-11). Paralleled in node 222 and in Aulixidens eugeniae, Distichodus maculatus, Markiana nigripinnis, and Nematocharax venustus.
Autapomorphies of Bryconamericus alpha:
Some trees:
1. Transitional vertebrae with haemal canal (229): (0 > 1) absent. (some trees under k9-14). Paralleled in nodes 195 and 212, and in Aulixidens eugeniae, Engraulisoma taeniatum, Metynnis maculatus, and Piabina argentea. Some trees: Paralleled in node 247 and in Paracheirodon axelrodi.
Autapomorphies of Bryconamericus agna:
Some trees:
1. Length of ascending process of premaxilla (104): (0 > 1) reaching just anterior end of nasal. (some trees under k9-14) . Paralleled in nodes 225 and 294 and in Charax stenopterus, Phenacogaster tegatus, and Stichonodon insignis.
2. Cusps of teeth on outer premaxillary row (125): (0 > 1) five or more cusps. (some trees under k9-14). Paralleled in nodes 265 and 294 and in Brycon orbignyanus, Bryconops melanurus, Gymnocharacinus bergii, Micralestes stormsi, and Nematocharax venustus.
Node 247: (100 / 100 / 76 / 10)
Bryconamericus exodon, other Bryconamericus?
Synapomorphies:
1. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162 and 253 and in Agoniates anchovia, Attonitus ephimeros, Characidium borellii, Iguanodectes geisleri, Moenkhausia cf. intermedia, Prionobrama paraguayensis, and Xenagoniates bondi.
Some trees:
2. Alignment of teeth on anterior premaxillary row (124): (0 > 1) not aligned, with one or two teeth situated anterior to remaining teeth. (k12-14).
3. Number of cusps of anterior maxillary teeth (139): (1 > 0) up to three. (k9-11). Reversal of synapomorphy 3 of node 246.
4. Rows of gill rakers on first ceratobranchial (192): (1 > 0) one. (k9-11).
5. Transitional vertebrae with haemal canal (229): (0 > 1) absent. (some trees under k9-14). Paralleled in nodes 195 and 212, and in Aulixidens eugeniae, Engraulisoma taeniatum, Metynnis maculatus, and Piabina argentea. Some trees: Paralleled in Bryconamericus alpha and Paracheirodon axelrodi.
6. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. (k12-14). Paralleled in nodes 162, 285, and 301 and in Aulixidens eugeniae, Creagrutus anary, Diapoma speculiferum, Moenkhausia cf. intermedia, and Pyrrhulina australis. Some trees: Paralleled in Knodus breviceps.
Autapomorphy of Bryconamericus cf. exodon:
1. Number of gill rakers on first hypobranchial and ceratobranchial (196): (1 > 0) 11 or more. Reversal of synapomorphy 5 of node 196. Paralleled in node 237 and in Bryconamericus cf. iheringii. Some trees: Paralleled in the Cheirodontinae and in Cyanocharax alburnus.
Autapomorphies of Bryconamericus exodon:
1. Rows of gill rakers on second ceratobranchial (193): (1 > 0) one. Paralleled in nodes 238 and 253. Some trees: Paralleled in Axelrodia lindeae.
2. Color of caudal-fin lobes (345): (0 > 3) both lobes dark brown or black. Paralleled in node 297.
Node 240: (-18 / 84 / - / 14)
Genera Acrobrycon, Argopleura, Attonitus, Aulixidens, Boehlkea?, Bryconacidnus?, Bryconadenos?, Caiapobrycon?, Ceratobranchia?, Chrysobrycon, Corynopoma, Creagrutus, Diapoma, Gephyrocharax, Glandulocauda, Hypobrycon?, Hysteronotus, Iotabrycon, Landonia, Lophiobrycon, Microgenys?, Mimagoniates, Monotocheirodon?, Nantis, Odontostoechus, Othonocheirodus?, Phallobrycon?, Phenacobrycon, Piabarchus?, Piabina, Planaltina, Pseudocorynopoma, Pterobrycon, Ptychocharax, Rhinobrycon?, Rhinopetitia?, Scopaeocharax, Tyttocharax, and Xenurobrycon; Bryconamericus iheringii, B. mennii, B. rubropictus, B. thomasi, other Bryconamericus?
The species of Bryconamericus included in this clade are separated from B. exodon, the type species of the genus, and then should be transferred to another genus according to this study.
Synapomorphy:
1. Bony hooks on last pelvic-fin ray of adult males (314): (0 > 1) as numerous as in other rays. Paralleled in nodes 232, 258, and 299 and in Axelrodia lindeae.
Node 239: (-20 / 59 / - / 22)
Genera Acrobrycon, Argopleura, Attonitus, Aulixidens, Boehlkea?, Bryconacidnus?, Bryconadenos?, Caiapobrycon?, Ceratobranchia?, Chrysobrycon, Corynopoma, Diapoma, Gephyrocharax, Glandulocauda, Hypobrycon?, Hysteronotus, Iotabrycon, Landonia, Lophiobrycon, Microgenys?, Mimagoniates, Monotocheirodon?, Othonocheirodus?, Phenacobrycon, Piabarchus?, Planaltina, Pseudocorynopoma, Pterobrycon, Ptychocharax, Rhinobrycon?, Rhinopetitia?, Scopaeocharax, Tyttocharax, and Xenurobrycon; Bryconamericus mennii, other Bryconamericus?
The single synapomorphy supporting this clade is the presence of insemination. This feature was reported for several genera of the Characidae, including the members of the former subfamilies Glandulocaudinae and Stevardiinae, which are included in this node (e. g. Weitzman et al., 2005). The presence or absence of insemination in many species was not evaluated, and this character (coded exclusively from literature) has many missing entries in the data matrix. The study of the reproductive biology of a higher number of species would serve as a better evaluation of both the phylogenetic informativeness of this character and the monophyly of this clade.
Synapomorphy:
1. Insemination (358): (0 > 1) present. Paralleled in Hollandichthys multifasciatus.
Node 245: (32 / 70 / 2 / 4)
Genera Attonitus, Aulixidens, Boehlkea?, Bryconacidnus?, Bryconadenos, Caiapobrycon?, Ceratobranchia?, Hypobrycon?, Microgenys?, Monotocheirodon?, Othonocheirodus?, Piabarchus?, Rhinobrycon?, Rhinopetitia?; some Bryconamericus?
The close relationship between Attonitus and Aulixidens was not previously proposed. The genus Attonitus was described from six synapomorphies which are unique or very unusual in Characidae (Vari & Ortega, 2000). Malabarba & Vari (2000) mentioned the similarity in the position of the mouth in Attonitus, Caiapobrycon, Ceratobranchia, Creagrutus, Hypobrycon, Othonocheirodus, Piabina, and Rhinobrycon, highlighting that all these genera have also four teeth in the inner premaxillary row. Almirón et al. (2001) listed a series of features related with the mouth position and the dentition which characterize the genera Attonitus, Caiapobrycon, and Hypobrycon. Weitzman et al. (2005) proposed the close relationship between Attonitus and Bryconadenos, based in the shared presence of glandular cells at the anal-fin base; as this is an unique feature among characids, Bryconadenos was included in this clade by those authors. The study of the reproductive biology of Aulixidens and the inclusion of members of Bryconadenos, Caiapobrycon, and Hypobrycon in a phylogeny of the family would undoubtedly serve to test the hypothesis herein proposed.
Synapomorphies:
1. Length of maxilla relative to dentary (100): (0 > 1) maxilla not reaching posterior end of Meckelian cartilage. Paralleled in node 234.
2. Form of teeth of inner premaxillary tooth row (128): (0 > 1) with cusps aligned in straight series and without anterior concavity. Paralleled in the Rhoadsiinae, in nodes 195 and 280, and in Hemigrammus bleheri and Odontostoechus lethostigmus.
3. Denticles on gill rakers (201): (0 > 1) absent. Paralleled in the Gymnocharacinae, in node 253, and in Axelrodia lindeae and Pseudochalceus kyburzi. Some trees: Paralleled in Hyphessobrycon elachys and H. herbertaxelrodi.
Autapomorphies of Aulixidens eugeniae:
1. Form of anterior process of lateral ethmoid (14): (1 > 0) broad in ventral view, contacting proximal region of vomer in its entire length. Reversal of synapomorphy 1 of node 179. Paralleled in Rhaphiodon vulpinus and Salminus brasiliensis.
2. Anterior convergence of ventral diverging lamellae with nasal septum of mesethmoid (31): (1 > 0) absent, or confluent near anterior end of nasal septum. Paralleled in Coptobrycon bilineatus and Knodus breviceps.
3. Anterior end of ascending process of maxilla (94): (1 > 0) with conspicuous notch. Paralleled in node 184.
4. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Carlana eigenmanni, Carnegiella strigata, Grundulus cochae, Odontostoechus lethostigmus, Paracheirodon axelrodi, Piabucus melanostomus, and Probolodus heterostomus.
5. Maxillary teeth (134): (1 > 0) absent. Paralleled in Coptobrycon bilineatus, Iguanodectes geisleri, Parecbasis cyclolepis, and Stichonodon insignis. Some trees: Paralleled in Hyphessobrycon elachys and Psellogrammus kennedyi.
6. Number of maxillary teeth (135): (1 > 0) only one, or absent. Paralleled in the Astyanax clade, in nodes 284 and 290, and in Cheirodon interruptus, Coptobrycon bilineatus, Hyphessobrycon bifasciatus, and Iguanodectes geisleri. Some trees: Paralleled in Hasemania nana, Paracheirodon axelrodi, and Parecbasis cyclolepis.
7. Number of maxillary teeth (136): (1 > 0) up to three. Reversal of synapomorphy 8 of node 205. Paralleled in nodes 232 and 246.
8. Separation between posterior dentary teeth (147): (0 > 1) more than width of these teeth. Paralleled in node 221 and in Astyanax cf. rutilus and Pristella maxillaris.
9. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
10. Shape of ectopterygoid (156): (0 > 1) triangular and much broadened anteriorly.
11. Form of anterior expansion of basihyal (191): (0 > 1) expanded, with anterior margin with two-thirds or more of its length. Paralleled in Cyphocharax spilotus, Prodontocharax melanotus, and Thayeria obliqua.
12. Interhyal (210): (0 > 1) absent. Paralleled in Engraulisoma taeniatum.
13. Transitional vertebrae with haemal canal (229): (0 > 1) absent. Paralleled in nodes 195 and 212, and in Engraulisoma taeniatum, Metynnis maculatus, and Piabina argentea. Some trees: Paralleled in node 247 and in Bryconamericus alpha and Paracheirodon axelrodi.
14. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. Paralleled in nodes 162, 285, and 301 and in Creagrutus anary, Diapoma speculiferum, Moenkhausia cf. intermedia, and Pyrrhulina australis. Some trees: Paralleled in node 247 and in Knodus breviceps.
15. Bony hooks on first pelvic-fin ray of adult males (315): (0 > 1) present. Paralleled in node 274 and in Aphyocharacidium bolivianum, Aphyocharax anisitsi, and Nantis indefessus.
16. Scales covering caudal-fin lobes (328): (0 > 1) covering one-third of their length. Paralleled in node 222 and in Distichodus maculatus, Markiana nigripinnis, and Nematocharax venustus. Some trees: Paralleled in Knodus breviceps.
Autapomorphies of Attonitus ephimeros:
1. Position of sphenotic spine relative to the orbit (12): (0 > 1) distinctly posterior to orbital margin. Paralleled in nodes 193 and 299 and in Acestrorhynchus pantaneiro, Cynopotamus argenteus, and Gymnocharacinus bergii.
2. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, 280, and 298 and in Aphyocharax nattereri, Bryconamericus scleroparius, Brycon orbignyanus, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
3. Position of opening on neurocranium communicating with laterosensory canal of sixth infraorbital (77): (0 > 1) in frontal. Paralleled in nodes 193 and 249 and in Micralestes stormsi.
4. Ventral margin of horizontal process of anguloarticular (109): (1 > 0) posteroventrally angled relative to laterosensory canal of dentary from medial view. Reversal of synapomorphy 1 of node 199. Paralleled in nodes 195, 280, and 298.
5. Form of anterior portion of ectopterygoid (157): (0 > 1) slender and articulating only to lateral margin of palatine, and lacking ligaments to neurocranium. Paralleled in the Alestidae, in node 170, and in Agoniates anchovia.
6. Bony lamellae between second and third basibranchials (184): (1 > 0) absent. Paralleled in the Serrasalmidae and in Axelrodia lindeae, Hollandichthys multifasciatus, Hoplocharax goethei, Jupiaba scologaster, Piabucus melanostomus, Pyrrhulina australis, Rhaphiodon vulpinus, and Xenagoniates bondi.
7. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162, 247, and 253 and in Agoniates anchovia, Characidium borellii, Iguanodectes geisleri, Moenkhausia cf. intermedia, Prionobrama paraguayensis, and Xenagoniates bondi.
8. Number of branched anal-fin rays (287): (1 > 0) 17 or less. Reversal of synapomorphy 6 of the Characoidea. Paralleled in the Alestidae, in nodes 280 and 290, and in Prodontocharax melanotus. Some trees: Paralleled in Hasemania nana.
9. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (1 > 0) on vertical through posterior half of Meckelian cartilage. Reversal of synapomorphy 1 of node 241. Paralleled in nodes 237 and 298.
Some trees:
10. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. (k12-14). Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Paralleled in node 249.
Node 238: (-13 / 87 / - / 22)
Genera Acrobrycon, Argopleura, Boehlkea?, Bryconacidnus?, Bryconadenos?, Caiapobrycon?, Ceratobranchia?, Chrysobrycon, Corynopoma, Diapoma, Gephyrocharax, Glandulocauda, Hypobrycon?, Hysteronotus, Iotabrycon, Landonia, Lophiobrycon, Microgenys?, Mimagoniates, Monotocheirodon?, Othonocheirodus?, Phenacobrycon, Piabarchus?, Planaltina, Pseudocorynopoma, Pterobrycon, Ptychocharax, Rhinobrycon?, Rhinopetitia?, Scopaeocharax, Tyttocharax, and Xenurobrycon; Bryconamericus mennii, other Bryconamericus?
This node includes Bryconamericus mennii and the Glandulocaudinae of Weitzman & Menezes (1998). This relationship, however, may change when some reproductive biology features of Bryconamericus mennii become known. The species lacks sexual dimorphism, and males even lack bony hooks, contrary to the Glandulocaudinae of Weitzman (2003), in which the sexual characters are much developed. Although Bryconamericus mennii has spermatozoa of the aquasperm type (Miquelarena et al., 2002), the evaluation of the presence of insemination in this species would be useful to test the monophyly of this clade.
Synapomorphy:
1. Rows of gill rakers on second ceratobranchial (193): (1 > 0) one. Paralleled in node 253 and in Bryconamericus exodon. Some trees: Paralleled in Axelrodia lindeae. Reversed in Diapoma terofali.
Autapomorphy of Bryconamericus mennii:
1. Bony hooks on fin rays (307): (1 > 0) absent. Paralleled in the Gymnocharacinae, in nodes 207, 283, 297, and 301, and in Astyanax paris, Exodon paradoxus, Inpaichthys kerri, Pseudochalceus kyburzi, and Rhoadsia altipinna. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
Node 237: (83 / 98 / 64 / 40)
Genera Acrobrycon, Chrysobrycon, Corynopoma, Diapoma, Gephyrocharax, Glandulocauda, Hysteronotus, Iotabrycon, Landonia, Lophiobrycon, Mimagoniates, Phenacobrycon, Planaltina, Pseudocorynopoma, Pterobrycon, Ptychocharax, Scopaeocharax, Tyttocharax, and Xenurobrycon.
This node corresponds to the Glandulocaudinae of Weitzman & Fink (1985) and Weitzman & Menezes (1998), and to the Glandulocaudinae plus Stevardiinae of Weitzman et al. (2005). The monophyly of this clade was proposed and tested by Weitzman & Menezes (1998), who advanced a phylogenetic diagnosis for the former Glandulocaudinae based principally on data from the reproductive biology. Some of these features were later discovered in non-glandulocaudin species, which weakened the original hypothesis of monophyly of this group and resulted in its splitting into two subfamilies, Glandulocaudinae and Stevardiinae (Weitzman et al., 2005). Although this is a highly supported node, its deeply nested position within the herein redefined Stevardiinae does not justify the use of a suprageneric name for this clade, notwithstanding the traditionally used name Glandulocaudinae for this group of taxa (e. g. Géry, 1977; Weitzman, 2003). According to the final hypothesis of this paper, maintaining the name Glandulocaudinae, extended to the base of this clade (to the node 239; also including Attonitus, Aulixidens, and Bryconamericus mennii, whose relationships are relatively poorly supported), would require the creation of four new categories of subfamilial level (for Cyanocharax, and the nodes 255, 246, and 249). Similarly, maintaining the original Glandulocaudinae as a separate subfamily would imply the creation of six new names at the subfamilial level (those listed above, plus one name for Bryconamericus mennii and one for the node 245). Stevardiinae also has priority over the remaining suprageneric names proposed in this clade (excepting the less inclusive Diapomini). This, and the probability that the number of necessary nomenclatural changes would increase with the inclusion of the many species not included in this analysis justify the synonymy of Glandulocaudinae and the redefinition of Stevardiinae. The composition of this clade follows Weitzman & Menezes (1998).
Synapomorphies:
1. Number of gill rakers on first hypobranchial and ceratobranchial (196): (1 > 0) 11 or more. Reversal of synapomorphy 5 of node 196. Paralleled in Bryconamericus cf. exodon and B. cf. iheringii. Some trees: Paralleled in the Cheirodontinae and in Cyanocharax alburnus.
2. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (1 > 0) on vertical through posterior half of Meckelian cartilage. Reversal of synapomorphy 1 of node 241. Paralleled in node 298 and in Attonitus ephimeros.
3. Hypertrophied ventral caudal-peduncle squamation (354): (0 > 1) present. Reversed in Mimagoniates rheocharis.
4. Caudal gland cells consisting of modified mucous cells (355): (0 > 1) present. Reversed in Mimagoniates rheocharis.
5. Type of spermatozoa (359): (0 > 1) introsperm. Paralleled in Hollandichthys multifasciatus.
Autapomorphies of Acrobrycon tarijae:
1. Articulation between second and third infraorbitals (62): (1 > 0) vertical. Reversal of synapomorphy 1 of node 196.
2. Relative length of palatine (172): (1 > 0) approximately one-half length of ectopterygoid, or less. Reversal of synapomorphy 4 of node 197. Paralleled in node 255 and in Aphyocharax nattereri, Gymnocharacinus bergii, Parecbasis cyclolepis, and Piabina argentea.
3. Palatine foramen (173): (0 > 1) present and very conspicuous. Paralleled in node 255.
4. Caudal-fin bony hooks in adult males of species bearing hooks on fins (312): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Hyphessobrycon luetkenii, H. socolofi, and Probolodus heterostomus.
Node 236: (16 / 91 / - / 5)
Genera Chrysobrycon, Corynopoma, Diapoma, Gephyrocharax, Glandulocauda, Hysteronotus, Iotabrycon, Landonia, Lophiobrycon, Mimagoniates, Phenacobrycon, Planaltina, Pseudocorynopoma, Pterobrycon, Ptychocharax, Scopaeocharax, Tyttocharax, and Xenurobrycon.
The basal position of Acrobrycon tarijae relative to the remaining members of the Glandulocaudinae of Weitzman & Menezes (1998) was not previously proposed. According to these authors Acrobrycon would be included in their tribe Diapomini, along with the genera Diapoma and Planaltina. That group would be paraphyletic in this analysis, given that Diapoma is related with Mimagoniates and Pseudocorynopoma. Furthermore, in this analysis, the Stevardiinae of Weitzman et al. (2005) are paraphyletic in terms of the "glandulocaudin" Mimagoniates. However, only a few species of this clade are analyzed here and a much more detailed phylogenetic analysis of this group was previously published by Weitzman & Menezes (1998). The position the of non-analyzed "glandulocaudins" follows Weitzman & Menezes (1998).
Synapomorphies:
1. First postcleithrum (247): (0 > 1) absent. Paralleled in node 170.
2. Number of branched pelvic-fin rays (258): (1 > 0) six or less. Paralleled in the Aphyocharacinae, in nodes 220, 280, and 302, and in Axelrodia lindeae, Cheirodon interruptus, Cyanocharax alburnus, Hollandichthys multifasciatus, Hoplocharax goethei, and Hyphessobrycon luetkenii. Some trees: Paralleled in Hasemania nana and Hyphessobrycon elachys.
3. Anal-fin position (284): (0 > 1) extended anteriorly ventral to dorsal fin. Paralleled in nodes 170, 208, and 212 and in Piabucus melanostomus.
4. Gill-derived gland on males (352): (1 > 0) absent. Reversal of synapomorphy 6 of node 196. Paralleled in node 251 and in Aphyocharax nattereri.
Node 235: (16 / 74 / - / 5)
Genera Chrysobrycon?, Corynopoma?, Gephyrocharax?, Glandulocauda?, Hysteronotus?, Iotabrycon?, Landonia?, Lophiobrycon, Mimagoniates, Phenacobrycon?, Planaltina?, Pseudocorynopoma, Pterobrycon?, Ptychocharax?, Scopaeocharax?, Tyttocharax?, and Xenurobrycon?
A sister-group relationship between Mimagoniates and Pseudocorynopoma has not previously been proposed. Indeed, Mimagoniates was included in the tribe Glandulocaudini and Pseudocorynopoma the in Hysteronotini by Weitzman & Menezes (1998). Later, most members of the Glandulocaudinae were transferred to the Stevardiinae, with the exceptions of Glandulocauda, Lophiobrycon, and Mimagoniates, which were the only members of the subfamily Glandulocaudinae, as redefined by Weitzman et al. (2005). The assessment of the internal relationships of the former members of the Glandulocaudinae were not a primary objective of this paper, and the scheme of relationships found here would likely change with the addition of more species or characters for this group.
Synapomorphies:
1. Number of teeth in inner premaxillary row (129): (0 > 1) five or more. Reversal of synapomorphy 3 of node 198. Paralleled in node 195 and in Grundulus cochae.
2. Number of branched-rays on dorsal-fin (270): (0 > 1) nine or more. Reversal of synapomorphy 2 of the Stevardiinae.
Autapomorphies of Pseudocorynopoma doriae:
1. Relative length of pterotic spine (46): (1 > 0) projected more posteriorly than attachment site of ligament from hyomandibula. Reversal of synapomorphy 4 of node 205.
2. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, 280, and 298 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Hollandichthys multifasciatus, and Salminus brasiliensis.
3. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, and Psellogrammus kennedyi. Some trees: Paralleled in node 302.
4. Foramen in posterior region of metapterygoid (168): (1 > 2) in form of incomplete arch, bordered posteriorly by hyomandibula. Reversal of synapomorphy 10 of node 205. Paralleled in Bryconamericus scleroparius.
5. Development of medial lamella of coracoid (238): (0 > 1) expanded as a keel. Paralleled in nodes 170 and 302 and in Paragoniates alburnus, Piabucus melanostomus, and Rhaphiodon vulpinus.
6. Second postcleithrum (248): (0 > 1) absent. Paralleled in the Gasteropelecidae, in node 302, and in Rhaphiodon vulpinus.
7. Anterior rays of dorsal fin of adult males (268): (0 > 1) elongate and reaching posteriorly to position close to adipose fin. Paralleled in Hyphessobrycon elachys.
8. Number of branched anal-fin rays (289): (0 > 1) 35 or more. Paralleled in nodes 207 and 212 and in Gymnocorymbus ternetzi, Metynnis maculatus, Piabucus melanostomus, Rhaphiodon vulpinus, Stethaprion erythrops, and Thoracocharax stellatus. Some trees: Paralleled in node 261 and in Markiana nigripinnis.
9. Proximal and medial radials of anal fins (294): (0 > 1) fused in most pterygiophores. Paralleled in nodes 184, 208, 218, and 221 and in Psellogrammus kennedyi. Some trees: Paralleled in node 295.
10. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent. Paralleled in Creagrutus anary, Hyphessobrycon eques, H. luetkenii, Phenacogaster tegatus, and Stethaprion erythrops. Some trees: Paralleled in Markiana nigripinnis and Psellogrammus kennedyi.
Autapomorphies of Mimagoniates rheocharis:
1. Synchondral articulation between lateral ethmoid and anterodorsal border of orbitosphenoid (35): (1 > 0) present. Paralleled in the Aphyocharacinae and in Leporinus striatus, Pristella maxillaris, and Rhaphiodon vulpinus.
2. Small foramen near posterior margin of pterosphenoid (44): (1 > 0) absent, or not pierced by nerves. Reversal of synapomorphy 1 of node 242.
3. Overlap of maxilla by second infraorbital (61): (0 > 1) present. Reversal of synapomorphy 6 of node 205. Paralleled in Hollandichthys multifasciatus.
4. Bony lamella dorsal to fourth basibranchial (185): (0 > 1) absent. Reversal of synapomorphy 3 of node 203. Paralleled in node 296 and in Axelrodia lindeae, Gymnocharacinus bergii, Hollandichthys multifasciatus, Nematocharax venustus, Paracheirodon axelrodi, and Prodontocharax melanotus.
5. Relative length of anterior dorsal-fin rays (271): (1 > 0) not reaching tip of posterior rays when adpressed. Reversal of synapomorphy 5 of node 179.
6. Number of dorsal pterygiophores (276): (0 > 1) 10 or more. Reversal of synapomorphy 3 of the Stevardiinae.
7. Anal-fin bony hooks in adult males of species bearing hooks on fins (308): (1 > 0) absent.
8. Hypertrophied ventral caudal-peduncle squamation (354): (1 > 0) absent. Reversal of synapomorphy 3 of node 237.
9. Caudal gland cells consisting of modified mucous cells (355): (1 > 0) absent. Reversal of synapomorphy 4 of node 237.
Node 244: (100 / 100 / 96 / 100)
Genera Chrysobrycon?, Corynopoma?, Diapoma, Gephyrocharax?, Glandulocauda?, Hysteronotus?, Iotabrycon?, Landonia?, Phenacobrycon?, Planaltina?, Pterobrycon?, Ptychocharax?, Scopaeocharax?, Tyttocharax?, and Xenurobrycon?
This node could be composed of other members of the Stevardiinae (sensu Weitzman et al., 2005) not included in this paper, in addition to Diapoma. Thus, the genera of the former Glandulocaudinae and Stevardiinae not included in this analysis are listed with question marks.
Synapomorphies:
1. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
2. Cartilages anterior to basihyal (188): (0 > 1) two well developed blocks of cartilage. Paralleled in node 299 and in Hasemania nana, Hyphessobrycon bifasciatus, Metynnis maculatus, Odontostilbe microcephala, and Roeboides descalvadensis. Some trees: Paralleled in node 265.
3. Position of last supraneural (283): (0 > 1) located more than two vertebrae in front of first dorsal pterygiophore. Paralleled in node 174 and in Engraulisoma taeniatum, Gymnocharacinus bergii and Xenagoniates bondi.
Autapomorphy of Diapoma terofali:
1. Rows of gill rakers on second ceratobranchial (193): (0 > 1) two.
Autapomorphies of Diapoma speculiferum:
1. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284, 289, and 298, and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
2. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. Paralleled in nodes 162, 285, and 301 and in Aulixidens eugeniae, Creagrutus anary, Moenkhausia cf. intermedia, and Pyrrhulina australis. Some trees: Paralleled in node 247 and in Knodus breviceps.
Node 249: (-9 / 77 / - / 4)
Genera Boehlkea?, Bryconacidnus?, Caiapobrycon?, Ceratobranchia?, Creagrutus, Hypobrycon?, Microgenys?, Monotocheirodon?, Nantis, Odontostoechus, Othonocheirodus?, Piabarchus?, Piabina, Rhinobrycon?, and Rhinopetitia?; Bryconamericus iheringii, B. rubropictus, B. thomasi, other Bryconamericus?
The monophyly of this clade was not proposed prior to Mirande (2009). As with the preceding nodes, the study of some features of the reproductive biology of the members of this clade are especially important. According to the present analysis, the species of Bryconamericus included in this clade are not closely related to B. exodon, the type species of the genus, and they should be transferred to, at least, two new genera. An alternative would be to include species in all this clade to the same genus, which, by priority would be Creagrutus Günther, 1864. This action, however, is unjustified until future studies including a higher proportion of the included species are carried out.
Synapomorphies:
1. Position of opening on neurocranium communicating with laterosensory canal of sixth infraorbital (77): (0 > 1) in frontal. Paralleled in node 193 and in Attonitus ephimeros and Micralestes stormsi.
Some trees:
2. Rows of gill rakers on first ceratobranchial (192): (0 > 1) two. (k12-14). Reversal of synapomorphy 3 of node 179. Paralleled in the Iguanodectinae, in node 280, and in Brycon orbignyanus, Bryconaethiops macrops, Carlana eigenmanni, Cheirodon interruptus, Hoplocharax goethei, Hyphessobrycon elachys, Odontostilbe microcephala, Parecbasis cyclolepis, Prodontocharax melanotus, and Rhaphiodon vulpinus. Some trees: Parelleled in Attonitus ephimeros. Reversed in node 253.
Node 248: (65 / 87 / - / 8)
Bryconamericus iheringii, B. thomasi, other Bryconamericus?
The morphological similarity between Bryconamericus iheringii and B. thomasi is so marked that Ringuelet et al. (1967), among others, considered these species as synonyms; however, the validity of B. thomasi was later demonstrated by Miquelarena & Aquino (1995). The relationships of these two species were previously unknown. Both species, but especially B. thomasi, have a marked sexual dimorphism, with strong and numerous bony hooks in males. The reproductive biology of these species is unknown and, as in the previous nodes, such information may be relevant to an assessment of the relationships of these species.
Synapomorphy:
1. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, Nematocharax venustus, and Probolodus heterostomus.
Autapomorphy of Bryconamericus thomasi:
1. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus rubropictus, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
Autapomorphies of Bryconamericus cf. iheringii:
1. Number of gill rakers on first hypobranchial and ceratobranchial (196): (1 > 0) 11 or more. Reversal of synapomorphy 5 of node 196. Paralleled in node 237 and in Bryconamericus cf. exodon. Some trees: Paralleled in the Cheirodontinae and in Cyanocharax alburnus.
2. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus rubropictus, Hyphessobrycon luetkenii, H. socolofi, and Phenacogaster tegatus.
Node 251: (-9 / 68 / - / 4)
Genera Boehlkea?, Bryconacidnus?, Caiapobrycon?, Ceratobranchia?, Creagrutus, Hypobrycon?, Microgenys?, Monotocheirodon?, Nantis, Odontostoechus, Othonocheirodus?, Piabarchus?, Piabina, Rhinobrycon?, and Rhinopetitia?; Bryconamericus rubropictus, other Bryconamericus?
This clade was not previously proposed. The presence of a gill gland, its single synapomorphy, must be confirmed by histological examination in some species. Studies about the presence and nature of this gland are desirable both to its use both in species-level systematics and in phylogenetic studies.
Synapomorphy:
1. Gill-derived gland on males (352): (1 > 0) absent. Reversal of synapomorphy 6 of node 196. Paralleled in node 236 and in Aphyocharax nattereri.
Node 250: (51 / 85 / - / 4)
Genera Boehlkea?, Bryconacidnus?, Caiapobrycon?, Ceratobranchia?, Hypobrycon?, Microgenys?, Monotocheirodon?, Othonocheirodus?, Piabarchus?, Rhinobrycon?, and Rhinopetitia?; Bryconamericus rubropictus, other Bryconamericus?
As is the case with characters involving glands and reproductive structures, the character supporting this clade should be studied in detail, especially in relation to its potential variation during the growth. The analyzed members of this clade may be populations of the same species, something that is currently under study by the author.
Synapomorphy:
1. Sclerotic bones (350): (0 > 1) two bones separated by cartilages. Paralleled in nodes 208, 210, 221, and 259.
Autapomorphies of Bryconamericus rubropictus:
1. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Reversal of synapomorphy 2 of node 198. Paralleled in nodes 280 and 298 and in Aphyodite grammica, Axelrodia lindeae, and Creagrutus cf. taphorni.
2. Length of laterosensory canal of dentary (79): (0 > 1) reduced or absent. Paralleled in node 279 and in Aphyocharax nattereri, Hyphessobrycon elachys, H. luetkenii, Nantis cf. indefessus, and Thayeria boehlkei.
3. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, 294, and 298, and in Aphyocharax nattereri, Bryconamericus thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
4. Pectoral-fin bony hooks in adult males of species bearing hooks on fins (310): (0 > 1) present. Paralleled in node 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Bryconamericus cf. iheringii, Hyphessobrycon luetkenii, H. socolofi, and Phenacogaster tegatus.
5. Dorsal-fin bony hooks in adult males of species bearing hooks on fins (311): (0 > 1) present. Paralleled in nodes 248 and 268 and in Astyanax cf. asuncionensis, A. lineatus, Bario steindachneri, Hyphessobrycon luetkenii, H. socolofi, Nematocharax venustus, and Probolodus heterostomus.
No autapomorphies found for Bryconamericus cf. rubropictus.
Node 254: (-9 / 60 / - / 22)
Genera Boehlkea?, Bryconacidnus?, Caiapobrycon?, Ceratobranchia?, Creagrutus, Hypobrycon?, Microgenys?, Monotocheirodon?, Nantis, Odontostoechus, Othonocheirodus?, Piabarchus?, Piabina, Rhinobrycon?, and Rhinopetitia?
The relationships of Nantis were unknown prior to the analysis of Mirande (2009). In the description of N. indefessus, this species was compared with the members of clade A (=Stevardiinae), although it was not originally included in this group due to the possession of five teeth in the inner premaxillary row (instead of four) (Mirande et al., 2004). The results of this analysis indicate that Nantis is a member of the herein redefined subfamily Stevardiinae. The genera Creagrutus, Odontostoechus, and Piabina have been included in the clade A (=Stevardiinae) by Malabarba & Weitzman (2003), but their relationships within this clade were previously unknown (Weitzman et al., 2005).
Synapomorphy:
1. Margins of toothed region of maxilla (96): (0 > 1) dorsally divergent. Paralleled in nodes 162, 209, and 282 and in Prodontocharax melanotus and Rhoadsia altipinna.
Autapomorphies of Odontostoechus lethostigmus:
1. Contact between frontals anteriorly to frontal fontanel (21): (0 > 1) present. Reversal of synapomorphy 4 of node 206. Paralleled in Bario steindachneri, Exodon paradoxus, Galeocharax humeralis, and Hyphessobrycon pulchripinnis. Some trees: Paralleled in Knodus breviceps.
2. Premaxillary, maxillary, and dentary teeth (119): (0 > 1) pedunculate and uniformly shaped. Paralleled in node 232 and in Gymnocharacinus bergii.
3. Number of rows of premaxillary teeth (122): (1 > 0) one. Paralleled in node 195 and in Aulixidens eugeniae, Carlana eigenmanni, Carnegiella strigata, Grundulus cochae, Paracheirodon axelrodi, Piabucus melanostomus, and Probolodus heterostomus.
4. Form of teeth of inner premaxillary tooth row (128): (0 > 1) with cusps aligned in straight series and without anterior concavity. Paralleled in the Rhoadsiinae, in nodes 195, 245, and 280, and in Hemigrammus bleheri.
5. Number of cusps of anterior maxillary teeth (139): (0 > 1) five or more cusps. Paralleled in the Rhoadsiinae, in nodes 273, 283, and 294, and in Bramocharax bransfordii, Brycon orbignyanus, Gymnocharacinus bergii, Hemibrycon dariensis, and Hyphessobrycon pulchripinnis. Some trees: Paralleled in node 246.
Node 298: (100 / 100 / 88 / 22)
Genus Nantis.
Synapomorphies:
1. Rhinosphenoid (47): (1 > 0) absent. Reversal of synapomorphy 2 of the Characidae. Paralleled in nodes 207, 260, and 280 and in Aphyocharax nattereri, Attonitus ephimeros, Brycon orbignyanus, Bryconamericus scleroparius, Hollandichthys multifasciatus, Pseudocorynopoma doriae, and Salminus brasiliensis.
2. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Reversal of synapomorphy 2 of node 198. Paralleled in node 280 and in Aphyodite grammica, Axelrodia lindeae, Bryconamericus rubropictus, and Creagrutus cf. taphorni.
3. Canal of lateral line on caudal-fin membrane (92): (1 > 0) absent. Reversal of synapomorphy 3 of the Characidae. Paralleled in the Aphyoditeinae and Bryconops clade, in nodes 227, 229, 244, 287, and 294, and in Aphyocharax nattereri, Bryconamericus rubropictus, B. thomasi, Charax stenopterus, Hyphessobrycon anisitsi, Inpaichthys kerri, and Phenacogaster tegatus.
4. Ventral margin of horizontal process of anguloarticular (109): (1 > 0) posteroventrally angled relative to laterosensory canal of dentary from medial view. Reversal of synapomorphy 1 of node 199. Paralleled in nodes 195 and 280 and in Attonitus ephimeros.
5. Posterior extent of ventral process of quadrate (151): (0 > 1) falling short of posterior margin of symplectic. Paralleled in the Hyphessobrycon luetkenii clade and Iguanodectinae, in nodes 284 and 289 and in Astyanax mexicanus, A. cf. rutilus, Aulixidens eugeniae, Cyphocharax spilotus, Diapoma speculiferum, Hemiodus cf. thayeria, Metynnis maculatus, Micralestes stormsi, Moenkhausia sanctaefilomenae, Nematocharax venustus, Probolodus heterostomus, Psellogrammus kennedyi, and Pseudocorynopoma doriae. Some trees: Paralleled in node 302.
6. Posterior margin of cleithrum (234): (1 > 0) without concavity ventral to first postcleithrum. Reversal of synapomorphy 2 of node 196. Paralleled in Cheirodon interruptus and Inpaichthys kerri.
7. Longitudinal position of insertion of adductor mandibulae tendon on dentary (330): (1 > 0) on vertical through posterior half of Meckelian cartilage. Reversal of synapomorphy 1 of node 241. Paralleled in node 237 and in Attonitus ephimeros.
Autapomorphy of Nantis cf. indefessus:
1. Length of laterosensory canal of dentary (79): (0 > 1) reduced or absent. Paralleled in node 279 and in Aphyocharax nattereri, Bryconamericus rubropictus, Hyphessobrycon elachys, H. luetkenii, and Thayeria boehlkei.
Autapomorphy of Nantis indefessus:
1. Bony hooks on first pelvic-fin ray of adult males (315): (0 > 1) present. Paralleled in node 274 and in Aphyocharacidium bolivianum, Aphyocharax anisitsi, and Aulixidens eugeniae.
Node 253: (100 / 100 / 96 / 32)
Genera Boehlkea?, Bryconacidnus?, Caiapobrycon?, Ceratobranchia?, Creagrutus, Hypobrycon?, Microgenys?, Monotocheirodon?, Othonocheirodus?, Piabarchus?, Piabina, Rhinobrycon?, and Rhinopetitia?
The monophyly of a clade composed of Creagrutus and Piabina was proposed by Vari & Harold (2001), supported by ten synapomorphies. Therefore, the study of relationships between these two genera is not a primary objective of this paper. The results herein obtained corroborate the hypothesis of Vari & Harold (2001). As several genera in the Stevardiinae were not analyzed, this clade could be actually more inclusive.
Synapomorphies:
1. Ventral projection of mesethmoid spine, forming a keel between premaxillae (26): (0 > 1) present. Paralleled in Roeboexodon geryi.
2. Epiphyseal branch of supraorbital canal (84): (1 > 0) present. Reversal of synapomorphy 1 of the Stevardiinae.
3. Length of ascending process of premaxilla (104): (1 > 0) reaching at least one-third of length of nasal.
4. Horizontal process of anguloarticular (108): (0 > 1) broadly covered by dentary which reaches posterior border of Meckelian cartilage. Reversal of synapomorphy 1 of node 206. Paralleled in nodes 246 and 261 and in Xenagoniates bondi.
5. Rows of gill rakers on first ceratobranchial (192): (1 > 0) one. Reversal of synapomorphy 2 of node 249.
6. Rows of gill rakers on second ceratobranchial (193): (1 > 0) one. Paralleled in node 238 and in Bryconamericus exodon. Some trees: Paralleled in Axelrodia lindeae.
7. Denticles on gill rakers (201): (0 > 1) absent. Paralleled in the Gymnocharacinae, in node 245, and in Axelrodia lindeae and Pseudochalceus kyburzi. Some trees: Paralleled in Hyphessobrycon elachys and H. herbertaxelrodi.
8. Posterior margin of cleithrum (235): (0 > 1) with markedly concave margin, almost forming straight angle. Paralleled in nodes 162 and 247 and in Agoniates anchovia, Attonitus ephimeros, Characidium borellii, Iguanodectes geisleri, Moenkhausia cf. intermedia, Prionobrama paraguayensis, and Xenagoniates bondi.
9. Insertion of adductor mandibulae tendon on dentary (331): (0 > 1) anterior to Meckelian cartilage. Paralleled in nodes 183 and 186 and in Xenagoniates bondi.
Autapomorphies of Piabina argentea:
1. Position of sphenotic spine relative to hyomandibula (11): (0 > 1) displaced anteriorly relative to anterior margin of hyomandibula. Paralleled in nodes 162 and 211 and in Acestrorhynchus pantaneiro, Salminus brasiliensis, and Serrasalmus maculatus.
2. Relative length of palatine (172): (1 > 0) approximately one-half length of ectopterygoid, or less. Reversal of synapomorphy 4 of node 197. Paralleled in node 255 and in Acrobrycon tarijae, Aphyocharax nattereri, Gymnocharacinus bergii, and Parecbasis cyclolepis.
3. Contact between lamella on anterior portion of first basibranchial with lamella on posterior portion of second basibranchial (183): (0 > 1) present. Paralleled in the Bryconops clade, in nodes 168, 177, and 216, and in Chalceus macrolepidotus, Distichodus maculatus, Hemiodus cf. thayeria, and Hoplias cf. malabaricus.
4. Transitional vertebrae with haemal canal (229): (0 > 1) absent. Paralleled in nodes 195 and 212, and in Aulixidens eugeniae, Engraulisoma taeniatum, and Metynnis maculatus. Some trees: Paralleled in node 247 and in Bryconamericus alpha and Paracheirodon axelrodi.
5. Posterior region of levator arcus palatini (337): (0 > 1) limited lateral and medially by A2 and A3 sections of adductor mandibulae. Paralleled in Rhaphiodon vulpinus.
Node 252: (100 / 100 / 89 / 70)
Genus Creagrutus.
As mentioned in the preceding node, a test for the monophyly of Creagrutus is beyond the objectives of this paper and this issue was studied in detail by Vari & Harold (2001), who diagnosed this genera supported by 11 synapomorphies. The two species herein analyzed were included in a large polytomy of 41 species of the genus in the hypothesis of Vari & Harold (2001).
Synapomorphies:
1. Longitudinal ridge in quadrate bordering adductor mandibulae muscle ventrally and, to some degree, laterally (152): (0 > 1) present. Paralleled in the Iguanodectinae and in node 209.
2. Shape of dentigerous plate of fifth ceratobranchial (204): (1 > 0) rounded, with posterior notch. Paralleled in the Iguanodectinae and in Axelrodia lindeae.
3. Articulation between ventral process of mesocoracoid and dorsal margin of scapula (245): (0 > 1) present and broad. Paralleled in Gymnocharacinus bergii.
4. Number of unbranched anal-fin rays (285): (1 > 0) three or fewer. Paralleled in Iguanodectes geisleri and Paracheirodon axelrodi.
5. Number of ventral procurrent caudal-fin rays (302): (0 > 1) 12 or more. Paralleled in the Bryconops clade, in node 229, and in Salminus brasiliensis.
Autapomorphies of Creagrutus cf. taphorni:
1. Ventral extent of third infraorbital (64): (0 > 1) not reaching horizontal arm of preopercle, at least anteriorly. Reversal of synapomorphy 2 of node 198. Paralleled in nodes 280 and 298 and in Aphyodite grammica, Axelrodia lindeae, and Bryconamericus rubropictus.
2. Length of medial bony ridge of opercle (170): (1 > 0) 60% or greater than opercular length. Paralleled in the Serrasalmidae, in node 210, and in Astyanax abramis, Hoplias cf. malabaricus, and Roeboides microlepis. Some trees: Paralleled in Acestrorhynchus pantaneiro and Salminus brasiliensis.
3. Process of scapula forming anterior border of scapular foramen (244): (0 > 1) absent. Paralleled in node 193 and in Aphyodite grammica, Deuterodon langei, Hoplias cf. malabaricus, Hyphessobrycon herbertaxelrodi, Leporinus striatus, Odontostilbe paraguayensis, and Thayeria obliqua.
Autapomorphies of Creagrutus anary:
1. Length of sphenotic spine (10): (0 > 1) extending ventrally to articulation between sphenotic and hyomandibula. Reversal of synapomorphy 1 of node 197. Paralleled in Inpaichthys kerri. Some trees: Paralleled in Aphyodite grammica.
2. Position of ventral margin of posttemporal (252): (1 > 0) anterior to lateral margin of epioccipital. Paralleled in nodes 162, 285, and 301 and in Aulixidens eugeniae, Diapoma speculiferum, Moenkhausia cf. intermedia, and Pyrrhulina australis. Some trees: Paralleled in node 247 and in Knodus breviceps.
3. Relative position of dorsal-fin anterior insertion (265): (1 > 0) anterior to or at vertical through pelvic-fin origin. Paralleled in node 282 and in Exodon paradoxus, Moenkhausia xinguensis, and Parecbasis cyclolepis.
4. Pelvic-fin bony hooks in adult males of species bearing hooks on fins (309): (1 > 0) absent. Paralleled in Hyphessobrycon eques, H. luetkenii, Phenacogaster tegatus, Pseudocorynopoma doriae, and Stethaprion erythrops. Some trees: Paralleled in Markiana nigripinnis and Psellogrammus kennedyi.
5. Contact between dorsal margin of adductor mandibulae and ventral margin of dilator operculi (335): (1 > 0) absent. Paralleled in Inpaichthys kerri, Piabucus melanostomus, Prionobrama paraguayensis, Pristella maxillaris, Prodontocharax melanotus, and Pyrrhulina australis. Some trees: Paralleled in Hyphessobrycon elachys.
6. Anterior extension of adductor arcus palatini (336): (1 > 0) covering most of dorsal surface of mesopterygoid. Paralleled in node 166 and in Markiana nigripinnis and Salminus brasiliensis. Some trees: Paralleled in Brycon orbignyanus.
Some trees:
7. Anterior convergence of ventral diverging lamellae with nasal septum of mesethmoid (31): (0 > 1) confluent at posterior end of nasal septum. (some trees under k9-14).
Incertae sedis genera
In strict sense, all the genera listed with question marks can be considered as incertae sedis within the Characidae; however, there are differences in the situation of that genera and those listed below. The genera listed with question marks have some evidence relating them to various clades obtained in this paper, and were listed in such a manner only because a corroboration from a phylogenetic study is lacking. Thus, these genera could be considered to be included, at least provisionally, in a clade defined here. The genera listed below, in contrast lack published evidence relating them to any clade herein recognized. Most of these genera were defined by autapomorphies, and their descriptions and published information does not include presumably apomorphic features shared with some clade of the present hypothesis. The available information of these genera is listed below in relation to the present analysis.
Astyanacinus Eigenmann, 1907
Astyanacinus was considered closely related to Astyanax, differing from that genus only by the form of the mouth. In Astyanacinus the maxilla is long and does not form an angle with the premaxilla (Eigenmann, 1921). This character state is present in other genera of the Characidae, as Dectobrycon, Hollandichthys, Oligosarcus, and Pseudochalceus. Among the presumably apomorphic features of Astyanacinus, the rounded humeral spot and the presence of circulii on the posterior field of scales [(pers. obs. in A. moorii (Boulenger)] and the presence of chromatophores densely concentrated in the focus of scales (pers. obs. in A. multidens Pearson), would relate this genus with the Astyanax bimaculatus-group, while the presence of chevron-shaped marks on the flanks is shared with Astyanax superbus Myers and Hyphessobrycon bifasciatus, among other species. These alternatives could relate Astyanacinus both with the Astyanax clade or with the Hyphessobrycon luetkenii clade and it is preferable to leave this genus as incertae sedis pending its inclusion in a phylogeny of the family.
Atopomesus Myers, 1927
Géry (1977) mentioned that this genus has seven aligned and strong premaxillary teeth; that author included this genus in his Aphyoditeina, without any phylogenetic analysis. Although the relatively high number of premaxillary teeth is rather similar to one of the synapomorphies of the Aphyoditeinae (eight or more premaxillary teeth), the teeth in members of this subfamily are slender and small. Therefore, it is preferable to leave Atopomesus as incertae sedis within the Characidae pending further analysis.
Bryconella Géry, 1965b
According to Géry (1977) this genus is a Hemigrammus - like tetra, with differences in the infraorbitals and the dentition. That author mentioned that the inner premaxillary tooth row of Bryconella has only two or three teeth, which are much close to the anterior row, and that in some individuals both rows merge in an irregular row of teeth. Among the species herein examined, a comparable situation was observed only in Inpaichthys kerri (Aphyocharacinae), but this feature could be a parallelism.
Brittanichthys Géry, 1965a
The phylogenetic position of this genus is uncertain. Géry (1965a, 1973, 1977) classified it among the Aphyoditeina, based principally on a numerical taxonomy analysis (Géry, 1965a). It shares the presence of one row of premaxillary teeth, among other features, with some members of Aphyoditeinae. The analysis of Géry (1965a) grouped Brittanichthys with Leptobrycon and these two species were grouped with Aphyodite and Parecbasis. Géry (1965a) mentioned also that Brittanichthys has ectopterygoid and mesopterygoid teeth and lacks a tongue; these conditions are unique or much unusual among characids. Moreira (2002), in his coding of this species, reported the frontal fontanel as margined anteriorly by the mesethmoid, thereby differing from the examined species of the Aphyoditeinae, in which the frontal fontanel is limited anteriorly by the frontals. This genus is inseminating (Malabarba, 1998a) and modifications of the medial caudal-fin rays (Géry, 1965a, 1977) resemble those of some Stevardiinae. The relationships of this genus, thus, should be tested in a phylogenetic framework.
Dectobrycon Zarske & Géry, 2006
Dectobrycon armeniacus Zarske & Géry shares some dentition features and the presence of an interrupted lateral line with Hollandichthys and Pseudochalceus (Pseudochalceus clade) and the presence of scales covering most of the length of the anal-fin with Markiana (Astyanax clade) (Zarske & Géry, 2006). The available data would indicate a higher affinity of this genus with Hollandichthys and Pseudochalceus, but it is preferable herein to leave this genus provisionally as incertae sedis within the Characidae.
Genycharax Eigenmann, 1912
The only species described for this genus, Genycharax tarpon Eigenmann, has a general appearance of an Astyanax, but with a clupeoid mouth and elongated and curved teeth. This genus was related with Astyanax and some Characinae by Géry (1977). According to the illustrations of Géry (1977) this species lacks a supraorbital bone, and it would not be included either in the subfamilies Acestrorhynchinae, Agoniatinae, Bryconinae, Cynodontinae, Iguanodectinae or Salmininae, nor in the Bryconops clade. Although this species shares some details of general appearance and the presence of unicuspidate teeth with the Characinae, such a proposed relationship would be speculative and it is maintained herein as incertae sedis.
Gymnotichthys Fernández-Yépez, 1950
The unique known species of this genus, Gymnotichthys hildae Fernández-Yépez has a naked predorsal line as occurs in Gymnocorymbus and the overall body shape and coloration are similar to some Moenkhausia of the lepidura-group (Géry, 1977). If this species is related with some of these genera, it should be included in the Tetragonopterinae. Indeed, if the Moenkhausia lepidura - group of Géry (1977) is monophyletic and includes G. hildae, all its species should be transferred to Gymnotichthys, according to the hypothesis herein proposed. However, all these conjectures are speculative in the current state of knowledge and further studies are necessary to assess the relationships of this genus.
Mixobrycon Eigenmann, 1915
Mixobrycon ribeiroi (Eigenmann) was originally described as a species of Cheirodon (Eigenmann & Ogle, 1907) and it was considered as part of the Cheirodontinae by Géry (1977) in having only one row of premaxillary teeth. Malabarba (1998a) mentioned that the holotype of this species, although much damaged, resembles some Hyphessobrycon species and that the unique feature relating this species with the Cheirodontinae is the dentition.
Oligobrycon Eigenmann, 1915
Oligobrycon was included in the Aphyoditeina by Géry (1977) by sharing some characters that are actually broadly distributed in Characidae (e. g. compressed body, adipose fin present, dorsal fin without dark markings). Géry stated in the identification key of the Aphyoditeina that O. microstomus Eigenmann has strong but not compressed teeth and only four teeth in the premaxilla. One of the synapomorphies of the Aphyoditeinae is the presence of eight or more slender premaxillary teeth in a single row; thus, this species is maintained as incertae sedis.
Parapristella Géry, 1964b
This genus, with two recognized species, is distinguishable from Pristella mainly in having two rows of premaxillary teeth, resembling a Hemigrammus species with a higher number of maxillary teeth (Géry, 1977). Although both Hemigrammus and Pristella are herein included in the Tetragonopterinae, Parapristella does not share presumably apomorphic character-states with any of the genera included in this clade and it is maintained herein as incertae sedis.
Schultzites Géry, 1964b
Schultzites axelrodi Géry, the unique species of this genus, is differentiated from Moenkhausia by having seven to ten maxillary teeth distributed along 2/3 of its length. The remaining characters of this genus resemble Moenkhausia dichroura or M. intermedia (Tetragonopterinae) (Géry, 1977). As in Parapristella, the limited available information for this genus would suggest its inclusion in the Tetragonopterinae, but given the lack of apomorphic characters supporting this relationship, it is maintained herein as incertae sedis.
Scissor Günther, 1864
This monotypic genus was described from a single specimen of uncertain origin, although it is supposed that it is from Suriname (Lima et al., 2003). According to Eigenmann (1917) this specimen has short gill-rakers, 29 anal-fin rays, conical teeth distributed along one half of the maxillary length, and premaxillary and dentary teeth similar to those of Tetragonopterus. Géry (1977) included Scissor macrocephalus Günther in his tribe Bramocharacini. The available information, as evident, is not sufficient to propose the inclusion of this genus in any of the clades herein proposed.
Serrabrycon Vari, 1986
Serrabrycon magoi Vari, the only species of this genus, shares the presence of teeth oriented outside the mouth and the lepidophagous habit with Probolodus (Tetragonopterinae), Bryconexodon, Exodon, Roeboexodon, and Roeboides (Characinae) (Vari, 1986). According to its description, this species could be related with the genera Hemigrammus and Pristella (Tetragonopterinae), or with some of the mentioned lepidophagous genera.
Stygichthys Brittan & Böhlke, 1965
The position of this genus within Characidae is completely enigmatic; even its assignment to the Characidae can not be considered to be well justified. The single species of this genus, Stygichthys typhlops Brittan & Böhlke, has hypogean habits and some unique or much unusual features among the Characidae (Géry, 1977). The presence in this species of a very short anal fin with only eight rays is unique within the Characidae. No information relevant to a hypothesis about the relationships of this genus is available and Stygichthys is maintained as incertae sedis within the Characidae.
Thrissobrycon Böhlke, 1953b
This genus, composed only of Thrissobrycon pectinifer Böhlke, was included in the Aphyoditeina by Géry (1973). This author mentioned that the clupeoid mouth of this genus resembles that of Leptobrycon and Oxybrycon. This feature differentiates these three genera from the genera herein included in the Aphyoditeinae.
Tucanoichthys Géry & Römer, 1997
The relationships of the miniature genus Tucanoichthys are unknown. Tucanoichthys tucano Géry & Römer, its single species, has one row of eight conical premaxillary teeth, resembling some members of the Aphyoditeinae or Characinae. The maxilla is completely toothed as in the Characinae, whereas the predorsal line is naked as in Gymnocorymbus (Tetragonopterinae). Its coloration resembles that of Hyphessobrycon herbertaxelrodi, with a broad black lateral band dorsally delineated by a clear margin (Géry & Römer, 1997). The resemblance of this species to the Aphyoditeinae and the miniature genus Priocharax was mentioned in its description (Géry & Römer, 1997).
Discussion of characters of Eigenmann (1917)
Most of the current systematics of the Characidae was delineated by the classification of Eigenmann (1917). This classification was proposed as provisional by that author, who highlighted that some of the genera were probably polyphyletic and noted the necessity of a classification that reflected the divergent phylogenetic history of the group. In a first attempt to use an evolutionary approach, he proposed alternative states for 17 characters which presence or absence, in different combinations, defined most genera of the Characidae. Eigenmann (1917) "optimized" these character-states in a radial scheme, recognizing the impossibility of producing a divergent scheme with his data. Eigenmann traced evolutionary lines for each character in his radial scheme, taking as primitive their most frequent states. In this manner, he considered Astyanax as the most primitive genus in having the most frequent states of all the characters he utilized. Eigenmann's classification was later followed by Géry (e. g. 1977) and others, but its shortcomings were subsequently noted in phylogenetically-oriented papers that questioned the value of the characters of Eigenmann (1917) (e. g. Weitzman & Fink, 1983). Most of these characters are analyzed here in a familial context, and their phylogenetic informativeness can be evaluated for first time based on a thorough phylogenetic analysis.
Ch. 64: Ventral coverage of third infraorbital
Defined as "cheeks partly naked" vs. "cheeks entirely covered by the third suborbital" by Eigenmann (1917). This character was not "optimized" in the hypothesis of Eigenmann (1917: fig. 1), although the absence of contact between the third infraorbital (second suborbital according to Eigenmann) was used to distinguish Knodus from Moenkhausia, and to characterize Astyanacinus, Astyanax, Ctenobrycon, Deuterodon, Hasemania, Hollandichthys, Hyphessobrycon, Landonia, Pseudochalceus, Pristella, and Psellogrammus, in his generic key. In the present analysis this character is highly homoplastic (28 steps). Considering only unambiguous transformations, this character is a synapomorphy for nine clades of this analysis and an autapomorphy for 14 species. The reduction of infraorbitals was proposed to be related with miniaturization events (e. g. Bührnheim et al., 2008). Although it is true that most miniature species usually have reduced infraorbitals (in number and/or development), a slight reduction producing a separation between the infraorbitals and the preopercle is also present in some non-miniature species. Indeed, only nine of the transformations proposed for this character are optimized as separation of the third infraorbital and the preopercle, while the remaining 14 are optimized as expansion of that infraorbital to reach the preopercle. This suggests that although highly homoplastic, this character has phylogenetic information which is independent from miniaturization events and it should be analyzed in phylogenies, especially in those considering the homoplasy degree as useful information (e. g. under implied weighting or self-weighted optimization; Goloboff, 1993, 1997).
Ch. 91: Lateral line
Defined as "lateral line complete" vs. "lateral line incomplete" by Eigenmann (1917). This character delineated much of the Eigenmann's characid scheme of relationships (Eigenmann, 1917: fig. 1), as an outer line separating the genera with an interrupted lateral line. An interrupted lateral line defined, according to Eigenmann (1917), Brycochandus (=Bryconops in part), Hasemania, Hemigrammus, Hollandichthys, Hyphessobrycon, Nematobrycon, Pristella, Psellogrammus, Pseudochalceus, and Thayeria. In that scheme the Cheirodontinae, Glandulocaudinae (=Stevardiinae in part, as herein recognized), and Rhoadsiinae lie over that line, showing variability within each of these subfamilies. It is noticeable, however, that these groups are not closely related each other, according to the figure of Eigenmann, but originated from different lines of evolution (e. g. Hemigrammus, Pristella, and Thayeria are related with Moenkhausia, whereas Psellogrammus is related to Ctenobrycon). Thus, Eigenmann (1917) was implicitly proposing several parallel reductions of the lateral line within the Characidae. This is in agreement with most subsequent discussions about that issue, especially regarding miniaturization (e. g. Weitzman & Fink, 1983). However, the high homoplasy and the parallel reduction (not regaining) of the lateral line was not previously tested in a phylogeny of the family. In the hypothesis herein proposed, the lateral line is optimized as having 11 reductions and only one reacquisition, in Xenagoniates bondi. The reductions are synapomorphies of five clades and six autapomorphies. Thus, the results of this paper agree with Eigenmann (1917) and subsequent authors in that the loss of a complete lateral line is more usual than reacquisition of a complete one. Miniaturization can not, however, be considered to be the only event producing a reduction of the lateral line, as some medium-sized genera (i. e. Hollandichthys) have reduced lateral lines.
Ch. 123: Number of rows of premaxillary teeth
Defined as "premaxillary teeth in two series" vs. "premaxillary teeth in three series" by Eigenmann (1917). The presence of three series of premaxillary teeth was reported by Eigenmann (1917) for Creagrutus and Piabina. As mentioned in the description of the character 123, Piabina argentea is coded as polymorphic. This character is herein optimized as synapomorphic for the Bryconinae and Creagrutus (or Piabina plus Creagrutus, given its polymorphism in Piabina) and autapomorphic for Bryconaethiops macrops and Chalceus macrolepidotus.
Ch. 137: Extent of dentition along maxilla
Defined as "maxillary with few teeth or none" vs. "maxillary with teeth along its entire edge" by Eigenmann (1917). Eigenmann (1917: fig. 1) implicitly proposed the parallel acquisition of teeth along the entire maxillary margin in Hemibrycon, (Hollandichthys + Pseudochalceus), Knodus, Nematobrycon, Phenacogaster, and Pristella. In this analysis this character has a moderate degree of homoplasy, with eight unambiguous acquisitions, and is a synapomorphy for three clades and autapomorphy for five species, along with two losses of teeth along the entire maxillary margin.
Ch. 148: Heterogeneity of dentary teeth
Defined as "teeth of the sides of the dentary abruptly smaller" vs. "teeth of the sides of the dentary graduated" by Eigenmann (1917). This character was not considered in the scheme of relationships of Eigenmann (1917: fig. 1), but was used in his generic key to distinguish Astyanacinus, Astyanax, Ctenobrycon, and Psellogrammus, with abruptly minute dentary teeth from Deuterodon and Landonia, with graduated teeth. Several intermediate states were, however, observed in this paper, and only the extreme cases are coded as having abruptly minute dentary teeth. Thus, the original character of Eigenmann and those herein used are not directly comparable. As coded here, this character is moderately homoplastic, with six parallel acquisitions of abruptly minute dentary teeth and only one reversion to graduate teeth.
Ch. 284: Anal-fin position
Defined as "origin of anal behind origin of dorsal" vs. "origin of anal under or in front of origin of dorsal" by Eigenmann (1917). This character was not considered in the scheme of relationships of Eigenmann (1917: fig. 1). In the present analysis, this character has five unambiguous transformations to a posteriorly situated dorsal-fin. Four of them are synapomorphies for specific clades, while the remaining one is an autapomorphy of Piabucus melanostomus. However, the posterior position of the dorsal-fin, above the anal fin, is shared by most of the Iguanodectinae, except Iguanodectes geisleri, among others (Moreira, 2002). Thus, although relatively homoplastic, this character has important phylogenetic information at some level.
Ch. 317: Ctenii or spines on scales
Ch. 318: Anterior margin of scales
Defined as "scales entire" vs. "scales ctenoid" and "scales crenate" by Eigenmann (1917). This character was considered as two separate characters in the present analysis. The presence of spinoid scales is a synapomorphy of a clade composed of Acestrocephalus, Cynopotamus, and Galeocharax, but both this state and these species were not studied by Eigenmann (1917). The presence of ctenoid scales was mentioned in the generic key of Eigenmann (1917) as present in Ctenobrycon and Psellogrammus; in the present hypothesis this state is an autapomorphy of Psellogrammus kennedyi, but no species of Ctenobrycon are analyzed here and this condition could be a synapomorphy of a clade composed of these two genera. The presence of crenate scales was mentioned in the generic key of Eigenmann (1917) for Entomolepis Eigenmann (=Bario). This character, however, is coded as polymorphic in this species given that it is variable during growth and is optimized in the current hypothesis as independent autapomorphies of Markiana nigripinnis and Tetragonopterus argenteus.
Ch. 324: Predorsal scales
Defined as "predorsal line scaled" vs. "predorsal line naked". The acquisition of a naked predorsal area has three unambiguous steps in the present hypothesis, two of them supporting the Gasteropelecidae and a clade composed of Gymnocorymbus and Stichonodon, respectively. The remaining is an autapomorphy of Lonchogenys ilisha. This character furthermore has ambiguous changes within the Gymnocharacinae which may support a clade when a greater number of species is analyzed.
Ch. 327: Scales covering anal-fin base
Defined as "anal naked except at the base" vs. "anal scaled to near its tip" by Eigenmann (1917). The presence of several rows of minute scales covering the anal-fin base has 11 parallel acquisitions in the present hypothesis. Three of these changes are synapomorphies, while eight of them are autapomorphies. The rather homoplastic nature of this character, however, is not a valid argument to ignore it in a phylogenetic analysis, especially when the degree of homoplasy of the characters is used as relevant information during tree searches.
Ch. 328: Scales covering caudal-fin lobes
Defined as "caudal fin naked" vs. "caudal fin scaled" by Eigenmann (1917). This character was the main feature in which the scheme of relationships of Eigenmann (1917: fig. 1) was based. Thus, this character had no parallelisms in his scheme and all the incongruence was resolved by proposing parallelisms in the characters conflicting with this one. However, as is to be expected according to the current state of knowledge, this character is homoplastic in this analysis, having eight unambiguous steps supporting one clade and being autapomorphic for seven species. As with the previous characters, this degree of homoplasy is not a valid reason to exclude this character from phylogenetic analyses, but it is clear that the characid classification can not be principally based in this character.
Ch. 356: Adipose fin
Defined as "adipose fin present" vs. "adipose fin absent" by Eigenmann (1917). The absence of an adipose fin is infrequent within the Characiformes and usually related with miniaturization events (Bührnheim et al., 2008). In this analysis the absence of an adipose fin is optimized as having four unambiguous steps, of which two are autapomorphies and the remaining two supports the clade composed of Hoplias and Pyrrhulina and the subfamily Gymnocharacinae, respectively.
On average, the characters of Eigenmann (1917) analyzed for this phylogeny have 8.7 homoplastic steps, ranging from 1 to 27 (consistency index: 11.5). This degree of homoplasy is slightly higher than the average for the whole matrix; in average the characters used for this phylogeny have 4.6 homoplastic steps (consistency index: 17.8). Also, the average retention index of Eigenmann's characters is slightly lower than the average for the whole matrix (60.5 vs. 67.1). This relatively high degree of homoplasy supports the idea that the systematics of the Characidae cannot be exclusively based on the characters of Eigenmann, as claimed in several papers (e. g. Weitzman & Fink, 1983). It is noticeable, however, that taking into account the degree of homoplasy during the parsimony searches through differentially weighted schemes (Goloboff, 1993; 1997), as in the present hypothesis, the influence of highly homoplastic characters is reduced proportionally to their degree of homoplasy. This leaves characters with low homoplasy (more congruent with each other) as driving most of the topology of trees. Particularly when using some of these weighting schemes, no degree of homoplasy is a valid argument to exclude information from any phylogenetic analysis.
Conclusions
This phylogeny is far from conclusive, but it is a starting point for specific studies in different clades and degrees of inclusiveness. The morphological description and documentation presented in this paper are intended to be a reference point for future studies in this group. However, the morphological evidence analyzed in this paper undoubtedly does not include all the anatomical variation suitable for analysis in a phylogenetic context and major contributions in this area are still possible and necessary. As evidenced, the characters classically used in the systematic of the family, as longly discussed in the literature (e. g. Eigenmann, 1917; Weitzman & Fink, 1983) are highly homoplastic, and although useful at some level, are not sufficient to diagnose generic or suprageneric clades. This phylogeny delineates future lines of research in almost every major clade, and may serve as a null hypothesis for many possible researches.
Acknowledgements
This paper is part of the Ph.D. thesis of the author. I am much indebted to Mercedes Azpelicueta and Pablo Goloboff for advice and continuous encouragement. Ueso Montero and Virginia Abdala encouraged me to go deep in morphological issues of this analysis; Rubén Barquez, Fernando Lobo, Martín Ramírez, Gastón Aguilera, Claudia Szumik, and Anyelo Vanegas-Ríos provided important suggestions during the development of this paper. Carlos Lucena generously assisted me, especially in the first stages of this paper. This paper was immensely benefited by very detailed and constructive suggestions of Richard P. Vari and two anonymous reviewers. Most specimens for this paper was generously lent or exchanged by Sonia Fisch-Muller (MHNG), Patrice Pruvost (MNHN), Margarete Lucena (MCP), Mark Sabaj and John Lundberg (ANSP), and Cristina Butí (FML). This paper was founded by the Argentinean Government institutions Fundación Miguel Lillo, CONICET (Consejo Nacional de Investigaciones Científicas y Tecnológicas), and FONCyT (Fondo para la Investigación Científica y Tecnológica) (PICT-2007-01314 to Pablo A. Goloboff and PICT-2008-1201 to Juan Marcos Mirande). Most of this paper was done in a Linux platform; I thank the Ubuntu, OpenOffice, Gimp, and InkScape communities. TNT was provided free by the Willi Hennig Society.
Literature Cited
Accepted June 7, 2010
Published September 24, 2010
Acestrocephalus sardina. MCP 17072: 2 ex. 56.6-63.0 mm. Brazil, Amazonas, upper Negro River basin, near mouth of Marauiá River. Oct 1979. Acestrorhynchus pantaneiro. CI-FML 3870: 1 ex. 127.8 mm. Argentina, Corrientes, Guayquiraró, Corriente River. Apr 2005. Acrobrycon tarijae. CI-FML 3890: 2 ex. 52.6-53.8 mm. Argentina, Tucumán, Monteros, Capitán Cáceres, Mandolo River. Feb 2004. Agoniates anchovia. MHNG 2388.93: 1 ex. 158.5 mm. Brazil, Negro River basin, Tupé Lake. Nov 1979. MCP 16969: 1 ex. 127.7 mm. Brazil, Negro River basin, Urubaxi River. Feb 1980. Alestes cf. macrophthalmus. MHNG 2203.21: 2 ex. 77.0-79.0 mm. Gabon, Loa Loa. Aug 1964. Apareiodon affinis. CI-FML 3862: 1 ex. 88.0 mm. Argentina, Entre Ríos, Uruguay River. Jan 2001. Aphyocharacidium bolivianum. MCP 37960: 3 ex. 23.1-26.3 mm. Brazil, Acre, Sena Madureira, Purus River basin, igarapé Taquari between Atimani River and Sena Madureira. Aphyocharax anisitsi. CI-FML 2951: 1 ex. 30.3 mm. Argentina, Salta, Cañada El Hogar, Pilcomayo River. May 1999. CI-FML 3875: 1 ex. 29.6 mm. Argentina, Salta, La Unión, Puesto de la Viuda, Bermejo River basin. Nov 2001. Aphyocharax dentatus. CI-FML 3035: 2 ex. 51.0-53.2 mm. Argentina, Salta, Cañada El Hogar, Misión La Paz, Pilcomayo River. 1999. Aphyocharax nattereri. CI-FML 3876: 2 ex. 19.6-21.7 mm. Argentina, Formosa, El Bagual, Bermejo River. Apr 1992. Aphyodite grammica. MHNG 2172.89: 2 ex. 21.5-22.5 mm. Brazil, Atauia, Negro River basin. Nov 1967. Astyanax abramis. CI-FML 3908: 1 ex. 65.1 mm. Argentina, Salta, Rivadavia, La Unión, Pozo del Toro, Bermejo River. Nov 2001. Astyanax cf. abramis. CI-FML 3909: 2 ex. 63.2-75.6 mm. Argentina, Tucumán, Monteros, Capitán Cáceres, Mandolo River. Jul 2005. CI-FML 3911: 2 ex. 75.1-81.6 mm. Argentina, Tucumán, Monteros, Pueblo Viejo River near Reserva La Florida. Mar 2005. Astyanax asuncionensis. CI-FML 3910: 2 ex. 46.0-46.3 mm. Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. Astyanax cf. asuncionensis. CI-FML 3912: 4 ex. 55.1-67.2 mm. Argentina, Tucumán, Monteros, Capitán Cáceres, Mandolo River. Nov 2003. Astyanax chico. CI-FML 3913: 2 ex. 60.2-75.2 mm. Argentina, Salta, Bermejo River basin, El Oculto stream. May 2002. Astyanax correntinus. CI-FML 3826: 1 ex. 66,7 mm. Argentina, Corrientes, Perichón, near Corrientes City, Paraná River. Apr 2005. Astyanax cf. eigenmanniorum1. CI-FML 3914: 2 ex. 45.5-47.5 mm. Argentina, Santiago del Estero, Los Quiroga dam, Dulce River. Oct 2001. Astyanax cf. eigenmanniorum2. CI-FML 3915: 3 ex. 45.8-54.6 mm. Argentina, Tucumán, Trancas, Loro River. Oct 2000. Astyanax endy. CI-FML 3916: 2 ex. 48.6-49.6 mm. Argentina, Salta, Orán, 4 km. from Aguas Blancas. Aug 2003; CI-FML 3279: 7 ex. (in alcohol) 33.7-53.6 mm. Argentina, Salta Orán, El Oculto, El Oculto stream. Oct 2001. Astyanax latens. CI-FML 3327: 2 ex. 40.6-44.0 mm. Argentina, Salta, Orán, El Oculto, El Oculto stream. Oct 2001. Astyanax lineatus. CI-FML 3884: 1 ex. 62.3 mm. Argentina, Salta, Orán, Bermejo River. Aug 2003. Astyanax mexicanus. ANSP 162587: 2 ex. 39.4-48.5 mm. USA, Texas, Victoria Co., río Guadalupe at Dupont pump. Oct 1987. Astyanax paris. CI-FML 3919: 2 ex. 45.7-48.6 mm. Argentina, Misiones, San Pedro, Parque Provincial Piñalito, Piñalito stream. Jul 2005. Astyanax pelegrini. CI-FML 3847: 3 ex. 59.1-63.8 mm. Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. Astyanax puka CI-FML 3850: 3 ex. 42.7-50.0 mm. Argentina, Tucumán, Monteros, Capitán Cáceres, Mandolo River. Jul 2005. Astyanax cf. rutilus CI-FML 3917: 1 ex. 51.8 mm. Argentina, Tucumán, Tafí Viejo, El Cadillal, Celestino Gelsi dam. Oct 2002. CI-FML 3918: 2 ex. 69.3-70.1 mm. Argentina, Tucumán, Monteros, Capitán Cáceres, Mandolo River. Jul 2005. Astyanax troya. CI-FML 3920: 2 ex. 74.7-80.9 mm. Argentina, Misiones, Aristóbulo del Valle, Cuñá-Pirú. Dec 2004. Attonitus ephimeros. CI-FML 3895 (Ex. ANSP 180682): 51.4 mm. Perú, Cuzco, Coribeni River, vicinity of Kiteni. Jul 2004. Aulixidens eugeniae. ANSP 134797: 39.7-41.7 mm. Venezuela, Matepalma beach, Orinoco River. Apr 1925. Axelrodia lindeae. MCP 37314: 4 ex. 18.7-23.8 mm. Brazil, Acre, Purus River basin, igarapé Marizinho, BR 364, Antimari River drainage. Bario steindachneri. MHNG 2184.46: 1 ex. 62.0 mm. Brazil, upper Solimões basin, Igarapé Preto near Leticia. Dec 1960. Bramocharax bransfordii. MHNG 2120.078, 086, 088: 59.0-66.5 mm. Nicaragua, Lake Managua, Momotobo. Feb 1983. Brycinus carolinae. MNHN 1982-0909: 1 ex. 109.8 mm. Guinea, Niandé, Níger River basin. May 1980. Brycon falcatus. MHNG 2677.085: not measured (disarticulated). Brazil, Pará, Maroni. Brycon orbignyanus. CI-FML 3874: 1 ex. 152.2 mm. Argentina, Corrientes, Guayquiraró, Corriente River. Apr 2005. Brycon pesu. MCP 23299: 2 ex. 67.0-67.5 mm. Brazil, Pará, Paragominas, Capim River ca. 56 Km. W from Paragominas. Jul 1998. Bryconaethiops macrops. MNHN 1979-0382: 1 ex. 97.1 mm. Centroafrican Republic, Bangui, Oubangi River, Congo River basin. 1930. Bryconamericus agna. CI-FML 3896: 2 ex. 42.1-48.9 mm. Argentina, Misiones, Aristóbulo del Valle, Cuñá-Pirú. Dec 2004. Bryconamericus alpha. ANSP 130512: 2 ex. 35.3-42.8 mm. Ecuador, Napo, Santa Cecilia, Aguarico River. Jun 1967. Bryconamericus exodon. CI-FML 3897: 2 ex. 39.0-41.1 mm. Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. Bryconamericus cf. exodon. CI-FML 3903: 1 ex. 41.2 mm. Argentina, Salta, Misión Chaqueña, Bermejo River. Aug 2003. Bryconamericus cf. iheringii. CI-FML 3898: 2 ex. 50.2-51.4 mm. Argentina, Tucumán, Monteros, Capitán Cáceres, Mandolo River. Nov 29 2003. CI-FML 3899: 2 ex. 42.3-42.8 mm. Argentina, Tucumán, Tafí Viejo, El Cadillal, Celestino Gelsi dam. Feb 2001. Bryconamericus mennii. CI-FML 3900: 2 ex. 39.5-41.4 mm. Argentina, Misiones, Aristóbulo del Valle, Cuñá-Pirú. Dec 2004. Bryconamericus rubropictus. CI-FML 3901 (Ex. MCNi 500): 1 ex. 49.1 mm. Argentina, Salta, Cachi, Calchaquí River. Aug 1996. Bryconamericus cf. rubropictus. CI-FML 3902: 34.3-47.3 mm. Argentina, Catamarca, tributary to Santa María River, Fuerte Quemado. Mar 2002. Bryconamericus scleroparius. ANSP 163169: 2 ex. 58.0-65.3 mm. Costa Rica, Limón, River on the road between Sixaola and Limón, 7 km NE from BriBri. Mar 1987. Bryconamericus thomasi. CI-FML 3348: 3 ex. 49.1-51.0 mm. Argentina, Salta, Orán, El Oculto, Blanco River. Feb 2002. CI-FML 3904: 2 ex. 46.6-53.5 mm. Argentina, Salta, Orán, Santa María River, tributary of Colorado River. Aug 2003. Bryconexodon juruenae. MCP 30657: 67.3 mm. Brazil, Mato Grosso, Porto dos Gaúchos, Tapajós River basin, igarapé Ribeirão Preto. Jan 2002. Bryconops affinis. MHNG 2184.28: 2 ex. 29.0-32.0 mm. Guayana Francesa, Balatée crick, Maroni basin. Oct 1979. Bryconops melanurus. MCP 15807: 3 ex. 41.2-83.4 mm. Brazil, Mato Grosso, Barra do Bugres. Aug 1991. Carlana eigenmanni. LACM 9230.020: 2 ex. 45.2-57.6 mm. Costa Rica, Heredia, Puerto Viejo River. Sep 1962. Carnegiella strigata. CI-FML 3868: 1 ex. 28.7 mm. Aquarium specimen. Chalceus macrolepidotus. MHNG 2189.13: 2 ex. 64.0-67.0 mm. French Guiana, Litany Maripasoula in its confluence with le Tampoe, upper Maroni River. Nov 1957. Characidium borellii. CI-FML 3865: 2 ex. 50.9-58.6 mm. Argentina, Tucumán, Monteros, Capitán Cáceres. Mandolo River. Nov 2003. Characidium rachovii. CI-FML 3866: 1 ex. 30.9 mm. Argentina, Tucumán, Monteros, Capitán Cáceres, Mandolo River. Jul 2005. Charax stenopterus. CI-FML 3878: 1 ex. 39.2 mm. Argentina, Corrientes, Guayquiraró, Corriente River. Apr 2005. Cheirodon interruptus. CI-FML 3825: 2 ex. 32.9-33.4 mm. Argentina, Santiago del Estero, río Hondo, río Hondo dam. Feb 2001. Coptobrycon bilineatus. MCP 39051: 2 ex. 28.9-31.5 mm. Brazil, São Paulo, Itatinga River. Creagrutus anary. CI-FML 3905 (Ex. ANSP 178135): 1 ex. 47.1 mm. Perú, Loreto, Maynas, Napo River, near town of Mazan. Aug 2001. Creagrutus cf. taphorni. MHNG 2183.34: 2 ex. 49.0-51.3 mm. Venezuela, Edo. Carabobo 5 km N from Guacara, Vigirima River. Mar 1968. Cyanocharax alburnus. CI-FML 3906 (Ex. MCP 7054): 2 ex. 37.5-46.4 mm. Brazil, Rio Grande do Sul, Porto Alegre, Praia das Pombas. Dec 1985. Cynopotamus argenteus. CI-FML 3879: 1 ex. 118.6 mm. Argentina, Corrientes, Guayquiraró, Corriente River. Apr 2005. Cyphocharax spilotus. CI-FML 3741: 1 ex. 52.2 mm. Argentina, Santiago del Estero, río Hondo, río Hondo dam. Mar 2001. Deuterodon iguape. MHNG 2183.6: 2 ex. 52.0-52.5 mm. Brazil, São Paulo, Iguapé da Ribeira River basin. May 1964. Deuterodon langei. MCP 12158: 1 ex. 80.8 mm. Brazil, Paraná, Morretes, São João River. Jul 1988. Diapoma speculiferum. CI-FML 3891: 1 ex. 33.1 mm. Brazil, Rio Grande do Sul, Barra do Ribeiro, BR-116 km 56, Açude dos García. Jun 1985. Diapoma terofali. CI-FML 3892: 2 ex. 46.7-57.3 mm. Argentina, Misiones, Aristóbulo del Valle, Moreno stream. Dec 2004. Distichodus maculatus. ANSP 77826: 1 ex. 43.0 mm. Centroafrican Republic, Ubangi-Shari, Fort Sibut, Tomi River, Ubangi River basin. Oct 1934. Engraulisoma taeniatum. CI-FML 3921 (Ex. ANSP 149324): 2 ejs 31.0-32.1 mm. Colombia, Meta, Metica River, Meta drainage. Mar 1975. Exodon paradoxus. MHNG 2188.47: 2 ex. 37.0-40.0 mm. Brazil, Ilha do Bananal, Araguaia River. Jul 1961. Galeocharax humeralis. CI-FML 3951: 1 ex. 94.6 mm. Argentina, Salta, Rivadavia, La Unión. Pozo de los Yacarés, Bermejo River. Nov 2001. Grundulus cochae. ANSP 134934: 4 ex. 35.8-47.1 mm. Colombia, north Bogotá, at Puente de Suba. Unknown date. Gymnocorymbus ternetzi. CI-FML 3826: 2 ex. 33.7-35.6 mm. Paraguay, Alto Paraguay, Fortín Patria, Negro River. Nov 2002. Gymnocharacinus bergii. CI-FML 3922: 2 ex. 39.0-43.8 mm. Argentina, rio Negro, Arroyo Valcheta. Hasemania nana. CI-FML 3923: 2 ex. 22.5-27.2 mm. Aquarium specimens. Hemibrycon dariensis. ANSP 104426. 2 ex. 36.6-43.4 mm. Panamá, Cocle, Creek of río Cocle about 5 mi. N of Penonome on road to La Pintada. Mar 1962. Hemibrycon surinamensis. MHNG 2182.63: 1 ex. 48.0 mm. French Guiana, Balatée creek, Comté River. Oct 1979. Hemigrammus erythrozonus. CI-FML 3827: 2 ex. 25.5-26.9 mm. Aquarium specimens. Hemigrammus bleheri. CI-FML 3924: 1 ex. 31.0 mm. Aquarium specimen. Hemigrammus ulreyi. CI-FML 3925: 1 ex. 31.5 mm. Unknown data. Hemigrammus unilineatus. ANSP 134904: 1 ex. 27.3 mm. Guiana, Mora Passage, or mud creek below Wismar. Year 1908. Hemiodus cf. thayeria. CI-FML 3867: 1 ex. 49.8 mm. Aquarium specimen. Heterocharax macrolepis. MCP 11457: 2 ex. 33.4-34.0 mm. Brazil, Amazonas, Boa Vista, upper basin of Negro River. Feb 1980. Hollandichthys multifasciatus. MHNG 2173.91: 46.0-52.0 mm. Brazil, Paranagua. Jul 1912. MCP 30560: 3 ex. 63.5-70.5 mm. Brazil, tributary of rio Quilombo, Quilombo, Cubatão. Hoplias cf. malabaricus. CI-FML 3871: 1 ex. 112.1 mm. Argentina, Salta, Orán, El Oculto. Blanco River. Feb 2002. CI-FML 3872: 1 ex. 99.8 mm. Argentina, Santiago del Estero, Salado River. Aug 2004. Hoplocharax goethei. MCP 11456: 2 ex. 21.0-25.0 mm. Brazil, Amazonas, Boa Vista, upper basin of Negro River. Feb 1980. Hyphessobrycon anisitsi. CI-FML 3926: 2 ex. 30.3-34.7 mm. Argentina, Santa Fe, Helvecia. Dec 1991. Hyphessobrycon bifasciatus. CI-FML 3927: 2 ex. 28.8-36.0 mm. Aquarium specimens. Hyphessobrycon elachys. CI-FML 3928: 2 ex. 13.9-16.5 mm. Unknown data. Hyphessobrycon eques. CI-FML 3929: 2 ex. 29.4-31.3 mm. Argentina, Santa Fe, San José del Rincón. Dec 1991. Hyphessobrycon herbertaxelrodi. CI-FML 3930: 2 ex. 23.2-24.0 mm. Aquarium specimens. Hyphessobrycon luetkenii. CI-FML 3931: 2 ex. 25.6-30.4 mm. Argentina, Santa Fe, San José del Rincón. Dec 1991. Hyphessobrycon pulchripinnis. CI-FML 3932: 2 ex. 28.4-33.2 mm. Aquarium specimens. Hyphessobrycon socolofi. CI-FML 3933: 1 ex. 37.7 mm. Aquarium specimen. Iguanodectes geisleri. MHNG 2177.10: 1 ex. 49.0 mm. Brazil, Igarapé do Pretinho, tributary of Negro River, Caurès River. Nov 1976. Inpaichthys kerri. CI-FML 3934: 3 ex. 25.4-26.9 mm. Aquarium specimens. Knodus breviceps. MHNG 2184.97: 2 ex. 43.5-47.0 mm. Perú, near Iquitos. Dec 1963. Jupiaba mucronata. ANSP 170182. 1 ex. 51.2 mm. Guiana, Region 8 (Maudia), Potaro River, sand beach at Tukeit Falls. Sep 1990. Jupiaba scologaster. CI-FML 3935: 2 ex. 31.8-34.2 mm. Perú, Iquitos, Pampa Chica, Amazonas basin, Nanay River. Feb 2001. Leporinus striatus. CI-FML 3864: 1 ex. 81.4 mm. Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. Lonchogenys ilisha. MCP 11460: 2 ex. 39.3-45.0 mm. Brazil, Amazonas, Anavilhanas, Negro River basin. Unknown date. Markiana nigripinnis. CI-FML 3936: 2 ex. 75.3-78.6 mm. Paraguay, Alto Paraguay, Parque Nacional Defensores del Chaco. Nov 2002. CI-FML 3937: 1 ex. 96.6 mm. Argentina. Salta, Rivadavia, La Unión, Pozo del Toro, Bermejo River basin. Nov 2001. Metynnis maculatus. CI-FML 3871: 1 ex. 55.7 mm. Aquarium specimen. Micralestes stormsi. ANSP 66943: 2 ex. 46.5-54.3 mm. Centroafrican Republic, Tomi River, tributary of Ubangi, at Fort Sibut, Ubangi-Shari. Oct 1934. Microschemobrycon casiquiare. ANSP 159704: 2 ex. 26.0-26.4 mm. Venezuela, Amazonas, Sipapo River; along beaches of sand and rock ca 1-4 km above Pendare. Nov 1985. Mimagoniates rheocharis. MCP 29273: 3 ex. 38.6-53.1 mm. Brazil, Santa Catarina, Praia Grande, Mampituba River basin, Molha Coco stream in Vila Rosa. Mar 2002. Moenkhausia dichroura. CI-FML 3938: 2 ex. 45.3-47.2 mm. Paraguay, Alto Paraguay, Bahía Negra. Paraguay River. Nov 2002. Moenkhausia cf. intermedia. CI-FML 3417: 4 ex. 31.5-35.4 mm. Argentina, Salta, Orán, El Oculto, El Oculto stream. Feb 2002. Moenkhausia sanctaefilomenae. CI-FML 3939: 4 ex. 28.3-31.8 mm. Paraguay, Alto Paraguay, Fortín Patria, Negro River. Nov 2002. Moenkhausia xinguensis. CI-FML 3942 (Ex. ANSP 161350). 2 ex. 46.9-48.7 mm. Venezuela, Amazonas, Orinoco River at sand island ca. 1-2 km upstream from Guachipana. Mar 1987. Nantis indefessus. CI-FML 3940: 1 ex. 41.6 mm. Argentina, Salta, Orán, Estancia Anta Muerta, Pescado River. Aug 2003. Nantis cf. indefessus. CI-FML 3941: 1 ex. 35.2 mm. Argentina, Jujuy, Pozo de los Sauces, between Purmamarca and Tilcara, Grande River. Sep 2004. Nematobrycon palmeri. MHNG 2182.86: 2 ex. 25.0-30.2 mm. Colombia, aquarium specimens. Nematocharax venustus. MCP 17987: 3 ex. 34.3-42.4 mm. Brazil, Bahía, Buerarema, Pratas River basin in São Jose. Jan 1995. Odontostilbe microcephala. CI-FML 3408: 2 ex. 41.9-44.1 mm. Argentina, Salta, Orán, El Oculto, Blanco River. Feb 2002. CI-FML 3886: 2 ex. 42.2-50.6 mm (43 ex. 31.2-53.6 mm in alcohol). Argentina, Salta, Orán, Finca Anta Muerta, Pescado River. Aug 2003. Odontostilbe paraguayensis. CI-FML 3885. 2 ex. 29.4-31.2 mm (115 ex. 21.6-31.7 mm in alcohol). Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. Odontostilbe pequira. CI-FML 3887: 2 ex. 34.4-37.8 mm. Argentina, Salta, La Unión, Pozo de los Yacarés. Oct 2001. Odontostoechus lethostigmus. MCP 10776: 1 ex. 61.3 mm. Brazil, Rio Grande do Sul, Maquiné, Maquiné River in Maquiné, Tramandaí River basin. May 1986. Oligosarcus bolivianus. CI-FML 3366: 1 ex. 83.4 mm. Argentina, Salta, Orán, El Oculto, El Oculto stream. Feb 2002. Oligosarcus cf. jenynsii. CI-FML 3771: 1 ex. 68.8 mm. Argentina, Santiago del Estero, río Hondo. Apr 2001. CI-FML 3943: 1 ex. 60.8 mm. Argentina, Tucumán, Trancas, Loro River. Oct 2000. Oligosarcus sp. CI-FML 3850: 1 ex. 61.4 mm. Argentina, Salta, San Martín, near Itau River. Nov 2005. Paracheirodon axelrodi. CI-FML 3944: 2 ex. 23.8-24.1 mm. Aquarium specimens. Paragoniates alburnus. MHNG 2188.67: 1 ex. 50.0 mm. Brazil, Ilha do Careiro near Manaus. Nov 1967. MHNG 2370.12: 1 ex. 61.5 mm. Perú, Ucayali-Pucallpa, Neshuya River. Jun 1983. Parecbasis cyclolepis. MHNG 2228: 2 ex. 60.0-61.5 mm. Bolivia, Chapare River. Jun 1982. Parodon nasus. CI-FML 3863: 1 ex. 42.7 mm. Argentina, Salta, Orán, Pescado River. Aug 2003. Phenacogaster tegatus. CI-FML 3880: 1 ex. 35.3 mm. Argentina, Misiones, Capital, Nemesio Parma. Unknown date. Phenagoniates macrolepis. ANSP 134909: 2 ex. 25.8-34.8 mm. Venezuela, tributary of Motatan River, 30 km. N of Trujillo. Mar 1938. Piabina argentea. CI-FML 3907 (Ex. ANSP 171965): 41.2 mm. Brazil, Minas Gerais, Riacho dos Poções, tributary of rio Coxá/rio Carinhanha. Jul 1993. Piabucus melanostomus. CI-FML 3894: 2 ex. 67.0-86.8 mm. Aquarium specimens. Piaractus mesopotamicus. CI-FML 3872: 1 ex. 69.7 mm. Aquarium specimens. Poptella paraguayensis. CI-FML 3882: 2 ex. 39.6-43.7 mm. Aquarium specimens. Prionobrama paraguayensis. CI-FML 3877: 1 ex. 35.9 mm. Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. Pristella maxillaris. CI-FML 3945: 2 ex. 28.0-28.6 mm. Aquarium specimens. Probolodus heterostomus. MHNG 2227.89: 2 ex. 54.5-67.0 mm. Brazil, Sta. Branca, Paraíba River. Aug 1982. Prochilodus lineatus. CI-FML 3781: 1 ex. 73.5 mm. Argentina, Santiago del Estero, río Hondo, río Hondo dam. Mar 2001. Prodontocharax melanotus. CI-FML 3888 (Ex. ANSP 143528): 1 ex. 32.9 mm. Perú, Madre de Dios, upper Madre de Dios basin, Shintuya. Aug 1977. Psellogrammus kennedyi. CI-FML 3946: 4 ex. 36.3-40.3 mm. Paraguay, Alto Paraguay, Fortín Patria, Negro River. Nov 2002. Pseudochalceus kyburzi. USNM 324462: 2 ex. 36.8-45.3 mm. Aquarium specimens. Pseudocorynopoma doriae. CI-FML 3893: 1 ex. 57.5 mm. Unknown data. Puntius tetrazona. CI-FML 3860: 1 ex. 26.2 mm. Aquarium specimen. Pyrrhulina australis. CI-FML 3873: 1 ex. 26.7 mm. Paraguay, Alto Paraguay, Fortín Patria, Negro River. Nov 2002. Rhaphiodon vulpinus. CI-FML 3871: 1 ex. 193.8 mm. Argentina, Corrientes, Guayquiraró, Corriente River. Apr 2005. Rhoadsia altipinna. MHNG 2173.31: 59.0-73.5 mm. Perú, El Caucho, Fai River at Zarumilla River. Nov 1978. Roeboexodon geryi. MHNG 2188.14: 1 ex. 37.0-41.0 mm. Suriname, Desiongkondre and Pokigron, Surinam River. Dec 1963. Roeboides descalvadensis. CI-FML 3859: 2 ex. 61.4-63.1 mm (23 ex. 57.5-81.1 mm in alcohol). Argentina, Salta, La Unión, Pozo de los Yacarés. Aug 2003. Roeboides microlepis. CI-FML 3881: 1 ex. 82.9 mm. Argentina, Corrientes, Guayquiraró, Corriente River. Apr 2005. Salminus brasiliensis. CI-FML 3784: 1 ex. 131.4 mm. Argentina, Santiago del Estero, río Salí basin, río Hondo dam. Aug 2000. Serrapinnus calliurus. CI-FML 3889: 2 ex. 23.1-23.2 mm. Argentina, Salta, La Unión, Puesto de la Viuda, Bermejo River basin. Nov 2001. Serrasalmus maculatus. CI-FML 3873: 1 ex. 69.2 mm. Argentina, Salta, La Unión, Bermejo River basin. Aug 2003. Stethaprion erythrops. MHNG 2187.33: 2 ex. 43.0-44.5 mm. Perú, Yarina Cocha near Pucallpa, Ucayali River. Jul 1980. Stichonodon insignis. MHNG 2173.85: 1 ex. 54.5 mm. Brazil, Solimões, Muddy Iguarape. Mar 1974. Tetragonopterus argenteus. CI-FML 3883: 1 ex. 62.2 mm. Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. CI-FML 3852: 1 ex. 53.0 mm; Argentina, Corrientes, Guayquiraró, Corriente River. Apr 2005. Thayeria boehlkei. CI-FML 3947: 2 ex. 25.2-26.6 mm. Aquarium specimens. Thayeria obliqua. MHNG 2173.55: 2 ex. 26.5-35.5 mm. Brazil, Ilha do Castanha, Aripuana River. Jul 1976. Thoracocharax stellatus. CI-FML 3869: 2 ex. 43.7-45.2 mm (13 ex. 38.8-44.9 mm in alcohol). Paraguay, Alto Paraguay, Bahía Negra, Paraguay River. Nov 2002. Triportheus nematurus. CI-FML 3948: 1 ex. 82.1 mm. Paraguay, Alto Paraguay, Fortín Patria, Negro River. Nov 2002. Triportheus pantanensis. CI-FML 3949: 1 ex. 77.4 mm. Paraguay, Alto Paraguay, Fortín Patria, Negro River. Nov 2002. Xenagoniates bondi. MHNG 2366.27: 1 ex. 44.5 mm. Venezuela, Portuguesa, Apuré River. Feb 1978.
Appendix 2
Appendix 2 - Click to enlarge Appendix 2
- Albert, J. S. A. & R. Campos-da-Paz. 1998. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. Pp. 419-446. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Almirón, A. E., J. R. Casciotta, M. M. Azpelicueta & A. L. Cione. 2001. A new species of Hypobrycon (Characiformes: Characidae) from Uruguay basin in Misiones, Argentina. Neotrópica, 47(1): 33-40.
- Arefjev, V. A. 1990a. Karyotypic diversity of characid families (Pisces, Characidae). Caryologia, 43(3-4): 291-304.
- Arefjev, V. A. 1990b. Problems of karyotypic variability in the family Characidae. Caryologia, 43(3-4): 305-319.
- Arratia, G. 1987. Description of the primitive family Diplomystidae (Siluriformes, Teleostei, Pisces): morphology, taxonomy and phylogenetic implications. Bonner Zoologische Monographien, 24: 1-123.
- Artoni, R. F. & L. A. C. Bertollo. 2002. Evolutionary aspects of the ZZ/ZW sex chromosome system in the Characidae fish, genus Triportheus. A monophyletic state and NOR location on the W chromosome. Heredity, 89: 15-19.
- Azpelicueta, M. M. 1994. Three east-Andean species of Diplomystes (Siluriformes: Diplomystidae). Ichthyological Exploration of Freshwaters, 5(3): 223-240.
- Azpelicueta, M. M. & J. O. García. 2000. A new species of Astyanax (Characiformes, Characidae) from Uruguay River basin in Argentina, with remarks on hook presence in Characidae. Revue suisse de Zoologie, 107(2): 245-257.
- Azpelicueta, M. M., J. R. Casciotta & A. E. Almirón. 2002. Two new species of the genus Astyanax (Characiformes, Characidae) from the Paraná River basin in Argentina. Revue suisse de Zoologie, 109(2): 243-259.
- Bellafronte, E., V. P. Margarido & O. Moreira-Filho. 2005. Cytotaxonomy of Parodon nasus and Parodon tortuosus (Pisces, Characiformes). A case of synonymy confirmed by cytogenetic analyses. Genetics and Molecular Biology, 28(4): 710-716.
- Benine, R. C. 2004. Análise Filogenética do Gênero Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) com uma Revisão dos Táxons do Alto rio Paraná. Unpublished Master Dissertation, Universidade Estadual Paulista, Botucatu, São Paulo, 317p.
- Bertaco, V. A. 2003. Taxonomia e Filogenia do Gênero Hollandichthys Eigenmann, 1909 (Teleostei: Characidae) do Sul e Sudeste do Brasil. Unpublished Master Dissertation, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, 160p.
- Bertaco, V. A. 2008. Taxonomy and Phylogeny of the Neotropical Fish Genus Hemibrycon Günther, 1864 (Ostariophysi: Characiformes: Characidae). Unpublished Ph.D. Dissertation, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, 296p.
- Bertaco, V. A. & P. H. F. Lucinda. 2005. Astyanax elachylepis, a new characid fish from the rio Tocantins drainage, Brazil (Teleostei: Characidae). Neotropical Ichthyology, 3(3): 389-394.
- Bertaco, V. A. & L. R. Malabarba. 2005. A new species of Hyphessobrycon (Teleostei: Characidae) from the upper rio Tocantins drainage, with bony hooks on fins. Neotropical Ichthyology, 3(1): 83-88.
- Bertollo, L. A. C., O. Moreira-Filho & P. M. Galetti Jr. 1986. Cytogenetics and taxonomy: considerations based on chromosome studies of freshwater fish. Journal of Fish Biology, 28(2): 153-159.
- Böhlke, J. E. 1953a. Studies on fishes of the family Characidae. Nş 4. A review of the genus Microschemobrycon with descriptions of two new species. Annals and Magazine of Natural History (Series 12), 6(71): 841-849.
- Böhlke, J. E. 1953b. Studies on fishes of the family Characidae. Nş 3. A minute new herring like characid fish genus adapted for plankton feeding, from the rio Negro. Stanford Ichthyological Bulletin, 5(2): 168-170.
- Bremer, K. 1994. Branch support and tree stability. Cladistics, 10(3): 295-304.
- Brittan, M. R. & J. E. Böhlke. 1965. A new blind characid fish from southeastern Brazil. Notulae Naturae (Philadelphia), 380: 1-4.
- Buckup, P. A. 1991. The Characidiinae: A Phylogenetic Study of the South American Darters and Their Relationships With Other Characiform Fishes. Unpublished Ph.D. Dissertation, University of Michigan, Ann Arbor, 391p.
- Buckup, P. A. 1993. Phylogenetic interrelationships and reductive evolution in Neotropical Characidiine fishes (Characiformes, Ostariophysi). Cladistics, 9(3): 305-341.
- Buckup, P. A. 1998. Relationships of the Characidiinae and phylogeny of characiform fishes (Teleostei, Ostariophysi). Pp. 123-143. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Bührnheim C. M. 2006. Sistemática de Odontostilbe Cope, 1870 com a Proposição de uma Nova Tribo Odontostilbini e Redefinição dos Gêneros Incertae Sedis de Cheirodontinae (Ostariophysi: Characiformes: Characidae). Unpublished Ph. D. Dissertation, Pontifícia Universidade Católica de Rio Grande do Sul, Porto Alegre, 315p.
- Bührnheim, C. M., T. P. Carvalho, L. R. Malabarba & S. H. Weitzman. 2008. A new genus and species of characid fish from the Amazon basin - the recognition of a relictual lineage of characid fishes (Ostariophysi: Cheirodontinae: Cheirodontini). Neotropical Ichthyology, 6(4): 663-678.
- Bührnheim, C. M. & L. R. Malabarba. 2006. Redescription of the type species of Odontostilbe Cope, 1870 (Teleostei: Characidae: Cheirodontinae), and description of three new species from the Amazon basin. Neotropical Ichthyology, 4(2): 167-196.
- Bührnheim, C. M. & L. R. Malabarba. 2007. Redescription of Odontostilbe pulchra (Gill, 1858) (Teleostei: Characidae: Cheirodontinae), and description of two new species from the río Orinoco basin. Neotropical Ichthyology, 5(1): 1-20.
- Burns, J. R., S. H. Weitzman, H. J. Grier & N. A. Menezes. 1995. Internal fertilization, testis and sperm morphology in Glandulocaudinae fishes (Teleostei: Characidae: Glandulocaudinae). Journal of Morphology, 224(2): 131-145.
- Burns, J. R., S. H. Weitzman & L. R. Malabarba. 1997. Insemination in eight species of Cheirodontinae fishes (Teleostei: Characidae: Cheirodontinae). Copeia, 1997(2): 433-438.
- Burns, J. R., S. H. Weitzman, K. R. Lange & L. R. Malabarba. 1998. Sperm ultrastructure in characid fishes (Teleostei: Ostariophysi). Pp. 223-232. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Burns, J. R. & S. H. Weitzman. 2005. Insemination in ostariophysan fishes. Pp. 107-134. In: Viviparous Fishes. Uribe, M. C. & H. J. Grier (Eds.). Florida, New Life Publishers, 603p.
- Bushmann, P. J., J. R. Burns & S. H. Weitzman. 2002. Gill-derived glands in glandulocaudine fishes (Teleostei: Characidae: Glandulocaudinae). Journal of Morphology, 253(2): 187-195.
- Calcagnotto, D., S. A. Schaefer & R. DeSalle. 2005. Relationships among characiform fishes inferred from analysis of nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution, 36(1): 135-153.
- Cardoso, A. 2003a. Subfamily Rhoadsiinae. Pp. 213-214. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 742p.
- Cardoso, A. 2003b. Sistemática e Filogenia da Subfamília Rhoadsiinae (Teleostei: Characiformes: Characidae). Unpublished Master Dissertation, Pontifícia Universidade Católica de Rio Grande do Sul, Porto Alegre, 81p.
- Carvalho, M. L., C. Oliveira & F. Foresti. 2002. Description of a ZZ/ZW sex chromosome system in Thoracocharax cf. stellatus (Teleostei, Characiformes, Gasteropelecidae). Genetics and Molecular Biology, 25(3): 299-303.
- Casciotta, J. R., A. E. Almirón, J. A. Bechara, J. P. Roux & F. Ruíz Díaz. 2003. Astyanax pynandi sp. n. (Characiformes, Characidae) from Esteros del Iberá wetland, Argentina. Revue suisse de Zoologie, 110(4): 807-816.
- Casciotta, J. R. & A. E. Almirón. 2004. Astyanax chico sp. n. - a new species from the río San Francisco basin, northwest of Argentina (Teleostei: Characiformes: Characidae). Zoologische Abhandlungen (Dresden), 54: 11-17.
- Castro, R. M. C. 1984. Osteologia e Relações Filogenéticas de Engraulisoma taeniatum Castro, 1981 (Ostariophysi, Characiformes, Characidae). Unpublished Master Dissertation, Universidade de São Paulo, 162p.
- Castro, R. M. C., A. C. Ribeiro, R. C. Benine & A. L. A. Melo. 2003. Lophiobrycon weitzmani, a new genus and species of glandulocaudine fish (Characiformes: Characidae) from the rio Grande drainage, upper rio Paraná system, southeastern Brazil. Neotropical Ichthyology, 1(1): 11-19.
- Centofante, L., L. A. C. Bertollo, P. A. Buckup & O. Moreira-Filho. 2003. Chromosomal divergence and maintenance of sympatric Characidium fish species (Crenuchidae, Characidiinae). Hereditas, 138(3): 213-218.
- Cestari, M. M. & P. M. Galetti Jr. 1992. Chromosome studies of Serrasalmus spilopleura (Characidae, Serrasalmidae) from the Paraná-Paraguay Rivers: Evolutionary and cytotaxonomic considerations. Copeia, 1992(1): 108-112.
- Chapman, W. M. 1944. The osteology of the Pacific deepbodied anchovia, Anchoa compressa Journal of Morphology, 74(2): 311-329.
- Cione, A. L., W. M. Dahdul, J. G. Lundberg & A. Machado-Allison. 2009. Megapiranha paranensis, a new genus and species of Serrasalmidae (Characiformes, Teleostei) from the upper Miocene of Argentina. Journal of Vertebrate Paleontology, 29(2): 350-358.
- de Pinna, M. C. C. 1991. Concepts and tests of homology in the cladistic paradigm. Cladistics 7(4), 367-394.
- Dimmick, W. W & A. Larson. 1996. A molecular and morphological perspective on the phylogenetic relationships of the otophysan fishes. Molecular Phylogenetics and Evolution, 6(1): 120-133.
- Eigenmann, C. H. 1907. Fowler's "heterognathous fishes" with a note on the Stethaprioninae. The American Naturalist, 41(492): 767-772.
- Eigenmann, C.H. 1909. Reports on the expedition to British Guiana of the Indiana University and the Carnegie Museum; 1908. Annals of the Carnegie Museum, 6(1): 4-54.
- Eigenmann, C. H. 1910. Catalogue of the fresh-water fishes of tropical and south temperate America. Pp: 375-511. In: Reports of the Princeton University Expeditions to Patagonia, 1896-1899, volume 3, part 4, Zoölogy. Scott, W. B. (Ed.). Stuttgart, Verlagshandlung E. Schweizerbart'sche .
- Eigenmann, C. H. 1911. New characins in the collection of the Carnegie Museum. Annals of the Carnegie Museum, 8(1): 164-180.
- Eigenmann, C. H. 1912. The freshwater fishes of British Guiana, including a study of the ecological grouping of species and the relation of the fauna of the plateau to that of the lowlands. Memoirs of the Carnegie Museum, 5(1): 1-578.
- Eigenmann, C. H. 1914. Some results from studies of South American fishes. IV. New genera and species of South American fishes. Indiana University Studies, 20: 44-48.
- Eigenmann, C. H. 1915. The Cheirodontinae, a subfamily of minute characid fishes of South America. Memoirs of the Carnegie Museum, 7(1): 1-99.
- Eigenmann, C. H. 1917. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43(1): 1-102.
- Eigenmann, C. H. 1918. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43(2): 103-208.
- Eigenmann, C. H. 1921. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43(3): 209-310.
- Eigenmann, C. H. 1927. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43(4): 311-428.
- Eigenmann, C. H. & G. S. Myers. 1929. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43(5): 429-558.
- Eigenmann, C. H. & F. Ogle. 1907. An annotated list of characin fishes in the United States National Museum and the Museum of Indiana University, with descriptions of new species. Proceedings of the United States National Museum, 33(1556): 1-36.
- Eschmeyer, W. N. & R. Fricke. 2009. Catalog of Fishes electronic version (updated 13 Mar. 2009). http://research.calacademy.org/ichthyology/catalog/fishcatsearch.html
- Falcão J. N. & L. A. C. Bertollo. 1985. Chromosome characterization in Acestrorhynchinae and Cynopotaminae (Pisces, Characidae). Journal of Fish Biology, 27(5): 603-610.
- Farris, J. S. 1969. A successive approximations approach to character weighting. Systematic Zoology, 18(4): 374-385.
- Farris, J. S. 1970. Methods for computer Wagner trees. Systematic Zoology, 19(1): 83-92.
- Farris, J. S. 1983. The logical basis of phylogenetic analysis. Pp: 7-36. In: Advances in Cladistics, Vol. 2. Platnick, N. I. & V. A. Funk (Eds.). New York, Columbia University Press, 218p.
- Fernández-Yépez, A. (1950) Algunos peces del Rio Autana. Novedades Científicas del Museo de Historia Natural La Salle (Série Zoológica), 2: 1-18.
- Fink, S. V. & W. L. Fink. 1981. Interrelationships of the ostariophysan fishes (Teleostei). Zoological Journal of the Linnean Society, 72(4): 297-353.
- Fink, S. V. & W. L. Fink. 1996. Interrelationships of the ostariophysan fishes (Teleostei). Pp: 209-249. In: Interrelationships of Fishes. Stiassny, M. L. J., L. R. Parenti & G. D. Johnson (Eds.). San Diego, Academic Press, 496p.
- Fink, W. L. & S. H. Weitzman. 1974. The so-called cheirodontin fishes of Central America with descriptions of two new species (Pisces: Characidae). Smithsonian Contributions to Zoology, 172: 1-46.
- Foresti, F., L. F. Almeida-Toledo & A. S. Moreira-Filho. 1989. Supranumerary chromosome system, C-banding pattern characterization and multiple nucleolus organizer regions in Moenkhausia sanctaefilomenae (Pisces, Characidae). Genetica, 79(2): 107-114.
- Franz-Odendaal, T. A. & B. K. Hall. 2006. Skeletal elements within teleost eyes and a discussion on their homology. Journal of Morphology, 267(11): 1326-1337.
- Galetti Jr., P. M., F. Foresti, L. A. C. Bertollo & O. Moreira-Filho. 1981. Heteromorphic sex chromosomes in three species of the genus Leporinus (Pisces, Anostomidae). Cytogenetics and Cell Genetics, 29(3): 138-142.
- Garutti, V. 1999. Descrição de Astyanax argyrimarginatus sp. n. (Characiformes, Characidae) procedente da bacia do Rio Araguaia, Brasil. Revista Brasileira de Biologia, 59(4): 585-591.
- Géry, J. 1963. Essai sur les affinités phylogénétiques des Agoniates et l'origine des Characidae, à propos de la description d'une forme nouvelle de l'Amazone peruvienne: Agoniates ladigesi Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, 60: 265-284.
- Géry, J. 1964a. Poissons characoides de l'Amazone peruvienne (resultats scientifiques de l'expedition Amazone-Ucayali du Dr. K. H. Luling, 1959-1960). Beiträge zur Neotropical Fauna, 4(1): 1-44.
- Géry, J. 1964b. Preliminary description of seven new species and two new genera of characoid fishes from the Upper rio Meta in Colombia. Tropical Fish Hobbyist, 13(4): 25-32, 41-48.
- Géry, J. 1965a. A new genus from Brazil - Brittanichthys Tropical Fish Hobbyist, 13(6): 13-23, 61-69.
- Géry, J. 1965b. Poissons characoïdes sud-américains du Senckenberg Muséum, II. Characidae et Crenuchidae de l'Igarapé Préto (Haute Amazonie). Senckenberg Biology, 46(1): 11-45.
- Géry, J. 1966. A review of certain Tetragonopterinae (Characoidei), with the description of two new genera. Ichthyological Aquarium Journal, 37(5): 211-236.
- Géry, J. 1972. Poissons des Guyanes. 1. Generalites. Zoologische Verhandelungen, 122(1): 1-133.
- Géry, J. 1973. New and little-known Aphyoditeina (Pisces, Characoidei) from the Amazon basin. Studies on Neotropical Fauna, 8: 81-137.
- Géry, J. 1977. Characoids of the World. Neptune City, TFH Publications, 672p.
- Géry, J. & W. J. Junk. 1977. Inpaichthys kerri n. g. n. sp., um novo peixe caracídeo do alto rio Aripuana, Mato Grosso, Brasil. Acta Amazonica, 7(3): 417-422.
- Géry, J. & U. Römer. 1997. Tucanoichthys tucano gen. n. sp. n., a new miniature characid fish (Teleostei: Characiformes: Characidae) from the rio Uaupes basin in Brazil. Aqua, Journal of Ichthyology and Aquatic Biology, 2(4): 65-72.
- Goloboff, P. A. 1993. Estimating character weights during tree search. Cladistics, 9(1): 83-91.
- Goloboff, P. A. 1997. Self-weighted optimization: tree searches and character state reconstructions under implied transformation costs. Cladistics, 13(3): 225-245.
- Goloboff, P. A. & J. S. Farris. 2001. Methods for quick consensus estimation. Cladistics, 17(1): S26-S34.
- Goloboff, P. A., J. S. Farris & K. C. Nixon. 2003a. TNT: Tree Analysis Using New Technology. Versión 1.1, February 2007. Software and documentation available from the authors and at www.zmuc.dk/public/phylogeny
- Goloboff, P. A., J. S. Farris, M. Källersjö, B. Oxelman, M. J. Ramírez & C. A. Szumik. 2003b. Improvements to resampling measures of group support. Cladistics, 19(4): 324-332.
- Goloboff, P. A., J. S. Farris & K. C. Nixon. 2008. TNT, a free program for phylogenetic analysis. Cladistics, 24(5): 774-786.
- Goloboff, P. A., C. I. Mattoni & A. S. Quinteros. 2006. Continuous characters analyzed as such. Cladistics, 22(6): 589-601.
- Greenwood, P. H., D. E. Rosen, S. H. Weitzman & G. S. Myers.1966. Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bulletin of the American Museum of Natural History, 131(4): 339-456.
- Günther, A. 1864. Catalogue of the Fishes in the British Museum, Volume 5. London, Trustees of the British Museum, 455p.
- Gyldenholm, A. O. & J. J. Scheel. 1971. Chromosome number of fishes. I. Journal of Fish Biology, 3(4): 479-486.
- Hadley, A. 2006. CombineZM software. Available from http://hadleyweb.pwp.blueyonder.co.uk/CZM/News.htm
- Harder, W. 1975. Anatomy of Fishes. Stuttgart, E. Schweizerbart'sche Verlagsbuchhandlung, 612p.
- Hennig, W. 1966. Phylogenetic systematics. Illinois, University of Illinois, Urbana, 263p.
- Hinegardner, R. & D. E. Rosen. 1972. Cellular DNA content and evolution of teleostean fishes. American Naturalist, 106(951): 621-644.
- Howes, G. J. 1976. The cranial musculature and taxonomy of characoid fishes of the tribes Cynodontini and Characini. Bulletin of the British Museum (Natural History), Zoology Series, 29(4): 203-248.
- Howes, G. J. 1978. The anatomy and relationships of the cyprinid fish Luciobrama macrocephalus (Lacepède). Bulletin of the British Museum (Natural History), Zoology Series, 34(1): 1-64.
- Howes, G. J. 1979. Notes on the anatomy of Macrochirichthys macrochirus (Valenciennes), 1844, with comments on the Cultrinae (Pisces, Cyprinidae). Bulletin of the British Museum (Natural History), Zoology Series, 36(3): 147-200.
- Howes, G. J. 1980. The anatomy, phylogeny and classification of Bariliinae cyprinid fishes. Bulletin of the British Museum (Natural History), Zoology Series, 37(3): 129-198.
- Hubert, N., C. Bonillo & D. Paugy. 2005. Does elision account for molecular saturation: Case study based on mitochondrial ribosomal DNA among Characiform fishes (Teleostei: Ostariophysii). Molecular Phylogenetics and Evolution, 35(1): 300-308.
- Jégu, M. 2003. Subfamily Serrasalminae. Pp. 182-196. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 742p.
- Kirby, R. F., K. W. Thompson & C. Hubbs. 1977. Karyotypic similarities between the mexican and blind tetras. Copeia, 1977(3): 578-580.
- Langeani, F. 1998. Phylogenetic study of the Hemiodontidae (Ostariophysi, Characiformes). Pp. 145-160. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Lima, F. C. T. 2003a. Subfamily Clupeacharacinae. P. 171. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Lima, F. C. T. 2003b. Subfamily Bryconinae. Pp. 174-181. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Lima, F. C. T. 2006. Revisão Taxonômica e Relações Filogenéticas do Gênero Salminus (Teleostei: Ostariophysi: Characiformes: Characidae). Unpublished Ph. D. Dissertation, Universidade de São Paulo, São Paulo, Brazil, 253p.
- Lima, F. C. T., L. R. Malabarba, P. A. Buckup, J. F. Pezzi da Silva, R. P. Vari, A. Harold, R. Benine, O. T. Oyakawa, C. S. Pavanelli, N. A. Menezes, C. A. S. Lucena, M. C. S. L. Malabarba, Z. M. S. Lucena, R. E. Reis, F. Langeani, L. Cassati, V. A. Bertaco, C. Moreira & P. H. F. Lucinda. 2003. Characidae, genera incertae sedis Pp. 106-169. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Lima, F. C. T. & A. M. Zanata. 2003. Subfamily Agoniatinae. P. 170. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Lima, R. S. 2003c. Subfamily Aphyocharacinae. Pp. 197-199. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Lucena, C. A. S. 1987. Revisão e redefinição do gênero neotropical Charax Scopoli, 1777 com a descrição de quatro espécies novas (Pisces, Characiformes, Characidae). Comunicações do Museu de Ciências e Tecnologia da PUCRS, 40: 5-124.
- Lucena, C. A. S. 1993. Estudo Filogenético da Família Characidae com uma Discussão dos Grupos Naturais Propostos (Teleostei, Ostariophysi, Characiformes). Unpublished Ph.D. Dissertation, Universidade de São Paulo, São Paulo, 158p.
- Lucena, C. A. S. 1998. Relações filogenéticas e definição do gênero Roeboides Günther (Ostariophysi: Characiformes: Characidae). Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia, 11: 19-59.
- Lucena, C. A. S. 2003. Revisão taxonômica e relações filogenéticas das espécies de Roeboides grupo-microlepis (Ostariophysi, Characiformes, Characidae). Iheringia, Série Zoologia, 93(3): 283-308.
- Lucena, C. A. S. 2007. Revisão taxonômica das espécies do gênero Roeboides grupo-affinis (Ostariophysi, Characiformes, Characidae). Iheringia, Série Zoologia, 97(2): 117-136.
- Lucena, C. A. S. & Z. M. S. Lucena. 2002. Redefinição do gênero Deuterodon Eigenmann (Ostariophysi: Characiformes: Characidae). Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia, 15(1): 113-135.
- Lucena, C. A. S. & N. A. Menezes. 1998. A phylogenetic analysis of Roestes Günther and Gilbertolus Eigenmann, with a hypothesis of the relationships of the Cynodontidae and Acestrorhynchidae (Teleostei: Ostariophysi: Characiformes). Pp. 261-277. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Lucena, C. A. S. & N. A. Menezes. 2003. Subfamily Characinae. Pp. 200-208. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Machado-Allison, A. 1982. Estudios sobre la subfamilia Serrasalminae (Teleostei-Characidae). Parte I. Estudio comparado de los juveniles de las "cachamas" de Venezuela (géneros Colossoma y Piaractus). Acta Biológica Venezuélica, 11(3): 1-102.
- Machado-Allison, A. 1983. Estudios sobre la subfamilia Serrasalminae (Teleostei-Characidae). Parte II. Discusión sobre la condición monofilética de la subfamilia. Acta Biológica Venezuélica, 11(4): 145-196.
- Machado-Allison, A. 1985. Estudios sobre la subfamilia Serrasalminae. Parte III: sobre el estatus genérico y relaciones filogenéticas de los géneros Pygopristis, Pygocentrus, Pristobrycon y Serrasalmus (Teleostei-Characidae-Serrasalminae). Acta Biológica Venezuélica, 12(3): 19-42.
- Machado-Allison, A. 1986. Osteología comparada del neurocráneo y branquicráneo en los géneros de la subfamilia Serrasalminae (Teleostei - Characidae). Acta Biológica Venezuélica, 12(Suppl. 2): 1-66.
- Malabarba, L. R. 1998a. Monophyly of the Cheirodontinae, characters and major clades (Ostariophysi, Characidae). Pp. 193-234. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Malabarba, L. R. 2003. Subfamily Cheirodontinae. Pp. 215-221. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 742p.
- Malabarba, L. R. & R. P. Vari. 2000. Caiapobrycon tucurui, a new genus and species of characid from the rio Tocantins basin, Brazil (Characiformes, Characidae). Ichthyological Exploration of Freshwaters, 11(4): 315-326.
- Malabarba, L. R. & S. H. Weitzman. 1999. A new genus and species of South American fishes (Teleostei: Characidae: Cheirodontinae) with derived caudal fin, including comments about inseminating cheirodontines. Proceedings of the Biological Society of Washington, 112(2): 410-432.
- Malabarba, L. R. & S. H. Weitzman. 2000. A new genus and species of inseminating fish (Teleostei: Characidae: Cheirodontinae: Compsurini) from South America with uniquely derived caudal-fin dermal papillae. Proceedings of the Biological Society of Washington, 113(1): 269-283.
- Malabarba, L. R. & S. H. Weitzman. 2003. Description of a new genus with six species from southern Brazil, Uruguay and Argentina, with a discussion of a putative characid clade (Teleostei: Characiformes: Characidae). Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia, 16(1): 67-151.
- Malabarba, M. C. S. L. 1998b. Phylogeny of fossil Characiformes and paleobiogeography of the Tremembé Formation, São Paulo, Brazil. Pp: 69-84. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Menezes, N. A. 1969. Systematics and evolution of the tribe Acestrorhynchini (Pisces, Characidae). Arquivos de Zoologia, 18(1-2): 1-150.
- Menezes, N. A. 1976. On the Cynopotaminae, a new subfamily of the Characidae (Osteichthyes, Ostariophysi, Characoidei). Arquivos de Zoologia, 28(2): 1-91.
- Menezes, N. A. 2003. Family Acestrorhynchidae. Pp. 231-233. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Menezes, N. A., K. M. Ferreira & A. L. Netto-Ferreira. 2009. A new genus and species of inseminating characid fish from the rio Xingu basin (Characiformes: Characidae). Zootaxa, 2167: 47-58.
- Menezes, N. A. & J. Géry. 1983. Seven new acestrorhynchin characid species (Osteichthyes, Ostariophysi, Characiformes) with comments on the systematics of the group. Revue suisse de Zoologie, 90(3): 563-592.
- Menezes, N. A. & S. H. Weitzman. 2009. Systematics of the neotropical fish subfamily Glandulocaudinae (Teleostei: Characiformes: Characidae). Neotropical Ichthyology, 7(3): 295-370.
- Menezes, N. A., S. H. Weitzman & J. R. Burns. 2003. A systematic review of Planaltina (Teleostei: Characiformes: Characidae. Glandulocaudinae: Diapomini) with a description of two new species from the upper rio Paraná, Brazil. Proceedings of the Biological Society of Washington, 116(3): 557-600.
- Miquelarena, A. M. 1982. Estudio comparado del esqueleto caudal en peces characoideos de la República Argentina II. Familia Characidae. Limnobios, 2(5): 277-304.
- Miquelarena, A. M. 1986. Estudio de la dentición en peces caracoideos de la República Argentina. Biología Acuática, 8: 1-60.
- Miquelarena, A. M. & A. E. Aquino. 1985. Situación taxonómica y geográfica de Bryconamericus thomasi Fowler, 1940 (Teleostei, Characidae). Revista Brasileira de Biologia, 55(4): 559-569.
- Miquelarena, A. M. & R. H. Arámburu. 1983. Osteología y lepidología de Gymnocharacinus bergi (Pisces, Characidae). Limnobios, 2(7): 491-512.
- Miquelarena, A. M. & R. C. Menni (2005) Astyanax tumbayaensis, a new species from northwestern Argentina highlands (Characiformes: Characidae) with a key to the Argentinean species of the genus and comments on their distribution. Revue suisse de Zoologie, 112(3): 661-676.
- Miquelarena, A. M., L. C. Protogino, R. Filiberto & H. L. López. 2002. A new species of Bryconamericus (Characiformes: Characidae) from the Cuña-Pirú creek in north-eastern Argentina, with comments on accompanying fishes. Aqua, Journal of Ichthyology and Aquatic Biology, 6(2): 69-82.
- Miquelarena, A. M., L. C. Protogino & H. L. López. 2005. Astyanax hermosus, a new species from the Primero River basin, Cordoba, Argentina (Characiformes: Characidae). Revue suisse de Zoologie, 112(1): 13-20.
- Mirande, J. M. 2008. Filogenia de la Familia Characidae (Teleostei, Characiformes). Unpublished Ph. D. Dissertation, Universidad Nacional de Tucumán, San Miguel de Tucumán, 520 p.
- Mirande, J. M. 2009. Weighted parsimony phylogeny of the family Characidae (Teleostei: Characiformes). Cladistics, 25(6): 574-613.
- Mirande, J. M., G. Aguilera & M. M. Azpelicueta. 2004. A new genus and species of small characid (Ostariophysi, Characidae) from the upper río Bermejo basin, northwestern Argentina. Revue suisse de Zoologie, 111(4): 715-728.
- Mirande, J. M., G. Aguilera & M. M. Azpelicueta. 2006a. Nomenclatural note on the genus Nans (Ostariophysi, Characidae). Revue suisse de Zoologie, 113(2): 305.
- Mirande, J. M., G. Aguilera & M. M. Azpelicueta. 2007. A new species of Astyanax (Characiformes: Characidae) from the endorheic rio Salí basin, Tucumán, northwestern Argentina. Zootaxa, 1646: 31-39.
- Mirande, J. M., M. M. Azpelicueta & G. Aguilera. 2006b. Redescription of Astyanax correntinus (Holmberg, 1891) (Teleostei: Characiformes: Characidae), more than one hundred years from original description. Zoologische Abhandlungen (Dresden), 55: 9-15.
- Morais Filho, M. B. & O. Schubart. 1955. Contribuição ao Estudo do Dourado (Salminus maxillosus Val.) do rio Mogi Guassu (Pisces, Characidae). São Paulo, Ministério da Agricultura, Divisão de Caça e Pesca, 130p.
- Moreira, C. R. 2002. Relações Filogenéticas em Iguanodectinae (Teleostei; Characiformes; Characidae). Unpublished Master Dissertation, Universidade de São Paulo, São Paulo, 276p.
- Moreira, C. R. 2003. Subfamily Iguanodectinae. Pp. 172-173. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Murray, A. M. 2004. Osteology and morphology of the characiform fish Alestes stuhlmannii Pfeffer, 1896 (Alestidae) from the Rufiji River basin, east Africa. Journal of Fish Biology, 65(5): 1412-1430.
- Murray, A. M. & K. M. Stewart. 2002. Phylogenetic relationships of the African genera Alestes and Brycinus (Teleostei, Characiformes, Alestidae). Canadian Journal of Zoology, 80(11): 1887-1899.
- Myers, G. S. 1927. Descriptions of new South American freshwater fishes collected by Dr. Carl Ternetz. Bulletin of the Museum of Comparative Zoology, 68(3): 107-135.
- Nelson, G. 1969. Gill arches and the phylogeny of fishes with notes on the classification of the vertebrates. Bulletin of the American Museum of Natural History, 141(4): 475-522.
- Ornelas-García, C. P., O. Domínguez-Domínguez & I. Doadrio. 2008. Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evolutionary Biology, 8: 340.
- Ortí, G. & A. Meyer. 1997. The radiation of characiform fishes and the limits of resolution of mitochondrial ribosomal DNA sequences. Systematic Biology, 46(1): 75-100.
- Ortí, G., P. Petry, J. I. R. Porto, M. Jégu & A. Meyer. 1996. Patterns of nucleotide change in mitochondrial ribosomal RNA genes and the phylogeny of piranhas. Journal of Molecular Evolution, 42(2): 169-182.
- Paintner-Marques, T. R., L. Giuliano-Caetano & A. L. Dias. 2002. Multiple NOR's in Bryconamericus aff. exodon (Osteichthyes, Characidae, Tetragonopterinae). Hereditas, 137: 107-112.
- Patterson, C. 1975. The braincase of pholidophorid and leptolepid fishes with a review of the Actinopterygian braincase. Philosophical Transactions of the Royal Society B, 269: 275-529.
- Pauls, E. & L. A. C. Bertollo. 1984. Caracterização cromossômica do gênero Prochilodus (Pisces, Prochilodontidae). Ciência e Cultura, 36(S): 787.
- Pflieger, W. L. 1997. The Fishes of Missouri. Missouri, Conservation Commission of the State of Missouri, 372p.
- Portela, A. L. B. S., P. M. Galetti Jr. & L. A. C. Bertollo. 1988. Considerations on the chromosome evolution of Tetragonopterinae (Pisces, Characidae). Revista Brasileira de Genética, 11: 307-316.
- Porto, J. I. R., E. Feldberg, C. Nakayama & J. N. Falcão. 1992. A checklist of chromosome numbers and karyotypes of Amazonian freshwater fishes. Revue d'Hydrobiologie Tropicale, 25: 287-299.
- Quevedo, R. 2006. Estudo Taxonômico e Filogenético da Subfamília Paragoniatinae Géry (Characiformes: Characidae). Unpublished Ph.D. Dissertation. Universidade Federal do Rio Grande do Sul, Porto Alegre, 288p.
- Reis, R. E. 1989. Systematic revision of the Neotropical characid subfamily Stethaprioninae (Pisces, Characiformes). Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia, 2(6): 3-86.
- Reis, R. E. 2003a. Subfamily Tetragonopterinae. P. 212. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Reis, R. E. 2003b. Subfamily Stethaprioninae. Pp. 209-211. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Reis, R. E., S. O. Kullander & C. J. Ferraris, Jr. (2003) Check List of the Freshwater Fishes of South and Central America. Porto Alegre, Edipucrs, 729p.
- Ringuelet, R. A., R. H. Arámburu & A. A. Arámburu. 1967. Los Peces Argentinos de Agua Dulce. Buenos Aires, Comisión de Investigaciones Científicas de la provincia de Buenos Aires, 602p.
- Roberts, C. D. 1993. Comparative morphology of spined scales and their phylogenetic significance in the Teleostei. Bulletin of Marine Science, 52(1): 60-113.
- Roberts, T. R. 1969. Osteology and relationships of characoid fishes particularly of the genera Hepsetus, Salminus, Hoplias, Ctenolucius and Acestrorhynchus Proceedings of the California Academy of Sciences, 36(15): 391-500.
- Roberts, T. R. 1973. Osteology and relationships of the Prochilodontidae, a South American family of characoid fishes. Bulletin of the Museum of Comparative Zoology, 145(4): 213-235.
- Roberts, T. R. 1974. Osteology and classification of the Neotropical fishes of the families Hemiodontidae (including Anodontidae) and Parodontidae. Bulletin of the Museum of Comparative Zoology, 146(9): 411-472.
- Román-Valencia, C. 2000. Tres nuevas especies de Bryconamericus (Ostariophysi: Characidae) de Colombia y diagnóstico del género. Revista de biología tropical, 48(2-3): 449-464.
- Román-Valencia, C. 2002. Revisión sistemática de las especies del género Bryconamericus (Teleostei: Characidae) de Centroamérica. Revista de biología tropical, 50(1): 173-192.
- Román-Valencia, C. 2003. Sistemática de las especies colombianas de Bryconamericus (Characiformes, Characidae). Dahlia, 6: 17-58.
- Román-Valencia, C. & J. A. Vanegas-Ríos. 2009. Análisis filogenético y biogeográfico de las especies del género Bryconamericus (Characiformes, Characidae) de la baja América Central. Caldasia, 31(2): 393-406.
- Rosen, D. E. 1972. Origin of the characid fish genus Bramocharax and a description of a second, more primitive, species in Guatemala. American Museum Novitates, 2500: 1-21.
- Ruiz-C., R. I. & C. Román-Valencia. 2006. Osteología de Astyanax aurocaudatus Eigenmann, 1913 (Pisces, Characidae), con notas sobre la validez de Carlastyanax Géry, 1972. Animal Biodiversity and Conservation, 29(1): 49-64.
- Serra, J. P. 2003. Análise filogenética e revisão taxonômica de Hasemania Ellis, 1911 (Characiformes, Characidae). Unpublished Master Dissertation, Universidade Estadual Paulista, São José do Rio Preto, 148p.
- Serra, J. P. & F. Langeani. 2006. Redescrição e osteologia de Bryconamericus exodon Eigenmann, 1907 (Ostariophysi, Characiformes, Characidae). Biota Neotropica, 6(3): 1-14.
- Silva, A. R. & E. L. Maistro. 2006. Cytogenetic divergence between two sympatric species of Characidium (Teleostei, Characiformes, Crenuchidae) from the Machado River, Minas Gerais, Brazil. Genetics and Molecular Biology, 29(3): 459-463.
- Starks, E. C. 1930. The primary shoulder girdle of the bony fishes. Stanford University Publications, University Series, Biological Sciences, 6(2): 149-239.
- Taylor, W. R. & G. C. van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9(2): 107-119.
- Toledo-Piza, M. 2000. The Neotropical fish subfamily Cynodontinae (Teleostei: Ostariophysi: Characiformes): a phylogenetic study and a revision of Cynodon and Rhaphiodon. American Museum Novitates, 3286, 1-88.
- Toledo-Piza, M. 2003. Family Cynodontidae. Pp. 234-237. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Toledo-Piza, M. 2007. Phylogenetic relationships among Acestrorhynchus species (Ostariophysi: Characiformes: Acestrorhynchidae). Zoological Journal of the Linnean Society, 151(4): 691-757.
- Trapani, J. 2001. Position of developing replacement teeth in teleosts. Copeia, 2001(1): 35-51.
- Uj, A. 1990. Etude Comparative de l'Osteologie Cranienne des Poissons de la Familie des Characidae et son Importance Phylogenetique. Unpublished Ph. D. Dissertation. Université de Geneve, Geneve, 247p.
- Vari, R. P. 1977. Notes on the characoid subfamily Iguanodectinae, with a description of a new species. American Museum Novitates, 2612: 1-6.
- Vari, R. P. 1979. Anatomy, relationships and classification of the families Citharinidae y Distichodontidae (Pisces, Characoidea). Bulletin of the British Museum (Natural History) Zoology series, 36(5): 261-344.
- Vari, R. P. 1983. Phylogenetic relationships of the families Curimatidae, Prochilodontidae, Anostomidae, and Chilodontidae (Pisces: Characiformes). Smithsonian Contributions to Zoology, 378: 1-60.
- Vari, R. P. 1986. Serrabrycon magoi, a new genus and species of scale-eating characid (Pisces: Characiformes) from the upper río Negro. Proceedings of the Biological Society of Washington, 99(2): 328-334.
- Vari, R. P. 1995. The Neotropical fish family Ctenoluciidae (Teleostei: Ostariophysi: Characiformes): supra and infrafamilial phylogenetic relationships, with a revisionary study. Smithsonian Contributions to Zoology, 564: 1-97.
- Vari, R. P., R. M. C. Castro & S. J. Raredon. 1995. The Neotropical fish family Chilodontidae (Teleostei: Characiformes): a phylogenetic study and a revision of Caenotropus Günther. Smithsonian Contributions to Zoology, 577: 1-32.
- Vari, R. P. & A. S. Harold. 1998. The genus Creagrutus (Teleostei: Characiformes: Characidae): Monophyly, relationships, and undetected diversity. Pp: 245-260. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Vari, R. P. & A. S. Harold. 2001. Phylogenetic study of the Neotropical fish genera Creagrutus Günther and Piabina Reinhardt (Teleostei: Ostariophysi: Characiformes), with a revision of the cis-Andean species. Smithsonian Contributions to Zoology, 613: 1-239.
- Vari, R. P. & H. Ortega. 2000. Attonitus, a new genus of sexually dimorphic characiforms (Ostariophysi, Characidae) from western Amazonia; a phylogenetic definition and description of three new species. Ichthyological Exploration of Freshwaters, 11(2): 113-140.
- Weitzman, S. H. 1954. The osteology and relationships of the South American characid fishes of the subfamily Gasteropelecinae. Stanford Ichthyological Bulletin, 4(1): 213-263.
- Weitzman, S. H. 1960. The phylogenetic relationships of Triportheus, a genus of South American characid fishes. Stanford Ichthyological Bulletin, 7(4): 239-244.
- Weitzman, S. H. 1962. The osteology of Brycon meeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyogical Bulletin, 8(1): 3-77.
- Weitzman, S. H. 1964. Osteology and relationships of South American characid fishes of subfamilies Lebiasininae and Erythrininae with special reference to subtribe Nannostomina. Proceedings of the United States National Museum, Smithsonian Institution, 116(3499): 127-169.
- Weitzman, S. H. 2003. Subfamily Glandulocaudinae. Pp. 222-230. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Weitzman, S. H. & S. V. Fink. 1985. Xenurobryconin phylogeny and putative pheromone pumps in Glandulocaudinae fishes (Teleostei: Characidae). Smithsonian Contributions to Zoology, 421: 1-119.
- Weitzman, S. H. & W. L. Fink. 1983. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei: Characidae), with comments on the phylogeny of New World characiforms. Bulletin of the Museum of Comparative Zoology, 150(6): 339-395.
- Weitzman, S. H. & L. R. Malabarba. 1998. Perspectives about the phylogeny and classification of the Characidae. Pp. 161-170. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Weitzman, S. H. & L. R. Malabarba. 1999. Systematics of Spintherobolus (Teleostei: Characidae: Cheirodontinae) from eastern Brazil. Ichthyological Exploration of Freshwaters, 10(1): 1-43.
- Weitzman, S. H. & N. A. Menezes. 1998. Relationships of the tribes and genera of the Glandulocaudinae (Ostariophysi, Characiformes, Characidae) with the description of a new genus, Chrysobrycon Pp. 171-192. In: Phylogeny and classification of Neotropical fishes. Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Porto Alegre, Edipucrs, 603p.
- Weitzman S. H., N. A. Menezes, H. G. Evers & J. R. Burns. 2005. Putative relationships among inseminating and externally fertilizing characids, with a description of a new genus and species of Brazilian inseminating fish bearing an anal-fin gland in males (Characiformes: Characidae). Neotropical Ichthyology, 3(3): 329-360.
- Weitzman, S. H. & L. Palmer. 1997. A new species of Hyphessobrycon (Teleostei: Characidae) from the Neblina region of Venezuela and Brazil, with comments on the putative 'rosy tetra clade'. Ichthyological Exploration of Freshwaters, 7(3): 209-242.
- Weitzman, S. H. & L. Palmer. 2003. Family Gasteropelecidae. Pp. 101-103. In: Check List of the Freshwater Fishes of South and Central America. Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (Eds.). Porto Alegre, Edipucrs, 729p.
- Wilkens, H. & U. Strecker. 2003. Convergent evolution of the cavefish Astyanax (Characidae, Teleostei): genetic evidence from reduced eye-size and pigmentation. Biological Journal of the Linnean Society, 80: 545-554.
- Winterbottom, R. 1974. A descriptive synonymy of the striated muscles of the Teleostei. Proceedings of the Academy of Natural Sciences of Philadelphia, 125(12): 225-317.
- Winterbottom, R. 1980. Systematics, osteology and phylogenetic relationships of fishes of the ostariophysan subfamily Anostominae (Characoidei, Anostomidae). Life Science Contribution, Royal Ontario Museum, 123: 1-112.
- Zanata, A. M. 1997. Jupiaba, um novo gênero de Tetragonopterinae com osso pélvico em forma de espinho (Characidae, Characiformes). Iheringia, Série Zoologia, 83: 99-136.
- Zanata, A. M. 2000. Estudo das Relações Filogenéticas do Gênero Brycon Muller & Troschel, 1844 (Characidae; Characiformes). Unpublished Ph.D. Dissertation, Universidade de São Paulo, São Paulo, Brazil, 276p.
- Zanata, A. M. & A. Akama. 2004. Myxiops aphos, new characid genus and species (Characiformes: Characidae) from the rio Lençóis, Bahia, Brazil. Neotropical Ichthyology, 2(2): 45-54.
- Zanata, A. M. & M. Toledo-Piza. 2004. Taxonomic revision of the South American fish genus Chalceus Cuvier (Teleostei: Ostariophysi: Characiformes) with the description of three new species. Zoological Journal of the Linnean Society, 140(1): 103-135.
- Zanata, A. M. & R. P. Vari. 2005. The family Alestidae (Ostariophysi, Characiformes): a phylogenetic analysis of a trans-Atlantic clade. Zoological Journal of the Linnean Society, 145(1): 1-144.
- Zarske, A. & J. Géry. 2006. Beschreibung einer neuen Salmler-Gattung und zweier neuer Arten (Teleostei: Characiformes: Characidae) aus Peru und Brasilien. Zoologische Abhandlungen (Dresden), 55: 31-49.
Appendix 2

Appendix 1. List of examined material. Only C&S and alcohol specimens are listed.
Publication Dates
-
Publication in this collection
04 Nov 2010 -
Date of issue
2010
History
-
Accepted
24 Sept 2010 -
Received
07 June 2010