Abstract
Cetengraulis edentulus is a broadely distributed engraulid in Southwest Atlantic, currently accounting for the main fish species commercially exploited at Guanabara Bay, Brazil. This study aimed to extend the knowledge on reproduction of C. edentulus at Guanabara Bay and to test whether some descriptors of reproductive activity, especially the gonadosomatic index (GSI), and the index of reproductive activity (IRA) changed among seasons. A total of 978 C. edentulus specimens were retrieved from purse seine commercial landings at Conservas Rubi S.A. company, in São Gonçalo city, RJ. Subsamples of 90-120 individuals were collected from bimonthly yields between July 2010 (winter) and June 2011 (autumn). Most fish were adults (120-170 mm TL), in response to the high selectivity of commercial fisheries. All descriptors indicated a broad spawning period (late winter to spring), peaking in November, suggesting this is the critical period to protect C. edentulus stocks from overfishing at Guanabara Bay. Fecundity averaged 12,720 oocytes and was positively related to fish size, GSI and fullness index, indicating that preserving larger individuals (TL > 160 mm) could contribute significantly to the reproductive success of C. edentulus, since they produce more oocytes. Cetengraulis edentulus é um engraulídeo amplamente distribuído no Atlântico Sudoeste, sendo um importante recurso comercialmente explorado na baía de Guanabara, Brasil. O presente estudo teve como objetivo ampliar o conhecimento sobre a reprodução de C. edentulus na baía de Guanabara e testar quais descritores da atividade reprodutiva, especialmente o índice gonadossomático (IGS) e o índice de atividade reprodutiva (IAR) se modificaram ao longo das estações do ano. Um total de 978 indivíduos de C. edentulus foram capturados pelo método de rede de cerco da frota comercial no cais da empresa Conservas Rubi S.A., na cidade de São Gonçalo, RJ. Subamostras de 90-120 indivíduos de C. edentulus foram coletados bimestralmente durante o período de julho de 2010 (inverno) e junho de 2011 (outono). A maioria dos indivíduos foram adultos (120-170 mm CT), em resposta a alta seletividade das capturas comerciais. Todos os índices indicaram um amplo período reprodutivo (final do inverno e primavera), com pico em novembro, sugerindo que este seria um período crítico para preservar os estoques de C. edentulusda sobrepesca na baía de Guanabara. A fecundidade média foi de 12720 ovócitos e foi positivamente relacionada com tamanho do peixe, IGS e índice de repleção, indicando que preservar maiores indivíduos (CT > 160 mm) poderia contribuir significativamente para o sucesso reprodutivo de C. edentulus na área, visto que esses indivíduos produzem um maior número de ovócitos.
Atlantic anchoveta; Engraulidae; Fecundity; Gonad maturation; Reproduction; Tropical bay
Introduction
Studies on reproductive biology of fishes provide important baselines for stock management and conservation (King & McFarlane, 2003King, J. R. & G. A. McFarlane. 2003. Marine fish life history strategies: applications to fishery management. Fisheries Management and Ecology, 10: 249-264.; Silva et al., 2005Silva, G. C., A. C. L. Castro & E. A. Gubiani. 2005. Estrutura populacional e indicadores reprodutivos de Scomberomorus brasiliensis no litoral ocidental maranhense. Acta Scientarium. Biological Sciences, 27: 383-389.). Most studies on fish reproduction rely on the characterization of gonad maturity stage, which are critical for the precise determination of a species reproductive strategy. Gonadosomatic index (GSI), condition factor (K), and hepatosomatic index (HSI) are traditional descriptors that have been applied individually or together to describe fish spawning period (Querol et al., 2002Querol, M. V. M., E. Querol & N. N. A. Gomes. 2002. Fator de condição gonadal, índice hepatossomático e recrutamento como indicadores do período de reprodução de Loricariichthys platymetopon (Osteichthyes, Loricariidae), Bacia do Rio Uruguai Médio, Sul do Brasil. Ilheringia, Série Zoologia, 92: 79-84.; Silva et al., 2005Silva, G. C., A. C. L. Castro & E. A. Gubiani. 2005. Estrutura populacional e indicadores reprodutivos de Scomberomorus brasiliensis no litoral ocidental maranhense. Acta Scientarium. Biological Sciences, 27: 383-389.; Araújo et al., 2008bAraújo, F. G., M. A. Silva, M. C. C. Azevedo & J. N. S. Santos. 2008b. Spawning season, recruitment and early life distribution of Anchoa tricolor (Spix and Agassiz, 1829) in a tropical bay in southeastern Brazil. Brazilian Journal of Biology, 68: 823-829.). In addition to those descriptors, Dei Tos et al. (2002)Dei Tos, C. , G. Barbieri, A. A. Agostinho, L. C. Gomes & H. I. Suzuki. 2002. Ecology of Pimelodus maculatus (Siluriformes) in the Corumbá reservoir, Brazil. Cybium, 26: 275-282. used the index of reproductive activity (IRA), a method that combines the proportion of reproducing individuals and values of gonadosomatic index to evaluate the reproductive activity for the whole fish assemblage or single populations. Although the IRA has been broadly applied for fresh water fish assemblages (Dias et al., 2005Dias, R. M., D. Bailly, R. R. Antônio, H. I. Suzuki & A. A. Agostinho. 2005. Colonization of the Corumbá Reservoir (Corumbá River, Paraná River Basin, Goiás State, Brazil) by the "lambari" Astyanax altiparanae (Tetragonopterinae; Characidae). Brazilian Archives of Biology and Technology, 48: 467-476.; Bailly et al., 2008Bailly, D., A. A. Agostinho & H. I. Suzuki. 2008. Influence of the flood regime on the reproduction of fish species with different reproductive strategies in the Cuiabá River, upper Pantanal, Brazil. River Research and Applications, 24: 1218-1229.; Braga et al., 2008Braga, M. R., J. M. R. Aranha & J. R. Vitule. 2008. Reproduction period of Mimagoniates microlepis, from an Atlantic forest stream in Southern Brazil. Brazilian Archives of Biology and Technology, 51: 345-351.), few studies used this index to describe reproductive activity of marine fish (Souza & Chaves, 2007Souza, L. M. & P. T. Chaves. 2007. Atividade reprodutiva de peixes e o defeso da pesca de arrasto no litoral norte de Santa Catarina, Brasil. Revista Brasileira de Zoologia, 24: 1113-1121.; Rodrigues-Filho et al., 2011Rodrigues-Filho, J. L., J. R. Verani, A. C. Peret, L. M. Sabinson & J. O. Branco. 2011. The influence of population structure and reproductive aspects of the genus Stellifer (Oken, 1817) on the abundance of species on the southern Brazilian coast. Brazilian Journal of Biology, 71: 991-1002.).
Engraulid fishes, commonly known as anchovies, are widely distributed in tropical and sub-tropical waters (Mcgowan & Berry, 1983Mcgowan, M. F. & F. H. Berry. 1983. Clupeiformes: Development and Relationships . Pp. 108-126. In: Blaxter J. H. S. (Ed.). Ontogeny and Systematics of Fishes, 8.). Anchovies are recognized as an important trophic link in coastal food chains, since they convert planktonic biomass into forage for higher level consumers (Hildebrand, 1963Hildebrand, S. F. 1963. Family Engraulidae. Memories Sears Foundation for Marine Research, 1: 152-249.). Most engraulids spawn at coastal areas in the inner continental shelf, and recruitment occurs often in protected, shallow areas that provide food and shelter against predators (Silva et al., 2003Silva, M. A., F. G. Araújo, M. C. C. Azevedo & P. Mendonça. 2003. Distribuição espacial e temporal de Cetengraulis edentulus (Cuvier, 1829) (Actinopterygii, Engraulidae) na Baía de Sepetiba, Rio de Janeiro, Brasil. Revista Brasileira de Zoologia, 20: 577-581.). Adults perform seasonal migrations between estuarine and oceanic areas (MacGregor & Houde, 1996Macgregor, J. M. & E. D. Houde. 1996. Onshore-Offshore pattern and variability in distribution and abundance of bay anchovy Anchoa mitchilli eggs and larvae in Cheasapeake Bay. Marine Ecology Progress Series, 138: 15-25.), aggregating in large shoals that are frequently targeted by commercial fisheries. Many species of anchovies are economically important in several regions in South America, namely the Anchoveta Engraulis ringens (Jenyns, 1842) in Peru, the Argentine anchovy Engraulis anchoita (Hubbs & Marini, 1935) in Argentina, and Cetengraulis edentulus (Cuvier, 1829) in Venezuela (Whitehead, 1977Whitehead, P. J. P. 1977. Engraulidae. FAO species identification sheets for fishery purposes. Western Central Atlantic (Fishing Area 31), 2 .) and Guanabara Bay, Brazil (Jablonski et al., 2006Jablonski, S., A. F. Azevedo & L. H. A. Moreira. 2006. Fisheries and conflicts in Guanabara Bay, Rio de Janeiro, Brazil. Brazilian Archives of Biology and Technology, 49: 9-91.).
Cetengraulis edentulus is widely distributed throughout the Southwest Atlantic, occurring from Antilles and Cuba to Southern Brazil (Whitehead, 1988Whitehead, P. J. P. 1988. Clupeoid of the world (Suborder Clupeoidei): an annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. FAO Fisheries Synopsis, Rome, 7 (125), 1-579 .). This planktivorous species is well tolerant to changes in environmental conditions, being often found at inner zones of coastal bays, where salinity is generally low, temperatures are high, and phytoplankton biomass is high due to the great input of organic waste (Sergipense et al., 1999Sergipense, S., E. P. Caramaschi & I. Sazima. 1999. Morfologia e hábitos alimentares de duas espécies de Engraulidae (Teleostei, Clupeiforme) na Baía de Sepetiba, Rio de Janeiro. Revista Brasileira de Oceanografia, 47: 173-188.; Silva et al., 2003Silva, M. A., F. G. Araújo, M. C. C. Azevedo & P. Mendonça. 2003. Distribuição espacial e temporal de Cetengraulis edentulus (Cuvier, 1829) (Actinopterygii, Engraulidae) na Baía de Sepetiba, Rio de Janeiro, Brasil. Revista Brasileira de Zoologia, 20: 577-581.; Araújo et al., 2008aAraújo, F. G, M. A. Silva, J. N. S. Santos & R. M. Vasconcellos. 2008a. Habitat selection by anchovies (Clupeiformes: Engraulidae) in a tropical bay at Southeastern Brazil. Neotropical Ichthyology, 6: 583-590.). Although the great importance of C. edentulus as fishery resource in coastal bays of Southeastern Brazil, especially on Guanabara Bay where accounted for 69% (ca.13,000 ton) of total landings in 2004, (Jablonski et al., 2006Jablonski, S., A. F. Azevedo & L. H. A. Moreira. 2006. Fisheries and conflicts in Guanabara Bay, Rio de Janeiro, Brazil. Brazilian Archives of Biology and Technology, 49: 9-91.; Araújo et al., 2008aAraújo, F. G, M. A. Silva, J. N. S. Santos & R. M. Vasconcellos. 2008a. Habitat selection by anchovies (Clupeiformes: Engraulidae) in a tropical bay at Southeastern Brazil. Neotropical Ichthyology, 6: 583-590.), the ecology of this species is barely known, especially on its reproductive biology (Souza-Conceição et al., 2005Souza-Conceição, J. M., M. Rodrigues-Ribeiro & M. A. Castro-Silva. 2005. Dinâmica populacional, biologia reprodutiva e o ictioplâncton de Cetengraulis edentulus na enseada do Saco dos Limões, Florianópolis, Santa Catarina, Brasil. Revista Brasileira de Zoologia, 22: 953-961.). The present study aimed to broaden the knowledge on C. edentulus reproduction in Guanabara Bay and test whether reproductive activity, gonadosomatic index and fecundity varied among seasons. The major implications of our findings for conservation and management of C. edentulus stocks in Guanabara Bay are also briefly discussed.
Material and Methods
Study area.
Guanabara Bay is a ca. 400km2 estuary located on the coast of Rio de Janeiro State (22°50'S 43°10'W; Fig. 1). The climate is tropical-humid with a warm-rainy season (December-March) and a cooler-dry season (July-August) (Paranhos & Mayr, 1993Paranhos, R. & L. M. Mayr. 1993. Seasonal Patterns of Temperature and Salinity in Guanabara Bay, Brazil. Fresenius Environment Bulletin, 2: 647-652.; Valentin et al., 1999Valentin, J. L., D. R. Tenenbaum, A. Bonecker, S. L. C. Bonecker, C. R. Nogueira, R. Paranhos & M. C. Villac. 1999. Caractéristiques hydrologiques de La Baie de Guanabara (Rio de Janeiro, Brésil). Journal de Recherche Oceanographique, 24: 33-41.). Guanabara Bay is 36 km long and has 3 x 109m3 of water volume, mean depth of 7.6 m, and maximum depth of 30 m at the entrance channel (Valentin et al., 1999Valentin, J. L., D. R. Tenenbaum, A. Bonecker, S. L. C. Bonecker, C. R. Nogueira, R. Paranhos & M. C. Villac. 1999. Caractéristiques hydrologiques de La Baie de Guanabara (Rio de Janeiro, Brésil). Journal de Recherche Oceanographique, 24: 33-41.). Tidal currents account for most water circulation in this bay, with a microtidal regime (i.e., 0.7 m of amplitude), semi-diurnal tides, and maximum currents of 1.6m s-1 that result in 23 days of water residence time (Kjerfve et al., 1997Kjerfve, B., C. H. A. Ribeiro, G. T. M. Dias, A. M. Filippo & V. S. Quaresma. 1997. Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, 17: 1609-1643.). Substratum is predominantly muddy, with increasingly contribution of sand toward the outer zone, and the salinity ranges from 21.0 to 34.5 (Kjerfve et al., 1997Kjerfve, B., C. H. A. Ribeiro, G. T. M. Dias, A. M. Filippo & V. S. Quaresma. 1997. Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, 17: 1609-1643.). Guanabara Bay is surrounded by one of the largest metropolitan areas in Brazil (i.e., Rio de Janeiro city), with more than 11 million inhabitants. As consequence, water quality is poorer at the inner zone, because of the mainland proximity and the restrict circulation, while better conditions (i.e., higher transparency and dissolved oxygen) are found toward the outer zone, more influenced by adjacent oceanic waters.
Sampling and data analysis.
Individuals of Cetengraulis edentulus were obtained from boat landings at Conservas Rubi S.A., a fish processing industry located at São Gonçalo city, bordering Guanabara Bay. Fish was caught by artisanal fishermen that traditionally have exploited C. edentulus stocks in the Bay through purse seine method. Subsamples (90-120 individuals) of C. edentulus were obtained every two months from July 2010 (winter) to June 2011 (autumn). All individuals were measured for total length (mm; TL) and total weight (g), of which 754 gonads were weighed and preserved in 10% buffered formalin. Individuals were classified into adults or juveniles according to the stage of the gonad development and the length at first maturity (L50) of 118 mm TL found by Souza-Conceição et al. (2005)Souza-Conceição, J. M., M. Rodrigues-Ribeiro & M. A. Castro-Silva. 2005. Dinâmica populacional, biologia reprodutiva e o ictioplâncton de Cetengraulis edentulus na enseada do Saco dos Limões, Florianópolis, Santa Catarina, Brasil. Revista Brasileira de Zoologia, 22: 953-961. for a population of C. edentulus at a Southern Brazilian estuary.
The stages of gonad maturation were macroscopically classified into five stages following Núñez & Duponchelle (2009)Núñez, J. & F. Duponchelle. 2009. Towards a universal scale to assess sexual maturation and relation life history traits in oviparous teleost fishes. Fish Physiology and Biochemistry, 35: 167-180.: 1-immature, 2-maturing, 3-mature, 4-spawned (for females) and spent (for males), 5-recovering and resting. GSI was calculated as: GSI = 100(Wg/Wt), where Wg is the gonad weight (g) and Wt is the total weight (g). The condition factor (K) was computed as: K = We/L3, where We is the eviscerated body weight (g) and L is the total length (mm). The hepatosomatic index (HSI) was estimated, except for winter, as: HIS = Wl/We, where Wl = liver weight (g) and We = eviscerated weight (g). These indexes were applied to link physiological condition and energy storage to C. edentulus reproduction. The fullness index (FI) was calculated to assess whether feeding activity of C. edentulus was affected by gonad development according to: FI = 100(Ws/We), where Ws = stomach weight (g) and We = eviscerated weight (g).
The index of reproductive activity (IRA) was calculated, only for females, to appraise the seasonal variation of reproductive activity, as follows: IAR = [lnNi((ni/Σ ni)+( ni /Ni)).GSIi/GSIe] / [lnNm(nm/Σ ni +1)].100, where Ni is the number of females in sample unit i; ni is the number of mature females in sample unit i; Nm is the number of females in the largest sample unit; nm is the number of mature females in the sample unit with the largest n; GSIi is the average gonadosomatic index of mature females in sample unit i; GSIe is the largest female value of GSI. IRA was classified as in Dei Tos et al. (2002)Dei Tos, C. , G. Barbieri, A. A. Agostinho, L. C. Gomes & H. I. Suzuki. 2002. Ecology of Pimelodus maculatus (Siluriformes) in the Corumbá reservoir, Brazil. Cybium, 26: 275-282., into three categories: incipient (0 < IRA < 5); moderate (5 < IRA < 10); and intense (IRA > 10).
An adaptation of the gravimetric method was used for estimation of fecundity to infer the potential production of new individuals in which the mature gonads were examined macroscopically with the aid of a binocular stereoscopic. Firstly, each gonad was weighed and divided into five sections, in order to consider possible variations in the number of oocytes along the gonad length. Then, all of these sections were weighed and a fraction of approximately 0.01 g was retrieved from each one. These fractions were then transferred to a Petri dish and all vitellogenic oocytes were recorded. Forty oocytes of each fraction were sorted and measured through an ocular micrometer (0.1 mm; 32x zoom). Finally, the number of oocytes in each fraction was adjusted to the total weight of the gonad, allowing an estimation of the fecundity.
Analysis of covariance (ANCOVA) was used to compare the gonadosomatic index, condition factor, hepatosomatic index and fullness index among sex and seasons, using fish length as the covariate. One way ANOVA was also applied to IRA to test differences among seasons, and Tukey post-hoc test was used for significant analyses (P < 0.05) to determine which means were different from each other. Non-generalized Additive Models (GAMs) were also applied to investigate the relationship of fecundity and oocytes diameter with GSI, TL, and FI. GAM is a non-parametric regression technique that is not limited to linear relationships, being sensitive to several types of data distribution. GAMs complexity was chosen using the stepwise selection procedure, through the Akaike Information Criterion (AIC). The AIC considers not only the degree of fit, but also uses the criterion of parsimony, penalizing very complex models (Burnham & Anderson, 1998Burnham, K. P. & D. R. Anderson. 1998. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd edition. Springer-Verlag, New York, USA..).
Results
A total of 978 Cetengraulis edentulus specimens were sampled, with size ranging between 114-179 mm TL (average = 147 mm TL), mostly adults. Males predominated within 120-150 mm TL size classes, while females were more frequent above 150 mm TL (Fig. 2). Males more than 165 mm TL and females less than 130 mm TL were not recorded.
Number of Cetengraulis edentulus per size classes (mm) of total length (white column = female; black column = male) in Guanabara Bay.
The relative abundance (%) of C. edentulus per maturation stage (GMS) shows the contribution of males and females at different levels of gonad development (as in Núñez & Duponchelle, 2009Núñez, J. & F. Duponchelle. 2009. Towards a universal scale to assess sexual maturation and relation life history traits in oviparous teleost fishes. Fish Physiology and Biochemistry, 35: 167-180.) throughout the seasons (Fig. 3). No individuals with immature gonads (stage 1) were found, agreeing with the results of size class structure. During winter, there was a greater frequency of mature gonads (stage 3), with 90.2% of males and 63.4% females at this stage. Also in this period, 32.7% of females had maturing gonads (stage 2), while 6.6% of males showed gonads at recovering/resting (stage 5). A higher frequency of mature gonads was recorded in spring, with 60.8% males and 47.1% females at this stage. Spawned (stage 4) and recovering/resting gonads were also recorded for 38.2% and 13.7% of females in this season. Maturing gonads predominated during summer and autumn, accounting for 68.2% of males and 64.5% females in the first season, and 59.4% of males and 81.2% females in the latter. Fish with recovering/resting gonads were also abundant in these seasons, contributing with 29.5% of males and 29% females in summer, and with 40.6% males and 18.9% females in autumn.
Seasonal variation in percent (%) frequency of occurrence of gonad maturation stages (GMS) for females (white column) and males (black column) of Cetengraulis edentulus in Guanabara Bay.
The gonadosomatic index (GSI) differed significantly between seasons (ANCOVA, F (429, 7) = 115.1; P < 0.001), with a significant sex × season interaction (F (429, 7) = 7.51; P < 0.001), with no significant differences among sexes. Male GSI peaked in late spring (Fig. 4), these values being significantly different from all others (Tukey post-hoctest; P < 0.05). The values of GSI for late spring did not differ significantly from the beginning of winter and summer, while the onset of winter did not differ from late summer and autumn as a whole. Female peaked in early spring, which did not differ between males and females in late winter and spring (Tukey post-hoctest; P < 0.05). Intermediate GSI values were observed for both sexes in early summer.
Mean values (± standard error) of gonadosomatic index (GSI) among months and seasons (black square = females; white circle = males) of Cetengraulis edentulus in Guanabara Bay.
GSI were correlated with the fullness index (FI) and the total length of the individuals (Fig. 5A-B). AIC selected a negative linear relationship between the GSI and FI (F (1, 24) = 7.94; P < 0.01), indicating that in fish with heavier stomachs, gonads occupied a small proportion of the abdominal cavity (Fig. 5A). A non-linear relationship was found between GSI and TL (AIC; F (2, 22) = 6.1; P < 0.01), revealing a linear increase of GSI until the individuals reach 160 mm in length, becoming constant in larger fish (Fig. 5B).
Relation between gonadosomatic index with fullness index (A) and with total length (B) of Cetengraulis edentulus in Guanabara Bay. Lines represent the generalized additive models selected by the Akaike information criterion.
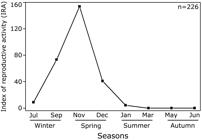
The index of reproductive activity was higher (IRA > 40; intense) from late winter (September) to spring (November and December; Fig. 6). The reproductive activity of C. edentulus decreased considerably at early summer (IRA = 4.3; incipient) and remained low until late autumn (IRA = 0; incipient). During early winter, IRA increased to moderate level, suggesting a recovering in the reproductive activity of C. edentulus.
The condition factor (K) showed significant differences between sexes (ANCOVA, F (531, 1) = 20.94; P < 0.001) and seasons (F (531, 7)= 21.19; P < 0.001), with no significant interaction between these two factors. Females reached higher K values than males, particularly due to higher contributions in early spring and autumn (Fig. 7). Greater values of K were recorded in early winter, followed by intermediate values in late winter and early spring, and minimum values in the other seasons (Tukey post-hoc test; P < 0.05).
Variation of the index of reproductive activity (IRA) for female Cetengraulis edentulus among months and seasons in Guanabara Bay.
Mean values (± standard error) of the condition factor among months and seasons (black square = females; white circle = males) of Cetengraulis edentulus in Guanabara Bay.
The hepatosomatic index (HSI) showed highly significant differences among sexes (ANCOVA, F (321, 1) = 27.78; P < 0.001), seasons (F (321, 5) = 27.63; P < 0.001) and interaction between these two factors (F (321, 5) = 9.24; P < 0.001). Males and females differed in the spring months (Fig. 8), as occurred with the GSI, and also in early summer (Tukey post-hoc test; P < 0.05). For females, the months of spring and early summer were significantly different from all others, representing the highest values of HSI (Fig. 9). For males, only the late spring was significantly different from late autumn (Tukey post-hoc test; P < 0.05). The fullness index (FI) showed significant differences only between seasons (F (531, 7) = 73.22; P < 0.0001) (Fig. 10). The winter months were significantly higher and different from those of all others seasons, which did not differ from each other (Tukey post-hoc test; P < 0.05).
Mean values (± standard error) of the hepatosomatic index among months and seasons (black square = females; white circle = males) of Cetengraulis edentulus in Guanabara Bay.
Mean values (± standard error) of the fullness index between months and seasons of Cetengraulis edentulus in Guanabara Bay.
The counting of vitellogenic oocytes revealed a mean fecundity of 12720.6 ± 823.5 occytes, with diameters ranging between 300 and 950 μm (mean = 619.3 µm) Fecundity was linearly and positively related to total length (TL) (AIC; linear F (1, 24) = 7.24; P = 0.01), but negatively related to FI (AIC; linear F (1, 24) = 3.58; P = 0.07) (Fig. 10A-B). After controlling for size effects, the standardized residuals of fecundity were non-linearly related with GSI (AIC; F(2, 22) = 2.1; P = 0.11), with few changes in the number of oocytes at low and intermediate GSI values, but increasing exponentially after that (Fig. 10C). A positive linear relationship was also selected for GSI (AIC; F (1, 24) = 39.17; P < 0.01) and condition factor (F (1, 24) = 3.96; P = 0.06) with the diameter of oocytes (Fig. 11A-B; respectively).
Relation between fecundity with total length (A) and fullness index (B) and between fecundity residuals (after controlling for the length effect) with gonadosomatic index (C) for Cetengraulis edentulus in Guanabara Bay. Lines represent the generalized additive models selected by the Akaike information criterion.
Relation between the oocytes mean diameter with the gonadosomatic index (A) and with the condition factor (B) of Cetengraulis edentulus in Guanabara Bay. Lines represent the generalized additive models selected by the Akaike information criterion.
Discussion
Our findings overall reveal that spawning season of Cetengraulis edentulus started in late winter and continued throughout the spring in Guanabara Bay. This pattern agrees with its congener Cetengraulis mysticetus (Günther, 1867) in the Gulf of Panama, which shares many morphological and ecological similarities (Beltrán-Leon, 2002), and also for Anchoa tricolor (Spix & Agassiz, 1829) in Sepetiba Bay (Araújo et al., 2008bAraújo, F. G., M. A. Silva, M. C. C. Azevedo & J. N. S. Santos. 2008b. Spawning season, recruitment and early life distribution of Anchoa tricolor (Spix and Agassiz, 1829) in a tropical bay in southeastern Brazil. Brazilian Journal of Biology, 68: 823-829.). Surprisingly, our results partially contrasted with those of Souza-Conceição et al. (2005)Souza-Conceição, J. M., M. Rodrigues-Ribeiro & M. A. Castro-Silva. 2005. Dinâmica populacional, biologia reprodutiva e o ictioplâncton de Cetengraulis edentulus na enseada do Saco dos Limões, Florianópolis, Santa Catarina, Brasil. Revista Brasileira de Zoologia, 22: 953-961. for Cetengraulis edentulus in a bay at Southern Brazil, which spawned in late spring and summer. These variations suggest a plasticity of C. edentulus to adjust their reproductive period in accordance with the local environmental characteristics, of which temperature, photoperiod and food availability have been suggested as determinants for engraulids reproduction in coastal bays (Silva et al., 2003Silva, M. A., F. G. Araújo, M. C. C. Azevedo & P. Mendonça. 2003. Distribuição espacial e temporal de Cetengraulis edentulus (Cuvier, 1829) (Actinopterygii, Engraulidae) na Baía de Sepetiba, Rio de Janeiro, Brasil. Revista Brasileira de Zoologia, 20: 577-581.; Araújo et al., 2008aAraújo, F. G, M. A. Silva, J. N. S. Santos & R. M. Vasconcellos. 2008a. Habitat selection by anchovies (Clupeiformes: Engraulidae) in a tropical bay at Southeastern Brazil. Neotropical Ichthyology, 6: 583-590., 2008bAraújo, F. G., M. A. Silva, M. C. C. Azevedo & J. N. S. Santos. 2008b. Spawning season, recruitment and early life distribution of Anchoa tricolor (Spix and Agassiz, 1829) in a tropical bay in southeastern Brazil. Brazilian Journal of Biology, 68: 823-829.). Comparing our results with those obtained for other C. edentulus or C. mysticetus populations, rising water temperatures seems to be the crucial factor for the spawning season of these species, which seems to start earlier (winter) for populations at lower latitudes (Beltrán-Leon, 2002; this study), and latter (spring) for those at higher latitudes (Souza-Conceição et al., 2005Souza-Conceição, J. M., M. Rodrigues-Ribeiro & M. A. Castro-Silva. 2005. Dinâmica populacional, biologia reprodutiva e o ictioplâncton de Cetengraulis edentulus na enseada do Saco dos Limões, Florianópolis, Santa Catarina, Brasil. Revista Brasileira de Zoologia, 22: 953-961.).
All of our reproductive descriptors converged to similar results for the spawning season of C. edentulus in Guanabara Bay, but with some variations. The gonadosomatic index (GSI) reached higher values between September and December, and coincided with the predominance of gonads in mature stage, indicating the spawning period.Those trends were supported by IRA, however this index has advantages by integrating information of the other two descriptors, providing thus more straightforward and accurate results. According to IRA values, for instance, the reproductive activity of C. edentulus also occurred between September and December, but peaked especially in November. The post-reproductive season (i.e., summer and autumn) was detected by all the three descriptors (i.e., IRA, GSI and stages of gonad maturation), but only IRA was able to reveal the pre-reproductive period at early winter. Our results, therefore, confirm IRA as a useful method that could be applied to accurately identify the temporal evolution of reproductive activity for tropical coastal fish.
Condition factor (K) is another descriptor widely used in studies on reproductive biology of fishes, predicting that individuals with higher K values are in better physiological conditions (Rodrigues-Filho et al., 2011Rodrigues-Filho, J. L., J. R. Verani, A. C. Peret, L. M. Sabinson & J. O. Branco. 2011. The influence of population structure and reproductive aspects of the genus Stellifer (Oken, 1817) on the abundance of species on the southern Brazilian coast. Brazilian Journal of Biology, 71: 991-1002.). In our study, C. edentulus of higher K values were recorded immediately before the periods of greatest reproductive activity, indicating that those individuals store reserves to withstand future losses of energy during gonad maturation. Condition factor did not decreased much during C. edentulus spawning, which may lead to low mortality of newly fertilized eggs and high rates of hatching and larvae survival (Laine & Rajasilta, 1999Laine, P. & M. Rajasilta. 1999. The hatching success of Baltic herring eggs and its relation to female condition. Journal of Experimental Marine Biology and Ecology, 237: 61-73.; Souza-Conceição et al., 2005Souza-Conceição, J. M., M. Rodrigues-Ribeiro & M. A. Castro-Silva. 2005. Dinâmica populacional, biologia reprodutiva e o ictioplâncton de Cetengraulis edentulus na enseada do Saco dos Limões, Florianópolis, Santa Catarina, Brasil. Revista Brasileira de Zoologia, 22: 953-961.). The sharp decrease in K values immediately after the reproductive peak suggests intense energy expenditure during spawning and gonad recovering (Rodrigues-Filho et al., 2011Rodrigues-Filho, J. L., J. R. Verani, A. C. Peret, L. M. Sabinson & J. O. Branco. 2011. The influence of population structure and reproductive aspects of the genus Stellifer (Oken, 1817) on the abundance of species on the southern Brazilian coast. Brazilian Journal of Biology, 71: 991-1002.). Corroborating with condition factor, HSI values were especially high in females throughout the reproductive period, suggesting a high protein synthesis and energy mobilization during gonad development and vitellogenesis (Querol et al., 2002Querol, M. V. M., E. Querol & N. N. A. Gomes. 2002. Fator de condição gonadal, índice hepatossomático e recrutamento como indicadores do período de reprodução de Loricariichthys platymetopon (Osteichthyes, Loricariidae), Bacia do Rio Uruguai Médio, Sul do Brasil. Ilheringia, Série Zoologia, 92: 79-84.).
Fecundity is an important life history trait, since high energy allocation to oocyte production may adversely affect the somatic maintenance and longevity of prolific species (Haag, 2013). Cetengraulis edentulus fecundity (5,973-20,372 oocytes) in Guanabara Bay was similar to other engraulids, such as Engraulis ringens (8,197-12,788 oocytes; Leal et al., 2009Leal, E. M., L. R. Castro & G. Claramunt. 2009. Variability in oocyte size and batch fecundity in anchoveta (Engraulis ringens, Jenyns 1842) from two spawning areas off the Chilean coast. Scientia Marina, 73: 59-66.), Engraulis encrasicolus(Linnaeus, 1758) (14,616 oocytes; Ouattara et al., 2008Ouattara, S., A. Fantodji & M. Ouattara. 2008. Quelques aspects reproductifs de l'anchois (Engraulis encrasicolus) de la pêche artisanale du littoral est ivoirien. Cybium, 32: 201-209.), and Engraulis anchoita (6,270-13,992; Pájaro et al., 2009Pájaro, M., G. J. Macchi, E. Leonarduzzi & J. E. Hansen. 2009. Spawning biomass of Argentine anchovy (Engraulis anchoita) from 1996 to 2004 using Daily Egg Production Method. Journal of the Marine Biological Association of the United Kingdom, 89: 829-837.). The linear and positive relationship between fecundity and the total length (GAM) observed for C. edentulusagrees with the general prediction that increased space in the abdominal cavity allows for a higher number of oocytes. On the other hand, fecundity was negatively correlated with the fullness index (FI), suggesting that C. edentulus decreases their feeding activity as the spawning season approaches. This may be related to the need of females for much space in the abdominal cavity to accommodate the increased gonads by oocyte maturation (Nunes & Hartz, 2006Nunes, D. M. & S. M. Hartz. 2006. Feeding dynamics and ecomorphology of Oligosarcus jenynsii (Gunther, 1864) and Oligosarcus robustus (Menezes, 1969) in the Lagoa Fortaleza, Southern Brazil. Brazilian Journal of Biology, 66: 121-132.). A negative linear relationship (GAM) between FI and GSI values confirm this hypothesis. Fecundity has changed little in fish of low and intermediate GSI, but increased exponentially toward GSI peaking values, indicating that fish with higher gonad contribution in relation to body weight showed a greater investment in number of oocytes. There were no relationship between fecundity and the diameter of oocytes, however the latter was positively correlated with GSI and K, reflecting the increased investment in yolk production. Since changes in condition factor can influence potential fecundity of pelagic fish (Lambert, 2008Lambert, Y. 2008. Why should we closely monitor fecundity in marine fish populations? Journal of Northwest Atlantic Fishery Science, 41: 93-106.), our results indicate that those C. edentulus in better physiological conditions probably produce oocytes with greater amount of yolk, leading to positive effects on GSI.
Our study has also applied contributions for management of C. edentulusstocks in Guanabara Bay. For instance, the reproductive traits found for C. edentulus, such as a broad spawning period (September to December) together with high fecundity can compensate eventual stock depletion by overfishing. The planktivorous habits of C. edentulus together with the prevalent eutrophic conditions in the inner regions of the bay (Lavrado et al., 1991Lavrado, H. P., L. M. Mayr, V. Carvalho & R. Paranhos. 1991. Evolution (1980-1990) of ammonia and dissolved oxygen in Guanabara Bay, RJ, Brazil. Pp. 3234-3245. In: Proceedings of the 7th Symposium on Coastal and Ocean Management. Coastal Zone, 91: 3234-3245.; Sergipense et al., 1999Sergipense, S., E. P. Caramaschi & I. Sazima. 1999. Morfologia e hábitos alimentares de duas espécies de Engraulidae (Teleostei, Clupeiforme) na Baía de Sepetiba, Rio de Janeiro. Revista Brasileira de Oceanografia, 47: 173-188.) could further lead to a fast recovering of C. edentulus stocks, since it is expected that the species do not face any constraints of food availability. However, if the current C. edentulus catches in Guanabara Bay, which is probably greater than the 20,000 tons / year (Jablonski et al., 2006Jablonski, S., A. F. Azevedo & L. H. A. Moreira. 2006. Fisheries and conflicts in Guanabara Bay, Rio de Janeiro, Brazil. Brazilian Archives of Biology and Technology, 49: 9-91.), is posing risks to stocks, our results indicate that early spring (November) is the peak of spawning and is therefore the most critical period that should be closed for fishing. The maintenance of larger individuals (TL > 160 mm), which had a high investment in number and size of oocytes, could have also an important positive effect on the reproductive success of this species in an unstable and eutrophic ecosystem. Therefore, further studies that evaluate the population size and appraise for the maximum sustainable catch levels are crucial to assure the conservation and recovering of C. edentulus stocks in Guanabara Bay.
Acknowledgments
We especially thank the Programa de Pós-graduação em Ciências Biológicas - Biodiversidade Neotropical and Laboratory of Theoretical and the Laboratório de Ictiologia Teórica e Aplicada for providing the logistic support, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarship grants to ACSF. We also thank to Conservas Rubi S.A. for financial and logistic support during the field work.
Literature Cited
- Araújo, F. G, M. A. Silva, J. N. S. Santos & R. M. Vasconcellos. 2008a. Habitat selection by anchovies (Clupeiformes: Engraulidae) in a tropical bay at Southeastern Brazil. Neotropical Ichthyology, 6: 583-590.
- Araújo, F. G., M. A. Silva, M. C. C. Azevedo & J. N. S. Santos. 2008b. Spawning season, recruitment and early life distribution of Anchoa tricolor (Spix and Agassiz, 1829) in a tropical bay in southeastern Brazil. Brazilian Journal of Biology, 68: 823-829.
- Bailly, D., A. A. Agostinho & H. I. Suzuki. 2008. Influence of the flood regime on the reproduction of fish species with different reproductive strategies in the Cuiabá River, upper Pantanal, Brazil. River Research and Applications, 24: 1218-1229.
- Braga, M. R., J. M. R. Aranha & J. R. Vitule. 2008. Reproduction period of Mimagoniates microlepis, from an Atlantic forest stream in Southern Brazil. Brazilian Archives of Biology and Technology, 51: 345-351.
- Burnham, K. P. & D. R. Anderson. 1998. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd edition. Springer-Verlag, New York, USA..
- Dei Tos, C. , G. Barbieri, A. A. Agostinho, L. C. Gomes & H. I. Suzuki. 2002. Ecology of Pimelodus maculatus (Siluriformes) in the Corumbá reservoir, Brazil. Cybium, 26: 275-282.
- Dias, R. M., D. Bailly, R. R. Antônio, H. I. Suzuki & A. A. Agostinho. 2005. Colonization of the Corumbá Reservoir (Corumbá River, Paraná River Basin, Goiás State, Brazil) by the "lambari" Astyanax altiparanae (Tetragonopterinae; Characidae). Brazilian Archives of Biology and Technology, 48: 467-476.
- Haag, W. R. 2013. The role of fecundity and reproductive effort in defining life-history strategies of North American freshwater mussels. Biological Reviews: 1-22.
- Hildebrand, S. F. 1963. Family Engraulidae. Memories Sears Foundation for Marine Research, 1: 152-249.
- Jablonski, S., A. F. Azevedo & L. H. A. Moreira. 2006. Fisheries and conflicts in Guanabara Bay, Rio de Janeiro, Brazil. Brazilian Archives of Biology and Technology, 49: 9-91.
- King, J. R. & G. A. McFarlane. 2003. Marine fish life history strategies: applications to fishery management. Fisheries Management and Ecology, 10: 249-264.
- Kjerfve, B., C. H. A. Ribeiro, G. T. M. Dias, A. M. Filippo & V. S. Quaresma. 1997. Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, 17: 1609-1643.
- Laine, P. & M. Rajasilta. 1999. The hatching success of Baltic herring eggs and its relation to female condition. Journal of Experimental Marine Biology and Ecology, 237: 61-73.
- Lambert, Y. 2008. Why should we closely monitor fecundity in marine fish populations? Journal of Northwest Atlantic Fishery Science, 41: 93-106.
- Lavrado, H. P., L. M. Mayr, V. Carvalho & R. Paranhos. 1991. Evolution (1980-1990) of ammonia and dissolved oxygen in Guanabara Bay, RJ, Brazil. Pp. 3234-3245. In: Proceedings of the 7th Symposium on Coastal and Ocean Management. Coastal Zone, 91: 3234-3245.
- Leal, E. M., L. R. Castro & G. Claramunt. 2009. Variability in oocyte size and batch fecundity in anchoveta (Engraulis ringens, Jenyns 1842) from two spawning areas off the Chilean coast. Scientia Marina, 73: 59-66.
- Macgregor, J. M. & E. D. Houde. 1996. Onshore-Offshore pattern and variability in distribution and abundance of bay anchovy Anchoa mitchilli eggs and larvae in Cheasapeake Bay. Marine Ecology Progress Series, 138: 15-25.
- Mcgowan, M. F. & F. H. Berry. 1983. Clupeiformes: Development and Relationships . Pp. 108-126. In: Blaxter J. H. S. (Ed.). Ontogeny and Systematics of Fishes, 8.
- Nunes, D. M. & S. M. Hartz. 2006. Feeding dynamics and ecomorphology of Oligosarcus jenynsii (Gunther, 1864) and Oligosarcus robustus (Menezes, 1969) in the Lagoa Fortaleza, Southern Brazil. Brazilian Journal of Biology, 66: 121-132.
- Núñez, J. & F. Duponchelle. 2009. Towards a universal scale to assess sexual maturation and relation life history traits in oviparous teleost fishes. Fish Physiology and Biochemistry, 35: 167-180.
- Ouattara, S., A. Fantodji & M. Ouattara. 2008. Quelques aspects reproductifs de l'anchois (Engraulis encrasicolus) de la pêche artisanale du littoral est ivoirien. Cybium, 32: 201-209.
- Pájaro, M., G. J. Macchi, E. Leonarduzzi & J. E. Hansen. 2009. Spawning biomass of Argentine anchovy (Engraulis anchoita) from 1996 to 2004 using Daily Egg Production Method. Journal of the Marine Biological Association of the United Kingdom, 89: 829-837.
- Paranhos, R. & L. M. Mayr. 1993. Seasonal Patterns of Temperature and Salinity in Guanabara Bay, Brazil. Fresenius Environment Bulletin, 2: 647-652.
- Querol, M. V. M., E. Querol & N. N. A. Gomes. 2002. Fator de condição gonadal, índice hepatossomático e recrutamento como indicadores do período de reprodução de Loricariichthys platymetopon (Osteichthyes, Loricariidae), Bacia do Rio Uruguai Médio, Sul do Brasil. Ilheringia, Série Zoologia, 92: 79-84.
- Rodrigues-Filho, J. L., J. R. Verani, A. C. Peret, L. M. Sabinson & J. O. Branco. 2011. The influence of population structure and reproductive aspects of the genus Stellifer (Oken, 1817) on the abundance of species on the southern Brazilian coast. Brazilian Journal of Biology, 71: 991-1002.
- Sergipense, S., E. P. Caramaschi & I. Sazima. 1999. Morfologia e hábitos alimentares de duas espécies de Engraulidae (Teleostei, Clupeiforme) na Baía de Sepetiba, Rio de Janeiro. Revista Brasileira de Oceanografia, 47: 173-188.
- Silva, M. A., F. G. Araújo, M. C. C. Azevedo & P. Mendonça. 2003. Distribuição espacial e temporal de Cetengraulis edentulus (Cuvier, 1829) (Actinopterygii, Engraulidae) na Baía de Sepetiba, Rio de Janeiro, Brasil. Revista Brasileira de Zoologia, 20: 577-581.
- Silva, G. C., A. C. L. Castro & E. A. Gubiani. 2005. Estrutura populacional e indicadores reprodutivos de Scomberomorus brasiliensis no litoral ocidental maranhense. Acta Scientarium. Biological Sciences, 27: 383-389.
- Souza-Conceição, J. M., M. Rodrigues-Ribeiro & M. A. Castro-Silva. 2005. Dinâmica populacional, biologia reprodutiva e o ictioplâncton de Cetengraulis edentulus na enseada do Saco dos Limões, Florianópolis, Santa Catarina, Brasil. Revista Brasileira de Zoologia, 22: 953-961.
- Souza, L. M. & P. T. Chaves. 2007. Atividade reprodutiva de peixes e o defeso da pesca de arrasto no litoral norte de Santa Catarina, Brasil. Revista Brasileira de Zoologia, 24: 1113-1121.
- Valentin, J. L., D. R. Tenenbaum, A. Bonecker, S. L. C. Bonecker, C. R. Nogueira, R. Paranhos & M. C. Villac. 1999. Caractéristiques hydrologiques de La Baie de Guanabara (Rio de Janeiro, Brésil). Journal de Recherche Oceanographique, 24: 33-41.
- Whitehead, P. J. P. 1977. Engraulidae. FAO species identification sheets for fishery purposes. Western Central Atlantic (Fishing Area 31), 2 .
- Whitehead, P. J. P. 1988. Clupeoid of the world (Suborder Clupeoidei): an annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. FAO Fisheries Synopsis, Rome, 7 (125), 1-579 .
Publication Dates
-
Publication in this collection
02 Dec 2014 -
Date of issue
Oct-Dec 2014
History
-
Received
05 Nov 2013 -
Accepted
30 June 2014