Abstracts
The Sternarchellini (Gymnotiformes, Apteronotidae) is a clade of 10 electric fish species that inhabit deep river channels of the Amazon and Orinoco basins, attain moderate adult body sizes (15-50 cm TL), and have a predatory life style. Here we trace the evolutionary origin and diversification of Sternarchellini using standard phylogenetic and biogeographic procedures and a dataset of 70 morphological characters. The main results are: 1) the genus Sternarchella includes both species currently assigned to the genus Magosternarchus; and 2) neither of the multi-species assemblages of Sternarchellini in the Amazon and Orinoco basins are monophyletic. Historical biogeographic analysis suggests that sternarchelline evolution was linked to the large-scale river capture event that formed the modern Amazon and Orinoco basins, i.e. the Late Miocene rise of the Vaupes structural arch and concomitant breaching of the Purus structural arch. This event is hypothesized to have contributed to formation of the modern sternarchelline species, and to the formation of the modern basin-wide sternarchelline species assemblages. The results indicate that cladogenesis (speciation) and anagenesis (adaptive evolution) were decoupled processes in the evolution of Sternarchellini.
Ecological specialization; Evolution; Historical Biogeography; Neotropical; Osteology
Sternarchellini (Gymnotiformes, Apteronotidae) é um clado de 10 espécies de peixes elétricos que habitam canais profundos de rios das bacias do Amazonas e Orinoco, que atingem um tamanho moderado quando adultos (15-50 cm CT), e possuem hábito predatório. Rastreamos a origem evolutiva e diversificação de Sternarchellini utilizando técnicas filogenéticas e biogeográficas padrões e um conjunto de dados de 70 caracteres morfológicos. Os principais resultados são: 1) o gênero Sternarchella inclui duas espécies atualmente atribuídas ao gênero Magosternarchus; e 2) as assembleias de multi-espécies de Sternarchellini nas bacias Amazônica e do Orinoco não formam grupos monofiléticos. A análise biogeográfica histórica sugere que a evolução do Sternarchellini esteve ligada ao evento de captura de rio de grande escala que formou as atuais bacias do Amazonas e Orinoco, i.e., o soerguimento do arco estrutural Vaupés no Mioceno Superior e o rompimento concomitante do arco estrutural Purus. É proposto que esse evento contribuiu para o surgimento das espécies atuais de Sternarchellini, e para a formação das assembleias modernas de espécies de Sternarchellini com ampla distribuição nas bacias. Os resultados indicam que cladogênese (especiação) e anagenêse (evolução adaptativa) foram processos desacoplados na evolução de Sternarchellini
Introduction
The Sternarchellini (Gymnotiformes: Apteronotidae) is a clade of medium-sized (15-50 cm TL) knife-shaped (culteriform) electric fishes that inhabit deep river channels in tropical South America (Lundberg et al., 1996Lundberg, J. & W. Lewis. 1987. A major food web component in the Orinoco River channel: evidence from planktivorous electric fishes. Science, 237: 81-83.; Albert, 2001Albert, J. S. 2001. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Museum of Zoology, University of Michigan, Ann Arbor. ). Sternarchellines inhabit the main stems of the Amazon and Orinoco rivers and some of their larger lowland tributaries, where they are predators of small aquatic animals (Albert & Crampton, 2005aAlbert, J. S. & W. G. R. Crampton. 2005a. Diversity and phylogeny of Neotropical electric fishes (Gymnotiformes). Pp. 360-409. In: Electroreception. T. H. Bullock, C. D. Hopkins, A. N. Popper & R. R. Fay (Eds.). Springer Handbook of Auditory Research, Volume 21, Springer-Verlag, Berlin. ). The genus Sternarchella includes some of the most abundant species of electric fishes that live in deep channels of the Amazon and Orinoco basins (Lundberg et al., 2013). Sternarchellines are an important food source for large river catfishes, and therefore contribute to the food web, supporting a major fishery of the region (Lundberg & Lewis, 1987; Crampton, 1996Crampton, W. G. R. 1996. Gymnotiform fish: an important component of Amazonian floodplain fish communities. Journal of Fish Biology, 48: 298-301. ).
Sternarchelline species possess a suite of morphological and behavioral phenotypes associated with active locomotion and foraging in swiftly flowing riverine water. These traits include: robust oral and pharyngeal jaws with many large conical teeth, a reticulated skeleton riddled with lipid filled cavities, and large lamellar attachment sites for axial and jaw muscles. Sternarchellines also produce a very high-frequency wave-type electric organ discharge (EOD) for use in object location and social communication, generating discharge frequencies ranging from about 940 to 2,180 cycles per second (Hz). Some sternarchellines produce the highest electrical discharge frequencies of all electric fishes, with the electrogenic system of S. schotti being the fastest known biological oscillator (Albert & Crampton, 2005bAlbert, J. S. & W. G. R. Crampton. 2005b. Electroreception and electrogenesis. Pp. 431-472. In: Evans, D. E. (Ed). The Physiology of Fishes. 3rd ed. CRC Press, New York. ; Crampton & Albert, 2006).
The Sternarchellini is a member of the Apteronotidae, the most species-rich family of Gymnotiformes, with 87 currently valid species. The Apteronotidae is an ancient group that originated in the early Cenozoic or late Cretaceous (Albert, 2001Albert, J. S. 2001. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Museum of Zoology, University of Michigan, Ann Arbor. ; Near et al., 2012Near, T. J., R. I. Eytan, A. Dornburg, K. L. Kuhn, J. A. Moore, M. P. Davis, P. C. Wainwright, M. Friedman & W. L. Smith. 2012. Resolution of ray-finned fish phylogeny and timing of diversification. Proceedings of the National Academy Sciences USA, 109: 13698-13703. ) and which ranges over the whole extent of the South American platform, from northern Argentina to Panamá (Eigenmann & Allen, 1922Eigenmann, C. H. & W. Allen. 1922. Fishes of Western South America. University of Kentucky, Lexington. ). Within Apteronotidae, Sternarchellini is a member of the Navajini, including the genera Compsaraia, Magosternarchus, Porotergus,Sternarchella, and Sternarchogiton (Albert, 2001). Members of the Navajini are highly specialized for living in the deep channels (10 - 50 meters) of large lowland Amazonian rivers (stream orders 610). The Navajini, from the Spanish word navaja, blade, is named for its highly derived body shape, strongly compressed laterally, semi-translucent with a pink hue in life, with few or no melanophores and few large scales over most of the body surface, large thin translucent and rhomboid-shaped scales along the lateral line, and a relatively deep body with long bony supports (anal-fin pterygiophores) used to anchor the muscles that undulate the elongate anal fin. The character definitions for Sternarchellini are from Albert (2001), amended herein.
Sternarchellini is currently known from nine valid species and one undescribed species allotted to three genera: Magosternarchus with two species (M. duccis and M. raptor, Lundberg et al., 1996), Pariosternarchus with one species, and Sternarchella with seven species (Albert, 2001; Albert & Crampton, 2006; Table 1). Magosternarchus is notable in having one of the most specialized head and jaw morphologies for grasping prey items within the Apteronotidae. Magosternarchus also exhibit extreme behaviors, such as feeding on the tails of other gymnotiform fishes (Lundberg et al., 1996), and discharging electric signals at frequencies up to 2,000 Hz (Albert & Crampton, 2005b). The monotypic Pariosternarchus amazonensis (Albert & Crampton, 2006) has a very broad and flat ventral surface of the head, with greatly expanded mandibular laterosensory canals, presumably used in object detection on the river benthos. Species of Magosternarchus and Pariosternarchus are rare in collections, and are presumably present in low densities in the wild. Sternarchella is known from six valid species: S. calhamazon (Lundberg et al., 2013), S. orinoco (Mago-Leccia, 1994Mago-Leccia, F. 1978. Los peces de la familia Sternopygidae de Venezuela. Acta Scientífica Venezolana, 29: 1-51. ), S. orthos (Mago-Leccia, 1994), S. schotti (Steindachner, 1868Steindachner, F. 1868. Die Gymnotidae des K. K. Hof-Naturaliencabinetes zu Wien. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien, 58: 249-264. ), S. sima (Starks, 1913Starks, E. C. 1913. The fishes of the Stanford Expedition to Brazil.Stanford University Publications . ), S. terminalis (Eigenmann & Allen, 1942), and one undescribed species (Sternarchella sp. A, Crampton, 2011). Most species of Sternarchella (except S. schotti and Sternarchella sp. A) are commonly taken in middle and bottom water trawls in large rivers of the Amazon and Orinoco basins, and these species are presumably present in high abundances in these habitats.
The genus Sternarchella was introduced by Eigenmann (in Eigenmann and Ward, 1905) to include S. schotti (Steindachner, 1868), originally described as Sternarchus schotti from Barra do Rio Negro (Manaus), Brazil. Steindachner (1868) also described S. capanemae from Manaus, but this is now treated as a junior synonym of S. schotti (Lundberg et al., 2013: 170-171). Sternarchella sima (Starks, 1913) was described from the vicinity of Pará, Brazil. Sternarchella terminalis (Eigenmann & Allen, 1922) was described from Iquitos, Peru, although originally placed in another apteronotid genus, Porotergus. Sternarchella curvioperculata (Godoy, 1968Godoy, M. P. de. 1968. Nova espécie de "Sternarchella" Eigenmann (Pisces, Gymnonoti, Sternarchidae). Revista Brasileira de Biologia, 28: 351-355. ) was described from the Rio Mogi-Guassu in the upper rio Paraná basin, and subsequently placed in the genus Porotergus by Mago-Leccia (1994). Sternarchella curvioperculata was not found to share characters with other Sternarchella species or Magosternarchus in a subsequent morphological study by Triques (2005)Triques, M. L. 1993. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia, 6: 85-130.. Sternarchella orthos and S. orinoco were described by Mago-Leccia (1994) from localities in Orinoco basin, Venezuela. Sternarchella calhamazon was recently described by Lundberg et al. (2013) as the most abundant species of apteronotid electric fish in the Amazonian river channels.
Understanding the phylogeny and historical biogeography of Sternarchellini will contribute to answering some important questions in Neotropical Ichthyology. How did so many apteronotid species come to inhabit the relatively small habitat space presented by deep river channels of lowland Amazonia? Does the deep channel fauna represent a case of adaptive radiation by means of ecological specialization? What are the relative roles of geographic isolation and ecological specialization in the origin of these species, and in the formation of the basin-wide assemblages? Here we provide a phylogenetic revision of the Sternarchellini from examination of specimens representing all known species, and use the results to help interpret its history of biogeographic and adaptive evolution.
Material and Methods
Material used in this study included 69 museum lots with 239 specimens, including 57 lots of ingroup taxa with 220 specimens. Specimens examined are listed in Appendix 1 APPENDIX 1 Specimens examined for morphometrics and osteology. . Museum abbreviations are: American Museum of Natural History (AMNH), Academy Natural Sciences, Philadelphia (ANSP), Field Museum of Natural History (FMNH), Museum University San Marcos (MUSM), California Academy of Sciences-Stanford University (CAS-SU), University of Florida (UF), University of Michigan Museum of Zoology (UMMZ), and United States National Museum (USNM).
External characters, morphometrics, and meristics, were examined from specimens of 16 apteronotid species, all originally fixed in 10% formalin and preserved in 70% ethanol. Morphometric methods were modified for Sternarchella species from Albert (2001). Morphometric measurements are: total length (TL), length to the end of the anal fin (LEA), anal fin length (AF), head length (HL), preorbital distance (PR), eye diameter (ED), postorbital distance (PO), interorbital distance (IO), mouth width (MW), head depth through the nape (HD1), head depth through the eye (HD2), head width (HW), length from anus to anal-fin origin (PA) (Fig. 1); body depth through end of the body cavity (BD), and body width at the end of the body cavity (BW) are not depicted in Fig. 1. Specimens with damaged or incompletely regenerated tails were not measured for TL. Due to proportion of specimens with incompletely regenerated tails, HL was used as the standard measure of overall specimen size. PC1 may also be used as a measure of overall body size. Morphometric data are summarized in Table 2.
Line drawing of the holotype of Sternarchella sima (SU 22220), illustrating landmarks used in morphometric analysis.
Principal Components Analyses (PCA) including specimens of all Sternarchella species were conducted with the software package PAST (Hammer et al., 2001 Hammer, Ø., D. Harper & P. Ryan. 2001. PAST-Palaeontological statistics. /~ pardomv/pe/2001_1/past/pastprog/past. ) in order to understand morphometric variation and to discover phenotypic discontinuities in a multivariate morphospace. Twelve log-transformed morphometric characters were used in the PCA. Total length, LEA, and AF measurements were not used due to the occurrence of incompletely regenerated tails in several specimens. The first principal axis (PC1) variable loadings were all positive and varied little in magnitude. PC1 was inferred as a general size factor (Jolicoeur & Mosimann, 1960Jolicoeur, P. 1963. The Multivariate Generalization of the Allometry Equation. Biometrics, 19: 497-499. ; Jolicoeur, 1963; McElroy & Douglas, 1995McElroy, D. & M. Douglas. 1995. Patterns of morphological variation among endangered populations of Gila robusta and Gila cypha (Teleostei: Cyprinidae) in the upper Colorado River basin. Copeia, 1995: 636-649. ).
Clearing and staining for bone and cartilage followed the procedure of Taylor & Van Dyke (1985)Taylor, W. R. & G. Van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9: 107-119.. The following modification to the clearing and staining procedure was utilized to manage the high lipid content of many Sternarchellini: before starting the protocol for clearing and staining, specimens were placed in xylene for one day. Specimens were then washed in three separate 95% ethanol baths to remove xylene from tissues. Specimens were subsequently transferred directly to Alcian Blue solution to maximize uptake of the hydrophobic stain. Cleared and stained specimens were stored in a final solution of 70% glycerol (with thymol). Dissection of cleared and stained specimens followed the method outlined by Weitzman (1974)Weitzman, S. 1974. Osteology and evolutionary relationships of the Sternoptychidae, with a new classification of stomiatoid families. Bulletin of the American Museum Natural History, 153: 327-478. using microdissection tools under an Olympus SZX - 12 dissecting stereomicroscope equipped with a camera lucida. Bones were disarticulated to functional groups (neurocranium, suspensorium, pectoral girdle) or to individual bony elements. Specimens from a total of 16 species were dissected and coded for phylogenetic analysis. Cleared and stained specimens were examined and illustrated following conventions for gymnotiform osteology (Albert, 2001). Outlines and standardized features of each bone were traced in lateral and medial views, and images were digitized using an Epson Perfection V300 scanner and edited in Adobe Photoshop and Illustrator. Illustrations of the neurocranium in dorsal, lateral, and ventral views are provided in Figs. 2-3, and of the suspensorium in lateral view in Figs. 4-5.
Diagrammatic representation of neurocranium of Sternarchella terminalis (MUSM 45236, 235 mm TL). A. Dorsal view. B. Lateral view. C. Ventral view. Scale bar = 5 mm.
Diagrammatic representations of neurocrania in sternarchelline species. Dorsal, lateral, and ventral views in left, middle and right columns, respectively. Species arranged from top to bottom: A. Pariosternarchus amazonensis, ANSP 192996. B. Magosternarchus raptor, UF 116762. C. Sternarchella terminalis, MUSM 45236. D. S. orthos, USNM 228725. E. Sternarchella n. sp. A. F. Sternarchella schotti, UF 11657. G. S. calhamazon, MUSM 45234. H. S. sima, ANSP 192107. I. S. orinoco, USNM 228727. Note neurocrania range from rounded and gracile (paedomorphic) at top, to elongate and robust (peramorphic) at bottom. Scale bars = 5 mm.
Lateral view of suspensorium in selected sternarchelline species. A. Sternarchella schotti, UF 116570. B. S. terminalis, MUSM 45236. C. Magosternarchus raptor, UF 116762. Scale bars = 3 mm.
Lateral view of suspensorium in selected sternarchelline species. A. Sternarchella calhamazon, MUSM uncat. B. S. sima, ANSP 192107. C. Pariosternarchus amazonensis, UF 129334. Scale bars = 3 mm.
Characters were selected based on their phylogenetic informativeness (Pimentel & Riggins, 1987Pimentel, R. & R. Riggins. 1987. The nature of cladistic data. Cladistics, 3: 201-209. ) and analyzed using Maximum Parsimony (MP). Osteological nomenclature follows Patterson (1975)Patterson, C. 1975. The braincase of pholidophorid and leptolepid fishes, with a review of the actinopterygian braincase. Philosophical Transactions of the Royal Society, London (B), 53: 275-579. and Albert (2001). All characters were coded from mature specimens, inferred from the degree of ossification in the sphenoid region of the neurocranium and coracoid region of the pectoral girdle (Albert, 2001). The data matrix is provided in Appendix 2 Appendix 2 Data matrix used in phylogenetic analysis (P indicates states 0&1). Characters and states defined in Appendix 4. . The general principles of phylogenetic systematics outlined by Hennig (1966)Hennig, W. 1966. Phylogenetic Systematics. University of Illinois Press, Urbana, IL. and Wiley (1981)Wiley, E. O. 1981. Phylogenetics. The theory and practice of phylogenetic systematics.. John Wiley & Sons New York. were employed during parsimony analysis. Microsoft Excel (2010) and MacClade 4.08 PPC (Madison & Madison, 2005Madison, W. P. & D. R. Madison. 2005. MacClade, Analysis of Phylogeny and Character Evolution, version 4.08. Sunderland, Sunderland Associated, Inc, Massachusetts.) software packages were used to assemble a data matrix of 16 taxa and 70 morphological characters. Character states were polarized using six apteronotid outgroup species, as per the tree topology of Albert (2001).
A heuristic search with Tree-Bisection-Reconnection (TBR) algorithm was performed using PAUP* v. 4.0 b10 (Swofford, 2003Swofford, D. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (* and Other Methods). Version 4. Sinauer Associates, Sunderland, MA.) in the parsimony analysis. All multistate characters were treated as unordered. Bremer Support (Bremer, 1988Bremer, K. 1988. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution, 42: 795-803. ) was conducted using TNT (Goloboff et al., 2008Goloboff, P., J. Farris & K. Nixon. 2008. TNT, a free program for phylogenetic analysis. Cladistics, 24: 774-786. ) to assess branch support, using 1000 replicates. A bootstrap analysis (resampling characters with replacement; Hillis & Bull, 1993Hillis, D. M. & J. J. Bull. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology, 42: 182-192. ) was also performed to assess branch support, using 1000 replicates. Consistency (CI) and retention indexes (RI) are provided as measures of character fit to a given tree topology (Farris, 1989Farris, J. 1989. The retention index and the rescaled consistency index. Cladistics, 5: 417-419. ).
Results
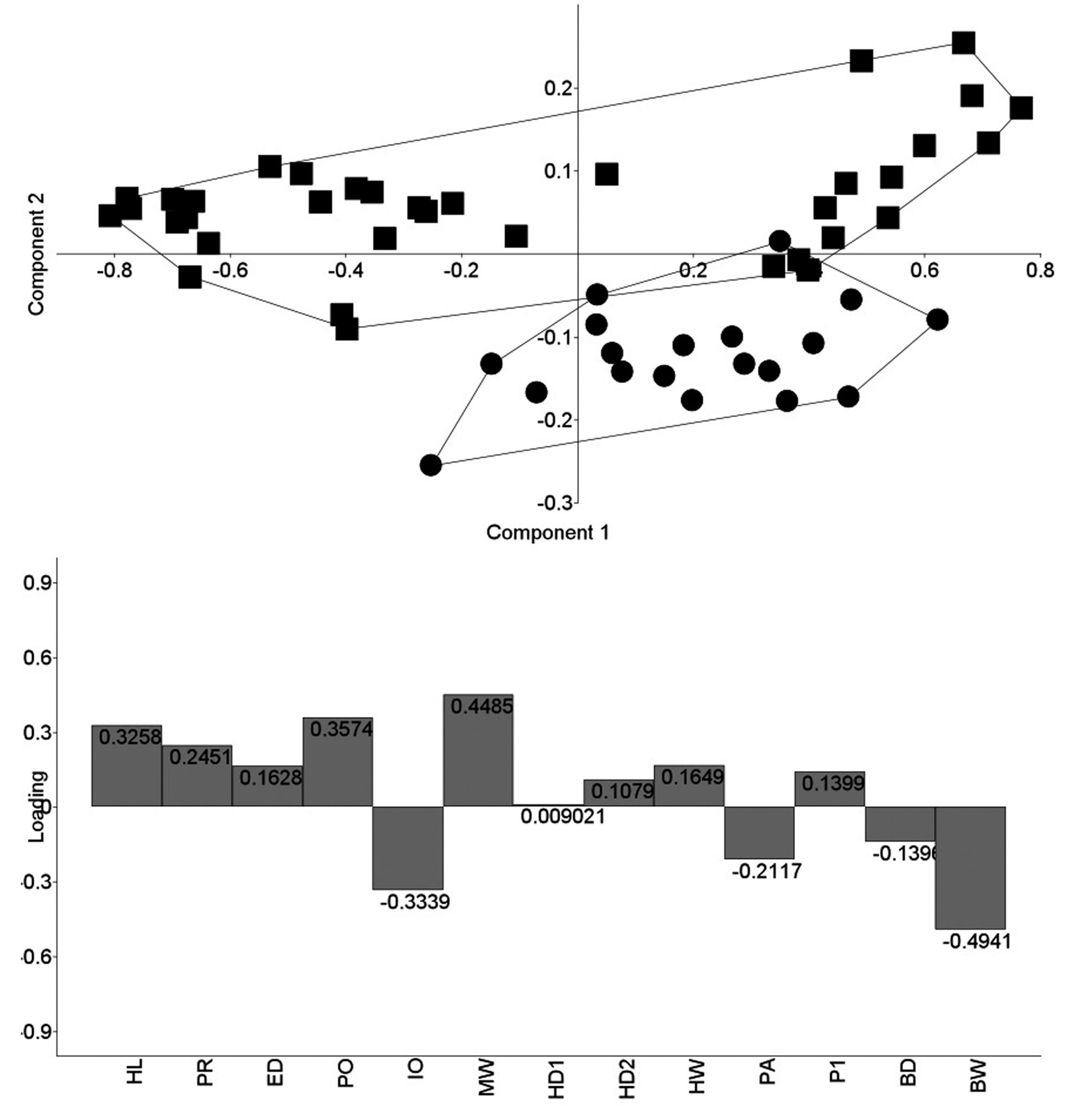
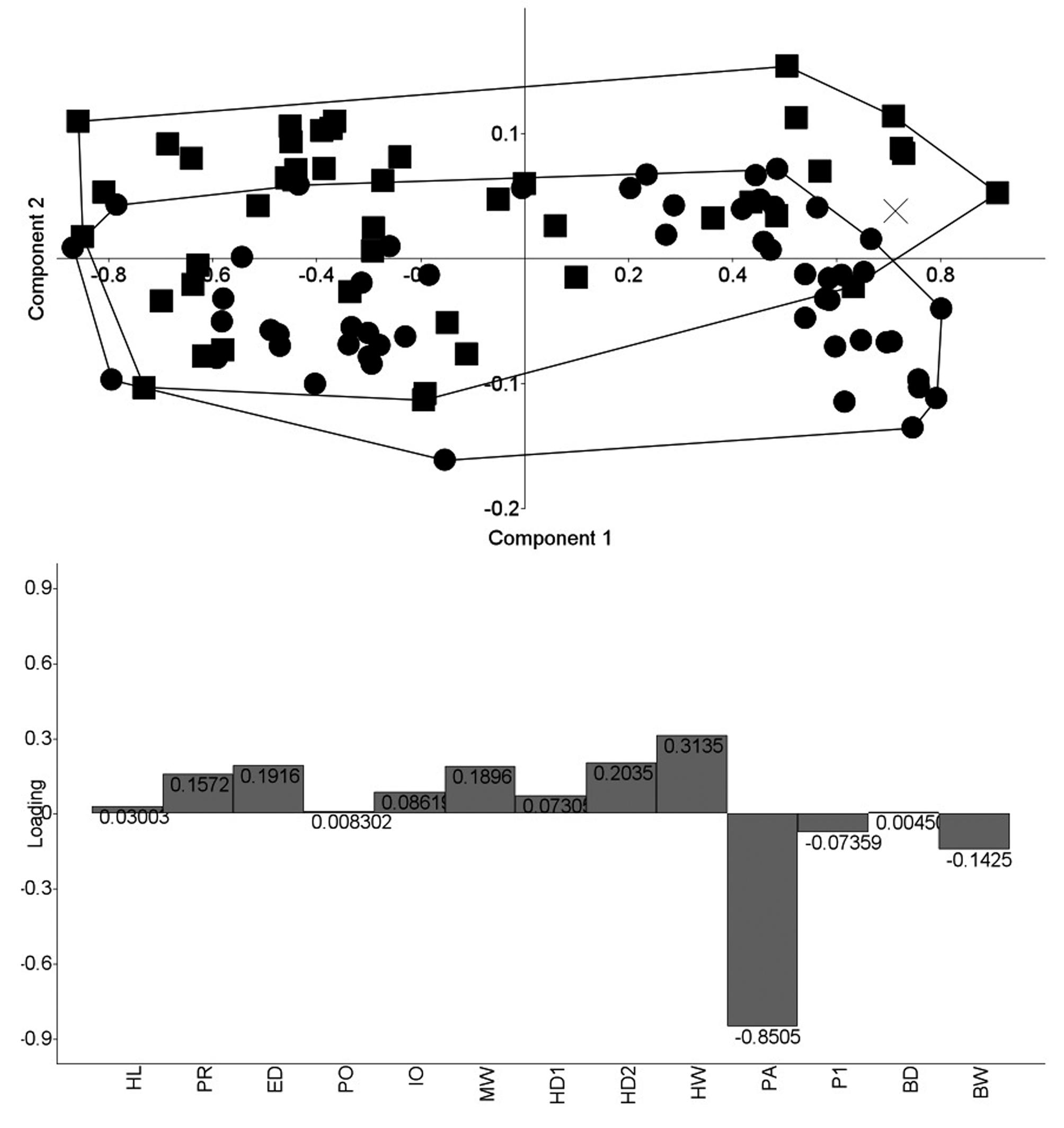
Three separate PCAs were run on the morphometric data set. The first analysis examined only the species present in the rio Amazon basin (Fig. 6). The results of this analysis indicate that Sternarchella sima, S. schotti, and S. calhamazon do not overlap in the multivariate morphospace. However, S. terminalis does partially overlap in morphospace with some sympatric species. Morphological differences between S. sima and S. orinoco are evident from an almost complete segregation of the two species on the second PCA axis (Fig. 7). These differences are observed in the loading factors, which is dominated by two traits of head and body width: S. sima has more widely-set eyes (IO) than S. orinoco, and a broader body at the posterior margin of the body cavity (BW) (see also Table 2). The third PCA examined S. terminalis and S. orthos and recovered no distinguishable differences in PC1 or PC2 (Fig. 8). Only a single character was found in this study to be useful in differentiating S. terminalis from the Amazon basin and S. orthos from the Orinoco basin: large scales above the lateral line (5-6 SAL) in S. orthos (see Mago-Leccia, 1994:85), vs. small scales (7-9 SAL) in S. terminalis.
Scatter plot of PC2 and PC3 for specimens representing four Sternarchella species from the Amazon basin (n= 126). Loadings of the 13 variables reported in lower panel. Sternarchella schotti represented as squares (n= 10), S. sima as circles (n= 19), S. terminalis as Xs (n=45), S. n. sp. 1 as triangles (n= 52). Note this assemblage is not monophyletic. Morphometric data reported in Tables.
Scatter plot of PC1 and PC2 for 56 specimens representing species in the S. sima group. Loadings of all 13 variables for PC2 reported in lower panel. Sternarchella sima represented as circles (n= 19), and S. orinoco as squares (n= 37). Note S. sima and S. orinoco are readily separated by IO, MW, and BW.
PCA scatter plot of species in the S. terminalis species group. Loadings of all 13 variables for PC2 reported in lower panel. Sternarchella terminalis represented as squares (n= 45), S. orthos as circles (n= 54), and Sternarchella sp. A represented as an X (n= 1).
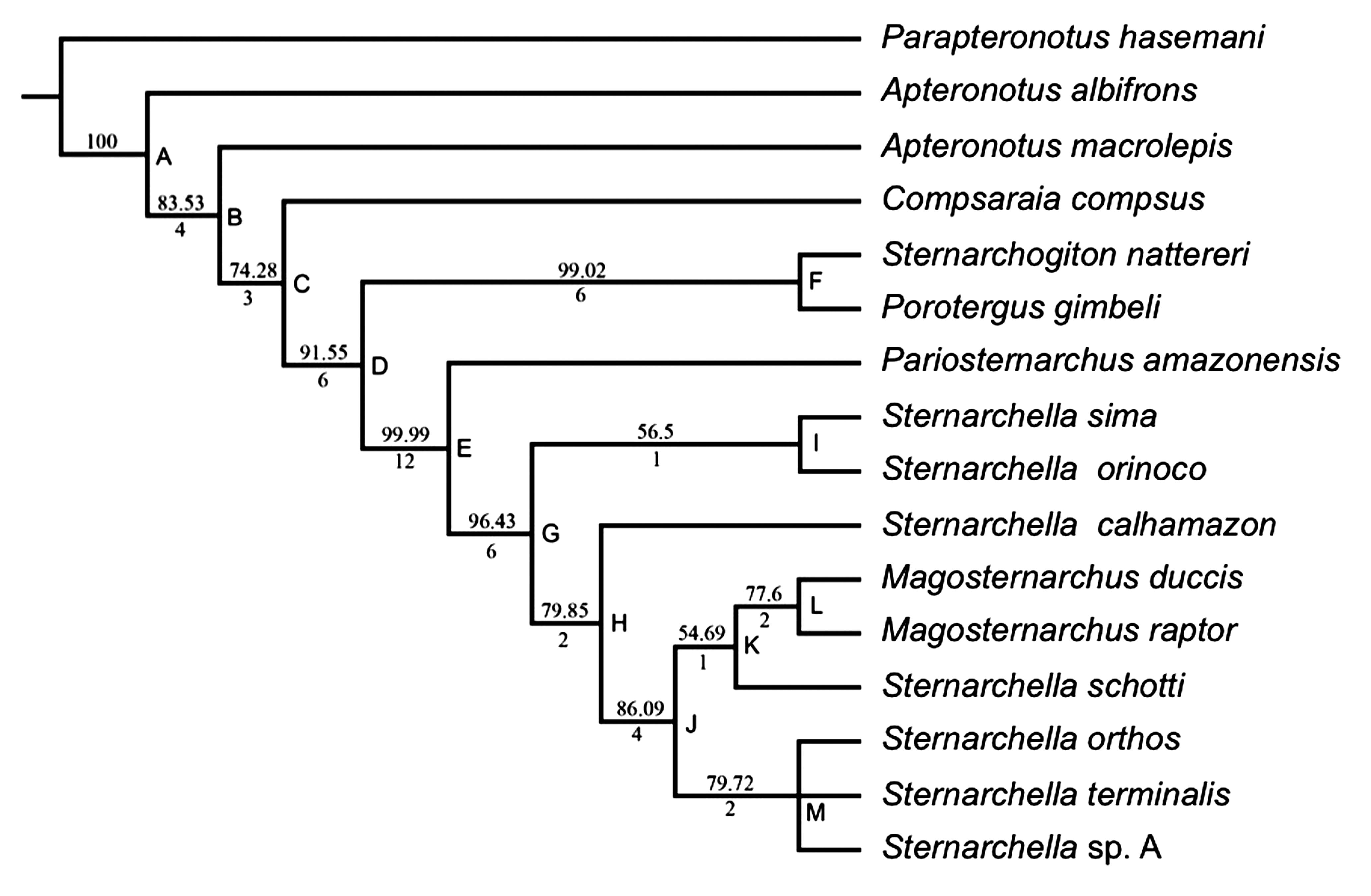
Two equally parsimonious trees of 138 steps were recovered in the analyses. In one of them, Sternarchella n. sp. A is sister to S. terminalis, and in the other Sternarchella sp. A is sister to S. orthos. A strict consensus places Sternarchella sp. A in a trichotomy with S. terminalis and S. orthos (Fig. 9). Sternarchella is paraphyletic according to the phylogenetic analysis, since it includes M. raptor and M. duccis. Relatively high Bremer support and bootstrap values, which provide further confidence in the monophyly of Sternarchellini and more inclusive clades, are presented in Fig. 9. A summary of character state changes is provided in Appendix 3 Appendix 3 Summary of character state changes on phylogeny of Figure 9. , and brief descriptions of the characters and character states are provided in Appendix 4 Appendix 4. Characters and character states descriptions. .
Phylogenetic tree of Sternarchellini and closely related apteronotine species resulting from MP analysis of the data matrix in Appendix 3 Appendix 3 Summary of character state changes on phylogeny of Figure 9. . Bootstrap values present above nodes and Bremer support values below nodes.
Clade diagnoses
Here we provide diagnoses for eight clades, with clades names referring to lettered nodes in Fig. 9.
Clade E is the Sternarchellini, which consists of all Sternarchella, Pariosternarchus, and Magosternarchus species. Monophyly of this clade is supported by 12 characters: MW at least 18% HL; anterior shelf of maxilla absent (except in Magosternarchus), dentary longer than deep, dorsal margin of dentary slightly concave, supraoccipital crest exceeding dorsal margin of parietals, supraorbital canal not fused to frontals, endopterygoid process long contacting or fused to frontals, dorsal margin of opercle concave, first basibranchial fan or rod shaped as opposed to hour-glassed shaped except in S. calhamazon, basibranchial two not ossified except in S. schotti, fourth epibranchial post-medial bridge present, urohyal blade unossified or poorly ossified except for S. terminalis species group.
Clade G is comprised of all Sternarchella and Magosternarchus species, and its monophyly is supported by six characters: premaxilla large in size, wider than maxilla, anterior hook of maxilla absent, ventral margin of descending blade of maxilla rounded, as opposed to straight (except in M. raptor), ventral ethmoid large and robust with a large fan shaped lateral process, dorsal-medial portion of orbitosphenoids in contact, seven or less large robust teeth present on hypobranchial 6.
Clade H is comprised of S. schotti, S. calhamazon, Magosternarchus, and the "Sternarchella terminal mouth species group", consisting of S. terminalis, S. orthos, and S. sp. A. The monophyly of this clade is supported by four characters: terminal or superior mouth placement, as opposed to subterminal, premaxilla triangular in shape, tip of endopterygoid process broad, and twelve or less teeth present on the pharyngobranchial, except in S. schottiand S. orthos. Unlike other Sternarchellini species, S. calhamazon possess an hour-glassed shaped first basibranchial. Sternarchella calhamazon also has a superior mouth.
Clade J consists of Magosternarchus, S. schotti, and the Sternarchella terminal mouth species group (Clade H), and its monophyly is supported by five characters: IO less than 20% HL, anterior portion of mesethmoid straight in lateral view, lateral ethmoid large and robust with an hourglass shape, supraoccipital crest deeply concave with a dorsally-oriented process at posterior margin, as opposed to an elongate blade, endopterygoid process forming an oblique angle with plane of endopterygoid.
Clade K is comprised of Magosternarchus and S. schotti and its monophyly is supported by a single unambiguous character state: presence of 14 or more precaudal vertebra. This clade is not well supported, with bootstrap values below 70%, and a Bremer support value of 1. Sternarchella schotti is unique among Sternarchellini species in having an eye diameter (EO) 8% or more of head length (HL), a mouth width (MW) less than 18% HL, a large flat space between reduced lateral parietal ridges, an ossified second basibranchial, 14 or more teeth present on the pharyngobranchial, and an elongate swim-bladder which extends posteriorly past the body cavity.
Clade I is the Sternarchella sima species group, comprised of S. sima and S. orinoco, and its monophyly is supported by four character states: PO large, over 67% HL, pectoral fin large, over 80% HL, four distinct rows of teeth present on the premaxilla, and three to four rows of teeth present on the dentary. Species of that clade also possesses ventrally placed mouths. Within the Sternarchella sima species group, S. sima and S. orinoco are distinguishable from one another by two characters: S. orinoco has a more narrow head (IO less than 20% HL), while S. sima has a broader head (IO larger than 20% HL). Sternarchella orinoco also has a longer body than S. sima, and a BW less than 21% HL, whereas S. sima has a wider body with a BW greater than 21% HL. The overall difference in morphology between S. sima and S. orinoco is represented in a PCA, which largely do not overlap in the morphospace (Fig. 7).
Clade L is comprised of the two Magosternarchus species. The monophyly of Clade L is supported by four characters: absence of a short gape, anterior shelf of maxilla present, anterior fontanel shorter than posterior fontanel, and a narrow orbitosphenoid. Magosternarchus raptor is diagnosed by six characters: PR greater than 35% HL, BW less than 21% HL, terminal mouth position, ventral margin of the descending blade of the maxilla strait, as opposed to curved (which is the state in other Sternarchellini species), ventral process of the pterosphenoid present, and width of opercle about half as deep. Magosternarchus raptor is easily distinguished from other sternarchelline species by larger, more robust oral jaws. Magosternarchus duccis possess a small PO, unlike any other sternarchelline species. Magosternarchus duccis is readily distinguished from M. raptor by a superior mouth.
Clade M is the Sternarchella terminal-mouth species group, and is comprised of S. terminalis, S. orthos, and S. calhamazon. The monophyly of Clade M is supported by three character states: terminal mouth position, three rows of teeth on premaxilla, and urohyal blade unossified or poorly ossified. A single character distinguishes S. terminalis from the other two species with a terminal mouth: a narrow orbitosphenoid.
Discussion
Taxonomic status of Sternarchella from the Orinoco basin
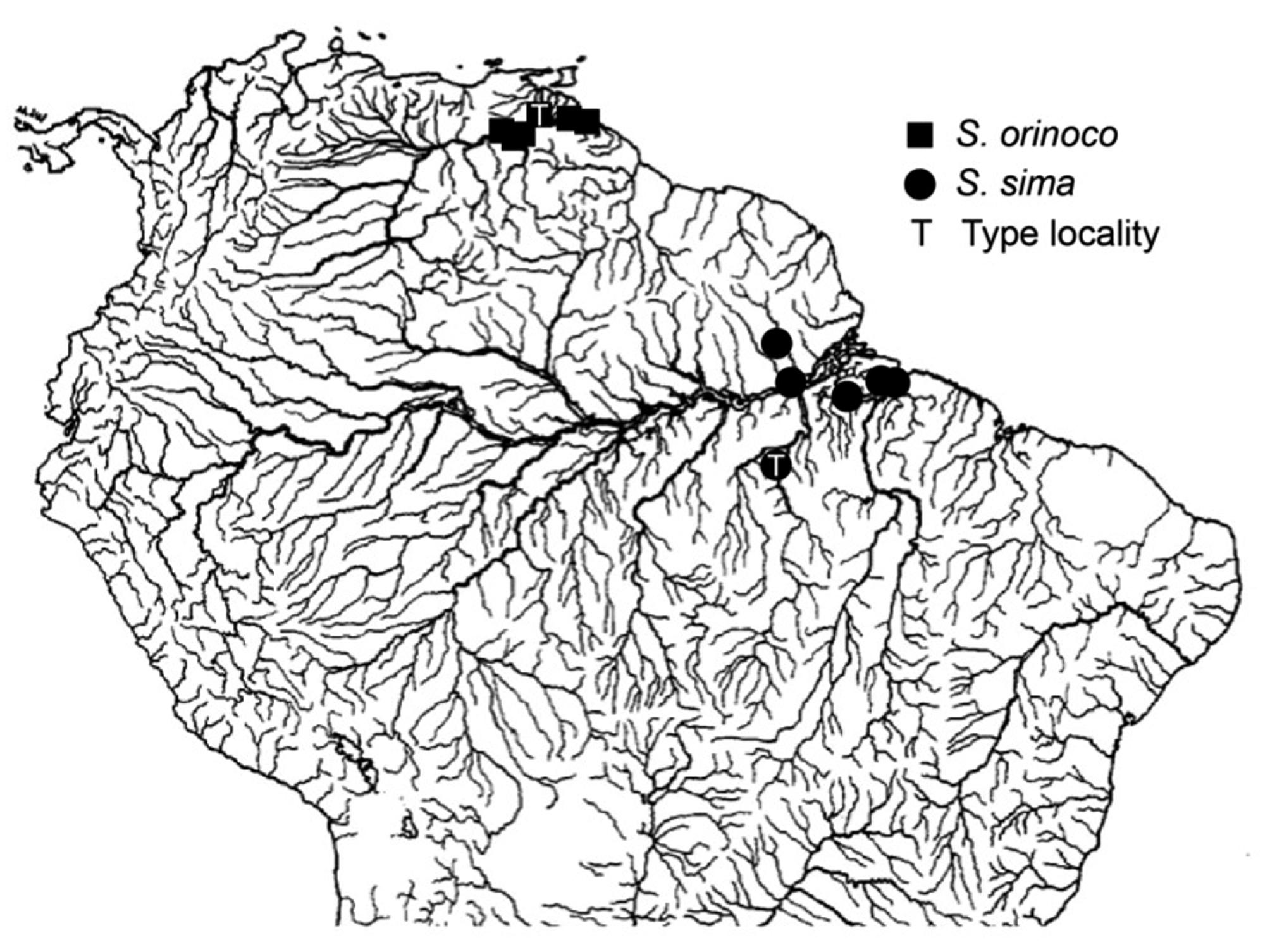
Results of the PCA analyses indicate that Sternarchella sima and S. orinoco exhibit distinct phenotypes (Fig. 7), whereas S. terminalis and S. orthos are morphologically indistinguishable according to the characters examined (Fig. 8). A preliminary analysis using geometric morphometric of head neurocranial shape in lateral views recovered similar results (K. Evans and J. Albert, pers. obs.), finding statistically significant differences between S. sima and S. orinoco (P < 0.001), and no significant differences between S. terminalis and S. orthos (P = 0.1037). The osteological data reviewed here indicate that S. orthos has a narrower orbitosphenoid (character 33) and more pharyngobranchial teeth (character 59) than S. terminalis (14 or more vs. 13 or less). However, both of these characters may have dubious taxonomic value, being variable within many apteronotid species (Albert, 2001), and exhibiting relatively high homoplasy on the tree of Fig. 9(CI = 0.25 and 0.20, respectively). The reliability of these characters as diagnostic traits for S. terminalis therefore needs to be tested with larger sample sizes. Given the relatively poor geographic sampling of individuals from across the large range of these species, it seems premature to advance any formal nomenclatural changes, and we provisionally recognize S. orinoco and S. orthos from the Orinoco basin as valid species.
Origin of the deep-channel electric fish fauna
The Sternarchellini represents an excellent taxon in which to study the contributing roles of geography and ecology in the formation of the diverse and specialized deep-channel Neotropical fish fauna. In some clades of deep-channel apteronotids, several closely-related species occur together in sympatry and syntopy, such as in Adontosternarchus, Porotergus, Sternarchella, Sternarchogiton, and Sternarchorhynchus (Crampton & Albert, 2006; Crampton et al., 2011). Other gymnotiform clades are also diverse in the deep-channel habitat, including the sternopygids Eigenmannia and Rhabdolichops, and the rhamphichthyid Rhamphichthys (Albert et al., 2011b; Carvalho, 2013Carvalho, T. P. 2013. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): diversification in South American freshwaters. Unpublished Ph.D. Dissertation, University of Louisiana at Lafayette, 516p. ). The presence of multiple sympatric congeners within this habitat, including several instances of sister-species pairs, is unusual among Neotropical fishes, where most sister-species pairs are distributed in allopatry (Albert & Crampton, 2001; Albert & Reis, 2011Albert, J. S., T. Carvalho, P. Petry, M. A. Holder, E. Maxime, J. Espino, I. Corahua, R. Quispe, B. Rengifo, H. Ortega & R. E. Reis. 2011a. Aquatic biodiversity in the Amazon: habitat specialization and geographic isolation promote species richness. Animals, 1: 205-241. ; Albert et al., 2011aAlbert, J. S., T. Carvalho, P. Petry, M. A. Holder, E. Maxime, J. Espino, I. Corahua, R. Quispe, B. Rengifo, H. Ortega & R. E. Reis. 2011a. Aquatic biodiversity in the Amazon: habitat specialization and geographic isolation promote species richness. Animals, 1: 205-241. ). The Sternarchellini is part of the diverse assemblage of apteronotid fishes that inhabits the large rivers of tropical South America (Mago-Leccia et al., 1985; Lundberg & Lewis, 1987). A high proportion (70 of 85, or 82%) of apteronotid species are restricted to the deep channels of the Amazon and Orinoco rivers and their large tributaries. This concentration of species in the deep channels is notable considering the small proportion (2.6%) of the total bottom area that these channels occupy in the tropical South America (Goulding et al., 2003Goulding, M., R. Barthem, E. Ferreira & R. Duenas. 2003. The Smithsonian Atlas of the Amazon. Smithsonian Books, Washington, D.C.; Winemiller & Willis, 2011 Winemiller, K. O. & S. Willis. 2011. The Vaupes Arch and Casiquiare Canal. Pp. 225-242. In: Albert, J. S. & R. E. Reis (Eds.). Historical Biogeography of Neotropical Freshwater Fishes. University of California Press, Berkeley.). Even accounting for the larger volume of large rivers (stream orders 6-10), the total amount of habitat space they occupy is small compared to that of all the small rivers and streams (stream orders 1-5) combined, that drain more than 11 million km2 (Albert & Crampton, 2005aAlbert, J. S. & W. G. R. Crampton. 2005a. Diversity and phylogeny of Neotropical electric fishes (Gymnotiformes). Pp. 360-409. In: Electroreception. T. H. Bullock, C. D. Hopkins, A. N. Popper & R. R. Fay (Eds.). Springer Handbook of Auditory Research, Volume 21, Springer-Verlag, Berlin. ; Crampton & Albert, 2006; Crampton et al., 2011).
The origin of deep-channel habits by the Navajini, including the Sternarchellini, may have occurred before the separation of the modern Amazon and Orinoco basins, an event that followed the rise of the Vaupes Arch in the Late Miocene (ca. 10-8 Ma; Dobson, 2001Dobson, D. M., G. R. Dickens, & D. K. Rea. 2001. Terrigenous sediment on Ceara Rise: a Cenozoic record of South American orogeny and erosion. Palaeogeography, Palaeoclimatology, Palaeoecology, 165: 215-229. ; Winemiller & Willis, 2011). The Navajini includes several clades with multiple sister-species pairs and other supraspecific taxa distributed across the Amazon-Orinoco divide. Clades with multiple multispecies assemblages co-exist in both basins (Albert et al., 2011; Albert & Carvalho, 2011). The Amazon and Orinoco basins are connected on the modern landscape via the Casiquiare Canal, but this waterway probably does not act as a dispersal corridor for most sternarchelline fishes. Sternarchellines are lowland species, and to date no sternarchelline species have been collected from within the Casiquiare Canal or the rivers connected to it above the rapids around the base of Guiana Shield, in either the upper rio Negro or upper río Orinoco basins (i.e., rapids at São Gabriel and Puerto Ayacucho, (Winemiller & Willis, 2011).
The phylogenetic position of Magosternarchus, nested within Sternarchellini, helps explain the evolution of the extreme phenotypic, behavioral, and ecological specializations of these deep-channel species. The large jaws and robust dentition of Magosternarchus are used in predation, in particular on the tails of other gymnotiforms (Lundberg et al., 1996). Previous studies concluded that Magosternarchus is the sister taxon of the clade composed of Sternarchella (Lundberg et al., 1996), or of Sternarchella and Pariosternarchus (Albert & Crampton, 2006Albert, J. S., N. R. Lovejoy & W.G.R. Crampton. 2006. Miocene tectonism and the separation of cis- and trans-Andean river basins: evidence from Neotropical fishes. Journal of South American Earth Sciences, 21: 14-27. ). Under these hypotheses the large jaws, terminal or superior mouth position, and aggressive behaviors of some Sternarchella species are interpreted to have independently evolved from the conditions observed in Magosternarchus. However, according to our phylogenetic results, the genus Sternarchella is found to be paraphyletic, including the two species currently assigned to the genus Magosternarchus (Fig. 9). This result suggests that the large jaws, terminal or superior mouth positions and aggressive predatory behaviors of some Sternarchella species and Magosternarchus are homologous. Five characters support a relationship between Magosternarchus, S. schotti and the S. terminalis species group: IO less than 20% HL (CI=0.25), anterior mesethmoid straight in lateral view (CI=0.50), lateral ethmoid large and hour-glass shaped (CI=1.00), supraoccipital crest dorsal process (CI=1.00), and endopterygoid process oblique in orientation (CI=0.50).
Paleogeography and the origin of species in the Sternarchellini
The paleogeography and geological timeframe over which sternarchelline species originated is poorly constrained, and a time-calibrated phylogeny for the group is not yet available. However, the tree topology (Fig. 9) and geographic distributions (Figs. 10-12) of sternarchelline species do invite inferences regarding aspects of diversification in this clade (Fig. 13). Divergence times of the two Amazon-Orinoco species pairs (S. sima + S. orinoco; S. terminalis + S. orthos) before 10 Ma would imply multiple vicariance events across Vaupes Arch (Fig. 13, right). Alternatively, divergence times of these species pairs after 10 Ma would imply multiple dispersal events across the newly formed Vaupes Arch (Fig. 13, left).
Distribution map of examined lots of two Sternarchella species. Sternarchella schotti represented as circles, and S. calhamazon as squares. Type locality denoted by T.
Distribution map of examined lots of the S. sima group. Sternarchella sima represented by circles, and S. orinoco, by squares. Type localities denoted by T.
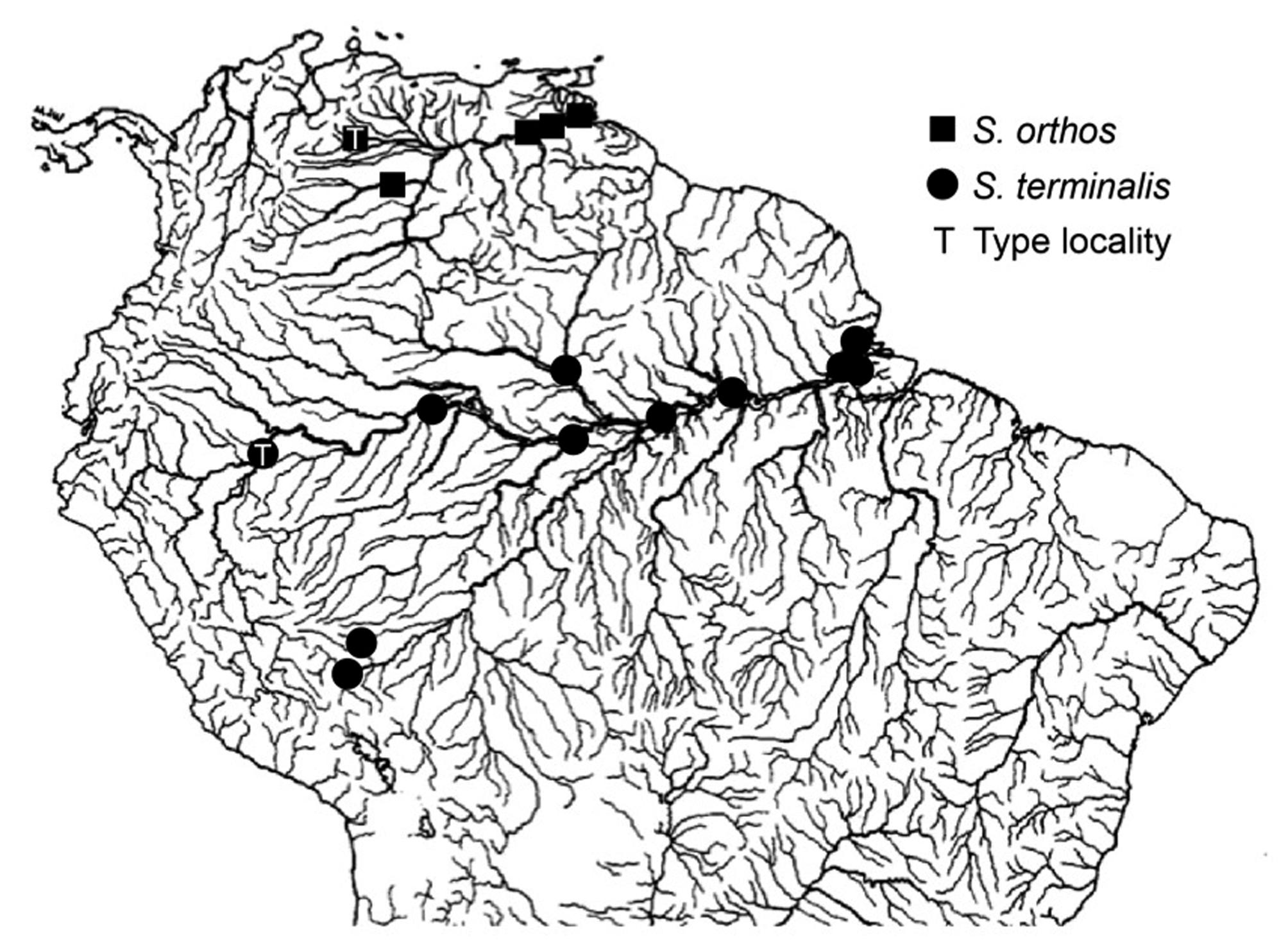
Distribution map of examined lots in the Sternarchella terminal-mouth species complex. Sternarchella terminalis represented as circles, S. orthos as squares. Type localities denoted by T.
Alternative time frames for divergences of sternarchelline taxa across the Vaupes Arch. A. Younger divergence (Late Miocene-Pliocene) with multiple dispersal events (red lines) across Vaupes Arch (dashed black line). B. Older divergences (Middle Miocene or older) with multiple vicariance events (red lines) across Vaupes Arch. Tree topology depicted in Fig. 9. Colored regions depict approximate limits of Middle Miocene Amazonian watersheds separated by Purus Arch. Blue: Proto- Orinoco Amazon; Green: Eastern Amazon. Base map by M. Weitzman.
Under a history with more ancient (Middle Miocene or older) divergence times (Fig. 13, right), the origin of the modern basin-wide sternarchelline species-assemblages accompanied the Late Miocene rise of the Vaupes Arch and concomitant breaching of the Purus Arch. If, as we hypothesize above, the Sternarchellini was already present by the Middle Miocene or before, its early divergences could have been affected by the breaching of the Purus Arch (ca. 10-8 Ma), in which the modern Eastern Amazon basin captured the modern Western Amazon basin from the lower proto-Amazon-Orinoco basin (= modern Orinoco basin). The north-flowing proto-Amazon-Orinoco (i.e., Subandean) basin, which drained into the Caribbean sea, was the major drainage system of northern South America for most of the early Cenozoic (Lundberg et al., 1998). The presence of two sister-species pairs in the lower portions of the modern Amazon and Orinoco rivers, but not in the Casiquiare Canal or other rivers above the fall line of the Guiana Shield, supports this more ancient, vicariance, hypothesis.
Under the more ancient divergence scenario, inferences can be made about possible extinctions in the area of the modern Orinoco basin. Several sternarchelline taxa (Pariosternarchus, Magosternarchus, S. schotti, and S. calhamazon) occur today only in the Amazon basin. Based on the tree topology presented in Fig. 9, it is possible that some or all of these taxa originated before the rise of the Vaupes Arch, the event that separated the modern Orinoco and Amazon basins ca. 10 Ma (Lovejoy et al., 2010 Lovejoy, N. R., S. Willis & J. S. Albert. 2010. Molecular signatures of Neogene biogeographical events in the Amazon fish fauna. Pp. 405-417. In: C. M. Hoorn & F. Wesselingh (Eds.). Amazonia: Landscape and Species Evolution. A Look into the Past. Wiley-Blackwell; Chichester, Oxford and Hoboken. ). Under these conditions, taxa currently absent from the modern Orinoco basin may have been present along the longitudinal extent of the proto-Amazon-Orinoco river system. The hypothesis of widespread extinction of fish taxa from the lower (northern) portions of the proto-Amazon-Orinoco is indicated by the presence of fossilized plates and fin spines of freshwater stingrays (Potamotrygon), lungfish (Lepidosiren), pirarucu (Arapaima), and several families of riverine catfishes from the La Venta Formation (río Magdalena) of Colombia (Lundberg et al., 1998; Albert et al., 2006) and Urumaco Formation (Maracaibo basin) of northern Venezuela. Both formations are located near the paleomouth of the protoAmazon-Orinoco River during the middle Miocene (10-12 Ma). Albert et al. (2011b: 53) reported 91 genera of fishes endemic to the modern Amazon basin that are, by definition, excluded from the modern Orinoco basin. Many of these genera are known as fossils from the Miocene Urumaco Formation, and are now entirely extinct in the modern Orinoco.
Several apteronotid taxa found only in the deep channels of the modern Amazon basin are candidates for having once been present, and later having become extinct, in the area of the modern Orinoco basin. These taxa include Parapteronotus, Pariosternarchus, Magosternarchus, and Orthosternarchus. The absence of these apteronotid taxa in the La Venta and Urumaco Formations should not to be taken as strong evidence that they were in fact absent from these regions. Amazonian lowlands are an exceptionally poor substrate for fossilization, with few ancient (non-floodplain) lakes, and high rates of bioturbation. Most groups of aquatic organisms that live in tropical rainforests are not represented in the fossil record (Lundberg et al., 1998; Lovejoy et al., 2010). Gymnotiformes in general are poorly ossified, lacking plates, spines, or other hard tissues that might become fossilized, and the order as a whole is known as fossils from a few body fragments in just a single locality in Bolivia (Albert & Fink, 2007). Finally, deep-channel apteronotids like Sternarchellini are especially unlikely to be preserved as fossils, with a highly demineralized endoskeleton that develops a finely reticulated mesh-like texture during growth (Albert, 2001).
Another possible interpretation for the absence of these sternarchelline taxa in the modern Orinoco basin is that they were simply never present in the northern (lower) portion of the proto-Amazon-Orinoco River, i.e., the llanos basin. There is sedimentological evidence for the presence of several marine incursions into the continental interior during the interval 20-10 M (Lovejoy et al., 2006; Wesselingh & Hoorn, 2011 Wesselingh, F. P. & C. Hoorn. 2011. Geological development of Amazon and Orinoco basins. Pp. 59-67. In: J. S. Albert & R. E. Reis (Eds.). Historical Biogeography of Neotropical Freshwater Fishes. UC Press, Berkeley. ). These marine incursions may have resulted in local extinctions of strictly freshwater taxa in the northern portion of the proto-Amazon-Orinoco basin, or they may have formed a biogeographic barrier or filter between the northern and southern portions of this basin.
However, a history with less ancient (Middle Miocene or younger) divergence times for the two sternarchelline sister-species pairs (i.e., S. sima and S. orinoco, S. terminalis, and S. orthos) would imply multiple dispersal events from the Amazon to Orinoco basins (Fig. 13, left). Under this time-frame there is no need to hypothesize extinction events in the Orinoco basin. The Vaupes Arch is indeed a permeable barrier to dispersal for some deep-channel gymnotiform species (e.g., Adontosternarchus balaenops, Sternarchorhamphus muelleri, Steatogenys elegans, Rhamphichthys rostratus), which are known to occur in both the Amazon and Orinoco rivers. Further, and quite aside from the modern connection of the Amazon and Orinoco via the Casiquiare Canal, these two basins are not well-separated physiographically, with a broad lowland corridor joining them on the modern landscape, and a hydrogeographic history of exchanging head-waters via river capture (Winemiller & Willis, 2011). In fact, the watershed divide between these basins may more profitably be viewed as a semipermeable dispersal filter rather than as an impermeable dispersal barrier for lowland riverine species (Lovejoy et al., 2010). The complete absence of S. sima from the Western Amazon (Gálvis et al., 2006Gálvis, G., J. I. Mojica, S. R. Duque, G. C. Castellanos, P. Sánchez-Duarte, M. A. Arce, A. Gutiérrez, L. F. Jimenez, M. Santos, S. Vejarano. 2006. Peces del Medio Amazonas. Región de Leticia. Conservation Internacional, Bogota. ; Crampton, 2011; Ortega et al., 2011Ortega, H., M. Hidalgo, E. Correa, J. Espino, L. Chocano, G. Trevejo, V. Meza, A. M. Cortijo & R. Quispe. 2011. Lista anotada de los peces de aguas continentales del Peru Estado actual del conocimiento, distribución, usos y aspectos de conservación. Museo de Historia Natural, UNMSM, Ministerio del Ambiente, Dirección General de Diversidad Biológica, Lima.; Albert et al., 2012), and the presence of this species in the Negro and upper Orinoco (Winemiller & Willis, 2011), is consistent with the less ancient dispersal scenario, rather than the more ancient vicariance scenario.
Ecological and phenotypic diversification
One of the remarkable features of diversity within Sternarchellini is that several closely related species have highly divergent trophic phenotypes (inferior, terminal, or superior mouths). These species coexist geographically (in sympatry) and ecologically (in syntopy), and several species are frequently caught in the same net (Albert & Crampton, 2005a; Lundberg et al., 2013), indicating that they all live closely together in the deep river channels (Crampton, 2011). Phenotypic disparity in these sternarchellines is observed in the extent of the development of skeletal structures in the head, oral jaws and branchial arches. Some sternarchelline species (i.e., P. amazonensis, S. sima, and S. orinoco) have short and gracile snouts, a round neurocranial vault, and less well-developed oral jaws with smaller conical teeth, all relatively paedomorphic phenotypes. At the other end of the spectrum are species (i.e., M. raptor, M. duccis, S. terminalis, and Sternarchella sp. A) with more peramorphic skeletal development, including robust oral jaw bones and dentition, an elongate neurocranium, few pharyngeal teeth, and small and less numerous gill rakers.
Does the diversity of sternarchelline fishes inhabiting deep Amazonian river channels represent the result of an 'adaptive radiation'? The term 'adaptive radiation' refers to the rapid diversication of a single lineage (i.e., a monophyletic clade) into many phenotypically and ecologically distinct species, usually in association with a substantial increase in morphological and ecological diversity (Simpson 1944Simpson, G. G. 1944. Tempo and Mode in Evolution. New York: Columbia University Press. ; Schluter, 2000Schluter, D. 2000. The ecology of adaptive radiation. Oxford, Oxford University Press. ). To be 'adaptive', the species divergence results from the action of natural selection, forcing lineages to diverge in functional aspects, like trophic or habitat use. Phenotypic changes can either accompany speciation (e.g., by natural selection in sympatry), or they can develop after lineage splitting in allopatry, subsequently followed by range expansion and ecological co-existence (e.g., Hunt et al., 2007Hunt, T., J. Bergsten, Z. Levkanicova, A. Papadopoulou, O. St John, R. Wild, P. Hammond, D. Ahrens, M. Balke, M. Caterino, J. Gomez-Zurita, I. Ribera, T. Barraclough, M. Bocakova, L. Bocak & A. Vogler. 2007. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science, 5858: 1913-1916. ). In an adaptive radiation, phenotypic changes accompany speciation because the speciation results from adaptation (Albert et al., 2011b).
A single sister-species pair in the sternarchelline, in the genus Magosternarchus, live in sympatry according to the phylogeny presented in Fig. 9. In the genus Sternarchella the two documented sister-species pairs are allopatric. However, the absence of Magosternarchus in the Orinoco basin could have resulted from extinction (see above), in which case the distribution of these species would not support a model of sympatric speciation. The morphological disparity between the two extant Magosternarchus species is greater than that of Sternarchella species pairs across the modern Orinoco-Amazon divide, perhaps suggesting a divergence time that pre-dates the ca. 10 MY estimated for the formation of the Vaupes Arch. The hypothesis that Magosternarchus is older than this divide could be tested using divergence time estimates from an analysis of phylogenetic relationships using molecular sequence data (Lovejoy et al., 2010).
There is to date little evidence suggesting habitat partitioning in the deep river channels. The benthic zone of large blackwater and whitewater Amazonian rivers is difficult to study and there is little limnological data on this environment (Val, 1995Val, A. 1995. Oxygen transfer in fish: morphological and molecular adjustments. Brazilian Journal of Medical and Biological Research, 28: 1119-1127. ). However, these deep river environments have no light, have a very swift current, stable oxygen and temperature profiles, low autochthonous production and presumably high predation pressures (Crampton et al., 2011). Biotic inventory data indicate high amounts of longitudinal connectivity (Albert et al., 2011b). Species richness has been shown to be elevated near tributary confluences in deep channel gymnotiforms (Fernandes et al., 2004Fernandes, C. C., J. Podos & J. G. Lundberg. 2004. Amazonian ecology: tributaries enhance the diversity of electric fishes. Science, 5692: 1960-1962.). None of these observations indicate a suitable set of circumstances for adaptive divergence along habitat or other ecological gradients. Very little is known about breeding in deep channel apteronotids, although several species (e.g., Sternarchorhamphus muelleri) appear to use floodplain (varzea) floating meadows as a nursery for eggs and larvae (Crampton, 1998), and this may also be true for some sternarchellines. The hypothesis that phenotypic diversity within the Sternarchellini represents an adaptive radiation would be supported by stable isotope data documenting distinct trophic positions of species within the Amazon river food web (e.g., Layman et al., 2005Layman, C. A., K. O. Winemiller, D. A. Arrington & D. B. Jepsen. 2005. Body size and trophic position in a diverse tropical food web. Ecology, 86: 2530-2535. ; Lujan et al., 2011Lujan, N. K., D. P. German & K. O. Winemiller. 2011. Do wood grazing fishes partition their niche? Morphological and isotopic evidence for trophic segregation in Neotropical Loricariidae. Functional Ecology, 25: 1327-1338. ).
Previous studies of diversity in other clades of deep channel apteronotids include taxonomic revisions of Adontosternarchus (Mago-Leccia et al., 1985), Sternarchogiton (de Santana & Crampton, 2007de Santana, C. D. & W. G. R Crampton. 2007. Revision of the deep-channel electric fish genus Sternarchogiton (Gymnotiformes: Apteronotidae). Copeia, 2007: 387-402.), Porotergus (de Santana & Crampton, 2010), and Sternarchorhynchus (de Santana & Vari, 2010). The first three of these studies did not directly address the ecological and evolutionary topics addressed here, and no formal hypotheses of interrelationships have yet been proposed for these clades (Mago-Leccia et al., 1985; de Santana & Crampton, 2007; de Santana & Crampton, 2010). However, multiple species in each of these clades are distributed sympatrically in the Amazon and Orinoco rivers, and stand as candidates for species-pairs that may have diverged in sympatry (Crampton, 2011).
De Santana & Vari (2010) interpreted diversification in Sternarchorhynchus to be the result of an adaptive radiation. Their phylogenetic results recovered two of eight sister-species pairs distributed in sympatry, in the main stems of the Amazon (S. cramptoni and S. rezteri) and Orinoco (S. roseni and S. mendesi) rivers (de Santana & Vari 2010, fig. 23). However, de Santana & Vari (2010) did not present functional or ecological data in support of the hypothesis of adaptive divergence among closely related species. More extensive taxonomic reviews of deep channel apteronotid species, in conjunction with species-dense time-calibrated molecular phylogenies, will help further understanding of fish diversity in large rivers of the Amazon and Orinoco basins.
We thank D. Catania (CAS), W. Crampton (UCF), J. Lundberg and M. Sabaj Pérez (ANSP), H. Ortega (MUSM), and R. Vari (USNM) for access to specimens, T. Carvalho, D. Green, E. Maxime, and V. Tagliacollo for discussions, and W. Crampton and an anonymous reviewer for helpful comments to the manuscript. This research was supported in part by National Science Foundation grants 0614334 and 0741450 to JSA.
Literature Cited
- Albert, J. S. 2001. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Museum of Zoology, University of Michigan, Ann Arbor.
- Albert, J. S. 2003. Family Apteronotidae. Pp. 503-508. In: Reis, R. E., S. O. Kullander & C. J. Ferraris Jr., (Eds.). Checklist of the Freshwater Fishes of South and Central America. Edipucrs, Porto Alegre.
- Albert, J. S., & T. P. Carvalho. 2011. Neogene assembly of modern faunas. Pp. 119-136. In: Historical Biogeography of Neotropical Freshwater Fishes. Albert, J. S. & R. E. Reis (Eds.). University of California Press, Berkeley.
- Albert, J. S. & W. G. R. Crampton. 2001. Five new species of Gymnotus (Teleostei: Gymnotiformes) from an Upper Amazon floodplain, with descriptions of electric organ discharges and ecology. Ichthyological Exploration of Freshwaters, 12: 241-266.
- Albert, J. S. & W. G. R. Crampton. 2005a. Diversity and phylogeny of Neotropical electric fishes (Gymnotiformes). Pp. 360-409. In: Electroreception. T. H. Bullock, C. D. Hopkins, A. N. Popper & R. R. Fay (Eds.). Springer Handbook of Auditory Research, Volume 21, Springer-Verlag, Berlin.
- Albert, J. S. & W. G. R. Crampton. 2005b. Electroreception and electrogenesis. Pp. 431-472. In: Evans, D. E. (Ed). The Physiology of Fishes. 3rd ed. CRC Press, New York.
- Albert, J. S. & W. G. R. Crampton. 2006. Pariosternarchus amazonensis: a new genus and species of Neotropical electric fish (Gymnotiformes: Apteronotidae) from the Amazon River. Ichthyological Exploration of Freshwaters, 17: 267-274.
- Albert, J. S. & W. L. Fink. 1996. Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus. Copeia, 1996: 85102.
- Albert, J. S. & W. L. Fink. 2007. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. Journal of Vertebrate Paleontology, 27: 17-25.
- Albert, J. S. & R. E. Reis. 2011. Historical Biogeography of Neotropical Freshwater Fishes. University of California Press.
- Albert, J. S., N. R. Lovejoy & W.G.R. Crampton. 2006. Miocene tectonism and the separation of cis- and trans-Andean river basins: evidence from Neotropical fishes. Journal of South American Earth Sciences, 21: 14-27.
- Albert, J. S., P. Petry & R. E. Reis. 2011b. Major biogeographic and phylogenetic patterns. Pp. 21-58. In: Albert, J. S. & R. E. Reis (Eds.). Historical Biogeography of Neotropical Freshwater Fishes. University of California Press, Berkeley.
- Albert, J. S., T. Carvalho, P. Petry, M. A. Holder, E. Maxime, J. Espino, I. Corahua, R. Quispe, B. Rengifo, H. Ortega & R. E. Reis. 2011a. Aquatic biodiversity in the Amazon: habitat specialization and geographic isolation promote species richness. Animals, 1: 205-241.
- Albert, J. S., T. P. Carvalho, J. A. Chuctaya, P. Petry, R. E. Reis, B. Rengifo, & H. Ortega. 2012. Fishes of the Fitzcarrald, Peruvian Amazon. Lulu Press, Raleigh, NC.
- Barthem, R. B. & M. Goulding. 1997. The Catfish Connection. Columbia University Press, New York.
- Bremer, K. 1988. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution, 42: 795-803.
- Carvalho, T. P. 2013. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): diversification in South American freshwaters. Unpublished Ph.D. Dissertation, University of Louisiana at Lafayette, 516p.
- Crampton, W. G. R. 1996. Gymnotiform fish: an important component of Amazonian floodplain fish communities. Journal of Fish Biology, 48: 298-301.
- Crampton, W. G. R. 1998. Effects of anoxia on the distribution, respiratory strategies and electric signal diversity of gymnotiform fishes. Journal of Fish Biology, 53: 307-330.
- Crampton, W. G. R. 2008. Diversity and adaptation in deep channel Neotropical electric fishes. Pp. 283-339. In: P. Sebert et al. (Eds.). Fish Life in Special Environments. Science Publishers, Enfield, N.H.
- Crampton, W. G. R. 2011. An ecological perspective on diversity and distributions. Pp. 165-189. In: Albert, J. S. & R. E. Reis (Eds.). Historical Biogeography of Neotropical Freshwater Fishes. University of California Press, Berkeley.
- Crampton, W. G. R. & J. S. Albert. 2006. Evolution of electric signal diversity in gymnotiform fishes. Pp. 641-725. In: F. Ladich, S. P. Collin, P. Moller & B. G. Kapoor, (Eds.). Communication in Fishes. Publishers Inc, Enfield, NH.
- Crampton, W. G. R., N. Lovejoy & J. Waddell. 2011. Reproductive character displacement and signal ontogeny in a sympatric assemblage of electric fish. Evolution, 65: 1650-1666.
- Dobson, D. M., G. R. Dickens, & D. K. Rea. 2001. Terrigenous sediment on Ceara Rise: a Cenozoic record of South American orogeny and erosion. Palaeogeography, Palaeoclimatology, Palaeoecology, 165: 215-229.
- Eigenmann, C. H. & W. Allen. 1922. Fishes of Western South America. University of Kentucky, Lexington.
- Eigenmann, C. H. & D. P. Ward. 1905. The Gymnotidae. Proceedings of the Washington Academy of Sciences, 7: 157-185.
- Farris, J. 1989. The retention index and the rescaled consistency index. Cladistics, 5: 417-419.
- Fernandes, C. C., J. Podos & J. G. Lundberg. 2004. Amazonian ecology: tributaries enhance the diversity of electric fishes. Science, 5692: 1960-1962.
- Fink, S. V. & Fink, W. L. 1996. Interrelationships of ostariophysan fishes (Teleostei). Pp. 209-249. In: M. L. Stiassny, L. R. Parenti & G. D. Johnson (Eds.). Interrelationships of Fishes. Academic Press.
- Gálvis, G., J. I. Mojica, S. R. Duque, G. C. Castellanos, P. Sánchez-Duarte, M. A. Arce, A. Gutiérrez, L. F. Jimenez, M. Santos, S. Vejarano. 2006. Peces del Medio Amazonas. Región de Leticia. Conservation Internacional, Bogota.
- Godoy, M. P. de. 1968. Nova espécie de "Sternarchella" Eigenmann (Pisces, Gymnonoti, Sternarchidae). Revista Brasileira de Biologia, 28: 351-355.
- Goloboff, P., J. Farris & K. Nixon. 2008. TNT, a free program for phylogenetic analysis. Cladistics, 24: 774-786.
- Goulding, M., R. Barthem, E. Ferreira & R. Duenas. 2003. The Smithsonian Atlas of the Amazon. Smithsonian Books, Washington, D.C.
- Hammer, Ø., D. Harper & P. Ryan. 2001. PAST-Palaeontological statistics. /~ pardomv/pe/2001_1/past/pastprog/past.
- Hennig, W. 1966. Phylogenetic Systematics. University of Illinois Press, Urbana, IL.
- Hillis, D. M. & J. J. Bull. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology, 42: 182-192.
- Hunt, T., J. Bergsten, Z. Levkanicova, A. Papadopoulou, O. St John, R. Wild, P. Hammond, D. Ahrens, M. Balke, M. Caterino, J. Gomez-Zurita, I. Ribera, T. Barraclough, M. Bocakova, L. Bocak & A. Vogler. 2007. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science, 5858: 1913-1916.
- Jolicoeur, P. 1963. The Multivariate Generalization of the Allometry Equation. Biometrics, 19: 497-499.
- Jolicoeur, P. & J. Mosimann. 1960. Size and shape variation in the painted turtle. A principal component analysis. Growth, 24: 339-354.
- Layman, C. A., K. O. Winemiller, D. A. Arrington & D. B. Jepsen. 2005. Body size and trophic position in a diverse tropical food web. Ecology, 86: 2530-2535.
- Lujan, N. K., D. P. German & K. O. Winemiller. 2011. Do wood grazing fishes partition their niche? Morphological and isotopic evidence for trophic segregation in Neotropical Loricariidae. Functional Ecology, 25: 1327-1338.
- Lovejoy, N. R., S. Willis & J. S. Albert. 2010. Molecular signatures of Neogene biogeographical events in the Amazon fish fauna. Pp. 405-417. In: C. M. Hoorn & F. Wesselingh (Eds.). Amazonia: Landscape and Species Evolution. A Look into the Past. Wiley-Blackwell; Chichester, Oxford and Hoboken.
- Lovejoy, N. R., J. S , Albert & W. G. R. Crampton. 2006. Miocene marine incursions and marine/freshwater transitions: evidence from Neotropical fishes. Journal of South American Earth Sciences, 21: 1-9.
- Lundberg, J. & W. Lewis. 1987. A major food web component in the Orinoco River channel: evidence from planktivorous electric fishes. Science, 237: 81-83.
- Lundberg, J. G. & F. Mago-Leccia. 1986. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proceedings of the Academy of Natural Sciences, 138: 53-85.
- Lundberg, J. G., C. C. Fernandes, J. S. Albert & M. Garcia. 1996. Magosternarchus, a new genus with two new species of electric fishes (Gymnotiformes: Apteronotidae) from the Amazon River Basin, South America. Copeia, 1996: 657-670.
- Lundberg, J. G., L. G. Marshall, J. Guerrero, B. Horton, M. C. S. L. Malabarba & F. Wesselingh. 1998. The stage for Neotropical fish diversification: a history of tropical South American rivers. Pp. 13-48. In: Phylogeny and Classification of Neotropical Fishes. Malabarba, L., R. E. Reis, R. P. Vari, C. A. S. de Lucena & Z. M. S. de Lucena (Eds.). Museu de Ciências e Tecnologia, Porto Alegre.
- Lundberg, J. G., C. C. Fernandes, R. Campos-da-Paz & J. P. Sullivan. 2013. Sternarchella calhamazon n. sp., the Amazon's most abundant species of apteronotid electric fish, with a note on the taxonomic status of Sternarchus capanemae Steindachner, 1868 (Gymnotiformes, Apteronotidae) . Proceedings of the Academy of Natural Sciences of Philadelphia, 162: 157-173.
- Madison, W. P. & D. R. Madison. 2005. MacClade, Analysis of Phylogeny and Character Evolution, version 4.08. Sunderland, Sunderland Associated, Inc, Massachusetts.
- Mago-Leccia, F. 1978. Los peces de la familia Sternopygidae de Venezuela. Acta Scientífica Venezolana, 29: 1-51.
- Mago-Leccia, F. 1994. Electric fishes of the continental waters of America. Biblioteca de la Academia de Ciencias Físicas, Matemáticas y Naturales, 29: 1-229.
- Mago-Leccia F., J. G. Lundberg & J. N. Baskin. 1985. Systematics of the South American freshwater genus Adontosternarchus (Gymnotiformes, Apteronotidae). Contributions in Science, Los Angeles County Museum of Natural History, 358:1-19.
- McElroy, D. & M. Douglas. 1995. Patterns of morphological variation among endangered populations of Gila robusta and Gila cypha (Teleostei: Cyprinidae) in the upper Colorado River basin. Copeia, 1995: 636-649.
- Myers, G. S. 1936. A new genus of gymnotid eels from the Peruvian Amazon. Proceedings of the Biological Society of Washington, 49: 115-116.
- Near, T. J., R. I. Eytan, A. Dornburg, K. L. Kuhn, J. A. Moore, M. P. Davis, P. C. Wainwright, M. Friedman & W. L. Smith. 2012. Resolution of ray-finned fish phylogeny and timing of diversification. Proceedings of the National Academy Sciences USA, 109: 13698-13703.
- Ortega, H., M. Hidalgo, E. Correa, J. Espino, L. Chocano, G. Trevejo, V. Meza, A. M. Cortijo & R. Quispe. 2011. Lista anotada de los peces de aguas continentales del Peru Estado actual del conocimiento, distribución, usos y aspectos de conservación. Museo de Historia Natural, UNMSM, Ministerio del Ambiente, Dirección General de Diversidad Biológica, Lima.
- Patterson, C. 1975. The braincase of pholidophorid and leptolepid fishes, with a review of the actinopterygian braincase. Philosophical Transactions of the Royal Society, London (B), 53: 275-579.
- Pimentel, R. & R. Riggins. 1987. The nature of cladistic data. Cladistics, 3: 201-209.
- de Santana, C. D. & W. G. R Crampton. 2007. Revision of the deep-channel electric fish genus Sternarchogiton (Gymnotiformes: Apteronotidae). Copeia, 2007: 387-402.
- de Santana, C. D. & W. G. R. Crampton. 2010. A review of the South American electric fish genus Porotergus (Gymnotiformes: Apteronotidae) with the description of a new species. Copeia, 2010: 165-175.
- de Santana, C. D. & R. P. Vari. 2010. Electric fishes of the genus Sternarchorhynchus (Teleostei, Ostariophysi, Gymnotiformes); phylogenetic and revisionary studies. Zoological Journal of the Linnean Society, 159: 223-371.
- Schluter, D. 2000. The ecology of adaptive radiation. Oxford, Oxford University Press.
- Simpson, G. G. 1944. Tempo and Mode in Evolution. New York: Columbia University Press.
- Starks, E. C. 1913. The fishes of the Stanford Expedition to Brazil.Stanford University Publications .
- Steindachner, F. 1868. Die Gymnotidae des K. K. Hof-Naturaliencabinetes zu Wien. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien, 58: 249-264.
- Swofford, D. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (* and Other Methods). Version 4. Sinauer Associates, Sunderland, MA.
- Taylor, W. R. & G. Van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9: 107-119.
- Triques, M. L. 1993. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia, 6: 85-130.
- Triques, M. L. 2005. Análise cladística de caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana, 6: 121-149.
- Val, A. 1995. Oxygen transfer in fish: morphological and molecular adjustments. Brazilian Journal of Medical and Biological Research, 28: 1119-1127.
- Wesselingh, F. P. & C. Hoorn. 2011. Geological development of Amazon and Orinoco basins. Pp. 59-67. In: J. S. Albert & R. E. Reis (Eds.). Historical Biogeography of Neotropical Freshwater Fishes. UC Press, Berkeley.
- Weitzman, S. 1974. Osteology and evolutionary relationships of the Sternoptychidae, with a new classification of stomiatoid families. Bulletin of the American Museum Natural History, 153: 327-478.
- Wiley, E. O. 1981. Phylogenetics. The theory and practice of phylogenetic systematics.. John Wiley & Sons New York.
- Winemiller, K. O. & S. Willis. 2011. The Vaupes Arch and Casiquiare Canal. Pp. 225-242. In: Albert, J. S. & R. E. Reis (Eds.). Historical Biogeography of Neotropical Freshwater Fishes. University of California Press, Berkeley.
APPENDIX 1 Specimens examined for morphometrics and osteology.


Appendix 2 Data matrix used in phylogenetic analysis (P indicates states 0&1). Characters and states defined in Appendix 4.


Appendix 3 Summary of character state changes on phylogeny of Figure 9.


Appendix 4. Characters and character states descriptions.


Publication Dates
-
Publication in this collection
26 Aug 2014 -
Date of issue
Jul-Sep 2014
History
-
Received
09 May 2013 -
Accepted
07 Jan 2014