ABSTRACT
We document for the first time the early ontogeny of Centropomus poeyi based on captive raised material representing 0-19 days posthatch (dph). The achievement of early developmental landmarks (i.e., yolk-sac depletion, flexion, development of fins) and changes in pigmentation are described (1.4 mm NL-10.6 mm SL; 0-19 dph) and documented for a subset of individuals using high quality photographs. The ontogeny of the viscerocranium is also described (2.4 mm NL-10.6 mm SL; 6-19 dph). Development in C. poeyi occurs over a short period with attainment of the juvenile stage (i.e., full complement of fin rays present in each fin) occurring by 6.9 mm SL. The ontogeny of external pigmentation in C. poeyi is marked by two trends throughout growth: (1) a decrease in pigmentation dorsally; and (2) an increase in pigmentation ventrally along the midline. Development of the viscerocranium begins with the appearance of the maxilla and dentary in individuals of 2.4 mm NL, coinciding with the depletion of the yolk-sac. By 10.6 mm SL all bones of the viscerocranium are present and teeth are present on all teeth-bearing bones of the adult. Aspects of early development in C. poeyi are compared with the congeners C. undecimalis and C. parallelus.
Keywords:
Aquaculture; Morphology; Ontogeny; Perciformes; Viscerocranium
RESUMEN
Se documentó por primera vez la ontogenia temprana de Centropomus poeyi basada en material obtenido de cautiverio a partir de embriones (0 días después de la eclosión, dde) hasta los 19 dde. Se registró la aparición de puntos de referencia en el desarrollo temprano (p.ej. consumo de saco vitelino, flexión, desarrollo de aletas) y los cambios de pigmentación han sido descritos (1.4 mm NL-10.6 mm SL; 0-19 dde), siendo documentado para un subgrupo de individuos utilizando fotografías de alta calidad. El desarrollo del viscerocráneo también fue descrita (2.4 mm NL-10.6 mm SL; 6-19 dde). El desarrollo en C. poeyi sucede en un periodo corto de tiempo hasta alcanzar una etapa juvenil (p.ej., aparición completa de los radios presentes en cada aleta), lo que se observa a los 6.9 mm SL. La pigmentación externa en C. poeyi esta marcada por dos tendencias en el crecimiento: (1) un descenso en la pigmentación dorsal; y (2) un incremento en la pigmentación ventral a lo largo de la línea media. El desarrollo del viscerocráneo comienza con el aparecimiento del maxilar y el dentario en individuos de 2.4 mm NL, lo cual coincide con el consumo del saco vitelino. A los 10.6 mm SL todos los huesos del viscerocráneo están presentes como se observa en los dientes en los adultos. Algunos aspectos del desarrollo temprano en C. poeyi son comparados con los congéneros C. undecimalis y C. parallelus.
Palabras-clave:
Acuicultura; Morfología; Ontogenia; Perciformes; Viscerocranium
Introduction
The family Centropomidae is a small group of perciform fishes (Order Perciformes), consisting of a single genus (Centropomus) with twelve species restricted to the tropical and subtropical waters of the New World (Nelson, 2006Nelson JS. Fishes of the World. 4th ed. Hoboken (NJ): J. Wiley; 2006.). Species of Centropomus are found on both sides of the Central American Isthmus, with six species distributed along the Pacific coast of Central and South America (from Northern Mexico to Peru) and six along the Atlantic coast of North, Central and South America (from peninsular Florida to Brazil) (Tringali et al., 1999Tringali MD, Bert TM, Seyoum S, Bermingham E, Bartolacci D. Molecular phylogenetics and ecological diversification of the transisthmian fish genus Centropomus (Perciformes: Centropomidae). Mol Phylogenet Evol. 1999; 13(1):193-207.). Commonly referred to as Robalo or Snook, members of this family are economically important throughout their range (Orrell, 2003Orrell TM. Centropomidae Snooks. In: Carpenter KE, ed. The living marine resources of the Western Central Atlantic (Bony Fishes part 1(Acipenseridae to Grammatidae). vol 2). FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. Rome: FAO. 2002. p. 601-1374.), eithercommercially as in Central and South America where they are the focus of artesian fisheries (Lemos et al., 2006Lemos D, Netto B, Germano A. Energy budget of juvenile fat snook Centropomus parallelus fed live food. Comp Biochem Physiol A Mol Integr Physiol. 2006; 144(1):33-40.; Díaz-Jaimes et al., 2007Díaz-Jaimes P, Sandoval-Castellanos E, Uribe-Alcocer M. Comparative population structure of three snook species (Teleostei: Centropomidae) from the eastern central Pacific. Ichthyol Res. 2007; 54(4):380-87.; Álvarez-Lajonchère, Tsuzuki, 2008Álvarez-Lajonchère L, Tsuzuki MY. A review of methods for Centropomus spp. (snooks) aquaculture and recommendations for the establishment of their culture in Latin America. Aquac Res. 2008; 39(7):684-700.) or recreationally as in the United States where they are targeted by sport fishers (Taylor et al., 2001Taylor RG, Whittington JA, Haymans DE. Catch-and-release mortality rates of common snook in Florida. NAm J Fish Manage. 2001; 21(1):70-75.; Pope et al., 2006Pope KL, Blankinship DR, Fisher M, Patiño R. Status of the common snook (Centropomus undecimalis) in Texas. Tex J Sci. 2006; 58(4):325-32.). All twelve species of Centropomus are similar in external appearance and are distinguished only by a few subtle morphological differences (Rivas, 1986Rivas LR. Systematic review of the perciform fishes of the genus Centropomus. Copeia . 1986; (3):579-611.; Orrell, 2003Orrell TM. Centropomidae Snooks. In: Carpenter KE, ed. The living marine resources of the Western Central Atlantic (Bony Fishes part 1(Acipenseridae to Grammatidae). vol 2). FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. Rome: FAO. 2002. p. 601-1374.). This overall similarity between different species of snook has rendered the delineation of geographic distributions for individual species challenging, creating problems for species-level management (Rivas, 1986Rivas LR. Systematic review of the perciform fishes of the genus Centropomus. Copeia . 1986; (3):579-611.; Díaz-Jaimes et al., 2007Díaz-Jaimes P, Sandoval-Castellanos E, Uribe-Alcocer M. Comparative population structure of three snook species (Teleostei: Centropomidae) from the eastern central Pacific. Ichthyol Res. 2007; 54(4):380-87.).
Centropomids have been of interest as a potential species for aquaculture since the early 1970’s after overexploitation of natural stocks resulted in decreased population sizes (Taylor et al., 1998Taylor RG, Grier HJ, Whittington JA. Spawning rhythms of common snook in Florida. J Fish Biol . 1998; 53(3):502-20.; Pope et al., 2006Pope KL, Blankinship DR, Fisher M, Patiño R. Status of the common snook (Centropomus undecimalis) in Texas. Tex J Sci. 2006; 58(4):325-32.; Álvarez-Lajonchère, Tsuzuki, 2008Álvarez-Lajonchère L, Tsuzuki MY. A review of methods for Centropomus spp. (snooks) aquaculture and recommendations for the establishment of their culture in Latin America. Aquac Res. 2008; 39(7):684-700.). Despite being able to readily obtain fertilized eggs, through either strip spawning or the induction of spawning using GnRH-a hormone injection, the captive propagation of centropomids has been generally hindered by low survival rates of larvae and juveniles (Álvarez-Lajonchère, Tsuzuki, 2008Álvarez-Lajonchère L, Tsuzuki MY. A review of methods for Centropomus spp. (snooks) aquaculture and recommendations for the establishment of their culture in Latin America. Aquac Res. 2008; 39(7):684-700.). For this reason, multiple studies aiming to improve the cultivation of these fishes have been conducted (Neidig et al., 2000Neidig CL, Skapura DP, Grier HJ, Dennis CW. Techniques for spawning common snook: broodstock handling, oocyte staging, and egg quality. N Am J Aquacult. 2000; 62(2):103-13.; Lemos et al., 2006Lemos D, Netto B, Germano A. Energy budget of juvenile fat snook Centropomus parallelus fed live food. Comp Biochem Physiol A Mol Integr Physiol. 2006; 144(1):33-40.; Wittenrich et al., 2009Wittenrich ML, Rhody NR, Turingan RG, Main KL. Coupling osteological development of the feeding apparatus with feeding performance in common snook, Centropomus undecimalis, larvae: identifying morphological constraints to feeding. Aquaculture. 2009; 294(3):221-27.; Yanes-Roca et al., 2015Yanes-Roca C, Rhody NR, Nystrom M, Wittenrich ML, Main KL. Embryonic and early larval development in hatchery-reared common snook. N Am J Aquacult . 2012; 74(4):499-511.). Surprisingly, however, there has been very little research focused on the early morphological development of centropomids. Currently only five studies have examined early morphological development in two species of snook, including, Centropomus undecimalis (Bloch, 1792), the common snook (Lau, Shafland, 1982Lau SR, Shafland PL. Larval development of snook, Centropomus undecimalis (Pisces: Centropomidae). Copeia. 1982; 1982(3):618-27.; Potthoff, Tellock, 1993Potthoff T, Tellock JA. Osteological development of the snook, Centropomus undecimalis (Teleostei, Centropomidae). B Mar Sci. 1993; 52(2):669-716.; Wittenrich et al., 2009Wittenrich ML, Rhody NR, Turingan RG, Main KL. Coupling osteological development of the feeding apparatus with feeding performance in common snook, Centropomus undecimalis, larvae: identifying morphological constraints to feeding. Aquaculture. 2009; 294(3):221-27.; Yanes-Roca et al., 2015Yanes-Roca C, Rhody NR, Nystrom M, Wittenrich ML, Main KL. Embryonic and early larval development in hatchery-reared common snook. N Am J Aquacult . 2012; 74(4):499-511.), and Centropomus parallelus Poey, 1860, the fat snook (Itagaki et al., 2013Itagaki MK, Katsuragawa M, Pimentel CMM, Oliveira IR, Ohkawara MH. Early development of fat snook, Centropomus parallelus (Poey 1860) (Teleostei, Centropomidae) from southeastern Brazil. Zootaxa. 2013; 3669(1):65-75.). Through the efforts of a fledgling captive propagation program for centropomids of the Gulf of Mexico at the Laboratorio de Acuicultura Tropical (Universidad Juárez Autónoma de Tabasco), early developmental material (larvae and juveniles) were obtained for a third species of centropomid, Centropomus poeyi Chávez, 1961, the Mexican snook. Centropomus poeyi is the second largest species of snook in the Atlantic, reaching a maximum size of 90 cm total length, but possesses the narrowest geographic range of any centropomid, ranging from southern Tamaulipas (Mexico) to Belize (Rivas, 1986Rivas LR. Systematic review of the perciform fishes of the genus Centropomus. Copeia . 1986; (3):579-611.). In this study we document aspects of the early development of C. poeyi, including changes in external appearance and the progression of skeletal development in the viscerocranium.
Materials and Methods
Adult specimens of Centropomus poeyi were maintained and spawned at the Estación de Marina de Acuicultura (Universidad Juarez Autónoma de Tabasco; UJAT) in Jalapita, Centla, Tabasco. Spawning of broodfish was induced using an implant of GnRH-a hormone (Argent Labs®) (200 μg/fish in females and 100 μg/fish in males) following the procedure described in Álvarez-Lajonchere, Hernández-Molejón (2001Álvarez-Lajonchère L, Hernández-Molejón OG. Producción de juveniles de peces estuarinos para un Centro en América Latina y el Caribe: diseño, operación y tecnologías. Baton Rouge, LA, USA: The World Aquaculture Society; 2001.). Once spawning occurred, eggs were collected and incubated until hatching at which point they were transferred to 300 L recirculating tanks and fed a mixture of rotifers, Artemia nauplii and commercial feed. Approximately 10-15 individuals were sampled daily from hatching (0 days posthatch [dph]) up to 19 dph. Once collected, larvae were euthanized via an overdose treatment of tricaine methanesulfonate (MS-222) and fixed in a solution of 4% buffered paraformaldehyde for 24 h. Specimens were then transferred through a series of graded ETOH solutions (30%, 50%) for 24 h each before being stored in a final solution of 70% ETOH. A total of 300 individuals of C. poeyi ranging in size from 1.4-10.6 mm (notochord length [NL] for preflexion larvae/standard length [SL] for postflexion larvae) were collected. Twenty-eight individuals ranging in size from 2.4-10.6 mm NL/SL were cleared and double-stained (C&S) for bone and cartilage investigation (Taylor, Van Dyke, 1985Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107-20.). Specimens were examined under a ZEISS SteReo Discovery V20 stereomicroscope. Select individuals were photographed in dorsal, lateral and ventral views using a ZEISS Axiocam MRc5 digital camera attached to the aforementioned microscope using the Z-stack option. Stacked images were processed using Adobe Photoshop CS5.1 and Illustrator CS5.1.
Material examined. All material examined is deposited in the Collection of Fishes at the Biodiversity Research and Teaching Collections of Texas A&M University (TCWC): TCWC 19680.01, 272 specimens, 1.4 mm NL-9.2 mm SL; TCWC 19679.01, 28 specimens (C&S), 2.4 mm NL-10.6 mm SL.
Results
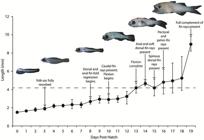
Overview of Development. An overview of growth in Centropomus poeyi in notochord length (pre-flexion) and standard length (post-flexion) is provided in Fig. 1. During the yolk-sac and pre-flexion stages, growth occurred gradually and uniformly across the cohort, with the sizes of individuals collected each day showing little to no variation in length. Post-flexion individuals showed an increase in the rate of growth with an average size increase per day of ~0.86 mm (range 3.2-9.8 mm; n=90). Additionally, during the post-flexion stage, variation in length between individuals increased dramatically, with differences reaching as high as 3.7 mm or 48.7% (range 3.9-7.6 mm; n=7) between individuals collected 18 dph.
Growth in length for Centropomus poeyi (TCWC 19680.01; 210, 1.4-9.8 mm SL) from 0 to 19 days posthatch with major developmental landmarks indicated. Dashed line represents completion of Flexion. Points represent average sizes of individuals in mm corresponding to days posthatch (notochord length below dashed line, standard length above dashed line), with error bars representing full range of sizes observed.
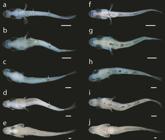
Freshly hatched larvae of C. poeyi ranged from 1.4 to 1.6 mm NL (n =4). At hatching (Fig. 2a), individuals possess a large oval shaped yolk-sac located ventral to the anterior third of the body with an oil globule positioned at the anterior region of the yolk-sac, ventral to the head. The gut, which appears as a thin tube, is beginning to develop and the body, excluding the head and yolk-sac, is surrounded by a thin, transparent fin fold. By 1.9 mm NL (Fig. 2c), the oil globule is completely resorbed, the yolk-sac is reduced to a fraction of its original size and the mouth appears to be fully formed. In individuals of 2.0 mm NL (Fig. 2d), the yolk-sac is fully resorbed and larvae have started feeding exogenously. The swim bladder is inflated and visible dorsal to the anterior region of the gut. By 2.7 mm NL (Figs. 2e, 3a, f), the dorsal-fin fold has started to regress in a rostrocaudal direction posterior to the head. At the same time, regression of both the dorsal- and anal-fin folds has started around the caudal peduncle and flexion of the notochord is underway in individuals of approximately 3 mm NL. At 4.0 mm SL flexion is complete and the dorsal- and anal-fin folds are fully regressed in individuals of 4.4 mm SL (Figs. 2g, 3c, h).
Larvae and juveniles of Centropomus poeyi (TCWC 19680.01) in lateral view. a. 1.6 mm NL; b. 1.7 mm NL; c. 1.9 mm NL; d. 2.2 mm NL; e. 2.7 mm NL; f. 3.1 mm NL; g. 4.4 mm SL; h. 4.8 mm SL; i. 9.2 mm SL. Scale bars equal to 0.4 mm. Arrows indicate location of dorsal melanophore clusters. O, oil globule; Y, yolk-sac.
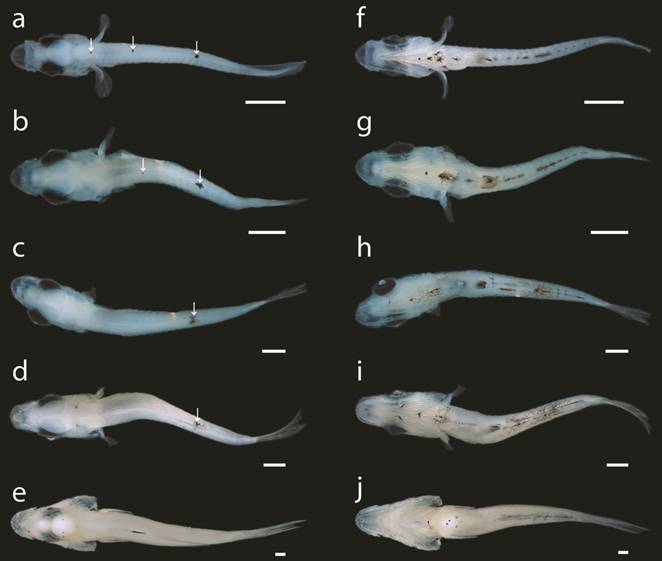
Larvae and juveniles of Centropomus poeyi (TCWC 19680.01) in dorsal and ventral view; a, f. 2.7 mm NL; b, g. 3.1 mm NL; c, h. 4.4 mm SL; d, i. 4.8 mm SL; e, j. 9.2 mm SL. Scale bars equal to 0.4 mm. Arrows indicate location of dorsal melanophore clusters.
The caudal fin is the first of the median fins to develop with the hypural cartilages starting to appear in individuals as small as 2.6 mm NL, prior to the start of flexion. By 3.0 mm NL, the first caudal-fin rays can be seen distal to the hypural cartilages. A full complement of 17 principal caudal-fin rays is present along with the first procurrent caudal-fin rays by 4.2 mm SL, after flexion is complete. The soft fin rays of the dorsal and anal fins are present in all individuals of 4.4 mm SL or larger but appear in individuals as small as 3.4 mm NL. Spinous dorsal-fin rays are present in individuals of 4.9 mm SL with the full complement of dorsal fin-rays (IX.9) present by 5.6 mm SL. The adult anal-fin ray count of III.6 is present in individuals as small as 5.0 mm SL.
The pectoral fins are the first of the paired fins to develop, with pectoral-fin buds first apparent in individuals of 1.7 mm NL. Despite the early development of the pectoral-fin buds, pectoral-fin rays do not appear until 4.4 mm SL and a full complement of 15-17 pectoral-fin rays is not present until at least 6.9 mm SL. Pelvic-fin buds appear in individuals as small as 4.3 mm SL with the first pelvic-fin rays present in individuals of 5.3 mm SL. The adult complement of 1 spinous and 5 soft pelvic-fin rays is seen by 6.9 mm SL.
Pigmentation. Newly hatched larvae (1.4 mm NL, Fig. 2a) possess two dorsal rows of melanophores that originate from the occipital region of the cranium and stop just short of the posterior tip of the notochord. Three melanophores sit along the anterior margin of the cranium and a cluster of melanophores can be seen laterally on either side of the body in line with the posterior margin of the yolk-sac. A large stellate cluster of melanophores is situated between the oil globule and yolk-sac. By 1.7 mm NL (Fig. 2b), the eye has started to develop pigmentation and the cranium is covered by pigmentation dorsally. The dorsal rows of melanophores have given way to three separate clusters, the first in line with the yolk-sac, the second in line with the mid-gut and the third located approximately two-thirds down the length of the body. The cluster located laterally along the body is gone and a row of melanophores extends along the ventral midline of the notochord, originating dorsal to the gut and stopping in line with the posterior cluster of dorsal melanophores. The eyes are fully pigmented at 1.9 mm NL (Fig. 2c) and the cranium has lost all pigmentation. The dorsal clusters of melanophores have become significantly reduced in size and the row along the ventral midline has expanded anteriorly, dorsal to the remnant of the yolk-sac, and posteriorly, almost to the tip of the notochord. With the complete resorption of the yolk-sac in individuals of 2 mm NL (Fig. 2d), the large stellate cluster of melanophores now sits ventrally along the body between the pectoral-fin buds. Two additional clusters of melanophores have appeared along the ventral margin of the gut, the first ventral to the mid-gut and the second almost at the posterior tip of the gut. In individuals of 2.7 mm NL (Figs. 2e, 3a, f), the large stellate cluster of melanophores located between the pectoral-fin buds has separated into three distinct centers and an additional cluster is now located anterior to the group of three. Additionally, a small melanophore appears on the posteroventral tip of the lower jaw on both sides. The anteriormost dorsal cluster of melanophores has disappeared by 3.1 mm NL (Figs. 2f, 3b, g) and pigmentation has appeared proximally on the caudal-fin rays. At 4.4 mm SL (Figs. 2g, 3c, h), only the posteriormost cluster of dorsal melanophores remains and those along the ventral midline are starting to take on a stellate form with melanin radiating from the centers. Pigmentation is present anteriorly on either side of the upper jaw by 4.8 mm SL (Figs. 2h, 3d, i) and two additional melanophores have appeared on the ventral midline of the jaw. A deep cluster of melanophores has appeared along the posterior margin of the occipital region and a small cluster has appeared at the posterodorsal tip of the preopercle. The pigmentation along the caudal fin extends further distally along the ventralmost principal caudal-fin rays and pigmentation has started to form anteriorly on the anal fin. By 9.2 mm SL (Figs. 2i, 3e, j), no pigmentation remains along the dorsal midline. Additional melanophores have appeared around the mouth and posterior margin of the cranium. Melanophores have spread across the caudal fin and pigmentation has formed along the posterodistal margin of the spinous dorsal fin. In the anal fin, pigmentation has spread to cover the length of the first two spinous fin rays. No pigmentation is present along the lateral portion of the body except for two small clusters on the caudal peduncle.
Development of the Viscerocranium. In the smallest C&S specimen (2.4 mm NL), the maxilla and the dentary are the only bones present in the jaws. In the hyoid arch the pars hyomandibularis and the pars symplectica are present but still in early development. The ceratohyal cartilage is bar shaped and articulates with the hypohyal cartilage anteriorly and the interhyal cartilage posteriorly. The pars quadrata and pars metapterygoidea are the only elements of the palatoquadrate present. At this size, development of the lower gill arches is underway with ceratobranchial cartilages 1-4 and hypobranchial cartilages 1-3 present.
By 2.8 mm NL the premaxilla is present and possesses two small conical teeth. Development of the pars hyomandibularis is complete with the foramen for the passage of the hyomandibular branch of the facial nerve now present on the dorsal head. The symplectic is present as a perichondral ossification around the midlength of the pars symplectica. The anterior ceratohyal is present as an endochondral ossification and the ceratohyal cartilage has started to expand dorsally and ventrally at the posterior end with two branchiostegal rays loosely associated with the developing cartilage. The pars autopalatina has appeared anterodorsal to the quadratometapterygoid portion of the palatoquadrate cartilage. All cartilaginous elements of the upper and lower gill arches except the basihyal are present. Ceratobranchials 1 and 2 are perichondrally ossifying and a few small conical teeth can be seen suspended in the epithelium near ceratobrabranchial 5 cartilage and pharyngobranchial 3 cartilage.
At 3.5 mm NL, a few small conical teeth are located anteriorly on the dentary and the basihyal cartilage is starting to develop. The anguloarticular can be seen lateral to the posterior end of Meckel’s cartilage. The hyomandibular is present as a perichondral ossification on the surface of the pars hyomandibularis just ventral to the foramen for the passage of the hyomandibular branch of the facial nerve. Three additional branchiostegal rays are present, five in total, with two associated with the posterior end of the ceratohyal cartilage and one associated with the anterior ceratohyal. The interhyal is a perichondral ossification and the urohyal can be seen as a small blade-like ossification ventral to the gill arches. The quadrate has begun to ossify endochondrally along the posteroventral margin of the pars quadrata and the endopterygoid is present as a thin sliver of bone resting along the dorsal margin of the pars autopalatina. Ceratobranchials 1-5 are ossifying endochondrally and epibranchials 1-4 are ossifying perichondrally. Additional teeth have appeared on both ceratobranchial 5 and pharyngobranchial 3 toothplate and pharygobranchial toothplates 2-4 each possess a single conical tooth.
At 4.3 mm SL, small conical teeth run the length of both the dentary and premaxilla. The retroarticular is endochondrally ossified at the posteroventralmost tip of Meckel’s cartilage. The posterior ceratohyal has started to ossify endochondrally posterodorsally on the ceratohyal cartilage and a full complement of 7 branchiostegal rays can be seen. The ventral hypohyal is present as a perichondral ossification at the ventralmost tip of the hypohyal cartilage. The palatine is perichondrally ossifying around the anterior portion of the pars autopalatina and the ectopterygoid can be seen along the ventral margin of the posterior end of the pars autopalatina and the pars quadrata. All elements of the upper and lower gill arches are ossifying perichondrally. Gill rakers have formed along the length of the anterior margin of ceratobranchials 1-3.
In the largest C&S specimen examined (10.6 mm SL), all bones of the viscerocranium are present. A row of six small teeth run along the anterior edge of the vomer and three small conical teeth can be seen on the ventromedial edge of the palatine. All teeth present in the jaws are still small, conical and similar in size. Two rows of gill rakers are found on ceratobranchials 1-3 and a single row is present on the anterior side of ceratobranchial 4. Gill rakers contributing to the anterior row on ceratobranchial 1 are twice the length of those contributing to the posterior row on ceratobranchial 1 or those found on ceratobranchials 2-4.
Discussion
Development of Centropomus poeyi. Development of Centropomus poeyi occurs during a relatively short period of growth with all bones of the viscerocranium present and attainment of the juvenile stage (i.e., full complement of fin rays present in all fins) by 6.1 mm SL and 6.9 mm SL respectively (~18-20dph).
Two trends were observed in the development of body pigmentation in C. poeyi. The first is a decrease in pigmentation along the dorsal midline, which becomes restricted to three distinct clusters that decrease in size as growth occurs. These clusters gradually fade with the anteriormost, middle and posteriormost clusters disappearing at 3.1 mm NL, 4.4 mm SL and 9.2 mm SL, respectively. The second trend observed is an increase in pigmentation along the ventral midline with no pigmentation present at hatching. A row of melanophores ventral to the notochord is the first to appear followed by a large cluster of melanophores that migrates ventrally with the resorption of the yolk-sac. As growth occurs, the large cluster splits into three smaller clusters and additional clusters form around the base of the anal fin, which persist up to 10.6 mm SL (the size of the largest individuals in our series).
Comparison to Centropomus undecimalis and C. parallelus. External development of C. poeyi is very similar to that previously described for both C. undecimalis (Potthoff, Tellock, 1993Potthoff T, Tellock JA. Osteological development of the snook, Centropomus undecimalis (Teleostei, Centropomidae). B Mar Sci. 1993; 52(2):669-716.) and C. parallelus (Itagaki et al., 2013Itagaki MK, Katsuragawa M, Pimentel CMM, Oliveira IR, Ohkawara MH. Early development of fat snook, Centropomus parallelus (Poey 1860) (Teleostei, Centropomidae) from southeastern Brazil. Zootaxa. 2013; 3669(1):65-75.) with most developmental landmarks occurring at approximately the same size in all three species and with only a few notable differences. The pectoral fins appear to develop at a smaller body size in C. poeyi than in the other two species, with fin ray development occurring between 4.4-6.9 mm SL. In C. undecimalis pectoral-fin ray development occurs at sizes approximately 2 mm larger (6.2-9.1 mm SL; Potthoff, Tellock, 1993Potthoff T, Tellock JA. Osteological development of the snook, Centropomus undecimalis (Teleostei, Centropomidae). B Mar Sci. 1993; 52(2):669-716.) when compared to C. poeyi and the pectoral-fin rays do not begin to appear until sizes greater than 5 mm SL in C. parallelus (Itagaki et al., 2013). Similarly, the fin rays of the pelvic fin start to appear at a smaller body size in C. poeyi (5.3 mm SL) when compared to that of C. undecimalis (6.6 mm SL) or C. parallelus (6.5 mm SL) although the full complement of pelvic-fin rays is present at approximately the same size in all three species. Overall, developmental landmarks of C. undecimalis were reached in individuals of slightly larger body sizes in comparison to C. poeyi or C. parallelus. This is not surprising since it has been previously observed that species of smaller body size tend to develop quicker than closely related larger species (Reiss, 1989Reiss JO. The meaning of developmental time: A metric for comparative embryology. Am Nat. 1989; 134(2):170-89.; Block, Mabee, 2012Block AJ, Mabee PM. Development of the mandibular, hyoid arch and gill arch skeleton in the Chinese barb Puntius semifasciolatus: comparisons of ossification sequences among Cypriniformes. J Fish Biol. 2012; 81(1):54-80.).
Ontogeny of the viscerocranium in C. poeyi is very similar to C. undecimalis except for the following differences: (1) the urohyal is the first element to appear in C. undecimalis but does not appear until after the anterior ceratohyal and first few branchiostegal rays in C. poeyi; and (2) the ossifications of the hyosymplectic cartilage appear before those of the palatoquadrate cartilage in C. poeyi, while the inverse occurs in C. undecimalis. The skeletal ontogeny of C. undecimalis is very well illustrated in Potthoff, Tellock (1993Potthoff T, Tellock JA. Osteological development of the snook, Centropomus undecimalis (Teleostei, Centropomidae). B Mar Sci. 1993; 52(2):669-716.) and the reader is directed to that work for further information on skeletal development in that species.
Several aspects of larval pigmentation in C. poeyi differ from that of C. undecimalis or C. parallelus. According to Itagaki et al. (2013Itagaki MK, Katsuragawa M, Pimentel CMM, Oliveira IR, Ohkawara MH. Early development of fat snook, Centropomus parallelus (Poey 1860) (Teleostei, Centropomidae) from southeastern Brazil. Zootaxa. 2013; 3669(1):65-75.), individuals between 2.6 mm NL and 7.0 mm SL of C. parallelus do not possess any pigmentation along the dorsal midline, an important character that can be used to distinguish larvae of C. parallelus from larvae of C. undecimalis, in which pigment is present along the dorsal midline at these sizes. Like C. undecimalis, C. poeyi also possesses clusters of melanophores at distinctive locations along the dorsal midline within this size range allowing it to be distinguished from C. parallelus. Based upon the features of pigmentation described for C. undecimalis by Lau, Shafland (1982Lau SR, Shafland PL. Larval development of snook, Centropomus undecimalis (Pisces: Centropomidae). Copeia. 1982; 1982(3):618-27.), these same clusters of pigmentation may be used to distinguish individuals of C. poeyi larger than 4.4 mm SL from C. undecimalis of a similar size. Between 4.4 and 9.2 mm SL two clusters of melanophores are present on the dorsal midline of C. undecimalis, one below the spinous portion of the dorsal fin and the other below the soft portion of the dorsal fin, compared to a single cluster located near the posterior end of the soft portion of the dorsal fin during this range for C. poeyi (Figs. 2f, 3c, h). At 9.2 mm SL the posteriormost cluster of melanophores along the dorsal midline disappears in C. poeyi (Figs. 2i, 3e, j) while pigmentation is maintained along the dorsal midline in C. undecimalis. It should be noted that the largest individual in our study was 10.6 mm SL and we were not able to observe changes in pigmentation beyond this size.
Implications for captive propagation. The high levels of daily variation in length (48.7%) observed for Centropomus poeyi are indicative of potential future problems with cannibalism. Cannibalism has previously been shown to increase significantly when a size ratio of 2:1 is reached and has also been a major problem with larviculture of both C. undecimalis and C. parallelus (Corrêa, Cerqueira, 2007Corrêa CF, Cerqueira VR. Effects of stocking density and size distribution on growth, survival and cannibalism in juvenile fat snook (Centropomus parallelus Poey). Aquac Res . 2007; 38(15):1627-34.; Álvarez-Lajonchère, Tsuzuki, 2008Álvarez-Lajonchère L, Tsuzuki MY. A review of methods for Centropomus spp. (snooks) aquaculture and recommendations for the establishment of their culture in Latin America. Aquac Res. 2008; 39(7):684-700.). Several methods have previously been implemented for the mitigation of cannibalism in larviculture, including size grading, reduced stocking density or alternative feeding regimes (Hecht, Pienaar, 1993Hecht T, Pienaar AG. A review of cannibalism and its implications in fish larviculture. J World Aquac Soc. 1993; 24(2):246-61.), and these will likely need to be implemented in future captive propagation efforts for C. poeyi.
Centropomids have been targeted as potential species for aquaculture since the early 1970’s after overexploitation of natural stocks resulted in declining populations (Taylor et al., 1998Taylor RG, Grier HJ, Whittington JA. Spawning rhythms of common snook in Florida. J Fish Biol . 1998; 53(3):502-20.; Pope et al., 2006Pope KL, Blankinship DR, Fisher M, Patiño R. Status of the common snook (Centropomus undecimalis) in Texas. Tex J Sci. 2006; 58(4):325-32.; Álvarez-Lajonchère, Tsuzuki, 2008Álvarez-Lajonchère L, Tsuzuki MY. A review of methods for Centropomus spp. (snooks) aquaculture and recommendations for the establishment of their culture in Latin America. Aquac Res. 2008; 39(7):684-700.). Despite the wealth of studies aimed towards improving the success of captive propagation and larviculture in snooks (Neidig et al., 2000Neidig CL, Skapura DP, Grier HJ, Dennis CW. Techniques for spawning common snook: broodstock handling, oocyte staging, and egg quality. N Am J Aquacult. 2000; 62(2):103-13.; Lemos et al., 2006Lemos D, Netto B, Germano A. Energy budget of juvenile fat snook Centropomus parallelus fed live food. Comp Biochem Physiol A Mol Integr Physiol. 2006; 144(1):33-40.; Wittenrich et al., 2009Wittenrich ML, Rhody NR, Turingan RG, Main KL. Coupling osteological development of the feeding apparatus with feeding performance in common snook, Centropomus undecimalis, larvae: identifying morphological constraints to feeding. Aquaculture. 2009; 294(3):221-27.; Yanes-Roca et al., 2015Yanes-Roca C, Rhody NR, Nystrom M, Wittenrich ML, Main KL. Embryonic and early larval development in hatchery-reared common snook. N Am J Aquacult . 2012; 74(4):499-511.) the majority of research has been focused only on two species, Centropomus undecimalis and C. parallelus. We have provided information on larval development in a third species of Centropomus (C. poeyi), including the attainment of external developmental landmarks considered important for larviculture. Though the process of larval development is similar across the three species of Centropomus that have been investigated to date, we have noted some minor differences (e.g., size at which a full complement of pectoral-fin rays is attained) in the development of C. poeyi in comparison to C. undecimalis and C. parallelus that could potentially result in minor differences in rearing protocols for different species. Larval rearing can be a major bottleneck hampering success in captive rearing of marine fishes for commercial/re-stocking purposes (Houde, 1972Houde ED. Some recent advances and unsolved problems in the culture of marine fish larvae. Proc Annu Work - J World Maric Soc. 1972; 3(1-4):83-112. ) and having information on the attainment of important developmental landmarks such as yolk-sac resorption and commencement of exogenous feeding is vital for successful rearing. The detailed information on early ontogeny compiled herein should be of direct benefit to the future propagation of C. poeyi.
Acknowledgments
This research was possible with funding from Texas A&M Agrilife Research (TEX09452 to KWC) and Texas A&M/CONACYT Collaborative Research Grant Program (project number 2012-023 to KWC & CAAG). This is publication number 1564 of the Biodiversity Research and Teaching Collections of Texas A&M University. The authors thank María J. Contreras-García, Alejandro Mcdonal-Vera and Leonardo Cruz-Rosado for their outstanding technical assistance during larval culture and George Mattox for critical comments that helped improve the contents of this paper.
References
- Álvarez-Lajonchère L, Hernández-Molejón OG. Producción de juveniles de peces estuarinos para un Centro en América Latina y el Caribe: diseño, operación y tecnologías. Baton Rouge, LA, USA: The World Aquaculture Society; 2001.
- Álvarez-Lajonchère L, Tsuzuki MY. A review of methods for Centropomus spp. (snooks) aquaculture and recommendations for the establishment of their culture in Latin America. Aquac Res. 2008; 39(7):684-700.
- Block AJ, Mabee PM. Development of the mandibular, hyoid arch and gill arch skeleton in the Chinese barb Puntius semifasciolatus: comparisons of ossification sequences among Cypriniformes. J Fish Biol. 2012; 81(1):54-80.
- Corrêa CF, Cerqueira VR. Effects of stocking density and size distribution on growth, survival and cannibalism in juvenile fat snook (Centropomus parallelus Poey). Aquac Res . 2007; 38(15):1627-34.
- Díaz-Jaimes P, Sandoval-Castellanos E, Uribe-Alcocer M. Comparative population structure of three snook species (Teleostei: Centropomidae) from the eastern central Pacific. Ichthyol Res. 2007; 54(4):380-87.
- Hecht T, Pienaar AG. A review of cannibalism and its implications in fish larviculture. J World Aquac Soc. 1993; 24(2):246-61.
- Houde ED. Some recent advances and unsolved problems in the culture of marine fish larvae. Proc Annu Work - J World Maric Soc. 1972; 3(1-4):83-112.
- Itagaki MK, Katsuragawa M, Pimentel CMM, Oliveira IR, Ohkawara MH. Early development of fat snook, Centropomus parallelus (Poey 1860) (Teleostei, Centropomidae) from southeastern Brazil. Zootaxa. 2013; 3669(1):65-75.
- Lau SR, Shafland PL. Larval development of snook, Centropomus undecimalis (Pisces: Centropomidae). Copeia. 1982; 1982(3):618-27.
- Lemos D, Netto B, Germano A. Energy budget of juvenile fat snook Centropomus parallelus fed live food. Comp Biochem Physiol A Mol Integr Physiol. 2006; 144(1):33-40.
- Neidig CL, Skapura DP, Grier HJ, Dennis CW. Techniques for spawning common snook: broodstock handling, oocyte staging, and egg quality. N Am J Aquacult. 2000; 62(2):103-13.
- Nelson JS. Fishes of the World. 4th ed. Hoboken (NJ): J. Wiley; 2006.
- Orrell TM. Centropomidae Snooks. In: Carpenter KE, ed. The living marine resources of the Western Central Atlantic (Bony Fishes part 1(Acipenseridae to Grammatidae). vol 2). FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. Rome: FAO. 2002. p. 601-1374.
- Pope KL, Blankinship DR, Fisher M, Patiño R. Status of the common snook (Centropomus undecimalis) in Texas. Tex J Sci. 2006; 58(4):325-32.
- Potthoff T, Tellock JA. Osteological development of the snook, Centropomus undecimalis (Teleostei, Centropomidae). B Mar Sci. 1993; 52(2):669-716.
- Reiss JO. The meaning of developmental time: A metric for comparative embryology. Am Nat. 1989; 134(2):170-89.
- Rivas LR. Systematic review of the perciform fishes of the genus Centropomus Copeia . 1986; (3):579-611.
- Taylor RG, Grier HJ, Whittington JA. Spawning rhythms of common snook in Florida. J Fish Biol . 1998; 53(3):502-20.
- Taylor RG, Whittington JA, Haymans DE. Catch-and-release mortality rates of common snook in Florida. NAm J Fish Manage. 2001; 21(1):70-75.
- Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107-20.
- Tringali MD, Bert TM, Seyoum S, Bermingham E, Bartolacci D. Molecular phylogenetics and ecological diversification of the transisthmian fish genus Centropomus (Perciformes: Centropomidae). Mol Phylogenet Evol. 1999; 13(1):193-207.
- Wittenrich ML, Rhody NR, Turingan RG, Main KL. Coupling osteological development of the feeding apparatus with feeding performance in common snook, Centropomus undecimalis, larvae: identifying morphological constraints to feeding. Aquaculture. 2009; 294(3):221-27.
- Yanes-Roca C, Rhody NR, Nystrom M, Wittenrich ML, Main KL. Embryonic and early larval development in hatchery-reared common snook. N Am J Aquacult . 2012; 74(4):499-511.
Publication Dates
-
Publication in this collection
2018
History
-
Received
23 May 2016 -
Accepted
22 Dec 2017