Abstracts
Two new species of Microglanis are described from tributaries of upper-middle rio Araguaia, States of Mato Grosso and Goiás, Brazil. Microglanis oliveirai differs from its congeners by the short supraoccipital process not reaching the small anterior nuchal plate, and reduced number of gill rakers, lateral line pores, ribs, vertebrae, and caudal-fin rays. Microglanis xylographicus differs from its congeners by the presence of melanophores surrounding the neuromasts of the superficial lines, snout length 11.4-12.9% SL and body color pattern consisting of horizontal brown stripes similar to a wooden bark pattern. An identification key of Microglanis species from the Amazon basin is presented.
Ecology; Neotropical; Rio Tocantins; Taxonomy
Duas espécies novas de Microglanis são descritas de tributários do alto-médio rio Araguaia, estados do Mato Grosso e Goiás, Brasil. Microglanis oliveirai difere de seus congêneres pelo processo supraoccipital curto não atingindo a pequena placa nucal anterior, e número reduzido de rastros branquiais, poros da linha lateral, costelas, vértebras e raios da nadadeira caudal. Microglanis xylographicus difere de seus congêneres pela presença de melanóforos ao redor dos neuromastos alinhados superficialmente, comprimento do focinho 11,4-12,9% CP e padrão de colorido do corpo, formado por listras horizontais castanhas como marcas de casca de madeira. Uma chave de identificação de espécies de Microglanis da bacia Amazônica é apresentada.
Two new species of Microglanis (Siluriformes: Pseudopimelodidae) from the upper-middle rio Araguaia basin, Central Brazil
William Benedito Gotto RuizI; Oscar Akio ShibattaII
IUniversidade de São Paulo, Programa de Pós-Graduação em Biologia Comparada, Laboratório de Ictiologia de Ribeirão Preto, Departamento de Biologia. Av. dos Bandeirantes, 3900, 14040-901 Ribeirão Preto, SP, Brazil. willigotto@yahoo.com.br
IIUniversidade Estadual de Londrina, Departamento de Biologia Animal e Vegetal, Centro de Ciências Biológicas, 86051-990 Londrina, PR, Brazil. shibatta@uel.br
ABSTRACT
Two new species of Microglanis are described from tributaries of upper-middle rio Araguaia, States of Mato Grosso and Goiás, Brazil. Microglanis oliveirai differs from its congeners by the short supraoccipital process not reaching the small anterior nuchal plate, and reduced number of gill rakers, lateral line pores, ribs, vertebrae, and caudal-fin rays. Microglanis xylographicus differs from its congeners by the presence of melanophores surrounding the neuromasts of the superficial lines, snout length 11.4-12.9% SL and body color pattern consisting of horizontal brown stripes similar to a wooden bark pattern. An identification key of Microglanis species from the Amazon basin is presented.
Key words: Ecology, Neotropical, Rio Tocantins, Taxonomy.
RESUMO
Duas espécies novas de Microglanis são descritas de tributários do alto-médio rio Araguaia, estados do Mato Grosso e Goiás, Brasil. Microglanis oliveirai difere de seus congêneres pelo processo supraoccipital curto não atingindo a pequena placa nucal anterior, e número reduzido de rastros branquiais, poros da linha lateral, costelas, vértebras e raios da nadadeira caudal. Microglanis xylographicus difere de seus congêneres pela presença de melanóforos ao redor dos neuromastos alinhados superficialmente, comprimento do focinho 11,4-12,9% CP e padrão de colorido do corpo, formado por listras horizontais castanhas como marcas de casca de madeira. Uma chave de identificação de espécies de Microglanis da bacia Amazônica é apresentada.
Introduction
Microglanis Eigenmann, 1912 is endemic to freshwaters of South America, where it has a wide geographic distribution that includes both sides of northern Andes, and also the region from Venezuela to Uruguay (Shibatta, 2003a). It is the most species-rich genus of the Pseudopimelodidae, comprising 19 species (Ruiz & Shibatta, 2010; Ottoni et al., 2010). Microglanis can be identified from the remaining pseudopimelodids by the following combination of characters: body size smaller than 80.0 mm SL, premaxillary tooth patch with a rounded lateral margin and no posterior projection, lateral line canal incomplete, a filamentous mesocoracoid arch, and no axillary pore (Mees, 1974; Shibatta, 1998; 2003b).
In the last few years several species of Microglanis were described (Shibatta & Benine, 2005; Sarmento-Soares et al., 2006; Mori & Shibatta, 2006; Alcaraz et al., 2008; Ruiz & Shibatta, 2010; Ottoni et al., 2010), which indicates that the genus is both more species-rich and widespread than previously supposed. The only species of Microglanis previously recorded from the rio Tocantins/Araguaia basin is M. robustus Ruiz & Shibatta, 2010. In the present paper, two additional species are described from the upper-middle rio Araguaia basin in Brazil.
Material and Methods
Specimens of all species of Microglanis were examined, with the exception of M. ater Ahl, 1936, M. zonatus Eigenmann & Allen, 1942, and M. minutus Ottoni, Mattos & Barbosa, 2010, of which photographs and data from literature were analyzed. Morphometric characters of 234 specimens were taken point-to-point with digital caliper with accuracy of 0.1 mm, under a stereomicroscope. Counts and measurements were taken on the left side of specimens, with a few exceptions, when the structures were damaged or absent. Measurements were taken following Ruiz & Shibatta (2010), and expressed as percents of standard length (SL), except subunits of the head, which are recorded as percents of head length (HL). Meristic data included counts of dorsal, pectoral, pelvic, anal and caudal-fin rays, gill rakers, serrations of pectoral-fin spine, lateral line pores, and superficial neuromasts. Roman numerals indicate unbranched rays (uppercases as spines and lowercases as soft rays) and Arabic numerals represent branched rays. The location and number of pores of lateral line and superficial neuromasts were analyzed according to Ruiz & Shibatta (2010). In the diagnoses and descriptions of species, the frequency of each meristic data was presented in brackets and the counts of the holotypes are identified by asterisks. Counts of branchiostegal rays, vertebrae, ribs, pterygiophores, and procurrent caudal-fin rays were obtained from two cleared and stained (c&s) specimens of each species, prepared according to Dingerkus & Uhler (1977). Vertebral counts included only free centra (elements of the anterior complex centrum were not included), with the compound caudal centra (preural 1 + ural 1) counted as a single element. For analysis of food items, the entire digestive tract contents of two specimens of each species herein described were examined. Gas bladder nomenclature followed Birindelli & Shibatta (2011).
Institutional abbreviations are: ANSP, Academy of Natural Sciences of Philadelphia, Philadelphia, PA; CAS, California Academy of Sciences, San Francisco, CA; INPA, Instituto Nacional de Pesquisas da Amazônia, Manaus, AM; LBP, Laboratório de Biologia e Genética de Peixes, Instituto de Biociências da Universidade Estadual Paulista, Botucatu, SP; MCP, Museu de Ciências e Tecnologia, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS; MEPN, Museo de la Escuela Politécnica Nacional de Quito, Ecuador; MHNG, Muséum D'Histoire Naturelle de Genève, Geneva; MNHNP, Museo Nacional de Historia Natural Del Paraguay, Assunción, Paraguay; MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ; MZUEL, Museu de Zoologia da Universidade Estadual de Londrina, Londrina, PR; MZUSP, Museu de Zoologia da Universidade de São Paulo, São Paulo, SP; NUP, Coleção Ictiológica do Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Universidade Estadual de Maringá, Maringá, PR; ROM, Royal Ontario Museum, Toronto; UFRGS, Departamento de Zoologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS USNM, National Museum of Natural History, Smithsonian Institution,Washington, DC.
Results
Microglanis oliveirai, new species
Holotype. INPA 35623, 26.3 mm SL, Brazil, Mato Grosso, Barra do Garças, Vale dos Sonhos District, BR-158 road, km 750, rio Corrente (right margin tributary of the rio das Mortes), rio Araguaia basin, 15°29'56.3"S 52°12'10.8"W, 31 Jul 2008, W. B. G. Ruiz, L. R. Jarduli & E. S. Silva.
Paratypes. All from Brazil, Mato Grosso State, rio Araguaia basin: MZUEL 5175, 11, 19.6-25.4 mm SL (2 c&s, 21.7-23.3 mm SL), same data as holotype. LBP 1852, 4, 21.0-22.2 mm SL, Barra do Garças, rio Insula (tributary of rio das Mortes), 15°32'54.2"S 52°12'17.7"W, 27 Aug 2003, C. Martin. MZUSP 86239, 3, 18.6-21.0 mm SL, Cocalinho, rio Cristalino, 47 km from Cocalinho, MT 326 road, 14°12'45"S 51°18'21"W, 14 Oct 2004, AXE Expedition [O. T. Oyakawa et al.]. MZUSP 86222, 10, 17.7-24.5 mm SL, Cocalinho, rio Corixo da Saudade (Corixinho) (tributary of rio Cristalino), 25 km North of Cocalinho, MT 326 road, 14°17'20.6"S 51°9'12.1"W, 13 Oct 2004, AXE Expedition [O. T. Oyakawa et al.]. MZUSP 86260, 2, 20.5-21.22 mm SL, Cocalinho, corixão do Meio (tributary of rio Cristalino), 42 km North of Cocalinho, MT-326 road, 14°11'14"S 51°14'58"W, 14 Oct 2004, AXE Expedition [O. T. Oyakawa et al.]. MZUSP 86253, 2, 17.6-22.1 mm SL, Cocalinho, ribeirão Água Preta (tributary of rio Cristalino), 79 km North of Cocalinho, MT 326 road, 14°08'57"S 51°32'21"W, 14 Oct 2004, AXE Expedition [O. T. Oyakawa et al.].
Diagnosis.Microglanis oliveirai is readily distinguished from all congeners in the possession of the following characters: short lateral line, reaching only the vertical line through base of dorsal-fin spine; 3-6 lateral line pores; 3-6 gill rakers in the first branchial arch; 10-11 branched caudal fin rays; 5 pleural ribs; 27-28 free vertebrae; large anterior fontanel; small supraoccipital process not contacting anterior nuchal plate; small nuchal shield.
Description. Small size; largest examined specimen 26.3 mm SL. Morphometric data presented in Table 1. Body elongate, depressed anteriorly, especially at head, compressed posteriorly from pectoral-fin insertion. Greatest body depth at dorsal-fin origin, approximately elliptical in cross section. Greatest body width at pectoral girdle. Dorsal profile slightly convex on predorsal region, almost straight or slightly concave from dorsal-fin spine to anterior region of adipose fin, and concave at caudal peduncle. Ventral profile slightly convex from mouth to pectoral-fin base, approximately straight from later point to anal-fin and concave from anal fin origin to ventral procurrent caudal-fin rays. Head as wide as long, strongly depressed, anteriorly rounded in dorsal view. Small eyes, situated dorso-laterally, covered by skin, without free orbital margin and positioned at midlength of HL. Snout short. Anterior nostril tubular, close to upper lip; posterior nostril larger, rounded, close to eye, with small flap on anterior portion. Mouth gape wide. Barbels thin, short, slightly flattened in cross section. Maxillary barbel surpassing base of pectoral-fin spine. Mental barbels arranged in arc along ventral surface of jaw; outer pair surpassing base of pectoral-fin spines; inner pair shorter, slightly less than one-half length of outer mental barbels.
Pectoral fin roughly triangular in dorsal and ventral views, rays I,5. Pectoral-fin spine long, strong, flat, recurved at midpoint, strongly serrated in both margins, covered by thin skin. Anterior margin of pectoral-fin spine with four to eight retrorse serrations proximally, followed by none to two Y-shaped serrations, and two to seven antrorse serrations distally (total = 9-13[32]). Posterior margin with six to 10[32] retrorse serrations, larger than those of anterior margin. Tip of pectoral-fin spine strongly ossified (Fig. 2). Pectoral fin not reaching pelvic-fin base. Dorsal fin II,6 (spinelet present); rounded margin, slightly deeper than long. Dorsal-fin spine straight, smooth, shorter than soft rays and covered by skin. Dorsal fin not reaching adipose-fin base when adpressed. Pelvic fin i,5, roughly semicircular in ventral view, its origin situated at midbody close to vertical line through two last dorsal-fin rays, when adpressed not reaching anal-fin base. Adipose fin relatively small, with rounded posterior margin free. Anal fin relatively large and deep, with rounded margin, 10[15] or 11*[17] total rays. Anal-fin base slightly smaller than adipose-fin base. Longest anal-fin rays reaching first ventral procurrent caudal-fin rays. Caudal fin large, deep, slightly forked; rounded lobes, with upper lobe well developed; 10[20] or 11*[12] branched rays, 15 dorsal procurrent rays, nine to 10 smaller ventral procurrent rays. Caudal peduncle relatively long (15.5-17.6 % SL).
Lateral line incomplete, not reaching the vertical through the first branched dorsal-fin ray; 3[1], 4[7], 5*[16], or 6[8] large pores, about half diameter of parieto-supraoccipital fontanel. Cephalic sensory canals with eight pores on mandibular canal; one on preopercle canal; four on infraorbital canal; one on antorbital branch of infraorbital canal; five on supraorbital canal; one on parietal branch of supraorbital canal; one on otic canal; and two on postotic canal. Parietal branch of supraorbital canal with one terminal pore. Cephalic neuromasts lines with three or four small neuromasts on nasal line, one on rostral line, four to six on mandibular line, two on anterior line, and two on supratemporal accessory line. Trunk neuromasts lines with two neuromasts on dorsal-trunk line, six to 12 on medium trunk line, five to eight on subdorsal-trunk line, and five to seven very small neuromasts on subventral-trunk line.
Premaxillary tooth patch narrow, long, weakly curved, lateral margin slightly rounded, without posterior projections, with notch at symphysis. Dentary tooth patch slightly narrower than, and about twice the length of, the premaxillary tooth patch, with notch on mandibular symphysis. Teeth small, villiform, pointing posteriorly, teeth larger on dentary patch. Gill rakers spiny, unbranched, and relatively small. First branchial arch bearing few rakers: 0+1+2=3[4], 0+1+3=4*[6], 1+1+1=3[5], 1+1+2=4[9], 1+1+3=5[5], or 1+1+4=6[2]. Branchiostegal membranes free from isthmus. Branchiostegal rays 9-10. Free vertebrae 27-28. Pleural ribs 5. Anal-fin proximal pterygiophores 9-10. Gas bladder large, cordiform, with simple inner T-shaped septum.
Color in alcohol. Ground color light brown. Ventral region of body and head pale with brown spots. Head dark brown, with lateral portions of head, margin of opercle, anterior and posterior nostrils, and pores of cephalic canals light colored. Wide, irregular light band passing through nape and anterior portion of trunk, immediately after opercular opening. Upper lip dark, lower lip light. Light barbels speckled with dark brown spots. Overall trunk color light brown, with faint vermiculated stripes. Three large dark brown blotches, appearing dorsally as saddle markings. First blotch similar to an inverted U, below dorsal fin, reaching horizontal line through axis of trunk. Second blotch more elongated, situated immediately below adipose fin, extending posteroventrally to reach the brown blotch at anal-fin base. In some specimens, this sub-adipose blotch is divided in two by a light band across the horizontal axis of the body. Third blotch positioned at middle to posterior portions of caudal peduncle, roughly triangular-shaped, with one of vertices pointing anteriorly. Middle trunk-line neuromasts faintly surrounded by melanophores. Pectoral and pelvic fins hyaline, slightly speckled with brown pigmentation over rays. Medial to distal portion of pectoral-fin spine with a large dark brown blotch. Dorsal fin mostly hyaline, a large dark blotch situated at anterior basal portion of fin, extending into dorsal-fin spine. A brown, narrow stripe running midway between dorsal-fin base and margin. Adipose fin with one oval light blotch on anterior portion, followed by narrow brown stripe, and light area slightly speckled with melanophores. Anal fin mostly hyaline, speckled with melanophores, concentrated as a small, relatively diffuse anterior basal portion and, in large specimens, a weak, brown arched stripe, on middle portion of anal fin between the 3rd and 6th rays. Caudal fin hyaline, speckled with brown cromatophores, with one straight, vertical dark brown stripe on the base. In larger specimens a narrow stripe shaped approximately like the numeral three, located at the middle of the fin.
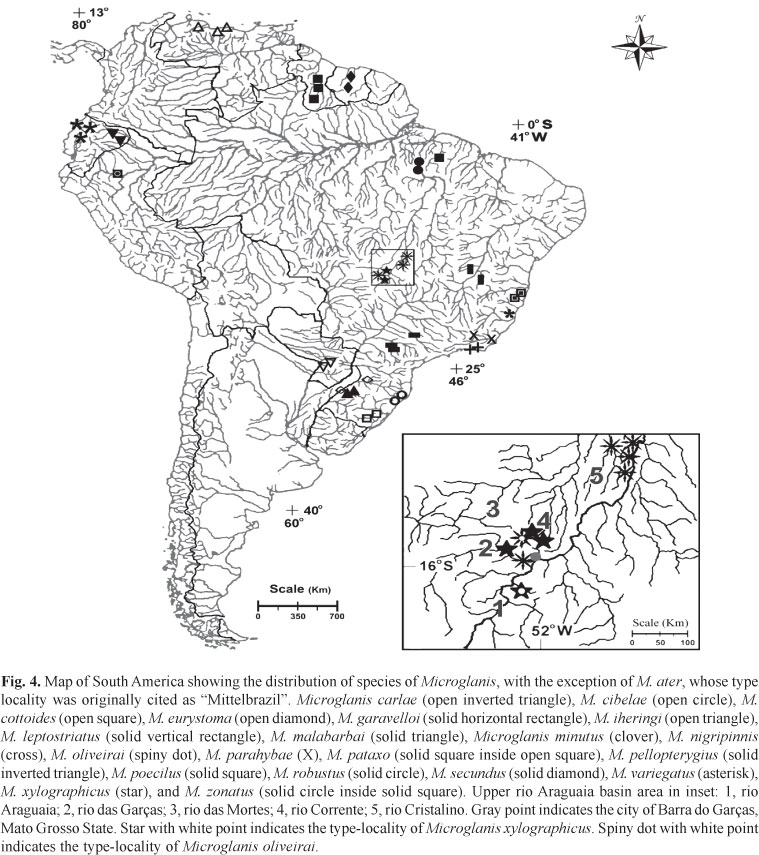
Distribution.Microglanis oliveirai is known from tributaries of rio das Mortes and rio Cristalino (Fig. 3A), upper-middle rio Araguaia basin, State of Mato Grosso, Brazil (Fig. 4).
Etymology.Microglanis oliveirai is named in honor of Claudio de Oliveira, from the Universidade Estadual Paulista "Júlio de Mesquita Filho", campus Botucatu (SP), in recognition of his extensive contribution to the knowledge of Neotropical fish evolution.
Remarks. Specimens collected at the rio Cristalino basin have larger interorbital width and possess an overall darker coloration when compared with specimens of the rio das Mortes basin.
Microglanis xylographicus, new species
Holotype. INPA 35624, 27.8 mm SL, Brazil, Goiás State, Aragarças, córrego Jaraguá (tributary of rio Araguaia), road to Torixoréu, 15°56'26.9"S 52°15'18.2"W, 31 Jul 2008, W. B. G. Ruiz, L. R. Jarduli & E. S. Silva.
Paratypes. All from Brazil, rio Araguaia basin. Goiás State. MZUEL 5174, 2, 23.1-26.2 mm SL, same data as holotype. Mato Grosso. MZUEL 5173, 4, 18.2-22.3 mm SL (1 c&s, 21.5 mm SL), rio Corrente (tributary of rio das Mortes), BR-158 road, km 750, Vale dos Sonhos, 15°29'56.29"S 52°12'10.75"W, 31 Jul 2008, W. B. G. Ruiz, L. R. Jarduli & E. S. Silva. LBP 11521, 10, 16.9-23.7 mm SL (1 c&s, not measured), Barra do Garças, rio Insula (tributary of rio das Mortes), 15°32'54.2"S 52°12'17.7"W, 27 Aug 2003, C. Martin. LBP 1684, 23.8 mm SL, rio das Garças, 15°54'18.1"S 52°19'24.2"W, 13 Jul 2002, C. Oliveira. Goiás. LBP 1852, 2, 18.7-20.9 mm SL, rio Insula, Barra do Garças, 15º32'54.2"S 52º12'17.7"W, 27 Aug 2003, C. Martin. UFRGS 13166, 1, 21.7 mm SL, Aragarças, stream tributary of rio Claro, between Aragarças and Jussara, 15° 52' 10.9"S 51° 32' 56.7"W, V. A. Bertaco, F. R. Carvalho & F. C. Jerep.
Diagnosis.Microglanis xylographicus is easily distinguished from its congeners by two characters related to color pattern: (1) trunk brown with horizontal light stripes that in conjunction impart a tree-bark pattern, and (2) absence of any light marks (blotch or transversal band) crossing the occipital region. The new species can be further distinguished from its congeners, except Microglanis robustus, by the neuromasts surrounded by melanophores, forming three series of aligned small black points laterally on the trunk, four series in the head, one series on the lateral of nape, and one series posterior to the nape. It is distinguished from M. robustus by having a relatively long snout 11.4-12.9% SL (vs. 9.1-10.7% SL), and five pleural ribs (vs. 6 or 7).
Description. Small size, largest examined specimen 27.8 mm SL. Morphometric data presented in Table 1. Dorsal profile convex on predorsal region, almost straight from dorsal-fin origin to anterior region of adipose fin, and concave from latter point to anteriormost procurrent caudal-fin ray. Ventral profile gently convex from lower lip to pelvic-fin origin, slightly concave from latter point to anal-fin origin, slightly convex at origin of anal-fin, and concave from latter point to anteriormost ventral procurrent caudal-fin ray. Body depressed in the region anterior to dorsal-fin origin, becoming gradually compressed posteriorly from pectoral-fin. Greatest body depth at dorsal-fin origin; body roughly triangular in cross section. Greatest body width at pectoral girdle. Head broad, depressed and slightly rounded in dorsal view. Small eyes, positioned dorsolaterally, covered by skin, without free orbital margin, closer to mouth than to opercular opening. Anterior nostril tubular, close to upper lip, situated just anterior to vertical line through base of maxillary barbel. Posterior nostril rounded, close to orbital margin, with conspicuous flap on anterior portion. Mouth wide, its gape occupying almost entire width of head. Barbels short, thin, flattened in cross section. Maxillary barbel reaching pectoral-fin spine base in some specimens. Two mental pairs, outer pair reaching, or falling short of, pectoral-spine base, inner pair about one-half length of outer mental barbel.
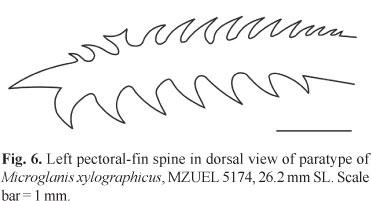
Pectoral fin roughly triangular in dorsal and ventral view, I,5, when adpressed not reaching pelvic-fin base. Pectoral-fin spine strong, flattened, slightly arched, completely covered by thick skin, with large serrations on both sides. Anterior margin of pectoral-fin spine with seven to 11 retrorse serrations proximally, followed by none to two slightly forked or rarely Y-shaped serrations, and none to four small antrorse serrations (total = 9-14[18]). Posterior margin of pectoral-fin spine with five to eight [18] retrorse serrations, larger than those of anterior margin. Tip of pectoral-fin spine ossified and pointed (Fig. 6). Dorsal fin II,6 (spinelet present), inserted almost at anterior midbody, deeper than longer, margin slightly rounded, when adpressed usually reaching adipose-fin base. Dorsal-fin spine straight, smooth, covered by skin, about the same length of last branched ray. Pelvic fin i,5, its insertion situated at midbody, margin rounded, and when adpressed, not reaching anal-fin base. Adipose fin moderately long and deep, with posterior margin free and angular, not confluent with caudal fin, and located above anal-fin origin. Anal fin large and deep, with 10[9], 11*[8], or 12[1] rays, and rounded margin. Anal-fin base shorter than adipose-fin base. Posterior anal-fin rays reaching first two ventral procurrent caudal-fin rays. Caudal fin large, deep, bilobed; upper lobe usually longer than lower lobe, with 12*[15] or 13[3] branched rays, 16 dorsal procurrent rays, and 10 smaller ventral procurrent rays. Caudal peduncle deep (12.3-14.4% SL) and long (16.8-18.5% SL).
Lateral line incomplete, but usually reaching or surpassing vertical line through posterior portion of dorsal-fin base; 7[3], 8[6], 9[5], 10*[2], 11[1], or 12[1] small pores. Cephalic sensory canals with eight pores on mandibular canal, one on preopercle canal, four on infraorbital canal, one on antorbital branch of infraorbital canal, five on supraorbital canal, one on parietal branch of supraorbital canal, one on otic canal, and two on postotic canal. Pterotic branch deprived of pores. Cephalic neuromasts lines with four or five small neuromasts on nasal line, one on rostral line, five to seven on mandibular line, two on anterior line, and two on supratemporal accessory line. Trunk neuromasts lines with two neuromasts on dorsal-trunk line, six to 13 on medium trunk line, five to eight on subdorsal-trunk line, and four to eight small neuromasts on subventral-trunk line.
Premaxillary tooth patch narrow, long, slightly curved, rounded lateral margin, without posterior projections, and with large notch at symphysis. Dentary tooth patch semicircular, narrow, of similar width of premaxillary tooth patch, but more than double its length, with notch on symphysis. Teeth small and villiform, pointing posteriorly. Gill rakers spiny and unbranched. Gill rakers on first branchial arch: 1+1+3=5[1], 1+1+4=6[13], 1+1+5=7[1], 2+1+3=6[2], or 2+1+5=8*[1]. Opercular membranes free from isthmus, supported by 8 or 9 branchiostegal rays. Free vertebrae 28. Pleural ribs 5. Anal-fin proximal pterygiophores 9. Gas bladder large, cordiform, with simple inner T-shaped septum.
Color in alcohol. Body almost entirely brown; anterior region darker; lateral portions of trunk and caudal peduncle with light brown horizontal streaks, imparting a pattern similar to tree bark, more concentrated on dorsal and median regions of body. Dorsal region of body completely dark brown, ventral region slightly mottled with brown spots. Head dark brown, with one lateral black stripe that extends to a region anterior to nostrils, uniting eye to upper lip. Upper lip dark, lower lip light. Barbels (mainly maxillaries) slightly mottled with brown. Melanophores surrounding neuromasts of all superficial lines, forming small, conspicuous black dots distributed in three horizontal rows along lateral portion of body, two dots above opercular opening, four dots on posterior top of head, and four dots on predorsal region. Caudal peduncle with a weak brown blotch of irregular shape. Pectoral, pelvic, anal and caudal fins hyaline, with speckled brown pigmentation roughly arranged in rows along fin rays. Dorsal fin with two conspicuous horizontal dark brown stripes, the first basal and wide, the second a little above dorsal-fin middle half, absent in small specimens. Dorsal-fin spine dark brown, pectoral-fin spine light brown, with dark brown speckles. Anterior portion of adipose fin light, followed by a dark brown blotch, central and posterior portions light-colored, with small irregular light brown blotches. Anal fin mostly hyaline, with dark speckling and with small brown blotches on anterior margin, absent in small specimens. Caudal fin hyaline, speckled with light brown pigmentation forming weak, vertical light brown stripes over fin rays; a wide, conspicuous, straight vertical stripe at caudal-fin base, and a second, considerably less conspicuous vertical stripe, situated slightly after caudal-fin middle half, roughly shaped like the numeral three, present only in large specimens.
Distribution.Microglanis xylographicus is known from tributaries of the rio das Mortes and small nearby direct tributaries of the rio Araguaia such as the córrego Jaraguá (Fig. 3B) and rio das Garças, in States of Mato Grosso and Goiás, Brazil (Fig. 4).
Etymology. The name xylographicus is derived from the Greek xylos referring to wood, and graphikos, of writing. It is a reference to the horizontally striated color pattern, like a tree bark. An adjective.
Remarks. Specimens from the type locality, the córrego Jaraguá, are larger than specimens collected at the rio Insula and rio Corrente (23.1-27.8 vs. 16.9-23.8 mm SL), have more neuromasts on median-trunk line (11-13 vs. 6-11), larger mouth width (48.0-49.7% vs. 44.2-47.1% HL), smaller orbital diameter (3.4-3.8% vs. 3.7-4.4% SL), smaller dorsal-fin spine length (11.8-13.1% vs. 12.9-16.2% SL), larger posterior cleithral process length (11.7-12.2% vs. 9.2-11.6% SL), smaller adipose-fin base length (17.8-19.8% vs. 19.5-21.1% SL), and a darker brown color. The two populations are separated (about 90 km) by the Serra do Roncador and Serra Azul, indicating that those differences might be geographically related. In the absence of additional evidence, we consider that the two populations belong to a single species.
Discussion
The two species of Microglanis described herein are apparently endemic to the tributaries of the upper-middle rio Araguaia. They occur sympatrically in the rio Corrente (Fig. 3C), which is also the type locality of M. oliveirai. The rio Corrente runs across a wide expanse of Cerrado vegetation (Central Brazil Savanna). It has a maximum depth of about 2.5 m, and a width of 6 to 11 m. The bottom of the river consists mostly of mud, with sandy patches and some gravel, and abundant submerged leaf-litter. Physical and chemical properties of the water at the moment of collection were: water transparency=0.15 m, pH=6.4, temperature=22.1ºC, and conductivity=1.132 µS/cm. Specimens of M. oliveirai and M. xylographicus were collected together in small peripheric pools formed by a shallow branch of the rio Corrente, amidst roots of the marginal vegetation, tree branches, and submerged leaf-litter. It is generally accepted that species with similar morphologies and ecologies can not coexist in the same portion of a habitat, according to Gause's competitive exclusion principle (Hardin, 1960). In the case of M. oliveirai and M. xylographicus, competition might be avoided by the partitioning of resources (sensu Ross, 1986). This is a subject that should be properly addressed in future studies, but in order to offer some initial insight into this question, the digestive tract of two specimens of M. oliveirai and two specimens of M. xylographicus were examined. The stomach contents of all four specimens included only autochthonous aquatic insects, a result expected for benthic catfishes. The analysis also revealed that the dissected specimens of M. oliveirai consumed 67% of larvae of Chironomidae and 33% of larvae of Trichoptera, while 100% of the stomach contents of M. xylographicus were composed of larvae of Chironomidae.
Other cases of sympatry involving species of Microglanis were reported in Mees (1974), which recorded sympatry among M. poecilus and M. secundus in two river systems of Surinam, and in Bizerril & Perez-Neto (1992), which recorded sympatry among M. nigripinnis Bizerril & Perez-Neto, 1992 and M. parahybae (Steindachner, 1880) at the rio Macacu, State of Rio de Janeiro, Brazil.
Small adult size is a feature that consistently distinguishes Microglanis from other genera of the Pseudopimelodidae. Among the six Amazonian species of Microglanis that have been described so far, the largest is M. pellopterygius, whose maximum recorded length is 54 mm SL. The species of Microglanis from the eastern South America, M. parahybae, M. pataxo Sarmento-Soares, Martins-Pinheiro, Aranda & Chamon, 2006, M. leptostriatus Mori & Shibatta, 2006, and M. minutus, are also small-sized, with a maximum recorded length of 44.7 mm SL in M. parahybae. The largest lengths recorded in species of Microglanis are those of M. cottoides, with 68 mm SL, from the rio Ribeira de Iguape (Oyakawa et al., 2006), and M. eurystoma, with 77.6 mm SL, from the rio Uruguay (Malabarba & Mahler, 1998), which also marks the southern limit of distribution of the genus. Following the trend observed in the previously known Amazonian species, the two species of Microglanis described herein are also extremely small-sized, with up to 27.8 mm SL in M. xylographicus.
Microglanis oliveirai shares several features with M. poecilus, the most widely distributed species in the genus, which occurs across several river systems in Guyana, French Guiana, Venezuela (rio Orinoco basin), Suriname, and Brazil (rio Amazon basin). The two species share (1) a low and elongated body, (2) a wide light transversal band crossing the nape, (3) a short lateral line canal, not surpassing the vertical line through the middle dorsal-fin base, (4) few pores in the lateral line, (5) rays of the pectoral and pelvic fins slightly brown pigmented, (6) the middle to distal portion of the pectoral-fin spine with a small oval brown blotch, (7) the anterior margin of the pectoral-fin spine with well-developed serrations, one of them Y-shaped between the series of retrorse and antrorse serrations, (8) a small brown blotch similar to an inverted U below the dorsal fin, reaching the horizontal line through the axis of the trunk, (9) a brown blotch below the adipose fin, reaching the anal-fin base, usually with a notch on its middle portion, (10) a roughly triangular blotch on the caudal peduncle, with one of its vertices directed anteriorly, and (11) the upper lobe of caudal fin larger than the lower lobe. However, M. oliveirai can be distinguished from M. poecilus by the relatively lower numbers of gill rakers, lateral line pores, vertebrae, and branched caudal-fin rays.
Microglanis xylographicus and M. robustus, from the lower rio Tocantins basin, are similar in a series of features, such as (1) the absence of a light stripe crossing the nape, (2) melanophores surrounding the neuromasts, together forming a series of small black dots aligned on the head, nape, predorsal area, and trunk, (3) rays of pectoral, pelvic, anal and caudal fins with speckled brown pigmentation roughly arranged in rows, and (4) upper lobe of caudal fin slightly larger than lower lobe. Microglanis xylographicus can be easily distinguished from M. robustus by the possession of a dark brown body color with narrow light stripes, together imparting a tree bark-like color pattern, the absence of a light area in the center of the dark brown blotch below the dorsal fin, and a longer snout length.
Key to the species of Microglanis from the rio Amazon basin
1. Absence of light transverse band on nape; presence of melanophores surrounding the neuromasts of superficial lines, forming series of black dots ....................................... 2
1'. Presence of a light transverse band on nape; absence of melanophores surrounding the neuromasts of superficial lines .......................................................................................... 3
2. Dark brown trunk with light brown stripes imparting a ground color similar to tree bark; absence of light marks on nape; snout length 11.4-12.9% SL ........ Microglanis xylographicus
2'. Light brown trunk with dark brown saddles below dorsal and adipose fins; light cordiform blotch on nape; snout length 9.1-10.7% SL .............................. Microglanis robustus
3. Very wide dark-brown stripes on fins ................... ...................................................... Microglanis pellopterygius
3'. Dark brown stripes on fins absent or narrow ..................... 4
4. Lateral line long, reaching the vertical line through middle of adipose fin; number of pores 14-20 ........................ .................................................................. Microglanis iheringi
4'. Lateral line short, not reaching the vertical line through middle of adipose fin; number of pores 3-9 ......................... 5
5. Tip of pectoral-fin spine bifurcated ...................... ............................................................... Microglanis secundus
5'. Tip of pectoral-fin spine undivided ................................... 6
6. Caudal fin rounded; all anterior serrae of the pectoral spine retrorse, except the last antrorse ........... Microglanis zonatus
6'. Caudal fin slightly bilobed; upper lobe of caudal fin more developed than lower lobe; anterior serrae of pectoral-fin spine antrorse and retrorse, with a single Y-shaped serration in between ............................................................ 7
7. Lateral line reaching the vertical through middle of dorsal-fin base; 5-7 total gill rakers in the first branchial arch; 6 pleural ribs; i,12,i caudal-fin rays ........................... ................................................................. Microglanis poecilus
7'. Lateral line reaching the vertical through base of dorsal-fin spine; 3-6 total gill rakers in the first branchial arch; 4-5 pleural ribs; i,10,i or i,11,i caudal-fin rays ..................... ................................................................. Microglanis oliveirai
Comparative material.Microglanis carlae. Paraguay. MNHNP 3667, holotype, 1, 33.8 mm SL, río Salado, río Paraguay basin; MZUSP 98255, paratypes, 5, 23.9-29.1 mm SL; MZUEL 5021, paratypes, 5, 25.1-30.3 mm SL; NUP 5362, paratype, 1 (c&s), 24.8 mm SL, same data as holotype. Microglanis cibelae: Brazil. Rio Grande do Sul. MCP 19822, paratypes, 3, 35.1-47.8 mm SL, arroio do Ouro, tributary of rio Maquiné; Santa Catarina. MCP 14686, 5, 37.5-67.1 mm SL, rio Canoas, rio Mampituba basin. Microglanis cottoides: Brazil. Rio Grande do Sul. MCP 16769, 3, 20.2-39.3 mm SL, rio Ijuizinho, rio Uruguay basin; MCP 17706, 4, 45.3-25.1 mm SL, arroio Quarizinho, rio Uruguai basin. Microglanis eurystoma, Brazil. Santa Catarina. MCP 13405, holotype, 1, 77.6 mm SL, rio Uruguai; Rio Grande do Sul. MCP 12698, paratypes, 12, 25.9-40.7 mm SL, arroio Passo Alto, rio Uruguai basin. Microglanis garavelloi, Brazil. Paraná. MZUSP 88006, holotype, 1, 31.7 mm SL, ribeirão Taquari, upper rio Paraná basin; MZUSP 1730, paratypes, 5, 24.0-31.2 mm SL, same data as holotype; MCP 1678, paratypes, 7 (3 c&s), 24.0-27.3 mm SL, ribeirão Taquari. Microglanis iheringi, Venezuela. Aragua. USNM 121985, paratype, 1, 30.1 mm SL, río Turmero; Portuguesa. CAS 64403, 3, 25.3-40.3 mm SL, río Orinoco; Putumayo. MHNG 1232.13, 1, 41.1 mm SL, Úmbria. Microglanis leptostriatus, Brazil. Minas Gerais. MZUSP 47456, paratypes, 2, 28.4-28.7 mm SL, rio Verde, rio São Francisco basin; MZUEL 3733, paratypes, 6, 19.2-28.2 mm SL, rio da Cruz, rio São Francisco basin. Microglanis malabarbai, Brazil. Rio Grande do Sul. MCP 37252, 1, 47.5 mm SL, arroio Alexandrino, rio Ijuí basin; MCP 37187, 1, 50.3 mm SL, arroio das Pedras, rio Ijuí basin. Microglanis nigripinnis, Brazil. Rio de Janeiro. MZUSP 80223, 1, 45.3 mm SL, tributary of the rio São João, Eastern basin; MZUSP 80229, 2, 38.8-47.3 mm SL, tributary of the rio São João, Eastern basin. Microglanis parahybae, Brazil. Rio de Janeiro. MNRJ 15989, 5, 29.6-33.6 mm SL, rio Dois Rios, rio Paraíba do Sul basin; MNRJ 16047, 5, 29.3-38.9 mm SL, rio Muriaé, rio Paraíba do Sul basin. Microglanis pataxo, Brazil. Bahia. MZUSP 54516, 10, 24.4-31.8 mm SL, rio Mucuri, Eastern basin. Microglanis pellopterygius, Ecuador. Napo. ANSP 130437, holotype, 1, 68.0 mm SL, río Aguarico; MEPN 88.4-12, 2, 22.6-22.9 mm SL, tributary of the río Aguarico. Microglanis poecilus, Guiana. Kurupukari. ROM 60738, 1, 22.5 mm SL, unknown stream of Essequibo river basin; ROM 62390, 1, 17.0 mm SL, Shimiri stream, Yawiri, Essequibo river basin; ROM 62391, 1, 17.6 mm SL, Essequibo river; Brazil. Pará. INPA 23863, 26, 16.7-24.9 mm SL, rio Capim, rio Capim basin. Microglanis robustus, Brazil. Pará. INPA 8053, 1, 20.3 mm SL, holotype, lower rio Tocantins, rio Tocantins-Araguaia basin; INPA 32885, paratypes, 11 (2 c&s), 18.4-23.3 mm SL, same data as holotype; INPA 7943, paratypes, 2, 20.0-22.2 mm SL; INPA 7957, paratypes, 3, 19.2-21.7 mm SL, Jatobal, lower rio Tocantins. Microglanis secundus, Suriname. Brokopondo. MHNG 2621.038, 6, 20.1-25.1 mm SL, rio Mindrineti. Microglanis variegatus, Ecuador. Vinces. USNM 083653, paratype, 1, 28.7 mm SL, pool in forests near the Vinces; Los Ríos. MHNG 2098.034, 2, 25.0-27.7 mm SL, río Palengue; MHNG 1232.11, 2, 24.0-25.2 mm SL, Hazienda Clementina.
Acknowledgements
The Universidade Estadual de Londrina and CAPES provided funds for a field expedition to the rio Araguaia basin, which resulted in the collection of a substantial portion of the type series of both species described herein. We also thank L. R. Jarduli and E. S. da Silva for the tireless help during the expedition. Special thanks are given to R. Luing and P. C. Venere (UFMT) for hosting the members of the expedition to the rio Araguaia, and also for help in the fieldwork. For loan of material, we are grateful to C. Oliveira (LBP), O. Oyakawa (MZUSP), S. Fisch-Muller (MHNG), S. L. Jewett (USNM), C. Ferraris Jr. (CAS), P. Bartsch (ZMB), Z. M. Lucena (MCP), L. R. Malabarba (UFRGS), J. C. Garavello (UFSCar), P. A. Buckup (MNRJ), Jansen Zuanon and Lucia Rapp Py-Daniel (INPA), and Ramiro Barriga (MEPN). We thank Marcelo Rocha (INPA), Fernando C. Jerep (MCP/PUCRS), Flávio C. T. Lima (ZUEC/MZUSP), Fernando R. Carvalho (UFRGS), José L. O. Birindelli (MZUSP), and an anonymous referee, for providing useful comments and suggestions. Sirlei T. Bennemann and Débora F. Silva (UEL) helped in the identification of alimentary items. José L. O. Birindelli provided the photograph of rio Cristalino. Financial support was provided by PCI/MCT/INPA Program (OAS travel to the INPA fish collection), CNPq (308624/2009-2), and the All Catfish Species Inventory Project (NSF DEB 0315963).
Literature Cited
Submitted June 15, 2011
Accepted September 9, 2011
Published December 26, 2011
- Alcaraz, H. S. V., W. J. Graça & O. A. Shibatta. 2008. Microglanis carlae, a new species of bumblebee catfish (Siluriformes: Pseudopimelodidae) from the rio Paraguay basin in Paraguay. Neotropical Ichthyology, 6: 425-432.
- Birindelli, J. L. O. & O. A. Shibatta. 2011. Morphology of the gas bladder in bumblebee catfishes (Siluriformes, Pseudopimelodidae). Journal of Morphology, 272: 890-896.
- Bizerril, C. R. S. F. & P. R. Perez-Neto. 1992. Description of a new species of Microglanis (Siluroidei, Pimelodidae) from eastern Brazil. Revue Française d'Aquariologie, 18: 97-100.
- Dingerkus, G. & L. Uhler. 1977. Enzyme clearing alcian blue stained whole vertebrates for demonstration of cartilage. Stain Technology, 52: 229-232.
- Hardin, G. 1960. The competitive exclusion principle. Science, 131: 1292-1297.
- Malabarba, L. R. & J. K. F. Mahler. 1998. Review of the genus Microglanis in the rio Uruguay and coastal drainages of southern Brazil (Ostariophysi: Pimelodidae). Ichthyological Exploration of Freshwaters, 9: 243-254.
- Mees, G. F. 1974. The Auchenipteridae and Pimelodidae of Suriname (Pisces, Nematognathi). Zoologische Verhandelingen, 132: 1-246.
- Mori, H. & O. A. Shibatta. 2006. A new species of Microglanis Eigenmann, 1912 (Siluriformes: Pseudopimelodidae) from Rio São Francisco basin, Brazil. Zootaxa, 1302: 31-42.
- Ottoni, F. P., J. L. O. Mattos & M. A. Barbosa. 2010. Description of a new species of Microglanis from the rio Barra Seca basin, southeastern Brazil (Teleostei: Siluriformes: Pseudopmelodidae). Vertebrate Zoology, 60: 187-192.
- Oyakawa, O. T., A. Akama, K. C. Mautari, & J. C. Nolasco. 2006. Peixes de riachos da Mata Atlântica. Editora Neotropica, São Paulo, 201p.
- Ross, S. T. 1986. Resource partitioning in fish assemblage: a review of field studies. Copeia, 1986: 352-388.
- Ruiz, W. B. G. & O. A. Shibatta. 2010. A new species of Microglanis (Siluriformes, Pseudopimelodidae) from lower rio Tocantins basin, Pará, Brazil, with description of superficial neuromasts and pores of lateral line system. Zootaxa, 2632: 53-66.
- Sarmento-Soares, L. M., R. F. Martins-Pinheiro, A. T. Aranda & C. C. Chamon. 2006. Microglanis pataxo, a new catfish from southern Bahia coastal rivers, northeastern Brazil (Siluriformes: Pseudopimelodidae). Neotropical Ichthyology, 4: 157-166.
- Shibatta, O. A. 1998. Sistemática e evolução da família Pseudopimelodidae (Ostariophysi, Siluriformes), com a revisão taxonômica do gênero Pseudopimelodus Unpublished PhD. Dissertation, Universidade Federal de São Carlos, São Paulo, 357p.
- Shibatta, O. A. 2003a. Family Pseudopimelodidae (Bumble-bee catfishes, dwarf marbled catfishes). Pp. 401-405. In: Reis, R. E., S. O. Kullander & C.J. Ferraris Jr. (Eds.). Check List of the Freshwater Fishes of South and Central America. Edipucrs, Porto Alegre, 729p.
- Shibatta, O. A. 2003b. Phylogeny and classification of 'Pimelodidae'. Pp. 385-400. In: Arratia, G., B. G. Kapoor, M. Chardon & R. Diogo (Eds.). Catfishes, Vol. 1. Sciences Publishers Inc., Enfield, 487p.
- Shibatta, O. A. & R. C. Benine. 2005. A new species of Microglanis (Siluriformes: Pseudopimelodidae) from upper Rio Paraná basin, Brazil. Neotropical Ichthyology, 3: 579-585.
Publication Dates
-
Publication in this collection
17 Jan 2012 -
Date of issue
2011
History
-
Received
15 June 2011 -
Accepted
09 Sept 2011