Abstracts
There is an increasing demand for inexpensive and safe anesthetics that can reduce fish stress caused by some procedures such as capture and handling. In this context, the present study evaluated the potential of essential oils (EO) of three Brazilian native plants (Hesperozygis ringens, Lippia sidoides and Ocotea acutifolia) as anesthetics for the silver catfish - Rhamdia quelen. Moreover, an analysis was made of the chemical composition of these oils and their influence on stress parameter. EO of H. ringens and O. acutifolia were effective as anesthetics, without behavioral side effects. EO of O. acutifolia (150 µL L-1) promoted an increase in blood glucose level. Regarding to the composition, pulegone accounts for 96.63% of the EO of H. ringens, and caryophyllene oxide amounts to 56.90% of the EO of O. acutifolia. Two chemotypes, thymol and carvacrol (68.40% and 67.89%, respectively) were verified for EO of L. sidoides. Both samples of EO of L. sidoides showed anesthetic activity in silver catfish, but exposure also caused loss of mucus and mortality. Thus, only the EO of H. ringens and O. acutifolia are advised for anesthetic use
Essential oil; Hesperozygis ringens; Lippia sidoides; Ocotea acutifolia; Stress
Existe uma crescente demanda por anestésicos baratos e seguros capazes de reduzir o estresse em peixes produzido durante procedimentos como captura e manuseio. Neste contexto, o presente estudo avaliou o potencial como anestésico dos óleos essenciais (EO) de três espécies vegetais nativas (Hesperozygis ringens, Lippia sidoides e Ocotea acutifolia) em jundiás - Rhamdia quelen. Adicionalmente, a composição química desses óleos e suas influências sobre o estresse também foram avaliadas. Os EO de H. ringens e O. acutifolia foram efetivos como anestésicos sem efeitos adversos detectáveis. EO de O. acutifolia (150 µL L-1) promoveu um aumento na glicemia. Em relação a sua composição, pulegona correspondeu a 96,63% do EO de H. ringens, e óxido de cariofileno a 56,90% do EO de O. acutifolia. Dois quimiotipos, timol e carvacrol (68,40% e 67,89%, respectivamente) foram verificados para os EO de L. sidoides. Ambas as amostras de EO de L. sidoides apresentaram atividade anestésica em jundiás, contudo a exposição produziu perda de muco e mortalidade. Desta forma, somente os EO de H. ringens e O. acutifolia têm seu uso recomendável como anestésicos
Introduction
Anesthetics can be useful in fisheries and fish biology procedures to immobilize the animals during handling, thus preventing physical injury and stress (Inoue et al., 2003; Bressler & Ron, 2004). In this context, some studies have been conducted with plant essential oils (EO) and their isolated compounds in order to find new anesthetics that are more effective, safer and less expensive than the currently available synthetic drugs (Inoue et al., 2003; Guénette et al., 2007). Examples of anesthetics obtained from natural sources with action upon different fish species are eugenol (Guénette et al., 2007; Cunha et al., 2010a), menthol (Façanha & Gomes, 2005), and EO of Eugenia caryophyllata and E. aromatica (Inoue et al., 2003; Bressler & Ron, 2004), Lippia alba (Cunha et al., 2010b, 2011), Cinnamomum cassia (Power et al., 2010), Melaleuca alternifolia (Hajek, 2011), and Ocimum gratissimum (Silva et al., 2012).
Brazil is considered to have one of the world's greatest plant diversities, with over 40,000 different plant species (Oliveira et al., 2012). Some plant species that were still not studied belong to Lamiaceae, Lauraceae, and Verbenaceae families. These botanical families were recognized in some reports by their contribution for the treatment of central nervous system diseases and high EO content (Brito & Brito, 1993; Gomes et al., 2009). Hesperozygis ringens (Benth.) Epling (Lamiaceae), known as "espanta-pulga", is one endangered and endemic plant of the southern of Brazil. Only acaricidal activity has been reported to date for its EO (Fracaro & Echeverrigaray, 2006; Ribeiro et al., 2010). Ocotea acutifolia (Nees) Mez (Lauraceae), known as "canela-branca", is a riparian species distributed in Uruguay and southern Brazil (Sobral et al., 2006), for which no reports of the presence of EO were found. Lippia sidoides Cham. (Verbenaceae) is a shrub native from northeastern Brazil, commonly called as "alecrim-pimenta", used in folk medicine as a spasmolytic, antimicrobial, and local anesthetic as well as a sedative (Brito & Brito, 1993).
The aim of this study was to evaluate the anesthetic activity in juvenile silver catfish and the chemical composition of EO obtained from three Brazilian native plants (H. ringens, O. acutifolia and L. sidoides) as well as to investigate the effect of the anesthesia produced with such EO on glucose levels.
Material and Methods
Animals
Silver catfish were purchased from fish culture sector of Universidade Federal de Santa Maria (UFSM) and transported to the laboratory, where they were maintained in continuously aerated 250 L tanks, with controlled water parameters. Dissolved oxygen (experiment 1: 8.97±0.39 mg L-1; experiment 2: 5.82±0.08 mg L-1) and temperature (experiment 1: 19.55±0.69°C; experiment 2: 20.71±0.09°C) were measured with an YSI oxygen meter (Model Y5512); pH (experiment 1: 7.8±0.03; experiment 2: 7.55±0.09) was determined with a DMPH-2 pH meter. Total ammonia levels (experiment 1: 0.90±0.04 mg L-1; experiment 2: 1.12±0.04 mg L-1) were measured by the salicylate method (Verdouw et al., 1978). All experiments used a semi-static system where 50% of the water volume was changed daily. Fish were fed once a day with commercial feed (28% crude protein). Juveniles were fasted for a period of 24 h prior to the experiments. The methodologies of the experiments were approved by the Ethical and Animal Welfare Committee of the Universidade Federal de Santa Maria (Process nº 46/2010).
Plant Materials
Leaves of H. ringens and O. acutifolia were respectively collected in São Francisco de Assis (Rio Grande do Sul, Brazil) in January and May 2011. The species were identified by Dr. Solon Jonas Longhi and voucher specimens (SMDB nº 13.427 and nº 13.450, respectively) were deposited in the herbarium of the Departamento de Biologia, UFSM. Two samples of aerial parts of L. sidoides were collected in May 2008, dried for three days in a ventilated drying oven at 45°C, and stored in closed, dark packages until extraction started. Sample 1 was grown in Araxá (Minas Gerais State, Brazil) and Sample 2 in Jardinópolis (São Paulo State, Brazil). Voucher specimens identified by Fátima Salimena were deposited in the Departamento de Biotecnologia (UNAERP), under numbers 1327 (Sample 1) and 1328 (Sample 2).
Essential oil extraction and analysis
The EO of H. ringens, O. acutifolia and L. sidoides were extracted by hydrodistillation using a Clevenger type apparatus for 2, 3, and 3 h, respectively (European Pharmacopoeia, 2007). The EO were stored at -4ºC in amber glass bottles until analysis by gas chromatography coupled with mass spectrometry (GC-MS) and biological tests. EO yields were calculated w/w (%). GC-MS TIC analysis was conducted using an Agilent-6890 gas chromatograph coupled with an Agilent 5973 mass selective detector, using an HP5-MS column (5% phenyl - 95% methylsiloxane, 30 m x 0.25 mm i. d. x 0.25 mm) and EI-MS of 70 eV. The operating conditions were: split inlet 1:100; temperature program, 40-320ºC at 4ºC min-1; carrier gas He; flow rate 1 mL min-1; injector and detector temperature 250ºC. The constituents of the EO were identified by comparison of the mass spectra with a mass spectral library (NIST, 2005), and the Kovats retention index with literature data (Adams, 2001).
Biological activity
Experiment 1: Anesthesia induction and recovery. Juvenile fish (12.2±0.5 g; 11.0±0.1 cm) were transferred to aquaria containing 1 L of water continuously aerated and the EO concentrations firstly diluted in ethanol 95% (1:10). Concentrations of 55, 111, 277 and 554 µL L-1 of the EO obtained from H. ringens, and 50, 100, 150, 300, 600, and 900 µL L-1 of the EO of O. acutifolia were used in this experiment. For L. sidoides, two EO samples from different chemotypes were tested at concentrations of 30, 70, 150, 300, and 600 µL L-1. Ethanol control was also performed at the same concentration used for dilution of the highest EO concentrations. To evaluate the time required for anesthesia induction, five (EO of H. ringens) or six (EO of O. acutifolia and L. sidoides) juveniles were used for each concentration tested, and each juvenile was used only once, according to Schoettger & Julin (1967). This method involves six stages, in which the following parameters were observed: light and deep sedation (stages 1 and 2, respectively), partial and total loss of equilibrium (stage 3a and b, respectively), deep anesthesia (stage 4) and medullar collapse (stage 5). The maximum observation time was 30 min. After induction, juveniles were transferred to anesthetic-free aquaria to measure recovery time. Animals were considered to be recovered when they showed normal swimming behavior in response to external stimuli. After recovery, the fish were grouped according to the anesthetic protocol and transferred into continuously aerated 40 L aquaria, where they were observed for 48 hours for any signs of abnormal behavior, diseases or mortality.
Experiment 2: Evaluation of blood glucose levels. This experiment was conducted to verify stress parameter of fish exposed to EO H. ringens (137 and 277 µL L-1) and O. acutifolia (150 and 300 µL L-1). Control groups of water and ethanol were also included for each sample, as well as an unhandled basal group. Silver catfish (N = 6; 61.7±2.7g; 19.3±0.3 cm) were transferred to 40 L aquaria three days before the experiment.
Fish were captured with a dip net and transferred in pairs to continuously aerated 2 L aquaria. The time between capture and release did not exceed 30 seconds. Juveniles remained in the aquarium until they reached stage 4 of anesthesia induction with EO while the controls of EO of H. ringens and O. acutifolia remained for 7 and 18 min, respectively. These times were chosen for the controls because they correspond to the highest induction time until stage 4, for each EO used in this experiment (see results). The fish in the basal control group were removed from the 40 L aquaria and immediately submitted to blood collection.
After the induction procedure, blood (0.1-0.5 mL) was collected from the caudal vein with 1 mL syringes and submitted to glucose determination with a digital Accu-Check(r) Advantage II apparatus. Following blood collection, all fish were handled for biometric measurements and transferred to anesthetic-free 40 L aquaria, where they were observed for 48 h for any signs of abnormal behavior, diseases or mortality.
Statistical analysis
All data are presented as mean + SEM. The relationship between the time required for anesthesia induction and the concentration of the anesthetic used was determined by means of software Slide Write Plus version 4.0. To verify the homogeneity of variances and normality, data were submitted to Levene and Kolmogorov-Smirnov tests, respectively. The results obtained for stages 2, 3a, and 3b of EO O. acutifolia and L. sidoides (sample 1 and 2) were Ln transformed previously to statistical analysis. One-way ANOVA and Tukey tests were used for data of anesthesia induction and recovery and also for blood glucose levels. Stage 3a of induction with EO of O. acutifolia was analyzed by the Kruskal-Wallis test followed by the Dunn test. Samples of EO of L. sidoides were analyzed by two-way ANOVA and the Tukey test or the Scheirer-Ray-Hare extension of the Kruskal-Wallis test, when appropriate. Water parameters between experiments were compared using t-test or Mann-Whitney test. Analyses were performed with software SigmaPlot version 11.0, and the minimum significance level was set at P<0.05.
Results
Chemical composition
A total of 67 compounds were identified for the EO of the three species studied, accounting for 97.3-99.5% of the total compositions of the analyzed samples (Table 1). H. ringens showed a high yield of EO (3.5%), whereas the yield of EO of O. acutifolia was 0.80%. EO of L. sidoides obtained from sample 1 (2.22%) showed a greater yield than the one from sample 2 (0.90%).
Chemical compositions and physical characteristics of the essential oils obtained from Hesperozygis ringens, Ocotea acutifolia and Lippia sidoides. *Retention index did not report; RI: Retention index; tr: Trace (<0.05%); a Adams (2001), NIST (2005).
The major chemical component of EO of H. ringens was pulegone (96.63%). Other mono and sesquiterpenoid derivatives were also found in this EO, but at low concentrations (<1.20%). A total of 24 substances were identified in the EO of O. acutifolia. Among the major constituents, the most significant ones are caryophyllene oxide (56.90%), calarene epoxide (11.74%) and τ-elemene (8.17%). The major constituents of EO of L. sidoides (sample 1) were thymol (68.40%), p-cymene (8.72%) and β-caryophyllene (5.90%), while the second sample had carvacrol (67.89%), p-cymene (21.76%) and β-caryophyllene (3.90%) as major compounds.
Biological activity
All water parameters evaluated differed statistically between experiments. Dissolved oxygen level and pH were higher in the experiment 1, while temperature and total ammonia levels were lower in the same experiment.
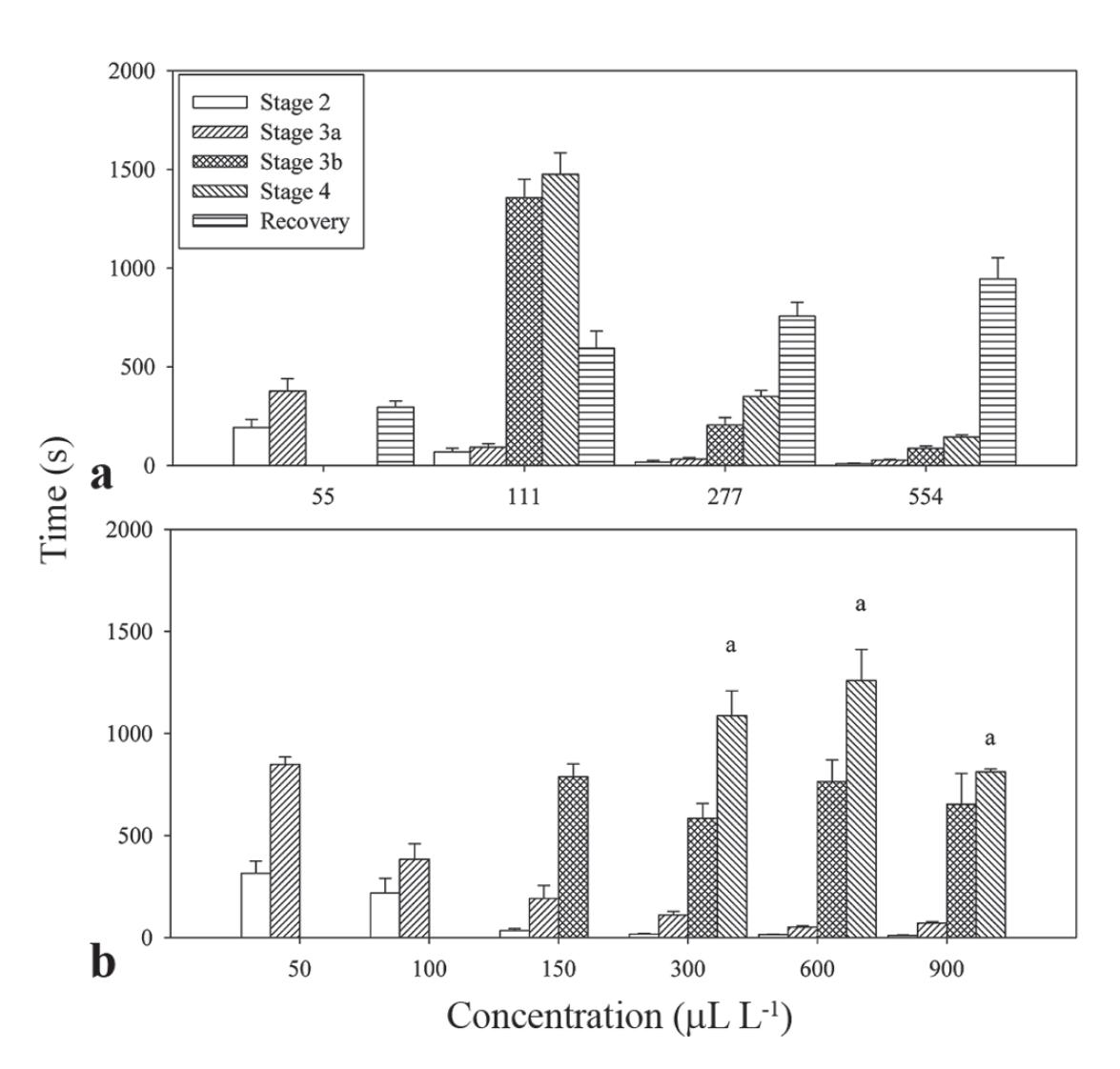
All EO tested in this study showed sedative and anesthetic effects in silver catfish through bath administration. Ethanol at the highest concentration used to dilute the samples did not produce any anesthetic effect when applied alone. Fish exposed to EO of H. ringens reached deep anesthesia in concentrations ranging from 111 (about 24 min) to 554 µL L-1 (about 2 min), while 55 µL L-1 induced up to partial loss of equilibrium (Fig. 1a). A clear reduction in the induction time occurred with the increase of EO concentration. The opposite pattern was verified at the time of recovery, where an increase in the concentration of the EO of H. ringens was followed by a correspondent elevation in the recovery time (Table 2). All juveniles recovered between 5-15 min without signs of toxicity or mortality until 48 h after exposure.
Induction time and recovery of essential oils in silver catfish juveniles: a = Hesperozygis ringens; b = Ocotea acutifolia. Stages of induction were observed according to Schoettger & Julin (1967). Maximum observation time for induction and recovery was 30 min. Data are presented as mean±SEM (N = 5-6). Different letters indicate significant differences among concentrations for the same induction stage (P<0.05). Recovery time was omitted of Fig. 1b because it was higher than 30 min for most fish tested (see results).
Relationship between the time required to reach the stages of induction and recovery from anesthesia and the concentration of the essential oils (EOs) of Hesperozygis ringens, Ocotea acutifolia and Lippia sidoides in silver catfish. Where x=concentration of essential oil (µL L-1); y=time to reach the stage of induction or recovery from anesthesia (Schoettger & Julin, 1967) in seconds (s).
Anesthesia was reached with 300-900 µL L-1 EO of O. acutifolia (between 13-18 min; Fig. 1B). A positive relationship between the concentration and the time required for the induction of anesthesia was observed at all stages, except for stage 4 (Table 2). Lower concentrations (50-150 µL L-1) did not induce anesthesia during the 30 min evaluation period. Only 50% of the animals exposed to 150 µL L-1 (970±75.7 s) and 17% of the fish exposed to 300 µL L-1 (1560 s) recovered during the time of observation, while all juveniles returned to normal behavior in 660.7±21 sec after exposure to 50 µL L-1. For the additional concentrations tested, recovery time was higher than 30 min. Mortality was not observed until 48 h after exposure.
There was no difference in anesthetic effect between the two samples of EO of L. sidoides as regards induction time until stages 3b and 4. Fish exposed to sample 2 took longer time to reach stage 2 and 3a with 70 µL L-1, and stage 3a with 150 µL L-1 when compared to sample 1. The opposite pattern occurred with 600 µL L-1, where stage 2 was reached sooner for sample 2 than for sample 1 (Fig. 2).
Anesthetic effect of essential oils obtained from Lippia sidoides in silver catfish juveniles: a = Stage 2; b = Stage 3a; c = Stage 3b; d = Stage 4, according to Schoettger & Julin (1967). Maximum observation time for induction was 30 min. Data are presented as mean±SEM (N = 6). Different letters indicate significant differences among concentrations within each sample and * describes significant differences among samples (P<0.05).
The relationship between the induction time of anesthesia and the concentration of the EO of L. sidoides was verified for sample 1 at stages 3a, 3b and 4 (Table 2). Juveniles exposed to 150-600 µL L-1 of both samples reached deep anesthesia at a statistically similar time (about 11-20 min) (Fig. 2d). Additionally, deep anesthesia was also verified in 33% of the fish exposed to 70 µL L-1 of sample 1. Concentrations of 30 µL L-1 of samples 1 and 2, and 70 µL L-1 of sample 2, promoted only partial loss of equilibrium in juveniles during the 30 min of exposition.
Fish exposed to both EO of L. sidoides did not recover normal behavior until 30 min after transference to anesthetic-free aquaria. Exceptions to this pattern occurred in all animals exposed to 30 µL/L of both samples and 33% of the fish exposed to 70 µL L-1 of sample 2 (1543.0±108.0 s). However, the recovery of the animals differed according to EO sample tested at 30 µL L-1. All animals presented normal behavior in 713.5±38.7 sec with sample 2, whereas 50% of the fish recovered in 1048.0±36.6 sec with sample 1.
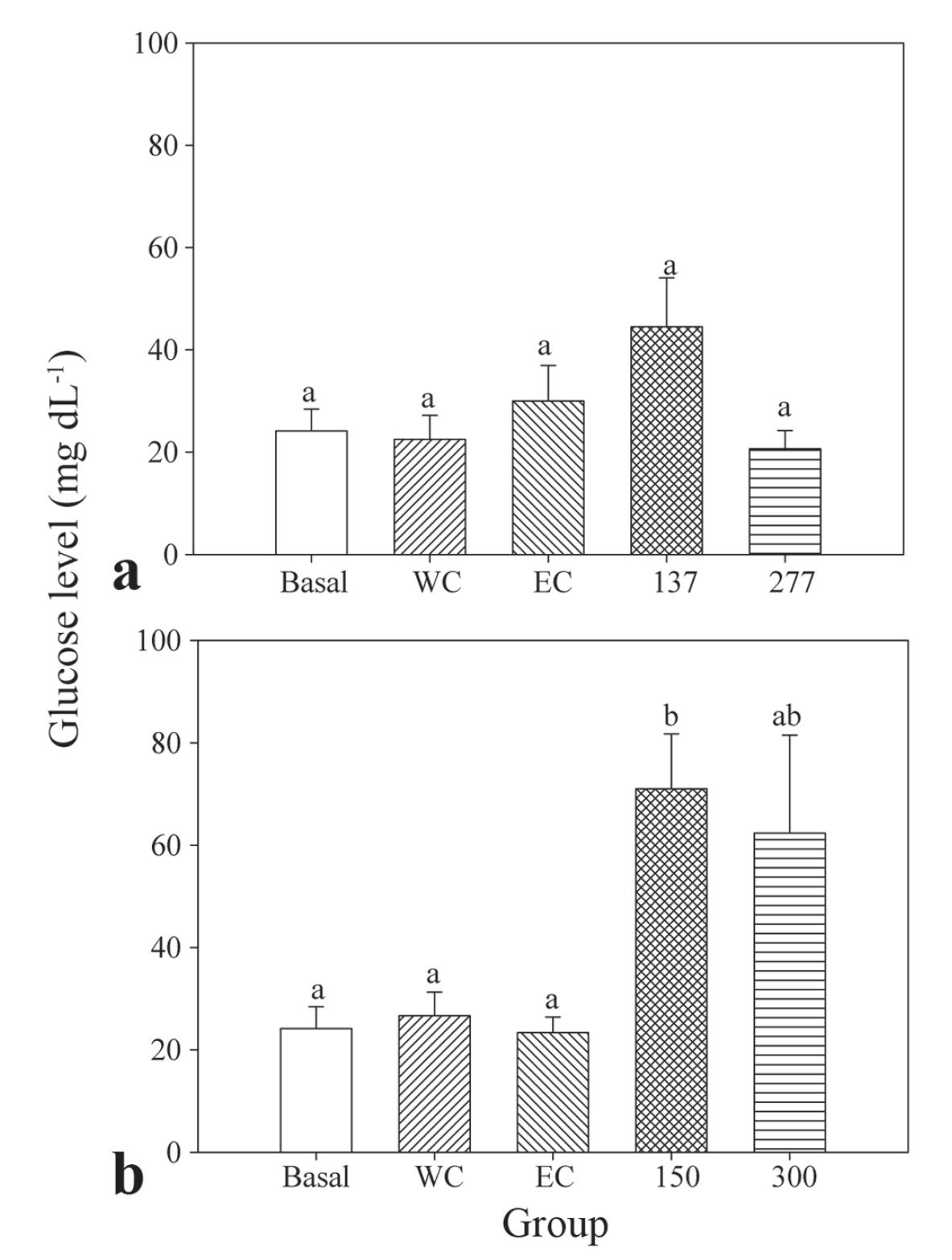
In the experiment to evaluate blood glucose level, deep anesthesia was obtained in 399.8±24.8 s and 307.2±22.4 s with, respectively, 137 and 277 µL L-1 EO of H. ringens. Concentrations of 150 and 300 µL L-1 EO of O. acutifolia were required to reach the same depression level in 735.3±67 s and 825.0±220.1 s, respectively. There was no statistical difference among the glucose levels of the basal group, water and ethanol controls (22-30 mg dL-1). Similar values were also detected in fish anesthetized with 277 µL L-1 EO of H. ringens. Significantly higher glucose levels were observed in silver catfish exposed to 150 µL L-1 EO of O. acutifolia compared to the basal and the two control groups (Fig. 3).
Blood glucose levels after anesthesia of silver catfish with essential oils: A = essential oil of Hesperozygis ringens; B = essential oil of Ocotea acutifolia; W = water control; EC = ethanol control. Data are presented as mean±SEM (N = 6). Different letters indicate significant differences among groups (P<0.05).
Discussion
Higher pulegone content and lower extractive yield were detected in this study for the EO of H. ringens, when compared to a previous report (Ribeiro et al., 2010). Similar pattern also was verified to both samples of the EO of L. sidoides in relation to the major compounds, thymol and carvacrol (Botelho et al., 2007; Lima et al., 2011). These differences could be due to genetic factors, and the vegetative period of the plants, as well as external factors such as height, water availability, temperature, light intensity, and soil fertility (Lima et al., 2003; Chalchat & Özcan, 2008).
Although there are no reports, to date, on the EO of O. acutifolia, the good yield achieved by this species is not surprising, since it belongs to the Lauraceae family. Compared with other Ocotea species, the composition of the EO of O. acutifolia differs greatly. The chemical profile verified to other Ocotea plants showed α-pinene, β-pinene, E-caryophyllene, α-humulene, germacrene D and ρ-cymene at high proportions (Takatu et al., 2007; Barbosa-Filho et al., 2008). However, some constituents present in leaf EO of O. acutifolia appear also in the leaf EO of O. brenesii (Chaverri & Cicció, 2005), and in the leaf EO of 10 species of Ocotea investigated in Costa Rica (Takatu et al., 2007).
The anesthetic activity for the EO of O. acutifolia and L. sidoides verified in this study was not completely unexpected. Previous reports showed analgesic and sedative properties of species of the genus Ocotea (Beirith et al., 1999; Zschocke et al., 2000b; Zhang, 2004). Regarding to L. sidoides, ethnopharmacological use as sedative and local anesthetic was described previously (Brito & Brito, 1993). To date, there are no reports about sedative and/or anesthetic activities for the Hesperozygis species.
According to Gilderhus & Marking (1987), the ideal anesthetic should induce fast anesthesia (3 min or less) with minimum hyperactivity or stress, and rapid recovery (within 10 min or less) after fish transference to anesthetic-free aquaria. These criteria were met for anesthesia of silver catfish with EO of H. ringens. Similar induction time until anesthesia could be obtained for this fish species with 200-500 mg L-1 EO of Lippia alba, 40-50 mg L-1 of eugenol, 70-300 mg L-1 EO of Ocimum gratissimum, 150-300 mg L-1 of MS-222 and 2.5-12 mg L-1 of propofol (Cunha et al., 2010a, 2010b; Gressler et al., 2012; Silva et al., 2012). Thus, the EO of H. ringens could be an alternative to the use of the anesthetics previously reported, since it showed activity without side effects and higher extractive yield than EO previously cited.
The long term induction and recovery times observed to fish exposed to the EO of O. acutifolia and L. sidoides may result from the hydrophobic characteristics of the compounds of these EO. Thymol/carvacrol and caryophyllene oxide (the major compounds of EO of L. sidoides and O. acutifolia, respectively) have a higher partition coefficient (log P) than pulegone, found in EO of H. ringens (Kang et al., 2007); hence, the former compounds can be considered to be more hydrophobic. Studies performed by Kiessling et al. (2009) indicated that isoeugenol, a lipophylic compound, had slower clearance than the hydrophilic drug MS-222 in Atlantic salmon (Salmo salar). A slow clearance may be associated to drug accumulation in the adipose tissue, which in turn would increase recovery time after long exposure time (Kiessling et al., 2009; Zahl et al., 2012).
The depressor effects of EO of H. ringens and L. sidoides may be partially due to their major compounds. Pulegone, thymol and carvacrol are positive allosteric modulators of the GABA receptor (Tong & Coats, 2010), which corresponds to one of the main targets of the action of sedative and anesthetics used in therapeutic (Johnston et al., 2006). Additionally, it should be noted that pulegone has a similar structure to menthol (Ringer et al., 2003), a recognized fish anesthetic (Façanha & Gomes, 2005).
Analgesic and sedative activity of extracts of the Ocotea species was associated with the presence of alkaloids (Zhang, 2004), triterpenes (Beirith et al., 1999) and sibyllenones (Zschocke et al., 2000b), which are not found in the EO of O. acutifolia. For caryophyllene oxide, the main compound of this EO, sedative effect in silver catfish at concentration ranges of 10-40 mg L-1 and loss of mucus at the highest concentration tested were detected (Benovit, 2012). Thus, EO of Ocotea acutifolia seems to contain other substances able to protect animals from the deleterious action of caryophyllene oxide. Secretion of mucus is a common side effect of some synthetic anesthetics currently used in aquaculture, such as 2-phenoxyethanol, quinaldine sulfate and benzocaine (Inoue et al., 2003; Velisek et al., 2007).
Side effects were observed during and after induction of anesthesia with both EO of L. sidoides. The fish exposed to all concentrations showed sudden jumping behavior towards the surface due to involuntary muscle contractions during induction. These events were independent of the presence of stimuli in caudal peduncles or inside the aquarium, and they were followed by a motionless period of the fish at the bottom. For animals exposed to sample 2, these events were observed more frequently. High loss of mucus during induction and total mortality after exposure occurred in all fish exposed to 300 and 600 µL L-1 of sample 1 and 600 µL L-1 of sample 2.
The above-mentioned mortality and adverse effects for EO of L. sidoides can result from acetylcholinesterase (AChE) inhibition. Similar behavior effects as those verified for this EO were described for Cyprinus carpio L. exposed to 2.4-D (2.4-dichlorophenoxyacetic acid) herbicide, a known AChE inhibitor (Sarikaya & Yýlmaz, 2003). AChE inhibition was previously reported in vitro for methanolic and ethanolic extracts of L. sidoides, as well as for thymol and carvacrol (Trevisan & Macedo, 2003; Jukic et al., 2007). Jukic et al. (2007) demonstrated that AChE inhibitory activity of carvacrol is 10 times stronger than the one for its isomer thymol, which could possibly explain the higher incidence of side effects in fish exposed to carvacrol-type EO (sample 2).
Glucose levels correspond to a common indicator for the stress response in teleost fish (Greenweel et al., 2003). In this study, fish of control groups did not change their glucose levels immediately after tank transference when compared to those in the basal group, which excludes this procedure as an agent able to influence the results. Thus, the hyperglycemic event detected after anesthesia with the EO of O. acutifolia corresponds to a stressor effect of this sample, which did not occur with the EO of H. ringens. Previous reports indicated that anesthesia with eugenol also promoted hyperglycemic effects in Nile tilapia (Oreochromis niloticus) and matrinxã (Brycon amazonicus) (Deriggi et al., 2006; Barbosa et al., 2007).
In conclusion, thymol and carvacrol chemotypes of EO of L. sidoides showed anesthetic effect (150-600 µL L-1) in silver catfish, but their use is not advised because of the mortality and the observed side effects. Nevertheless, pulegone-rich EO of H. ringens and caryophyllene oxide-rich EO of O. acutifolia can be used as anesthetics in this fish species at concentration ranges of 111-554 and 300-600 µL L-1, respectively. Regarding to the stress parameter evaluated, EO of O. acutifolia was shown to be a slight stressor agent, while EO of H. ringens showed no effect itself.
This study was supported by research funds from the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/PRONEX, Process No. 10/0016-8) and Conselho Nacional de Pesquisa e Desenvolvimento Científico (CNPq, Process No. 470964/2009-0). B. Baldisserotto, C. A. Mallmann, A. M. S. P. and S. J. Longhi are grateful to CNPq for research fellowships; L. L. Silva, D. T. Silva, M. A. Cunha are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their postgraduate fellowships. Q. I. Garlet is grateful to FIT/FIPE/UFSM for her undergraduate scholarship.
Literature Cited
- Adams, R. P. 2001. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Illinois, Allured Publishing Corporation.
- Barbosa, L. G., G. Moraes & L. A. K. A. Inoue. 2007. Metabolic responses of matrinxã to eugenol in anesthetic baths. Acta Scientiarum Biological Sciences, 29: 255-260.
- Barbosa-Filho, J. M., R. M. Cunha, C. S. Dias, P. F. Athayde-Filho, M. S. Silva, E. V. L. Cunha, M. I. L. Machado, A. A. Craveiro & I. A. Medeiros. 2008. GC-MS analysis and cardiovascular activity of the essential oil of Ocotea duckei. Revista Brasileira de Farmacognosia, 18: 37-41.
- Beirith, A., A. R. S. Santos, J. B. Calixto, S. C. Hess, I. Messana, F. Ferrari & R. A. Yunes. 1999. Study of the antinociceptive action of the ethanolic extract and the triterpene 24-hydroxytormentic acid isolated from the stem bark of Ocotea suaveolens. Planta Medica, 65: 50-55.
- Benovit, S. C. 2012. Composição e atividade sedativa e anestésica do óleo essencial de Aloysia gratissima (Gillies & Hook.) Troncoso (Verbenaceae) em jundiás (Rhamdia quelen). Unpublished Ph.D. or MS.c? Dissertation, Universidade Federal de Santa Maria, Santa Maria, número de páginas?.
- Botelho, M. A., N. A. P. Nogueira, G. M. Bastos, S. G. C. Fonseca, T. L. G. Lemos, F. J. A. Matos, D. Montenegro, J. Heukelbach, V. S. Rao & G. A. C. Brito. 2007. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Brazilian Journal of Medical and Biological Research, 40: 349-356.
- Bressler, K. & B. Ron. 2004. Effect of anesthetics on stress and the innate immune system of gilthead seabream (Sparus aurata). The Israeli Journal of Aquaculture - Bamidgeh, 56: 5-13.
- Brito, A. R. M. & A. A. S. Brito. 1993. Forty years of Brazilian medicinal plant research. Journal of Ethnopharmacology, 39: 53-67.
- Chalchat, J. C. & M. M. Özcan. 2008. Comparative essential oil composition of flowers, leaves and steam of basil (Ocimum basilicum L.) used as herb. Food Chemistry, 110: 501-503.
- Chaverri, C. & J. F. Cicció. 2005. Essential oils of trees of the genus Ocotea (Lauraceae) in Costa Rica. I. Ocotea brenesii. Revista de Biología Tropical, 53: 431-436.
- Cunha, M. A., F. M. C. Barros, L. O. Garcia, A. P. L. Veeck, B. M. Heinzmann, V. L. Loro, T. Emanuelli & B. Baldisserotto. 2010b. Essential oil of Lippia alba: a new anesthetic for silver catfish, Rhamdia quelen. Aquaculture, 306: 403- 406.
- Cunha, M. A., C. C. Zeppenfeld, L. O. Garcia, V. L. Loro, M. B. Fonseca, T. Emanuelli, A. P. L. Veeck, C. E. Copatti & B. Baldisserotto. 2010a. Anesthesia of silver catfish with eugenol: time of induction, cortisol response and sensory analysis of fillet. Ciência Rural, 40: 2107-2114.
- Cunha, M. A., B.F. Silva, F.A.C. Delunardo, S.C. Benovit, L.C. Gomes, B.M. Heinzmann & B. Baldisserotto. 2011. Anesthetic induction and recovery of Hippocampus reidi exposed to the essential oil of Lippia alba. Neotropical Ichthyology, 9: 683-688.
- Deriggi, F. G., L. A. K. A. Inoue & G. Moraes. 2006. Stress responses to handling in Nile tilapia (Oreochromis niloticus Linnaeus): assessment of eugenol as an alternative anesthetic. Acta Scientiarum Biological Sciences, 28: 269-274.
- European Pharmacopoeia. 2007. 6th ed. Strassbourg, European Directorate for the Quality of Medicines.
- Façanha, M. F. & L. C. Gomes. 2005. Efficacy of menthol as an anesthetic for tambaqui (Colossoma macropomum, Characiformes: Characidae). Acta Amazonica, 35: 71-75.
- Fracaro, F. & S. Echeverrigary. 2006. Genetic variability in Hesperozygis ringens Benth. (Lamiaceae), an endangered aromatic and medicinal plant of Southern Brazil. Biochemical Genetics, 44: 479-490.
- Gomes, N. G. M., M. G. Campos, J. M. C. Órfão & C. A. F. Ribeiro. 2009. Plants with neurobiological activity as potential targets for drug discovery. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 33: 1372-1389.
- Gilderhus, P. A. & L. L. Marking. 1987. Comparative efficacy of 16 anesthetic chemicals on rainbow trout. North American Journal of Fisheries Management, 7: 288-292.
- Greenwell, M. G., J. Sherrill & L. A. Clayton. 2003. Osmoregulation in fish mechanisms and clinical implications. Veterinary Clinics of North America: Exotic Animal Practice, 6: 169-189.
- Guénette, S. A., F. C. Uhland, P. Hélie, F. Beaudry & P. Vachon. 2007. Pharmacokinetics of eugenol in rainbow trout (Oncorhynchus mykiss). Aquaculture, 266: 262-265.
- Gressler, L. T., T. V. Parodi, A. P. K. Riffel, S. T. daCosta & B. Baldisserotto. 2012. Immersion anaesthesia with tricaine methanesulfonate or propofol on different sizes and strains of silver catfish Rhamdia quelen. Journal of Fish Biology, 81: 1436-1445.
- Hajek, G. J. 2011. The anaesthetic-like effect of tea tree oil in common carp Cyprinus carpio L. Aquaculture Research, 42: 296-300.
- Inoue, L. A. K. A., C. S. Neto & G. Moraes. 2003. Clove oil as anesthesic for juveniles of matrinxã Brycon cephalus (Gunther, 1969). Ciência Rural, 33: 943-947.
- Johnston, G. A., J. R. Hanrahan, M. Chebib, R. K. Duke & K. N. Mewett. 2006. Modulation of ionotropic GABA receptors by natural products of plant origin. Advances in Pharmacology, 54: 285-316.
- Jukic, M., O. Politeo, M. Maksimovic, M. Milos & M. Milos. 2007. In Vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytotheraphy Research, 21: 259-261.
- Kang, L., C. W. Yap, P. F. C. Lim, Y. Z. Chen, P. C. Ho, Y. W. Chan, G. P. Wong & S. Y. Chan. 2007. Formulation development of transdermal dosage forms: Quantitative structure-activity relationship model for predicting activities of terpenes that enhance drug penetration through human skin. Journal of Controlled Release, 120: 211-219.
- Kiessling, A., D. Johansson, I. H. Zahl & O. B. Samuelsen. 2009. Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration. Aquaculture, 286: 301-308.
- Lima, H. R. P., M. A. C. Kaplan & A. V. M. Cruz. 2003. Influence of abiotic factors on terpenoids production and variability in the plant. Floresta e Ambiente, 10: 71-77.
- Lima, R. K., M. G. Cardoso, J. C. Moraes, S. M. Carvalho, V. G. Rodrigues & L. G. L. Guimarães. 2011. Chemical composition and fumigant effect of essential oil of Lippia sidoides Cham, and monoterpenes against Tenebrio molitor (L.) (Coleoptera: Tenebrionidae). Ciência e Agrotecnologia, 35: 664-671.
- NIST/ EPA/ NIH mass spectral library and search/ analysis programs. 2005. J. Wiley and Sons, Hoboken, NJ.
- Oliveira, V. B., L. T. Yamada, C. W. Fagg & M. G. L. Brandão. 2012. Native foods from Brazilian biodiversity as source of bioactive compounds. Food Research International, 48: 170-179.
- Power, D. M., J. Fuentes & A. P. Harrison. 2010. A noninvasive monitoring device for anesthetics in fish. Open Access Animal Physiology, 2: 17-23.
- Ribeiro, V. L. S., J. C. Santos, S. A. L. Bordignon, M. A. Apel, A. T. Henriques & G. Von Poser. 2010. Acaricidal properties of the essential oil Hesperozygis ringens (Lamiaceae) on the cattle tick Riphicephalus (Boophilus) microplus. Bioresource Technology, 101: 2506-2509.
- Ringer, K. L., M. E. McConkey, E. M. Davis, G. W. Rushing & R. Croteau. 2003. Monoterpene double-bond reductases of the (-)-menthol biosynthetic pathway: isolation and characterization of cDNAs encoding (-)-isopiperitenone reductase and (+)-pulegone reductase of peppermint. Archives of Biochemistry and Biophysics, 418: 80-92.
- Sacchetti, G., A. Guerrini, P. Noriega, A. Bianchi & R. Bruni. 2006. Essential oil of wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) leaves from Amazonia Ecuador. Flavour and Fragrance Journal, 21: 674-676.
- Sarikaya, R. & M. Yilmaz. 2003. Investigation of acute toxicity and the effect of 2,4-D (2,4-dichlorophenoxyacetic acid) herbicide on the behavior of the common carp (Cyprinus carpio L., 1758; Pisces, Cyprinidae). Chemosphere, 52: 195-201.
- Schoettger, R. A. & M. Julin. 1967. Efficacy of MS-222 as an anesthetic on four salmonids. Investigations in Fish Control, United States Department of the Interior, 13: 1-15.
- Silva, L. L., T. V. Parodi, P. Rekcziegel, V. O. Garcia, M. E. Bürger, B. Baldisserotto, C. A. Mallmann, A. M. S. Pereira & B. M. Heinzmann. 2012. Essential oil of Ocimum gratissimum L.: anesthetic effects, mechanism of action and tolerance in silver catfish, Rhamdia quelen. Aquaculture, 350-353: 91-97.
- Sobral, M., J. A. Jarenkow, P. Brack, J. Lorocca & R. S. Rodrigues. 2006. Flora Arbórea e Arborescente do Rio Grande do Sul, Brasil. São Carlos, RiMa.
- Takatu, S., W. A. Haber & W. N. Setzer. 2007. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochemical Systematics and Ecology, 35: 525-532.
- Tong, F. & J. R. Coats. 2010. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl uptake in American cockroach ventral nerve cord. Pesticide Biochemistry and Physiology, 98: 317-324.
- Trevisan, M. T. S. & F. V. V. Macedo. 2003. Screening for acetylcholinesterase inhibitors from plants to treat Alzheimer's disease. Química Nova, 26: 301-304.
- Velisek, J., T. Wlasow, P. Gomulka, Z. Svobodova & L. Novotny. 2007. Effects of 2-phenoxyethanol anaesthesia on sheatfish (Silurus glanis L.). Veterinarni Medicina, 52: 103-110.
- Verdouw, H., C. J. A. van Echteld & E. M. J. Dekkers. 1978. Ammonia determination based on indophenol formation with sodium salicylate. Water Research, 12: 399-402.
- Zahl, I. H., O. Samuelsen & A. Kiessling. 2012. Anesthesia of farmed fish: implications for welfare. Fish Physiology and Biochemistry, 38: 201-218.
- Zhang, Z. J. 2004. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sciences, 75: 1659-1600.
- Zschocke, S., S. E. Drewes, K. Paulus, R. Bauer & J. van Staden. 2000a. Analytical and pharmacological investigation of Ocotea bullata (black stinkwood) bark and leaves. Journal of Ethnopharmacology, 71: 219-230.
- Zschocke, S., J. van Staden, K. Paulus, R. Bauer, M. M. Horn, O. Q. Munro, N. J. Brown & S. E. Drewes. 2000b. Stereostructure and anti-inflammatory activity of three diastereomers of ocobullenone from Ocotea bullata. Phytochemistry, 54: 591-595.
-
Published June 28, 2013
Publication Dates
-
Publication in this collection
June 2013
History
-
Received
05 Oct 2012 -
Accepted
18 Mar 2013