Abstracts
The aim of this work was to study two aspects of phenotypic plasticity in the Patagonian pejerrey Odontesthes hatcheri (Teleostei: Atherinopsidae) the dependence of the early morphology on developmental time and temperature, and the induction of morphological changes by controlled feeding in juveniles. Newly hatched free embryos, incubated at two different temperatures (13 and 18oC), and juveniles were used for the study and induction of phenotypic plasticity. Body and head shapes were analyzed with geometric morphometrics and linear measurements. Our results showed that shape variation at hatching was related to the bending of the embryo head on the yolk sac, increasing the head-trunk angle due to progressive straightening of the embryo. The head-trunk angle was related with temperature at incubation, with embryos incubated at higher temperature being more bent. Embryos that hatched earlier had bigger yolk sacs than those that hatched later. In juveniles, controlled feeding experiments added new morphological variation to that of wild juveniles. In all comparisons, the slenderness of the head, the size of premaxilla and jaw, and the position of the eye showed an enlarged variation due to controlled feeding. These results will contribute to comprehending the complexity of the morphological variation of O. hatcheri.
Atherinopsidae; Development; Feeding; Morphometry; Odontesthes hatcheri
O objetivo deste trabalho foi estudar a variação morfológica e plasticidade fenotípica do peixe-rei da Patagônia Odontesthes hatcheri (Teleostei: Atherinopsidae), a dependência da morfologia inicial no tempo de desenvolvimento e temperatura, e a indução de alterações morfológicas pela alimentação controlada em juvenis. Embriões recém-nascidos, incubados a duas temperaturas diferentes (13 e 18oC) e juvenis foram utilizados para o estudo de indução de plasticidade fenotípica. Formas do corpo e cabeça foram analisadas com técnicas de morfometria geométrica e medições lineares. Os nossos resultados mostraram que a variação da forma no nascimento foi relacionada com a curvatura da cabeça do embrião no saco vitelino, aumentando o ângulo de cabeça-tronco devido ao endireitamento progressivo do embrião. O ângulo da cabeça-tronco relacionou-se com a temperatura de incubação, com os embriões incubados na temperatura elevada sendo mais curvados. Os embriões que eclodiram mais cedo tinham sacos vitelinos maiores do que aqueles que eclodiram tardiamente. Em juvenis, os experimentos de alimentação controlada adicionaram nova variação morfológica àquela dos juvenis selvagens. Em todas as comparações, a espessura da cabeça, o tamanho da pré-maxila e mandíbula, e a posição do olho mostraram uma maior variação devido à alimentação controlada. Estes resultados irão contribuir para a compreensão da complexidade da variação morfológica de O. hatcheri.
Early morphological variation and induction of phenotypic plasticity in Patagonian pejerrey
Sonia A. Crichigno; Miguel A. Battini; Víctor E. Cussac
Universidad Nacional del Comahue, Instituto de Investigaciones en Biodiversidad y Medioambiente (INIBIOMA), Consejo Nacional de Investigaciones Científicas y Técnicas. Quintral 1250, Bariloche, 8400 Río Negro, Argentina. soacri@yahoo.com.ar (SAC)
ABSTRACT
The aim of this work was to study two aspects of phenotypic plasticity in the Patagonian pejerrey Odontesthes hatcheri (Teleostei: Atherinopsidae) the dependence of the early morphology on developmental time and temperature, and the induction of morphological changes by controlled feeding in juveniles. Newly hatched free embryos, incubated at two different temperatures (13 and 18ºC), and juveniles were used for the study and induction of phenotypic plasticity. Body and head shapes were analyzed with geometric morphometrics and linear measurements. Our results showed that shape variation at hatching was related to the bending of the embryo head on the yolk sac, increasing the head-trunk angle due to progressive straightening of the embryo. The head-trunk angle was related with temperature at incubation, with embryos incubated at higher temperature being more bent. Embryos that hatched earlier had bigger yolk sacs than those that hatched later. In juveniles, controlled feeding experiments added new morphological variation to that of wild juveniles. In all comparisons, the slenderness of the head, the size of premaxilla and jaw, and the position of the eye showed an enlarged variation due to controlled feeding. These results will contribute to comprehending the complexity of the morphological variation of O. hatcheri.
Key words: Atherinopsidae, Development, Feeding, Morphometry, Odontesthes hatcheri.
RESUMO
O objetivo deste trabalho foi estudar a variação morfológica e plasticidade fenotípica do peixe-rei da Patagônia Odontesthes hatcheri (Teleostei: Atherinopsidae), a dependência da morfologia inicial no tempo de desenvolvimento e temperatura, e a indução de alterações morfológicas pela alimentação controlada em juvenis. Embriões recém-nascidos, incubados a duas temperaturas diferentes (13 e 18ºC) e juvenis foram utilizados para o estudo de indução de plasticidade fenotípica. Formas do corpo e cabeça foram analisadas com técnicas de morfometria geométrica e medições lineares. Os nossos resultados mostraram que a variação da forma no nascimento foi relacionada com a curvatura da cabeça do embrião no saco vitelino, aumentando o ângulo de cabeça-tronco devido ao endireitamento progressivo do embrião. O ângulo da cabeça-tronco relacionou-se com a temperatura de incubação, com os embriões incubados na temperatura elevada sendo mais curvados. Os embriões que eclodiram mais cedo tinham sacos vitelinos maiores do que aqueles que eclodiram tardiamente. Em juvenis, os experimentos de alimentação controlada adicionaram nova variação morfológica àquela dos juvenis selvagens. Em todas as comparações, a espessura da cabeça, o tamanho da pré-maxila e mandíbula, e a posição do olho mostraram uma maior variação devido à alimentação controlada. Estes resultados irão contribuir para a compreensão da complexidade da variação morfológica de O. hatcheri.
Introduction
There is abundant evidence of phenotypic plasticity in fishes (Balon, 2004). It is considered as the ability of an organism to react to environmental input with a change in form, state, movement, or rate of activity (West-Eberhard, 2003), or as the property of individual genotypes to produce different phenotypes when exposed to different environmental conditions (Pigliucci, 2001; Pigliucci et al., 2006; Pfennig et al., 2010).
Induced phenotypic plasticity has been studied extensively (Grünbaum et al., 2007). During embryonic development, water temperature is the most important environmental factor that influences fish (Chambers & Leggett, 1987; Blaxter, 1992).
Temperature modulates the amount of time required to complete embryonic development, within a specific range for each species (Kunz, 2004; Kamler, 2008), and has effects on the morphology, physiology, and behavior of fish during development (Martell et al., 2005). Environmental effects other than temperature can also act along the ontogeny, inducing a certain morphology. For example, morphological reversion in the head of Micropterus salmoides floridanus (= Micropterus floridanus) could has been experimentally related to food quality (Wintzer & Motta, 2005). Also, diet induced body and head shape variation has been observed in Cichlasoma managuense (= Parachromis managuensis) and Lepomis humilis (Meyer, 1987; Hegrenes, 2001).
The distribution area of the Patagonian pejerrey Odontesthes hatcheri (Eigenmann), Patagonia, was signed by old and new processes that shaped the landscape and fauna: a Gondwanan heritage, the Andes uplifting, Pleistocene ice, volcanic activity, introduction of exotic fishes, and climate change (Pascual et al., 2007). Larvae and juveniles of O. hatcheri perform ontogenetic habitat and diet shifts in Patagonian lakes. After hatching in the littoral zone, free embryos (sensu Balon, 1999) migrate to the limnetic zone where the exogenous feeding begins. Later, these larvae return to the littoral zone (Cussac et al., 1992). They feed mainly on both nauplii of Cyclopoida and the rotifer Pompholix sulcata in the limnetic and in the littoral zones, up to their juvenile period, when their diet changes (Cervellini et al., 1993). Larval Patagonian pejerrey showed marked shape changes after few days of fasting (Battini et al., 1995). In adults, relationships between body shape and environmental factors such as total phosphorus, coastline development, and altitude (Conte-Grand, 2012), as well as between cephalic shape and mean depth, content of Chlorophyll-a, and mean summer air temperature were found (Crichigno, 2012).
The aim of this work was to study two aspects of phenotypic plasticity in the Patagonian pejerrey Odontesthes hatcheri; the dependence of the early morphology on developmental time and temperature, and the induction of morphological changes by controlled feeding in juveniles.
Material and Methods
Morphology of newly hatched sibling free embryos. Adult Odontesthes hatcheri were captured using gillnets (15, 30, and 40 mm bar mesh) in a shallow lake in the Patagonian plateau, Carrilafquen Chica (41º12'S 69º25'W, see Reissig et al., 2006 for details). Individuals were anaesthetized with benzocaine solution (0.05 g . L-1). Ovocytes and sperm of four parental couples (PC) were obtained by stripping, and dry fertilization (Barnabé, 1990) was then performed. At the laboratory (Centro de Salmonicultura Bariloche, Universidad Nacional del Comahue), the eggs corresponding to two PC were incubated into small baskets into a 200 L aquarium with aeration and a daily 20% water exchange, at 18ºC, and those corresponding to the other two at 13ºC, always maintaining a 0.5% NaCl level. Both temperatures are included within the summer (breeding season) range of surface water temperature in lakes and reservoirs where the species is present. Water supply came from Gutierrez River, 4 km downstream an oligotrophic lake (Gutierrez Lake, 41º09'59"S 71º24'35"W, Quirós, 1988).
When the eggs began to hatch, and during three consecutive days, newly hatched free embryos were anaesthetized with benzocaine (0.05 g . L-1) and photographed (NIKON D70) under stereomicroscope (Leica Wild M3C). Two images of each individual were recorded, left side and cephalic dorsal view, taking care to minimize parallax error. In this way, newly hatched free embryos of different ages (AH = 1, 2, and 3, Table 1), in terms of days after fertilization (DAF), were obtained.
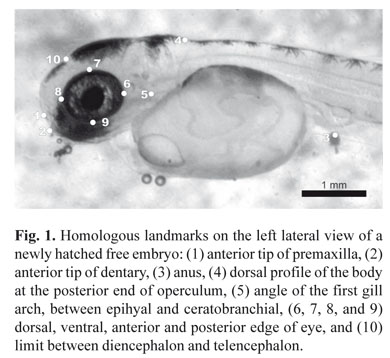
Ten landmarks were digitized on images, on the fish's left side: (1) anterior tip of the premaxilla, (2) anterior tip of dentary, (3) anus, (4) dorsal profile of the body at the posterior end of the operculum, (5) angle of the first gill arch, between epihyal and ceratobranchial, (6, 7, 8, and 9) dorsal, ventral, anterior, and posterior edge of the eye, and (10) limit between diencephalon and telencephalon (Fig. 1).
Body shape was quantified using the Geometric Morphometric Analysis (GMA) approach of thin-plate splines (TPS; Bookstein, 1991; Rohlf & Marcus, 1993; Parsons et al., 2003; Adams et al., 2004). Images were first scaled and rotated to a common size and orientation using a generalized Procrustes superimposition approach (Bookstein, 1991; Adams et al., 2004). The mean (or consensus) body shape for each population was estimated and quantified as partial warp scores using tpsRelw (Morphometrics at Sunny Stony Brook, 2012). The description of shape variation was performed visualizing the deformation grids and direction vectors, at the extreme of each Relative Warp (RW) axis from left to right and from bottom to top. Discriminant Analysis (DA, SPSS Inc.) was performed employing the Partial Warps (PW), including uniform and non-uniform coordinates (weight matrix), in order to discriminate free embryos according to AH, PC and incubation temperature (Table 1).
Linear measures of the dorsal view images were taken with Image Pro-plus. These measures were (Fig. 2): mouth width (MW), head length (HL), yolk sac width (YW), yolk sac length (YL), eye diameter (ED), and total length (TL). The residuals of the double logarithmic regression of linear measurements on TL were obtained in order to avoid size dependence and used to perform DA among ages.
Cephalic morphological variation of juveniles. Juveniles were captured with seine net in the same lake (Carrilafquen Chica) and transported to the laboratory in 1% NaCl. One subset (N= 22) was anaesthetized with benzocaine (0.05 g. L-1) and photographed (left side). Captured fish were separated into two groups and put in 150 L aquaria, at room temperature (mean 12.4ºC, ranging from 10 to 15ºC). One group was fed wild zooplankton (coming from Lake Los Juncos, 41º03'S 71º00'W) and the other was fed Tubifex sp. Both groups were fed ad libitum twice a day. After 60 days, individuals were photographed again. Individuals fed with Tubifex sp. were photographed also at day 240.
In all cases, 11 landmarks were digitized with TpsDig v2.10 software (Morphometrics at Sunny Stony Brook, 2012): (1) anterior dorsal tip of premaxilla, (2) anterior ventral tip of premaxilla, (3) anterior tip of dentary, (4) posterior ventral tip of premaxilla, (5) posterior ventral tip of maxillary, (6) ventral contact point between symplectic and preopercle, (7) upper tip of pelvic fin base, and (8, 9, 10, and 11) upper, lower, anterior and posterior edge of the eye. Identity of landmarks was confirmed using an X-ray image (Fig. 3).
The following analyses and comparisons were performed: a) variation of head shape among just captured individuals (N = 22); b) comparison of head shape among individuals fed with zooplankton for 60 days, individuals fed with Tubifex sp. for 60 days, and just captured individuals of similar size (N = 64); and c) comparison of head shape between individuals fed with Tubifex sp., at 60 and 240 days, and just captured individuals of similar size (N = 71). The size ranges of the compared groups showed overlap (Table 2).
Results
Newly hatched free embryos, lateral view. The first two RWs explained 75.29% of variation (N = 128, RW1 = 61.07%, and RW2= 14.22%) and deformation grids showed the bending of the embryo over the yolk sac.
DA among AH (at 18ºC and within the same PC, Table 1) showed one significant Discriminant Function (DF) that correctly classified 88.7% of cases and explained 82.4% of variation (DF1, N = 71, Wilks´ lambda = 0.285, P< 0.001). Deformation grids showed how the head of the embryo, initially curved over the yolk sac, lifts dorsalwards and straightens itself out (Fig. 4).
DA between PC with different AH and the same DAF and temperature (PCAH, Table 1) showed a significant DF (DF1, N = 49, Wilks´ lambda = 0.239, P< 0.001) that correctly classified 100% of cases and explained 100% of variation.
DA between PC with different DAF and the same temperature and AH (PCDAF, Table 1) failed to showed a significant DF (DF1, N = 39, Wilks´ lambda = 0.405, P = 0.051). However, considering the combined effect of temperature, PC, and DAF at AH 1 (comparison TPCDAF in Table 1), DA showed one significant DF that correctly classified 97.5% of cases and explained 100% of variation (DF1, N = 40, Wilks´ lambda = 0.249, P< 0.001). In the same way, combined effects of temperature, PC, and DAF at AH 2 (comparison TPCDAF in Table 1), showed one significant DF that correctly classified 92.2 % of cases and explained 100% of variation (DF1, N = 64, Wilks´ lambda = 0.344, P< 0.001). Deformation grids from both comparisons showed that the individuals incubated at 18ºC had their heads curved over the yolk sac, unlike individuals incubated at 13ºC (Fig. 5).
Newly hatched free embryos, linear measures in dorsal view.
DA among AH (at 18ºC and within the same PC, Table 1) showed one significant DF that correctly classified 54.7% and explained 98.4% of variation (DF1, Wilks´ lambda = 0.598, P< 0.001). The two main measurements included in the DF were the length and width of the yolk sac. AH 1 individuals had the widest and shortest yolk sacs (Fig. 6).
Juvenile cephalic morphological variation and feeding experiment. Recently captured individuals: the first two RWs explained 52.21% of variation (N = 22; RW1 = 32.63%; and RW2 = 19.58%). Deformation grids in RW1 show individuals with elongated head, longer premaxilla, longer lower jaw, and slightly lower eyes in the negative extreme. The opposite morphology was observed in the positive extreme. In RW2, individuals with longer head, longer premaxilla, longer lower jaw, and bigger eyes, shifted forward and downward, were observed in the negative extreme. The opposite morphology was observed in the positive extreme.
Feeding with zooplankton and Tubifex sp. for 60 days: DA (N = 64) among just caught individuals, individuals fed with zooplankton and individuals fed with Tubifex sp. showed two significant DFs, correctly classifying 98.4% of cases and explaining 100% of variation (DF1, Wilks´ lambda = 0.062, P< 0.001, and DF2, Wilks´ lambda = 0.339, P< 0.001, Fig. 7). Deformation grids showed, for individuals fed with zooplankton compared to just caught individuals, higher mouth (anterior tips of premaxilla and dentary), and more anterior isthmus. Individuals fed with Tubifex sp., when compared to just caught individuals, had bigger eyes in a higher and more posterior position, wider anterior portion of premaxilla, lower mouth (anterior tips of premaxilla and dentary), more anterior isthmus, and more posterior pectoral fin base.
Feeding with Tubifex sp.: DA (N = 71) among times (0, 60, and 240 days) correctly classified 94.4% of cases and explained 100% of variation (DF1, Wilks´ lambda = 0.083, P< 0.001, and DF2, Wilks´ lambda = 0.339, P< 0.001). DF1 separated just captured individuals from treated individuals and DF2 separated 60 days of feeding from 240 days of feeding groups (Fig. 8). The morphological variations along time showed an increase of eye size, an elongation of premaxilla and lower jaw, and an upward and forward movement of the isthmus.
Discussion
Hatching is not a fixed threshold, but is triggered by environmental cues at different times during the embryonic period, thus even sibling individuals can hatch at very different stages of development (Yamagami, 1988; Balon, 1999). Our results showed that morphological variation at hatching in O. hatcheri is related to the bending of the embryo over the yolk sac, increasing the head-trunk angle due to the straightening of the embryo along time, as was described by Kimmel et al. (1995) in zebrafish. In the fish's dorsal view, it became apparent that earlier hatched O. hatcheri embryos (AH 1) had bigger yolk sacs than individuals that hatched later.
The effect of parental couples seems to be less conspicuous than AH. Although DA between PC with different AH and the same DAF and temperature (PCAH, Table 1) brought a significant DF, the DA between PC with different DAF and the same temperature and AH (PCDAF, Table 1) failed. Our data (comparison TPCDAF in Table 1) suggest that, at hatching, O. hatcheri embryos incubated at higher temperature were more bent. Probably, the size of the yolk sac and the bending of the embryo after hatching will be major factors operating on the beginning of exogenous feeding (Battini et al., 1995). This is consistent with the differential consumption of yolk observed in Anarhichas minor (Sund & Falk-Petersen, 2005), where decreased consumption occurred at higher temperature and thus the embryos had more yolk at hatching and were more bent over the yolk sac. Sund & Falk-Petersen (2005) and Peterson et al. (2004) also observed in Anarhichas minor and Gadus morhua that embryos incubated at lower temperature hatched with larger length. Furthermore, water temperature directs the sexual differentiation process in the pejerrey Odontesthes bonariensis (Valenciennes) and affects larval condition and growth rate (Strüssmann & Patiño, 1995; Ito et al., 2005; Chalde et al., 2011).
Regarding juvenile feeding, morphological variation increased when, in addition to just captured wild individuals, those subjected to controlled feeding were considered. In all comparisons, the slenderness of the head, size of premaxilla and jaw, and position of the eyes showed appreciable variation in the RW1. Both individuals fed only with zooplankton and those fed only with Tubifex sp. had slenderer heads than just captured wild individuals. Furthermore, shapes obtained throughout feeding with Tubifex sp. during increasing time lapses included bigger eyes and slenderer heads. In the same way, Grünbaum et al. (2007) found different levels of developmental plasticity in critical ontogenetic periods of Salvelinus alpinus that are most responsive to environmental constraints. Shape variation has been also induced by controlled diet in other fishes. Cichlasoma managuense (= Parachromis managuensis) (Meyer, 1987) and Lepomis humilis (Hegrenes, 2001) fed with Artemia nauplii or Tenebrio larvae, showed that individuals fed with large prey (Tenebrio larvae) developed an elongated, fusiform shape with a sharply angled snout, after eight months of treatment. In the same way, another native Patagonian fish, Percichthys trucha (Valenciennes), showed juveniles fed with zooplankton as having slenderer head and body, and longer jaw than individuals fed with Tubifex sp. (large prey) after 70 days of treatment (Crichigno, 2012).
Head shape, size and position of the eyes, and size of premaxilla and jaws are likely to have performance consequences for prey capture and consumption (Carson & Wainwright, 2010). Patagonian lakes show a wide range of photic habitats (Lattuca et al., 2007) and, particularly, the abundance of zooplanktophagous fish like O. hatcheri can modify planktonic food webs and water transparency (Reissig et al., 2006). Although an overview of the diet of O. hatcheri indicates an omnivorous diet, a more detailed observation shows that diet changes greatly between lakes, with most of the intralacustrine variation ascribed to the ontogenetic shift (Ferriz, 1987; Grosman & Rudzik, 1990; Cervellini et al., 1993; Macchi et al., 1999). This succession of more or less stenophagous ontogenetic periods could be imposing precise requirements for the oropharyngeal apparatus of the fish.
In conclusion, temperature and hatching timing could act on the head shape during the early life of O. hatcheri. Later on, juvenile feeding adds another variable to head shape variation.
Plastic induction of head shape of O. hatcheri would provide, by mechanistic, epigenetic processes, additional morphological variation and in consequence a menu of capabilities for prey catching under changing selection selective pressures. In this context, our results could contribute to the comprehension of the plastic component of the morphological variation of the species.
Acknowledgements
We would like to acknowledge the following institutions for granting the present project: Universidad Nacional del Comahue (04B147), CONICET (PIP 112-200801-00282), and FONCYT (PICT2005-35241) of Argentina. This work would not have been possible without the cooperation of Centro de Salmonicultura Bariloche, Universidad Nacional del Comahue.
Literature Cited
Submitted August 17, 2011
Accepted May 8, 2012
- Adams, D. C., F. J. Rohlf & D. E. Slice. 2004. Geometric morphometrics: ten years of progress following the 'revolution'. Italian Journal of Zoology, 71: 5-16.
- Balon, E. K. 1999. Alternative ways to become a juvenile or a definitive phenotype (and on some persisting linguistic offenses). Environmental Biology of Fishes, 56: 17-38.
- Balon, E. K. 2004. Evolution by epigenesis: farewell to Darwinism, neo- and otherwise. Rivista di Biologia / Biology Forum, 97: 269-312.
- Barnabé, G. 1990. Aquaculture (Volume 1 and 2). England, Ellis Horwood Limited, 1104p.
- Battini, M. A., M. F. Alonso & V. E. Cussac. 1995. Growth and nutritional condition of the larvae of Odontesthes microlepidotus (Atherinidae): An experimental approach. Environmental Biology of Fishes, 42: 391-399.
- Blaxter, J. H. S. 1992. The effect of temperature on larval fishes. Netherlands Journal of Zoology, 42: 336-357.
- Bookstein, F. L. 1991. Morphometric tools for landmark data. Cambridge, Cambridge University Press, 436p.
- Carlson, R. L. & P. C. Wainwright. 2010. The ecological morphology of darter fishes (Percidae: Etheostomatinae). Biological Journal of the Linnean Society, 100: 30-45.
- Cervellini, P. M., M. A. Battini & V. E. Cussac. 1993. Ontogenetic shifts in the feeding of Galaxias maculatus (Galaxiidae) and Odontesthes microlepidotus (Atherinidae). Environmental Biology of Fishes, 36: 283-290.
- Chalde, T., D. A. Fernández, V. E. Cussac & G. M. Somoza. 2011. The effect of rearing temperature in larval development of pejerrey, Odontesthes bonariensis Morphological indicators of development. Neotropical Ichthyology, 9: 747-756.
- Chambers, R. C. & W. C. Leggett. 1987. Size and age at metamorphosis in marine fishes: an analysis of laboratory-reared winter flounder (Pseudopleuronectes americanus) with a review of variation in other species. Canadian Journal of Fisheries and Aquatic Sciences, 44: 1936-1947.
- Conte-Grand, C. 2012. El pejerrey patagónico, Odontesthes hatcheri: biología y potencialidades para su cultivo. Unpublished Ph.D. Dissertation, Universidad Nacional del Sur, Bahía Blanca. 174p.
- Crichigno, S. 2012. Variación morfológica y plasticidad fenotípica del aparato bucofaríngeo de Odontesthes hatcheri (Eigenmann, 1909) y Percichthys trucha (Cuvier & Valenciennes, 1840). Unpublished Ph.D. Dissertation, Universidad Nacional del Comahue, Bariloche. 95p.
- Cussac, V. E., P. M. Cervellini & M. A. Battini. 1992. Intralacustrine movements of Galaxias maculatus (Galaxiidae) and Odontesthes microlepidotus (Atherinidae) during their early life history. Environmental Biology of Fishes, 35: 141-148.
- Ferriz, R. A. 1987. Alimentación del pejerrey patagónico Patagonina hatcheri (Eigenmann, 1909) en el embalse Ramos Mexia, Neuquén, Argentina. Hydrobiologia, 6: 61-66.
- Grosman, F. & G. Rudzik. 1990. Análisis de la dieta del pejerrey patagónico Patagonina hatcheri Eigenmann, 1909, de la Laguna Terraplén, Chubut, Argentina. Biota, 6: 71-88.
- Grünbaum T., R. Cloutier, P. M. Mabee & N. R. Le François. 2007. Early developmental plasticity and integrative responses in arctic charr (Salvelinus alpinus): effects of water velocity on body size and shape. Journal of Experimental Zoology (Molecular and Developmental Evolution), 308B: 396-408.
- Hegrenes, S. 2001. Diet-induced phenotypic plasticity of feeding morphology in the orangespotted sunfish, Lepomis humilis Ecology of Freshwater Fish, 10: 35-42.
- Ito, L. S., M. Yamashita, F. Takashima & C. A. Strüssmann. 2005. Dynamics and histological characteristics of gonadal sex differentiation in pejerrey (Odontesthes bonariensis) at feminizing and masculinizing temperatures. Journal of Experimental Zoology 303A: 504-514.
- Kamler, E. 2008. Resource allocation in yolk-feeding fish. Reviews in Fish Biology and Fisheries, 18: 143-200.
- Kimmel, C. B., W. W. Ballard, S. R. Kimmel, B. Ullmann & T. F. Schilling. 1995. Stages of embryonic development of the zebrafish. Developmental Dynamics, 203: 255-310.
- Kunz, Y. W. 2004. Developmental Biology of Teleost Fishes. Netherlands, Springer, 636p.
- Lattuca, M. E., S. Ortubay, M. A. Battini, J. P. Barriga & V. E. Cussac. 2007. Presumptive environmental effects on body shape of Aplochiton zebra (Pisces, Galaxiidae) in Northern Patagonian lakes. Journal of Applied Ichthyology, 23: 25-33.
- Macchi P. J., V. E. Cussac, M. F. Alonso & M. A. Denegri. 1999. Predation relationships between introduced salmonids and the native fish fauna in lakes and reservoirs in Northern Patagonia. Ecology of Freshwater Fish, 8: 227-236.
- Martell, D. J., J. D. Kieffer & E. A. Trippel. 2005. Effects of temperature during early life history on embryonic and larval development and growth in haddock. Journal of Fish Biology, 66: 1558-1575.
- Meyer, A. 1987. Phenotypic plasticity and heterochrony in Cichlasoma managuense (Pisces, Chichlidae) and their implications for speciation in Cichlid fishes. Evolution, 6: 1357-1369.
- Morphometrics at Sunny Stony Brook. 2012. Available from: http:// life.bio.sunysb.edu/morph/index.html (Date of access: Jan 30, 2012).
- Parsons, K. J., B. R. Robinson & T. Herbert. 2003. Getting into shape: an empirical comparison of truss-based morphometric methods with a newer geometric method applied to new world cichlids. Environmental Biology of Fishes, 67: 417-431.
- Pascual, M. A., V. Cussac, B. Dyer, D. Soto, P. Vigliano, S. Ortubay & P. Macchi. 2007. Freshwater fishes of Patagonia in the 21st century after a hundred years of human settlement, species introductions, and environmental change. Aquatic Ecosystem Health and Management, 10: 212-227.
- Peterson, R. H., D. J. Martin-Robichaud & P. Harmon. 2004. Influence of incubation temperature on body movements of Atlantic cod (Gadus morhua L.) embryos and on size at hatch. Aquaculture Research, 35: 453-458.
- Pfennig, D. W., A. W. Matthew, E. C. Snell-Rood, T. Cruickshank, C. D. Schlichting & A. P. Moczek. 2010. Phenotypic plasticity's impacts on diversification and speciation. Trends in Ecology and Evolution, 25: 459-467.
- Pigliucci, M. 2001. Phenotypic Plasticity: Beyond Nature and Nurture. Baltimore, Johns Hopkins University Press, 333p.
- Pigliucci, M., C. J. Murren & C. D. Schlichting. 2006. Phenotypic plasticity and evolution by genetic assimilation. Journal of Experimental Biology, 209: 2362-2367.
- Quirós, R. 1988. Relationships between air temperature, depth, nutrients and chlorophyll in 103 Argentinian lakes. Verhandlungen des Internationalen Verein Limnologie, 23: 647-658.
- Reissig, M., C. Trochine, C. Queimaliños, E. Balseiro & B. Modenutti. 2006. Impact of fish introduction on planktonic food webs in lakes of the Patagonian Plateau. Biological Conservation, 132: 437-447.
- Rohlf, F. J. & L. F. Marcus. 1993. A revolution in morphometrics. Trends in Ecology and Evolution, 8: 129-132.
- Sund, T. & I. Falk-Petersen. 2005. Effects of incubation temperature on development and yolk sac conversion efciencies of spotted wolfsh (Anarhichas minor Olafsen) embryos until hatch. Aquaculture Research, 36: 1133-1143.
- Strüssmann, C. A. & R. Patiño 1995. Temperature manipulation of sex differentiation in fish. Pp. 153-157. In: F. Goetz & P. Thomas (Eds.) Proceedings of the Fifth International Symposium on the Reproductive Physiology of Fish. The University of Texas at Austin, Texas, 389p.
- Strüssmann, C. A., T. Akaba, K. Ijima, K. Yamaguchi, G. Yoshizaki & F. Takashima. 1997. Spontaneous hybridization in the laboratory and genetic markers for the identification of hybrids between two atherinid species, Odontesthes bonariensis (Valenciennes, 1835) and Patagonina hatcheri (Eigenmann, 1909). Aquaculture Research, 28: 291-300.
- West-Eberhard, M. J. 2003. Developmental Plasticity and Evolution, Oxford University Press, New York, 618p.
- Wintzer, A. P. & P. J. Motta. 2005. Diet-induced phenotypic plasticity in the skull morphology of hatchery-reared Florida largemouth bass, Micropterus salmoides floridanus Ecology of Freshwater Fish, 14: 311-318.
- Yamagami, K. 1988. Mechanisms of hatching in fish. Pp. 447-500. In: Hoar, W. S. & D. J. Randall (Eds.) Fish physiology. Volume XIA. The physiology of the developing fish. New York, Academic Press Inc., 546p.
Publication Dates
-
Publication in this collection
14 June 2012 -
Date of issue
2012
History
-
Received
17 Aug 2011 -
Accepted
08 May 2012