Abstract
Photo-identification allows individual recognition of animal species based on natural marks, being an alternative to other more stressful artificial tagging/marking techniques. An increasing number of studies with different animal groups has shown that photo-identification can successfully be used in several situations, but its feasibility to study freshwater fishes is yet to be explored. We demonstrate the potential use of photo-identification for intraspecific recognition of individuals in the stream-dwelling loricariid Rineloricaria aequalicuspis . We tested photo-identification in laboratory and field conditions based on the interindividual variability in abdominal bony plates. Our test yielded high correct matches in both laboratory (100%) and field conditions (> 97%), comparable to other reliable techniques and to studies that successfully used photo-identification in other animals. In field conditions, the number of correct matches did not differ statistically between computer-assisted and naked-eye identification. However, the average time expended to conclude computer-assisted photo evaluations was about half of the time expended to conclude naked-eye evaluations. This result may be exacerbated when using database with large number of images. Our results indicate that photo-identification can be a feasible alternative technique to study freshwater fish species, allowing for a wider use of mark-recapture in ecological and behavioral studies.
Keywords:
Individual identification; Loricariid; Marking; Mark-recapture; Tagging
Resumo
A foto-identificação permite o reconhecimento individual de espécies de animais baseando-se em marcas naturais, sendo uma alternativa a outras técnicas de marcação artificial mais estressantes comumente usadas. O número crescente de estudos que usam foto-identificação em diferentes grupos animais mostra que esta técnica pode ser utilizada com sucesso, mas a viabilidade em estudos com peixes de água doce ainda não foi avaliada. Nós demonstramos o uso potencial da foto-identificação para o reconhecimento individual de peixes com indivíduos do loricarídeo Rineloricaria aequalicuspis. Nós testamos foto-identificação em condições de laboratório e de campo com base na variabilidade inter-individual das placas ósseas abdominais. O teste resultou em elevada porcentagem de acerto nas comparações, tanto para a condição de laboratório (100%) quanto para a de campo (> 97%), o que é comparável com outras técnicas confiáveis e com outros estudos que empregaram foto-identificação com sucesso. No teste de campo, o número de acertos não diferiu estatisticamente entre auxílio de computador e olho nu. Entretanto, o tempo médio despendido para concluir as avaliações com o auxílio de computador foi cerca da metade do tempo despendido para as avaliações a olho nu. Esse resultado pode ser exacerbado em avaliações com um grande número de imagens. Nossos resultados indicam que a foto-identificação pode ser uma técnica alternativa viável para estudar peixes de água doce e possibilita um uso mais amplo da marcação e recaptura para estudos ecológicos e comportamentais.
Introduction
The recognition of individual specimens has been essential to scientific discoveries in several fields of ecology, evolution and behavior (Monteiro et al., 2014Monteiro, N. M., R. M. Silva, M. Cunha, A. Antunes, A. G. Jones & M. N. Vieira. 2014. Validating the use of colouration patterns for individual recognition in the worm pipefish using a novel set of microsatellite markers. Molecular Ecology Resources, 14: 150-156.), although it still remains a challenge for research in animal ecology. Several techniques have been developed and successfully used to mark individuals or groups of individuals for later identification (Silvy et al., 2012Silvy, N. J., R. R. Lopez & M. J. Peterson. 2012. Techniques for marking wildlife. v.1 Pp. 230-257. In:. Silvy, N. J (Ed.). The wildlife techniques manual. 7th ed. Baltimore, Johns Hopkins University Press.). Such markings have been used for example to estimate population size (Haines & Modde, 1996Haines, G. B. & T. Modde. 1996. Evaluation of marking techniques to estimate population size and first-year survival of Colorado squawfish. North American Journal of Fisheries Management, 16: 905-912.; Moore et al., 2010Moore, J. A., T. Grant, D. Brown, S. N. Keall & N. J. Nelson. 2010. Mark-recapture accurately estimates census for tuatara, a burrowing reptile. The Journal of Wildlife Management, 74: 897-901.), growth (Linnane et al., 2012Linnane, A., D. Hobday, S. Frusher & C. Gardner. 2012. Growth rates of juvenile southern rock lobster (Jasus edwardsii ) estimated through a diver-based tag-recapture program. Marine and Freshwater Research, 63: 110-118.), survival (Monk et al., 2011Monk, M. H., J. Berkson & P. Rivalan. 2011. Estimating demographic parameters for loggerhead sea turtles using mark-recapture data and a multistate model. Population Ecology, 53: 165-174.) and recruitment rates (Pearson & Munro, 1991Pearson, R. G. & J. L. Munro. 1991. Growth, mortality and recruitment rates of giant clams, Tridacna gigas and T . derasa , at Michaelmas Reef, central Great Barrier Reef, Australia. Marine and Freshwater Research, 42: 241-262.), to monitor populations for conservation management (Dutton et al., 2005Dutton, D. L., P. H. Dutton, M. Chaloupka & R. H. Boulon. 2005. Increase of a Caribbean leatherback turtle Dermochelys coriacea nesting population linked to long-term nest protection. Biological Conservation, 126: 186-194. ; Biggins et al., 2006Biggins, D. E., J. L. Godbey, B. J. Miller & L. R. Hanebury. 2006. Radio telemetry for black-footed ferret research and monitoring. Pp. 175-189. In: Roelle, J. E., B. J. Miller, J. L. Godbey& D. E. Biggins (Eds.). Recovery of the black-footed ferret: progress and continuing challenges. Proceedings of the symposium on the status of the Black-footed Ferret and its habitat, Fort Collins, Colorado, January 28-29, 2004. [Reston, VA], U.S. Dept. of the Interior, U. S. Geological Survey.) and to identify individuals for natural history studies (Franz & Fontana, 2013Franz, I. & C. S. Fontana. 2013. Breeding biology of the Tawny-Bellied seedeater (Sporophila hypoxantha ) in southern Brazilian upland grasslands. The Wilson Journal of Ornithology, 125: 280-292.). However, using artificial marks has limitations that may affect the results or even preclude studies on some species.
Many of the commonly used marking techniques are invasive (e .g ., subcutaneous chemical markings, tattoos, amputations, insertion of transponders and subcutaneous tags) and may pose a risk to animal health or survival (Silvy et al., 2012Silvy, N. J., R. R. Lopez & M. J. Peterson. 2012. Techniques for marking wildlife. v.1 Pp. 230-257. In:. Silvy, N. J (Ed.). The wildlife techniques manual. 7th ed. Baltimore, Johns Hopkins University Press.). Even the use of non-invasive artificial marks such as tags, collars or external colorants, may cause behavioral alteration, increasing the risk of predation and reducing fitness (Gauthier-Clerc et al., 2004Gauthier-Clerc, M., J. -P. Gendner, C. A. Ribic, W. R. Fraser, E. J. Woehler, S. Descamps, C. Gilly, C. Le Bohec & Y. Le Maho. 2004. Long-term effects of flipper bands on penguins. Proceedings of the Royal Society of London. Series B, Biological Society, 271(suppl. 6): S423-S426.; Wilson et al., 2011Wilson, C. D., G. Arnott, N. Reid & D. Roberts. 2011. The pitfall with PIT tags: marking freshwater bivalves for translocation induces short-term behavioural costs. Animal Behaviour, 81: 341-346.; Carlson & Langkilde, 2013Carlson, B. E. & T. Langkilde. 2013. A common marking technique affects tadpole behavior and risk of predation. Ethology, 119: 167-177.). Additionally, the possible loss of artificial marks may represent the loss of desired data (Reisser et al., 2008Reisser, J., M. Proietti, P. Kinas & I. Sazima. 2008. Photographic identification of sea turtles: method description and validation, with an estimation of tag loss. Endangered Species Research, 5: 73-82.) and a series of ethical and animal welfare issues must be taken into consideration, whether the marks are permanent or not (Wilson & McMahon, 2006Wilson, R. P. & C. R. McMahon. 2006. Measuring devices on wild animals: what constitutes acceptable practice? Frontiers in Ecology and the Environment, 4: 147-154.).
An alternative to using artificial or invasive marks is the identification of individuals based on their natural marks, such as colors, spots, blotches or stripes patterns, which has been used for different vertebrate groups (e .g . Speed et al., 2007Speed, C. W., M. G. Meekan & C. J. A. Bradshaw. 2007. Spot the match -wildlife photo-identification using information theory. Frontiers in Zoology, 4 (jan.).; Martin-Smith, 2011Martin-Smith, K. M. 2011. Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. Journal of Fish Biology, 78: 1757-1768.; Caorsi et al., 2012Caorsi, V. Z., R. R. Santos & T. Grant. 2012. Clip or snap? An evaluation of toe-clipping and photo-identification methods for identifying individual Southern Red-Bellied Toads, Melanophryniscus cambaraensis . South American Journal of Herpetology, 7: 79-84.; Reisser et al., 2008Reisser, J., M. Proietti, P. Kinas & I. Sazima. 2008. Photographic identification of sea turtles: method description and validation, with an estimation of tag loss. Endangered Species Research, 5: 73-82.; Anderson et al., 2010Anderson, C. J. R., N. D. V. Lobo, J. D. Roth & J. M. Waterman. 2010. Computer-aided photo-identification system with an application to polar bears based on whisker spot patterns. Journal of Mammalogy, 91: 1350-1359.). In addition, the shape of fins and scars has also been used to recognize photographed individuals (Marshall & Pierce, 2012Marshall, A. D. & S. J. Pierce. 2012. The use and abuse of photographic identification in sharks and rays. Journal of Fish Biology, 80: 1361-1379.; Giglio et al., 2014Giglio, V. J., J. Adelir-Alves & A. A. Bertoncini. 2014. Using scars to photo-identify the goliath grouper, Epinephelus itajara . Marine Biodiversity Records, 7: e108(4 p).). In this sense, photo-identification has become an important tool, because it avoids physical marking by using only natural features of the body (Speed et al ., 2007Speed, C. W., M. G. Meekan & C. J. A. Bradshaw. 2007. Spot the match -wildlife photo-identification using information theory. Frontiers in Zoology, 4 (jan.).), it also reduces animal stress if live specimen manipulation is necessary. An additional advantage of photo-identification is that a digital image database can be compiled and then used for a careful identification process based on the examination of several images of one specimen, allowing eventual comparisons with other specimens or with previous images from the same specimen. This digital image database also allows the validation of images in mark-recapture experiments, and comparison of identifications made by different observers, reducing the chance for observer bias.
Photo-identification has been successfully applied in studies of large vertebrates (e .g . Anderson et al., 2010Anderson, C. J. R., N. D. V. Lobo, J. D. Roth & J. M. Waterman. 2010. Computer-aided photo-identification system with an application to polar bears based on whisker spot patterns. Journal of Mammalogy, 91: 1350-1359.; Bolger et al., 2012Bolger, D. T., T. A. Morrison, B. Vance, D. Lee & H. Farid. 2012. A computer‐assisted system for photographic mark-recapture analysis. Methods in Ecology and Evolution, 3: 813-822.), but also on smaller animals like amphibians (e .g . Kenyon et al., 2009Kenyon, N., A. D. Phillott & R. A. Alford. 2009. Evaluation of the photographic identification method (PIM) as a tool to identify adult Litoria genimaculata (Anura: Hylidae). Herpetological Conservation and Biology, 4: 403-410.; Caorsi et al., 2012Caorsi, V. Z., R. R. Santos & T. Grant. 2012. Clip or snap? An evaluation of toe-clipping and photo-identification methods for identifying individual Southern Red-Bellied Toads, Melanophryniscus cambaraensis . South American Journal of Herpetology, 7: 79-84.), lizards (e .g . Knox et al., 2013Knox, C. D., A. Cree & P. J. Seddon. 2013. Accurate identification of individual geckos (Naultinus gemmeus ) through dorsal pattern differentiation. New Zealand Journal of Ecology, 37: 60-66.; Sreekar et al., 2013Sreekar, R., C. B. Purushotham, K. Saini, S. N. Rao, S. Pelletier & S. Chaplod. 2013. Photographic capture-recapture sampling for assessing populations of the Indian gliding lizard Draco dussumieri . PLoS ONE, 8: e55935 (15p.).) and even invertebrates (e .g . Chim & Tan, 2012Chim, C. K. & K. S. Tan. 2012. Recognition of individual knobby sea stars Protoreaster nodosus (L., 1758) using aboral surface characteristics. Journal of Experimental Marine Biology and Ecology, 430-431: 48-55.; Caci et al., 2013Caci, G., A. B. Biscaccianti, L. Cistrone, L. Bosso, A. P. Garonna & D. Russo. 2013. Spotting the right spot: computer-aided individual identification of the threatened cerambycid beetle Rosalia alpina . Journal of Insect Conservation, 17: 787-795.). Photo-identification has been extensively applied for studying marine mammals (e .g . Hastings et al., 2008Hastings, K. K., L. A. Hiby & R. J. Small. 2008. Evaluation of a computer-assisted photograph-matching system to monitor naturally marked harbor seals at Tugidak Island, Alaska. Journal of Mammalogy, 89: 1201-1211.; Nery et al., 2008Nery, M. F., M. A. Espécie & S. M. Simão. 2008. Site fidelity of Sotalia guianensis (Cetacea: Delphinidae) in Sepetiba Bay, Rio de Janeiro, Brazil. Revista Brasileira de Zoologia, 25: 182-187.), sharks and rays (Marshall & Pierce, 2012Marshall, A. D. & S. J. Pierce. 2012. The use and abuse of photographic identification in sharks and rays. Journal of Fish Biology, 80: 1361-1379.) and, more recently, has been demonstrated a feasible technique for identification of marine teleost fishes (e .g . Perrig & Goh, 2008Perrig, M. & B. P. L. Goh. 2008. Photo-ID on reef fish: avoiding tagging-induced biases. Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida, 22: 7-11; Giglio et al., 2014Giglio, V. J., J. Adelir-Alves & A. A. Bertoncini. 2014. Using scars to photo-identify the goliath grouper, Epinephelus itajara . Marine Biodiversity Records, 7: e108(4 p).). Studies with teleost fishes include mainly species from the Family Syngnathidae (pipefishes, seahorses and seadragons) due to their distinguishable patterns of spots and blotches on the lateral surface of the abdomen (Martin-Smith, 2011Martin-Smith, K. M. 2011. Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. Journal of Fish Biology, 78: 1757-1768.; Correia et al., 2014Correia, M., J. Palma, H. Koldewey & J. P. Andrade. 2014. The use of a non‐invasive tool for capture-recapture studies on a seahorse Hippocampus guttulatus population. Journal of Fish Biology, 84: 872-884.).
The reliable use of photo-identification in mark-recapture studies requires that at least two assumptions must be met (modified from Bolger et al., 2012Bolger, D. T., T. A. Morrison, B. Vance, D. Lee & H. Farid. 2012. A computer‐assisted system for photographic mark-recapture analysis. Methods in Ecology and Evolution, 3: 813-822.). First, individuals must bear patterns on some region of the external surface of their body that are sufficiently variable to discriminate among individuals. Second, an individual's pattern should be stable over the study period (and preferably along the individual adult life span) and unambiguously photographed under differing conditions. Although these assumptions may not be attainable for all fish species, photo-identification can be a useful alternative to traditional marking techniques for many species. In fact, photo-identification has already been used for individual identification of marine teleost fish (e .g . Martin-Smith, 2011Martin-Smith, K. M. 2011. Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. Journal of Fish Biology, 78: 1757-1768.; Correia et al., 2014Correia, M., J. Palma, H. Koldewey & J. P. Andrade. 2014. The use of a non‐invasive tool for capture-recapture studies on a seahorse Hippocampus guttulatus population. Journal of Fish Biology, 84: 872-884.; Giglio et al., 2014Giglio, V. J., J. Adelir-Alves & A. A. Bertoncini. 2014. Using scars to photo-identify the goliath grouper, Epinephelus itajara . Marine Biodiversity Records, 7: e108(4 p).), but the potential use of photo-identification to study freshwater fish is yet to be explored.
In this paper we test the feasibility of using photo-identification as a technique for recognition of individual specimens of a Neotropical freshwater fish species, Rineloricaria aequalicuspis Reis & Cardoso (Loricariidae). The body of loricarid fishes is typically covered by bony plates and the number, size, arrangement and shape of these plates are frequently variable among individuals of the same species. In Rineloricaria species, the abdominal plates are quietly variable and sometime used to diagnose species. We expected that photo-identification would be a feasible technique because some species of Rineloricaria have a particularly clear arrangement of abdominal plates which is variable among individuals of the same species. We tested recognition of individual fish using images taken both in laboratory and in field conditions. A good performance under field conditions is desirable for this technique to be used in mark-recapture studies. We also tested whether performance of a computer-assisted individual recognition (using an algorithm for image comparison) would be better than direct naked-eye photo-identification and examined if performance would be affected by variability in assessment between different users, which could be a problem if photo-identification of a large number of samples is to be performed by different people.
Material and Methods
Studied species. Rineloricaria aequalicuspis (Fig. 1) is a siluriform fish endemic to streams of coastal drainages in southern Brazil (Reis & Cardoso, 2001Reis, R. E. & A. R. Cardoso. 2001. Two new species of Rineloricaria from southern Santa Catarina and northeastern Rio Grande do Sul, Brazil (Teleostei: Loricariidae). Ichthyological Exploration of Freshwaters, 12: 319-332.). It is usually found in piedmont streams with clear waters and rocky channel substrate. Its maximum total length (TL) reaches ca . 20 cm, and its body is completely covered by bony plates, except for the abdominal surface, where these plates are irregularly interspersed by naked areas (Fig. 1). Abdominal plates show inter-individual variation in number, size, shape and arrangement, so that it is possible to distinguish different specimens based on these characteristics. However, configuration or size of these abdominal plates could change along the ontogenetic development and thus we used only individuals larger than 10 cm of TL in our tests. All analyzed specimens were caught in streams of the rio Maquiné basin, a coastal drainage located in the State of Rio Grande do Sul, Southern Brazil. Although there are two other Rineloricaria species in the rio Maquiné basin (R. quadrensis Reis, 1983 and R. maquinensisReis & Cardoso, 2001Reis, R. E. & A. R. Cardoso. 2001. Two new species of Rineloricaria from southern Santa Catarina and northeastern Rio Grande do Sul, Brazil (Teleostei: Loricariidae). Ichthyological Exploration of Freshwaters, 12: 319-332.), these are readily distinguishable in the field (see Reis & Cardoso, 2001Reis, R. E. & A. R. Cardoso. 2001. Two new species of Rineloricaria from southern Santa Catarina and northeastern Rio Grande do Sul, Brazil (Teleostei: Loricariidae). Ichthyological Exploration of Freshwaters, 12: 319-332.).
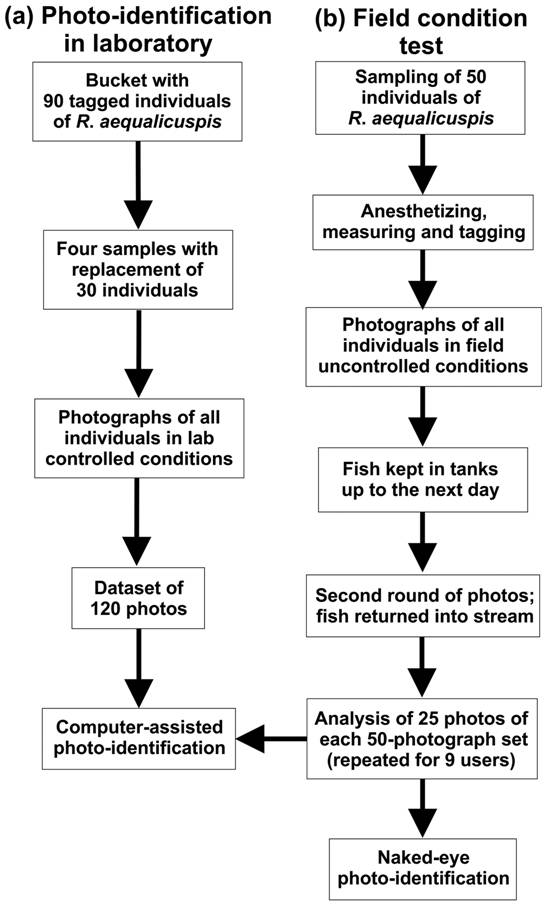
Photo-identification in laboratory. In this test, we aimed to assess the accuracy of individual photo-identification technique using photographs taken at a comfortable setting (laboratory vs . field situation) and under a well-defined photographing protocol. The results were used as a bench-mark to be compared to results under field conditions. We used 90 R. aequalicuspis specimens collected for a previous study in the rio Maquiné basin. These specimens had been fixed in 10% formalin and stored in 70% ethanol. Specimens used in this test were deposited at the Departamento de Zoologia of the Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre (UFRGS 20661 to 20671).
Lateral, dorsal and ventral views of a Rineloricaria aequalicuspis individual (110 mm TL). Ventral view shows the arrangement of the abdominal plates. Photograph courtesy of L. R. Malabarba.
Firstly, all specimens were tagged with individual numbers in plastic tags attached to the caudal fin, so that we could control the identity of each individual and validate later recognition attempts made by photo-identification based on abdominal plate patterns. Subsequently, we put all individuals inside a 20-L plastic bucket and took four random samples of 30 individuals with replacement. This means that, the same individual could have been captured more than once. After each sample was taken, we took standardized photos of the specimens, in a total of 120 photos. We used always the same digital camera (a common point-and-shoot digital camera) and a tripod stabilizer to take photos of the fish abdomen. Pictures were taken from a ventral view and from a standardized distance of 5-6 cm. All photographs were taken from a 90° angle and were performed in an ordinary fish laboratory room under common fluorescent light (Fig. 2).
Diagram showing the steps employed to assess the performance of photo-identification technique in laboratory (a) and field (b) conditions for Rineloricaria aequalicuspis .
The photographs were computer-edited in order to cut out only the focal area for individual recognition, i.e .,the abdominal pattern of bony plates. This area is defined with reference to a straight line from the urogenital pore to the most anterior edge of the pectoral spine base (Fig. 3). Occasionally, we lightly corrected brightness and contrast in darker images. Subsequently, we used the 120 processed images (4 samples of 30 specimens) in pairwise comparisons in order to evaluate the efficacy of photo-identification technique to the individual recognition of R . aequalicuspis based on abdominal plates patterns.
Variation in number, shape, size and organization of the bony plates covering the abdominal surface of six different Rineloricaria aequalicuspis individuals with more than 10 cm total length. These are examples of photographs taken during the field test. (a) 175 mm TL; (b) 138 mm TL; (c) 156 mm TL; (d) 145 mm TL; (e) 141 mm TL; (f) 151 mm TL.
In laboratory condition, we tested individual photo-identification using only computer-assisted identification. Computer-assisted identification consisted of pairwise comparisons of the 120 photographs using the software Wild-ID 1.0 (Bolger et al., 2012Bolger, D. T., T. A. Morrison, B. Vance, D. Lee & H. Farid. 2012. A computer‐assisted system for photographic mark-recapture analysis. Methods in Ecology and Evolution, 3: 813-822.). This software uses the Scale Invariant Feature Transform (SIFT) algorithm to extracts distinctive features invariant to image scale, rotation, viewpoint, local distortion and illumination (Lowe, 2004Lowe, D. G. 2004. Distinctive image features from scale-invariant keypoints. International Journal of Computer Vision, 60: 91-110.). For each image, SIFT features are extracted by the following steps: 1) difference-of-Gaussian function is used in a grey-scale transformed image to identify potential interest points; 2) at each location, Taylor expansions are used to interpolate the subpixel location of the actual extremum; 3) one or more dominant orientations (angles relative to image axes) are assigned to each keypoint location based on the gradient in pixel intensity around the keypoint; 4) additional local image gradients are measured in four regions immediately surrounding each keypoint to generate a keypoint descriptor (Bolger et al ., 2012Bolger, D. T., T. A. Morrison, B. Vance, D. Lee & H. Farid. 2012. A computer‐assisted system for photographic mark-recapture analysis. Methods in Ecology and Evolution, 3: 813-822.). In the end, SIFT feature refers to a keypoint location together with its scale, orientation and descriptor (Bolger et al ., 2012Bolger, D. T., T. A. Morrison, B. Vance, D. Lee & H. Farid. 2012. A computer‐assisted system for photographic mark-recapture analysis. Methods in Ecology and Evolution, 3: 813-822.). In pairs of images being compared, the program locates for each feature in image 1, the feature in image 2 that minimizes the Euclidean distance between the feature descriptors.
In each computer-assisted comparison event, a target picture is fixed for comparison and the software selects the twenty most similar images ranked according to Euclidian distances relative to the target image. The user is then responsible for deciding if one of the ranked images matched the target image, i .e . whether the two different photos were taken from the same individual. If the user decides that two images were taken from the same individual it means that a recapture event was found. Because each fish was individually tagged and we had documented the picture file name corresponding to each tag number, we could check whether each comparison event yielded correct results (these were our control data). Correct results could be either true positives (correctly finding that two photos were taken from the same fish), or true negatives (correctly finding that none of the pictures belong to the same individual).
In order to evaluate the efficacy of the computer-assisted photo-identification technique for R . aequalicuspis , only one user conducted the complete identification procedure. The percentage of correct matches, including false negatives (incorrectly determining that none of the pictures are similar to the target image) and false positives (incorrectly finding that any picture is similar to the target image) was determined by comparing user decisions with control data.
Field condition test. We sampled 50 individuals of R. aequalicuspis in a stream of the rio Maquiné basin located in Southern Brazil (29o38'18"S, 50o13'30"W). The sampled stream segment was a 300 m long riffle with rocky bed, averaged wetted width of 9.3 m and averaged depth of 22.7 cm. Specimens were sampled using the kick-sampling technique with two dip-nets of same sizes (80x40x45 cm and mesh of 2 mm). Immediately after we caught fishes we transferred them to five plastic boxes (each with 72 L capacity) filled with water from the sampled site. Individuals smaller than 10 cm of TL individuals were returned to the sampled site immediately after catch.
The photographs were taken in situ , at the edge of the stream, in order to simulate general conditions that one can experience during field work (e .g . lack of a comfortable setting for taking pictures, necessity of quick fish manipulation for avoiding stress or mortality, varied light conditions and limited time for completing the whole procedure). Before an individual fish was photographed, it was submitted to the following procedure: 1) anesthesia with Eugenol (clove oil); 2) total length measurement; and 3) tagging. The tagging consisted in slightly tying a numbered tag using nylon string at the most distal portion of caudal peduncle. This last procedure allowed us to obtain a known individual id-number for later evaluating the efficacy of our field photo-identification test. Photographs of the abdominal plates of each specimen were freely taken during the field work, by holding the fish with one hand and photographing with the other hand, without strictly controlling for distance between the digital camera and the fish and without using any artificial light or tripod stabilizer. We subsequently transferred all individuals to other plastic boxes filled up with water of the sampled stream, but without anesthetic. Fishes were kept alive in these boxes with portable air pumps for 14 hours, and then we performed a second round of photographs, simulating a second sampling at the same site. The same aforementioned process was performed during the second round of photographs. Tags were removed from all individuals before we returned them alive to the stream (Fig. 2).
For field work conditions, we tested whether computer-assisted photo-identification efficacy and expended time differ from naked-eye photo-identification. Field photographs were also computer-edited in the same way as described in the laboratory testing (Fig. 3). From the set of photographs obtained under field conditions, we simulated two sampling events by randomly selecting 25 photographs from the 50 photographs taken in the first day and more 25 photographs from the set of 50 photographs taken in the second day. We repeated this process for nine different users to compare photo-identification by using computer-assisted individual recognition (Wild-ID software) with direct naked-eye photo-identification. The nine users made their evaluations independently and at different moments, according to their availability. Time expended to complete the entire evaluation was recorded by each user using the same criteria. For naked-eye photo-identification, the timer started when the first pair of image files was opened and ended when the last pair of image files was closed. For computer-assisted photo-identification, the software timer started as soon as the software was started and progressed until the last pair images had been evaluated. A combination of different numbers was used as code names for the image files in order to allow posterior checking with original tag numbers and to compute number of correct matches (true positive and true negative) and misidentifications (false positive and false negative).
Data analysis. The results of the laboratory test were expressed as the percentage number of correct matches in the total number of comparisons and as the number of times the software classified true positives as the most similar images. In the field test, the percentage of correct pairwise recognitions and the average time (min) expended in assessing the images were calculated for both computer-assisted and naked-eye photo-identification procedures based on evaluations made by nine different users. Potential differences in the efficacy of both procedures were also evaluated by performing t-test using percentage of correct pairwise recognition and expended minutes as response variables. We also recorded the percentage of times that true positives were ranked in the first position by the software in computer-assisted evaluations in order to address other measure for computer-assisted reliability. Statistical analyses were performed in R environment (R Development Core Team, 2014R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing. Available from: Available from: https://www.r-project.org/
. (21 November 2014).
https://www.r-project.org/...
).
Results
Laboratory test. Computer-assisted pairwise comparisons of 120 photos taken from 69 individuals of R. aequalicuspis resulted in 100% of correct matches. Within the total number of pairwise comparisons, we recorded 51 recaptures (true positives) in which 82.2% were ranked in first position by the computer-assisted software, i .e . had higher similarity with the target photo. The remaining recaptures were ranked in second position by the software. Time expended to finish the computed-assisted evaluation was 73 min.
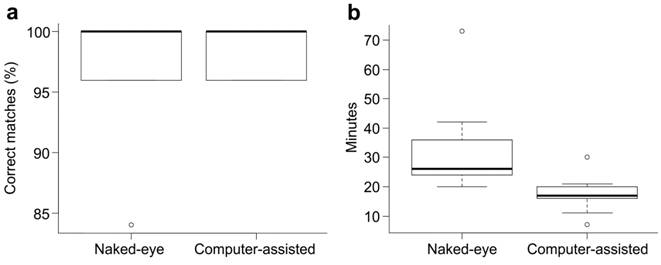
Field test. The average percentage of correct matches for the nine users was 97% (six misidentifications in a total of 225) when with naked-eye and 99% (three misidentifications in a total of 225) when computer-assisted (Table 1). The percentage of correct matches between naked-eye and computer-assisted identification did not differ statistically (t1, 16 = 0.71; p = 0.49). There was a total of 96 recaptures in computer-assisted pairwise matches in which 90 were ranked in first position by the computer-assisted software and three, two and one were ranked in second, third and fourth position, respectively . The average time expended in pairwise comparisons was 33 min and 50 sec for naked-eye identification and 17 min and 20 sec for computer-assisted identification, and both techniques differed statistically (t1, 16 = 2.78; p = 0.013) (Fig. 4).
Data on the field test for individuals of Rineloricaria aequalicuspis in which photo-identification efficacy was compared between naked-eye and computer-assisted technique. Data include number of photos compared by nine different users, number of correct matches (true positive and true negative) and misidentifications (false positive and false negative) and average percentage of correct matches and time expended.
Percentage of correct matches (a) and expended minutes (b) between naked-eye and computer-assisted field test photo-identification for individual recognition of Rineloricaria aequalicuspis (n = 9). Boxplots show median (central thicker line), first and third quartile (box limits), 95% confidence interval of median (whiskers), and outliers.
Discussion
Our photo-identification test for Rineloricaria aequalicuspis individuals yielded high proportion of correct matches, both for pictures taken in laboratory (100%) and in field conditions (average ≥ 97%). Although we did not directly compared photo-identification with other tagging or marking methods, accuracy was similar or higher than in other studies that directly compared photo-identification to other methods. For example, Caorsi et al. (2012Caorsi, V. Z., R. R. Santos & T. Grant. 2012. Clip or snap? An evaluation of toe-clipping and photo-identification methods for identifying individual Southern Red-Bellied Toads, Melanophryniscus cambaraensis . South American Journal of Herpetology, 7: 79-84.) found 99.4% of correct matches in naked-eye photo-identification of an anuran species based on ventral color pattern, 90.9% using computer-assisted photo-identification and 95.3% using toe-clipping method. Furthermore, our results are comparable with Chim & Tan (2012Chim, C. K. & K. S. Tan. 2012. Recognition of individual knobby sea stars Protoreaster nodosus (L., 1758) using aboral surface characteristics. Journal of Experimental Marine Biology and Ecology, 430-431: 48-55.) study, which found respectively 87% and 100% of correct matches for computer-assisted and naked-eye photo evaluations of a sea star species based on tubercle patterns. Our results therefore indicate that photo-identification is a suitable method for individual identification in R. aequalicuspis . However, in our field test, nine people did the photograph evaluations and the percentage of correct matches varied from 84% to 100% depending on the user (average 97%). The lower percentage of correct matches in a single user occurred in naked-eye evaluation. This result may suggest that the user training is important to photo-identification efficacy, but also that computer-assisted may help to reduce the potential human mistakes in evaluations.
We used photo-identification data based on pictures taken under field condition to compare accuracy and performance (time expended in photo-identification) of naked-eye and computer-assisted photo-identification. The percentage of correct matches was high in both techniques (i .e . average 97% and 99% for naked-eye and computer-assisted, respectively), and did not differ statistically, probably because inter-individual differences in abdominal plates of R . aequalicuspis are clearly distinguishable and thus individuals could be easily recognized. However, the average time expended to conclude photo evaluations was substantially lower when using computer-assisted photo-identification; computer-assisted evaluation was about half of the time expended to conclude naked-eye photo evaluations. Consequently, computer-assisted photo-identification could greatly reduce the expended time when using large data sets. Although not tested in our study, it is possible that the percentage of correct matches is also affected when using large data sets (i .e . >hundreds of photos) when comparing computer-assisted versus naked-eye evaluations.
Individual recognition of fishes by markings and tags has been made in several ways for many years (Pine et al., 2003Pine, W. E., K. H. Pollock, J. E. Hightower, T. J. Kwak & J. A. Rice. 2003. A review of tagging methods for estimating fish population size and components of mortality. Fisheries, 28(10): 10-23.), but the potential of using photo-identification with teleost species has only recently started to be demonstrated (e .g . Correia et al., 2014Correia, M., J. Palma, H. Koldewey & J. P. Andrade. 2014. The use of a non‐invasive tool for capture-recapture studies on a seahorse Hippocampus guttulatus population. Journal of Fish Biology, 84: 872-884.). Most of the commonly used methods are invasive and can have adverse effects on fishes, increasing the risk of sequelae or mortality (Ombredane et al., 1998Ombredane, D., J. L. Baglinière & F. Marchand. 1998. The effects of Passive Integrated Transponder tags on survival and growth of juvenile brown trout (Salmo trutta L.) and their use for studying movement in a small river. Hydrobiologia, 371/372: 99-106.; Murray & Fuller, 2000Murray, D. L. & M. R. Fuller. 2000. A critical review of the effects of marking on the biology of vertebrates. Pp. 15-64. In: Boitani, L. & T. K. Fuller (Eds.). Research techniques in animal ecology: controversies and consequences. New York, Columbia University Press.), mainly for small and sensitive fishes as most of the stream fish species. An added mark or tag can also affect fish behavior (Mesa & Schreck, 1989Mesa, M. G. & C. B. Schreck. 1989. Electrofishing mark-recapture and depletion methodologies evoke behavioral and physiological changes in cutthroat trout. Transactions of the American Fisheries Society, 118: 644-658.), which is an undesirable side effect. Moreover, tagged individuals can often lose their tags compromising data uptake (Arnason & Mills, 1981Arnason, A. N. & K. H. Mills. 1981. Bias and loss of precision due to tag loss in Jolly-Seber estimates for mark-recapture experiments. Canadian Journal of Fisheries and Aquatic Sciences, 38: 1077-1095.). In addition to the accuracy of photo-identification demonstrated herein, we also noted that an anesthetized individual can be handled and photographed in less than 30 seconds if researchers are trained, reducing even more the probability of stress, death or subsequent sequelae.
Photo-identification of individual fish may be based on color pattern at the body surface, e .g . Syngnathidae (Martin-Smith, 2011Martin-Smith, K. M. 2011. Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. Journal of Fish Biology, 78: 1757-1768.; Correia et al., 2014Correia, M., J. Palma, H. Koldewey & J. P. Andrade. 2014. The use of a non‐invasive tool for capture-recapture studies on a seahorse Hippocampus guttulatus population. Journal of Fish Biology, 84: 872-884.), and pattern of spots and scarring marks, e .g . whale sharks (Arzoumanian et al., 2005Arzoumanian, Z., J. Holmberg & B. Norman. 2005. An astronomical pattern-matching algorithm for computer‐aided identification of whale sharks Rhincodon typus . Journal of Applied Ecology, 42: 999-1011.; Holmberg et al., 2008Holmberg, J., B. Norman& Z. Arzoumanian. 2008. Robust, comparable population metrics through collaborative photo-monitoring of whale sharks Rhincodon typus . Ecological Applications, 18: 222-233.). We found that the abdominal bony plate pattern of loricariids can be also a reliable characteristic to distinguish individuals of the same species. Several stream loricariids are benthic species with cryptic habits, living under or between cobbles and boulders, inside submerged logs and in wholes at clay banks (Power, 2003Power, M. E. 2003. Life cycles, limiting factors, and behavioral ecology of four loricariid catfishes in a Panamanian stream. v.2, Pp. 581-600. In: Arratia, G., B. G. Kapoor, M. Chardon & R. Diogo (Eds.). Catfishes. Enfield, New Hampshire, Science Publishers. ). This behavior implies that they must be capture for photographs of individuals to be taken. This is the main difference between our study and the aforementioned studies, in which fish photo-identification was performed without capturing fish (underwater photographs). Therefore, we also demonstrated that photo-identification may be a feasible method even when it is necessary to capture fish. However, we highlight that the feasibility of photo-identification when stream fishes need to be captured will greatly depend on each species sensitivity to this procedure, and may be quite variable between fish groups (e.g . loricariid vs. characid fishes).
Fish species are often not easily identifiable during field work because they are very similar to each other in external characters, such as in Rineloricaria , for which species identification in the field requires well trained collectors. We thus emphasize that field personnel involved in photo-identification studies must determine in advance what are the potentially similar species occurring in the study site and know how to identify those species. Also, photo-identification must be validated for any species before eventual application in research, since its use for a particular species does not guarantee application to other species.
Several fish, such as small characids, do not present distinguishable characteristics that allow researchers to easily distinguish individuals and thus, do not meet assumptions for the use of photo-identification. In these cases, other marking or tagging techniques are necessary. On the other hand, using a stream-dwelling armored catfish as a study case, we suggest that photo-identification is a suitable alternative technique for a large number of species that meet the assumptions for photo-identification (see Bolger et al., 2012Bolger, D. T., T. A. Morrison, B. Vance, D. Lee & H. Farid. 2012. A computer‐assisted system for photographic mark-recapture analysis. Methods in Ecology and Evolution, 3: 813-822.).
The possibility of using photo-identification for individual recognition of freshwater fish opens an ample opportunity for research based on mark-recapture methods. Photo-identification is cheap, less stressful for the fish and accurate (at least for R. aequalicuspis ). A further advantage is that digital images may be easily stored in association to other data (sex, size, and weight) and used for reassessments, new research questions and long-term investigations.
Acknowledgements
We thank to Mateus Camana, Laís Mozzaquattro, Matheus Dalmolin, Taís Guimarães, Michelle Abadie and Caroline Pinheiro who collaborated in performing photo-identification for the field test. We also thank to Mateus Camana and Laís Mozzaquattro for their collaboration during the field work and also to Michele Abadie for her help with initial Wild-ID software training and in discussing computer-assisted evaluations. We are also grateful to Antônio dos Santos Júnior for his help with organizing and tagging fish specimens for the laboratory test and to Luiz R. Malabarba (UFRGS) for provide a photographof R . aequalicuspis . JBM received a scholarship from CNPq/UFRGS.
References
- Anderson, C. J. R., N. D. V. Lobo, J. D. Roth & J. M. Waterman. 2010. Computer-aided photo-identification system with an application to polar bears based on whisker spot patterns. Journal of Mammalogy, 91: 1350-1359.
- Arnason, A. N. & K. H. Mills. 1981. Bias and loss of precision due to tag loss in Jolly-Seber estimates for mark-recapture experiments. Canadian Journal of Fisheries and Aquatic Sciences, 38: 1077-1095.
- Arzoumanian, Z., J. Holmberg & B. Norman. 2005. An astronomical pattern-matching algorithm for computer‐aided identification of whale sharks Rhincodon typus . Journal of Applied Ecology, 42: 999-1011.
- Biggins, D. E., J. L. Godbey, B. J. Miller & L. R. Hanebury. 2006. Radio telemetry for black-footed ferret research and monitoring. Pp. 175-189. In: Roelle, J. E., B. J. Miller, J. L. Godbey& D. E. Biggins (Eds.). Recovery of the black-footed ferret: progress and continuing challenges. Proceedings of the symposium on the status of the Black-footed Ferret and its habitat, Fort Collins, Colorado, January 28-29, 2004. [Reston, VA], U.S. Dept. of the Interior, U. S. Geological Survey.
- Bolger, D. T., T. A. Morrison, B. Vance, D. Lee & H. Farid. 2012. A computer‐assisted system for photographic mark-recapture analysis. Methods in Ecology and Evolution, 3: 813-822.
- Caci, G., A. B. Biscaccianti, L. Cistrone, L. Bosso, A. P. Garonna & D. Russo. 2013. Spotting the right spot: computer-aided individual identification of the threatened cerambycid beetle Rosalia alpina . Journal of Insect Conservation, 17: 787-795.
- Caorsi, V. Z., R. R. Santos & T. Grant. 2012. Clip or snap? An evaluation of toe-clipping and photo-identification methods for identifying individual Southern Red-Bellied Toads, Melanophryniscus cambaraensis . South American Journal of Herpetology, 7: 79-84.
- Carlson, B. E. & T. Langkilde. 2013. A common marking technique affects tadpole behavior and risk of predation. Ethology, 119: 167-177.
- Chim, C. K. & K. S. Tan. 2012. Recognition of individual knobby sea stars Protoreaster nodosus (L., 1758) using aboral surface characteristics. Journal of Experimental Marine Biology and Ecology, 430-431: 48-55.
- Correia, M., J. Palma, H. Koldewey & J. P. Andrade. 2014. The use of a non‐invasive tool for capture-recapture studies on a seahorse Hippocampus guttulatus population. Journal of Fish Biology, 84: 872-884.
- Dutton, D. L., P. H. Dutton, M. Chaloupka & R. H. Boulon. 2005. Increase of a Caribbean leatherback turtle Dermochelys coriacea nesting population linked to long-term nest protection. Biological Conservation, 126: 186-194.
- Franz, I. & C. S. Fontana. 2013. Breeding biology of the Tawny-Bellied seedeater (Sporophila hypoxantha ) in southern Brazilian upland grasslands. The Wilson Journal of Ornithology, 125: 280-292.
- Gauthier-Clerc, M., J. -P. Gendner, C. A. Ribic, W. R. Fraser, E. J. Woehler, S. Descamps, C. Gilly, C. Le Bohec & Y. Le Maho. 2004. Long-term effects of flipper bands on penguins. Proceedings of the Royal Society of London. Series B, Biological Society, 271(suppl. 6): S423-S426.
- Giglio, V. J., J. Adelir-Alves & A. A. Bertoncini. 2014. Using scars to photo-identify the goliath grouper, Epinephelus itajara . Marine Biodiversity Records, 7: e108(4 p).
- Haines, G. B. & T. Modde. 1996. Evaluation of marking techniques to estimate population size and first-year survival of Colorado squawfish. North American Journal of Fisheries Management, 16: 905-912.
- Hastings, K. K., L. A. Hiby & R. J. Small. 2008. Evaluation of a computer-assisted photograph-matching system to monitor naturally marked harbor seals at Tugidak Island, Alaska. Journal of Mammalogy, 89: 1201-1211.
- Holmberg, J., B. Norman& Z. Arzoumanian. 2008. Robust, comparable population metrics through collaborative photo-monitoring of whale sharks Rhincodon typus . Ecological Applications, 18: 222-233.
- Kenyon, N., A. D. Phillott & R. A. Alford. 2009. Evaluation of the photographic identification method (PIM) as a tool to identify adult Litoria genimaculata (Anura: Hylidae). Herpetological Conservation and Biology, 4: 403-410.
- Knox, C. D., A. Cree & P. J. Seddon. 2013. Accurate identification of individual geckos (Naultinus gemmeus ) through dorsal pattern differentiation. New Zealand Journal of Ecology, 37: 60-66.
- Linnane, A., D. Hobday, S. Frusher & C. Gardner. 2012. Growth rates of juvenile southern rock lobster (Jasus edwardsii ) estimated through a diver-based tag-recapture program. Marine and Freshwater Research, 63: 110-118.
- Lowe, D. G. 2004. Distinctive image features from scale-invariant keypoints. International Journal of Computer Vision, 60: 91-110.
- Marshall, A. D. & S. J. Pierce. 2012. The use and abuse of photographic identification in sharks and rays. Journal of Fish Biology, 80: 1361-1379.
- Martin-Smith, K. M. 2011. Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. Journal of Fish Biology, 78: 1757-1768.
- Mesa, M. G. & C. B. Schreck. 1989. Electrofishing mark-recapture and depletion methodologies evoke behavioral and physiological changes in cutthroat trout. Transactions of the American Fisheries Society, 118: 644-658.
- Monk, M. H., J. Berkson & P. Rivalan. 2011. Estimating demographic parameters for loggerhead sea turtles using mark-recapture data and a multistate model. Population Ecology, 53: 165-174.
- Monteiro, N. M., R. M. Silva, M. Cunha, A. Antunes, A. G. Jones & M. N. Vieira. 2014. Validating the use of colouration patterns for individual recognition in the worm pipefish using a novel set of microsatellite markers. Molecular Ecology Resources, 14: 150-156.
- Moore, J. A., T. Grant, D. Brown, S. N. Keall & N. J. Nelson. 2010. Mark-recapture accurately estimates census for tuatara, a burrowing reptile. The Journal of Wildlife Management, 74: 897-901.
- Murray, D. L. & M. R. Fuller. 2000. A critical review of the effects of marking on the biology of vertebrates. Pp. 15-64. In: Boitani, L. & T. K. Fuller (Eds.). Research techniques in animal ecology: controversies and consequences. New York, Columbia University Press.
- Nery, M. F., M. A. Espécie & S. M. Simão. 2008. Site fidelity of Sotalia guianensis (Cetacea: Delphinidae) in Sepetiba Bay, Rio de Janeiro, Brazil. Revista Brasileira de Zoologia, 25: 182-187.
- Ombredane, D., J. L. Baglinière & F. Marchand. 1998. The effects of Passive Integrated Transponder tags on survival and growth of juvenile brown trout (Salmo trutta L.) and their use for studying movement in a small river. Hydrobiologia, 371/372: 99-106.
- Pearson, R. G. & J. L. Munro. 1991. Growth, mortality and recruitment rates of giant clams, Tridacna gigas and T . derasa , at Michaelmas Reef, central Great Barrier Reef, Australia. Marine and Freshwater Research, 42: 241-262.
- Perrig, M. & B. P. L. Goh. 2008. Photo-ID on reef fish: avoiding tagging-induced biases. Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida, 22: 7-11
- Pine, W. E., K. H. Pollock, J. E. Hightower, T. J. Kwak & J. A. Rice. 2003. A review of tagging methods for estimating fish population size and components of mortality. Fisheries, 28(10): 10-23.
- Power, M. E. 2003. Life cycles, limiting factors, and behavioral ecology of four loricariid catfishes in a Panamanian stream. v.2, Pp. 581-600. In: Arratia, G., B. G. Kapoor, M. Chardon & R. Diogo (Eds.). Catfishes. Enfield, New Hampshire, Science Publishers.
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing. Available from: Available from: https://www.r-project.org/ (21 November 2014).
» https://www.r-project.org/ - Reis, R. E. & A. R. Cardoso. 2001. Two new species of Rineloricaria from southern Santa Catarina and northeastern Rio Grande do Sul, Brazil (Teleostei: Loricariidae). Ichthyological Exploration of Freshwaters, 12: 319-332.
- Reisser, J., M. Proietti, P. Kinas & I. Sazima. 2008. Photographic identification of sea turtles: method description and validation, with an estimation of tag loss. Endangered Species Research, 5: 73-82.
- Silvy, N. J., R. R. Lopez & M. J. Peterson. 2012. Techniques for marking wildlife. v.1 Pp. 230-257. In:. Silvy, N. J (Ed.). The wildlife techniques manual. 7th ed. Baltimore, Johns Hopkins University Press.
- Speed, C. W., M. G. Meekan & C. J. A. Bradshaw. 2007. Spot the match -wildlife photo-identification using information theory. Frontiers in Zoology, 4 (jan.).
- Sreekar, R., C. B. Purushotham, K. Saini, S. N. Rao, S. Pelletier & S. Chaplod. 2013. Photographic capture-recapture sampling for assessing populations of the Indian gliding lizard Draco dussumieri . PLoS ONE, 8: e55935 (15p.).
- Wilson, C. D., G. Arnott, N. Reid & D. Roberts. 2011. The pitfall with PIT tags: marking freshwater bivalves for translocation induces short-term behavioural costs. Animal Behaviour, 81: 341-346.
- Wilson, R. P. & C. R. McMahon. 2006. Measuring devices on wild animals: what constitutes acceptable practice? Frontiers in Ecology and the Environment, 4: 147-154.
Publication Dates
-
Publication in this collection
2016
History
-
Received
28 May 2015 -
Accepted
23 Oct 2015