ABSTRACT
The goliath catfish Brachyplatystoma rousseauxii has crucial economical and ecological functions in the Amazon basin. Although its life history characteristics have been studied in the Amazon, there is little information in the Madeira River basin, which holds genetically distinct populations and where dams were recently built. Using fish collected in Bolivia, Brazil and Peru, this study provides a validation of growth rings deposition and details the growth patterns of B. rousseauxii in the Madeira before the dams’ construction. Age structure and growth parameters were determined from 497 otolith readings. The species exhibits two growth rings per year and sampled fish were between 0 and 16 years old. In the Brazilian portion of the basin, mainly young individuals below 5 years old were found, whereas older fish (> 5 years) were caught only in the Bolivian and Peruvian stretches, indicating that after migrating upstream to reproduce, adults remain in the headwaters of the Madeira River. Comparing with previous publications, B. rousseauxii had a slower growth and 20 cm lower maximum standard length in the Madeira River than in the Amazon River. This study provides a baseline for future evaluation of changes in population dynamics of the species following dams closure.
Key words:
Amazon; Biannual rings; Goliath catfish; Life cycle; Otolith
RESUMO
Brachyplatystoma rousseauxii é um bagre de importante papel econômico e ecológico na bacia amazônica. Embora existam estudos acerca de sua história de vida na Amazônia, há pouca informação para a bacia do rio Madeira, onde existem populações geneticamente distintas e recentemente foram construídas duas usinas hidrelétricas. Este estudo validou a deposição das marcas de crescimento e detalhou os padrões de desenvolvimento dessa espécie no rio Madeira, antes da construção das barragens. As coletas abrangeram os territórios brasileiro, boliviano e peruano, com estrutura etária e parâmetros de crescimento determinados a partir de 497 otólitos. Foram observadas duas marcas de crescimento por ano e indivíduos entre 0 e 16 anos. Na porção brasileira foram encontrados principalmente jovens menores de 5 anos, enquanto que os peixes mais velhos (> 5 anos) foram capturados apenas na Bolívia e Peru, indicando que após a migração reprodutiva, os adultos permanecem nas cabeceiras do rio Madeira. Comparando com estudos prévios realizados na calha principal do rio Amazonas, B. rousseauxii apresentou crescimento mais lento e comprimento padrão máximo inferior de 20 cm no rio Madeira. Este estudo fornece uma base para a avaliação futura das mudanças na dinâmica populacional desse espécie após o implementação das barragens.
Palavras-chave:
Amazônia; Bagre gigante; Ciclo de vida; Marcas biannual; Otólito
Introduction
Apart from deforestation, habitat degradation, overexploitation and invasive species, hydroelectric impoundments and the resulting disruption of river connectivity are one of the main threats to freshwater biodiversity worldwide (Winemiller et al., 2016Winemiller KO, McIntyre PB, Castello L, Fluet- Chouinard E, Giarrizzo T, Nam S et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science . 2016; 351(6269):128-29.). In the Amazon Basin, where more than 175 hydroelectric dams are under construction or in operation, there is growing evidence that planned and current hydroelectric development will likely have massive impacts on the ecosystem and its exceptional biodiversity (Finer, Jenkins, 2012Finer M, Jenkins CN. Proliferation of hydroelectric dams in the Andean Amazon and implications for Andes-Amazon connectivity. PLoS ONE [serial on the Internet]. 2012; 7(4):e35126. Available from: http://dx.doi:10.1371/journal.pone.0035126
http://dx.doi:10.1371/journal.pone.00351...
; Castello et al., 2013Castello L, McGrath DG, Hess LL, Coe MT, Lefebvrel PA, Petry P, Macedo MN, Renó VF, Arantes CC. The vulnerability of Amazon freshwater ecosystems. Conserv Lett. 2013; 6(4):217-29.; Castello, Macedo, 2016Castello L, Macedo MN. Large-scale degradation of Amazonian freshwater ecosystems. Glob Change Biol [serial on the Internet]. 2016; 22(3):990-1007. Available from: http://dx.doi.org/10.1111/gcb.13173
http://dx.doi.org/10.1111/gcb.13173...
; Lees et al., 2016Lees AC, Peres CA, Fearnside PM, Schneider M, Zuanon JAS. Hydropower and the future of Amazonian biodiversity. Biodiversity Conserv. 2016; 25(3):451-66., Winemiller et al., 2016Winemiller KO, McIntyre PB, Castello L, Fluet- Chouinard E, Giarrizzo T, Nam S et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science . 2016; 351(6269):128-29.; Latrubesse et al., 2017Latrubesse EM, Arima EY, Dunne T, Park E, Baker VR, d’Horta FM, Wight C, Wittmann F, Zuanon J, Baker PA et al. Damming the rivers of the Amazon basin. Nature. 2017; 546(7658):363-69.).
Most Amazonian commercial fish species perform seasonal migrations, ranging from a few km to several thousands km, for reproductive and/or feeding purposes (Carolsfeld et al., 2003Carolsfeld J, Harvey B, Ross C, Baer A. Migratory fishes of South America: Biology, Fisheries and Conservation Status World Fisheries Trust. Washington: IDRC, World Bank; 2003.) that could be disrupted or imperilled by hydroelectric impoundments (Agostinho et al., 2007Agostinho AA, Gomes LC, Pelicice FM. Ecologia e Manejo de Recursos Pesqueiros em Reservatórios do Brasil. Maringá: Eduem; 2007., 2008Agostinho AA, Pelicice FM, Gomes LC. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol [serial on the Internet]. 2008; 68(4):1119-32. Available from: http://dx.doi.org/10.1590/S1519-69842008000500019
http://dx.doi.org/10.1590/S1519-69842008...
; He et al., 2017He F, Zarfl C, Bremerich V, Henshaw A, Darwall W, Tockner K, Jähnig SC. Disappearing giants: a review of threats to freshwater megafauna. WIREs Water [serial on the Internet]. 2017; 4:e1208. Available from: http://dx.doi:10.1002/wat2.1208
http://dx.doi:10.1002/wat2.1208...
). The large migratory Pimelodid catfishes of the genus Brachyplatystoma, also known as goliath catfishes, alone support annual landings above 30,000 tons.year-1 (FAO-COPESCAL, 2000Food and Agriculture Organization of the United Nations (FAO/Noruega), Comisíon de pesca continental para a América Latina (COPESCAL). Informe del taller regional sobre el manejo de las pesquerías de bagres migratórios del Amazonas. Roma: FAO; 2000.). Among these goliath catfishes, Brachyplatystoma rousseauxii (Castelnau, 1855), popularly known as dourada in Brazil and as dorado or plateado in the other Amazonian countries, is one of the most important species marketed throughout the Amazon basin. It represents nearly 9% of total landings in Bolivia, Brazil, Colombia and Peru, supporting, alone, annual landings of ~ 15,000 tons.year-1 (Gonzalez et al., 2009Gonzalez JCA, Garcia KAC, Núñez-Avellaneda M, Córdova EA, Galarza E, Oliveros LA, Natagani K. Recursos Hídricos y Ecosistemas Acuáticos. In: Perspectivas del Medio Ambiente en la Amazonía. Lima: PNUMA/OTCA/CIUP; 2009. p.146-161.).
This species also performs the most extensive freshwater migration ever described, from the spawning areas in the Andean piedmont of Bolivia, Colombia, Ecuador and Peru, to the nursery areas in the Amazon estuary (Barthem, Goulding, 1997Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.; Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
; Barthem et al., 2017Barthem RB, Goulding M, Leite RG, Cañas C, Forsberg B, Venticinque E, Petry P, Ribeiro MLB, Chuctaya J, Mercado A. Goliath catfish spawning in the far western Amazon confirmed by the distribution of mature adults, drifting larvae and migrating juveniles. Sci Rep [serial on the Internet]. 2017; 7:41784. Available from: https://www.nature.com/articles/srep41784.pdf DOI: 101038/srep41784
https://www.nature.com/articles/srep4178...
). Barthem, Goulding (1997Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.) first deduced this exceptional life cycle from size frequency and ripe gonad distributions between the estuary and the headwaters of the main Amazon tributaries. Duponchelle et al. (2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
) confirmed this life cycle at the individual level using otolith 87
S
r /86
S
r ratios, and further demonstrated natal homing behaviour. Using data from spawning adults, drifting larvae and juveniles, Barthem et al. (2017Barthem RB, Goulding M, Leite RG, Cañas C, Forsberg B, Venticinque E, Petry P, Ribeiro MLB, Chuctaya J, Mercado A. Goliath catfish spawning in the far western Amazon confirmed by the distribution of mature adults, drifting larvae and migrating juveniles. Sci Rep [serial on the Internet]. 2017; 7:41784. Available from: https://www.nature.com/articles/srep41784.pdf DOI: 101038/srep41784
https://www.nature.com/articles/srep4178...
) recently showed that the life cycle of B. rousseauxii involves a round trip migration of over 11,000 km.
Besides its economic importance, B. rousseauxii also plays key ecological functions as top predator of the Amazon main river channels (Barthem, Goulding, 1997Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.; Angelini et al., 2006Angelini R, Fabré NN, Silva-JR UL. Trophic analysis and fishing simulation of the biggest Amazonian catfish. Afr J Agric Res. 2006; 1(5):151-58.). Disruption of migration routes for B. rousseauxii and its congeners could have profound impacts as loss of apex consumers reduces length of trophic food webs in ecosystems, impacting greatly the abundance and composition of other species through the trophic cascades (Paine, 1966Paine RT. Food Web Complexity and Species Diversity. Am. Nat. 1966; 100(910):65-75., 1980Paine RT. Food Webs: Linkage, Interaction Strength and Community Insfrasctruture. J Animal Ecol.1980; 49(3):666-85.; Fretwell, 1987Fretwell DS. Food chain dynamics: the central theory of ecology? Oikos [serial on the Internet]. 1987; 50(3):291-301. Available from: http://www.jstor.org/stable/3565489
http://www.jstor.org/stable/3565489...
; Bauer, Hoye, 2014Bauer S, Hoye BJ. Migratory animals cou-ple biodiversity and ecosystem functioning worldwide. Science [serial on the Internet]. 2014; 344(6179):1242552. Available from: http://dx.doi.org/10.1126/science.1242552
http://dx.doi.org/10.1126/science.124255...
; Estes et al., 2011Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ et al. Trophic downgrading of planet earth. Science [serial on the Internet]. 2011; 333(6040):301-06. http://dx.doi.org/10.1126/science.1205106
http://dx.doi.org/10.1126/science.120510...
).
A more precise understanding of the impact of hydroelectric development in the Amazon basin on the exceptional migratory behaviour of this species would require linking individual movements and age patterns. Knowledge of age and growth plays a key role in understanding fish population dynamics, hence in fisheries management and also provides crucial insights for the conservation of threatened species (Campana, 2001Campana SE. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol [serial on the Internet]. 2001; 59(2):197-242. Available from: http://dx.doi.org/10.1111/j.1095-8649.2001.tb00127.x
http://dx.doi.org/10.1111/j.1095-8649.20...
; Hutchinson, TenBrink, 2011Hutchinson CE, TenBrink TT. Age determination of the Yellow Irish Lord: management implications as a result of new estimates of maximum age North American. North Am J Fish Mana [seril on the Internet]. 2011; 31(6):1116-22. Available from: https://doi.org/10.1080/02755947.2011.646453
https://doi.org/10.1080/02755947.2011.64...
). Until now, all studies about age and growth characteristics of B. rousseauxii have been carried out along of the Amazon River mainstem using both otoliths (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.) and size frequency distributions (García Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
; Córdoba et al., 2013Córdoba EA, Joven-León AV, Bonilla-Castillo CA, Petrere M Jr, Peláez M, Duponchelle F. Breeding, growth and exploitation of Brachyplatystoma rousseauxii in the Caqueta River, Colombia. Neotrop Ichthyol [serial on the Internet]. 2013; 11(3):637-47. Available from: http://dx.doi.org/10.1590/S1679-62252013000300017
http://dx.doi.org/10.1590/S1679-62252013...
). However, the existence of a clear genetic differentiation between B. rousseauxii from the western Amazon and from the Madeira River (Carvajal-Vallejos et al., 2014Carvajal-Vallejos FM, Duponchelle F, Desmarais E, Cerqueira F, Querouil S, Nuñez J, García C, Renno JF. Genetic structure in the Amazonian catfish Brachyplatystoma rousseauxii: influence of life history strategies. Genetica [serial on the Internet]. 2014; 142(4):323-36. Available from: http://dx.doi.org/10.1007/s10709-014-9777-2
http://dx.doi.org/10.1007/s10709-014-977...
) together with a natal homing behaviour in the latter (Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
), called for a specific study of its growth patterns within the Madeira basin. The present work therefore aimed at validating the periodicity of growth rings formation in otoliths and at testing hypotheses of regional variation in age and growth patterns of B. rousseauxii in the Madeira River basin using samples collected in Brazil, Bolivia and Peru before the construction of the Madeira dams.
Materials and Methods
Fish sampling and study area. In Bolivia, fish were sampled between February 2005 and March 2009 in Puerto Villaroel (n= 70, Mamoré River), Rurrenabaque (n=5), Cachuela Esperanza (n=37) (both on the Beni River), from local fishermen directly on the fishing ground (which often required several weeks of travel with fishermen) (see Carvajal-Vallejos et al., 2014Carvajal-Vallejos FM, Duponchelle F, Desmarais E, Cerqueira F, Querouil S, Nuñez J, García C, Renno JF. Genetic structure in the Amazonian catfish Brachyplatystoma rousseauxii: influence of life history strategies. Genetica [serial on the Internet]. 2014; 142(4):323-36. Available from: http://dx.doi.org/10.1007/s10709-014-9777-2
http://dx.doi.org/10.1007/s10709-014-977...
for details). In Peru, fish were bought to local fishermen in the main landing site of Puerto Maldonado (n=6, Madre de Dios River). Fishing grounds are located within a few dozen kilometres from the city and are usually landed the same day or the following morning. In the middle and lower Madeira basin (i.e. the Brazilian portion of the river), fish were sampled between April 2009 and July 2012, in seven different landing sites (Surpresa n= 11; Iata/Vila Murtinho n=18, Teotônio fall n=7, São Sebastião n =196, São Carlos n=103, Calama n=13 and Humaitá n=11), by Fish Conservation Programs under the covenant of UNIR and RIOMAR and Santo Antônio Energia and Energia Sustentável do Brasil (Fig. 1). During fish landings a local trained collector or a technician from the Ichthyology and Fisheries Laboratory (LIP) of the Federal University of Rondônia (UNIR) recorded the standard length (SL) (mm), date, fishing locality and then extracted the heads of each individual, from which the otoliths were later removed in the LIP/UNIR. To improve growth modeling, juvenile individuals (between 11 cm and 30 cm standard length LS) were collected from the reservoir of the UHE Santo Antônio Energia (place where previously was the Teotonio Fall) with gill nets (mesh size from 30 to 200 mm between opposite knots). Voucher specimens were deposited in the fish collection of Federal University of Rondônia, Porto Velho, Brazil (UFRO-I 14016, UFRO-I 15044, UFRO-I 15175).
Map of the sampling sites of Brachyplatystoma rousseauxii in the Madeira River basin. 1- Puerto Maldonado, 2- Rurrenabaque, 3-Puerto Villarroel, 4-Surpresa, 5- Cachuela Esperanza, 6-Iata/Vila Murtinho, 7-São Sebastião, 8-São Carlos, 9- Calama and 10- Humaitá.
Biological sample analysis. Otolith preparation and interpretation. On each fish, L
S (cm) was measured. The lapillus otoliths were extracted, washed in water, dried and stored in labelled envelopes for later laboratory processing. The otoliths were then embedded in polyester resin and sectioned transversally to a thickness of approximately 0.7 mm using a low-speed metallographic saw (Buehler Isomet and Isomet 1000). The thin sections were then polished (using 1200 and 2400 μm paper, then 1 μm alumin powder) until the core was visible, as detailed in Duponchelle et al. (2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
). Age and growth characteristics were determined from the examination of 497 individual transverse thin otolith sections. Otolith sections were observed using a stereo microscope and photographed using a Zeiss AxioCam camera under transmitted light. Distances between the core and the growth rings were measured using Axiovision software.
Each growth ring consisted of a pair of one narrow opaque band (dark aspect) and one wide translucent band (light aspect). Opaque bands, or rings, which correspond to seasonal increments, were counted between the core and the edge of the otolith. Photographs were examined twice by two independent readers to determine the number of opaque rings. When there was disagreement between readers about the number of rings, the otolith was re-interpreted and discarded if the readers did not agree. The distance (mm) between the core and the edge of the otolith (otolith radius) and between consecutive rings was measured along the otolith at a pre-determined 110° angle (Fig. 2c).
Transverse thin sections of Brachyplatystoma rousseauxii’s otoliths showing: a. one ring; b. and c. two rings; d. eight rings; e. ten rings; and f. twenty rings. The yellow line marks the rings.
The individual age in months was then calculated taking into account the date of capture, the number of growth rings and the mean hatching date for the populations: January (Van Damme et al., 2011Van Damme PA, Carvajal-Vallejos FM, Molina-Carpio J. Los peces y delfines de la Amazonía boliviana: hábitats, potencialidades y amenazas. Cochabamba: INIA; 2011.). For the estimation of the mean observed length-at-age, age groups were determined as follows: age-group 0 corresponded to fish whose calculated age was between 0.1 and 0.9 years, age-group 1 corresponded to fish whose calculated age was between 1.0 and 1.9 years, and so on.
Hydrological data. Data on the hydrological cycles of the Madeira river basin were provided by the Geological Survey of Brazil/CPRM (Companhia de Pesquisa de Recursos Minerais). The data came from the Porto Velho station.
Statistical analysis. Validation of ring formation. The periodicity of translucent ring deposition was determined through the monthly relative marginal increment ratio (RMI):
where R T is the total radius of the otolith, R N is the distance from the core of the otolith to the last ring and R N1 is the distance from the core to the penultimate ring (Haimovici, Reis, 1984Haimovici M, Reis EG. Determinação de idade e crescimento da castanha Umbrina canosai (Pisces, Sciaeinidae) do Sul do Brasil. Atlântica. 1984; 7:25-46.; Fabré, Saint-Paul, 1998Fabré NN, Saint-Paul U. Annulus formation on scales and seasonal growth of the Central Amazonian anostomid Schizodon fasciatus. J Fish Biol . 1998; 53(1):1-11.). RMI mean monthly values were compared using one-way-ANOVA with Tukey’s post hoc test. A significant decrease followed by an increase in RMI values was interpreted as the formation of a seasonal translucent ring.
Considering the differences in sampling period and hydrological cycle between the upper and the middle/lower Madeira basin, only individuals sampled in the Brazilian Amazon (see Fig. 1) were used in this analysis. The validation analysis was first carried out for two consecutive years between 2010 and 2012, but as the same tendency was observed in both years, the data were pooled into a single annual cycle to increase the number of specimens analysed at each month.
The von Bertalanffy growth function (VBGF) was calculated using a non-linear estimation (quasi-Newton method), which was calculated as equation (2):
where L and t are L S (cm) and age t (years) of the fish respectively, L ∞ is the asymptotic L S ; K is the growth coefficient representing how fast L ∞ is reached and t 0 is the theoretical age at which L S =0.
The age at first sexual maturity (A50) was calculated from the VBGF as follows (Duponchelle et al., 2007Duponchelle F, Lino F, Hubert N, Panfili J, Renno JF, Baras E, Torrico JP, Dugué R, Nuñez J. Environment-related life history trait variations of the red-bellied piranha, Pygocentrus nattereri, in two river basins of the Bolivian Amazon. J Fish Biol [serial on the Internet]. 2007; 71(4):1113-34. Available from: http://dx.doi.org/10.1111/j.1095-8649.2007.01583.x
http://dx.doi.org/10.1111/j.1095-8649.20...
; García-Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
):
where LS50 is the size at first sexual maturity and Loo and K are parameters from the VBGF. Size at first sexual maturity for the females of this species in the Madeira River was previously estimated at 73 cm LS from the same data set (Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
).
For comparison purposes, VBGF parameters of B. rousseauxii in the Amazon River mainstem (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.), expressed in fork length (L
F ), were converted to L
S using the equation provided in García-Vásquez et al. (2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
):
The growth parameters of the VBGF curves were compared between sexes using the likelihood ratio test (Tomassone et al., 1993Tomassone R, Dervin C, Masson JP. Biométrie: Modélisation de Phénomènes biologiques. Paris: Masson; 1993.) and applying the weighted sum of squares of Kimura (1980Kimura DK. Likelihood methods for the von Bertalanffy growth curve. Fish Bull. 1980; 77(4):765-76.). For k populations, the likelihood ratio test S ML was compared with χ2 using 3 degrees of freedom (3 parameters):
where n i is the number of individuals of the k th population, S 2 c is the residual variance of the pooled model (for all populations), and S 2 k is the residual variance of the models of the k populations, with k = 2 here.
Results
Over the study period, 562 otoliths of B. rousseauxii (11-120 cm L S ) were analysed, of which 65 (11.6 %) were discarded because they could not be interpreted. Of the 497 fish used, 142 were females (L S range 67-120 cm, mean ± SD; 93 ± 9.6 cm), 95 were males (L S range 49-112 cm, mean ± SD, 81± 12.2 cm), and 260 could not be sexed (L S range 11-119 cm, mean ± SD, 79 ± 19.2 cm).
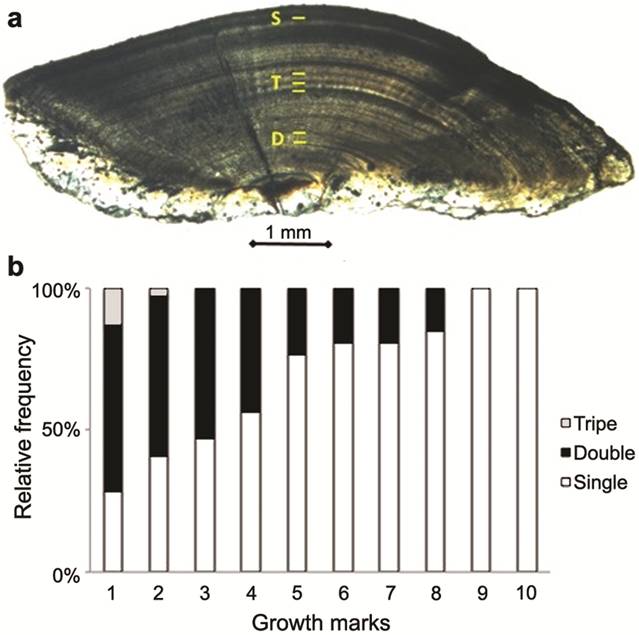
Interpretation of growth rings and validation. The alternation of a narrow opaque (dark) band with a wide translucent (light) band constituted a growth ring, for age estimation (Fig. 2). Otoliths with more than four growth rings exhibited two development patterns: the first with wide translucent bands up to the fourth or fifth growth rings and then the second, with a progressively decreasing width of the translucent bands until they became approximately of the same size as the opaque bands (Fig. 2).
The first growth ring formed on mean±SD of 0.85 ± 0.106 mm from the core, the second at 1.3 ± 0.097 mm, the third at 1.7 ± 0.088 mm, and despite overlap in the ring radius distributions, a clear modal progression could be observed, with the mean inter-rings radius distances progressively decreasing (Fig. 3).
Mean and standard deviation (SD) of each growth ring radius in otoliths of Brachyplatystoma rousseauxii from the Madeira River basin.
Three different types of rings could be observed: single (S), double (D) and triple (T) (Fig. 4a). D rings were observed in large proportions in the first four growth rings and their occurrence decreased afterwards until the 8th ring. From the 9th ring onwards, all rings were S rings. T rings were observed only in the first two growth rings and were most frequent in the first one (Fig. 4b).
Different types of growth rings; and b. their relative proportions, in transverse thin sections of Brachyplatystoma rousseauxii from the Madeira River basin. S-single, D-double, T-triple rings.
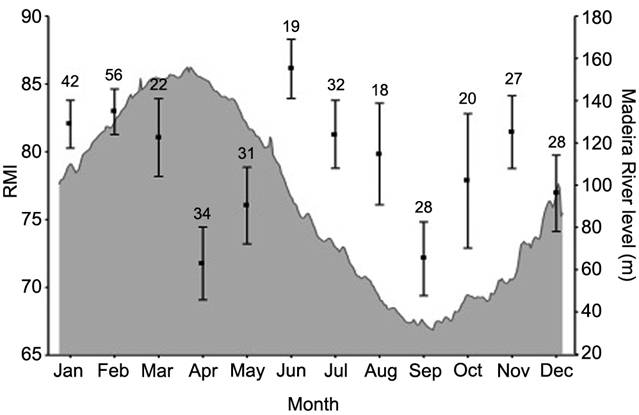
RMI (carried out on 357 individuals) significantly varied among months (one-way ANOVA, F11, 345= 2.07, P < 0.01), with lowest mean values in April (mean = 71.76 ± SD) and September (mean =72.10 ± SD) (Fig. 5). This indicates the formation of two opaque rings per year, hence two periods of reduced growth: one during the high-waters in April and the second during the low water period in September.
Mean monthly relative marginal increment (RMI ± S.D.) of 357 Brachyplatystoma rousseauxii’s otoliths in relation to the hydrological cycle in the Madeira River basin. The values above bars indicate the number of otoliths analysed each month.
Growth and age at maturity. Although more than half the fish used in this study could not be sexed, a gender specific analysis was still possible (Fig. 6a-b), with the following VBGF parameters for females (L ∞ = 108.3 cm, K = 0.55, t 0 = 0.029) and males (L ∞ = 96.2 cm, K = 0.57, t 0 = -0.065), using the same unsexed individuals under 60 cm L S to improve modelling for each sex. Females grew significantly faster than males (S ML = 111.5, P < 0.001). The difference between sexes ranged, on average, from ~ 5 cm at two years old, to ~12 cm at 10 years old and greater (Tab. 1).
Length-at age distribution and von Bertalanffy growth function (solid lines) of Brachyplatystoma rousseauxii: a. females (N = 142); b. males (N = 95); and c. females, males and unsexed individuals pooled (N = 497) in the lower / middle (Brazil) and in the upper (Bolivia and Peru) Madeira River basin. In order to improve modelling for females (a) and males (b), unsexed individuals < 60 cm (black dots) were also used.
Standard length-at-age of Brachyplatystoma rousseauxii in the Madeira River basin and along the Amazon River mainstem (from the Estuary to Iquitos; Alonso 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.), calculated from the VBGF. Total refers to females + males + unsexed individuals. *Although Alonso (2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.) did not observe fish older than 8 years, we know from other studies using length-frequency analyses (García-Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20... , Córdoba et al., 2013Córdoba EA, Joven-León AV, Bonilla-Castillo CA, Petrere M Jr, Peláez M, Duponchelle F. Breeding, growth and exploitation of Brachyplatystoma rousseauxii in the Caqueta River, Colombia. Neotrop Ichthyol [serial on the Internet]. 2013; 11(3):637-47. Available from: http://dx.doi.org/10.1590/S1679-62252013000300017
http://dx.doi.org/10.1590/S1679-62252013... ) that this species grow at least as old as 13 years in the Amazonas, hence we calculated length-at-age up to 15 years old as well, using VBG parameters taken from Alonso.
Considering females, males and unsexed individuals together, the VBGF for B. rousseauxii in the Madeira River basin yielded the following parameter estimates: L ∞ = 102.84 cm L S , K = 0.57 and t 0 = 0.021 (Fig. 6c). The species grew quickly during the first three years and the asymptotic phase of the growth curve was reached after five years.
Remarkably, there was only a small overlap in age distribution (3-5 years) between the middle and lower Madeira (Brazil) and the upper Madeira (Bolivia and Peru). In Brazil, fish were mainly young, between 0 and 5 years old, whereas older fish (> 6 years old) were only captured in Bolivia and Peru. Interestingly, as growth reached a plateau after 5 years, fish were not really larger in the upper basin, but were much older at a given length than in the middle and lower portion of the basin in Brazil. In fact, fish above ~ 80 cm could have any age between 3 and 15 years (Fig. 6c).
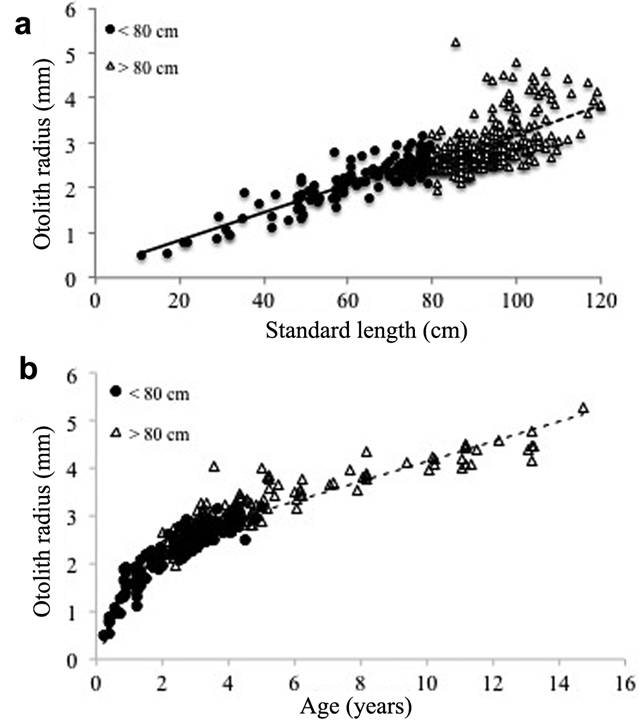
This also reflected in the relationship between fish standard length and otolith radius, with a strongly increased variance in otolith radius above 80 cm (Fig. 7a). Although otolith radius grew proportionately with age, its variability also tended to increase with age (Fig. 7b). The oldest fish sampled was a 15 years old male, rather small for its age, 85.4 cm L S , whereas the largest fish sampled (a female of 120 cm) was only 8 years old. Female B. rousseauxii reached the mean size at first sexual maturity (L 50 = 73 cm L S ) at 2.2 years old in the Madeira basin.
Relationship between fish standard length and otolith radius for Brachyplatystoma rousseauxii below 80 cm (black circles, black line: y = 0.031x + 0.209, r² = 0.805, P < 0.001) and above 80 cm (white triangles, broken line: y = 0.034x - 0.223, r² = 0.328, P < 0.001); and b. relationship between fish age and otolith radius for B. rousseauxii below 80 cm (black circles, black line: y = 0.887ln(x) + 1.567, r² = 0.879, P < 0.001) and above 80 cm (white triangles, broken line: y = 0.209ln(x) + 2.055, r² = 0.822, P < 0.001), both in Madeira River basin.
Discussion
Interpretation of growth rings and validation. The identification of the first growth ring is relatively difficult in B. rousseauxii, owing to many intermediate opaque bands (Fig. 2), along with D or T rings (Fig. 4). Intermediate bands could be due to fluctuating environmental conditions (mainly salinity) in the Amazon estuary (the species’ nursery area, Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.) and to the potential variations in competition for food with marine species (Barthem, Goulding, 1997Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.). Furthermore, the formation of the very first growth ring would be associated with the stress caused by increased salinity in the estuary during incursions of oceanic waters in August-September when freshwater flows decline (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.). Indeed, although B. rousseauxii is a potamodromous species, it can be observed down to the 20 m isobath in the estuary, where the salinity reaches almost 35 (psu). Under stress conditions, animals often divert growth energy to maintain the homostatic equilibrium (Fuzzen et al., 2011Fuzzen MLM, Bernier NJ, Kraak GVD. Stress and reproduction. In: Norris DO, Lopez KH, editors. Hormones and reproduction of vertebrates, vol 1. Academic Press; 2011. p.103-118.), which for teleost fish represents between 20 and 50% of the total energy budget (Boeuf, Payan, 2001Boeuf G, Payan P. How should salinity influence fish growth? Comp Biochem Physiol Part C [serial on the Internet]. 2001; 130(4):411-23. Available from: https://doi.org/10.1016/S1532-0456(01)00268-X
https://doi.org/10.1016/S1532-0456(01)00...
).
Most otoliths exhibited two clearly defined development patterns: the alternation of a large translucent band and a small opaque band until the fourth or fifth opaque band, followed afterwards by opaque and translucent bands of approximately equal width. This reduction in growth rate after the fourth or fifth ring was also observed in B. rousseauxii from the Amazon River mainstem, and interpreted as an energetic consequence of leaving the rich estuary area followed by the cost of the first upstream migration (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.), and ultimately, life in a fluvial system. This behaviour was recently confirmed by microchemical otolith analyses (Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
; Hermann et al., 2016Hermann TW, Stewart DJ, Limburg KE, Castello L. Unravelling the life history of Amazonian fishes through otolith microchemistry. Royal Soc Open Sci [serial on the Internet]. 2016; 3(6):160206 DOI: 101098/rsos160206.
https://doi.org/101098/rsos160206...
), and probably also applies to fish from the Madeira basin.
The present study demonstrates a clear biannual formation of growth rings for B. rousseauxii in the Madeira River basin, as already reported in the Amazon River mainstem (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.). One ring formed during the high waters and the other during the low water period, emphasizing the close relationship between the seasonal hydrologic cycle, controlled by the flood pulse (Junk et al., 1989Junk WJ, Bayley PB, Sparks RE. The flood pulse concept in river-floodplain-systems. In: Canada: Proceedings of International Large River Symposium. 1989. Can Spec Publ Fish Aquat Sci. (Canadian Special Publication of Fisheries and Aquatic Science s; No. 106:110-127).), and the life cycle of tropical freshwater fishes (Lowe-Mc Connell, 1999Lowe-McConnel RH. Estudos ecológicos de comunidades de peixes tropicais: introdução e diversidade: sua manutenção e evolução. São Paulo: EDUSP; 1999.). Although some biases can be associated with the use of RMI analyses in age validation (Campana, 2001Campana SE. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol [serial on the Internet]. 2001; 59(2):197-242. Available from: http://dx.doi.org/10.1111/j.1095-8649.2001.tb00127.x
http://dx.doi.org/10.1111/j.1095-8649.20...
), other methods such as mark recapture are impossible to implement in a species whose life cycle encompasses almost the whole Amazon basin. The fact that the results are consistent with previous, independent, studies in the Amazon mainstem using both otoliths (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.) and length-frequency analyses (García-Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
; Córdoba et al., 2013Córdoba EA, Joven-León AV, Bonilla-Castillo CA, Petrere M Jr, Peláez M, Duponchelle F. Breeding, growth and exploitation of Brachyplatystoma rousseauxii in the Caqueta River, Colombia. Neotrop Ichthyol [serial on the Internet]. 2013; 11(3):637-47. Available from: http://dx.doi.org/10.1590/S1679-62252013000300017
http://dx.doi.org/10.1590/S1679-62252013...
), tend to support the credibility of the validation carried out in the present study.
The number of growth rings formed during a complete annual cycle in the otoliths and other body hard parts (mainly scales and vertebrae) of Amazonian fishes is particularly interesting. All fish studied in western Amazonia, close to the Andes, display a single growth ring per year during the low water period, whether they belong to the Characiformes, Prochilodus nigricans Spix & Agassiz, 1829 in Bolivia (Loubens, Panfili, 1992Loubens G, Panfili J. Estimation de l’âge individuel de Prochilodus nigricans (Teleostei: Prochilodidae) dans le Béni (Bolivie): protocole d’étude et application. Aquat Living Resour .1992; 5(1):41-56.) and Ecuador (Silva, Stewart, 2006Silva EA, Stewart DJ. Age structure, growth and survival rates of the commercial fish Prochilodus nigricans (bocachico) in North-eastern Ecuador. Environ Biol Fish [serial on the Internet]. 2006; 77(1):63-77. Available from: http://dx.doi.org/10.1007/s10641-006-055-y
http://dx.doi.org/10.1007/s10641-006-055...
), Colossoma macropomum (Cuvier, 1816), Piaractus brachypomus (Cuvier, 1818), and Pygocentrus nattereri Kner, 1858 in Bolivia (Loubens, Panfili 1997Loubens G, Panfili J. Biologie de Colossoma macropomum (Teleostei: Serrasalmidae) dans le bassin du Mamoré (Amazonie bolivienne). Ichthyol Explor Fresh. 1997; 8(1):1-22., 2001Loubens G, Panfili J. Biologie de Piaractus brachypomus (Teleostei: Serrasalmidae) dans le bassin du Mamoré (Amazonie bolivienne). Ichthyol Explor Fresh . 2001; 12(1):51-64.; Duponchelle et al., 2007Duponchelle F, Lino F, Hubert N, Panfili J, Renno JF, Baras E, Torrico JP, Dugué R, Nuñez J. Environment-related life history trait variations of the red-bellied piranha, Pygocentrus nattereri, in two river basins of the Bolivian Amazon. J Fish Biol [serial on the Internet]. 2007; 71(4):1113-34. Available from: http://dx.doi.org/10.1111/j.1095-8649.2007.01583.x
http://dx.doi.org/10.1111/j.1095-8649.20...
, respectively), the Siluriformes, Pseudoplatystoma fasciatum (Linnaeus, 1766) and Pseudoplatystoma tigrinum (Valenciennes, 1840) in Bolivia (Loubens, Panfili, 2000Loubens G, Panfili J. Biologie de Pseudoplatystoma fasciatum et P. tigrinum (Teleostei: Pimelodidae) dans le bassin du Mamoré. Ichthyol Explor Fresh . 2000; 11(1):13-34.), the Perciformes, Plagioscion squamosissimus (Heckel, 1840) in Bolivia (Loubens, 2003Loubens G. Biologie de Plagioscion squamosissimus (Teleostei: Scianidae) dans le bassin du Mamoré (Amazonie bolivienne). Ichthyol Explor Fresh . 2003; 14(4):335-52.) or the Osteoglossiformes, Osteoglossum bicirrhosum (Cuvier, 1829) in Peru (Duponchelle et al., 2012Duponchelle F, Arce AR, Waty A, Panfili J, Renno JF, Farfan F, García-Vásquez A, Koo FC, Davila CG, Vargas G, Ortiz A, Pinedo R, Nuñez J. Contrasted hydrological systems of the Peruvian Amazon induce differences in growth patterns of the silver arowana, Osteoglossum bicirrhosum. Aquat Living Resour [serial on the Internet]. 2012; 25(1):55-66. Available from: https://doi.org/10.1051/alr/2012005
https://doi.org/10.1051/alr/2012005...
).
On the other hand, fish of these same orders, and sometimes the same species, tend to present two growth rings per annual cycle in central Amazonia, Brazil: in Characiformes, P. nigricans (Oliveira, 1996Oliveira MIB. Determinação de idade e aspectos da dinâmica populacional do curimatã Prochilodus nigricans (Pisces: Prochilodontidae) da Amazônia Central. [Master Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 1996.), C. macropomum (Villacorta-Correa, 1997Villacorta-Correa MA. Estudo de idade e crescimento do Tambaqui Colossoma macropomum (Characiformes: Characidae) no Amazonas central, pela analise de marcas sazonais nas estructuras mineralizadas e microestructuras nos otolitos. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 1997.), Semaprochilodus insignis (Jardine, 1841) (Viera, 1999Vieira EF. Determinação da idade e crescimento do jaraqui-de-escamagrossa (Semaprochilodus insignis) na Amazônia. [Master Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 1999.); in Siluriformes: Calophysus macropterus (Lichtenstein, 1819) (Pérez, Fabré, 2009Pérez A, Fabré NN. Seasonal growth and life history of the catfish Calophysus macropterus (Lichtenstein, 1819) (Siluriformes: Pimelodidae) from the Amazon floodplain. J Appl Ichthyol [serial on the Internet]. 2009; 25(3):343-49. Available from: http://dx.doi.org/10.1111/j.1439-0426.2008.01104.x
http://dx.doi.org/10.1111/j.1439-0426.20...
), Hypophthalmus marginatus Valenciennes, 1840 (Cutrim, Batista, 2005Cutrim L, Batista VS. Determinação de idade e crescimento do mapará (Hypophthalmus marginatus) na Amazônia Central. Acta Amaz [serial on the Internet]. 2005; 35(1):85-92. Available from: http://dx.doi.org/10.1590/S0044-59672005000100013
http://dx.doi.org/10.1590/S0044-59672005...
); and in Osteoglossiformes: Arapaima sp. (Arantes et al., 2010Arantes CC, Castello L, Stewart DJ, Cetra M, Queiroz HL. Population density, growth and reproduction of Arapaima in an Amazonian river-floodplain. Ecol Fresh Fish [serial on the Internet]. 2010; 19(3):455-65. Available from: http://dx.doi.org/10.1111/j.1600-0633.2010.00431.x). One of these biannuali usually forms during the low water season as well, similar to the only annual ring formed in western Amazonian fishes, and the other ring forms during the flood. The interpretation of these two periods of reduced growth varies according to studies or species: it could be associated with food limitations during the low water period and with reproductive activities during the flood, according to Pérez, Fabré (2009Pérez A, Fabré NN. Seasonal growth and life history of the catfish Calophysus macropterus (Lichtenstein, 1819) (Siluriformes: Pimelodidae) from the Amazon floodplain. J Appl Ichthyol [serial on the Internet]. 2009; 25(3):343-49. Available from: http://dx.doi.org/10.1111/j.1439-0426.2008.01104.x
http://dx.doi.org/10.1111/j.1439-0426.20...
) and Arantes et al. (2010Arantes CC, Castello L, Stewart DJ, Cetra M, Queiroz HL. Population density, growth and reproduction of Arapaima in an Amazonian river-floodplain. Ecol Fresh Fish [serial on the Internet]. 2010; 19(3):455-65. Available from: http://dx.doi.org/10.1111/j.1600-0633.2010.00431.x), or to reproductive migrations during the low waters and food limitations during the flood according to Cutrim, Batista (2005Cutrim L, Batista VS. Determinação de idade e crescimento do mapará (Hypophthalmus marginatus) na Amazônia Central. Acta Amaz [serial on the Internet]. 2005; 35(1):85-92. Available from: http://dx.doi.org/10.1590/S0044-59672005000100013
http://dx.doi.org/10.1590/S0044-59672005...
). Notable exceptions in central Amazonia are Schizodon fasciatus Spix & Agassiz, 1829, which forms a single ring per year during the flood (Fabré, Saint Paul, 1998Fabré NN, Saint-Paul U. Annulus formation on scales and seasonal growth of the Central Amazonian anostomid Schizodon fasciatus. J Fish Biol . 1998; 53(1):1-11.) and Cichla temensis Humboldt, 1821, which forms a single ring during the receding water period (Campos et al., 2015Campos CP, Freitas CEC, Amadio S. Growth of the Cichla temensis Humboldt, 1821 (Perciformes: Cichlidae) from the middle rio Negro, Amazonas, Brazil. Neotrop Ichthyol [serial on the Internet]. 2015; 13(2):413-20. Available from: http://dx.doi.org/10.1590/1982-0224-20140090
http://dx.doi.org/10.1590/1982-0224-2014...
).
The nature of B. rousseauxii’s life cycle, however, prevents its categorization as either from central or western Amazonia, as it encompasses both and also includes the estuary. Besides the particular environmental conditions of the estuary and their influence on the formation of the first few growth rings (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.), subsequent periods of growth rings formation appear to reflect two annual periods of reduced growth in B. rousseauxii’s life cycle. One of these periods is likely associated to the low availability of its main prey fishes, which move into the floodplains during the high-waters (Goulding, 1979Goulding M. Ecologia da pesca do rio Madeira. Manaus: CNPq/INPA; 1979.; Barthem, Goulding, 1997Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.; Junk et al., 1997Junk WJ. The Central Amazon floodplains Ecology of pulsing system. Berlin: Springer; 1997.).
The reason why a second annual ring forms during the low water season in B. rousseauxii could also relate to food limitation, although not for the same causes. During the low water season, the concentration in the main river channels of fish upon which B. rousseauxii preys is supposed to be maximum and should therefore provide optimum growth conditions. This is also the period of the hydrological cycle, however, when all the other large predatory species, including all large catfishes (Brachyplatystoma spp., Pseudoplatystoma spp. and Zungaro zungaro (Humboldt, 1821)) (Doria, Lima, 2015Doria CRC, Lima MAL. Rio Madeira: seus peixes e sua pesca. Porto Velho: EDUFRO/RIMa Editora; 2015.) and river dolphins (Silva et al., 2008Silva V, Goulding M, Barthem R. Golfinhos da Amazônia. Manaus: Editora INPA; 2008.; Crema et al., 2014Crema LC, Quaresma AC, Silva VMF. Nem tudo que nada é peixe: os mamíferos aquáticos amazônicos. Recursos Hídricos y Ecosistemas Acuáticos. In: Lopes A, Piedade MTF, editors. Conhecendo as áreas úmidas amazônicas: uma viagem pelas várzeas e igapós. Manus: INPA Editora; 2014. p.85-94.) are concentrated in the river channels and compete over the same resources. Although B. rousseauxii is an apex predator, this competitive situation could result in an unfavourable ratio of energy expenditure over food availability and hence, in a reduced growth rate. Similar patterns of reduced growth rate in fishes under increased densities and related competition has been widely reported in the literature, for Chinook Salmon (Mazur et al., 1993Mazur CF, Tillapaugh D, Brett JR, Iwama GK. The effect of feeding level and rearing density on growth Feed conversion and survival in Chinook salmon (Oncorhynchus tshawytscha) reared in salt water. Aquaculture [serial on the Internet]. 1993; 117(1):129-40. Available from: https://doi.org/10.1016/0044-8486(93)90129-M
https://doi.org/10.1016/0044-8486(93)901...
), Brown Trout (Vøllestad et al., 2002Vøllestad LA, Olsen EM, Forseth T. Growth-rate variation in brown trout in small neighbouring streams: evidence for density-dependence? J Fish Biol [serial on the Internet]. 2002; 61(6):1513-27. Available from: http://dx.doi.org/10.1111/j.1095-8649.2002.tb02494.x
http://dx.doi.org/10.1111/j.1095-8649.20...
) and other salmonids (Taniguchi, Nakano, 2000Taniguchi Y, Nakano S. Condition-Specific competition: Implications for the altitudinal distribution of stream fishes. Ecology [serial on the Internet]. 2000; 81(7):2027-39. Available from: http://dx.doi.org/10.1890/0012-9658(2000)081[2027:CSCIFT]2.0.CO;2
http://dx.doi.org/10.1890/0012-9658(2000...
; Puffer et al., 2015Puffer M, Berg OK, Huusko A, Vehanen T, Einum S. Effects of intra- and interspecific competition and hydropeaking on growth of juvenile Atlantic salmon (Salmo salar). Ecol Freshw Fish [serial on the Internet]. 2015; 26(1):99-107. Available from: http://dx.doi.org/10.1111/eff.12258
http://dx.doi.org/10.1111/eff.12258...
).
Growth. Despite the fact that more than half the fish could not be sexed, the growth dimorphism in favour of females, already reported in the Amazon River mainstem (Alonso, 2002Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.; García-Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
; Córdoba et al., 2013Córdoba EA, Joven-León AV, Bonilla-Castillo CA, Petrere M Jr, Peláez M, Duponchelle F. Breeding, growth and exploitation of Brachyplatystoma rousseauxii in the Caqueta River, Colombia. Neotrop Ichthyol [serial on the Internet]. 2013; 11(3):637-47. Available from: http://dx.doi.org/10.1590/S1679-62252013000300017
http://dx.doi.org/10.1590/S1679-62252013...
) was also observed in the Madeira basin. Females did grow faster than males. Fish from the Madeira, however, had an overall slower growth that fish from the Amazon River mainstem (Tab. 1). The difference ranged, on average, from about 10 cm at one year old to 25 cm at 15 years old for females, from over 5 cm at one year old to ~ 30 cm at 15 years old for males, and from 7 cm at one year old to nearly 30 cm at 15 years old for females, males and unsexed individuals together. This important difference also translates in the maximum observed lengths between the two systems: B. rousseauxii of 150 cm L
S are, or at least were, regularly observed in the upper Amazon (García-Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
; Córdoba et al., 2013Córdoba EA, Joven-León AV, Bonilla-Castillo CA, Petrere M Jr, Peláez M, Duponchelle F. Breeding, growth and exploitation of Brachyplatystoma rousseauxii in the Caqueta River, Colombia. Neotrop Ichthyol [serial on the Internet]. 2013; 11(3):637-47. Available from: http://dx.doi.org/10.1590/S1679-62252013000300017
http://dx.doi.org/10.1590/S1679-62252013...
), whereas they barely reach 130 cm LS in the upper Madeira (Van Damme et al., 2011Van Damme PA, Carvajal-Vallejos FM, Molina-Carpio J. Los peces y delfines de la Amazonía boliviana: hábitats, potencialidades y amenazas. Cochabamba: INIA; 2011.; Carvajal-Vallejos et al., 2014Carvajal-Vallejos FM, Duponchelle F, Desmarais E, Cerqueira F, Querouil S, Nuñez J, García C, Renno JF. Genetic structure in the Amazonian catfish Brachyplatystoma rousseauxii: influence of life history strategies. Genetica [serial on the Internet]. 2014; 142(4):323-36. Available from: http://dx.doi.org/10.1007/s10709-014-9777-2
http://dx.doi.org/10.1007/s10709-014-977...
). Out of the ~ 500 individuals analysed for the present study none was larger than 120 cm L
S . This growth difference is further emphasized by the differences in level of exploitation between the two systems. Indeed, fisheries usually harvest the largest specimens and often induce a decrease in the maximum size of exploited populations (Rochet, Trenckel, 2003Rochet MJ, Trenkel VM. Which community indicators can measure the impact of fishing? A review and proposals. Can J Fish Aquat Sci [serial on the Internet]. 2003; 60(1):86-99. Available from: https://doi.org/10.1139/f02-164
https://doi.org/10.1139/f02-164...
). Fishery exploitation is close to over-exploitation in both the Peruvian (García-Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
) and Colombian (Córdoba et al., 2013Córdoba EA, Joven-León AV, Bonilla-Castillo CA, Petrere M Jr, Peláez M, Duponchelle F. Breeding, growth and exploitation of Brachyplatystoma rousseauxii in the Caqueta River, Colombia. Neotrop Ichthyol [serial on the Internet]. 2013; 11(3):637-47. Available from: http://dx.doi.org/10.1590/S1679-62252013000300017
http://dx.doi.org/10.1590/S1679-62252013...
) waters, whereas it started later and remains relatively weak in the Bolivian Amazon (Goulding, 1979Goulding M. Ecologia da pesca do rio Madeira. Manaus: CNPq/INPA; 1979.; Van Damme et al., 2011Van Damme PA, Carvajal-Vallejos FM, Molina-Carpio J. Los peces y delfines de la Amazonía boliviana: hábitats, potencialidades y amenazas. Cochabamba: INIA; 2011.). Yet, in spite of a lower exploitation pressure, the maximum sizes are smaller in the upper Madeira.
Growth differences could also result from genetic determinism or phenotypic plasticity in response to environmental differences. Although three genetically distinct populations of B. rousseauxii are present in admixture in the Madeira, the numerically dominant genotype is the same in the Madeira as that in the Peruvian Amazon (Carvajal-Vallejos et al., 2014Carvajal-Vallejos FM, Duponchelle F, Desmarais E, Cerqueira F, Querouil S, Nuñez J, García C, Renno JF. Genetic structure in the Amazonian catfish Brachyplatystoma rousseauxii: influence of life history strategies. Genetica [serial on the Internet]. 2014; 142(4):323-36. Available from: http://dx.doi.org/10.1007/s10709-014-9777-2
http://dx.doi.org/10.1007/s10709-014-977...
). Yet all three genotypes attain smaller maximum lengths in the Madeira than in the upper Amazon, suggesting that the observed growth differences are rather a consequence of less favourable environmental conditions in the Madeira.
It is the river with the highest sediment load in the Amazon basin (Latrubesse et al., 2005Latrubesse EM, Stevaux JC, Sinha R. Tropical rivers. Geomorph [serial on the Internet]. 2005; 70(3):187-206. Available from: https://doi.org/10.1016/j.geomorph.2005.02.005
https://doi.org/10.1016/j.geomorph.2005....
), which might impact primary production and ultimately ecosystem productivity, resulting in less favourable trophic conditions. A high sediment load could also affect gills efficiency by reducing oxygen intake and metabolism (Val et al., 2005Val AL, Almeida-Val VMF, Randal DJ. Fish Physiology: The Physiology of Tropical Fishes. Academic Press; 2005.). Bolivia has one of the largest floodplains of the Amazon basin (Hamilton et al., 2004Hamilton SK, Sippel SJ, Melack JM. Seasonal inundation patterns in two large savanna floodplains of South America: the Llanos de Moxos (Bolivia) and the Llanos del Orinoco (Venezuela and Colombia). Hydrol Process [serial on the Internet]. 2004; 18(11):2103-16. Available from: http://dx.doi.org/10.1002/hyp.5559
http://dx.doi.org/10.1002/hyp.5559...
), but unlike the flooded forest of the floodplain in Central Amazonia (Goulding, 1990Goulding M. Amazon: The Flooded Forest. New York: Sterling Publishing; 1990.), it consists of a flooded savannah, which might not be as productive as the flooded rainforests of Central Amazonia.
Another explanation could lie in a poorer nutritious value of the prey fishes consumed in the Madeira vs. the Amazon systems. Whereas B. rousseauxii predominantly feeds upon detritivorous-herbivorous (Brycon spp., Mylossoma spp.) and omnivorous (Triportheus spp.) migratory Characiformes in the Amazon River mainstem (Barthem, Goulding, 1997Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.; García-Vásquez et al., 2009García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
http://dx.doi.org/10.1111/j.1095-8649.20...
), its main prey fishes in the Madeira River are the carnivorous Pimelodina flavipinnis Steindachner, 1876 and Hypophthalmus marginatus (Cella-Ribeiro et al., 2016Cella-Ribeiro A, Torrente-Vilara G, Lima-Filho JA, Doria CRC. Ecologia e Biologia de Peixes do Rio Madeira. Porto Velho: EDUFRO; 2016.). These last two species are invertivorous (Santos et al., 2006Santos GM, Ferreira EJG, Zuanon JAS. Peixes Comerciais de Manaus. Manaus: Ibama/ProVárzea; 2006.) and planktivorous (Carvalho, 1980Carvalho FM. Alimentação de Mapará (Hypophthalmus edentatus Spix 1829) do Lago Castanho, Amazonas (Siluriformes Hypophthalmidae). Acta Amazonica. 1980; 10(3):545-55.; Cutrim, Batista, 2005Cutrim L, Batista VS. Determinação de idade e crescimento do mapará (Hypophthalmus marginatus) na Amazônia Central. Acta Amaz [serial on the Internet]. 2005; 35(1):85-92. Available from: http://dx.doi.org/10.1590/S0044-59672005000100013
http://dx.doi.org/10.1590/S0044-59672005...
; Cella-Ribeiro et al., 2016Cella-Ribeiro A, Torrente-Vilara G, Lima-Filho JA, Doria CRC. Ecologia e Biologia de Peixes do Rio Madeira. Porto Velho: EDUFRO; 2016.), respectively. At each transfer from one level of the food web to the upper level, a large part of the energy is lost in heat (Odum, 1988Odum EP. Fundamentos de ecologia. 4th ed. Lisboa: Fundação Calouste Gulbenkian; 1998.), which should reduce the energy content of species higher in the food web. Vismara et al. (2004Vismara MR, Benedito-Cecilio E, Faria ACEA. Efeito da maturação gonadal sobre o conteúdo calórico e condição geral de peixes da planície de inundação do alto rio Paraná. Acta Sci Biol Sci [serial on the Internet]. 2004; 26(2):189-99. Available from: http://dx.doi.org/10.4025/actascibiolsci.v26i2.1635
http://dx.doi.org/10.4025/actascibiolsci...
) indeed observed a decreased caloric content from herbivorous to carnivorous fish species in the upper Paraná River floodplain. Hence, foraging on carnivorous species in the Madeira River instead of on detritivorous-herbivorous species in the Amazon River mainstem might partly account for the slower growth of B. rousseauxii in the Madeira.
A complementary potential explanation is that the Bolivian Amazon is the southernmost region of the Amazon basin. As such, it is subjected to frequent episodes of cold fronts from the south occurring during approximately 40% of winter days and 10% of summer days (Ronchail, 1989Ronchail J. Advections polaires en Bolivie: mise en évidence et caractérisation des effets climatiques. Hydrol Continent. 1989; 4(1):49-56.). These cold fronts result in important temperature decreases of up to 20°C from one day to another and lasting a few days (Ronchail, 1989Ronchail J. Advections polaires en Bolivie: mise en évidence et caractérisation des effets climatiques. Hydrol Continent. 1989; 4(1):49-56.; Lupo et al., 2001Lupo AR, Nocera JJ, Bosart LF, Hoffman EG, Knight DJ. South American Cold Surges: Types, Composites, and Case Studies. Mon Weather Rev [serial on the Internet]. 2001; 129:1021-41. Available from: https://doi.org/10.1175/1520-0493(2001)129<1021:SACSTC>2.0.CO;2
https://doi.org/10.1175/1520-0493(2001)1...
), that are likely to affect fish growth. The potential explanations listed in this last paragraph are not mutually exclusive and could add up to explain the slower growth of B. rousseauxii in the Madeira.
A slower growth was also detected in the first year of life, which is supposed to be passed in the Amazon estuary for all fish. The geographic separation between the adults in the headwaters and the juveniles in the estuary for B. rousseauxii is believed to reduce competition over food and space with other young large catfish stages and to provide particularly favourable trophic conditions for the young stages (Barthem, Goulding, 1997Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.). Recent studies using 87
S
r /86
S
r ratios in B. rousseauxii otoliths (Hegg et al., 2015Hegg JC, Giarrizzo T, Kennedy BP. Diverse early life-history strategies in migratory Amazonian catfish: implications for conservation and management. PLoS ONE [serial on the Internet]. 2015; 10(7):e0129697. Available from: https://doi.org/10.1371/journal.pone.0129697
https://doi.org/10.1371/journal.pone.012...
; Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
), however, suggested that not all fish enter the estuary and that some could use upstream areas within the Amazon as nursery. Although there is no evidence to support this hypothesis, nursery areas for Brachyplatystoma young stages hatched in the upper Madeira might, in general, be located upstream of the estuary, resulting in slower growth and higher competition with other catfish young stages compared to those that reside in the estuary.
In addition to growth differences found in the two systems, one of the most interesting results of this study was the clear age segregation between the lower and upper Madeira. Apart from one specimen, all fish caught in the Brazilian portion of the Madeira were less than 5 years old, including the larger ones, whereas most large individuals caught in Bolivia and Peru were between 5 and 15 years old. This clearly confirms that after their upstream reproductive runs in the upper Mamoré, Béni and Madre de Dios Rivers, B. rousseauxii specimens do not go back down to central Amazonia, which had already been suggested by recent otolith microchemistry analyses (Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
). Furthermore, this result also indicates that once they enter the Madeira, or home back to the Madeira, they do not just stay in the Madeira basin, they remain within the upper portion of the basin, within Bolivia and Peru.
This crucial information for fisheries management should actually be put in a past tense as the construction of two hydroelectric dams in the Brazilian portion of the Madeira, Santo Antônio and Jirau dams, have profoundly modified the situation. The fishways in Santo Antonio and Jirau have been found ineffective for accommodating the upstream passage of large catfishes, including B. rousseauxii, and many fishing communities above the dams report that these species have disappeared from the catches (Fearnside, 2014Fearnside PM. Brazil’s Madeira River dams: A setback for environmental policy in Amazonian development. Water Alternatives. 2014; 7(1):256-69., 2015Fearnside PM. Hidrelétricas na Amazônia: impactos ambientais e sociais na tomada de decisões sobre grandes obras. Manaus: Editora do INPA; 2015.). The adverse environmental conditions in the upstream reservoirs of the dams are also expected to compromise severely the downstream migration and survival of B. rousseauxii larvae and juveniles (Baras, Lucas, 2001Baras E, Lucas MC. Impacts of man’s modifications of river hydrology on the migration of freshwater fishes: a mechanistic perspective. Ecohydrol Hydrobiol. 2001; 1(3):291-304.; Carolsfeld et al., 2003Carolsfeld J, Harvey B, Ross C, Baer A. Migratory fishes of South America: Biology, Fisheries and Conservation Status World Fisheries Trust. Washington: IDRC, World Bank; 2003.; Pelicice et al., 2015Pelicice FM, Pompeu PS, Agostinho AA. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish Fish [serial on the Internet]. 2015; 16(4):697-715. Available from: http://dx.doi.org/10.1111/faf.12089
http://dx.doi.org/10.1111/faf.12089...
), especially in view of the small size of migrants (Barthem et al., 2014Barthem R, Costa MC, Cassemiro F, Leite RG, Silva N Jr. Diversity and Abundance of Fish Larvae Drifting in the Madeira River, Amazon Basin: Sampling Methods Comparison. In: Grillo O, editor. Biodiversity - The Dynamic Balance of the Planet. Croatica: Intech; 2014. p.137-158.; Cella-Ribeiro et al., 2015Cella-Ribeiro A, Assakawa LF, Torrente-Vilara G, Zuanon J, Leite RG, Doria C, Duponchelle F. Temporal and spatial distribution of young Brachyplatystoma spp (Siluriformes: Pimelodidae) along the rapids stretch of the Madeira River (Brazil) before the construction of two hydroelectric dams. J Fish Biol [serial on the Internet]. 2015; 86(4):1429-37. Available from: http://dx.doi.org/10.1111/jfb.12630
http://dx.doi.org/10.1111/jfb.12630...
; Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
).
This study is the first to describe the growth patterns of B. rousseauxii’s in the Madeira River. It provides important new information about the life-history characteristics of this species and contributes to a better understanding of its complex life cycle. It will serve as a base line for monitoring the development of B. rousseauxii’s life-history dynamics in the upper Madeira basin after the dams. These results have profound consequences for the fisheries management, especially considering the current and planned hydropower development scenario in the Amazon basin. As already warned by Duponchelle et al. (2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
http://dx.doi.org/10.1111/1365-2664.1266...
) and re-emphasized here, the Madeira dams threaten the life cycle of this flagship top predator species, which may cause deleterious cascading effects through the Amazonian aquatic food webs.
Acknowledgments
This study was financed by Santo Antônio Energia (SAE) partnership with the Universidade Federal de Rondônia (UNIR) and Instituto de Estudos e Pesquisas Agroambientais e Organizações Sustentáveis (IEPAGRO). We thank M. A. L. Lima and S. T. Brazil for providing otoliths from Brasil. M. Hauser received a scholarship from CAPES (Proc. Nº 1402376, Pro-Amazon Program: Biodiversity and Sustainability 047/2012) and CNPq (Proc. Nº 204344/2015-8). G. Torrente-Vilara received a grant from Foundation of Support to Research of the Amazon (PAREV/FAPEAM 019/2010), CAPES (Pro-Amazon Program: Biodiversity and Sustainability, process 6632/14-9), and FAPESP (São Paulo Research Foundation #2016/07910-0). The authors declare that have no conflict of interest.
References
- Agostinho AA, Gomes LC, Pelicice FM. Ecologia e Manejo de Recursos Pesqueiros em Reservatórios do Brasil. Maringá: Eduem; 2007.
- Agostinho AA, Pelicice FM, Gomes LC. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol [serial on the Internet]. 2008; 68(4):1119-32. Available from: http://dx.doi.org/10.1590/S1519-69842008000500019
» http://dx.doi.org/10.1590/S1519-69842008000500019 - Alonso JC. Padrão espaço-temporal da estrutura populacional e estado atual da exploração pesqueira da dourada Brachyplatystoma flavicans, Castelnau, 1855 (Siluriformes: Pimelodidae), no sistema estuário-Amazonas-Solimões. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2002.
- Angelini R, Fabré NN, Silva-JR UL. Trophic analysis and fishing simulation of the biggest Amazonian catfish. Afr J Agric Res. 2006; 1(5):151-58.
- Arantes CC, Castello L, Stewart DJ, Cetra M, Queiroz HL. Population density, growth and reproduction of Arapaima in an Amazonian river-floodplain. Ecol Fresh Fish [serial on the Internet]. 2010; 19(3):455-65. Available from: http://dx.doi.org/10.1111/j.1600-0633.2010.00431.x
- Baras E, Lucas MC. Impacts of man’s modifications of river hydrology on the migration of freshwater fishes: a mechanistic perspective. Ecohydrol Hydrobiol. 2001; 1(3):291-304.
- Barthem R, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. New York, NY: Columbia University Press; 1997.
- Barthem R, Costa MC, Cassemiro F, Leite RG, Silva N Jr. Diversity and Abundance of Fish Larvae Drifting in the Madeira River, Amazon Basin: Sampling Methods Comparison. In: Grillo O, editor. Biodiversity - The Dynamic Balance of the Planet. Croatica: Intech; 2014. p.137-158.
- Barthem RB, Goulding M, Leite RG, Cañas C, Forsberg B, Venticinque E, Petry P, Ribeiro MLB, Chuctaya J, Mercado A. Goliath catfish spawning in the far western Amazon confirmed by the distribution of mature adults, drifting larvae and migrating juveniles. Sci Rep [serial on the Internet]. 2017; 7:41784. Available from: https://www.nature.com/articles/srep41784.pdf DOI: 101038/srep41784
» https://www.nature.com/articles/srep41784.pdf - Bauer S, Hoye BJ. Migratory animals cou-ple biodiversity and ecosystem functioning worldwide. Science [serial on the Internet]. 2014; 344(6179):1242552. Available from: http://dx.doi.org/10.1126/science.1242552
» http://dx.doi.org/10.1126/science.1242552 - Boeuf G, Payan P. How should salinity influence fish growth? Comp Biochem Physiol Part C [serial on the Internet]. 2001; 130(4):411-23. Available from: https://doi.org/10.1016/S1532-0456(01)00268-X
» https://doi.org/10.1016/S1532-0456(01)00268-X - Campana SE. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol [serial on the Internet]. 2001; 59(2):197-242. Available from: http://dx.doi.org/10.1111/j.1095-8649.2001.tb00127.x
» http://dx.doi.org/10.1111/j.1095-8649.2001.tb00127.x - Campos CP, Freitas CEC, Amadio S. Growth of the Cichla temensis Humboldt, 1821 (Perciformes: Cichlidae) from the middle rio Negro, Amazonas, Brazil. Neotrop Ichthyol [serial on the Internet]. 2015; 13(2):413-20. Available from: http://dx.doi.org/10.1590/1982-0224-20140090
» http://dx.doi.org/10.1590/1982-0224-20140090 - Córdoba EA, Joven-León AV, Bonilla-Castillo CA, Petrere M Jr, Peláez M, Duponchelle F. Breeding, growth and exploitation of Brachyplatystoma rousseauxii in the Caqueta River, Colombia. Neotrop Ichthyol [serial on the Internet]. 2013; 11(3):637-47. Available from: http://dx.doi.org/10.1590/S1679-62252013000300017
» http://dx.doi.org/10.1590/S1679-62252013000300017 - Carolsfeld J, Harvey B, Ross C, Baer A. Migratory fishes of South America: Biology, Fisheries and Conservation Status World Fisheries Trust. Washington: IDRC, World Bank; 2003.
- Carvajal-Vallejos FM, Duponchelle F, Desmarais E, Cerqueira F, Querouil S, Nuñez J, García C, Renno JF. Genetic structure in the Amazonian catfish Brachyplatystoma rousseauxii: influence of life history strategies. Genetica [serial on the Internet]. 2014; 142(4):323-36. Available from: http://dx.doi.org/10.1007/s10709-014-9777-2
» http://dx.doi.org/10.1007/s10709-014-9777-2 - Carvalho FM. Alimentação de Mapará (Hypophthalmus edentatus Spix 1829) do Lago Castanho, Amazonas (Siluriformes Hypophthalmidae). Acta Amazonica. 1980; 10(3):545-55.
- Castello L, Macedo MN. Large-scale degradation of Amazonian freshwater ecosystems. Glob Change Biol [serial on the Internet]. 2016; 22(3):990-1007. Available from: http://dx.doi.org/10.1111/gcb.13173
» http://dx.doi.org/10.1111/gcb.13173 - Castello L, McGrath DG, Hess LL, Coe MT, Lefebvrel PA, Petry P, Macedo MN, Renó VF, Arantes CC. The vulnerability of Amazon freshwater ecosystems. Conserv Lett. 2013; 6(4):217-29.
- Cella-Ribeiro A, Assakawa LF, Torrente-Vilara G, Zuanon J, Leite RG, Doria C, Duponchelle F. Temporal and spatial distribution of young Brachyplatystoma spp (Siluriformes: Pimelodidae) along the rapids stretch of the Madeira River (Brazil) before the construction of two hydroelectric dams. J Fish Biol [serial on the Internet]. 2015; 86(4):1429-37. Available from: http://dx.doi.org/10.1111/jfb.12630
» http://dx.doi.org/10.1111/jfb.12630 - Cella-Ribeiro A, Torrente-Vilara G, Lima-Filho JA, Doria CRC. Ecologia e Biologia de Peixes do Rio Madeira. Porto Velho: EDUFRO; 2016.
- Crema LC, Quaresma AC, Silva VMF. Nem tudo que nada é peixe: os mamíferos aquáticos amazônicos. Recursos Hídricos y Ecosistemas Acuáticos. In: Lopes A, Piedade MTF, editors. Conhecendo as áreas úmidas amazônicas: uma viagem pelas várzeas e igapós. Manus: INPA Editora; 2014. p.85-94.
- Cutrim L, Batista VS. Determinação de idade e crescimento do mapará (Hypophthalmus marginatus) na Amazônia Central. Acta Amaz [serial on the Internet]. 2005; 35(1):85-92. Available from: http://dx.doi.org/10.1590/S0044-59672005000100013
» http://dx.doi.org/10.1590/S0044-59672005000100013 - Doria CRC, Lima MAL. Rio Madeira: seus peixes e sua pesca. Porto Velho: EDUFRO/RIMa Editora; 2015.
- Duponchelle F, Lino F, Hubert N, Panfili J, Renno JF, Baras E, Torrico JP, Dugué R, Nuñez J. Environment-related life history trait variations of the red-bellied piranha, Pygocentrus nattereri, in two river basins of the Bolivian Amazon. J Fish Biol [serial on the Internet]. 2007; 71(4):1113-34. Available from: http://dx.doi.org/10.1111/j.1095-8649.2007.01583.x
» http://dx.doi.org/10.1111/j.1095-8649.2007.01583.x - Duponchelle F, Arce AR, Waty A, Panfili J, Renno JF, Farfan F, García-Vásquez A, Koo FC, Davila CG, Vargas G, Ortiz A, Pinedo R, Nuñez J. Contrasted hydrological systems of the Peruvian Amazon induce differences in growth patterns of the silver arowana, Osteoglossum bicirrhosum Aquat Living Resour [serial on the Internet]. 2012; 25(1):55-66. Available from: https://doi.org/10.1051/alr/2012005
» https://doi.org/10.1051/alr/2012005 - Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno JF, Panfili J et al Trans-Amazonian natal homing in giant catfish. J Appl Ecol [serial on the Internet]. 2016; 53(5):1511-20. Available from: http://dx.doi.org/10.1111/1365-2664.12665
» http://dx.doi.org/10.1111/1365-2664.12665 - Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ et al Trophic downgrading of planet earth. Science [serial on the Internet]. 2011; 333(6040):301-06. http://dx.doi.org/10.1126/science.1205106
» http://dx.doi.org/10.1126/science.1205106 - Food and Agriculture Organization of the United Nations (FAO/Noruega), Comisíon de pesca continental para a América Latina (COPESCAL). Informe del taller regional sobre el manejo de las pesquerías de bagres migratórios del Amazonas. Roma: FAO; 2000.
- Fabré NN, Saint-Paul U. Annulus formation on scales and seasonal growth of the Central Amazonian anostomid Schizodon fasciatus J Fish Biol . 1998; 53(1):1-11.
- Fearnside PM. Brazil’s Madeira River dams: A setback for environmental policy in Amazonian development. Water Alternatives. 2014; 7(1):256-69.
- Fearnside PM. Hidrelétricas na Amazônia: impactos ambientais e sociais na tomada de decisões sobre grandes obras. Manaus: Editora do INPA; 2015.
- Finer M, Jenkins CN. Proliferation of hydroelectric dams in the Andean Amazon and implications for Andes-Amazon connectivity. PLoS ONE [serial on the Internet]. 2012; 7(4):e35126. Available from: http://dx.doi:10.1371/journal.pone.0035126
» http://dx.doi:10.1371/journal.pone.0035126 - Fretwell DS. Food chain dynamics: the central theory of ecology? Oikos [serial on the Internet]. 1987; 50(3):291-301. Available from: http://www.jstor.org/stable/3565489
» http://www.jstor.org/stable/3565489 - Fuzzen MLM, Bernier NJ, Kraak GVD. Stress and reproduction. In: Norris DO, Lopez KH, editors. Hormones and reproduction of vertebrates, vol 1. Academic Press; 2011. p.103-118.
- García-Vásquez A, Alonso JC, Carvajal F, Moreau J, Nuñez J, Renno JF, Tello S, Montreuil V, Duponchelle F. Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J Fish Biol [serial on the Internet]. 2009; 75(10):2527-51. Available from: http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x
» http://dx.doi.org/10.1111/j.1095-8649.2009.02444.x - Gonzalez JCA, Garcia KAC, Núñez-Avellaneda M, Córdova EA, Galarza E, Oliveros LA, Natagani K. Recursos Hídricos y Ecosistemas Acuáticos. In: Perspectivas del Medio Ambiente en la Amazonía. Lima: PNUMA/OTCA/CIUP; 2009. p.146-161.
- Goulding M. Ecologia da pesca do rio Madeira. Manaus: CNPq/INPA; 1979.
- Goulding M. Amazon: The Flooded Forest. New York: Sterling Publishing; 1990.
- Haimovici M, Reis EG. Determinação de idade e crescimento da castanha Umbrina canosai (Pisces, Sciaeinidae) do Sul do Brasil. Atlântica. 1984; 7:25-46.
- Hamilton SK, Sippel SJ, Melack JM. Seasonal inundation patterns in two large savanna floodplains of South America: the Llanos de Moxos (Bolivia) and the Llanos del Orinoco (Venezuela and Colombia). Hydrol Process [serial on the Internet]. 2004; 18(11):2103-16. Available from: http://dx.doi.org/10.1002/hyp.5559
» http://dx.doi.org/10.1002/hyp.5559 - He F, Zarfl C, Bremerich V, Henshaw A, Darwall W, Tockner K, Jähnig SC. Disappearing giants: a review of threats to freshwater megafauna. WIREs Water [serial on the Internet]. 2017; 4:e1208. Available from: http://dx.doi:10.1002/wat2.1208
» http://dx.doi:10.1002/wat2.1208 - Hegg JC, Giarrizzo T, Kennedy BP. Diverse early life-history strategies in migratory Amazonian catfish: implications for conservation and management. PLoS ONE [serial on the Internet]. 2015; 10(7):e0129697. Available from: https://doi.org/10.1371/journal.pone.0129697
» https://doi.org/10.1371/journal.pone.0129697 - Hermann TW, Stewart DJ, Limburg KE, Castello L. Unravelling the life history of Amazonian fishes through otolith microchemistry. Royal Soc Open Sci [serial on the Internet]. 2016; 3(6):160206 DOI: 101098/rsos160206.
» https://doi.org/101098/rsos160206 - Hutchinson CE, TenBrink TT. Age determination of the Yellow Irish Lord: management implications as a result of new estimates of maximum age North American. North Am J Fish Mana [seril on the Internet]. 2011; 31(6):1116-22. Available from: https://doi.org/10.1080/02755947.2011.646453
» https://doi.org/10.1080/02755947.2011.646453 - Junk WJ, Bayley PB, Sparks RE. The flood pulse concept in river-floodplain-systems. In: Canada: Proceedings of International Large River Symposium. 1989. Can Spec Publ Fish Aquat Sci. (Canadian Special Publication of Fisheries and Aquatic Science s; No. 106:110-127).
- Junk WJ. The Central Amazon floodplains Ecology of pulsing system. Berlin: Springer; 1997.
- Kimura DK. Likelihood methods for the von Bertalanffy growth curve. Fish Bull. 1980; 77(4):765-76.
- Latrubesse EM, Stevaux JC, Sinha R. Tropical rivers. Geomorph [serial on the Internet]. 2005; 70(3):187-206. Available from: https://doi.org/10.1016/j.geomorph.2005.02.005
» https://doi.org/10.1016/j.geomorph.2005.02.005 - Latrubesse EM, Arima EY, Dunne T, Park E, Baker VR, d’Horta FM, Wight C, Wittmann F, Zuanon J, Baker PA et al Damming the rivers of the Amazon basin. Nature. 2017; 546(7658):363-69.
- Lees AC, Peres CA, Fearnside PM, Schneider M, Zuanon JAS. Hydropower and the future of Amazonian biodiversity. Biodiversity Conserv. 2016; 25(3):451-66.
- Loubens G, Panfili J. Estimation de l’âge individuel de Prochilodus nigricans (Teleostei: Prochilodidae) dans le Béni (Bolivie): protocole d’étude et application. Aquat Living Resour .1992; 5(1):41-56.
- Loubens G, Panfili J. Biologie de Colossoma macropomum (Teleostei: Serrasalmidae) dans le bassin du Mamoré (Amazonie bolivienne). Ichthyol Explor Fresh. 1997; 8(1):1-22.
- Loubens G, Panfili J. Biologie de Pseudoplatystoma fasciatum et P. tigrinum (Teleostei: Pimelodidae) dans le bassin du Mamoré. Ichthyol Explor Fresh . 2000; 11(1):13-34.
- Loubens G, Panfili J. Biologie de Piaractus brachypomus (Teleostei: Serrasalmidae) dans le bassin du Mamoré (Amazonie bolivienne). Ichthyol Explor Fresh . 2001; 12(1):51-64.
- Loubens G. Biologie de Plagioscion squamosissimus (Teleostei: Scianidae) dans le bassin du Mamoré (Amazonie bolivienne). Ichthyol Explor Fresh . 2003; 14(4):335-52.
- Lowe-McConnel RH. Estudos ecológicos de comunidades de peixes tropicais: introdução e diversidade: sua manutenção e evolução. São Paulo: EDUSP; 1999.
- Lupo AR, Nocera JJ, Bosart LF, Hoffman EG, Knight DJ. South American Cold Surges: Types, Composites, and Case Studies. Mon Weather Rev [serial on the Internet]. 2001; 129:1021-41. Available from: https://doi.org/10.1175/1520-0493(2001)129<1021:SACSTC>2.0.CO;2
» https://doi.org/10.1175/1520-0493(2001)129<1021:SACSTC>2.0.CO;2 - Mazur CF, Tillapaugh D, Brett JR, Iwama GK. The effect of feeding level and rearing density on growth Feed conversion and survival in Chinook salmon (Oncorhynchus tshawytscha) reared in salt water. Aquaculture [serial on the Internet]. 1993; 117(1):129-40. Available from: https://doi.org/10.1016/0044-8486(93)90129-M
» https://doi.org/10.1016/0044-8486(93)90129-M - Odum EP. Fundamentos de ecologia. 4th ed. Lisboa: Fundação Calouste Gulbenkian; 1998.
- Oliveira MIB. Determinação de idade e aspectos da dinâmica populacional do curimatã Prochilodus nigricans (Pisces: Prochilodontidae) da Amazônia Central. [Master Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 1996.
- Pelicice FM, Pompeu PS, Agostinho AA. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish Fish [serial on the Internet]. 2015; 16(4):697-715. Available from: http://dx.doi.org/10.1111/faf.12089
» http://dx.doi.org/10.1111/faf.12089 - Paine RT. Food Web Complexity and Species Diversity. Am. Nat. 1966; 100(910):65-75.
- Paine RT. Food Webs: Linkage, Interaction Strength and Community Insfrasctruture. J Animal Ecol.1980; 49(3):666-85.
- Pérez A, Fabré NN. Seasonal growth and life history of the catfish Calophysus macropterus (Lichtenstein, 1819) (Siluriformes: Pimelodidae) from the Amazon floodplain. J Appl Ichthyol [serial on the Internet]. 2009; 25(3):343-49. Available from: http://dx.doi.org/10.1111/j.1439-0426.2008.01104.x
» http://dx.doi.org/10.1111/j.1439-0426.2008.01104.x - Puffer M, Berg OK, Huusko A, Vehanen T, Einum S. Effects of intra- and interspecific competition and hydropeaking on growth of juvenile Atlantic salmon (Salmo salar). Ecol Freshw Fish [serial on the Internet]. 2015; 26(1):99-107. Available from: http://dx.doi.org/10.1111/eff.12258
» http://dx.doi.org/10.1111/eff.12258 - Rochet MJ, Trenkel VM. Which community indicators can measure the impact of fishing? A review and proposals. Can J Fish Aquat Sci [serial on the Internet]. 2003; 60(1):86-99. Available from: https://doi.org/10.1139/f02-164
» https://doi.org/10.1139/f02-164 - Ronchail J. Advections polaires en Bolivie: mise en évidence et caractérisation des effets climatiques. Hydrol Continent. 1989; 4(1):49-56.
- Santos GM, Ferreira EJG, Zuanon JAS. Peixes Comerciais de Manaus. Manaus: Ibama/ProVárzea; 2006.
- Silva EA, Stewart DJ. Age structure, growth and survival rates of the commercial fish Prochilodus nigricans (bocachico) in North-eastern Ecuador. Environ Biol Fish [serial on the Internet]. 2006; 77(1):63-77. Available from: http://dx.doi.org/10.1007/s10641-006-055-y
» http://dx.doi.org/10.1007/s10641-006-055-y - Silva V, Goulding M, Barthem R. Golfinhos da Amazônia. Manaus: Editora INPA; 2008.
- Taniguchi Y, Nakano S. Condition-Specific competition: Implications for the altitudinal distribution of stream fishes. Ecology [serial on the Internet]. 2000; 81(7):2027-39. Available from: http://dx.doi.org/10.1890/0012-9658(2000)081[2027:CSCIFT]2.0.CO;2
» http://dx.doi.org/10.1890/0012-9658(2000)081[2027:CSCIFT]2.0.CO;2 - Tomassone R, Dervin C, Masson JP. Biométrie: Modélisation de Phénomènes biologiques. Paris: Masson; 1993.
- Val AL, Almeida-Val VMF, Randal DJ. Fish Physiology: The Physiology of Tropical Fishes. Academic Press; 2005.
- Van Damme PA, Carvajal-Vallejos FM, Molina-Carpio J. Los peces y delfines de la Amazonía boliviana: hábitats, potencialidades y amenazas. Cochabamba: INIA; 2011.
- Vieira EF. Determinação da idade e crescimento do jaraqui-de-escamagrossa (Semaprochilodus insignis) na Amazônia. [Master Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 1999.
- Villacorta-Correa MA. Estudo de idade e crescimento do Tambaqui Colossoma macropomum (Characiformes: Characidae) no Amazonas central, pela analise de marcas sazonais nas estructuras mineralizadas e microestructuras nos otolitos. [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 1997.
- Vismara MR, Benedito-Cecilio E, Faria ACEA. Efeito da maturação gonadal sobre o conteúdo calórico e condição geral de peixes da planície de inundação do alto rio Paraná. Acta Sci Biol Sci [serial on the Internet]. 2004; 26(2):189-99. Available from: http://dx.doi.org/10.4025/actascibiolsci.v26i2.1635
» http://dx.doi.org/10.4025/actascibiolsci.v26i2.1635 - Vøllestad LA, Olsen EM, Forseth T. Growth-rate variation in brown trout in small neighbouring streams: evidence for density-dependence? J Fish Biol [serial on the Internet]. 2002; 61(6):1513-27. Available from: http://dx.doi.org/10.1111/j.1095-8649.2002.tb02494.x
» http://dx.doi.org/10.1111/j.1095-8649.2002.tb02494.x - Winemiller KO, McIntyre PB, Castello L, Fluet- Chouinard E, Giarrizzo T, Nam S et al Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science . 2016; 351(6269):128-29.
Publication Dates
-
Publication in this collection
2018
History
-
Received
17 Oct 2017 -
Accepted
07 Feb 2018