Abstracts
Eigenmannia species are widely distributed in the Neotropics, with eight valid species currently recognized. Populations of Eigenmannia from three locations in the eastern Amazon were investigated using cytogenetic and morphological techniques, revealing two taxa designated here as Eigenmannia sp. "A" and Eigenmannia sp. "B". The species differ in three morphometric characters, two meristic characters, and one osteological character. Eigenmannia sp. "A" presents 2n = 34 (22 m/sm+12 st/a) and Eigenmannia sp. "B" presents 2n = 38 (14 m/sm+24st/a) and simple differentiated sex chromosomes of the type XX/XY. In both species the Constitutive Heterochromatin (CH) rich in A-T bases is distributed in the centromeric region of all chromosomes. Eigenmannia sp. "B" also presents CH blocks in the interstitial region of chromosome pairs 8, 9 and X which are positively stained with CMA3, indicating G-C rich regions. The NOR is located on the short arm of chromosome pair 17 of Eigenmannia sp. "A" and on the short arm of pair 14 of Eigenmannia sp. "B". FISH with rDNA probes hybridized to different-sized regions between homologs, suggesting heteromorphism. The differentiation of the X chromosome in Eigenmannia sp. "B" could be the result of amplification of repetitive DNA sequences.
Cytogenetics; FISH; Knife fishes; Morphology; Sex chromosomes
Espécies de Eigenmannia estão amplamente distribuídas na região Neotropical, com oito espécies válidas atualmente reconhecidas. Populações de Eigenmannia de três localidades do leste da Amazônia foram investigadas usando técnicas citogenéticas e morfológicas, revelando dois táxons designados aqui como Eigenmannia sp. "A" e Eigenmannia sp. "B". As espécies diferem em três caracteres morfométricos, dois merísticos e um osteológico. Eigenmannia sp. "A" apresenta 2n = 34 (22 m/sm+12st/a) e Eigenmannia sp. "B" apresenta 2n = 38 (14 m/sm+24st/a) e cromossomos sexuais de diferenciação simples, do tipo XX/XY. Em ambas espécies a Heterocromatina Constitutiva (HC) rica em bases A-T está distribuída na região centromérica de todos os cromossomos. Eigenmannia sp. "B" também apresenta blocos de HC na região intersticial dos pares cromossômicos 8, 9 e X que coraram positivamente para CMA3, indicando regiões ricas em G-C. A NOR está localizada no braço curto do par 17 em Eigenmannia sp. "A" e no braço curto do par 14 em Eigenmannia sp. "B". FISH com sondas de rDNA hibridizaram em regiões de tamanhos diferentes entre os homólogos, sugerindo heteromorfismo. A diferenciação do cromossomo X em Eigenmannia sp. "B" pode ser o resultado de amplificação de sequências repetitivas de DNA.
Introduction
Neotropical electric fishes of the genus Eigenmannia Jordan & Evermann, 1896, popularly known as "knife fishes", "tuviras", "peixe-faca" or "sarapós", present an ample distribution from Panama to northern Argentina (Mago Leccia, 1978Mago Leccia, F. 1978. Los peces de la familia Sternopygidae de Venezuela. Acta Cientifica Venezolana, 29(suppl. 1): 1-91.; Albert, 2001Albert, J. S. 2001. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Miscellaneous Publications Museum of Zoology, University of Michigan, 190: 1-127.; Albert & Crampton, 2005Albert, J. S. & W. G. R. Crampton. 2005. Diversity and phylogeny of Neotropical electric fishes (Gymnotiformes). Pp. 360-409. In: Bullock, T. H., C. D. Hopkins & R. R. Fay (Eds.). Electroreception. New York, Springer.). Eight valid species of Eigenmannia are recognized: E. humboldtii (Steindachner, 1878), E. limbata (Schreiner & Miranda Ribeiro, 1903), E. macrops (Boulenger, 1897), E. microstoma (Reinhardt, 1852), E. nigra Mago-Leccia, 1994, E. trilineata López & Castello, 1966, E. vicentespelaeaTriques, 1996Triques, M. L. 1996. Eigenmannia vicentespelaea, a new species of cave dwelling electrogenic Neotropical fish (Ostariophysi: Gymnotiformes: Sternopygidae). Revue Française D'aquariologie et Herpetologie, 23: 1-4. and E. virescens (Valenciennes, 1842) (Albert, 2003Albert, J. S. 2003. Family Sternopygidae (Glass Knifefishes, Rattail Knifefishes). Pp. 487-491. In: Reis, R. E., S. O. Kullander & C. J. Ferraris, Jr. (Orgs.). Check list of the freshwater fishes of South and Central America. Porto Alegre, Edipucrs.).
Albert (2001)Albert, J. S. 2001. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Miscellaneous Publications Museum of Zoology, University of Michigan, 190: 1-127. proposed a phylogeny for Gymnotiformes, though did not identify synapomophies for Eigenmannia, classifying the genus in three groups of species (1) Eigenmannia gr. microstoma, containing E. microstoma, E. humboldtii, E. limbata and E. nigra; (2) Eigenmannia gr. virescens, containing E. virescens, E. trilineata and E. vicentespelaea; and (3) Eigenmannia gr. macrops, as monospecific. All groups are diagnosed by morphological characters except Eigenmannia gr. macrops, which was delimited without presenting any further information on diagnostic characters.
Cytogenetic studies of Eigenmannia demonstrate a high level of karyotypic diversity (Table 1). Eigenmannia gr. virescens is the most studied group, presenting karyotypic variation which ranges from 2n = 28 in Eigenmannia sp. 1 (Almeida-Toledo et al., 1988Almeida Toledo, L. F., E. Viegas-Péquignot, F. Foresti, S. A. Toledo Filho & B. Dutrillaux. 1988. BrdU replication patterns demonstrating chromosome homoeologies in two fish species, genus Eigenmannia. Cytogenetics and Cell Genetics, 48: 117-120.), to 2n = 38 in Eigenmannia virescens (Almeida-Toledo et al., 2001Almeida-Toledo, L. F., F. Foresti, E. V. Péquignot & M. F. Z. Daniel-Silva. 2001. XX:XY sex chromosome system with X heterochromatinization: an early stage of sex chromosome differentiation in the Neotropical electric eel Eigenmannia virescens. Cytogenetics and Cell Genetics, 95: 73-78., 2002Almeida-Toledo, L. F., M. F. Z. Daniel-Silva, C. B. Moysés, S. B. A. Fonteles, C. E. Lopes, A. Akama & F. Foresti. 2002. Chromosome evolution in fish: sex chromosome variability in Eigenmannia virescens (Gymnotiformes: Sternopygidae). Cytogenetic and Genome Research, 99: 164-169.; Silva et al., 2009Silva, D. S., S. S. R. Milhomem, J. C. Pieczarka & C. Y. Nagamachi. 2009. Cytogenetic studies in Eigenmannia virescens (Sternopygidae, Gymnotiformes) and new inferences on the origin of sex chromosomes in the Eigenmannia genus. BMC Genetics, 10: 74 (1-8).). Previous results demonstrate the existence of differentiated sex chromosomes in the species of the E. gr. virescens, with two simple sex chromosome systems XX/XY (Almeida-Toledo et al., 2001Almeida-Toledo, L. F., F. Foresti, E. V. Péquignot & M. F. Z. Daniel-Silva. 2001. XX:XY sex chromosome system with X heterochromatinization: an early stage of sex chromosome differentiation in the Neotropical electric eel Eigenmannia virescens. Cytogenetics and Cell Genetics, 95: 73-78.) and ZZ/ZW (Almeida-Toledo et al., 2002Almeida-Toledo, L. F., M. F. Z. Daniel-Silva, C. B. Moysés, S. B. A. Fonteles, C. E. Lopes, A. Akama & F. Foresti. 2002. Chromosome evolution in fish: sex chromosome variability in Eigenmannia virescens (Gymnotiformes: Sternopygidae). Cytogenetic and Genome Research, 99: 164-169.; Silva et al., 2009Silva, D. S., S. S. R. Milhomem, J. C. Pieczarka & C. Y. Nagamachi. 2009. Cytogenetic studies in Eigenmannia virescens (Sternopygidae, Gymnotiformes) and new inferences on the origin of sex chromosomes in the Eigenmannia genus. BMC Genetics, 10: 74 (1-8).), as well as the existence of multiple sex chromosome systems X1X1X2X2/X1X2Y, in Eigenmannia sp. "2" (Almeida-Toledo et al., 1984Almeida-Toledo, L. F., M. F. Z. Daniel-Silva, C. B. Moysés, S. B. A. Fonteles, C. E. Lopes, A. Akama & F. Foresti. 2002. Chromosome evolution in fish: sex chromosome variability in Eigenmannia virescens (Gymnotiformes: Sternopygidae). Cytogenetic and Genome Research, 99: 164-169.) and E. trilineata (Fernandes et al., 2010Fernandes, C. A., D. Bailly, V. F. B. Silva & I. C. Martins-Santos. 2010. System of multiple sex chromosomes in Eigenmannia trilineata López & Castello, 1966 (Sternopygidae, Gymnotiformes) from Iguatemi River Basin, MS, Brazil. Cytologia, 75: 463-466.).
Although the taxa described in Eigenmannia are considered valid, various authors suggest that the species in this genus represent a complex, which is difficult to resolve (Shibatta, 1993Shibatta, O. A. 1993. Estudo comparativo ao nível intra-específico de Salminus hilarii, Pimelodus cf. maculatus, Leporinus cf. elongatus e Eigenmannia cf. virescens (Pisces, Ostariophysi) das bacias do Alto Paraná e São Francisco, através da análise morfométrica multivariada. Unpublished Ph. D. Dissertation, Universidade Federal de São Carlos, São Carlos.; Campos-da-Paz, 1997Campos-da-Paz, R. 1997. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). Unpublished Ph. D. Dissertation, Universidade de São Paulo, São Paulo , 336p.). This is corroborated by cytogenetic studies that demonstrate karyotypic variation among samples both within and between hydrological basins (Moysés et al., 2005Moysés, C. B., S. Mockford, L. F. Almeida-Toledo & J. M. Wright. 2005. Nine polymorphic microsatellite loci in the Neotropical electric eel Eigenmannia (Teleostei, Gymnotiformes). Molecular Ecology Notes 5: 7-9.).
We investigated three populations of Eigenmannia from streams of eastern Amazonia, using cytogenetic, morphometric, meristic and osteological analyses. The results reveal the existence of two cryptic species, and the description of a new occurrence of sex chromosomes of the simple XX/XY type for Eigenmannia in the Amazon basin.
Karyotypic studies in the genus Eigenmannia. Legend: 2n = diploid number; SC = Sex Chromosomes; ND = Not differentiated; KF = Karyotypic Formula; NOR = Nucleolar Organizing Region; CH = Constitutive Heterochromatin; CB = C band; p = short arm; q = long arm; m = metacentric; sm = submetacentric; st = subtelocentric; a = acrocentric. Symbols: (♀) = Female; (♂) = Male; (+) = technique performed on karyotype; (-) = technique not performed on karyotype.
Material and Methods
Samples. Fifty three samples were collected from three localities in eastern Amazonia, Pará State, Brazil (Fig. 1): two localities in the municipality of Capanema, rio Quatipuru basin and one in the municipality of São Francisco, rio Marapanim basin. All specimens were deposited in the ichthyology collection of the Museu Paraense Emílio Goeldi, Pará State, Brazil (MPEG) (Table 2). Samples were collected using seine nets, and kept alive with portable aeration in thermally protected receptacles for transport to the laboratory. Sample collection was made under licence 020/2005 (ICMBio Registration: 207419).
Localities of the samples of Eigenmannia in north-eastern Pará State. ? = undetermined sex.
Morphometry, meristics and osteology. Measurements were made on the left hand side of each specimen using a digital calliper to a precision of 0.1 mm while viewed under a stereomicroscope. Morphometric analyses were based on Mago Leccia (1978), Triques (1996) and Crampton et al. (2004)Milhomem, S. S. R., W. G. R. Crampton, J. C. Pieczarka, G. H. Shetka, D. S. Silva & C. Y. Nagamachi. 2012b. Gymnotus capanema, a new species of electric knife fish (Gymnotiformes, Gymnotidae) from eastern Amazonia, with comments on an unusual karyotype. Journal of Fish Biology, 80: 802-815.. Taxonomic parameters for Gymnotiformes were followed where measures of the body are described in proportion to the length as measured from the tip of the snout to the end of the anal fin (LEA - Length to the end of the anal fin) and measures of parts of the head described in proportion to the Head Length (HL). Meristic analyses included: number of rays of the pectoral fin, number of lateral line scales (counting from the first scale with a lateral line tube to the end of the anal fin) and the number of scales above the lateral line (counted at the highest point along the body, approximately in line with the distal portion of the longest ray of the anal fin). Specimens were cleared and stained following Taylor & Van Dyke (1985)Taylor, W. R. & G. C. Van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9: 107-119.. The osteological nomenclature of the maxilla follows Lundberg & Mago-Leccia (1986)Lundberg, J. G. & F. Mago-Leccia. 1986. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with description of four new species. Proceedings of the Academy Natural Sciences of Philadelphia, 138: 53-85. and de Santana & Crampton (2011)de Santana, C. D. & W. G. R. Crampton. 2011. Phylogenetic interrelationships, taxonomy, and reductive evolution in the Neotropical electric fish genus Hypopygus (Teleostei, Ostariophysi, Gymnotiformes). Zoological Journal of the Linnean Society, 163: 1096-1156..
Cytogenetic methods. Metaphase chromosomes were obtained following the protocol of Bertollo et al. (1978)Bertollo, L. A. C., C. S. Takahashi & O. Moreira Filho. 1978. Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Revista Brasileira de Genética, 1: 103-120.. Conventional analyses were performed, including staining with Giemsa (Merck), C-banding (Sumner, 1972Sumner, A. T. 1972. A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research, 75: 304-306.), impregnation with silver nitrate (Ag-NOR) (Howell & Black, 1980Howell, W. M. & D. A. Black. 1980. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia, 36: 1014-1015.), staining with the fluorochrome DAPI (Pieczarka et al., 2006Pieczarka, J. C., C. Y. Nagamachi, A. C. P. de Souza, S. S. R. Milhomem, R. R. Castro & A. L. Nascimento. 2006. An adaptation of DAPI- Banding to fishes chromosomes. Caryologia, 59: 43-46.), and staining with Chromomycin A3 (CMA3) (Schweizer, 1980Schweizer, D. 1980. Simultaneous fluorescent staining of R bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenetics and Cell Genetics, 27: 190-193.). The gonads were removed and prepared as a smear with a 32x20mm glass slide and observed under a stereomicroscope to determine the sex of the individuals.
Fluorescence in situ Hybridization (FISH) was performed with probes for 18S ribossomal DNA (rDNA 18S) obtained from the species Prochilodus argenteus Agassiz, 1829, marked with biotin or digoxigenin by nick translation (Hatanaka & Galetti Jr., 2004Hatanaka, T. & P. M. Galetti, Jr. 2004. Mapping of the18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica, 122: 239-244.). The in situ hybridization was detected using avidin (Cy3 or FITC) or anti-digoxigenin (FITC). The chromosomes were classified and measured following Guerra (1986)Guerra, M. S. 1986. Reviewing the chromosome nomenclature of Levan et al. Revista Brasileira de Genética, 9: 741-743..
Abbreviations used in text are: HL - head length, LEA - length to the end of the anal fin, DAPI - 4',6-diamidino-2-phenylindole, Vector Laboratories, Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO).
Results
Morphometric, meristic and osteological analyses. Morphometry, meristics and osteology revealed the existence of two distinct taxa, identified as belonging to the Eigenmannia virescens group, based on the characters proposed by Albert (2001)Albert, J. S. 2001. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Miscellaneous Publications Museum of Zoology, University of Michigan, 190: 1-127.. Forty-nine individuals from balneário Marapanim, lago do Segredo and Igarapé do Açaiteuazinho were designated as Eigenmannia sp. "A" (Fig. 2), and four individuals from do Açaiteuazinho were designated as Eigenmannia sp. "B" (Fig. 3). The populations here analyzed, although allocated in the species group Eigenmannia virescens, do not fit diagnoses of the species already described.
Left lateral view of fixed specimen (a), MPEG 27068, 102.7 mm LEA, and live specimen (b) of Eigenmannia sp. "A", lago Segredo, rio Quatipuru, municipality of Capanema, Pará, Brazil.
Left lateral view of fixed specimen (a) and live specimen (b) of Eigenmannia sp. "B", MPEG 27066, 131.8 mm LEA, from the igarapé do Açaiteuazinho, rio Quatipuru, municipality of Capanema, Pará, Brazil.
Eigenmannia sp. "A" presents a greater distance between the eye and naris compared to Eigenmannia sp. "B" (8.5-11.1% vs. 7.5% HL), a smaller orbital diameter (13.6-17.2% vs. 21.5-24.3% HL) and a shorter superior maxilla (16.3-19.0% vs. 22.3-24.0% HL), respectively. Two meristic characters also differ between the two species: the number of pectoral-fin rays, 15-16 in Eigenmannia sp. "A" vs. 17 in Eigenmannia sp. "B", and the number of lateral line scales, 122-129 in Eigenmannia sp. "A" vs. 115-119 in Eigenmannia sp. "B" (Table 3).
Morphometric and meristic data for Eigenmannia sp. "A" and Eigenmannia sp. "B". Legend: Min: Minimum; Max: Maximum; SD: Standard deviation; N: number of specimens analysed.
The morphology of the maxilla in Eigenmannia sp. "A" presents a small antero-dorsal process which is about half the size of the anterior naris, and the descending process is large, approximately the same width as the anterior naris (Fig. 4a). In Eigenmannia sp. "B", the antero-dorsal process is hypertrophied, being about the same size as the anterior naris, and the descending process is narrow (about half the width of the anterior naris) (Fig. 4b).
The two species present a yellow colouration when preserved, with three longitudinal stripes, one above the lateral line, one along the proximal portion of the pterygiophores of the anal fin and one along the base of the anal fin. They also present a dark band along the body localized between the lateral line and the stripe, which runs along the proximal portion of the pterygiophores of the anal fin.
Inverted lateral view, of the right maxilla of (a) Eigenmannia sp. "A" (MPEG 27061, 111.8 mm LEA) and (b) Eigenmannia sp. "B" (MPEG 27066, 117.6 mm LEA). Arrow indicates antero-dorsal process. Scale bar of 1 mm.
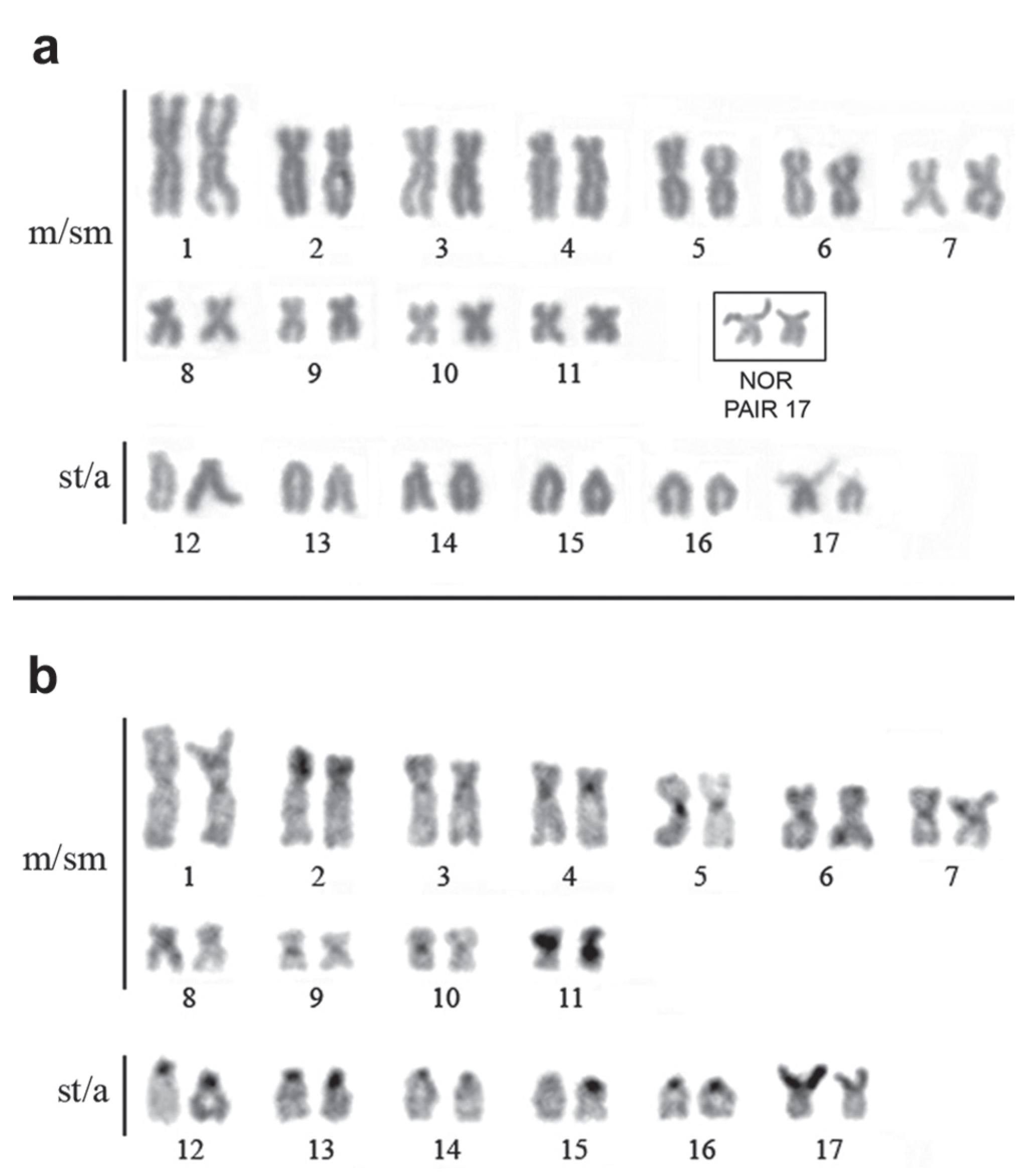
Cytogenetic analyses. Approximately 30 metaphase cells of each specimen were examined, revealing a difference in the karyotype of the two species. The species Eigenmannia sp. "A" presents 2n = 34 chromosomes and a karyotypic formula (KF) of 22 m/sm and 12 st/a without the presence of differentiated sex chromosomes (Fig. 5). Eigenmannia sp. "B" presents 2n = 38 and a KF of 14 m/sm and 24 st/a (Fig. 6). In males, a heteromorphic pair of acrocentric chromosomes was observed, with one of the homologous chromosomes distinctly larger than the other (Fig. 6b). This heteromorphism was not observed in the females, where the homologous chromosomes were both large (Fig. 6a).
Karyotype of Eigenmannia sp. "A" with 34 chromosomes. Conventional staining (a) and C banding (b). Box inset: chromosome 17 (p) with NOR. Legend: m/sm = metacentric/submetacentric and st/a = subtelocentric/acrocentric.
Karyotype of Eigenmania sp. "B" with 2n = 38, XX/XY from the rio Quatipuru. Male karyotype stained with Giemsa (a) and C banding (c). Box inset: pair 14, with NOR. Female karyotype stained with Giemsa (b) and C banding (d). Legend: m/sm = metacentric/submetacentric and st/a = subtelocentric/acrocentric.
The Constitutive Heterochromatin (CH) is mainly distributed in the centromeric region of all chromosomes of both species (Figs. 5b, 6c-d). In Eigenmannia sp. "B" CH blocks were also observed in the interstitial regions of acrocentric chromosomes 8, 9 and 10. It was also possible to note a large CH block in the interstitial region of the X chromosome, which was absent in the Y (Figs. 6c-d).
The NOR was localized on the short arm of chromosome pair 17 in Eigenmannia sp. "A" and on the short arm of chromosome pair 14 in Eigenmannia sp. "B" (Figs. 5-6 box), which was also found to present size heteromorphism between the homologous chromosomes of the two species.
DAPI fluorescence was found in the centromeric regions of all chromosome pairs of both species, consistent with the C-banding results (Figs.7a, 8a-b). CMA3 marked the short arm of chromosome pair 17 most intensely in Eigenmannia sp. "A" and the short arm of chromosome pair 14 most intensely in Eigenmannia sp. "B". Additionally, in Eigenmannia sp. "B" it was possible to observe intense marking of chromosome pairs 8 and 9, coincident with the CH blocks (Figs. 8c-d). FISH with 18S rDNA probes hybridized the region of the short arm of chromosome pair 17 in Eigenmannia sp. "A", and the region of the short arm of chromosome pair 14 in Eigenmannia sp. "B" (Figs. 8e-f).
(a) DAPI fluorescent banding coinciding with C banding. (b) CMA3 fluorescent banding is coincident with the NOR region on short arm of pair 17 of Eigenmannia sp. "A". (c) FISH with 18S rDNA probes hybridizing on the short arm of chromosome 17 (arrows).
DAPI fluorescent banding showing centromeric regions of both males (a) and females (b) of Eigenmannia sp. "B". CMA3 fluorescent banding hybridizing areas, which coincide with the NOR (Fig. 6) on chromosome 14 of males (c) and females (d), and strong signal on pairs 8, 9 and the X - inset boxes. FISH with 18S rDNA probes of Eigenmannia sp. "B", hybridizing on the short arm of chromosome 14 (arrows) of males (e, f).
Discussion
Taxonomic considerations. The morphometric, meristic and osteological divergences among Eigenmannia sp. "A" and Eigenmannia sp. "B" indicate the occurrence of two distinct lineages, corroborated by the karyotypic differences. The karyotypic differences (2n = 34 vs. 2n = 38, XX/XY) are sufficient to act as a post-zygotic reproductive isolation mechanism (King, 1993King, M. 1993. Species evolution: the role of chromosomal change. Cambridge, UK, Cambridge University Press, 336p.). As such, we find that the two cryptic species currently occur in sympatry in the rio Quatipuru basin (specifically at igarapé do Açaiteuazinho), but that Eigenmannia sp. "A" also occurs allopatrically in the rio Marapanim basin.
The species Eigenmannia sp. "A" and Eigenmannia sp. "B" are distinct from E. virescens, E. macrops, E. humboldtii, E. limbata and E. nigra by the presence of three longitudinal stripes (vs. uniform colouration without stripes). Additionally, they are distinct from E. humboldtii, E. limbata and E. nigra due to the colouration pattern of the anal fin, which is hyaline (vs. darkened along the distal margin in E. humboldtii and E. limbata or uniformly darkened in E. nigra).
Eigenmannia sp. "A" shares the presence of stripes along the body with E. trilineata, E. vicentespelaea and E. microstoma. It differs from E. trilineata and E. microstoma in the size of the antero-dorsal process of the maxilla, being equivalent to half the size of the posterior naris (vs. equivalent to the size of the posterior naris) and in the suborbital height, 22.3-29.9% HL (vs. 32.5-46.6%; 29.9-40.8%, respectively). It is distinguished from E. vicentespelaea by the number of pectoral fin rays, 15-16 vs. 17-19 and by the number of scales above the lateral line, 12-14 vs. 7-8.
Similarly, Eigenmannia sp. "B" shares the presence of longitudinal stripes on the body with E. trilineata, E. vicentespelaea and E. microstoma. It differs from E. trilineata and E. microstoma in the suborbital height, 22.6-25.2% HL (vs. 32.5-46.6%; 29.9-40.8%, respectively), and can be distinguished from E. microstoma by the length of the superior maxilla, 22.3-24.0% HL vs. 17.5-19.2%; and by the head height at the supraoccipital, 72.1-72.5% HL vs. 76.1-85.1%. Finally, it differs from E. vicentespelaea in the number of scales above the lateral line, 12 vs. 7-8 and in the body height, 15.4-15.9% LEA vs. 10.5-14.5%.
Cytogenetic considerations. The analysis of Gymnotiformes has revealed significant cytogenetic variation between populations (Milhomem et al., 2008Milhomem, S. S. R., J. C. Pieczarka, W. G. R. Crampton, D. S. Silva, A. C. P. Souza, J. R. Carvalho Jr. & C. Y. Nagamachi. 2008. Chromosomal evidence for a putative cryptic species in the Gymnotus carapo species-complex (Gymnotiformes, Gymnotidae). BMC Genetics, 9: 75 (1-10).; Nagamachi et al., 2010Nagamachi, C. Y., J. C. Pieczarka, S. S. R. Milhomem, P. C. M. O'Brien, A. C. P. de Souza & M. A. Ferguson-Smith. 2010. Multiple rearrangements in cryptic species of electric knifefish, Gymnotus carapo (Gymnotidae, Gymnotiformes) revealed by chromosome painting. BMC Genetics, 11: 28 (1-9).). The karyotypic diversity encountered in the genus Eigenmannia, reflects to some extent the population structure where the formation of small aggregations with low dispersion favours the fixation of chromosome rearrangements (Moysés et al., 2005Moysés, C. B., S. Mockford, L. F. Almeida-Toledo & J. M. Wright. 2005. Nine polymorphic microsatellite loci in the Neotropical electric eel Eigenmannia (Teleostei, Gymnotiformes). Molecular Ecology Notes 5: 7-9.; Silva et al., 2009). The karyotype 2n = 34 (22 m/sm+12st/a) described by Moysés et al. (2010)Moysés, C. B., M. F. Z. Daniel-Silva, C. E. Lopes & L. F. Almeida-Toledo. 2010. Cytotype-specific ISSR profiles and karyotypes in the Neotropical genus Eigenmannia (Teleostei: Gymnotiformes). Genetica, 138: 179-189. for Eigenmannia sp. in the rio São Francisco basin is different from that of Eigenmannia sp. "A" (24 m/sm+10st/a) based on KF. This difference in KF could be a result of a pericentric inversion event, which would also act as a post-zygotic isolation mechanism (King, 1993).
The CH encountered in the centromeric region of chromosomes of Eigenmannia sp. "A" and Eigenmannia sp. "B" is typical of Gymnotiformes (Silva et al., 2008Silva, D. S., S. S. R. Milhomem, A. C. P. de Souza, J. C. Pieczarka & C. Y. Nagamachi. 2008. A conserved karyotype of Sternopygus macrurus (Sternopygidae, Gymnotyformes) in the Amazon region: differences from other hydrographic basins suggest cryptic speciation. Micron, 39: 1251-1254.; Milhomem et al., 2012aMilhomem, S. S. R., W. G. R. Crampton, J. C. Pieczarka, D. S. Silva, A. L. Cardoso, P. C. da Silva, J. A. Oliveira & C. Y. Nagamachi. 2012a. Chromosomal and electric signal diversity in three sympatric electric knifefish species (Gymnotus, Gymnotidae) from the Central Amazon floodplain. Reviews in Fish Biology and Fisheries, 22: 485-497.), as well as being observed in other vertebrate species (Sumner, 2003Sumner, A. T. 2003. Chromosomes: organization and function. Oxford, Blackwell 287p.; Gomes et al., 2012Gomes, A. J. B, C. Y. Nagamachi, L. R. R. Rodrigues, S. G. Farias, J. D. Rissino & J. C. Pieczarka. 2012. Karyotypic variation in Rhinophylla pumilio Peters, 1865 and comparative analysis with representatives of two subfamilies of Phyllostomidae (Chiroptera). Comparative Cytogenetics, 6: 213-225.). C bands are usually positively marked by DAPI as they are composed predominantly of A-T bases (Silva et al., 2009Silva, D. S., S. S. R. Milhomem, J. C. Pieczarka & C. Y. Nagamachi. 2009. Cytogenetic studies in Eigenmannia virescens (Sternopygidae, Gymnotiformes) and new inferences on the origin of sex chromosomes in the Eigenmannia genus. BMC Genetics, 10: 74 (1-8).). However, in Eigenmannia sp. "B" the CH blocks in the interstitial regions of chromosomes 8, 9 and X were marked strongly with CMA3 (indicating G-C rich regions), demonstrating that this region presents a distinct composition compared to other CH classes present in the autosomes.
In the genus Eigenmannia, the NOR is simple, frequently located on the short arm of a subtelocentric/acrocentric chromosome (Table 1), as was observed in both Eigenmannia sp. "A" and sp. "B" (17p and 14p, respectively). However, the species Eigenmannia sp. 1 and Eigenmannia sp. 2 (Almeida-Toledo et al., 1984Almeida Toledo, L. F., H. Foresti & S. de Almeida Toledo Filho. 1984. Complex sex chromosome system in Eigenmannia sp. (Pisces, Gymnotiformes). Genetica, 64: 165-169., 1988Almeida Toledo, L. F., E. Viegas-Péquignot, F. Foresti, S. A. Toledo Filho & B. Dutrillaux. 1988. BrdU replication patterns demonstrating chromosome homoeologies in two fish species, genus Eigenmannia. Cytogenetics and Cell Genetics, 48: 117-120., 2000Almeida-Toledo, L. F., F. Foresti, M. F. Z. Daniel & S. A. Toledo-Filho. 2000. Sex chromosome evolution in fish: the formation of the neo-Y chromosome in Eigenmannia (Gymnotiformes). Chromosoma, 109: 197-200.), presented the NOR on the long arm of a metacentric chromosome, pair 10 (Table 1). As such, in order to confirm that the simple NOR is a shared character among the different species it is important to know whether the rDNA sequence sites are always located on the same chromosome in the different species, as demonstrated for Gymnotus gr. carapo (Milhomem et al., 2013Milhomem, S. S. R., P. C. Scacchetti, J. C. Pieczarka, M. A. Ferguson-Smith, J. C. Pansonato-Alves, P. C. M. O'Brien, F. Foresti & C. Y. Nagamachi. 2013. Are NORs always located on homeologous chromosomes? A FISH investigation with rDNA and whole chromosome probes in Gymnotus fishes (Gymnotiformes). PloS One, 8: e55608 (1-6).).
In both species the NOR is positively stained by CMA3, indicating that this region is interlaced with sequences rich in G-C bases (Nascimento et al., 2006Nascimento, A. L., A. C. P. Souza, E. Feldberg, J. R. Carvalho Jr, R. M. S. Barros, J. C. Pieczarka & C. Y. Nagamachi. 2006. Cytogenetic analysis on Pterophyllum scalare (Perciformes, Cichlidae) from Jari River, Pará State. Caryologia, 59: 138-143.; Silva et al., 2008Silva, D. S., S. S. R. Milhomem, A. C. P. de Souza, J. C. Pieczarka & C. Y. Nagamachi. 2008. A conserved karyotype of Sternopygus macrurus (Sternopygidae, Gymnotyformes) in the Amazon region: differences from other hydrographic basins suggest cryptic speciation. Micron, 39: 1251-1254.; Milhomem et al., 2007, 2008, 2012a, 2012bMilhomem, S. S. R., J. C. Pieczarka, W. G. R. Crampton, A. C. P. Souza, J. R. Carvalho Jr. & C. Y. Nagamachi. 2007. Differences in karyotype between two sympatric species of Gymnotus (Gymnotiformes: Gymnotidae) from the eastern amazon of Brazil. Zootaxa, 1397: 55-62.). FISH with rDNA probes hybridized to different-sized regions between homologs, suggests that heteromorphism in the size of the NOR could either be associated with differences in transcription activity of the ribosomal genes or may be the result of differences in the copy numbers of the ribosomal genes (Oliveira et al., 2009Oliveira, C., F. Foresti & A. W. S. Hilsdorf. 2009. Genetics of Neotropical fish: from chromosomes to populations. Fish Physiology and Biochemistry, 35: 81-100.; Milhomem et al., 2013Milhomem, S. S. R., P. C. Scacchetti, J. C. Pieczarka, M. A. Ferguson-Smith, J. C. Pansonato-Alves, P. C. M. O'Brien, F. Foresti & C. Y. Nagamachi. 2013. Are NORs always located on homeologous chromosomes? A FISH investigation with rDNA and whole chromosome probes in Gymnotus fishes (Gymnotiformes). PloS One, 8: e55608 (1-6).).
Sex chromosomes inEigenmannia. In fishes, sex chromosomes are not present in basal taxa and their origin within genera or families is probably convergent. Additional information suggests that the origin of sex chromosomes in Neotropical fishes is recent, with diversification in some taxa approximately 7-10 Ma (Charlesworth et al., 2005Charlesworth, D., B. Charlesworth & G. Marais. 2005. Steps in the evolution of heteromorphic sex chromosomes. Heredity, 95: 118-128.; Cioffi et al., 2011Cioffi, M. B., A. Sánchez, J. A. Marchal, N. Kosyakova, T. Liehr, V. Trifonov & L. A. C. Bertollo. 2011. Whole chromosome painting reveals independent origin of sex chromosomes in closely related forms of a fish species. Genetica, 139: 1065-1072.; Henning et al., 2011Henning, F., C. B. Moysés, D. Calcagnotto, A. Meyer & L. F. Almeida-Toledo. 2011. Independent fusions and recent origins of sex chromosomes in the evolution and diversification of glass knife fishes (Eigenmannia). Heredity, 106: 391-400.).
Different sex chromosome systems have previously been described in Eigenmannia, including both simple XX/XY and ZZ/ZW systems and a multiple X1X1X2X2/X1X2Y system (Table 1), suggesting that these different systems do not have a common origin. The species Eigenmannia sp. 2 (Almeida-Toledo et al., 1984Almeida Toledo, L. F., H. Foresti & S. de Almeida Toledo Filho. 1984. Complex sex chromosome system in Eigenmannia sp. (Pisces, Gymnotiformes). Genetica, 64: 165-169., 1988Almeida Toledo, L. F., E. Viegas-Péquignot, F. Foresti, S. A. Toledo Filho & B. Dutrillaux. 1988. BrdU replication patterns demonstrating chromosome homoeologies in two fish species, genus Eigenmannia. Cytogenetics and Cell Genetics, 48: 117-120., 2000Almeida-Toledo, L. F., F. Foresti, M. F. Z. Daniel & S. A. Toledo-Filho. 2000. Sex chromosome evolution in fish: the formation of the neo-Y chromosome in Eigenmannia (Gymnotiformes). Chromosoma, 109: 197-200.) and E. trilineata (2n = 31/32) present the multiple X1X1X2X2/X1X2Y system, where a centric fusion between two acrocentric chromosomes (pairs 6 and 11) in the male karyotype, resulted in a metacentric Y chromosome (neo-Y) in these species (Almeida-Toledo et al., 1984Almeida Toledo, L. F., H. Foresti & S. de Almeida Toledo Filho. 1984. Complex sex chromosome system in Eigenmannia sp. (Pisces, Gymnotiformes). Genetica, 64: 165-169., 1988Almeida-Toledo, L. F., M. F. Z. Daniel-Silva, C. B. Moysés, S. B. A. Fonteles, C. E. Lopes, A. Akama & F. Foresti. 2002. Chromosome evolution in fish: sex chromosome variability in Eigenmannia virescens (Gymnotiformes: Sternopygidae). Cytogenetic and Genome Research, 99: 164-169., 2000Almeida-Toledo, L. F., M. F. Z. Daniel-Silva, C. B. Moysés, S. B. A. Fonteles, C. E. Lopes, A. Akama & F. Foresti. 2002. Chromosome evolution in fish: sex chromosome variability in Eigenmannia virescens (Gymnotiformes: Sternopygidae). Cytogenetic and Genome Research, 99: 164-169.; Fernandes et al. 2010Crampton, W. G. R., K. G. Hulen & J. S. Albert. 2004. Sternopygus branco: a new species of Neotropical electric fish (Gymnotiformes: Sternopygidae) from the lowland Amazon basin, with descriptions of osteology, ecology, and electric organ discharges. Copeia, 2: 245-259.).
Karyotypes described for E. virescens (2n = 38) from the rio Mogi-Guaçu without sex chromosomes and karyotypes from the rio Tietê, with a simple XX/XY system (Table 1) (Almeida-Toledo et al., 2001Almeida-Toledo, L. F., F. Foresti, E. V. Péquignot & M. F. Z. Daniel-Silva. 2001. XX:XY sex chromosome system with X heterochromatinization: an early stage of sex chromosome differentiation in the Neotropical electric eel Eigenmannia virescens. Cytogenetics and Cell Genetics, 95: 73-78.), are similar to that described here for Eigenmannia sp. "B". Karyotypes of Eigenmannia virescens from the rio São Francisco, middle rio Amazonas, Island of Marajó, Abaetetuba, Belém and Benevides, present the ZZ/ZW system (Almeida-Toledo et al., 2002Almeida-Toledo, L. F., M. F. Z. Daniel-Silva, C. B. Moysés, S. B. A. Fonteles, C. E. Lopes, A. Akama & F. Foresti. 2002. Chromosome evolution in fish: sex chromosome variability in Eigenmannia virescens (Gymnotiformes: Sternopygidae). Cytogenetic and Genome Research, 99: 164-169.; Silva et al., 2009Silva, D. S., S. S. R. Milhomem, J. C. Pieczarka & C. Y. Nagamachi. 2009. Cytogenetic studies in Eigenmannia virescens (Sternopygidae, Gymnotiformes) and new inferences on the origin of sex chromosomes in the Eigenmannia genus. BMC Genetics, 10: 74 (1-8).). Silva et al. (2009)Silva, D. S., S. S. R. Milhomem, A. C. P. de Souza, J. C. Pieczarka & C. Y. Nagamachi. 2008. A conserved karyotype of Sternopygus macrurus (Sternopygidae, Gymnotyformes) in the Amazon region: differences from other hydrographic basins suggest cryptic speciation. Micron, 39: 1251-1254. suggest that the mechanisms involved in the differentiation of the W chromosome are the result of a pericentric inversion event in the proximal region of the short arm of an acrocentric chromosome, followed by a heterochromatization event. These events would have occurred in a distinct manner in independent populations, derived from an ancestral karyotype without differentiated sex chromosomes (Henning et al., 2011). It is highly possible that the sex chromosomes differentiation also contributed to species differentiation/divergence and worked as a reproductive isolation mechanism.
The events that occur during differentiation of sex chromosomes are still not well described. During the differentiation of sex chromosomes, the suppression or partial restriction of recombination between the sex determining pair of chromosomes should occur. This phenomenon is associated with the accumulation of heterochromatin on the sex chromosomes (Ohno, 1967Ohno, S. 1967. Sex chromosomes and sex-linked genes. Berlin, Springer-Verlag. 192p.). The absence of recombination favours the accumulation of repetitive sequences of DNA, permitting the morphological differentiation of the sex chromosomes (Almeida-Toledo et al., 2001Almeida-Toledo, L. F., F. Foresti, E. V. Péquignot & M. F. Z. Daniel-Silva. 2001. XX:XY sex chromosome system with X heterochromatinization: an early stage of sex chromosome differentiation in the Neotropical electric eel Eigenmannia virescens. Cytogenetics and Cell Genetics, 95: 73-78.; Cioffi et al., 2012Cioffi, M. B., O. Moreira-Filho, L. F. Almeida-Toledo & L. A. C. Bertollo. 2012. The contrasting role of heterochromatin in the differentiation of sex chromosomes: an overview from Neotropical fishes. Journal of Fish Biology, 80: 2125-2139.). Such events are expected to have been involved in the morphological differentiation of the X and Y chromosomes of Eigenmannia sp. "B".
The investigation of the organization of repetitive sequences can provide evidence for the origin and evolution of sex chromosomes. Studies with chromosome painting, using sex chromosome specific probes and FISH with probes for repetitive DNA sequences, combined with phylogenetic analyses are fundamental to elucidate the mechanisms involved in the origin and differentiation of sex chromosomes. Eigenmannia represents a potential comparative model within the Gymnotiformes for such comparative analyses. The inclusion of diverse analytical methods using morphological, osteological and cytogenetic data can be extremely useful to clarify taxonomic problems in Neotropical fishes such as those encountered in Eigenmannia, and may well indicate further cryptic species.
Acknowledgments
Most of this research was support by Fundação Amazônia de Amparo a Estudos e Pesquisas do Pará (FAPESPA ) through the Programa de Apoio a Núcleos de Excelência (PRONEX, TO 011/2008) project coordinated by JCP. Additional funding and support was provided by UFPA, CNPq and CAPES. This study is part of the Doctoral thesis of DSS in PPG em Genética e Biologia Molecular, under a CNPq Doctoral Scholarship. LAWP was also supported by a CAPES Masters Scholarship in Zoology and FAPESP (2013/09926-3) on a Doctoral Scholarship. JCP (307071/2009-1) and CYN (306989/2009-3) are grateful to CNPq for Productivity Grants. We thank Mr. Francisco, Ms. Josélia and Mr. José Guimarães for logistic support collecting samples at Tauari, Capanema (Pará). We thank Dr. Susana Milhomem for suggestions and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for the collection permit 020/2005 (Registration: 207419).
References
- Albert, J. S. 2001. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Miscellaneous Publications Museum of Zoology, University of Michigan, 190: 1-127.
- Albert, J. S. 2003. Family Sternopygidae (Glass Knifefishes, Rattail Knifefishes). Pp. 487-491. In: Reis, R. E., S. O. Kullander & C. J. Ferraris, Jr. (Orgs.). Check list of the freshwater fishes of South and Central America. Porto Alegre, Edipucrs.
- Albert, J. S. & W. G. R. Crampton. 2005. Diversity and phylogeny of Neotropical electric fishes (Gymnotiformes). Pp. 360-409. In: Bullock, T. H., C. D. Hopkins & R. R. Fay (Eds.). Electroreception. New York, Springer.
- Almeida-Toledo, L. F., M. F. Z. Daniel-Silva, C. B. Moysés, S. B. A. Fonteles, C. E. Lopes, A. Akama & F. Foresti. 2002. Chromosome evolution in fish: sex chromosome variability in Eigenmannia virescens (Gymnotiformes: Sternopygidae). Cytogenetic and Genome Research, 99: 164-169.
- Almeida Toledo, L. F., H. Foresti & S. de Almeida Toledo Filho. 1984. Complex sex chromosome system in Eigenmannia sp. (Pisces, Gymnotiformes). Genetica, 64: 165-169.
- Almeida Toledo, L. F., F. Foresti & S. de Almeida Toledo Filho. 1985. Spontaneous triploidy and NOR activity in Eigenmannia sp. (Pisces, Sternopygidae) from the Amazon basin. Genetica, 66: 85-88.
- Almeida-Toledo, L. F., F. Foresti, M. F. Z. Daniel & S. A. Toledo-Filho. 2000. Sex chromosome evolution in fish: the formation of the neo-Y chromosome in Eigenmannia (Gymnotiformes). Chromosoma, 109: 197-200.
- Almeida-Toledo, L. F., F. Foresti, E. V. Péquignot & M. F. Z. Daniel-Silva. 2001. XX:XY sex chromosome system with X heterochromatinization: an early stage of sex chromosome differentiation in the Neotropical electric eel Eigenmannia virescens. Cytogenetics and Cell Genetics, 95: 73-78.
- Almeida-Toledo, L. F., A. J. Stocker, F. Foresti & S. Almeida Toledo-Filho. 1996. Fluorescence in situ hybridization with rDNA probes on chromosomes of two nucleolus organizer region phenotypes of a species of Eigenmannia (Pisces, Gymnotoidei, Sternopygidae). Chromosome Research, 4: 301-305.
- Almeida Toledo, L. F., E. Viegas-Péquignot, F. Foresti, S. A. Toledo Filho & B. Dutrillaux. 1988. BrdU replication patterns demonstrating chromosome homoeologies in two fish species, genus Eigenmannia. Cytogenetics and Cell Genetics, 48: 117-120.
- Bertollo, L. A. C., C. S. Takahashi & O. Moreira Filho. 1978. Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Revista Brasileira de Genética, 1: 103-120.
- Campos-da-Paz, R. 1997. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). Unpublished Ph. D. Dissertation, Universidade de São Paulo, São Paulo , 336p.
- Cioffi, M. B., O. Moreira-Filho, L. F. Almeida-Toledo & L. A. C. Bertollo. 2012. The contrasting role of heterochromatin in the differentiation of sex chromosomes: an overview from Neotropical fishes. Journal of Fish Biology, 80: 2125-2139.
- Cioffi, M. B., A. Sánchez, J. A. Marchal, N. Kosyakova, T. Liehr, V. Trifonov & L. A. C. Bertollo. 2011. Whole chromosome painting reveals independent origin of sex chromosomes in closely related forms of a fish species. Genetica, 139: 1065-1072.
- Charlesworth, D., B. Charlesworth & G. Marais. 2005. Steps in the evolution of heteromorphic sex chromosomes. Heredity, 95: 118-128.
- Crampton, W. G. R., K. G. Hulen & J. S. Albert. 2004. Sternopygus branco: a new species of Neotropical electric fish (Gymnotiformes: Sternopygidae) from the lowland Amazon basin, with descriptions of osteology, ecology, and electric organ discharges. Copeia, 2: 245-259.
- Fernandes, C. A., D. Bailly, V. F. B. Silva & I. C. Martins-Santos. 2010. System of multiple sex chromosomes in Eigenmannia trilineata López & Castello, 1966 (Sternopygidae, Gymnotiformes) from Iguatemi River Basin, MS, Brazil. Cytologia, 75: 463-466.
- Gomes, A. J. B, C. Y. Nagamachi, L. R. R. Rodrigues, S. G. Farias, J. D. Rissino & J. C. Pieczarka. 2012. Karyotypic variation in Rhinophylla pumilio Peters, 1865 and comparative analysis with representatives of two subfamilies of Phyllostomidae (Chiroptera). Comparative Cytogenetics, 6: 213-225.
- Guerra, M. S. 1986. Reviewing the chromosome nomenclature of Levan et al. Revista Brasileira de Genética, 9: 741-743.
- Hatanaka, T. & P. M. Galetti, Jr. 2004. Mapping of the18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica, 122: 239-244.
- Henning, F., C. B. Moysés, D. Calcagnotto, A. Meyer & L. F. Almeida-Toledo. 2011. Independent fusions and recent origins of sex chromosomes in the evolution and diversification of glass knife fishes (Eigenmannia). Heredity, 106: 391-400.
- Howell, W. M. & D. A. Black. 1980. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia, 36: 1014-1015.
- King, M. 1993. Species evolution: the role of chromosomal change. Cambridge, UK, Cambridge University Press, 336p.
- Lundberg, J. G. & F. Mago-Leccia. 1986. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with description of four new species. Proceedings of the Academy Natural Sciences of Philadelphia, 138: 53-85.
- Mago Leccia, F. 1978. Los peces de la familia Sternopygidae de Venezuela. Acta Cientifica Venezolana, 29(suppl. 1): 1-91.
- Milhomem, S. S. R., W. G. R. Crampton, J. C. Pieczarka, G. H. Shetka, D. S. Silva & C. Y. Nagamachi. 2012b. Gymnotus capanema, a new species of electric knife fish (Gymnotiformes, Gymnotidae) from eastern Amazonia, with comments on an unusual karyotype. Journal of Fish Biology, 80: 802-815.
- Milhomem, S. S. R., W. G. R. Crampton, J. C. Pieczarka, D. S. Silva, A. L. Cardoso, P. C. da Silva, J. A. Oliveira & C. Y. Nagamachi. 2012a. Chromosomal and electric signal diversity in three sympatric electric knifefish species (Gymnotus, Gymnotidae) from the Central Amazon floodplain. Reviews in Fish Biology and Fisheries, 22: 485-497.
- Milhomem, S. S. R., J. C. Pieczarka, W. G. R. Crampton, D. S. Silva, A. C. P. Souza, J. R. Carvalho Jr. & C. Y. Nagamachi. 2008. Chromosomal evidence for a putative cryptic species in the Gymnotus carapo species-complex (Gymnotiformes, Gymnotidae). BMC Genetics, 9: 75 (1-10).
- Milhomem, S. S. R., J. C. Pieczarka, W. G. R. Crampton, A. C. P. Souza, J. R. Carvalho Jr. & C. Y. Nagamachi. 2007. Differences in karyotype between two sympatric species of Gymnotus (Gymnotiformes: Gymnotidae) from the eastern amazon of Brazil. Zootaxa, 1397: 55-62.
- Milhomem, S. S. R., P. C. Scacchetti, J. C. Pieczarka, M. A. Ferguson-Smith, J. C. Pansonato-Alves, P. C. M. O'Brien, F. Foresti & C. Y. Nagamachi. 2013. Are NORs always located on homeologous chromosomes? A FISH investigation with rDNA and whole chromosome probes in Gymnotus fishes (Gymnotiformes). PloS One, 8: e55608 (1-6).
- Moysés, C. B., M. F. Z. Daniel-Silva, C. E. Lopes & L. F. Almeida-Toledo. 2010. Cytotype-specific ISSR profiles and karyotypes in the Neotropical genus Eigenmannia (Teleostei: Gymnotiformes). Genetica, 138: 179-189.
- Moysés, C. B., S. Mockford, L. F. Almeida-Toledo & J. M. Wright. 2005. Nine polymorphic microsatellite loci in the Neotropical electric eel Eigenmannia (Teleostei, Gymnotiformes). Molecular Ecology Notes 5: 7-9.
- Nagamachi, C. Y., J. C. Pieczarka, S. S. R. Milhomem, P. C. M. O'Brien, A. C. P. de Souza & M. A. Ferguson-Smith. 2010. Multiple rearrangements in cryptic species of electric knifefish, Gymnotus carapo (Gymnotidae, Gymnotiformes) revealed by chromosome painting. BMC Genetics, 11: 28 (1-9).
- Nascimento, A. L., A. C. P. Souza, E. Feldberg, J. R. Carvalho Jr, R. M. S. Barros, J. C. Pieczarka & C. Y. Nagamachi. 2006. Cytogenetic analysis on Pterophyllum scalare (Perciformes, Cichlidae) from Jari River, Pará State. Caryologia, 59: 138-143.
- Ohno, S. 1967. Sex chromosomes and sex-linked genes. Berlin, Springer-Verlag. 192p.
- Oliveira, C., F. Foresti & A. W. S. Hilsdorf. 2009. Genetics of Neotropical fish: from chromosomes to populations. Fish Physiology and Biochemistry, 35: 81-100.
- Pieczarka, J. C., C. Y. Nagamachi, A. C. P. de Souza, S. S. R. Milhomem, R. R. Castro & A. L. Nascimento. 2006. An adaptation of DAPI- Banding to fishes chromosomes. Caryologia, 59: 43-46.
- de Santana, C. D. & W. G. R. Crampton. 2011. Phylogenetic interrelationships, taxonomy, and reductive evolution in the Neotropical electric fish genus Hypopygus (Teleostei, Ostariophysi, Gymnotiformes). Zoological Journal of the Linnean Society, 163: 1096-1156.
- Schweizer, D. 1980. Simultaneous fluorescent staining of R bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenetics and Cell Genetics, 27: 190-193.
- Shibatta, O. A. 1993. Estudo comparativo ao nível intra-específico de Salminus hilarii, Pimelodus cf. maculatus, Leporinus cf. elongatus e Eigenmannia cf. virescens (Pisces, Ostariophysi) das bacias do Alto Paraná e São Francisco, através da análise morfométrica multivariada. Unpublished Ph. D. Dissertation, Universidade Federal de São Carlos, São Carlos.
- Silva, D. S., S. S. R. Milhomem, J. C. Pieczarka & C. Y. Nagamachi. 2009. Cytogenetic studies in Eigenmannia virescens (Sternopygidae, Gymnotiformes) and new inferences on the origin of sex chromosomes in the Eigenmannia genus. BMC Genetics, 10: 74 (1-8).
- Silva, D. S., S. S. R. Milhomem, A. C. P. de Souza, J. C. Pieczarka & C. Y. Nagamachi. 2008. A conserved karyotype of Sternopygus macrurus (Sternopygidae, Gymnotyformes) in the Amazon region: differences from other hydrographic basins suggest cryptic speciation. Micron, 39: 1251-1254.
- Sumner, A. T. 1972. A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research, 75: 304-306.
- Sumner, A. T. 2003. Chromosomes: organization and function. Oxford, Blackwell 287p.
- Taylor, W. R. & G. C. Van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9: 107-119.
- Triques, M. L. 1996. Eigenmannia vicentespelaea, a new species of cave dwelling electrogenic Neotropical fish (Ostariophysi: Gymnotiformes: Sternopygidae). Revue Française D'aquariologie et Herpetologie, 23: 1-4.
Publication Dates
-
Publication in this collection
Apr-Jun 2015
History
-
Received
25 Apr 2014 -
Accepted
08 Apr 2015